Abstract
Aims
Accumulating evidence for the influence of the gut microbiota on the bidirectional communication along the gut-brain axis suggests a role of the gut microbiota in eating disorders (EDs) and alcohol and substance use disorders. The potential influence of altered gut microbiota (dysbiosis) on behaviors associated with such disorders may have implications for developing therapeutic interventions.
Methods
A systematic review of preclinical and clinical studies evaluating the gut microbiota, EDs and alcohol and substance use disorders was conducted using MEDLINE, Embase and Web of Science databases with the objective being to examine the role of the gut microbiota in behavioral correlates of these disorders. Original papers focused on the gut microbiota and potential behavioral implications were deemed eligible for consideration.
Results
The resulting 12 publications were limited to gut microbiota studies related to EDs and alcohol and substance use disorders. Some studies suggest that dysbiosis and gut microbial byproducts may influence the pathophysiology of EDs via direct and indirect interference with peptide hormone signaling. Additionally, dysbiosis was shown to be correlated with alcohol use disorder-related symptoms, i.e. craving, depression and anxiety. Finally, a mouse study suggests that manipulations in the gut microbiota may affect cocaine-related behaviors.
Conclusions
Promising, albeit preliminary, findings suggest a potential role of the gut microbiota in behavioral correlates of EDs and alcohol and substance use disorders.
Short summary
Preliminary evidence exists supporting the role of the gut microbiota in eating disorders and alcohol and substance use disorders, although additional investigation is needed to determine what is causative versus epiphenomenological.
INTRODUCTION
The gut microbiota is a collection of over 100 trillion microorganisms residing in the gastrointestinal tract (Savage, 1977). Their collective genome, the microbiome, encodes 100 times more genes than the human genome (Qin et al., 2010). The gut microbiota plays several roles in digestion, inflammation and immunity (Li et al., 2008; Abt and Artis, 2009; Nicholson et al., 2012; Leclercq et al., 2017). The microbiota–host relationship is mutualistic. Imbalances or alterations in microbial composition or activity – dysbiosis – can influence health and is implicated in various diseases, e.g. irritable bowel disease, colorectal cancer and diabetes (Kim et al., 2013). Gut dysbiosis is associated with damages to the intestinal barrier, which negatively impacts the host’s ability to respond to stressors (Mutlu et al., 2012).
There is a bidirectional pathway of communication along the microbiome-gut-brain axis. On one hand, bottom-up signaling pathways influence health through vagal, neural, endocrine and/or immune pathways, and may impact behavior, brain activity and levels of neurotransmitters, receptors and neurotrophic factors (Cryan and Dinan, 2012; Forsythe et al., 2012; Forsythe and Kunze, 2013). Additionally, microbial-derived metabolites can activate these pathways. On the other hand, neural signaling through top-down pathways can influence gut function, disrupt the intestinal barrier (‘leaky gut’) and alter the composition and function of the gut microbiota.
The influence of the microbiota on stress, autism, anxiety, depression, Parkinson’s disease and schizophrenia has been shown in various preclinical and clinical work (Sudo et al., 2004; Adams et al., 2011; Bravo et al., 2011; Diaz Heijtz et al., 2011; Neufeld et al., 2011; Bendtsen et al., 2012; Cryan and Dinan, 2012; Forsythe et al., 2012; Hsiao et al., 2013; Burokas et al., 2015). Similarly, recent research suggests that the gut microbiota may impact behaviors related to problematic alcohol drinking and eating. Notably, eating disorders (EDs) and alcohol and substance use disorders share neurobiological mechanisms that regulate these pathological behaviors and both disorders involve altered reward processing and related neural circuitries (Goodman, 2008; Schreiber et al., 2013). Behaviors related to EDs and alcohol and substance use disorders (e.g. urges, cravings, binge episodes) are associated with altered orbitofrontal and prefrontal cortex activity and reduced inhibition and self-control. EDs and alcohol and substance use disorders present with high mortality and morbidity (Table 1), but limited treatment options (Smink et al., 2012; Grant et al., 2015).
Table 1.
Overview of the disorders of interest examined in this systematic review
| Overview | Main features | Estimated prevalence |
|---|---|---|
| Eating disorders | ||
|
|
|
| Alcohol and substance use disorders | ||
|
Alcohol and substance use disorders
|
Substance use disorders
|
|
Alcohol use disorders
|
|
Recent preclinical studies have shown that the gut microbiota can influence key neural pathways involved in EDs and alcohol and substance use disorders. Mice lacking microbes have marked changes in striatal (Diaz Heijtz et al., 2011), amygdala (Stilling et al., 2015), hippocampal (Neufeld et al., 2011; Clarke et al., 2013) and cortical (Hoban et al., 2016) gene expression, in addition to alterations at the morphological and spine density level (Luczynski et al., 2016b). Such findings are coupled with an exaggerated hypothalamic–pituitary–adrenal axis response and changes in anxiety, cognitive and social behaviors (Luczynski et al., 2016a). These brain areas and behaviors are involved in ED and alcohol and substance use disorders pathology, thus it is plausible that the gut microbiota plays a role in such disorders. This systematic review examines the literature on, and discusses the relationship between, behaviors exhibited in these disorders and gut dysbiosis.
METHODS
A systematic review was conducted on the gut microbiota, EDs and alcohol and substance use disorders. We considered ‘behaviors’ in this context as any action or comportment correlated to the clinical manifestation of the disorder, and specifically we searched for craving. Both preclinical and clinical published studies were included without limitations for the year or language. Both reviews and original research articles were included in the search, but only full-length original articles were reviewed and included.
