Abstract
Hypoglycemia is associated with increased cardiovascular mortality in trials of intensive therapy in type 2 diabetes mellitus (T2DM). We previously observed an increase in arrhythmias during spontaneous prolonged hypoglycemia in patients with T2DM. We examined changes in cardiac autonomic function and repolarization during sustained experimental hypoglycemia. Twelve adults with T2DM and 11 age- and BMI-matched control participants without diabetes underwent paired hyperinsulinemic clamps separated by 4 weeks. Glucose was maintained at euglycemia (6.0 mmol/L) or hypoglycemia (2.5 mmol/L) for 1 h. Heart rate, blood pressure, and heart rate variability were assessed every 30 min and corrected QT intervals and T-wave morphology every 60 min. Heart rate initially increased in participants with T2DM but then fell toward baseline despite maintained hypoglycemia at 1 h accompanied by reactivation of vagal tone. In control participants, vagal tone remained depressed during sustained hypoglycemia. Participants with T2DM exhibited greater heterogeneity of repolarization during hypoglycemia as demonstrated by T-wave symmetry and principal component analysis ratio compared with control participants. Epinephrine levels during hypoglycemia were similar between groups. Cardiac autonomic regulation during hypoglycemia appears to be time dependent. Individuals with T2DM demonstrate greater repolarization abnormalities for a given hypoglycemic stimulus despite comparable sympathoadrenal responses. These mechanisms could contribute to arrhythmias during clinical hypoglycemic episodes.
Introduction
Two large trials have shown that intensive glycemic control does not reduce cardiovascular death in patients with type 2 diabetes mellitus (T2DM), a population at high cardiovascular risk (1,2), and in one trial was associated with increased mortality (3). Severe hypoglycemia was increased several fold in the intensive control arm of all three trials and was a strong independent predictor of mortality in post hoc analyses (4). Hypoglycemia is a plausible explanation for the observed excess mortality, but evidence for a direct mechanistic link remains unclear. Studies of insulin therapy in T2DM have shown an increased risk of fatal arrhythmic death associated with severe hypoglycemia (5). One mechanism by which hypoglycemia could promote arrhythmias is through changes in cardiac autonomic activity and repolarization.
In a study examining electrocardiogram (ECG) responses to spontaneous hypoglycemia in patients with T2DM using ambulatory glucose and Holter monitoring (6), we observed an increase in bradycardia and atrial ectopic activity during hypoglycemia compared with euglycemia. During nocturnal hypoglycemic episodes when glucose was generally lower and episodes more prolonged, we observed a phased response whereby initial increases in heart rate were followed by a relative bradycardia. We speculated that diurnal differences in the depth and duration of hypoglycemia lead to differential sympathetic and parasympathetic stimulation that vary over time. However, in spontaneous clinical episodes, neither the depth nor the duration can be controlled, and the measurement of circulating catecholamines or electrolytes, which are relevant to these responses, is not possible.
In the same study, we observed a higher rate of ventricular ectopic activity during hypoglycemia than during euglycemia (6); other studies have also reported higher rates of ventricular tachyarrhythmias during spontaneous hypoglycemic episodes (7). This higher rate could be related to abnormal cardiac repolarization, which we have previously demonstrated during experimental and spontaneous hypoglycemia (8). Individuals with T2DM (with varying degrees of autonomic dysfunction) exhibit longer baseline QT intervals than those without diabetes, which is associated with increased mortality (9). In our ambulatory study, QT prolongation was in excess of 500 ms, with gross morphological changes in T waves in some individuals (6).
The primary aim of this study was to examine changes in cardiac autonomic regulation and repolarization during controlled sustained experimental hypoglycemia as a potential mechanism to provoke cardiac arrhythmias. We hypothesized that phasic changes in cardiac autonomic response occur during sustained hypoglycemia. Responses in patients with T2DM were compared against control participants without diabetes.
Research Design and Methods
Participants
Twelve patients with T2DM and no known cardiovascular disease were recruited from Sheffield Teaching Hospitals outpatient diabetes clinics. Patients had been prescribed one or more oral hypoglycemic agents and/or glucagon-like peptide 1 analog or insulin for <2 years. Eleven age- and BMI-matched individuals without diabetes were recruited from University of Sheffield and Sheffield Teaching Hospitals staff. These control participants had a fasting plasma glucose of <7 mmol/L and HbA1c of <6.5% (<48 mmol/mol) as measured by ion exchange high-performance liquid chromatography. Participants taking β-blockers or QT-prolonging medications were excluded. Written informed consent was obtained from all participants, and the study received local ethics committee approval (National Research Ethics Service [NRES], Leeds, U.K.).
Baseline Assessment
Cardiovascular autonomic reflex tests were performed as previously described (10) by following a consensus statement (11). All patients were euglycemic at the time of autonomic function testing. Those with two or more abnormal cardiovagal tests were regarded as having definite cardiac autonomic neuropathy and were excluded. Spontaneous cardiovagal baroreceptor sensitivity (BRS) was obtained by using a Portapres (Finapres Medical Systems, Amsterdam, the Netherlands). BRS analysis was based on the sequence method and performed with the use of dedicated software (Nevrokard version 5.1.3; Nevrokard Kiauta, Izola, Slovenia) (12). All participants had a normal 12-lead ECG at baseline.
Hyperinsulinemic Clamp Protocol
All subjects participated in paired hyperinsulinemic-euglycemic and hypoglycemic studies separated by at least 4 weeks. Participants were fasted from midnight and instructed to avoid caffeine and vigorous exercise 24 h before the clamps. None of the participants experienced symptomatic hypoglycemia or capillary blood glucose <3.0 mmol/L in the previous 24 h.
Participants attended the clinical research facility at 8:00 a.m. after an overnight fast. In the patients with T2DM, blood glucose was initially stabilized between 6 and 7 mmol/L by using a variable low-dose intravenous insulin infusion. An intravenous cannula was inserted into the antecubital fossa of the nondominant arm for insulin and dextrose infusion. A retrograde cannula was inserted in the nondominant hand placed in a warming chamber at 55°C for blood glucose and catecholamine sampling. During euglycemic and hypoglycemic clamps in both groups, a primed continuous intravenous insulin infusion was administered at 120 mU/m2/min along with 20% dextrose at a variable rate, adjusted according to blood glucose concentrations every 5 min. Arterialized whole-blood glucose was measured in duplicate by using a glucose oxidase method (YSI 2300 STAT; Yellow Springs Instruments, Yellow Springs, OH). During the hypoglycemic clamp, glucose was lowered from euglycemia to 2.5 mmol/L over 60 min (T0–T60); thereafter, it was maintained at 2.5 mmol/L for a further 60 min (T60–T120). During the euglycemic clamp, arterialized whole-blood glucose was maintained at 6 mmol/L for the duration of the study (120 min). Participants were blinded to blood glucose values.
