Abstract
We sought to determine the relationship of fibroids to pregnancy loss in a prospective cohort in which fibroid status was uniformly documented in early pregnancy. Participants had an intake interview, transvaginal ultrasonography, computer-assisted telephone interview, and follow-up assessment of outcomes. We recruited diverse participants for the Right From the Start study from 8 metropolitan areas in 3 states in the United States during 2000–2012. Participants were at least 18 years of age, trying to become pregnant or at less than 12 weeks’ gestation, not using fertility treatments, fluent in English or Spanish, and available for telephone interviews. Miscarriage was defined as loss before 20 weeks’ gestation. Fibroid presence, number, type, and volume were assessed using standardized ultrasonography methods. We used proportional hazards models to estimate associations. Among 5,512 participants, 10.4% had at least 1 fibroid, and 10.8% experienced a miscarriage. Twenty-three percent had experienced a prior miscarriage and 52% prior births. Presence of fibroids was associated with miscarriage in models without adjustments. Adjusting for key confounders indicated no increase in risk (adjusted hazard ratio = 0.83, 95% confidence interval: 0.63, 1.08). No characteristic of fibroids was associated with risk. Prior evidence attributing miscarriage to fibroids is potentially biased. These findings imply that surgical removal of fibroids to reduce risk of miscarriage deserves careful scrutiny.
Keywords: fibroids, first trimester, miscarriage, pregnancy, reproductive epidemiology, spontaneous abortion, uterine leiomyoma
Although up to 1 in 5 women of reproductive age has uterine fibroids (1–3), little is known about the consequences of fibroids in pregnancy (4, 5). Fibroids have been reported to increase risk of miscarriage by approximately 60% (6–12), with some estimates of risk as much as 3-fold greater (13). Excluding studies of patients seeking fertility treatment, risk estimates have come from retrospective studies with participants selected from prenatal care or ultrasonography clinics. Most are limited by enrollment later in gestation than many miscarriages occur and by the predominance of participants from tertiary care centers, often with a history of infertility, high-risk pregnancy, or both (7–21). Methods used to assign fibroid status have included physical exam, participant self-report, birth certificates, and ultrasound databases not intended to uniformly identify all fibroids.
Imaging protocols also need updating. The ultrasonography criteria that were originally used to define the presence of a fibroid for research were established in the 1980s at the advent of obstetric ultrasonography and required the structure being identified to measure 3 cm or greater in mean diameter (22). Some studies have continued to use size requirements well above those routinely measured in clinical care with conventional ultrasonography equipment even though smaller fibroids are readily identifiable as a result of advances in ultrasonography technology (23–26). Updating the research definition to include smaller fibroids reduces misclassification of fibroid status and allows better assessment of the relationship between fibroid characteristics and pregnancy outcomes.
To advance understanding of the influence of fibroids on pregnancy, we sought to recruit participants who better represented the general population, to do so early in pregnancy or while participants were planning a pregnancy, and to document the presence or absence of fibroids uniformly by imaging all women. We prospectively enrolled women planning pregnancies or in early pregnancy from selected areas in 3 states using recruitment from the community. All had standardized research ultrasound examinations that included systematic repeated measurement of all fibroids. Specifically we aimed to determine whether the presence of 1 or more fibroids of 0.5 cm or greater in maximum diameter was independently associated with increased risk of miscarriage. We also assessed whether fibroid type, size, number, or total volume modified risk.
METHODS
Study population
With institutional review board approval, we used multiple methods to recruit women early in pregnancy or planning to become pregnant for Right From the Start: A Study of Early Pregnancy Health. Participants were recruited from 8 metropolitan areas in 3 states: Chapel Hill, Durham, and Raleigh in North Carolina; Galveston, Texas; and Knoxville, Memphis, Chattanooga, and Nashville in Tennessee (27). Recruitment materials were circulated through businesses, community groups, paid advertising, and direct mail, among other methods. Private practices that provide prenatal care and public clinics also helped inform patients. Interested women called a toll-free number and were screened for eligibility: aged 18 years or older, planning to live in the study area for 18 months, trying to become pregnant or pregnant less than 12 weeks’ completed gestation from last menstrual period, no use of fertility treatments, and fluency in English or Spanish, with access to a telephone for interviews. Pregnant women were enrolled after providing their last menstrual period date and date of first positive pregnancy test. Those trying to become pregnant were “pre-enrolled” and provided with free pregnancy test kits for up to 6 months. When they conceived, they were formally enrolled in the study.
At enrollment, participants provided basic demographic information and baseline data about selected risk factors for adverse pregnancy outcomes. Participants were later contacted by telephone for an extensive interview about potential influences on pregnancy, including prior reproductive history, medical history, symptoms and events during the current pregnancy, health behaviors (caffeine, tobacco, and alcohol use), medication and supplement use, and physical activity. The interview was completed at a mean gestational age of 12.2 (standard deviation, 3.1) weeks. Two-hundred eight-five (5.2%) women did not finish the first-trimester interview and had missing information for some covariates. Among them, 196 (69%) had live births, 1 (<1%) had a stillbirth, 39 (14%) had miscarriages, and 49 were censored (17%).
Transvaginal ultrasonography was scheduled at a research ultrasonography site to assess embryonic development and systematically examine the uterus for fibroids. The fibroid measurement protocol required 3 separate sets of measurements of each fibroid. Each set documented 3 perpendicular diameters. Multiple images of each fibroid with caliper markings of each diameter were recorded and a fibroid map completed. The map indicated the location of the fibroid in the uterus (cervix, corpus, fundus) and type of fibroid. Type was defined using mutually exclusive categories (submucous: any fibroid in contact with or distorting the uterine cavity without identifiable myometrium between the fibroid and the endometrium; subserous: distorting the external contour of the uterus; intramural: within the myometrium, neither distorting contour nor cavity; and pedunculated: attached to the uterus with an identifiable stalk). Study sonographers had 5 or more years of experience in obstetric ultrasonography, and images were reviewed by one of 2 obstetrician investigators masked to outcome.