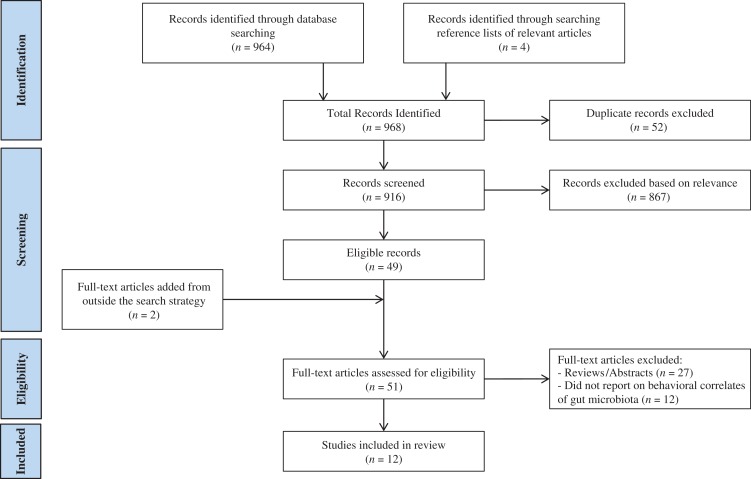
The MEDLINE, Embase and Web of Science databases were searched for records indexed up until April 2016 (see Table 2). In addition to the studies found by the systematic searches, citations within those articles were also evaluated, if relevant. The titles and abstracts were screened by two independent reviewers (J.E.T. and S.B.) for their relevance to the objective of this review. Articles focused on microbiota of body areas other than the gut, or on microbial profiles and/or metabolic factors, but not on host behaviors, were excluded. Inclusion criteria involved the use of behavioral outcomes or measures and a focus on the disorders of interest. In the event of a disagreement over relevance, the full texts of articles were examined and discussed to determine if the inclusion criterion for behavioral outcomes related to the disorders of interest was satisfied. The full texts of those deemed relevant were examined thoroughly. The selection followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al., 2009) (Fig. 1).
Table 2.
Search strategy of this systematic review
The terms were searched both as medical subject heading terms and text words in MEDLINE; the strategy was modified for the additional databases (Embase and Web of Science) to conform to their controlled vocabularies and database field structures:
|
Fig. 1.
Flow diagram of the systematic review.
RESULTS
The selection process for the studies included in this review is detailed in Fig. 1. Consistent with the exclusion criteria involving metabolic outcomes, or microbial profiling without behavioral measures or focusing on microbiota from alternative body areas, all microbiome-related studies on substance use disorders without behavioral correlations were deemed outside of the scope for this review. Because the only explicit behavioral outcome searched was ‘craving,’ many articles were read in full to assess for additional examples of behavioral outcomes, such as anxiety behavior. Of the initial 916 records, 10 studies satisfied the criteria of this systematic review: Two additional papers (Breton et al., 2016a; Kiraly et al., 2016) were identified and included following the culmination of the formal search due to their relevance to the review objective. Therefore, a total of 12 papers were included: 8 papers on EDs and 4 papers on alcohol and substance use disorders. These studies are reviewed next.
Gut microbiota, dysbiosis and EDs
Broad overview of the field
The impact of diet and nutrition on the gut microbiota led researchers to investigate the role of gut bacteria modulation in the central nervous system control of food intake (Flint et al., 2012). Gut bacteria perform host metabolic functions, facilitate energy extraction from food, increase nutrient availability (Backhed et al., 2005) and alter taste receptors (Duca et al., 2012).
Investigations on the gut-brain axis in EDs are sparse, despite correlations with microbial changes. Indeed, Anorexia Nervosa (AN) patients have significantly lower amounts of total intestinal bacteria and higher prevalence of specific bacterial strains, which may reflect altered metabolic capacity resulting from the disorder (Armougom et al., 2009; Morita et al., 2015; Bulik, 2016; Carr et al., 2016; Mack et al., 2016). Gut bacteria may play a role in cravings for specific foods or induce dysphoria to motivate the ingestion of foods (Alcock et al., 2014). An evolutionary pathway has been proposed, such that gut bacteria act to enhance their own survival or hinder that of competitive gut bacteria. Diminished microbial diversity may be associated with unhealthy eating patterns that can lead to the debilitating weight loss associated with AN (Alcock et al., 2014). Alterations to gut microbial profiles in AN patients persist after weight gain, although some increase in microbial richness, including elevated numbers of operational taxonomic units and increased diversity of population, is observed (Mack et al., 2016). Additionally, the presence of specific strains of gut bacteria have been linked to ED-related clinical features, e.g.Escherichia coli K12 and Body Mass Index (Million et al., 2013). Bulimia nervosa (BN) and binge-eating disorder (BED) diagnoses on the other hand have been correlated to antimicrobial medication use prior to ED treatment (Raevuori et al., 2016).
Central controls of satiety and food intake takes place through the integration of information from sensory, behavioral, physiological and neurohormonal signals (Morton et al., 2006). Orexigenic hormones like ghrelin are released in the fasting state and cross the blood–brain barrier to promote food intake by activating neurons that produce neuropeptides like neuropeptide Y and agouti-related peptide (AgRP) in the arcuate nucleus (Pimentel et al., 2012) . Anorexigenic hormones like leptin are released after food ingestion and, via similar mechanisms, activate neuropeptides such as glucagon-like peptide 1 and α-melanocyte-stimulating hormone (α-MSH) to promote satiety (Pimentel et al., 2012). When bound to melanocortin receptor 4 (MC4R), the final pathway in satiety signaling (Cone, 2005), α-MSH is involved in the regulation of mood and emotion as well (Kokare et al., 2010).
Immunoglobulins (Igs) can cross the blood–brain barrier and access the arcuate nucleus (Fetissov and Dechelotte, 2008). EDs may stem from the production of Igs, or auto-antibodies (auto-Abs), that modulate biological responses to appetite-regulating neuropeptides by binding to hormone receptors (Inui et al., 2015), and affect appetite and emotion. Rodent studies show a role of α-MSH-reactive auto-Abs, stemming from altered immune functioning and food-restriction-induced stress, in the regulation of appetite and emotion (Sinno et al., 2009).
Though causation has not been established, mechanisms explaining the link between gut microbiome and EDs have been proposed, including signaling via vagal tone (Kollai et al., 1994) and changes in immune functions (Kleiman et al., 2015a). EDs have also been linked to the gut microbiota through a process called ‘molecular mimicry’. Whereby, microbial proteins—with amino-acid sequence homology to appetite-regulating neuropeptides—are made available as a result of altered intestinal permeability and cross-react with auto-Abs, of which production have also been linked to intestinal inflammation (Coquerel et al., 2012). Indeed, increased levels of α-MSH auto-Abs were observed after food restriction (mild stressor). A subsequent significant increase in food intake and anxiety levels was observed in food-restricted rats when compared to a non-stressed control group, who exhibited acute bulimic and anxiolytic responses when exposed to α-MSH auto-Abs (Coquerel et al., 2012). Thus, evidence suggests that auto-Abs are dynamic, influenced by stress,and likely involved in neuroendocrine control of eating (Fetissov et al., 2008b). Because appetite-regulating neuropeptides are important signaling molecules in hunger and satiety, the ability of colonic microbiota to impact appetite and emotion via molecular mimicry may be involved in the development of EDs.