Heart Rate Variability and Blood Pressure
ECG signals were obtained by using a three-lead ECG monitor (Ivy Cardiac 3000 Trigger Monitor; Ivy Biomedical Systems, Branford, CT), digitized at a sampling frequency of 200 Hz, and recorded with the use of WR-TestWorks software version 2.4.0 (WR Medical Electronics). ECG recordings for heart rate variability (HRV) were performed at 30-min intervals during the clamp study (baseline, T30, T60, T90, T120). HRV was determined from 5-min resting recordings with the participant lying in supine and free breathing. Manual editing of R-R intervals was performed along with visual inspection of QRS complexes to exclude ectopic beats and artifacts. Normal R-R intervals were extracted in the time domain, and frequency domain HRV analysis was performed in accordance with published recommendations (13). The fast Fourier transform algorithm was applied to 5-min segments of R-R intervals for frequency domain analysis, and the power of HRV was calculated within the low frequency (LF) (0.04–0.15 Hz) and high frequency (HF) bands (0.15–0.4 Hz) (13). The power in the HF band reflects parasympathetic activity. The ratio between the LF power and total power (normalized LF power [LFnorm] = LF power + HF power) was calculated and indicates the level of sympathetic modulation in HRV (14,15).
Blood pressure was measured every 30 min by using an automated oscillometric sphygmomanometer (DINAMAP; GE Healthcare) after at least 5 min with the participant lying in supine. Pulse pressure was calculated as systolic blood pressure (SBP) minus diastolic blood pressure (DBP).
Cardiac Repolarization
To assess cardiac repolarization, high-resolution 12-lead ECGs were recorded for 5 min at the onset (T60) and end of hypoglycemia or euglycemia (T120) in a Mason-Likar configuration (16) with the participant lying supine. Signals were sampled at 1,200 Hz and amplified by using a g.USBamp amplifier and recorded with g.Recorder software (g.tec Medical Engineering, Schiedlberg, Austria). Preprocessing and data analysis were performed with custom-built software in MATLAB (MathWorks). The ECG signals were bandpass filtered between 0.2 and 40 Hz. Beat averaging was then performed on 5-min segments by using template matching to improve the signal-to-noise ratio. The repolarization analysis was based on a composite wave calculated from averaged beats from leads I, II, and V5 (17).
Measurement of the QT interval (i.e., from Q onset to T end) was based on the tangent method, and Bazett correction for heart rate was applied. Because the Bazett correction may overcorrect at higher heart rates, the QT interval was also corrected according to the nomogram method (18), which has been validated for heart rates between 40 and 120 beats/min (bpm). In addition to the QT interval, which represents duration of repolarization, conventional measures of T-wave morphology (T-wave symmetry and T-wave amplitude) were calculated based on the composite waveform as a measure of heterogeneity of repolarization. T-wave symmetry was defined as the area under the T wave from T onset to T peak divided by the T-wave area between T peak and T end (19). The median T-wave symmetry in normal individuals is 1.5, and for T symmetry, close to 1.0 is abnormal and associated with an increasing risk for arrhythmias (20). The normalized T-wave amplitude was calculated as the ratio of the T-wave amplitude during clamp at each time point relative to the T-wave amplitude at baseline.
Additional measures of T-wave morphology were calculated by using principal component analysis (PCA) and derived from averaged beats from eight ECG leads (I, II, and V1–V6) as previously described (21). These measures provide more-complex information on cardiac repolarization that is unaffected by heart rate. The PCA ratio was calculated as the height and width of the T-wave loop. An increased PCA ratio indicates a fatter T-wave loop and a more complex T-wave morphology, which is predictive of all-cause and cardiovascular mortality in the general population (22,23) after myocardial infarction (24,25) and in patients with diabetes (26). The T-wave loop dispersion (TWLD) represents the length of the loop and describes the temporal variation of interlead relationships during cardiac repolarization. The total cosine R-to-T (TCRT) was calculated as the global angle between the main QRS complex and T-wave vector and describes the difference between the depolarization and repolarization wavefronts. Decreased TWLD and TCRT have been shown to be predictive of cardiac death and associated with arrhythmias after myocardial infarction (24,25). Recordings were made at baseline, T60, and T120. Analysis of recordings was blinded to the glucose concentration.
Biochemical Analysis
For measurement of catecholamines, 6 mL of whole blood was collected into chilled lithium heparin tubes containing 50 μL EGTA/glutathione preservative and centrifuged at 3,000 rpm at 4°C for 10 min. The resulting supernatant was stored at −80°C until assayed by high-performance liquid chromatography. Plasma-free insulin was analyzed by an immunometric assay (Invitron Insulin ELISA; Invitron, Monmouth, U.K.) after precipitation with polyethylene glycol. Serum potassium was analyzed with an automated system (Roche cobas; Roche Diagnostics, Burgess Hill, U.K.) by using the direct ion selective electrode method. Biochemical parameters were measured at baseline and T120 during all clamps.
Statistical Analysis
Data that followed an approximate normal distribution were summarized as mean ± SE unless otherwise stated. Skewed data were summarized as median (interquartile range [IQR]).
Within each participant group, autonomic and repolarization parameters were analyzed by two-way repeated-measures ANOVA, where both time and glycemic arm were specified as repeated factors. The Greenhouse-Geisser correction was applied where sphericity was violated. Planned contrasts were made versus baseline and between euglycemia and hypoglycemia at equivalent time points with Šidák correction for multiple comparisons. To compare changes in repolarization in participants with versus those without diabetes, two-way repeated-measures ANOVA was performed with glycemic arm as a repeated factor. Planned contrasts were made for the effect of group and glycemic arm with Šidák correction for multiple comparisons. Catecholamines, glucose, and potassium at T120 were compared under euglycemic and hypoglycemic conditions by using a two-way ANOVA with planned contrasts for the effect of the group (with vs. without diabetes) and glycemic condition, with Šidák correction for multiple comparisons. A nonparametric Kruskal-Wallis test was used to compare free insulin levels at T120 under euglycemia versus hypoglycemia and between groups. Missing data were dealt with using casewise deletion. Analysis was performed with SPSS version 20.0 (IBM Corporation, Chicago, IL) and GraphPad Prism version 6.04 (GraphPad Software) statistical software. P < 0.05 was deemed statistically significant.
Results
Participant Characteristics
Participant characteristics are shown in Table 1. Patients with T2DM were similar in age and BMI to the control participants without diabetes. Five patients were taking oral hypoglycemic agents only, five were taking a combination of oral hypoglycemic agents and a glucagon-like peptide 1 analog, and two had been taking an oral hypoglycemic agent and basal insulin for <2 years. Two patients were taking ACE inhibitors and remained on them throughout the study. The patients tended to have higher baseline heart rates, blood pressure, and lower HRV and BRS than the control participants. Parameters of T-wave repolarization, including T-wave amplitude, TWLD, PCA ratio, and TCRT, tended to be lower in the patients at baseline than in the control participants; however, other measures, including corrected QT interval (QTc) and T-wave symmetry, were similar.
Table 1.