A follow-up telephone interview was conducted or paper form returned at 20–25 weeks of pregnancy to update pregnancy status, acquire additional pregnancy information, confirm site(s) of clinical care, and to confirm the intended birth hospital. Pregnancy losses were generally identified by a participant call to the study office or at times by self-report during the first-trimester interview. Medical record review was conducted for all losses. Ongoing pregnancies had review of birth records and/or confirmation of birth greater than 20 weeks through vital records.
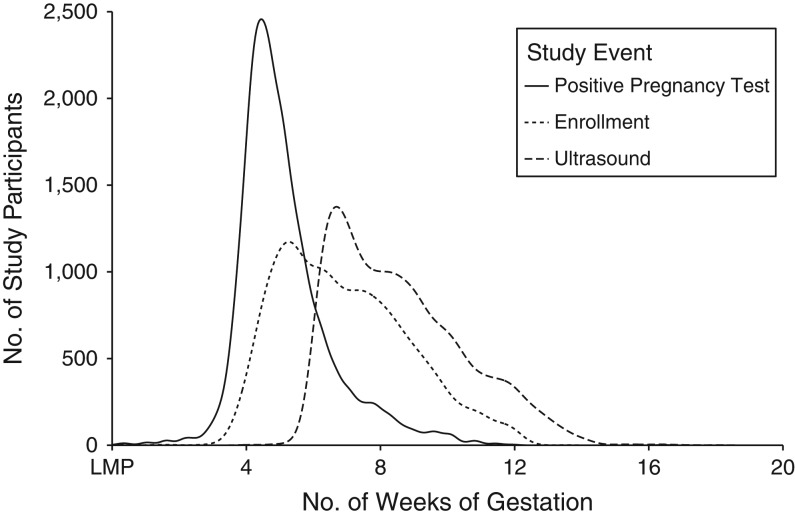
Self-report of pregnancy onset has been documented to be reliable for this population with a mean and median difference in gestational age of less than 1 day between estimates from dating by last menstrual period and ultrasound (28). We classified pregnancies with loss at less than 20 weeks’ gestation as miscarriages. The comparison group consisted of women who had births or censoring dates beyond 140 days’ gestation. The distribution of gestational age for participants at the time of key study events is illustrated in Figure 1.
Figure 1.
Distribution of gestational age at the time of study events, Right From the Start: A Study of Early Pregnancy Health, Southern United States, 2000–2012. The x-axis represents gestational age in weeks, and the y-axis is for the number of study participants with the related event over time. LMP, last menstrual period.
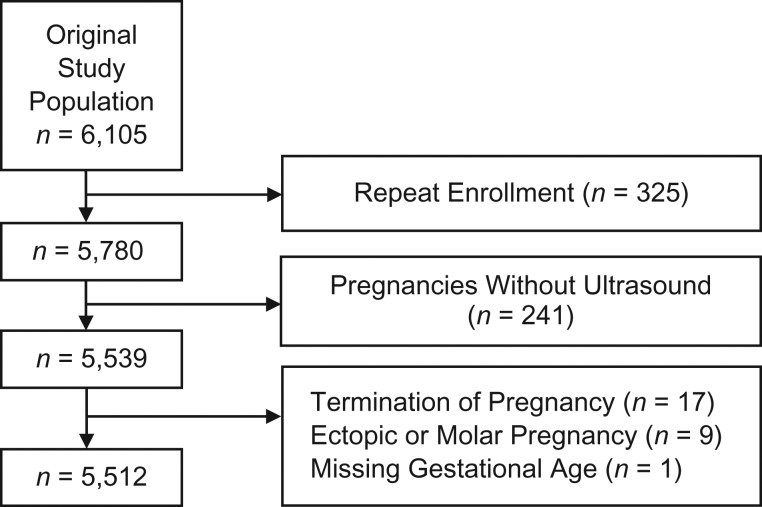
We enrolled a total of 6,105 pregnancies. This analysis is limited to the participants’ first participation if she enrolled for more than 1 pregnancy. We excluded women with induced abortions and ectopic pregnancies. We had data from 5,512 pregnancies for analysis (Figure 2).
Figure 2.
Flow diagram showing selection of study population, sample size, and exclusions, Right From the Start: A Study of Early Pregnancy Health, Southern United States, 2000–2012.
Our initial power calculations projected an increase in miscarriage risk and planned for a smaller study. With 3,300 participants and 10% anticipated to have fibroids, we had 85% power with α = 0.05 for a minimum detectable relative risk of 1.27 for an association of fibroids with miscarriage. We continued to collect ultrasound measurements of fibroids into subsequent phases of Right From the Start to enhance the precision of estimates.
Analytic methods
We used Cox proportional hazards survival models to characterize pregnancy loss in association with the presence of fibroids. Models adjusted for left truncation, allowing us to correctly estimate risk of miscarriage, conditioning on the fact that each subject had not had pregnancy loss before entering the cohort (29). For this analysis, women were followed, tracking days from enrollment through 20 weeks’ gestation, the occurrence of a pregnancy loss, or loss to follow up, whichever came first.