This review led to eight papers investigating the potential link between the gut microbiota and ED-related behaviors, as outline in Table 3 and discussed next.
Table 3.
Summary of findings on eating disorders
| Sample studied | Main findings | Sample size | Reference |
|---|---|---|---|
| Germ-free and Specific-pathogen free rats |
|
n = 6/group | Fetissov et al. (2008a) |
| Healthy female participants |
|
n = 15 | |
| Male C57Bl/6 mice |
|
n = 8/group | Tennoune et al. (2014) |
| Female patients with AN, BN, or BED |
|
|
|
| Adult Wistar rats |
|
n = 48 | Tennoune et al. (2015) |
| Female patients with AN or BN |
|
|
Fetissov et al. (2005) |
| Female patients with AN or BN and male Sprague-Dawley rats |
|
|
Fetissov et al. (2005) |
| Female patients with AN, BN or BED |
|
|
Breton et al. (2016a) |
| Female patients with AN |
|
|
Kleiman et al. (2015b) |
| Female patients with AN and/or BN and Sprague-Dawley rats |
|
|
Fetissov et al. (2002) |
Preclinical studies
Serum levels of auto-Abs against appetite-regulating neuropeptides were tested in both germ-free (GF; sterile of bacterial colonization) and specific-pathogen free (SPF; lacking only particular bacteria) rats to assess the dependence of auto-Ab production on the gut microbiota (Fetissov et al., 2008a). Serum auto-Abs against 14 appetite-regulating peptides were detected in both GF and SPF rats. However, observed levels differed, e.g. significantly lower levels of auto-Abs against AgRP were found in GF than SPF rats, whereas ghrelin auto-Abs were significantly higher in GF than SPF rats (Fetissov et al., 2008a).
A proteomics mouse study was conducted to validate the role of the gut microbiota and molecular mimicry in the pathophysiology of EDs (Tennoune et al., 2014). E. coli K12 was selected for its sequence homology with α-MSH. Specifically, a Caseinolytic protease B (ClpB) heat-shock disaggregation chaperone protein from E. coli K12 can mimic α-MSH. ClpB-immunized mice produced anti-ClpB auto-Abs that also demonstrated cross-reactivity with α-MSH, and demonstrated lower MC4R signaling, which is involved in energy balance regulation (Tennoune et al., 2014). ClpB-immunization of mice did not contribute to a significant change in locomotor behavior. However, immunized mice demonstrated reduced time spent, and shorter distance traveled, in closed arms of the O-maze test, suggesting decreased anxiety. Mean daily food intake was higher in immunized mice operating via interference with satiation mechanisms (i.e. constant number of meals, but increased meal size) rather than operating through hunger. Thus, anti-ClpB auto-Abs influence anxiety behavior and food intake by reduced sensitivity to the anorexigenic effects of α-MSH (Tennoune et al., 2014).
As an immediate follow-up study, the role of E. coli-related auto-Abs against α-MSH was further investigated as a possible explanation for sex-related differences in ED prevalence (Tennoune et al., 2015). E. coli K12 was more abundant in female than male rat feces. When administered by gavage over 3 weeks, the strain led to higher food and water intake, and weight gain, in females (Tennoune et al., 2015). Male rats trended towards increased anti-ClpB IgM, whereas plasma levels of α-MSH-reactive IgG were elevated in females. This study suggests that at least a part of sex-differences in food intake, immune response, kinetics and energy balance are related to differences in the prevalence of E. coli K12 in male and female rats, which contributes to differences in satiety, feeding, and emotional behavior in rats (Tennoune et al., 2015).
Clinical studies
Fetissov et al. (2005) investigated correlations of auto-Ab levels [against α-MSH, adrenocorticotropic hormone (ACTH), oxytocin (OT) and arginine vasopressin (AVP)] with ED-related psychological traits in both control and ED participants. Although auto-Abs were found in both groups, suggesting possible homeostatic functions, their levels differed. AN patients had significantly increased auto-Abs against α-MSH, OT and AVP, compared to BN and controls. These changes correlated with differences in ED-related cognitive and behavioral traits [assessed by Eating Disorder Inventory-Two (EDI-2)] among the three groups; AN and BN patients had similar total EDI-2 scores, which were higher than that of the control group. Auto-Abs against the peptides of interest showed correlations with EDI-2 scores in the ED groups; α-MSH demonstrated the highest correlation. Furthermore, auto-Abs against adrenocorticotropic hormone (ACTH) correlated positively (in AN) and negatively (in BN patients) with maturity fears, a wish for the security of pre-adolescent years in response to the demands of adulthood (Fetissov et al., 2005). A translational component of this study is discussed below.
To better understand if the presence of auto-Abs was involved in the etiology of ED pathology, or if auto-Ab dysregulation was a consequence of pathology, the presence of auto-Abs against 14 appetite-regulating peptides in the sera of healthy women was tested (Fetissov et al., 2008a). Auto-Abs against all peptides were identified, suggesting a general role of auto-Abs in normal physiology, not just in EDs. Sequence homology of at least 5 consecutive amino-acids between microorganisms and peptides of interest was confirmed, a criterion for molecular mimicry (Fetissov et al., 2008a).
To extend preclinical findings, Tennoune and colleagues (2014) took plasma samples from participants with EDs who also completed the EDI-2. Patients demonstrated elevated α-MSH cross-reactive anti-ClpB antibodies when compared to healthy female controls. Auto-Ab levels positively correlated to EDI-2 scores, demonstrating a potential link between the gut microbiota and ED behaviors.
Using similar methods, Breton and colleagues (Breton et al., 2016a) tested for plasma ClpB-levels in both ED and control participants, and administered the EDI-2 and the Montgomery-Åsberg Depression Rating Scale (MADRS), confirming previous findings (Tennoune et al., 2014). The plasma ClpB protein was present in all groups, with higher levels in the ED groups, that were not different from each other (Breton et al., 2016a). In ED patients, plasma ClpB positively correlated with α-MSH auto-Abs and significantly correlated with EDI-2 subscales: interpersonal distrust, social insecurity, and bulimia in AN patients; bulimia, maturity fears, interceptive awareness, and EDI-2 total score in BN patients; and ineffectiveness, maturity fears, and drive for thinness in BED patients. Significant correlations between ClpB and MADRS total scores were found only in AN patients (Breton et al., 2016a).
