Abstract
The role of interactions between intestinal pathogens in diarrheal disease is uncertain. From August 2010 to July 2011, we collected stool samples from 723 children admitted with diarrhea (cases) to 3 major hospitals in Dar es Salaam, Tanzania, and from 564 nondiarrheic children (controls). We analyzed the samples for 17 pathogens and assessed interactions between coinfections in additive and multiplicative models. At least one pathogen was detected in 86.9% of the cases and 62.8%, of the controls. Prevalence of coinfections was 58.1% in cases and 40.4% in controls. Rotavirus, norovirus genogroup II, Cryptosporidium, and Shigella species/enteroinvasive Escherichia coli were significantly associated with diarrhea both as monoinfections and as coinfections. In the multiplicative interaction model, we found 2 significant positive interactions: rotavirus + Giardia (odds ratio (OR) = 23.91, 95% confidence interval (CI): 1.21, 470.14) and norovirus GII + enteroaggregative E. coli (OR = 3.06, 95% CI: 1.17, 7.98). One significant negative interaction was found between norovirus GII + typical enteropathogenic E. coli (OR = 0.09, 95% CI: 0.01, 0.95). In multivariate analysis, risk factors for death were presence of blood in stool and severe dehydration. In conclusion, coinfections are frequent, and the pathogenicity of each organism appears to be enhanced by some coinfections and weakened by others. Severity of diarrhea was not affected by coinfections.
Keywords: biological interactions, coinfections, diarrheal disease, monoinfections, pathogenicity
It is estimated that 6.3 million children worldwide die every year, with nearly half of deaths occurring in sub-Saharan Africa alone (1). Despite the substantial decrease in mortality due to diarrhea (2, 3), diarrhea morbidity is still high, with an estimated 1.731 billion episodes of diarrhea for the year 2010 (3). The majority of deaths from diarrheal disease are of children below 2 years of age (3).
The etiology and epidemiology of infectious diarrheal disease in young children has been widely documented in different parts of the world (4, 5). In addition, the causative viruses, bacteria, and parasites have been well characterized (4, 5).
Each diarrhea pathogen is capable of causing disease alone, but diarrhea also occurs in the presence of 2 or more pathogens, herein referred to as coinfections. Several studies have reported diarrhea caused by coinfections in both developed (6–8) and developing countries (4, 9–12).
The association of coinfections and clinical severity is controversial. Some studies have found that coinfections may cause more severe diarrhea than infection with either pathogen alone (7, 9, 10). Other studies reported no differences in clinical severity between monoinfection and coinfections (13–15).
We previously described the epidemiology of viral (16–18) and parasitic (19) intestinal pathogens in Dar es Salaam, Tanzania. In this study, particular attention was paid to reporting coinfections, in order to give comprehensive analysis of common bacterial, viral, and parasitological causes of diarrhea in children and to assess the role of coinfections. In addition, by estimating pathogen interaction using the additive and multiplicative models, we have described possible biological interactions between coinfecting pathogens.
METHODS
Ethics statement
We received ethical approval from the Senate Research and Publications Committee of Muhimbili University of Health and Allied Sciences in Dar es Salaam, Tanzania, and from the Regional Committee for Medical and Health Research Ethics in Western Norway. Parents/guardians provided written informed consent on behalf of each child participating in the study. Permission was also obtained from the authorities of study hospitals.
Study population
This unmatched case-control study was conducted between August 2010 and July 2011. The study enrolled 1,287 children less than 2 years of age. Cases (n = 723) were children admitted with diarrhea, and controls (n = 564) were children attending child health clinics for immunization and growth monitoring or who were admitted due to diseases other than diarrhea. Cases enrolled in this study were admitted to 3 major hospitals of Dar es Salaam: Muhimbili National Hospital, Amana Regional Referral Hospital, and Temeke Regional Referral Hospital.
Case and control definitions
Cases were defined as children having 3 or more loose stools in a 24-hour period. This included children with acute diarrhea, persistent diarrhea, and presence of blood in stool. Controls were eligible if they were free of diarrhea in the previous 1 month.
Stool specimen collection
We collected a single stool specimen in sterile plastic containers from all cases and controls on the day of enrollment. A portion of stool specimen was frozen at −70°C on the same day that it was collected.
Collection of demographic and clinical information
Demographic and clinical information was obtained using a standardized questionnaire, as detailed previously (17). Briefly, we collected information on age (date of birth), sex, place of residence, parent/guardian level of education, and history of antibiotic use prior to admission. Consistency of stool (watery, bloody) and duration of diarrhea was also recorded.
Detection of enteric pathogens
Rotavirus and adenoviruses were detected using enzyme-linked immunosorbent assay while norovirus genogroups I (GI) and II (GII), Cryptosporidium, Giardia, and Entameoba histolytica were detected using real-time polymerase chain reaction (PCR) techniques as previously published (16–19).