Potential confounding factors consisted of known and suspected influences on miscarriage, including maternal age, race/ethnicity, smoking, alcohol use, caffeine consumption, vitamin use, first-trimester progesterone use, diabetes status, first-trimester body mass index (BMI), prior termination of pregnancy, parity, marital status, maternal education, maternal employment status, household income, calendar year of conception, and study site. Restricted cubic splines with 4 knots (at 0.05, 0.35, 0.65, and 0.95 quantiles) were applied to allow for a nonlinear relationship of the subjects’ age and BMI (at study entry) with miscarriage risk (30). Confounders that resulted in at least a 10% change to the associations between fibroids and miscarriage were included in final models. Potential effect modification by age, BMI, smoking status, and race/ethnicity was assessed separately with a likelihood ratio test but not found (P > 0.3). Age and BMI were treated as continuous variables to evaluate effect modification. Using a Cox proportional hazards model, we generated adjusted hazard ratios for presence compared with absence of fibroids, fibroid number (none (referent), 1, ≥2), fibroid type (none (referent), any submucous, any intramural, any subserous), total fibroid volume (none (referent), first quartile (0.003–0.922 cc), second quartile (0.922–4.608 cc), third quartile (4.608–19.729 cc), and fourth quartile (19.729–987.229 cc)), and largest fibroid diameter (none (referent), first quartile (0.51–1.36 cm), second quartile (1.36–2.35 cm), third quartile (2.35–3.62 cm), and fourth quartile (3.62–13.20 cm)), with 95% confidence intervals. Assumptions of proportional hazards were met.
We conducted sensitivity analyses adjusting for demographic, behavioral, and socioeconomic factors in various regression models. We also conducted a sensitivity analysis adjusting for prior history of miscarriage in our final regression model. All analyses were repeated excluding the women whose last contact was prior to 20 weeks of gestation and who were thus censored in the full models at time of last contact with a viable pregnancy. A total of 375 women had missing data for the covariates included in model adjustments. We performed multiple imputation with 5 imputed data sets to impute missing covariates based on demographic factors, outcome status, and other covariates potentially associated with missing data.
We conducted 2 sets of sensitivity analyses including the 237 women without ultrasounds in the regression models. First, we performed multiple imputation of 5 data sets to assign fibroid status based on both demographic factors and outcome status. Second, we assessed the possibility that women with a pregnancy loss disproportionally missed the ultrasonography visit. For the 105 women with pregnancy loss and unknown fibroid status, we randomly assigned a probability of having at least 1 fibroid of 26.6%—twice the probability among women with losses in the cohort. For the remaining 132 women with unknown fibroid status, we randomly assigned a probability of having at least 1 fibroid of 10.5%—the same probability as in the cohort. Generated data were combined and analyzed with those whose fibroid status was known. We repeated this random assignment analysis 10 times to obtain average miscarriage risk and corresponding 95% confidence intervals. All analyses used R, version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria), or STATA, version 13 (StataCorp LP, College Station, Texas). We used 2-sided tests with a significance level of 0.05 for all statistical inferences.
RESULTS
Of the women enrolled in Right From the Start during 2000–2012, 5,512 had complete ultrasound data: 571 (10.4%) had at least 1 fibroid, and 595 (10.8%) experienced miscarriage before 20 completed weeks’ gestation. Forty-seven percent (n = 229) of miscarriages occurred by the end of the 10th week of pregnancy. In bivariate analysis, maternal age, black non-Hispanic race/ethnicity, greater educational attainment, higher household income, history of prior miscarriage, history of prior termination of pregnancy, higher BMI, and consumption of alcohol were associated with having at least 1 fibroid (Table 1).
Table 1.
Participant Characteristics According to Fibroid Status, Right From the Start: A Study of Early Pregnancy Health, Southern United States, 2000–2012
| Characteristic | No Fibroids (n = 4,941) |
At Least 1 Fibroid (n = 571) |
ORa | 95% CI | ||
|---|---|---|---|---|---|---|
| No. of Participants | % | No. of Participants | % | |||
| Maternal age, years | ||||||
| <25 | 1,065 | 21.55 | 48 | 8.41 | 1.00 | Referent |