In an observational study of patients undergoing treatment for AN, stool samples were collected at admission and discharge (Kleiman et al., 2015b). Lower microbial diversity was observed in AN patients compared to healthy controls (n = 12). Microbial diversity had a significant negative association with self-reported depression, anxiety and ED psychopathology, and was negatively correlated with more severe ED symptoms. Slight improvements in diversity were made throughout the course of weight-restoration treatment (Kleiman et al., 2015a).
Translational studies
Human sera were applied to sections of rat brains (Fetissov et al., 2002). Approximately 74% of human sera bound to rat pituitary melanotropes and corticotropes, and approximately 20% selectively bound to α-MSH projections. When adsorbed with α-MSH and ACTH peptides, reduced staining was observed for both peptides, demonstrating that plasma auto-Abs from ED individuals and administered peptides share similar binding sites and can cross-react (Fetissov et al., 2002, 2005).
In summary, the ability of auto-Abs to interfere in appetite-related peptide signaling, and the influence of gut microbial proteins in modulating this signaling, demonstrates the potential impact of the microbiome-gut-brain axis on behaviors related to EDs.
GUT MICROBIOTA, DYSBIOSIS AND ALCOHOL AND SUBSTANCE USE DISORDERS
Broad overview of the field
Until recently, little was known about the specific effects of alcohol on the gut microbiome. Dysbiosis was reported in only a subset of patients with alcohol use disorder (AUD) who showed lower and higher median abundances of Bacteroidetes and Proteobacteria, respectively, such that microbial alterations correlated with elevated serum endotoxin levels (Mutlu et al., 2012). Alterations in microbial composition were not correlated to the duration of sobriety, suggesting alcohol-related dysbiosis is long-lasting and persists despite abstinent periods (Mutlu et al., 2012), though abstinence was measured primarily via self-report assessments. Chronic alcohol consumption can also induce bacterial overgrowth in the small intestine, mucosal damage in the large intestine, and subsequent elevations in intestinal permeability (Keshavarzian et al., 2009). Intestinal permeability increases with chronic stress, causing immunomodulatory bacterial cell wall components, such as lipopolysaccharides (LPS), to escape into the circulation and impact the host. For example, by altering the immune system (Cryan and Dinan, 2012) or lowering pain sensitivity thresholds (Benson et al., 2012). Although gut microbial alterations related to chronic alcohol use are supported, the mechanism of action is still unclear (Vassallo et al., 2015). A recent preclinical study evaluating the effect of vaporized ethanol administration in mice demonstrated elevated alpha diversity, significantly increased Alistipes genus, and significantly diminished genra Clostridium IV and XIVb, Dorea, and Coprococcus between mice exposed to ethanol vapor and controls (Peterson et al., 2017). Additionally, current understandings of the effect of drugs abuse on the gut microbiota are sparse, although differences have been demonstrated between the commensal gut microbiota of cocaine users and control subjects (Volpe et al., 2014).
This review led to the identification of three clinical papers that investigated the potential link between gut microbiome and AUD-related behaviors (Table 4). No preclinical studies were identified in the timeframe of the literature review. However, one preclinical paper was published investigating cocaine-related behaviors, and was included (Table 4).
Table 4.
Summary of findings on alcohol and substance use disorders
| Sample studied | Main finding | Sample size | Reference |
|---|---|---|---|
| Alcohol Dependent (AD) patients undergoing 3-week abstinence |
|
|
Leclercq et al. (2012) |
| Actively drinking AD patients undergoing alcohol detoxification |
|
|
Leclercq et al. (2014a) |
| AD patients undergoing 3-week abstinence |
|
Preliminary:
|
Leclercq et al. (2014b) |
| Male C57Bl/6 Mice |
|
n = 5–22/group | Kiraly et al. (2016) |
Preclinical study
A recent study by Kiraly and colleagues (2016) represents the first published investigation, to our knowledge, of the direct effects of gut microbiota manupulation on cocaine-related behaviors. Mice treated with antibiotics to diminish their gut microbiota demonstrated enhanced cocaine-reward and locomotive sensitivity. Antibiotic-treated mice exhibited conditioned place preference at lower doses than control mice, revealing a shifted dose-response curve in mice with reduced gut bacteria. Effects were unrelated to drug metabolism, the experimental methods, or increased intestinal permeability. The alterations were correlated to changes in the encoding of proteins important in reward circuitry transcription, and the repletion of bacterial products reversed behavioral outcomes.
Clinical studies
A study by Leclercq and colleagues (2012) evaluated the relationships between abstinence and intestinal permeability, inflammation, and symptoms relevant to alcohol relapse (i.e. alcohol craving, depression, anxiety, selective attention, or reaction time measured through a response inhibition task). Patients were tested the day after admission to a detoxification ward (T1) and at discharge (T2) for those who remained abstinent over the 18–19 days stay. Though significantly elevated at T1, alcohol dependent patients intestinal permeability and LPS concentrations recovered completely by T2. Alcohol dependent individuals were characterized by low-grade inflammation and greater psychological symptomology, with significantly elevated pro-inflammatory cytokines and higher depression, anxiety, and craving. A slight recovery in cytokine levels and a significant decrease in psychobehavioral outcomes was observed throughout short-term abstinence, though levels were still higher than controls. Additionally, alcohol dependent individuals performed worse than controls on the selective attention task, but demonstrated improvements in reaction time at T2 compared to T1. Importantly, cytokine levels were significantly positively associated with craving; selective attention was correlated with intestinal permeability; and cytokines such as interleukin-6 (IL-6) and IL-10 were positively correlated with anxiety, and negatively correlated with depression.
In another study, the same research team investigated whether gut-derived bacterial products influence peripheral blood mononuclear cells (PBMCs), important components of the immune system (Leclercq et al., 2014a). By comparing alcohol dependent patients to healthy controls at T1 and T2, links between immune, inflammatory, and psychobehavioral changes were assessed (Leclercq et al., 2014a). Gut bacteria-derived LPS and peptidoglycan (PGN) were elevated in alcohol dependent patients, and were accompanied by enhanced expression and activation of Toll-like receptor 4 (TLR4) and TLR2 complexes in PBMCs, which bind LPS and PGN, respectively (Leclercq et al., 2014a). Additionally, plasma levels of pro-inflammatory cytokines were elevated in alcohol dependent versus control patients, and were positively correlated with alcohol consumption and craving scores at T1. Changes in craving scores, which decreased over the course of detoxification, correlated significantly with the changes in cytokine levels. IL-8 levels best predicted alcohol craving in alcohol dependent patients (Leclercq et al., 2014a).
