Abstract
Multimorbidity is prevalent, but its optimal quantification and associations with mortality rate and physical functioning in young through older adults are uncertain. We used data collected using the Short Form-36 in the Nurses’ Health Study (enrollment started in 1976), Nurses’ Health Study II (begun in 1989), and Health Professionals Follow-up Study (begun in 1986) to identify associations of a multimorbidity-weighted index (MWI) and common alternative indices with mortality and future physical functioning. We used Cox proportional hazard ratios to determine incident 10-year mortality and general linear models to obtain coefficients for the associations of MWI with 4- and 8-year physical functioning. At baseline, mean values for the 219,950 participants were 55.0 (standard deviation, 3.7) years for age; 3.8 (range, 0–51) for MWI; 2.7 (range, 0–23) for disease count, and 0.43 (range, 0–13) for Charlson Comorbidity Index (CCI). During follow-up, 23,709 deaths (10.8%) occurred. CCI, MWI, and disease count were 0 for 77%, 12%, and 19% of participants, respectively. When comparing persons in the highest quartiles with those in the lowest, the hazard ratios for mortality were 6.04 (95% confidence interval (CI): 6.00, 6.09; P for trend < 0.0001) for the MWI, 4.86 (95% CI: 4.81, 4.91; P for trend < 0.0001) for disease count, and 3.29 (95% CI: 3.26, 3.32; P for trend < 0.0001) for the CCI. For future physical functioning, MWI had the best model fit and explained the greatest variance. Multimorbidity has important associations with future physical functioning and mortality that are easily captured with a readily measured index.
Keywords: Charlson Comorbidity Index, comorbidity, mortality, multimorbidity, multiple chronic conditions, physical functioning, Short Form-36, simple disease count
Among adults with chronic disease, multimorbidity (i.e., having multiple chronic conditions) is more common than having a single disease in isolation (1), and it is becoming increasingly recognized as its own entity rather than as comorbidity in the context of an index disease. Prior measures of multimorbidity have been limited by disease inventory, adjustment for disease severity, generalizability to nonhospitalized (2) and younger and middle-aged adults (3), and disease clusters that may not be replicable in other populations (3).
We recently developed and validated a multimorbidity-weighted index (MWI) that weights conditions by their concurrent impact on physical health–related quality of life in community-dwelling adults (4, 5). The MWI is easily assessed using simple disease inventories and to our knowledge is among the first indices to weight disease severity by an extensively validated and widely used health survey instrument for physical functioning (6, 7). Physical functioning is a universal patient-centered outcome consistent with values of patients’ well-being (6, 8) and is also strongly associated with adverse health outcomes, including increased mortality risk, hospital readmission, and emergency department return visits (9, 10).
Although the MWI weights disease severity by current physical functioning, its ability to predict mortality and future physical functioning is unknown. In the present study, our goal was to examine the associations of the MWI with mortality and future physical functioning and compare the performance of the MWI with those of 2 of the most frequently used alternatives: simple disease count and the Charlson Comorbidity Index (CCI), which weights diseases by 1-year mortality risk among hospitalized patients (11).
METHODS
Study population
The study population included community-dwelling participants in the United States from the nationally sampled prospective cohorts of 3 studies: the Nurses’ Health Study (NHS), Nurses’ Health Study II (NHS II), and Health Professionals Follow-Up Study (HPFS). NHS enrolled 121,701 female nurses who were aged 30–55 years when data collection began in 1976. The NHS II cohort comprised 116,686 female nurses who ranged in age from 25 to 42 years when the participants enrolled in 1989. The HPFS enrolled a cohort of 51,530 male health professionals who were aged 40–75 years when the study began in 1986. Participants receive biennial questionnaires regarding newly diagnosed medical conditions, medication use, diet, and health behaviors (e.g., tobacco use, physical activity level), and the follow-up rate has been greater than 90% for each cycle. To be included, participants must have reported the occurrence (or absence) of chronic diseases and conditions in 2000 (for the NHS and HPFS) or 2001 (for the NHS II) and had follow-up through 2004 (NHS) or 2008 (HPFS) for physical functioning and 2010 for death.
Multimorbidity assessment
Multimorbidity was measured using a MWI, as described previously (4). Briefly, we established weights for 81 of 374 self-reported, clinician-diagnosed conditions based on cross-sectional associations with physical functioning items on the Short Form-36 among 216,890 participants contributing 612,592 observations repeatedly between 1992 and 2008. Diseases varied widely in their associations with physical functioning, with the worst impact due to progressive neurologic and end-stage organ diseases. A straightforward interpretation of the MWI is that a 1-point increase in MWI represents a 1-point decrease in current physical functioning, where declines of 2–3 points in the Short Form-36 score may be considered clinically meaningful. The interpretability of the MWI is 2-fold: It provides estimates of the burdens of average diseases and of expected physical functioning decline. For these analyses, we applied our previously established weights to conditions reported in 2000–2001 to create a baseline MWI for each participant. A step-by-step guide on how to calculate the MWI, including a table of the MWI disease weightings, is shown in Web Appendix 1 and Web Table 1 (available at https://academic.oup.com/aje).
Simple disease count included the same summed conditions as the MWI; however, conditions were unweighted. The CCI included 19 chronic conditions weighted 1, 2, 4, or 6 based on their associations with predicted 1-year mortality in administrative data from hospitalized patients, and it was computed using methods described previously (11). For example, dementia and diabetes were weighted 1, whereas metastatic solid tumor and AIDS were weighted 6, and a participant’s comorbidity index was the sum of their weighted conditions.
Mortality assessment
At the end of each 2-year follow-up cycle, we sent to the National Death Index a list of participants that included older participants with a prolonged period of no response to questionnaires, participants with confirmed cancer who did not respond to a subsequent questionnaire, and participants reported as deceased but for whom a cause had not been identified (12, 13). Death certificates were sought if all attempts to contact next of kin were unsuccessful or death was not recorded in the National Death Index. The most comprehensive and completed mortality ascertainment was available through December 31, 2010.