Participant characteristics
| No T2DM (n = 11) | T2DM (n = 12) | |
|---|---|---|
| Age (years) | 52 (34–63) | 53.5 (37–64) |
| Sex, n | ||
| Male | 5 | 9 |
| Female | 6 | 3 |
| BMI (kg/m2) | 31 ± 2 | 34 ± 1 |
| Duration of diabetes (years) | NA | 11 ± 2 |
| HbA1c | ||
| % | 5.5 ± 0.3 | 7.8 ± 0.4 |
| mmol/mol | 34 ± 3 | 62 ± 4 |
| SBP (mmHg) | 131 ± 5 | 135 ± 4 |
| DBP (mmHg) | 72 ± 3 | 82 ± 3 |
| HR (bpm) | 64 ± 2 | 78 ± 2 |
| Repolarization | ||
| QT (ms) | 406 ± 9 | 381 ± 8 |
| QTcB (ms) | 412 ± 8 | 417 ± 6 |
| QTcN (ms) | 410 ± 7 | 406 ± 4 |
| T-wave amplitude (μV) | 466 ± 84 | 399 ± 35 |
| T-wave symmetry | 1.44 ± 0.06 | 1.55 ± 0.06 |
| TWLD | 1,415 ± 257 | 1,279 ± 75 |
| TCRT | 0.78 ± 0.06 | 0.69 ± 0.08 |
| PCA ratio | 0.10 ± 0.03 | 0.08 ± 0.02 |
| HRV | ||
| SDNN (ms) | 48.2 ± 4.4 | 27.4 ± 3.4 |
| RMSSD (ms) | 32.9 ± 4.6 | 15.5 ± 7.94 |
| Log total power (ms2) | 2.90 ± 0.11 | 2.34 ± 0.10 |
| Log HF (ms2) | 2.32 ± 0.14 | 1.85 ± 0.11 |
| LFnorm | 0.70 ± 0.04 | 0.65 ± 0.04 |
| BRS (ms/mmHg) | 11.51 ± 2.31 | 8.59 ± 1.18 |
Data are mean ± SE or median (IQR) unless otherwise indicated. NA, not applicable; SDNN, SD of normal R-R intervals.
Hyperinsulinemic Clamp
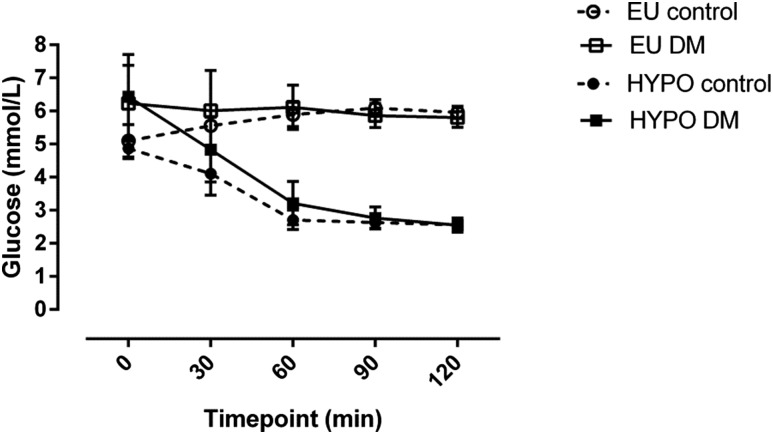
Target arterialized blood glucose levels are shown in Fig. 1. Blood glucose concentrations were 5.81 ± 0.29 and 5.96 ± 0.18 mmol/L at the end of euglycemic clamp in patients with T2DM and control participants, respectively, with no significant differences between groups (mean difference −0.15 [95% CI −0.85 to 0.55] mmol/L; P = 0.86). At the end of hypoglycemia, these values were 2.56 ± 0.22 and 2.56 ± 0.09 mmol, respectively (mean difference 0.0 [95% CI −0.70 to 0.70] mmol/L; P > 0.99). Median (IQR) free insulin levels at T120 were 576 (468–627) pmol/L during euglycemia and 689 (477–1076) pmol/L during hypoglycemia in patients and 865 (509–952) pmol/L during euglycemia and 665 (468–967) pmol/L during hypoglycemia in control participants, comparable across all four conditions (P = 0.23).
Figure 1.
Arterialized blood glucose during hyperinsulinemic-euglycemic and hypoglycemic clamps. Data are mean (SE). EU control, euglycemic clamp, control participants without diabetes; EU DM, euglycemic clamp, patients with T2DM; HYPO control, hypoglycemic clamp, control participants without diabetes; HYPO DM, hypoglycemic clamp, patients with T2DM.
Cardiac Autonomic Function
Heart Rate
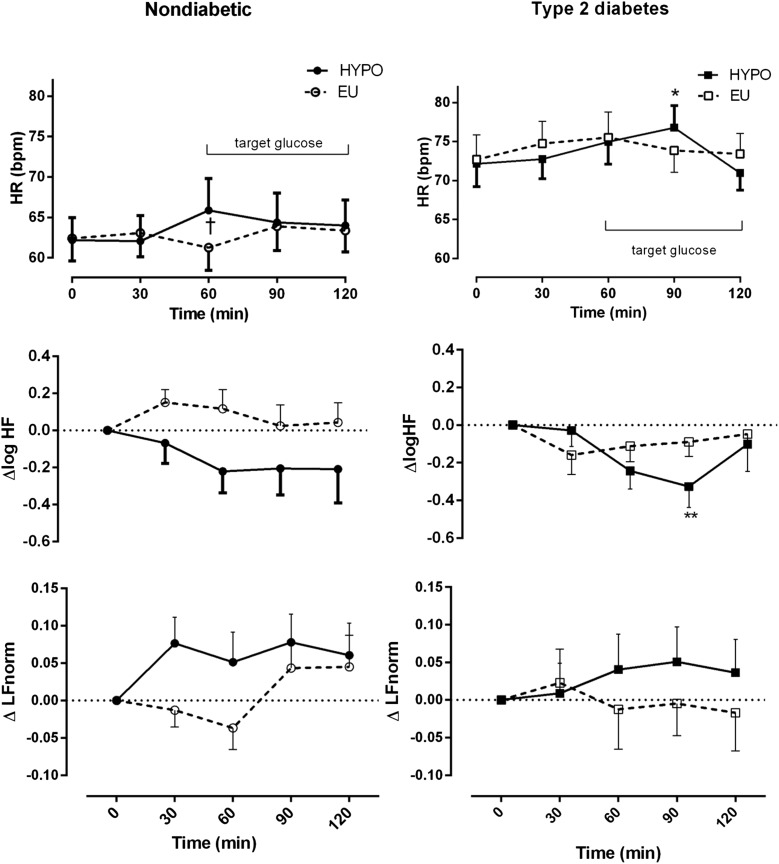
Baseline heart rates were higher among patients with T2DM. No significant changes in heart rate were observed during euglycemia in either participant group (Fig. 2, top panel, and Table 2). In control participants, heart rate increased from T60 and up to the end of the hypoglycemic clamp. Patients showed a delayed maximal increase in heart rate at T90 after 30 min at hypoglycemic levels. However, a subsequent fall in heart rate toward baseline at T120 was observed after sustained hypoglycemia of 1 h (Fig. 2, top panel).
Figure 2.