| 25–29 | 1,770 | 35.82 | 139 | 24.34 | 1.74 | 1.24, 2.44 |
| 30–34 | 1,525 | 30.86 | 225 | 39.40 | 3.27 | 2.37, 4.51 |
| ≥35 | 581 | 11.76 | 159 | 27.85 | 6.07 | 4.33, 8.52 |
| Race/ethnicity | ||||||
| White, non-Hispanic | 3,564 | 72.13 | 324 | 56.74 | 1.00 | Referent |
| Black, non-Hispanic | 822 | 16.64 | 188 | 32.92 | 2.52 | 2.07, 3.06 |
| Hispanic ethnicity | 341 | 6.90 | 32 | 5.60 | 1.03 | 0.71, 1.51 |
| Other | 210 | 4.25 | 27 | 4.73 | 1.41 | 0.93, 2.15 |
| Refused to answer | 4 | 0.08 | 0 | 0.00 | ||
| Education | ||||||
| High school or less | 921 | 18.64 | 69 | 12.08 | 1.00 | Referent |
| Some college | 916 | 18.54 | 102 | 17.86 | 1.49 | 1.08, 2.04 |
| College or more | 3,103 | 62.80 | 400 | 70.05 | 1.72 | 1.32, 2.24 |
| Missing | 1 | 0.02 | 0 | 0.00 | ||
| Marital status | ||||||
| Married/cohabiting | 4,364 | 88.32 | 501 | 87.74 | 1.00 | Referent |
| Other | 577 | 11.68 | 70 | 12.26 | 1.06 | 0.81, 1.38 |
| Employment | ||||||
| No | 1,388 | 28.09 | 146 | 25.57 | 1.00 | Referent |
| Yes | 3,268 | 66.14 | 408 | 71.45 | 1.19 | 0.97, 1.45 |
| Missing | 285 | 5.77 | 17 | 2.98 | ||
| Household income, $/year | ||||||
| ≤40,000 | 1,430 | 28.94 | 137 | 23.99 | 1.00 | Referent |
| 40,001–80,000 | 1,672 | 33.84 | 214 | 37.48 | 1.34 | 1.07, 1.67 |
| ≥80,001 | 1,386 | 28.05 | 188 | 32.92 | 1.42 | 1.12, 1.79 |
| Missing | 453 | 9.17 | 32 | 5.60 | ||
| Parity | ||||||
| 0 | 2,202 | 44.57 | 270 | 47.29 | 1.00 | Referent |
| 1 | 1,604 | 32.46 | 185 | 32.40 | 0.94 | 0.77, 1.15 |
| ≥2 | 798 | 16.15 | 93 | 16.29 | 0.95 | 0.74, 1.22 |
| Missing | 337 | 6.82 | 23 | 4.03 | ||
| Miscarriage history | ||||||
| None | 3,602 | 72.90 | 386 | 67.60 | 1.00 | Referent |
| Any | 1,002 | 20.28 | 162 | 28.37 | 1.51 | 1.24, 1.84 |
| Missing | 337 | 6.82 | 23 | 4.03 | ||
| Prior termination of pregnancy | ||||||
| None | 3,963 | 80.21 | 434 | 76.01 | 1.00 | Referent |
| Any | 641 | 12.97 | 114 | 19.96 | 1.62 | 1.30, 2.03 |
| Missing | 337 | 6.82 | 23 | 4.03 | ||
| Body mass indexb | ||||||
| <18.5 | 126 | 2.55 | 11 | 1.93 | 0.94 | 0.50, 1.77 |
| 18.5–24.9 | 2,641 | 53.45 | 244 | 42.73 | 1.00 | Referent |
| 25.0–29.9 | 1,139 | 23.05 | 159 | 27.85 | 1.51 | 1.22, 1.87 |
| ≥30 | 945 | 19.13 | 153 | 26.80 | 1.75 | 1.41, 2.17 |
| Missing | 90 | 1.82 | 4 | 0.70 | ||
| Diabetes | ||||||
| No | 4,516 | 91.40 | 536 | 93.87 | 1.00 | Referent |
| Type 1 | 16 | 0.32 | 1 | 0.18 | 0.53 | 0.07, 3.98 |
| Type 2 | 11 | 0.22 | 4 | 0.70 | 3.06 | 0.97, 9.66 |
| Gestational diabetes | 101 | 2.04 | 13 | 2.28 | 1.08 | 0.60, 1.95 |
| Multiple types | 1 | 0.02 | 0 | 0.00 | ||
| Missing | 296 | 5.99 | 17 | 2.98 | ||
| Progesterone use in first trimester | ||||||
| No | 4,805 | 97.25 | 552 | 96.67 | 1.00 | Referent |
| Yes | 136 | 2.75 | 19 | 3.33 | 1.22 | 0.75, 1.98 |
| Missing | 0 | 0.00 | 0 | 0.00 | ||
| Smoking during pregnancy | ||||||
| No | 4,475 | 90.57 | 537 | 94.05 | 1.00 | Referent |
| Yes | 177 | 3.58 | 17 | 2.98 | 0.80 | 0.48, 1.33 |
| Missing | 289 | 5.85 | 17 | 2.98 | ||
| Alcohol drinking during pregnancy | ||||||
| No | 4,423 | 89.52 | 514 | 90.02 | 1.00 | Referent |
| Yes | 229 | 4.63 | 39 | 6.83 | 1.47 | 1.03, 2.08 |
| Missing | 289 | 5.85 | 18 | 3.15 | ||
| Vitamin use in pregnancy | ||||||
| No | 148 | 3.00 | 13 | 2.28 | 1.00 | Referent |
| Yes | 4,492 | 90.91 | 541 | 94.75 | 1.37 | 0.77, 2.43 |
| Missing | 301 | 6.09 | 17 | 2.98 | ||
| Caffeine use in pregnancy | ||||||
| No | 1,436 | 29.06 | 173 | 30.30 | 1.00 | Referent |
| Yes | 3,220 | 65.17 | 381 | 66.73 | 0.98 | 0.81, 1.19 |
| Missing | 285 | 5.77 | 17 | 2.98 | ||
| Pregnancy intention | ||||||
| No | 1,259 | 25.48 | 148 | 25.92 | 1.00 | Referent |
| Yes | 3,061 | 61.95 | 360 | 63.05 | 1.00 | 0.82, 1.22 |
| Missing | 621 | 12.57 | 63 | 11.03 | ||
| Study site | ||||||
| North Carolina | 2,709 | 54.83 | 368 | 64.45 | 1.00 | Referent |
| Tennessee | 1,847 | 37.38 | 191 | 33.45 | 0.76 | 0.63, 0.92 |
| Texas | 385 | 7.79 | 12 | 2.10 | 0.23 | 0.13, 0.41 |
| Missing | 0 | 0.00 | 0 | 0.00 | ||
Abbreviations: CI, confidence interval; OR, odds ratio.
a Unadjusted odds ratios from logistic regression.
b Body mass index was calculated as weight (kg)/height (m)2 and was categorized as underweight: <18.5; normal weight: 18.5–24.9; overweight: 25.0–29.9; or obese: ≥30.
Among women with fibroids, 29% had more than 1 (Table 2). The most common type was intramural (46%), followed by subserous (41%) and submucous (20%). Median fibroid diameter of the largest fibroid per woman was 2.3 cm (interquartile range, 1.4–3.6 cm). Median total fibroid volume per woman was 4.6 cc (interquartile range, 0.9–19.4 cc).
Table 2.