The effects of short-term abstinence on inflammatory pathways were further investigated (Leclercq et al., 2014a). Pro-inflammatory cytokine messenger ribonucleic acid (mRNA) levels were reduced while TLR4, but not TLR2, mRNA levels decreased significantly towards levels similar to controls. Thus, short-term withdrawal was associated with recovery of LPS-dependent receptors, but not PGN-dependent receptors (Leclercq et al., 2014a).
In a follow-up study, the relationships between altered intestinal permeability, gut microbial composition and activity, and AD-related clinical symptoms were evaluated using similar methods as described above (Alcock et al., 2014). Only 43% of patients had elevated intestinal permeability at T1. The remaining 57% had intestinal permeability levels similar to controls. The patients were split into high and low intestinal permeability groups. Differences in the family and genus levels of bacteria were observed between high intestinal permeability, low intestinal permeability, and control participants. Some degree of microbiota composition recovery was observed following abstinence. Specifically, increases in Ruminococcaceae in high intestinal permeability individuals. Additional assessments revealed elevated cytokine and pro-inflammatory levels dependent on intestinal permeability status, such that significantly high IL-8 levels were observed when intestinal permeability was high. Positive correlations between psychological symptoms and intestinal permeability suggest a role for the gut-barrier in the clinical presentation of alcohol dependence. High intestinal permeability was associated with a more severe clinical alcohol dependence profile, with persistent symptoms despite slight recovery of intestinal permeability observed at T2. In contrast, psychological symptoms of those with low intestinal permeability recovered at T2 (Leclercq et al., 2014a).
DISCUSSION
Critical view of the studies reported
Though preliminary and limited, existing literature suggests a role of the gut microbiota in behaviors associated with EDs and alcohol and substance use disorder. Evidence of molecular mimicry and auto-Abs against appetite-regulating neuropeptides highlight a potential mechanism by which gut level activity impacts neural signaling and ED-related host behavior. The gut microbiota is not necessary for the production of auto-Abs, but can modulate their levels and may serve as a potential target for ED interventions (Fetissov et al., 2008a). Auto-abs against central, appetite-regulating peptides like α-MSH alter melanocortin system functioning, which can contribute to non-metabolic alterations in eating behavior and food craving, and may explain the clinical correlations between auto-Abs and self-reported ED symptoms (Fetissov et al., 2005; Tennoune et al., 2014; Kleiman et al., 2015b). Though the presence of auto-Abs is insufficient to cause EDs (Fetissov et al., 2005), high blood–brain-barrier permeability, which can result from stress, may be involved in triggering pathogenesis. The research presented identifies auto-Abs against key peptides as not only treatment targets, but also as markers for the function of the gut-brain axis and for response to microbiota-related ED treatments (Inui et al., 2015) and as potential means of identifying ED risk factors (Tennoune et al., 2015). By checking auto-Ab levels and sequence homology comparisons between ED and healthy participants, the studies reviewed support the possibility that gut microbiota-driven dysregulation of neuroendocrine control of appetite leads to behavioral profiles associated with food intake in patients with EDs.
With respect to AUD, chronic alcohol use induces dysbiosis and increased intestinal permeability in only a subset of alcohol dependent patients (Bode and Bode, 2003; Mutlu et al., 2012), allowing microbial metabolites to enter peripheral and central circulation and impact behavioral correlates such as alcohol craving (Leclercq et al., 2014b). Thus, chronic excessive alcohol use is necessary, but not sufficient to cause gut dysfunction in alcohol dependent patients (Mutlu et al., 2012; Leclercq et al., 2014b). Still, gut microbial and peripheral metabolite level alterations were linked to alcohol craving, anxiety, and depression, which are important factors associated with relapse (de Timary et al., 2015). Interestingly, the level of intestinal permeability was differentially associated with behaviors, with recovery in depression, anxiety, and craving levels observed in individuals with low intestinal permeability, but not in those with high intestinal permeability. This further suggests a role of the microbiome-gut-brain axis in AUD. The low intestinal permeability group demonstrated reduced severity and duration of psychological symptoms, while the high intestinal permeability group showed persistent AD-related behavioral changes throughout short-term abstinence, despite intestinal permeability recovery (Leclercq et al., 2014b). Two possible conclusions regarding the persistence of symptoms in the high intestinal permeability group were proposed (de Timary et al., 2015). On one hand, perhaps the symptomatology is not related to the amount of alcohol consumed, as both groups demonstrated comparable use, but is instead related to the composition and function of the gut microbiota, which differed between high and low intestinal permeability patients. On the other hand, perhaps the observed levels of anxiety and depression in the low intestinal permeability group are due to ethanol rather than direct influence of the gut microbiota, as these symptoms decrease during abstinence. Still, craving persists in both the intestinal permeability groups despite abstinence, suggesting alcohol alone is an insufficient explanation. Altered microbial measures associated with altered peripheral signaling and low-grade systemic inflammation that were observed at T2 suggest that alcohol use is likely the primary effector of how the inflammatory response affects alcohol craving (Leclercq et al., 2014a). Nonetheless, the influence of a possible interaction between the gut microbiota and ethanol in impacting the psychological profiles of AUD patients must be considered. Finally, a promising preliminary preclinical investigation demonstrating altered behavioral responses to repeated cocaine administration in mice with diminished gut microbiota profiles provides further validation for this area of research and must be replicated, as well as tested in other animal models and with additional drugs of abuse (Kiraly et al. 2016).
Both EDs and AUD seem to be linked with some form of barrier imbalance: elevated blood–brain barrier in the case of ED and elevated intestinal barrier permeability in the case of AUD. This opens the door for gut microbial products to reach the central and peripheral circulations to contribute to pathogenesis in both disorders. Moreover, binge eating and binge drinking are associated with forms of negative affect, such as anxiety and depression (Ferriter and Ray, 2011). The studies reviewed above demonstrate that alterations to the gut microbiota in clinical presentations of both ED and AUD have been correlated with altered anxiety and depressive behavior, potentially indicating a shared mechanism of influence exerted by the gut microbiota on these behavioral disorders.
Although this review facilitates the identification of promising directions for future research, the paucity of existing research on the topic of behavioral outcomes associated with EDs and alcohol and substance use disorders is a limitation of this assessment. Moreover, the majority of relevant studies produced by our search are concentrated in specific labs, and the results need additional validation.