Loss to follow-up was minimal (<1%) because of comprehensive searches that included contacting family members, the National Death Index, and state tumor registries. Individuals who were lost to follow-up and who survived beyond 2010 were censored from the analysis based on the last date of known contact.
Physical functioning assessment
The Medical Outcomes Study Short Form-36 physical functioning scale covers a broad range of physical functioning applicable across all age groups, ranging from activities of daily living such as bathing and dressing to rigorous activities such as running and lifting heavy objects (6). It is the most extensively validated, standardized, and widely used health survey instrument to assess health-related quality of life (7). The sum of 10 items forms a continuous measure from 0 to 100 for lowest to highest functioning. In the NHS, physical functioning was assessed in 2004; in the HPFS, it was assessed in 2008.
Statistical analysis
Our primary outcome was 10-year mortality rate. We used Cox proportional hazards models to determine the associations of the MWI, simple disease count, and CCI with mortality. We modeled the association between multimorbidity and mortality in several ways, including using continuous, transformed, and categorical variables, and we used the Akaike Information Criterion to compare model fit, where the lowest Akaike Information Criterion was most favorable. The model with the lowest Akaike Information Criterion and best clinical interpretability was selected for the final analyses.
To further assess for a potential nonlinear association specifically between the MWI and mortality, we used restricted cubic splines (14). The top 0.5% MWI values were winsorized (15) to reduce the possible effect of spurious outliers. Nonlinearity was tested with the likelihood ratio test to compare models with 1 linear term to those with both linear and cubic spline terms.
The hazard ratios and 95% confidence intervals for multimorbidity in unadjusted and mutually adjusted models were estimated through Cox proportional hazards modeling (16); the Efron (17) method was used to handle ties. The proportional hazards assumption was formally assessed through Schoenfeld residuals (18). P > 0.05 was used to indicate that there was a nonsignificant correlation and that the proportional hazards model assumption was not violated.
To quantify how accurately the models discriminated between survival outcomes, we computed the concordance (C) statistic (or area under the receiver operating characteristic curve) for survival (19). We used Pearson χ2 statistics to assess differences in the associations of quantiles of multimorbidity indices with mortality relative to the null hypothesis of no difference between the distributions.
A second outcome, future physical functioning, was measured continuously. We used general linear models to determine the associations of the MWI, simple disease count, and CCI in quartiles with future physical functioning. We compared the magnitude of the regression coefficients along with P and T values to indicate the strength of the association between each index with future physical functioning. To indicate how well each index predicted statistically observed physical functioning, we used the coefficient of determination (R2) to measure the proportion of total variation of physical functioning explained by each model.
We compared indices at baseline using the Pearson correlation coefficient. Given the high correlation between the MWI and simple disease count (and lack of variability in the CCI), we examined the mutually adjusted associations of the MWI and simple disease count with mortality and physical functioning in quartiles as a sensitivity analysis. To assess potential multicollinearity in the mutually adjusted models, we computed the variance inflation factor in the regression models (20) and assessed inflation of the standard error terms in the mutually adjusted models versus models of each independent index.
For robustness, we tested for potential effect modification by age (<55.0, 55.0–64.9, 65.0–74.9, or≥75.0 years), race (white; nonwhite, including black; Asian/Pacific Islander; American Indian/Alaska Native; Hispanic; or other), body mass index (measured as weight (kg)/height (m)2; <25.0, 25.0–29.9, ≥30.0), smoking status (never, former, or current smoker), and geographic region (West, Midwest, Northeast, or South) in associations of multimorbidity with mortality and physical functioning. Heterogeneity across participant characteristics was assessed using the I2 statistic, which measures the proportion of total variation across estimates due to heterogeneity (21). We also examined models adjusted for all the mentioned covariates plus alcohol intake (none and tertiles of intake in grams per day). Forest plots were created using Stata (release 14.0; StataCorp LP, College Station, Texas). All other analyses were performed using SAS (release 9.4; SAS Institute, Inc., Cary, North Carolina).
RESULTS
Participant characteristics
We included 219,950 participants (92,352 from NHS, 95,122 from NHS II, and 32,476 from HPFS) who completed the biennial questionnaire in 2000 or 2001 and had complete data on disease prevalence and demographics. At baseline in 2000 and 2001, the mean (standard deviation) age was 55 (3.7) years (Table 1). We documented death in 23,709 participants (10.8%) between 2000–2001 and December 31, 2010.
Table 1.
Multimorbidity Characteristics of Participants at Baseline, Nurses’ Health Study (2000), Health Professionals Follow-Up Study (2000), and Nurses’ Health Study II (2001)
| Participant Characteristic | Nurses’ Health Study (n = 92,352) |
Health Professionals Follow-Up Study (n = 32,476) |
Nurses’ Health Study II (n = 95,122) |
Meta-Analyzed Combined Cohorts (n = 219,950) |
|||
|---|---|---|---|---|---|---|---|
| Mean (SD) | IQR | Mean (SD) | IQR | Mean (SD) | IQR | Mean (SD) | |
| Age, years | 66.03 (7.1) | 66.62 (9.2) | 46.42 (5.0) | 57.60 (3.7) | |||
| MWI | 4.46 (4.3) | 4.36 (4.0) | 2.93 (3.3) | 3.76 (2.2) | |||
| MWI quartile | |||||||
| 4 | 6.43–48.00 | 6.10–50.80 | 4.27–35.10 | ||||
| 3 | 3.54–6.42 | 3.16–6.09 | 1.80–4.26 | ||||
| 2 | 1.20–3.53 | 1.52–3.15 | 0.34–1.79 | ||||
| 1 | <1.20 | <1.52 | <0.34 | ||||
| Disease count | 3.31 (2.4) | 2.93 (2.3) | 2.19 (1.9) | 2.71 (1.2) | |||
| Disease count quartile | |||||||
| 4 | 5.00–2300 | 4.00–19.00 | 3.00–18.00 | ||||
| 3 | 3.00–4.99 | 2.00–3.99 | 2.00–2.99 | ||||
| 2 | 2.00–2.99 | 1.00–1.99 | 1.00–1.99 | ||||
| 1 | <2.00 | <1.00 | <1.00 | ||||
| CCI | 0.54 (1.0) | 0.54 (1.0) | 0.20 (0.6) | 0.43 (0.6) | |||
| CCI quartile | |||||||
| 4 | 1.00–13.00 | 1.00–12.00 | N/Aa | ||||
| 3 | 0.00–0.99 | 0.00–0.99 | |||||
| 2 | 0 | 0 | |||||
| 1 | 0 | 0 | |||||
Abbreviations: CCI, Charlson Comorbidity Index; IQR, interquartile range; MWI, multimorbidity-weighted index; N/A, not calculable in quartiles; SD, standard deviation.