Heart rate and change in HRV during hypoglycemia (HYPO) in patients with T2DM and control participants without diabetes. ○, EU clamp, control participants without diabetes; □, EU clamp patients with T2DM; ●, HYPO clamp, control participants without diabetes; ■, HYPO clamp, patients with T2DM. Data are mean (SE). *P < 0.05, **P < 0.01 compared with baseline; †P < 0.05 euglycemia (EU) vs. HYPO. HR, heart rate.
Table 2.
HRV and blood pressure during euglycemia and hypoglycemia in patients with T2DM and control participants without diabetes
| Control |
T2DM |
|||
|---|---|---|---|---|
| Time point | Euglycemia | Hypoglycemia | Euglycemia | Hypoglycemia |
| HR (bpm) | ||||
| T0 | 62 ± 3 | 62 ± 3 | 73 ± 3 | 72 ± 3 |
| T30 | 63 ± 3 | 62 ± 3 | 75 ± 3 | 73 ± 3 |
| T60 | 61 ± 3 | 67 ± 4 | 76 ± 3 | 75 ± 3 |
| T90 | 64 ± 3 | 64 ± 4 | 74 ± 3 | 77 ± 3 |
| T120 | 63 ± 3 | 64 ± 3 | 73 ± 3 | 71 ± 2 |
| Log HF | ||||
| T0 | 2.18 ± 0.16 | 2.36 ± 0.13 | 1.87 ± 0.14 | 1.89 ± 0.18 |
| T30 | 2.33 ± 0.14 | 2.29 ± 0.13 | 1.71 ± 0.17 | 1.86 ± 0.17 |
| T60 | 2.30 ± 0.13 | 2.14 ± 0.15 | 1.75 ± 0.15 | 1.65 ± 0.19 |
| T90 | 2.20 ± 0.13 | 2.15 ± 0.15 | 1.78 ± 0.15 | 1.56 ± 0.18 |
| T120 | 2.22 ± 0.12 | 2.15 ± 0.15 | 1.82 ± 0.14 | 1.79 ± 0.23 |
| SBP (mmHg) | ||||
| T0 | 128 ± 6 | 121 ± 4 | 136 ± 5 | 136 ± 5 |
| T30 | 129 ± 8 | 125 ± 6 | 134 ± 4 | 135 ± 5 |
| T60 | 126 ± 5 | 126 ± 6 | 138 ± 6 | 141 ± 5 |
| T90 | 128 ± 5 | 128 ± 7 | 139 ± 5 | 144 ± 7 |
| T120 | 126 ± 5 | 124 ± 7 | 138 ± 6 | 134 ± 4 |
| DBP (mmHg) | ||||
| T0 | 75 ± 4 | 74 ± 3 | 77 ± 2 | 77 ± 2 |
| T30 | 75 ± 4 | 69 ± 4 | 75 ± 3 | 77 ± 2 |
| T60 | 75 ± 3 | 68 ± 3 | 77 ± 3 | 73 ± 3 |
| T90 | 73 ± 3 | 67 ± 3 | 76 ± 2 | 71 ± 4 |
| T120 | 73 ± 3 | 62 ± 3 | 78 ± 4 | 72 ± 3 |
Data are mean ± SE.
HRV
In control participants, the frequency domain measure of vagal activity log HF decreased from T60 (coincident with the rise in heart rate) and remained decreased until the end of the hypoglycemic clamp (Fig. 2, middle panel). In the patients with T2DM during hypoglycemia, log HF decreased maximally at T90. However, at T120, log HF returned to baseline levels, suggesting reactivation of vagal tone coincident with a decrease in heart rate toward baseline, but this did not occur in control participants (Fig. 2, middle panel, and Table 2). Similar trends were observed by time domain analyses. Root mean square of the successive differences (RMSSD), a time domain measure of vagal activity, decreased at T90 after hypoglycemia but returned to baseline levels at T120 in the patients (Supplementary Table 1). In control participants, LFnorm, a marker of relative sympathetic contribution, increased at T30 during hypoglycemia compared with a decrease during the euglycemic clamp. In patients, LFnorm did not change significantly over time in either glycemic condition (Fig. 2, bottom panel).
Blood Pressure
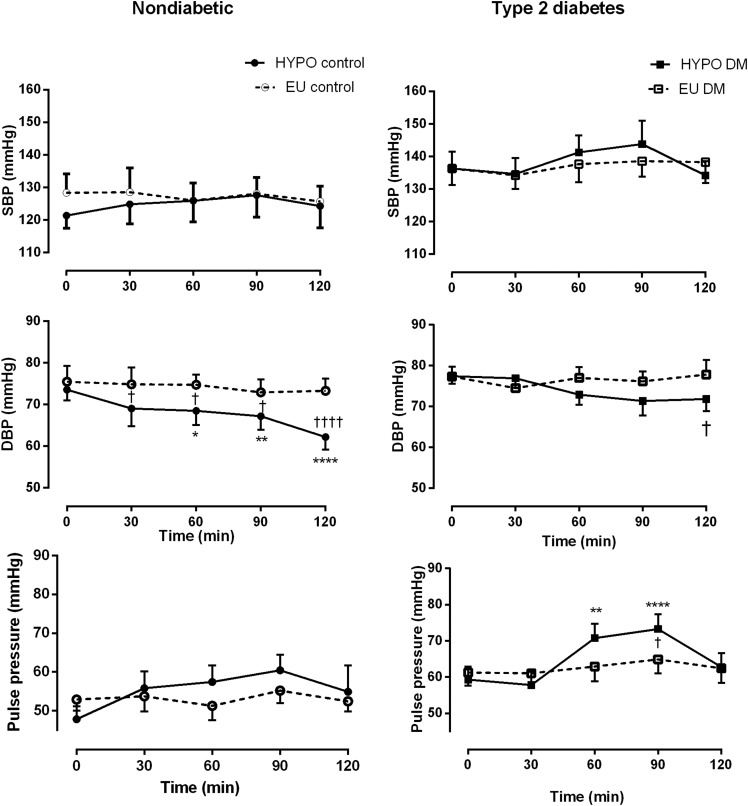
SBP, DBP, and pulse pressure did not change significantly during euglycemia in either group (Fig. 3, top panel, and Table 2). SBP did not change during hypoglycemia in control participants but tended to rise in patients with T2DM (Table 2). A smaller decline in DBP was observed among patients than among control participants (maximum change Δ −6 ± 10 vs. Δ −11.3 ± 5.93 mmHg, respectively) (Fig. 3, middle panel). DBP continued to decline until the end of the hypoglycemic clamp in control participants, with the minimum DBP occurring at median (IQR) 120 (105–120) min compared with 90 (82.5–90) min in the patients. During hypoglycemia, a significant increase in pulse pressure in patients (P = 0.02 for interaction between time and glycemic arm) was more abrupt than in control participants (Fig. 3, bottom panel).
Figure 3.
Blood pressure during hypoglycemia (HYPO) and euglycemia (EU) in patients with T2DM and control participants without diabetes. ○, EU clamp, control participants without diabetes; □, EU clamp patients with T2DM; ●, HYPO clamp, control participants without diabetes; ■, HYPO clamp, patients with T2DM. Data are mean (SE). *P < 0.05, **P < 0.01, ****P < 0.0001 compared with baseline; †P < 0.05, ††††P < 0.0001 EU vs. HYPO at equivalent time points.