Association of Fibroid Status and Fibroid Characteristics With Pregnancy Loss, Right From the Start: A Study of Early Pregnancy Health, Southern United States, 2000–2012
| Fibroid Characteristic | Births (n = 4,710a) | Pregnancy Loss (n = 595) | Unadjusted HR | 95% CI | Adjusted HRb | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | |||||
| Fibroids present | ||||||||
| No | 4,225 | 89.70 | 516 | 86.72 | 1.00 | Referent | 1.00 | Referent |
| Yes | 485 | 10.30 | 79 | 13.28 | 1.29 | 1.02, 1.64 | 0.83 | 0.63, 1.08 |
| No. of fibroids | ||||||||
| 0 | 4,225 | 89.70 | 516 | 86.72 | 1.00 | Referent | 1.00 | Referent |
| 1 | 344 | 7.30 | 54 | 9.08 | 1.26 | 0.95, 1.67 | 0.84 | 0.62, 1.15 |
| ≥2 | 141 | 2.99 | 25 | 4.20 | 1.37 | 0.92, 2.05 | 0.79 | 0.51, 1.23 |
| Fibroid type | ||||||||
| None | 4,225 | 89.70 | 516 | 86.72 | 1.00 | Referent | 1.00 | Referent |
| Any subserous | 206 | 4.37 | 28 | 4.71 | 1.07 | 0.73, 1.56 | 0.64 | 0.42, 0.98 |
| Any intramural | 216 | 4.59 | 43 | 7.23 | 1.52 | 1.11, 2.07 | 1.05 | 0.75, 1.47 |
| Any submucous | 95 | 2.02 | 21 | 3.53 | 1.70 | 1.10, 2.63 | 0.92 | 0.56, 1.50 |
| Total fibroid volume, cc | ||||||||
| No fibroids | 4,225 | 89.70 | 516 | 86.72 | 1.00 | Referent | 1.00 | Referent |
| Quartile 1: 0.003–0.922 | 112 | 2.38 | 30 | 5.04 | 1.89 | 1.31, 2.73 | 1.23 | 0.82, 1.83 |
| Quartile 2: 0.922–4.608 | 121 | 2.57 | 20 | 3.36 | 1.27 | 0.81, 1.99 | 0.82 | 0.51, 1.32 |
| Quartile 3: 4.608–19.729 | 124 | 2.63 | 16 | 2.69 | 1.08 | 0.66, 1.78 | 0.57 | 0.33, 1.01 |
| Quartile 4: 19.729–987.229 | 128 | 2.72 | 13 | 2.18 | 0.88 | 0.51, 1.53 | 0.64 | 0.36, 1.13 |
| Largest fibroid diameter, cm | ||||||||
| No fibroids | 4,225 | 89.70 | 516 | 86.72 | 1.00 | Referent | 1.00 | Referent |
| Quartile 1: 0.51–1.36 | 114 | 2.42 | 29 | 4.87 | 1.79 | 1.23, 2.60 | 1.12 | 0.74, 1.68 |
| Quartile 2: 1.36–2.35 | 117 | 2.48 | 23 | 3.87 | 1.50 | 0.99, 2.28 | 1.02 | 0.65, 1.59 |
| Quartile 3: 2.35–3.62 | 125 | 2.65 | 16 | 2.69 | 1.05 | 0.64, 1.73 | 0.52 | 0.29, 0.91 |
| Quartile 4: 3.62–13.20 | 129 | 2.74 | 11 | 1.85 | 0.77 | 0.42, 1.39 | 0.62 | 0.34, 1.14 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Data for 207 subjects who were lost to follow-up before 20 weeks of gestation were not presented in the table, but they were included in the Cox regression models censored at the time of last known status.
b Multivariate Cox proportional hazards models adjusted for maternal age, race/ethnicity, alcohol use, history of prior termination of pregnancy, and parity.
Models without adjustments of the association between the presence of any fibroid and miscarriage risk suggested a potential association (hazard ratio (HR) = 1.29, 95% confidence interval (CI): 1.02, 1.64) (Table 2). After adjustment for maternal age, race/ethnicity, alcohol use, prior termination of pregnancy, and parity, the effect on risk was null (adjusted HR = 0.83, 95% CI: 0.63, 1.08), with most of the impact of adjustment due to the association of more advanced age with increased risk of fibroids and miscarriage. Our null findings were not influenced by timing of enrollment. Stratifying by whether participants enrolled prior to pregnancy (n = 1,454) or during early pregnancy (n = 4,058), the adjusted hazard ratios were 0.62 (95% CI: 0.37, 1.05) and 0.91 (95% CI: 0.66, 1.25), respectively, for the association of fibroids with miscarriage.
Increased risk of miscarriage by fibroid type was not supported by adjusted analyses (intramural, adjusted HR = 1.05, 95% CI: 0.75, 1.47; submucous, adjusted HR = 0.92, 95% CI: 0.56, 1.50). Effects of total fibroid volume and largest fibroid diameter from adjusted analyses also did not suggest increased risk (lowest quartile of total fibroid volume, adjusted HR = 1.23, 95% CI: 0.82, 1.83; lowest quartile of largest-fibroid diameter, adjusted HR = 1.12, 95% CI: 0.74, 1.68) (Table 2). All unadjusted associations between fibroid characteristics and miscarriage were explained by confounding.
Additional adjustment for prior history of miscarriage did not influence the association between the presence of fibroid and the risk of miscarriage (adjusted HR = 0.82, 95% CI: 0.63, 1.08). When we adjusted for common reproductive epidemiology covariates in the models as part of sensitivity analysis, we found that after age and maternal race/ethnicity entered the models, few other covariates exerted notable influence on the estimate. All remained close to the null (Table 3). Multiple imputation of missing covariates for 375 participants generated consistent results (data not shown).
Table 3.