a In the Nurses’ Health Study II, the CCI was not computable in quartiles because of a strong left skew. Decile 10 corresponds to a CCI of 1–7; decile 9 corresponds to a CCI of 0–1; and deciles 1–8 correspond to a CCI of 0.
Multimorbidity characteristics
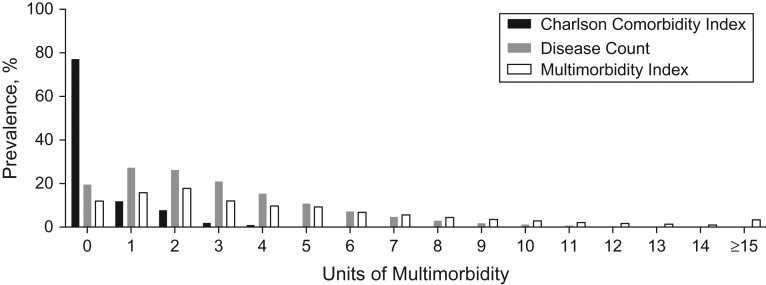
At baseline, participants had a mean MWI of 3.76 (range, 0–51), disease count of 2.71 (range, 0–23), and CCI of 0.43 (range, 0–13) (Figure 1). The MWI spanned the widest distribution of multimorbidity through low and high ranges and better distinguished between participants at the low range of multimorbidity. For example, 77% of adults had a CCI of 0 compared with 12% with an MWI of 0 and 19% with a disease count of 0. The MWI was strongly but not perfectly correlated with simple disease count (Pearson r = 0.88, 0.87, and 0.90 for NHS, NHS II, and HPFS, respectively; P < 0.0001 for all) and only moderately correlated with the CCI (Pearson r = 0.56, 0.44, and 0.59 for NHS, NHS II, and HPFS, respectively; P < 0.0001 for all).
Figure 1.
Distribution of multimorbidity indices at baseline in pooled cohorts from the Nurses’ Health Study (2000), Health Professionals Follow-Up Study (2000), and Nurses’ Health Study II (2001). Units of multimorbidity are as follows: Charlson Comorbidity Index units, determined by summing conditions weighted by risk of 1-year mortality; simple disease count units, which are the summation of unweighted conditions; and multimorbidity-weighted index units, determined by summing conditions weighted by their impact on the Short Form-36 physical functioning scale.
Multimorbidity and mortality
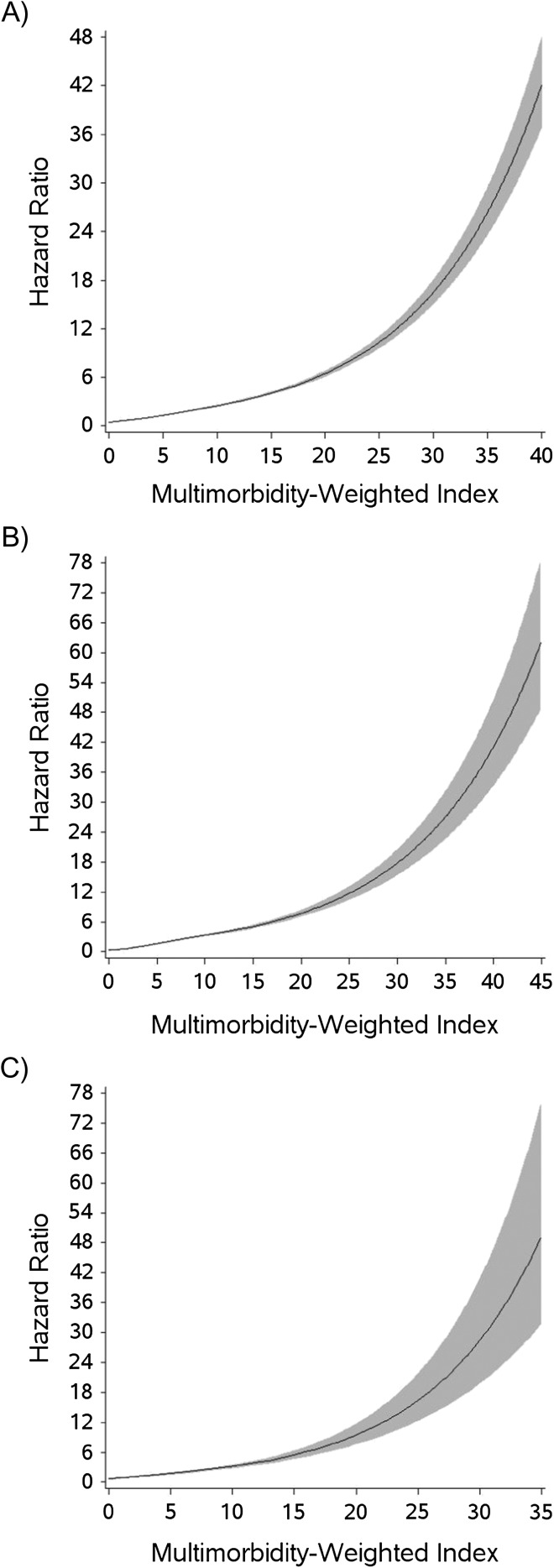
We first examined the association of the MWI with mortality using restricted cubic splines in each cohort (Figure 2). Nonlinearity occurred at high values of the MWI, but we observed an essentially linear relationship with mortality through MWI values of 20–25 in the NHS and NHS II cohorts and an MWI of 30 in HPFS. Thus, although extreme values of the MWI were associated with disproportionately high hazard ratios for mortality, the MWI appeared to have an essentially linear relationship with mortality throughout values commonly observed in community-dwelling adults.