Cardiac Repolarization
Cardiac repolarization data are presented for 10 patients with T2DM and 9 control participants (Table 3 and Fig. 4). In two patients and two control participants, repolarization analyses could not be performed at T120 because of technical issues related to the ECG data, which occurred at random.
Table 3.
Change in cardiac repolarization among patients with T2DM and control participants without diabetes during euglycemia and hypoglycemia
| Change from baseline |
Euglycemia vs. hypoglycemia adjusted P value | ||
|---|---|---|---|
| Euglycemia | Hypoglycemia | ||
| QTc (ms) | |||
| Control | Δ 21 ± 4 | Δ 57 ± 5 | 0.08 |
| T2DM | Δ 14 ± 4 | Δ 76 ± 20 | 0.0009 |
| T-wave symmetry | |||
| Control | Δ −0.22 ± 0.04 | Δ −0.20 ± 0.06 | 0.98 |
| T2DM | Δ −0.21 ± 0.06 | Δ −0.52 ± 0.14* | 0.09 |
| Tamp norm | |||
| Control | Δ −0.41 ± 0.06 | Δ −0.32 ± 0.18 | 0.74 |
| T2DM | Δ −0.43 ± 0.04 | Δ −0.57 ± 0.06 | 0.42 |
| TWLD | |||
| Control | Δ −476 ± 64 | Δ −404 ± 243 | 0.90 |
| T2DM | Δ −452 ± 41 | Δ −576 ± 53 | 0.70 |
| PCA ratio | |||
| Control | Δ 0.03 ± 0.02 | Δ 0.01 ± 0.05 | 0.90 |
| T2DM | Δ 0.04 ± 0.02 | Δ 0.16 ± 0.05* | 0.03 |
| TCRT | |||
| Control | Δ −0.01 ± 0.04 | Δ −0.002 ± 0.08 | 0.93 |
| T2DM | Δ −0.05 ± 0.17 | Δ 0.02 ± 0.15 | 0.99 |
Data are mean ± SE. Change in measures of repolarization at T120 compared with baseline. Two-way repeated-measures ANOVA with multiple comparisons between glycemic arms (euglycemia vs. hypoglycemia) and between participant groups. Data are from 9 control participants and 10 patients with T2DM. Tamp norm, normalized T-wave amplitude.
*Significant difference between patients with T2DM and control participants without diabetes within each glycemic arm.
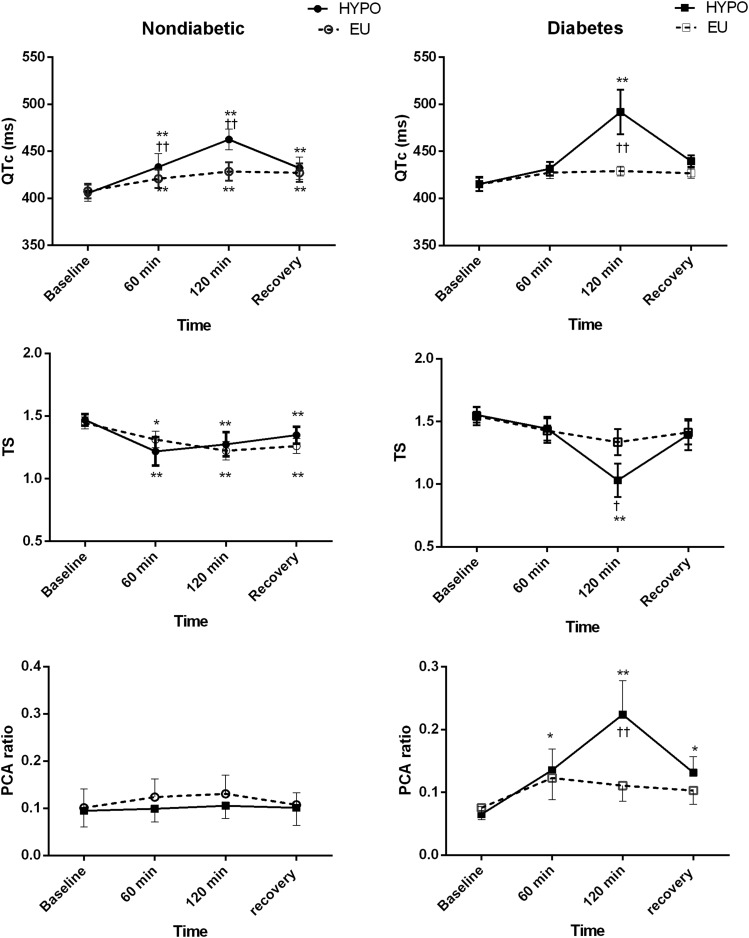
Figure 4.
Changes in cardiac repolarization during hypoglycemic (HYPO) and euglycemic (EU) clamps in patients with T2DM and control participants without diabetes. The T-wave symmetry (TS) is an index of T-wave morphology. The PCA ratio is the index of complexity of the T-wave morphology among the 12 leads. Data are mean (SE). Data from 9 control participants and 10 patients. *P < 0.05, **P < 0.01 compared with baseline; †P < 0.05, ††P < 0.01 EU vs. HYPO at equivalent time points.
QTc
Significant, relatively small increases were observed in QTc duration by Bazett formula (QTcB) in both participant groups during euglycemic clamp at T120 (Table 3 and Fig. 4). Compared with euglycemia, QTcB increased significantly more during hypoglycemia in both groups (Fig. 4). QTcB increased by Δ 57 ± 5 ms in control participants and Δ 76 ± 20 ms in patients with T2DM during hypoglycemia. Although QTcB tended to increase to a larger extent in patients, the difference was not significance (mean difference 19 [95% CI −22 to 59] ms; P = 0.50). The maximum increase occurred at T120 in both groups. QTc by the nomogram method (QTcN) exhibited similar trends, increasing by Δ 12 ± 8 vs. Δ 68 ± 21 ms during euglycemia and hypoglycemia, respectively, among patients (P = 0.003 for the glycemic arm). QTcN increased by Δ 18 ± 11 vs. Δ 56 ± 13 ms during euglycemia and hypoglycemia, respectively, in control participants (P = 0.06 for the glycemic arm), with no significant differences between groups in euglycemic or hypoglycemic responses.
T-Wave Morphology
T-wave symmetry, an index of T-wave morphology, fell by a similar extent in control participants during euglycemia and hypoglycemia (Fig. 4, middle panel). However, in patients with T2DM, T symmetry decreased significantly more during hypoglycemia than during euglycemia, resulting in more abnormally shaped symmetrical T waves. A significant difference was found in the change in T symmetry during hypoglycemia between groups (mean difference Δ –0.33 [95% CI −0.62 to −0.03]; P = 0.03). The amplitude of the T waves, normalized to the baseline fell by a similar extent during euglycemia and hypoglycemia in both groups (Table 3). Repolarization and wavefront propagation parameters derived from PCA are shown in Table 3. The PCA ratio, which describes the complexity of the T-wave morphology across the 12 ECG leads, did not change during euglycemia in either group. The PCA ratio significantly increased during hypoglycemia in patients (Δ 0.16 ± 0.05), indicating higher complexity, compared with no change in control participants (Δ 0.01 ± 0.05; P = 0.03 for difference between groups). The TWLD, which indicates temporal variation in interlead relationships, decreased similarly across glycemic conditions in both groups. No significant changes occurred for the wavefront direction descriptor (TCRT) in either glycemic arm for both groups.