Future directions
Limitations from the studies reviewed here call for additional research. Only one of the above studies involved inpatient admission for ED treatment, the duration of which was not mentioned (Kleiman et al., 2015b). Additionally, preclinical work found that the prevalence of E. coli-related auto-Ab is different between female and male rats, while most of the clinical investigations were conducted with female participants.
Investigating the potential causal relationship between gut microbiota and the pathological behaviors here reviewed is another important future direction. Though establishing causality in human models may prove challenging, translational models transplanting human gut microbiota samples to rodent models could be utilized to clarify the directionality of the microbiota-behavior relationship. Moreover, given the relative independence of the amount of ethanol consumed from the presence or absence of dysbiosis and intestinal permeability (de Timary et al., 2015), it will be important to determine whether altered gut microbial profiles can be viewed as a precursor to AUD. Greater awareness of the causal relationship between dysbiosis and alcohol intake may shed light on the mechanisms of communication between the gut microbiota and the brain. Understanding whether the effects are carried out via metabolites released into peripheral circulation (Mutlu et al., 2012), altered vagal nerve signaling (Forsythe et al., 2012), changes in addiction-related neurotransmitter systems (Cryan and Dinan, 2012), and/or inflammatory mechanisms (Leclercq et al., 2014a) will better inform potential intervention strategies. Given existing evidence, it is possible these mechanisms occur simultaneously and complement each other, but this must be confirmed in targeted studies. Treatments to improve intestinal integrity and modify serum metabolites are possible intervention strategies that should be investigated, as has been proposed for treatment of autistic behavior and related gut microbial dysregulation (Vuong and Hsiao, 2016). Finally, recent evidence suggests that the gut microbiota may influence host appetite via both short-term, local effects, and long-term changes (Breton et al., 2016b). Further investigation into the role of molecular mimicry in the longevity and mechanism of appetite regulation is needed.
Both EDs and AUDs affect nutritional intake or exert caloric effects. Given the known role of diet and caloric intake in driving gut microbiota composition, it must be noted that none of the studies included here controlled for the diet-microbiota interactions. Such experimental controls would be valuable areas of future research. Moreover, consumption of different types of alcohol was found to lead to different permeability profiles. For example, high intestinal permeability individuals tended to consume less wine and more spirits than low intestinal permeability patients (Leclercq et al., 2014b). Additionally, red wine consumption has been associated with higher microbial diversity and enhancement of bacterial species that convey anti-inflammatory properties (Zhernakova et al., 2016). Thus, perhaps types of alcoholic beverages, or specific components of certain alcohols, have differential effects on behaviors associated with AUD and must therefore be controlled for in future investigations.
Finally, molecular mimicry has been implicated in a number of disease states, including autoimmune disorders (Hornig, 2013), Alzheimer’s disease, and Parkinson’s disease (Friedland, 2015), and it is associated with some health-promoting effects (Friedland, 2015). Given the shared reward mechanisms associated with EDs and alcohol and substance use disorders, and the proven relevance of hormonal signaling in alcohol and substance use disorders (Kenna et al., 2012; Engel and Jerlhag, 2014), perhaps molecular mimicry plays a role in the pathology of behavioral disorders other than EDs. Additionally, molecular mimicry involving auto-Abs against other neuronal peptides relevant to alcohol and substance use disorders and ED may be involved through interference with reward pathways (Morris et al., 2016).
A better understanding of the factors contributing to, or causing, persistent craving for alcohol, cocaine and/or other drugs of abuse, ED-related behavior, depression, and anxiety symptoms can better inform clinicians and scientists to develop targeted treatment strategies. As a recent review suggests, perhaps the gut microbiota can be targeted to mitigate alcohol relapse behavior (Gorky and Schwaber, 2016). More generally, psychobiotics, organisms that beneficially impact the health of patients with psychiatric illnesses, have been demonstrated to impact depression and anxiety (Dinan et al., 2013; Fondo et al., 2015) and could be tested in future studies on ED and alcohol and substance use disorders.
CONCLUSION
This systematic review supports preliminary evidence for the role of the gut microbiota in EDs and alcohol and substance use disorders and suggests that additional investigation is needed to determine what is causative versus epiphenomenological. Should the promising findings outlined here be replicated and expanded towards causal and mechanistic relationships, manipulations of the microbiome-gut-brain axis may represent novel approaches for the treatment of EDs and/or alcohol and substance use disorders.
ACKNOWLEDGMENTS
The authors would like to thank Ms Karen Smith, from the National Institutes of Health Library, for bibliographic assistance.
FUNDING
This work was supported by National Institutes of Health (NIH) intramural funding ZIA-AA000218 (Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology; PI: Dr Leggio), jointly supported by the Division of Intramural Clinical and Biological Research of the National Institute on Alcohol Abuse and Alcoholism and the Intramural Research Program of the National Institute on Drug Abuse; and by a Peter G. Dodge Foundation (PGDF) grant award (PI: Dr Leggio). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHORS’ CONTRIBUTIONS
L.L. thought of the scientific basis and rationale for this systematic review. J.E.T. and S.B. performed the literature search and screened titles and abstracts for their relevance to the objective of this work. J.E.T. wrote the first draft of the manuscript. L.L. supervised the manuscript preparation. S.B., M.F., M.R.L. and J.F.C. contributed to the interpretation of the results and manuscript preparation. All authors critically reviewed the contents and approved the final version of the manuscript.