Figure 2.
Restricted cubic splines for 10-year incident mortality in the Nurses’ Health Study (2000–2010; A), Health Professionals Follow-Up Study (2000–2010; B), and Nurses’ Health Study II (2001–2010; C). Gray area, 95% confidence interval.
We next compared the MWI with simple count and the CCI in quantile-based categories. The hazard ratio of mortality was 6-fold greater (hazard ratio (HR) = 6.04, 95% CI: 6.00, 6.09; P for trend < 0.0001) for individuals in the highest quartile of MWI compared with those in the lowest (Table 2). There was a dose-response relationship between hazard ratios for mortality and increasing quartiles of the MWI (P for trend < 0.0001) in the unadjusted (Table 2; Web Figure 1) and adjusted (Web Table 1) models. The dose-response association between the MWI and mortality persisted even after CCI diseases were removed from the MWI, highlighting the limitations of restricting the index to a few key conditions based on inpatient mortality data. Among those with a CCI of 0, the hazard ratio for mortality was 5.96 (95% CI: 5.95, 5.97; P < 0.0001) for individuals in the highest quartile of the MWI compared with those in the lowest.
Table 2.
Hazard Ratios for Mortality of Participants After Follow-Up, Nurses’ Health Study, Health Professionals Follow-Up Study, and Nurses’ Health Study II, 2000–2010a
| Model and Quartiles | Nurses’ Health Studyb (n = 92,352) | Health Professionals Follow-Up Studyb (n = 32,476) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | AIC | C Statistic | 95% CI | HR | 95% CI | AIC | C Statistic | 95% CI | |
| MWI | 303,944 | 0.67 | 0.66, 0.67 | 132,203 | 0.70 | 0.69, 0.70 | ||||
| 4 vs. 1 | 5.29 | 5.00, 5.60 | 8.95 | 8.10, 9.89 | ||||||
| 3 vs. 1 | 2.19 | 2.06, 2.33 | 3.83 | 3.45, 4.26 | ||||||
| 2 vs. 1 | 1.50 | 1.41, 1.61 | 2.45 | 2.19, 2.73 | ||||||
| 1 | 1.00 | Referent | 1.00 | Referent | ||||||
| P for trend | <0.001 | <0.001 | ||||||||
| Simple disease count | 306,777 | 0.65 | 0.65, 0.66 | 134,279 | 0.68 | 0.67, 0.68 | ||||
| 4 vs. 1 | 4.50 | 4.26, 4.61 | 7.75 | 6.77, 8.88 | ||||||
| 3 vs. 1 | 2.05 | 1.93, 2.17 | 2.95 | 2.57, 3.40 | ||||||
| 2 vs. 1 | 1.39 | 1.30, 1.49 | 1.61 | 1.38, 1.88 | ||||||
| 1 | 1.00 | Referent | 1.00 | Referent | ||||||
| P for trend | <0.001 | <0.001 | ||||||||
| CCIc | 306,466 | 0.64 | 0.64, 0.65 | 134,279 | 0.64 | 0.64, 0.65 | ||||
| 4 vs. 1–3 | 3.27 | 3.16, 3.38 | 3.34 | 3.18, 3.51 | ||||||
| 1–3 | 1.00 | 1.00 | ||||||||
| P for trend | <0.001 | <0.001 | ||||||||
| Nurses’ Health Study IIb (n = 95,122) | Meta-Analyzed Combined Cohorts (n = 219,950) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | AIC | C Statistic | 95% CI | HR | 95% CI | AIC | C Statistic | 95% CI | |
| MWI | 27,555 | 0.64 | 0.62, 0.65 | N/A | 0.68 | 0.68, 0.68 | ||||
| 4 vs. 1 | 3.78 | 3.12, 4.58 | 6.04 | 6.00, 6.09 | ||||||
| 3 vs. 1 | 1.96 | 1.58, 2.41 | 2.32 | 2.29, 2.36 | ||||||
| 2 vs. 1 | 1.44 | 1.14, 1.80 | 1.73 | 1.68, 1.79 | ||||||
| 1 | 1.00 | Referent | 1.00 | Referent | ||||||
| P for trend | <0.001 | <0.001 | ||||||||
| Simple disease count | 28,516 | 0.62 | 0.61, 0.64 | N/A | ||||||
| 4 vs. 1 | 3.33 | 2.74, 4.04 | 4.86 | 4.81, 4.91 | 0.66 | 0.66, 0.67 | ||||
| 3 vs. 1 | 1.65 | 1.32, 2.06 | 2.15 | 2.10, 2.21 | ||||||
| 2 vs. 1 | 1.42 | 1.13, 1.78 | 1.43 | 1.37, 1.49 | ||||||
| 1 | 1.00 | Referent | 1.00 | Referent | ||||||
| P for trend | <0.001 | <0.001 | ||||||||
| CCId | N/A | N/A | N/A | N/A | 0.64 | 0.64, 0.64 | ||||
| 4 vs. 1–3 | 3.29 | 3.26, 3.32 | ||||||||
| 1–3 | 1.00 | Referent | ||||||||
| P for trend | <0.001 | |||||||||
Abbreviations: AIC, Akaike Information Criterion; CCI, Charlson Comorbidity Index; CI, confidence interval; HR, hazard ratio; MWI, multimorbidity-weighted index; N/A, not calculable in quartiles.