Influence of Model Covariates on Results, Right From the Start: A Study of Early Pregnancy Health, Southern United States, 2000–2012
| Model Focus | Covariates in Model | HR | 95% CI |
|---|---|---|---|
| No adjustments | Fibroid status only | 1.29 | 1.02, 1.64 |
| Age | Age | 1.00 | 0.79, 1.28 |
| Basic demographics | Age, race/ethnicity | 0.94 | 0.73, 1.21 |
| Basic behavioral | Age, race/ethnicity, smoking, alcohol use | 0.85 | 0.66, 1.11 |
| Expanded behavioral | Age, race/ethnicity, smoking, alcohol use, caffeine use, prenatal vitamin use, progesterone supplementation | 0.86 | 0.66, 1.12 |
| Expanded behavioral with metabolic | Age, race/ethnicity, smoking, alcohol use, caffeine use, prenatal vitamin use, progesterone supplementation, diabetes status, body mass index | 0.87 | 0.66, 1.13 |
| Control of confoundinga | Age, race/ethnicity, alcohol use, prior termination of pregnancy, parity | 0.83 | 0.63, 1.08 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Final model presented in text.
Last, in formal sensitivity analyses, we included the 237 women who otherwise met criteria for this analysis but did not have ultrasound information. Having a fibroid was not associated with increased risk of miscarriage in multiple imputation analysis or when prevalence of having a fibroid was doubled among women who had a pregnancy loss and had unknown fibroid status (adjusted HR = 1.03, 95% CI: 0.81, 1.30).
DISCUSSION
In retrospective analyses of ultrasound databases and in tertiary care center studies, fibroids have been linked to pregnancy complications including miscarriage, preterm birth, growth restriction, placental abruption, postpartum hemorrhage, malpresentation, and unplanned cesarean (7–21). Hypotheses about how fibroids harm pregnancies are often based on architectural explanatory models: fibroids distort the uterine cavity in ways that are hostile to the developing pregnancy, they prevent robust placentation, or their presence in the uterine wall causes abnormalities in uterine contractility that lead to complications. Our findings from a prospective, multicity, community-recruited cohort do not support the hypothesis that the presence of fibroids, including large fibroids or higher total fibroid volume, causes miscarriage.
Considerations
Before considering implications of our findings, let us first examine the degree of confidence we should have in the results. Our imaging and quality measures relied on experienced clinical sonographers implementing a research protocol, not on a limited pool of research sonographers. We used still images for 6 years. These may be inferior to real-time images or digital video clips subsequently obtained for secondary review. However, our findings were not changed when examining those time periods separately (data not shown). Likewise, although all sonographers had 5 or more years of experience in pelvic and obstetric sonography and perform thousands of clinical ultrasounds each year that require extreme precision—such as measurement of fetal nuchal fold thickness to assess risk of aneuploidy (in which fractions of millimeters change diagnostic categories)—mistakes were inevitably made in exact placement of calipers and in orientation of planes for measures. Such errors would be distributed across sonographers, who could not be aware of future pregnancy outcome and were therefore not likely to be systematically biased. Because sonographers were based at multiple sites, they were unlikely to have shared distinctive practice-based measurement styles that might create a consistent direction of bias as might occur if a small, colocated group made assessments. Comparing clinical sonography with magnetic resonance imaging in validation studies, measures are comparable with the exception that fibroids in a large uterus with multiple fibroids (greater than 4 fibroids) and large total volume are more accurately counted and individually measured using magnetic resonance imaging (23). In this population of young participants with few fibroids, this is unlikely to be an important source of measurement error. Our analysis relied most on classification of fibroid size and location into broad categories that can be achieved with accuracy (24–26).
We do not have a definitive mechanism to know whether fibroids were missed, although review of video surveys suggests that this was very rare. The net effect of errors in detection would be the misclassification of individuals as having fewer fibroids or as having no fibroids, meaning the associations we investigated could be diluted by such errors and may underestimate the influence of fibroids. Furthermore, in all imaging, misidentification is a concern. Smaller structures identified as fibroids might represent a focal myometrial contraction or an adenomyosis lesion rather than a uterine fibroid. Although risk of misidentification is low, we may not have been able to identify all such errors through review of still images. We implemented our protocol of repeated measures separated in time in order to increase the probability that the structure being reidentified and measured would indeed be a fibroid. To address the concern that focal contractions may be sustained for extended periods of time and could be more common in a uterus that was developing increased contractile activity prior to the onset of signs and symptoms of miscarriage, we conducted additional analyses and restricted the comparisons to women whose loss was more than 3, 5, or 7 days after ultrasonography, with comparable overall results (data not shown).
We used medical records to document the fact and timing of 98.8% of losses. We do not have pathology reports for miscarriages. This prevents our assessing the chromosomal and developmental status of the losses. We have no theoretical concern that fibroids cause chromosomal abnormalities. The association of fibroids with advancing maternal age and of advancing age with risk of genetic abnormalities has been addressed through inclusion of age as a confounder in multivariable models, along with other covariates with similar relationships, namely race/ethnicity, parity, history of termination or pregnancy, and alcohol use.
This analysis may underestimate the association of fibroids with loss in 3 ways. One is that some women who had pregnancy losses did not return for ultrasonography after the loss, and their fibroid status is unknown (n = 105). If fibroids increase risk of miscarriage then women who did not have ultrasounds because of early loss are disproportionately likely to have had 1 or more fibroids, and our estimate of risk is biased towards the null. However, the association remained null in 2 sets of sensitivity analyses using multiple imputation.
A second scenario turns on reverse causality. If fibroid growth is promoted by the hormonal milieu of early pregnancy, it is theoretically concerning that failing pregnancies, with lower levels of some hormones, such as human chorionic gonadotropin or progesterone, might not experience comparable fibroid growth. This could mean some very small fibroids did not reach our measurement threshold in women with losses but did in healthy pregnancies. Because the lowest quartiles of size and volume are associated with point estimates above 1, we judge this is unlikely.