REFERENCES
- Abt MC, Artis D (2009) The intestinal microbiota in health and disease: The influence of microbial products on immune cell homeostasis. Curr Opin Gastroenterol 25:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JB, Johansen LJ, Powell LD, et al. (2011) Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. Bmc Gastroenterol 11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcock J, Maley CC, Aktipis CA (2014) Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. Bioessays 36:940–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, (DSM-5). Washington, DC: American Psychiatric Association. [Google Scholar]
- Andersen AE. (2014) A proposed mechanism underlying eating disorders and other disorders of motivated behaviour In Andersen AE (ed). Males With Eating Disorders. London: Routledge, 221–54. [Google Scholar]
- Armougom F, Henry M, Vialettes B, et al. (2009) Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS One 4:e7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Ley RE, Sonnenburg JL, et al. (2005) Host-bacterial mutualism in the human intestine. Science 307:1915–20. [DOI] [PubMed] [Google Scholar]
- Barry D, Clarke M, Petry NM (2009) Obesity and its relationship to addictions: Is overeating a form of addictive behavior. Am J Addict 18:439–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker AE, Grinspoon SK, Klibanski A, et al. (1999) Eating disorders. N Engl J Med 340:1092–8. [DOI] [PubMed] [Google Scholar]
- Bendtsen KMB, Krych L, Sorensen DB, et al. (2012) Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PLoS One 7:e46231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S, Kattoor J, Wegner A, et al. (2012) Acute experimental endotoxemia induces visceral hypersensitivity and altered pain evaluation in healthy humans. Pain 153:794–9. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, et al. (2010) The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Res 1350:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode C, Bode JC (2003) Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol 17:575–92. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, et al. (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 108:16050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton J, Legrand R, Akkermann K, et al. (2016. a) Elevated plasma concentrations of bacterial ClpB protein in patients with eating disorders. Int J Eat Disord 49:805–8. [DOI] [PubMed] [Google Scholar]
- Breton J, Tennoune N, Lucas N, et al. (2016. b) Gut commensal E. coli proteins activate host satiety pathways following nutrient-induced bacterial growth. Cell metab 23:324–34. [DOI] [PubMed] [Google Scholar]
- Bulik CM. (2016) Towards a science of eating disorders: Replacing myths with realities: The fourth Birgit Olsson lecture. Nord J Psychiatry 70:224–30. [DOI] [PubMed] [Google Scholar]
- Burokas A, Moloney RD, Dinan TG, et al. (2015) Microbiota regulation of the mammalian gut-brain axis. Adv Appl Microbiol 91:1–62. [DOI] [PubMed] [Google Scholar]
- Carr J, Kleiman SC, Bulik CM, et al. (2016) Can attention to the intestinal microbiota improve understanding and treatment of anorexia nervosa. Expert Rev Gastroenterol Hepatol 10:565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, et al. (2013) The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 18:666–73. [DOI] [PubMed] [Google Scholar]
- Cone RD. (2005) Anatomy and regulation of the central melanocortin system. Nat Nurosci 8:571–78. [DOI] [PubMed] [Google Scholar]
- Coquerel Q, Sinno MH, Boukhettala N, et al. (2012) Intestinal inflammation influences alpha-MSH reactive autoantibodies: Relevance to food intake and body weight. Psychoneuroendocrinology 37:94–106. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG (2012) Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13:701–12. [DOI] [PubMed] [Google Scholar]
- de Timary P, Leclercq S, Starkel P, et al. (2015) A dysbiotic subpopulation of alcohol-dependent subjects. Gut Microbes 6:388–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, et al. (2011) Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA 108:3047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Stanton C, Cryan JF (2013) Psychobiotics: A novel class of psychotropic. Biol Psychiatry 74:720–6. [DOI] [PubMed] [Google Scholar]
- Duca FA, Swartz TD, Sakar Y, et al. (2012) Increased oral detection, but decreased intestinal signaling for fats in mice lacking gut microbiota. PLoS One 7:e39748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JA, Jerlhag E (2014) Role of appetite-regulating peptides in the pathophysiology of addiction: Implications for pharmacotherapy. CNS Drugs 28:875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriter C, Ray LA (2011) Binge eating and binge drinking: An integrative review. Eat Behav 12:99–107. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Dechelotte P (2008) The putative role of neuropeptide autoantibodies in anorexia nervosa. Curr Opin Clin Nutr Metab Care 11:428–34. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Hallman J, Oreland L, et al. (2002) Autoantibodies against alpha -MSH, ACTH, and LHRH in anorexia and bulimia nervosa patients. Proc Natl Acad Sci USA 99:17155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetissov SO, Hamze Sinno M, Coeffier M, et al. (2008. a) Autoantibodies against appetite-regulating peptide hormones and neuropeptides: Putative modulation by gut microflora. Nutrition 24:348–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetissov SO, Hamze Sinno M, Coquerel Q, et al. (2008. b) Emerging role of autoantibodies against appetite-regulating neuropeptides in eating disorders. Nutrition 24:854–9. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Harro J, Jaanisk M, et al. (2005) Autoantibodies against neuropeptides are associated with psychological traits in eating disorders. Proc Natl Acad Sci USA 102:14865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint HJ, Scott KP, Louis P, et al. (2012) The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 9:577–89. [DOI] [PubMed] [Google Scholar]
- Fondo EN, Chaloupka M, Heymans JJ, et al. (2015) Banning fisheries discards abruptly has a negative impact on the population dynamics of charismatic marine megafauna. PLoS One 10:e0144543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe P, Kunze WA (2013) Voices from within: Gut microbes and the CNS. Cell Mol Life Sci 70:55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe P, Kunze WA, Bienenstock J (2012) On communication between gut microbes and the brain. Curr Opin Gastroenterol 28:557–62. [DOI] [PubMed] [Google Scholar]
- Friedland RP. (2015) Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J Alzheimers Dis 45:349–62. [DOI] [PubMed] [Google Scholar]
- Goodman A. (2008) Neurobiology of addiction: An integrative review. Biochem Pharmacol 75:266–322. [DOI] [PubMed] [Google Scholar]
- Gorky J, Schwaber J (2016) The role of the gut-brain axis in alcohol use disorders. Prog Neuropsychopharmacol Biol Psychiatry 65:234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, et al. (2015) Epidemiology of DSM-5 alcohol use disorder: Results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry 72:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Saha TD, Ruan WJ, et al. (2016) Epidemiology of DSM-5 drug use disorder: Results from the national epidemiologic survey on alcohol and related conditions-III. JAMA Psychiatry 73:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoban A, Stilling R, Ryan F, et al. (2016) Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry 6:e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig M. (2013) The role of microbes and autoimmunity in the pathogenesis of neuropsychiatric illness. Curr Opin Rheumatol 25:488–795. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, et al. (2013) Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155:1451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui A, Chen CY, Meguid M (2015) Microbiome, peptide autoantibodies, and eating disorders: A missing link between gut and brain. Nutrition 31:544–5. [DOI] [PubMed] [Google Scholar]