a Baseline was 2001 in the Nurses’ Health Study II.
b Number of deaths in study cohorts: Nurses’ Health Study, n = 13,728; Health Professionals Follow-Up Study, n = 6,444; Nurses’ Health Study II, n = 1,320; and combined, n = 23,709.
c Because of a strong left skew, quartiles 1–3 served as the reference group, and the CCI cannot be directly compared with the MWI and simple disease count, for which quartile 1 served as the reference group.
d The CCI is not computable in quartiles because of a strong left skew, but it is calculable for deciles as follows, using deciles 1–8 as the reference group: for decile 10, HR = 7.30, 95% CI: 6.40, 8.34; for decile 9, HR = 2.81, 95% CI: 2.41, 3.28. P-trend < 0.0001; AIC, 28,090.
Simple disease count was also significantly associated with mortality. For every additional disease, the HR was 1.23 (95% CI: 1.22, 1.24; P < 0.0001). Using the count definition of multimorbidity as 2 or more diseases, individuals with multimorbidity had a 2-fold higher hazard ratio for mortality (HR = 2.02, 95% CI: 1.84, 2.22; P < 0.0001) than did disease-free individuals. There was no difference between individuals with 1 disease and those with no diseases (HR = 1.01, 95% CI: 0.90, 1.14; P = 0.84). As observed in Table 2, MWI was more strongly associated with mortality than was simple count for all quartiles of multimorbidity in all cohorts. MWI and simple disease count were more strongly associated with mortality than was the CCI. In the model mutually adjusted for MWI and simple disease count, the association of MWI with mortality continued to be of greater magnitude (HR = 4.09, 95% CI: 4.00, 4.18; P < 0.0001). The hazard ratio was more than double that of simple disease count (HR = 1.60, 95% CI: 1.52, 1.69; P < 0.0001) in the pooled NHS and HPFS cohorts; simple disease count, reflecting its left distributional skew, could not be aggregated into quartiles in NHS II. Standard error terms in the mutually adjusted models were 4%–40% higher than in the isolated index models, suggesting mild multicollinearity. However, the standard error terms remained several-fold less than the regression coefficients and were nonoverlapping, suggesting intact precision.
The C statistics for mortality prediction were greatest for the MWI in all cohorts and the meta-analyzed combined cohorts (C = 0.67, 0.70, and 0.64 for NHS, HPFS, and NHS II, respectively, and 0.68 for the combined cohorts), followed by disease count (C = 0.65, 0.68, and 0.62 for NHS, HPFS, and NHS II, respectively, and 0.66 for the combined cohorts) and the CCI when using the lowest combined reference group given its strong left distributional skew (C = 0.64 for NHS, HPFS, and the combined NHS and HPFS cohorts; however, the C-statistic was noncomputable in NHS II due to the limited skewed distribution; Table 2).
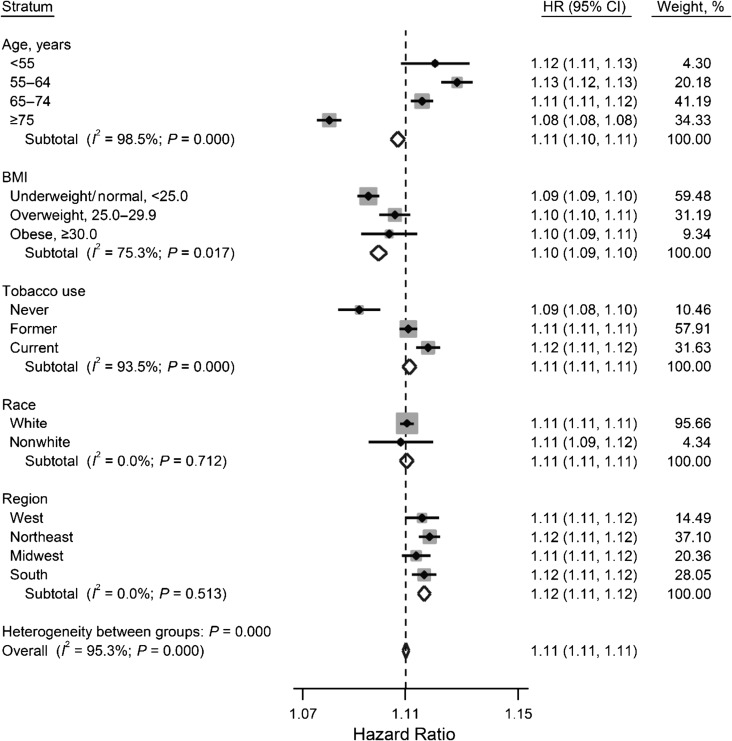
Potential effect modification of the association between the MWI and mortality by age, race, body mass index, smoking status, and geographic region was examined. There were no differences in the magnitudes of the positive association of MWI with mortality within strata of these risk factors (Figure 3). Statistical heterogeneity was observed for age, body mass index, and tobacco use, but the hazard ratios in every stratum were nearly identical, and each had a significant association. The overall hazard ratio for mortality after adjustment for all the aforementioned covariates plus alcohol consumption was 1.11 (95% CI: 1.11, 1.11; P < 0.0001) for each point increase in the MWI (Web Table 2).
Figure 3.