Biochemical Measurements
Baseline levels of catecholamines were similar between participant groups (P = 0.99). Catecholamines were significantly higher at the end of hypoglycemia versus euglycemia in both groups (all P < 0.01) (Table 4). Epinephrine levels at the end of hypoglycemia were 3.05 ± 0.71 and 3.83 ± 0.85 nmol/L in patients with T2DM and control participants, respectively, a difference that was not statistically significant (P = 0.54). Peak norepinephrine values at the end of hypoglycemia were 2.45 ± 0.23 and 2.69 ± 0.44 nmol/L in patients and control participants, respectively, a difference that was not statistically significant (mean difference −0.24 [95% CI −0.11 to 0.58] nmol/L; P = 0.75).
Table 4.
Biochemical parameters at end of clamp in patients with T2DM and control participants without diabetes
| Euglycemia | Hypoglycemia | Euglycemia vs. hypoglycemia adjusted P value | |
|---|---|---|---|
| Potassium (mmol/L) | |||
| Control | 3.69 ± 0.14 | 3.48 ± 0.11 | 0.40 |
| T2DM | 3.72 ± 0.13 | 3.27 ± 0.09 | 0.02 |
| Epinephrine (nmol/L) | |||
| Control | 0.14 ± 0.02 | 3.83 ± 0.85 | 0.0002 |
| T2DM | 0.16 ± 0.05 | 3.05 ± 0.71 | 0.003 |
| Norepinephrine (nmol/L) | |||
| Control | 1.56 ± 0.12 | 2.69 ± 0.44 | 0.002 |
| T2DM | 1.27 ± 0.09 | 2.45 ± 0.23 | 0.009 |
Data are mean ± SE. P value comparison between euglycemia and hypoglycemia by two-way ANOVA with multiple comparisons between glycemic arms and between participant groups. No significant differences were found between control participants without diabetes and patients with T2DM under either glycemic condition.
In control participants, potassium levels were not significantly different during hypoglycemia versus euglycemia (3.48 ± 0.11 vs. 3.69 ± 0.14 mmol/L, respectively; P = 0.40). However, in patient with T2DM, potassium was significantly lower at the end of the hypoglycemic clamp compared with euglycemia (3.27 ± 0.09 vs. 3.72 ± 0.13 mmol/L; P = 0.02). Potassium levels at the end of the hypoglycemic clamp tended to be lower in patients, but the difference was not significant (mean difference −0.21 [95% CI −0.60 to 0.17] mmol/L; P = 0.39).
Discussion
In individuals with T2DM, this study shows that hypoglycemia results in transient increases in heart rate with coincident vagal withdrawal followed by a relative decrease in heart rate during more sustained hypoglycemia of 1 h and accompanied by vagal reactivation (shown by increased HF power and RMSSD). In individuals without T2DM, vagal inhibition continues throughout hypoglycemia. Furthermore, greater repolarization abnormalities as shown by QTc and T-wave morphology are present in individuals with T2DM despite similar levels of hypoglycemia to those without T2DM.
The patients with T2DM exhibited impaired baseline autonomic function compared with control participants, with higher resting heart rates and lower HRV, although none had frank autonomic neuropathy by formal testing of cardiovascular reflexes. In the control group, participants demonstrated continued vagal withdrawal as evidenced by decreased HF HRV throughout hypoglycemia. This finding is consistent with that of previous studies of experimental hypoglycemia in healthy participants and patients with type 1 diabetes (27,28). In contrast, after initial increases in heart rate and vagal withdrawal, we observed a slowing of heart rate in patients with T2DM after 60 min of hypoglycemia (T120) with vagal reactivation. We observed a similar phasic response in heart rate during spontaneous prolonged hypoglycemia in patients with T2DM in our previous ambulatory study, with some patients demonstrating profound bradycardia accompanied by ectopic activity (6). Reasons for the differences between the participant groups in the current study are unclear. The arterial baroreflex is a negative feedback reflex that regulates arterial pressure around an operating point. BRS describes the degree to which the heart rate increases or decreases in response to a given change in mean arterial pressure as a consequence of the baroreflex. In detailed experimental studies of participants with and without type 1 diabetes, BRS has been shown to fall during experimental hypoglycemia along with a resetting of the working range to higher heart rates (29,30). In patients with diabetes, a failure of baroreceptors to reset to higher heart rates could lead to an increase in vagal restraint in the face of sustained sympathetic stimulation, rising SBP, and pulse pressure because the operating point remains at baseline levels. Impaired acute resetting of the baroreceptor operating point has also been reported among individuals with hypertension (31). Future studies could examine this hypothesis with concurrent measurements of baroreceptor function during experimental hypoglycemia in patients with T2DM.
During hypoglycemia, maximal changes in heart rate and decline in HF HRV occurred later in patients with T2DM at T90 than in control participants, in whom they occurred at T30. Delayed increments in heart rate and cardiac output responses during hypoglycemia have been previously reported in intensively treated individuals with type 1 diabetes and may reflect blunted counterregulatory hormonal responses (32). However, in the current study, epinephrine and norepinephrine levels during hypoglycemia were similar between participant groups.
We have also shown greater abnormalities in duration and heterogeneity of repolarization during hypoglycemia in patients with T2DM. Changes in the morphology of the T wave (both symmetry [T-wave symmetry] and complexity across the leads [PCA ratio]) were greater during hypoglycemia in the patients with T2DM, suggesting greater dispersion of repolarization, which is proarrhythmic and has been linked with increased cardiovascular risk (22,23,33). These T-wave changes during hypoglycemia have not been previously described in the T2DM population, and the differences compared with individuals without diabetes are noteworthy.
A stronger sympathoadrenal response is unlikely to be the primary explanation because the peak epinephrine levels were similar in both groups, but it might be the result of greater declines in serum potassium, which we observed during hypoglycemia in the patients with T2DM. In individuals with diabetes, autonomic dysfunction might, through denervation metabolic adrenergic hypersensitivity, lead to a greater β-adrenoreceptor–mediated fall in potassium and would be analogous to that observed in other responses, such as free fatty acid production during experimental hypoglycemia among individuals with type 1 diabetes and autonomic dysfunction (34). This mechanism could be explored in future experimental studies involving potassium clamping or adrenergic blockade.
Previous studies have shown a relationship between potassium and T-wave amplitude during hyperinsulinemic hypoglycemia (35,36). Our group has previously shown that replacement of potassium during hyperinsulinemic clamps reversed increases in QT dispersion but not QTc duration during hypoglycemia in individuals without diabetes (37). However, we cannot exclude the possibility that intrinsic abnormalities in repolarization substrate among the patients with T2DM are exacerbated by hypoglycemia. Of note, differences have also been reported in animal models of experimental hypoglycemia where diabetic rats were at greater risk for arrhythmias than their nondiabetic counterparts (38). The underlying mechanisms require further testing in experimental studies.