A third concern is that some reproductive time is not observed. Although women were enrolled in early pregnancy and provided with pregnancy test kits for early use, we note that our findings are relevant to recognized losses and do not assess time between implantation and first pregnancy testing. To examine this concern, our team has investigated time to conception in this cohort. We found that women with fibroids do not experience delay in conception (31). Because losses before recognition are reflected in aggregate data as delays in time to conception, this supports the conclusion that fibroids are not associated with prerecognition reproductive failure.
Conclusions and implications
In summary, if the preponderance of structures identified by ultrasonography were uterine fibroids, and we accurately documented losses, our study indicated that the presence of 1 or more fibroids had no discernible effect on risk of miscarriage. This challenges common beliefs of women and care providers who are confident that presence of a fibroid, numerous fibroids, larger fibroids, or those located in crucial positions in the uterus are harmful. Current clinical wisdom purports that fibroids are a cause of miscarriage, and prominent professional organizations, such as the American Society of Reproductive Medicine endorse intervention (32):
“The management of uterine fibroids depends on your physician's recommendation. In certain cases, surgery to remove the fibroids prior to conception can reduce a woman's chance of miscarriage by 50%.”
However, several meta-analyses of trials, such as that by Metwally et al. (4, p. 1) for the Cochrane database in 2012 have concluded that there is:
“…no evidence for a significant effect of myomectomy for any of the described types of fibroids on the miscarriage rate.”
Clinical trials are the appropriate vehicle for implicating or rejecting causality, although trials to address this question pose significant logistical challenges. Future trials might consider only a specific type of fibroid, such as submucous location, or a uterine characteristic, such as fibroids comprising greater than 50% of total uterine volume. But these studies must be approached with caution as effect size may be small and not applicable as a general principle of care. Until such answers are available for specific subpopulations of women, the data are insufficient to guide clinical care, and some common recommendations, such as myomectomy for large fibroids to enhance reproductive performance, should be reevaluated.
If fibroids harm early pregnancy, the mechanism is comparatively rare, and the net effect is such that we cannot counsel women that having fibroids may have led to a miscarriage. As we provide care for women known to have fibroids, it would be prudent to temper prior recommendations that encouraged surgical intervention in order to reduce miscarriage risk.
ACKNOWLEDGMENTS
Author affiliations: Vanderbilt Epidemiology Center, Institute for Medicine and Public Health, Vanderbilt University Medical Center, Nashville, Tennessee (Katherine E. Hartmann, Digna R. Velez Edwards, Alexandra C. Sundermann); Department of Obstetrics and Gynecology, Vanderbilt University School of Medicine, Nashville, Tennessee (Katherine E. Hartmann, Digna R. Velez Edwards, Alexandra C. Sundermann); Department of Epidemiology, Brown School of Public Health, Brown University, Providence, Rhode Island (David A. Savitz); Department of Obstetrics and Gynecology, Brown Alpert Medical School, Brown University, Providence, Rhode Island (David A. Savitz); Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina (Michele L. Jonsson-Funk); Department of Biostatistics, Institute for Medicine and Public Health, Vanderbilt University Medical Center, Nashville, Tennessee (Pingsheng Wu); and Epidemiology Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, North Carolina (Donna D. Baird).
This research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (grants R01HD043883 and R01HD049675) and the American Water Works Association Research Foundation (grants 2579). Salary support for D.R.V.E. was provided in part by the NIH Building Interdisciplinary Research Careers in Women's Health Career Development Program (grant K12HD04383); stipend support was provided in part by the National Institute of General Medical Studies award for the Vanderbilt Medical-Scientist Training Program (grant T32GM07347). Additional infrastructure resources were provided by a CTSA award (grant UL1TR000445) from the National Center for Advancing Translational Sciences.
We thank doctoral student Nicholas J. Strayer for creating the R code to generate Figure 1.
Conflict of interest: none declared.