Forest plot displaying adjusted Cox proportional hazards ratios (HR) for mortality by participant characteristics and the overall estimate for each point increase in the multimorbidity-weighted index, Nurses’ Health Study (2000–2010), Health Professionals Follow-Up Study (2000–2010), and Nurses’ Health Study II (2001–2010). The overall model estimate was adjusted for all covariates, including age, body mass index (BMI; calculated as weight (kg)/height (m)2), tobacco use, race, and geographic region; models within each stratum were adjusted for all other covariates. Boxes represent the hazard ratios by strata of participant characteristics, whereby the size of the box is proportional to the weight assigned to the characteristic (i.e., larger boxes represent larger sample sizes). The dotted vertical line represents the combined overall estimate. The horizontal lines represent the 95% confidence intervals (CI) around the estimates. The width of the diamond represents the 95% confidence interval around the overall estimate.
Multimorbidity and future physical functioning
The association between multimorbidity and future physical functioning was strongest and best explained by the MWI, followed by simple disease count (Table 3). The CCI was less strongly associated with and explained only 5%–7% of the variance in 4-year physical functioning in women only and 8-year physical functioning in men only. After mutual adjustment for MWI and simple disease count across quartiles, the association of the MWI with future physical functioning was still that of the greatest magnitude and the statistically strongest in the NHS (β = −10.55, 95% CI: −10.92, −10.18; P < 0.0001) and HPFS (β = −8.60, 95% CI: −9.24, −7.97; P < 0.0001) cohorts. Multicollinearity was mild in the mutually adjusted models, with variance inflation factors between 1.3 and 3.5 for the CCI, disease count, and the MWI.
Table 3.
Change in Short Form-36 Physical Functioning Scale by Standardized Metrics After Follow-Up, Nurses’ Health Study (2000–2004) and Health Professionals Follow-Up Study (2000–2008)
| Model and Quartiles | Short Form-36 Physical Functioning Scale β Coefficient | 95% CI | P Value | T Value | R2 |
|---|---|---|---|---|---|
| 4-Year Physical Functioning in the NHS | |||||
| MWI | 0.21 | ||||
| 4 vs. 1 | −14.13 | −14.34, −13.92 | <0.001 | −129.33 | |
| 3 vs. 1 | −7.44 | −7.64, −7.23 | <0.001 | −70.25 | |
| 2 vs. 1 | −3.04 | −3.24, −2.83 | <0.001 | −29.05 | |
| 1 | 1.00 | Referent | |||
| Simple disease count | 0.17 | ||||
| 4 vs. 1 | −12.86 | −13.07, −12.64 | <0.001 | −117.23 | |
| 3 vs. 1 | −6.25 | −6.45, −6.05 | <0.001 | −60.90 | |
| 2 vs. 1 | −2.45 | −2.69, −2.22 | <0.001 | −21.08 | |
| 1 | 1.00 | Referent | |||
| CCI | 0.07 | ||||
| 4 vs. 1–3 | −0.94 | −1.13, −0.75 | <0.001 | −9.63 | |
| 1–3 | 1.00 | Referent | |||
| 8-Year Physical Functioning in the HPFS | |||||
| MWI | 0.15 | ||||
| 4 vs. 1 | −11.29 | −11.65, −10.93 | <0.001 | −61.69 | |
| 3 vs. 1 | −5.38 | −5.71, −5.05 | <0.001 | −31.71 | |
| 2 vs. 1 | −2.52 | −2.84, −2.20 | <0.001 | −15.31 | |
| 1 | 1.00 | Referent | |||
| Simple disease count | 0.13 | ||||
| 4 vs. 1 | −10.04 | −10.44, −9.63 | <0.001 | −48.66 | |
| 3 vs. 1 | −4.29 | −4.67, −3.90 | <0.001 | −21.81 | |
| 2 vs. 1 | −1.66 | −2.08, −1.24 | <0.001 | −7.72 | |
| 1 | 1.00 | Referent | |||
| CCI | 0.05 | ||||
| 4 vs. 1–3 | −5.50 | −5.80, −5.20 | <0.001 | −35.90 | |
| 1–3 | 1.00 | Referent | |||
Abbreviations: CCI, Charlson Comorbidity Index; CI, confidence interval; HPFS, Health Professionals Follow-Up Study; MWI, multimorbidity-weighted index; NHS, Nurses’ Health Study.
The magnitude of the association of MWI with physical functioning was more than double that of simple disease count (NHS: β = −4.33, 95% CI: −4.70, −3.97, P < 0.0001; HPFS: β = −3.36, 95% CI: −4.04, −2.68, P < 0.0001). Future physical functioning was worse in women at 4 years of follow-up than in men at 8 years of follow-up, was consistent across all metrics, and may be, in part, due to higher MWI scores among women.
Heterogeneity in the association of the MWI with future physical functioning by age was suggested by the I2 statistic in the HPFS cohort but not NHS cohort. For all characteristics, the regression coefficients were significant and nearly equal within each stratum (Web Figure 2).
DISCUSSION
In the present study of 219,950 community-dwelling adults ranging in age from 25 to 75 years at study enrollment, we found that compared with the CCI and simple disease count, a health-related quality-of-life–weighted multimorbidity index covering a comprehensive set of chronic conditions had the broadest distribution of multimorbidity and uniquely captured the low-end distribution of multimorbidity. The MWI was strongly associated with lower long-term physical functioning and a higher mortality rate, with a significant dose-response relationship. The MWI provided the best-fitting model and discrimination for long-term mortality prediction compared with the CCI and disease count, thus validating this easily computed measure of multimorbidity.