One strength of the current study is that cardiovascular variables were measured at multiple time points during hypoglycemia. Previous studies have shown increases (39), decreases (35), or no change (36) in vagal power during hyperinsulinemic hypoglycemia in individuals with and without type 1 diabetes. These discrepant data may reflect measurements at a single, often variable time point, an explanation supported by the current study indicating that changes in cardiac autonomic tone appear phasic and depend on duration of hypoglycemia.
This study had some limitations. Controlling for the depth and frequency of breathing during HRV recordings proved challenging and may have affected the HF component of HRV, an index of vagal activity (14). In the original protocol, participants were asked to pace their breathing at 12 breaths/minute (0.2 Hz) by following a timed visual display, but they failed to comply consistently during hypoglycemia perhaps because of cognitive impairment. However, the mean spontaneous respiratory frequencies across 5 min of recording were consistently situated in the middle of the HF band, ∼0.25 Hz. Differences between experimental conditions were small and unlikely to significantly affect the HRV spectra and the estimation of vagal activity and its changes during the protocol (Supplementary Table 2).
Relevant differences exist between our experimental model of sustained hyperinsulinemic hypoglycemia and spontaneous clinical episodes. In this study, although heart rate tended to fall toward baseline during sustained hypoglycemia with vagal reactivation, we did not observe profound bradycardia as we previously reported during spontaneous prolonged nocturnal episodes in an observational clinical study (6). These differences could be related to circadian variation because experiments were conducted during the day. During the night, background vagal tone tends to be greater, and sympathoadrenal responses to hypoglycemia are suppressed during sleep, which may lead to greater vagal predominance (40). Spontaneous nocturnal episodes were generally more prolonged and reached lower glucose values than those induced during the current study (which might also explain the relatively modest changes in heart rate). We limited the duration (60 min) and depth (2.5 mmol/L) of experimental hypoglycemia for ethical reasons, so both may have also played a role. Experimental hypoglycemia is associated with a greater decline in potassium and catecholamine flux, which could lead to exaggerated repolarization abnormalities. However, the magnitude of QT prolongation observed here has been reported in clinical settings. In a retrospective cohort study of patients with T2DM presenting to the emergency department with severe hypoglycemia, 14% had QTc in excess of 500 ms on presentation and up to one-third had hypokalemia (41).
The current study suggests that abnormal repolarization and autonomic tone interact differentially depending on the duration of hypoglycemia. Thus, autonomic dysfunction in the patients with T2DM may have contributed to the different time course in vagal tone between the participant groups as well as the more pronounced abnormalities in some measures of cardiac repolarization. This interaction might also explain the different patterns of arrhythmias we previously observed during our ambulatory clinical study (6). With use of a rodent model of experimental hypoglycemia, Reno et al. (38) observed arrhythmias ranging from QT prolongation, to ventricular ectopy, to heart block that depended on duration and severity of hypoglycemia. In the current study, sustained experimental hypoglycemia was associated with later reactivation of vagal tone in patients with T2DM despite sustained increases in circulating catecholamines, which occurred when QT prolongation and T-wave changes were maximal.
In conclusion, the data indicate that cardiac autonomic regulation during hypoglycemia appears to be time dependent and different between patients with T2DM and control participants without diabetes. In T2DM, the initial heart rate increment to hypoglycemia was delayed, and vagal activity was reactivated during sustained hypoglycemia. Patients with T2DM also exhibited greater repolarization abnormalities than participants without diabetes, despite a similar hypoglycemic stimulus and comparable catecholamine levels. These mechanisms could contribute to the arrhythmias that have been reported during clinical hypoglycemic episodes. The data provide further support of a possible relationship between hypoglycemia and increased cardiovascular mortality in T2DM and highlight the potential contribution of autonomic dysfunction.
Supplementary Material
Article Information
Acknowledgments. The authors thank the Diabetes Department and Clinical Research Facility of Sheffield Teaching Hospitals for assistance and all patients who gave time toward this study.
Funding. This article is a summary of independent research funded in part by the National Institute for Health Research (NIHR) (grant number BRF-2011-004) and carried out at the NIHR Sheffield Clinical Research Facility.
The views expressed are those of the authors and not necessarily those of the National Health Service, NIHR, or Department of Health.
Duality of Interest. S.R.H. has served as a consultant for Sanofi Aventis and Boehringer Ingelheim; has served as an advisory board panel member for Eli Lilly, Novo Nordisk, LifeScan, and Takeda; and has attended speakers’ bureaus for AstraZeneca, Novo Nordisk, Eli Lilly, and MSD. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. E.C. designed the study, collected and analyzed the data, and wrote the manuscript. A.B. developed the ECG-related methodology and software, analyzed the data, helped to collect the data, and edited the manuscript. E.W. and A.L.-S. helped to collect the data and edited the manuscript. J.F. advised on the statistical analysis and reviewed the manuscript. I.A.M. analyzed the catecholamine data and reviewed the manuscript. P.J.S. and S.R.H. designed the study, reviewed the data, and edited and redrafted the manuscript. E.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-1310/-/DC1.