REFERENCES
- 1. Wise LA, Palmer JR, Stewart EA, et al. . Age-specific incidence rates for self-reported uterine leiomyomata in the Black Women's Health Study. Obstet Gynecol. 2005;105(3):563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laughlin SK, Baird DD, Savitz DA, et al. . Prevalence of uterine leiomyomas in the first trimester of pregnancy: an ultrasound-screening study. Obstet Gynecol. 2009;113(3):630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baird DD, Dunson DB, Hill MC, et al. . High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107. [DOI] [PubMed] [Google Scholar]
- 4. Metwally M, Cheong YC, Horne AW. Surgical treatment of fibroids for subfertility. Cochrane Database Syst Rev. 2012;11:CD003857. [DOI] [PubMed] [Google Scholar]
- 5. Segars JH, Parrott EC, Nagel JD, et al. . Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: comprehensive review, conference summary and future recommendations. Hum Reprod Update. 2014;20(3):309–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benson CB, Chow JS, Chang-Lee W, et al. . Outcome of pregnancies in women with uterine leiomyomas identified by sonography in the first trimester. J Clin Ultrasound. 2001;29(5):261–264. [DOI] [PubMed] [Google Scholar]
- 7. Eldar-Geva T, Meagher S, Healy DL, et al. . Effect of intramural, subserosal, and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertil Steril. 1998;70(4):687–691. [DOI] [PubMed] [Google Scholar]
- 8. Farhi J, Ashkenazi J, Feldberg D, et al. . Effect of uterine leiomyomata on the results of in-vitro fertilization treatment. Hum Reprod. 1995;10(10):2576–2578. [DOI] [PubMed] [Google Scholar]
- 9. Mollica G, Pittini L, Minganti E, et al. . Elective uterine myomectomy in pregnant women. Clin Exp Obstet Gynecol. 1996;23(3):168–172. [PubMed] [Google Scholar]
- 10. Ramzy AM, Sattar M, Amin Y, et al. . Uterine myomata and outcome of assisted reproduction. Hum Reprod. 1998;13(1):198–202. [DOI] [PubMed] [Google Scholar]
- 11. Seoud MA, Patterson R, Muasher SJ, et al. . Effects of myomas or prior myomectomy on in vitro fertilization (IVF) performance. J Assist Reprod Genet. 1992;9(3):217–221. [DOI] [PubMed] [Google Scholar]
- 12. Stovall DW, Parrish SB, Van Voorhis BJ, et al. . Uterine leiomyomas reduce the efficacy of assisted reproduction cycles: results of a matched follow-up study. Hum Reprod. 1998;13(1):192–197. [DOI] [PubMed] [Google Scholar]
- 13. Guillaume AJ, Benjamin F, Spitzer M. Myomectomy performed concurrently with tuboplasty. J Reprod Med. 1998;43(6): 483–486. [PubMed] [Google Scholar]
- 14. Cavallotti D, Casilla G, Piantelli G, et al. . Early complications of prenatal invasive diagnostics: perspective analysis. Acta Biomed. 2004;75(suppl 1):23–26. [PubMed] [Google Scholar]
- 15. Chen YH, Lin HC, Chen SF, et al. . Increased risk of preterm births among women with uterine leiomyoma: a nationwide population-based study. Hum Reprod. 2009;24(12):3049–3056. [DOI] [PubMed] [Google Scholar]
- 16. Coronado GD, Marshall LM, Schwartz SM. Complications in pregnancy, labor, and delivery with uterine leiomyomas: a population-based study. Obstet Gynecol. 2000;95(5):764–769. [DOI] [PubMed] [Google Scholar]
- 17. Exacoustòs C, Rosati P. Ultrasound diagnosis of uterine myomas and complications in pregnancy. Obstet Gynecol. 1993;82(1):97–101. [PubMed] [Google Scholar]
- 18. Salvador E, Bienstock J, Blakemore KJ, et al. . Leiomyomata uteri, genetic amniocentesis, and the risk of second-trimester spontaneous abortion. Am J Obstet Gynecol. 2002;186(5):913–915. [DOI] [PubMed] [Google Scholar]
- 19. Sheiner E, Bashiri A, Levy A, et al. . Obstetric characteristics and perinatal outcome of pregnancies with uterine leiomyomas. J Reprod Med. 2004;49(3):182–186. [PubMed] [Google Scholar]
- 20. Stout MJ, Odibo AO, Graseck AS, et al. . Leiomyomas at routine second-trimester ultrasound examination and adverse obstetric outcomes. Obstet Gynecol. 2010;116(5):1056–1063. [DOI] [PubMed] [Google Scholar]
- 21. Vergani P, Ghidini A, Strobelt N, et al. . Do uterine leiomyomas influence pregnancy outcome? Am J Perinatol. 1994;11(5):356–358. [DOI] [PubMed] [Google Scholar]
- 22. Muram D, Gillieson M, Walters JH. Myomas of the uterus in pregnancy: ultrasonographic follow-up. Am J Obstet Gynecol. 1980;138(1):16–19. [DOI] [PubMed] [Google Scholar]
- 23. Dueholm M, Lundorf E, Hansen ES, et al. . Accuracy of magnetic resonance imaging and transvaginal ultrasonography in the diagnosis, mapping, and measurement of uterine myomas. Am J Obstet Gynecol 2002;186(3):409–415. [DOI] [PubMed] [Google Scholar]
- 24. Fedele L, Bianchi S, Dorta M, et al. . Transvaginal ultrasonography versus hysteroscopy in the diagnosis of uterine submucous myomas. Obstet Gynecol. 1991;77(5):745–748. [PubMed] [Google Scholar]
- 25. Hurley V. Imaging techniques for fibroid detection. Baillieres Clin Obstet Gynaecol. 1998;12(2):213–224. [DOI] [PubMed] [Google Scholar]
- 26. Riccabona M, Nelson TR, Pretorius DH. Three-dimensional ultrasound: accuracy of distance and volume measurements. Ultrasound Obstet Gynecol. 1996;7(6):429–434. [DOI] [PubMed] [Google Scholar]
- 27. Promislow HE, Makarushka CM, Gorman JR, et al. . Recruitment for a community-based study of early pregnancy: the Right from the Start study. Paediatr Perinat Epidemiol. 2004;18(2):143–152. [DOI] [PubMed] [Google Scholar]
- 28. Hoffman CS, Messer LC, Mendola P, et al. . Comparison of gestational age at birth based on last menstrual period and ultrasound during the first trimester. Paediatr Perinat Epidemiol. 2008;22(6):587–596. [DOI] [PubMed] [Google Scholar]
- 29. Dupont W. Statistical Modeling for Biomedical Researchers: A Simple Introduction to the Analysis of Complex Data. 2nd edCambridge, UK: Cambridge University Press, Publishers; 2009. [Google Scholar]
- 30. Harrell F. Regression Modeling Strategies. 1st ed New York, NY: Springer, Publishers; 2002. [Google Scholar]
- 31. Johnson G, MacLehose RF, Baird DD, et al. . Uterine leiomyomata and fecundability in the Right from the Start study. Hum Reprod. 2012;27(10):2991–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. American Society for Reproductive Medicine Fibroid tumors (transcript of patient education materials; video timestamp 10:07). http://www.reproductivefacts.org/resources/educational-videos/full-length-videos/fibroid-tumors/. Accessed May 3, 2017.