Our study has implications for the scope and measurement of multimorbidity. For the scope of multimorbidity, our study confirms and extends results from prior studies of multimorbidity and increased long-term mortality (22) by including young and middle-aged adults. Although multimorbidity is often considered a geriatric phenomenon, several young and middle-aged adults had accumulated a high MWI decades earlier than older peers. The youngest participants in the present study had a lower MWI, on average, than did older adults, which is likely due to a combination of younger age (and hence, less time to acquire multiple morbid chronic diseases) and a shorter disease duration compared with older adults. Nonetheless, their multimorbidity burden ranged broadly. In the NHS II cohort of young and middle-aged adults, a higher MWI was associated with a higher mortality risk in a persistent dose-response association that was similar to those observed in the NHS I and HPFS cohorts of middle-aged and older adults. Whereas prior studies on multimorbidity prevalence have included young and middle-aged adults (23, 24), those in which multimorbidity and short- and long-term mortality have been examined were limited to older adults (25). Our study also included older women and men (up to 79 and 89 years of age at baseline, respectively) who were followed for 10 years. There have been few studies in which investigators have examined physical functioning in younger adults with multimorbidity, including a systematic review in which a consistent association between multimorbidity and functional decline was reported (26).
Regarding the measurement of multimorbidity, the present study provides direct comparisons of the performance of the MWI with those of simple disease count and the CCI in long-term physical functioning and mortality prediction. The CCI (11) was developed and validated to predict inpatient mortality and is 1 of the most cited methods for comorbidity adjustment. Although the CCI was weighted to mortality, whereas the MWI was weighted to physical functioning, the MWI outperformed the CCI even in mortality prediction. Furthermore, the dose-response association between the MWI and mortality persisted even after removing CCI conditions from the MWI, verifying that additional conditions captured in the MWI contribute informatively to mortality risk. The MWI also predicted long-term physical functioning, for which the CCI and simple disease count performed marginally. Although both the MWI and disease count outperformed the CCI for mortality and physical functioning predictions, the MWI persisted in its outperformance of disease count in prediction of future physical functioning and mortality, even in mutually adjusted models. Finally, whereas several other measures using administrative data have been weighted to inpatient mortality, cost, or use (27–29), the MWI was weighted to a patient-centered outcome (i.e., current physical health-related quality of life), and yet it also demonstrated strong associations with mortality.
Our study has potential limitations. First, rare diseases such as acquired immunodeficiency syndrome and cirrhosis were less prevalent than were other conditions. However, the numbers of observations in these cohorts still exceeded those in other cohorts used to create similar indices, and they remained sufficiently prevalent to generate weighted estimates for these conditions. Second, multimorbidity was assessed as the sum of individual weighted chronic diseases, a assumption similar to those in other commonly used indices (11, 27). In prior studies with smaller disease inventories, investigators have reported additive and multiplicative effects of several conditions (30). However, the potential combination of disease interactions in large inventories becomes increasingly infeasible and would require enormous data sets with exceptional computing power to evaluate and confirm with validity. Our results, using flexible splines, suggest this limitation was likely to be most problematic only for those with extreme multimorbidity. Third, our sample included participants from all geographic regions but was not explicitly nationally representative. However, disease prevalence rates and participant characteristics, such as obesity and tobacco use, were similar to those in US and other populations (31–33). If disease prevalence rates differ by race, ethnicity, or socioeconomic factors, conditional associations of the MWI with mortality are likely robust to these factors. Our results did not suggest effect modification by age, race, sex, body mass index, smoking status, or geographic region, but further studies are needed to assess generalizability. Fourth, this study did not include young men. Young women experience active changes in hormonal status, parity, and potential dysfunctions of the reproductive organs; there are no equivalent biologic exposures in men. Finally, we examined mortality and future physical functioning to assess the criterion validity of the MWI, but other outcomes available in other data sets, such as all-cause hospitalization, use, and cost, may be of interest to further compare across indices.
An easily measured multimorbidity index weighted to current physical functioning with comprehensive capture of chronic conditions in community-dwelling adults was strongly associated with higher mortality risk and lower long-term physical functioning. Compared with other indices, the MWI had the best model fit, had the strongest association with mortality and future physical functioning, and spanned the widest distribution of multimorbidity in both directions—distinctively at the low end, where multimorbidity onset and progression may be identified and targeted earlier for intervention.
The MWI has potential clinical and research implications. It may be used to quantify the burden of multimorbidity and predict future outcomes, such as physical functioning, mortality, and health economic outcomes, in community-dwelling adults. The MWI weights diseases by a robust and standardized instrument for physical functioning that is not routinely captured in clinical settings, though its application requires only self-reported conditions. Future studies should include translation of the MWI to codes from the International Classification of Diseases, Clinical Modification to expand its use to administrative studies and electronic health records, and identification of modifiable risk factors to prevent the onset and progression of multimorbidity. Potential applications include risk adjustment and incorporation into automated electronic health records to systematically stratify adults who may benefit from complex care management and multidisciplinary team–based resources and to identify and target high-risk adults early to prevent and delay multimorbidity progression and complications.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of General Medicine, University of Michigan, Ann Arbor, Michigan (Melissa Y. Wei); Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, Michigan (Melissa Y. Wei); and Division of General Medicine and Primary Care, Beth Israel Deaconess Medical Center, Harvard Medical School, Brookline, Massachusetts (Kenneth J. Mukamal).
This study was supported by the National Institutes of Health (grants UM1 CA186107 for the Nurses’ Health Study, UM1 CA176726 for the Nurses’ Health Study II, and UM1 CA167552 for the Health Professionals Follow-up Study). M.Y.W. was supported through a National Research Service Award from the National Institutes of Health National Center for Research Resources (T32 HP12706-06).
This work was presented in part at the Society of General Internal Medicine 2015 New England Regional Meeting, March 27–28, 2015, Boston, Massachusetts, and the Society of General Internal Medicine 38th Annual Meeting, April 22–25, 2015, Toronto, Ontario, Canada, and published in abstract form (J Gen Intern Med. 2015;30(suppl 2):S212).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: none declared.