References
- 1.Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 2.Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoungas S, Patel A, Chalmers J, et al.; ADVANCE Collaborative Group . Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–1418 [DOI] [PubMed] [Google Scholar]
- 5.Mellbin LG, Rydén L, Riddle MC, et al.; ORIGIN Trial Investigators . Does hypoglycaemia increase the risk of cardiovascular events? A report from the ORIGIN trial. Eur Heart J 2013;34:3137–3144 [DOI] [PubMed] [Google Scholar]
- 6.Chow E, Bernjak A, Williams S, et al. . Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes 2014;63:1738–1747 [DOI] [PubMed] [Google Scholar]
- 7.Stahn A, Pistrosch F, Ganz X, et al. . Relationship between hypoglycemic episodes and ventricular arrhythmias in patients with type 2 diabetes and cardiovascular diseases: silent hypoglycemias and silent arrhythmias. Diabetes Care 2014;37:516–520 [DOI] [PubMed] [Google Scholar]
- 8.Marques JL, George E, Peacey SR, et al. . Altered ventricular repolarization during hypoglycaemia in patients with diabetes. Diabet Med 1997;14:648–654 [DOI] [PubMed] [Google Scholar]
- 9.Cox AJ, Azeem A, Yeboah J, et al. . Heart rate-corrected QT interval is an independent predictor of all-cause and cardiovascular mortality in individuals with type 2 diabetes: the Diabetes Heart Study. Diabetes Care 2014;37:1454–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien IA, O’Hare P, Corrall RJ. Heart rate variability in healthy subjects: effect of age and the derivation of normal ranges for tests of autonomic function. Br Heart J 1986;55:348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tesfaye S, Boulton AJ, Dyck PJ, et al.; Toronto Diabetic Neuropathy Expert Group . Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parati G, Di Rienzo M, Bertinieri G, et al. . Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension 1988;12:214–222 [DOI] [PubMed] [Google Scholar]
- 13.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996;93:1043–1065 [PubMed] [Google Scholar]
- 14.Bernardi L, Spallone V, Stevens M, et al.; Toronto Consensus Panel on Diabetic Neuropathy . Methods of investigation for cardiac autonomic dysfunction in human research studies. Diabetes Metab Res Rev 2011;27:654–664 [DOI] [PubMed] [Google Scholar]
- 15.Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 1994;90:1826–1831 [DOI] [PubMed] [Google Scholar]
- 16.Mason RE, Likar I. A new system of multiple-lead exercise electrocardiography. Am Heart J 1966;71:196–205 [DOI] [PubMed] [Google Scholar]
- 17.Badilini F, Maison-Blanche P, Childers R, Coumel P. QT interval analysis on ambulatory electrocardiogram recordings: a selective beat averaging approach. Med Biol Eng Comput 1999;37:71–79 [DOI] [PubMed] [Google Scholar]
- 18.Karjalainen J, Viitasalo M, Mänttäri M, Manninen V. Relation between QT intervals and heart rates from 40 to 120 beats/min in rest electrocardiograms of men and a simple method to adjust QT interval values. J Am Coll Cardiol 1994;23:1547–1553 [DOI] [PubMed] [Google Scholar]
- 19.Merri M, Benhorin J, Alberti M, Locati E, Moss AJ. Electrocardiographic quantitation of ventricular repolarization. Circulation 1989;80:1301–1308 [DOI] [PubMed] [Google Scholar]
- 20.di Bernardo D, Murray A. Explaining the T-wave shape in the ECG. Nature 2000;403:40. [DOI] [PubMed] [Google Scholar]
- 21.Acar B, Yi G, Hnatkova K, Malik M. Spatial, temporal and wavefront direction characteristics of 12-lead T-wave morphology. Med Biol Eng Comput 1999;37:574–584 [DOI] [PubMed] [Google Scholar]
- 22.Porthan K, Viitasalo M, Jula A, et al. Predictive value of electrocardiographic QT interval and T-wave morphology parameters for all-cause and cardiovascular mortality in a general population sample. Heart Rhythm 2009;6:1202–1208 [DOI] [PubMed]
- 23.Okin PM, Devereux RB, Fabsitz RR, Lee ET, Galloway JM, Howard BV. Principal component analysis of the T wave and prediction of cardiovascular mortality in American Indians: the Strong Heart Study. Circulation 2002;105:714–719 [DOI] [PubMed] [Google Scholar]
- 24.Perkiömäki JS, Hyytinen-Oinas M, Karsikas M, et al. . Usefulness of T-wave loop and QRS complex loop to predict mortality after acute myocardial infarction. Am J Cardiol 2006;97:353–360 [DOI] [PubMed] [Google Scholar]
- 25.Zabel M, Acar B, Klingenheben T, Franz MR, Hohnloser SH, Malik M. Analysis of 12-lead T-wave morphology for risk stratification after myocardial infarction. Circulation 2000;102:1252–1257 [DOI] [PubMed] [Google Scholar]
- 26.Okin PM, Devereux RB, Lee ET, Galloway JM, Howard BV; Strong Heart Study . Electrocardiographic repolarization complexity and abnormality predict all-cause and cardiovascular mortality in diabetes: the Strong Heart Study. Diabetes 2004;53:434–440 [DOI] [PubMed] [Google Scholar]
- 27.Koivikko ML, Salmela PI, Airaksinen KE, et al. . Effects of sustained insulin-induced hypoglycemia on cardiovascular autonomic regulation in type 1 diabetes. Diabetes 2005;54:744–750 [DOI] [PubMed] [Google Scholar]
- 28.Limberg JK, Farni KE, Taylor JL, et al. . Autonomic control during acute hypoglycemia in type 1 diabetes mellitus. Clin Auton Res 2014;24:275–283 [DOI] [PubMed] [Google Scholar]
- 29.Limberg JK, Dube S, Kuijpers M, et al. . Effect of hypoxia on heart rate variability and baroreflex sensitivity during hypoglycemia in type 1 diabetes mellitus. Clin Auton Res 2015;25:243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao AD, Bonyhay I, Dankwa J, et al. . Baroreflex sensitivity impairment during hypoglycemia: implications for cardiovascular control. Diabetes 2016;65:209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie PL, McDowell TS, Chapleau MW, Hajduczok G, Abboud FM. Rapid baroreceptor resetting in chronic hypertension. Implications for normalization of arterial pressure. Hypertension 1991;17:72–79 [DOI] [PubMed] [Google Scholar]
- 32.Russell RR 3rd, Chyun D, Song S, et al. . Cardiac responses to insulin-induced hypoglycemia in nondiabetic and intensively treated type 1 diabetic patients. Am J Physiol Endocrinol Metab 2001;281:E1029–E1036 [DOI] [PubMed] [Google Scholar]
- 33.Kardys I, Kors JA, van der Meer IM, Hofman A, van der Kuip DA, Witteman JC. Spatial QRS-T angle predicts cardiac death in a general population. Eur Heart J 2003;24:1357–1364 [DOI] [PubMed] [Google Scholar]
- 34.Hilsted J, Richter E, Madsbad S, et al. . Metabolic and cardiovascular responses to epinephrine in diabetic autonomic neuropathy. N Engl J Med 1987;317:421–426 [DOI] [PubMed] [Google Scholar]
- 35.Koivikko ML, Karsikas M, Salmela PI, et al. . Effects of controlled hypoglycaemia on cardiac repolarisation in patients with type 1 diabetes. Diabetologia 2008;51:426–435 [DOI] [PubMed] [Google Scholar]
- 36.Laitinen T, Lyyra-Laitinen T, Huopio H, et al. . Electrocardiographic alterations during hyperinsulinemic hypoglycemia in healthy subjects. Ann Noninvasive Electrocardiol 2008;13:97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson RT, Harris ND, Ireland RH, Lee S, Newman C, Heller SR. Mechanisms of abnormal cardiac repolarization during insulin-induced hypoglycemia. Diabetes 2003;52:1469–1474 [DOI] [PubMed] [Google Scholar]
- 38.Reno CM, Daphna-Iken D, Chen YS, VanderWeele J, Jethi K, Fisher SJ. Severe hypoglycemia-induced lethal cardiac arrhythmias are mediated by sympathoadrenal activation. Diabetes 2013;62:3570–3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schächinger H, Port J, Brody S, et al. . Increased high-frequency heart rate variability during insulin-induced hypoglycaemia in healthy humans. Clin Sci (Lond) 2004;106:583–588 [DOI] [PubMed] [Google Scholar]
- 40.Jones TW, Porter P, Sherwin RS, et al. . Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med 1998;338:1657–1662 [DOI] [PubMed] [Google Scholar]
- 41.Tsujimoto T, Yamamoto-Honda R, Kajio H, et al. . Vital signs, QT prolongation, and newly diagnosed cardiovascular disease during severe hypoglycemia in type 1 and type 2 diabetic patients. Diabetes Care 2014;37:217–225 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.