- Kenna GA, Swift RM, Hillemacher T, et al. (2012) The relationship of appetitive, reproductive and posterior pituitary hormones to alcoholism and craving in humans. Neuropsychol Rev 22:211–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A, Farhadi A, Forsyth CB, et al. (2009) Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol 50:538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Jeon YS, Chun J (2013) Current status and future promise of the human microbiome. Pediatr Gastroenterol Hepatol Nutr 16:71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Walker DM, Calipari ES, et al. (2016) Alterations of the host microbiome affect behavioral responses to cocaine. Sci Rep 6:35455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman SC, Carroll IM, Tarantino LM, et al. (2015. a) Gut feelings: A role for the intestinal microbiota in anorexia nervosa. Int J Eat Disord 48:449–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman SC, Watson HJ, Bulik-Sullivan EC, et al. (2015. b) The intestinal microbiota in acute anorexia nervosa and during renourishment: Relationship to depression, anxiety, and eating disorder psychopathology. Psychosom Med 77:969–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokare DM, Dandekar MP, Singru PS, et al. (2010) Involvement of alpha-MSH in the social isolation induced anxiety- and depression-like behaviors in rat. Neuropharmacology 58:1009–18. [DOI] [PubMed] [Google Scholar]
- Kollai M, Bonyhay I, Jokkel G, et al. (1994) Cardiac vagal hyperactivity in adolescent anorexia nervosa. Eur Heart J 15:1113–8. [DOI] [PubMed] [Google Scholar]
- Leclercq S, Cani PD, Neyrinck AM, et al. (2012) Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun 26:911–8. [DOI] [PubMed] [Google Scholar]
- Leclercq S, De Saeger C, Delzenne N, et al. (2014. a) Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biol Psychiatry 76:725–33. [DOI] [PubMed] [Google Scholar]
- Leclercq S, de Timary P, Delzenne NM, et al. (2017) The link between inflammation, bugs, the intestine and the brain in alcohol dependence. Transl Psychiatry 7:e1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq S, Matamoros S, Cani PD, et al. (2014. b) Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci USA 111:E4485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Wang B, Zhang M, et al. (2008) Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci USA 105:2117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall Dahlgren C, Wisting L (2016) Transitioning from DSM-IV to DSM-5: A systematic review of eating disorder prevalence assessment. Int J Eat Disord 49:975–97. [DOI] [PubMed] [Google Scholar]
- Luczynski P, McVey Neufeld KA, Oriach CS, et al. (2016. a) Growing up in a bubble: Using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int J Neuropsychopharmacol 19. pii: pyw020. doi:10.1093/ijnp/pyw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynski P, Whelan SO, O’Sullivan C, et al. (2016. b) Adult microbiota‐deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Eur J Neurosci 44:2654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack I, Cuntz U, Grämer C, et al. (2016) Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci Rep 6:26752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Million M, Angelakis E, Maraninchi M, et al. (2013) Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int J Obes (Lond) 37:1460–66. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Moher D, Liberati A, Tetzlaff J, et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita C, Tsuji H, Hata T, et al. (2015) Gut dysbiosis in patients with anorexia nervosa. PLoS One 10:e0145274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Broughton SJ, Wessels Q (2016) Microbes, molecular mimicry and molecules of mood and motivation. Med Hypotheses 87:40–3. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, et al. (2006) Central nervous system control of food intake and body weight. Nature 443:289–95. [DOI] [PubMed] [Google Scholar]
- Mutlu EA, Gillevet PM, Rangwala H, et al. (2012) Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol 302:G966–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Mental Health (NIMH) (2016) Eating Disorders, Vol. 2016 https://www.nimh.nih.gov/health/topics/eating-disorders/index.shtml?utm_source=rss&utm_medium=rss (13 April 2017, date last accessed). [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, et al. (2011) Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 23:255–64. e119. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Holmes E, Kinross J, et al. (2012) Host-gut microbiota metabolic interactions. Science 336:1262–7. [DOI] [PubMed] [Google Scholar]
- Peterson VL, Jury NJ, Cabrera-Rubio R, et al. (2017) Drunk bugs: Chronic vapour alcohol exposure induces marked changes in the gut microbiome in mice. Behav Brain Res 323:172–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel GD, Micheletti TO, Pace F, et al. (2012) Gut-central nervous system axis is a target for nutritional therapies. Nutr J 11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Li R, Raes J, et al. (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raevuori A, Lukkariniemi L, Suokas JT, et al. (2016) Increased use of antimicrobial medication in bulimia nervosa and binge eating disorder prior to the eating disorder treatment. Int J Eat Disord 49:542–52. [DOI] [PubMed] [Google Scholar]
- Savage DC. (1977) Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 31:107–33. [DOI] [PubMed] [Google Scholar]
- Schreiber LR, Odlaug BL, Grant JE (2013) The overlap between binge eating disorder and substance use disorders: Diagnosis and neurobiology. J Behav Addict 2:191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte EM, Grilo CM, Gearhardt AN (2016) Shared and unique mechanisms underlying binge eating disorder and addictive disorders. Clin Psychol Rev 44:125–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinno MH, Do Rego JC, Coeffier M, et al. (2009) Regulation of feeding and anxiety by alpha-MSH reactive autoantibodies. Psychoneuroendocrinology 34:140–9. [DOI] [PubMed] [Google Scholar]
- Smink FR, van Hoeken D, Hoek HW (2012) Epidemiology of eating disorders: Incidence, prevalence and mortality rates. Curr Psychiatry Rep 14:406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilling RM, Ryan FJ, Hoban AE, et al. (2015) Microbes & neurodevelopment–absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav Immun 50:209–20. [DOI] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, et al. (2004) Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 558:263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennoune N, Chan P, Breton J, et al. (2014) Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide alpha-MSH, at the origin of eating disorders. Transl Psychiatry 4:e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennoune N, Legrand R, Ouelaa W, et al. (2015) Sex-related effects of nutritional supplementation of Escherichia coli: Relevance to eating disorders. Nutrition 31:498–507. [DOI] [PubMed] [Google Scholar]
- Vassallo G, Mirijello A, Ferrulli A, et al. (2015) Review article: Alcohol and gut microbiota – the possible role of gut microbiota modulation in the treatment of alcoholic liver disease. Aliment Pharmacol Ther 41:917–27. [DOI] [PubMed] [Google Scholar]
- Volpe GE, Ward H, Mwamburi M, et al. (2014) Associations of cocaine use and HIV infection with the intestinal microbiota, microbial translocation, and inflammation. J Stud Alcohol Drugs 75:347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong HE, Hsiao EY (2016) Emerging roles for the gut microbiome in autism spectrum disorder. Biol Psychiatry 81:411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, et al. (2013) Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet 382:1575–86. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2011) Global Status Report on Alcohol and Health. Geneva: WHO. [Google Scholar]
- Zhernakova A, Kurilshikov A, Bonder MJ, et al. (2016) Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 352:565–69. [DOI] [PMC free article] [PubMed] [Google Scholar]