Abbreviations
- CCI
Charlson Comorbidity Index
- HPFS
Health Professionals Follow-up Study
- MWI
multimorbidity-weighted index
- NHS
Nurses’ Health Study
- NHS II
Nurses’ Health Study II
REFERENCES
- 1. Ornstein SM, Nietert PJ, Jenkins RG, et al. . The prevalence of chronic diseases and multimorbidity in primary care practice: a PPRNet report. J Am Board Fam Med. 2013;26(5):518–524. [DOI] [PubMed] [Google Scholar]
- 2. Radner H, Yoshida K, Mjaavatten MD, et al. . Development of a multimorbidity index: impact on quality of life using a rheumatoid arthritis cohort. Semin Arthritis Rheum. 2015;45(2):167–173. [DOI] [PubMed] [Google Scholar]
- 3. Foguet-Boreu Q, Violan C, Rodriguez-Blanco T, et al. . Multimorbidity patterns in elderly primary health care patients in a south Mediterranean European region: a cluster analysis. PLoS One. 2015;10(11):e0141155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wei MY, Kawachi I, Okereke OI, et al. . Diverse cumulative impact of chronic diseases on physical health-related quality of life: implications for a measure of multimorbidity. Am J Epidemiol. 2016;184(5):357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wei MY, Kabeto MU, Langa KM, et al. . Multimorbidity and physical and cognitive function in nationally-representative US adults: performance of a new multimorbidity-weighted index [published online ahead of print June 9, 2017]. J Gerontol A Biol Sci Med Sci. (doi: 10.1093/gerona/glx114). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ware JE Jr, Snow KK, Kosinski M, et al. . SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: The Health Institute; 1993. [Google Scholar]
- 7. Contopoulos-Ioannidis DG, Karvouni A, Kouri I, et al. . Reporting and interpretation of SF-36 outcomes in randomised trials: systematic review. BMJ. 2009;338:a3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fried TR, McGraw S, Agostini JV, et al. . Views of older persons with multiple morbidities on competing outcomes and clinical decision-making. J Am Geriatr Soc. 2008;56(10):1839–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedmann PD, Jin L, Karrison TG, et al. . Early revisit, hospitalization, or death among older persons discharged from the ED. Am J Emerg Med. 2001;19(2):125–129. [DOI] [PubMed] [Google Scholar]
- 10. Rodríguez-Artalejo F, Guallar-Castillón P, Pascual CR, et al. . Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med. 2005;165(11):1274–1279. [DOI] [PubMed] [Google Scholar]
- 11. Charlson ME, Pompei P, Ales KL, et al. . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 12. Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax nationwide death search. Am J Epidemiol. 1994;140(11):1016–1019. [DOI] [PubMed] [Google Scholar]
- 13. Stampfer MJ, Willett WC, Speizer FE, et al. . Test of the National Death Index. Am J Epidemiol. 1984;119(5):837–839. [DOI] [PubMed] [Google Scholar]
- 14. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. [DOI] [PubMed] [Google Scholar]
- 15. Hastings C, Mosteller F, Tukey JW, et al. . Low moments for small samples: a comparative study of order statistics. Ann Math Stat. 1947;18(3):413–426. [Google Scholar]
- 16. Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34(2):187–220. [Google Scholar]
- 17. Efron B. The efficiency of Cox’s likelihood function for censored data. J Am Stat Assoc. 1977;72(359):557–565. [Google Scholar]
- 18. Schoenfeld D. Chi-squared goodness if fit test for the proportional hazards regression model. Biometrika. 1981;67(1):145–153. [Google Scholar]
- 19. Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23(13):2109–2123. [DOI] [PubMed] [Google Scholar]
- 20. Belsley DA, Kuh E, Welsch RE. Detecting and assessing collinearity In: Belsley DA, Kuh E, Welsch RE, eds. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. Hoboken, NJ: John Wiley & Sons, Inc.; 1980:85–191. [Google Scholar]
- 21. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 22. Menotti A, Mulder I, Nissinen A, et al. . Prevalence of morbidity and multimorbidity in elderly male populations and their impact on 10-year all-cause mortality: the FINE study (Finland, Italy, Netherlands, Elderly). J Clin Epidemiol. 2001;54(7):680–686. [DOI] [PubMed] [Google Scholar]
- 23. Barnett K, Mercer SW, Norbury M, et al. . Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. [DOI] [PubMed] [Google Scholar]
- 24. Pefoyo AJ, Bronskill SE, Gruneir A, et al. . The increasing burden and complexity of multimorbidity. BMC Public Health. 2015;15:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marengoni A, Angleman S, Melis R, et al. . Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430–439. [DOI] [PubMed] [Google Scholar]
- 26. Ryan A, Wallace E, O’Hara P, et al. . Multimorbidity and functional decline in community-dwelling adults: a systematic review. Health Qual Life Outcomes. 2015;13:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elixhauser A, Steiner C, Harris DR, et al. . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 28. Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079. [DOI] [PubMed] [Google Scholar]
- 29. Quan H, Li B, Couris CM, et al. . Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. [DOI] [PubMed] [Google Scholar]
- 30. Emerging Risk Factors Collaboration, Di Angelantonio E, Kaptoge S, et al. . Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schnell K, Weiss CO, Lee T, et al. . The prevalence of clinically-relevant comorbid conditions in patients with physician-diagnosed COPD: a cross-sectional study using data from NHANES 1999–2008. BMC Pulm Med. 2012;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schäfer I, von Leitner EC, Schön G, et al. . Multimorbidity patterns in the elderly: a new approach of disease clustering identifies complex interrelations between chronic conditions. PLoS One. 2010;5(12):e15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Baal PH, Engelfriet PM, Boshuizen HC, et al. . Co-occurrence of diabetes, myocardial infarction, stroke, and cancer: quantifying age patterns in the Dutch population using health survey data. Popul Health Metr. 2011;9(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.