For detection of enteric bacteria, real-time multiplex PCR with primers and probes, described previously (20), was used for detection of ipaH, invA, aggR, eae, STh, STp, and LT genes to detect Shigella species/enteroinvasive E. coli (EIEC), Salmonella species, enteroaggregative E. coli (EAEC), atypical enteropathogenic E. coli (EPEC), STh and STp heat-stable toxin–producing enterotoxigenic E. coli (ST-ETEC), and heat-labile toxin–producing ETEC (LT-ETEC). We modified primers published by Iida et al. (21) and designed new probes to detect bfpA genes for typical EPEC. For detection of cadF genes for Campylobacter jejuni, primers and probes published by Elfving et al. (22) were used. PCR conditions used were as previously described (20). In order to determine whether the pathogen was present at a level that might cause diarrhea, we used cycle thresholds. Cycle thresholds show the number of cycles needed to detect a signal from the sample and are inverse to the amount of nucleic acid that is in the sample. For all bacteria and for norovirus GI and GII, cycle-threshold values above 33 were defined as negative. For protozoans Giardia and Cryptosporidium species, a cycle-threshold value of above 37 was defined as negative. Stool was also cultured on thiosulfate-citrate-bile salts-sucrose agar (TCBS) media within 6 hours after collection for detection of Vibrio cholerae, and identification was done locally by conventional methods. All other laboratory analysis was performed at the University of Bergen.
Statistical analysis
Statistical analysis was performed using Stata, version 13 (StataCorp LP, College Station, Texas). A binary logistic regression model was used to estimate pathogenicity for each pathogen (i.e., odds ratios) (23) in any infection (i.e., when all pathogens (monoinfection and coinfections) detected in each child were included). In order to see the effect of coinfection, we also calculated odds ratios separately for monoinfection and coinfections detected for each child. To account for potential confounding by age and sex, all models were adjusted for age in months and sex of the child. Except for V. cholerae, which was detected in only 1 child, we included all detected pathogens from stool samples for each child in the regression model. In order to control and check for stability of results, the 95% confidence intervals were estimated by bootstrapping the data (by repeating the process of random sampling and replacement of data 1,000 times) to produce estimates associated with each statistic.
To estimate pathogen-specific disease burden, we calculated attributable fraction (AF) by using odds ratios derived from the multivariate analysis with 95% confidence intervals and pathogen prevalence among patients with diarrhea (24) using the STATA-punafcc package. Because this was a case-control study, odds ratios were used as an approximation of risk ratios to calculate AF (25). AF was derived from a multiple logistic regression model adjusting for presence of other pathogens significantly associated with diarrhea. Age and sex were also included in the model. AF was calculated across ages and in 2 age strata (children aged ≤11 months and >11 months).
The biologic interaction between coinfecting pathogens associated with diarrhea was assessed using additive- and multiplicative-scale models, as previously described (26). VanderWeele (26) found that in case-control studies of rare outcomes, odds ratio can approximate risk ratios to calculate relative excess risk due to interaction (RERI) as well as attributable proportion (AP).
For assessment of biological interactions between coinfecting pathogens in the additive model, STATA/IC (StataCorp LP) was used to produce odds ratios, RERI, AP, and their 95% confidence intervals (see Web Appendix 1, available at https://academic.oup.com/aje). The model adjusted for the effect of age and sex. Interaction existed if the RERI value was not equal to zero. To assess biological interactions in the multiplicative model, we used a logistic regression model with an interaction term containing 2 coinfecting pathogens. Interactions existed if the multiplicative interactions value was not equal to 1.
Univariate and multivariate regression analyses were used to assess the risk factors for death. The factors included in the multivariate model were 3 pathogens significantly associated with diarrhea in univariate analysis and clinical characteristics (age group, presence of blood in stool, lack of breastfeeding, presence of dehydration, duration of diarrhea, malnutrition, and human immunodeficiency virus (HIV) status) for children admitted with diarrhea. Breastfeeding was recorded only for children aged 7–12 months, who are expected to breastfeed. The model was adjusted for age, sex, and history of antibiotic use during the previous 14 days.
To assess the differences in clinical characteristics between monoinfection and coinfections, we performed multivariate analysis that included all the clinical characteristics, nutritional status, type of diarrhea, presence of dehydration, HIV status, presence of blood in stool, and death.
RESULTS
The study included 1,287 children: 723 cases and 564 controls. Malnutrition was more prevalent among children aged >11 months than among children aged ≤11 months as follows: underweight, 60.1% (298/496) versus 43.0% (340/791) (odds ratio (OR) = 2.0, 95% confidence interval (CI):1.59, 2.51); stunting, 64.3% (319/496) versus 60.4% (478/791) (OR = 1.2, 95% CI: 0.94, 1.49); wasting, 34.7% (172/496) versus 20.6% (163/791) (OR = 2.05, 95% CI: 1.59, 2.63). Persistent diarrhea was more prevalent among children who were HIV-positive than among those who were HIV-negative (8/26 for 30.8% versus 7/80 for 8.8%; OR = 4.64, 95% CI: 1.49, 14.46). Demographic and clinical characteristics of the study population are shown in Web Table 1.
Detection of pathogens
We searched for 17 pathogens, and at least 1 pathogen was detected in 982 (76.0%) of the 1,287 samples and in 86.9% (628/723) of cases and 62.8% (354/564) of controls. No pathogens were detected in 13.1% cases and 37.2% controls.
Prevalence of monoinfection and coinfections
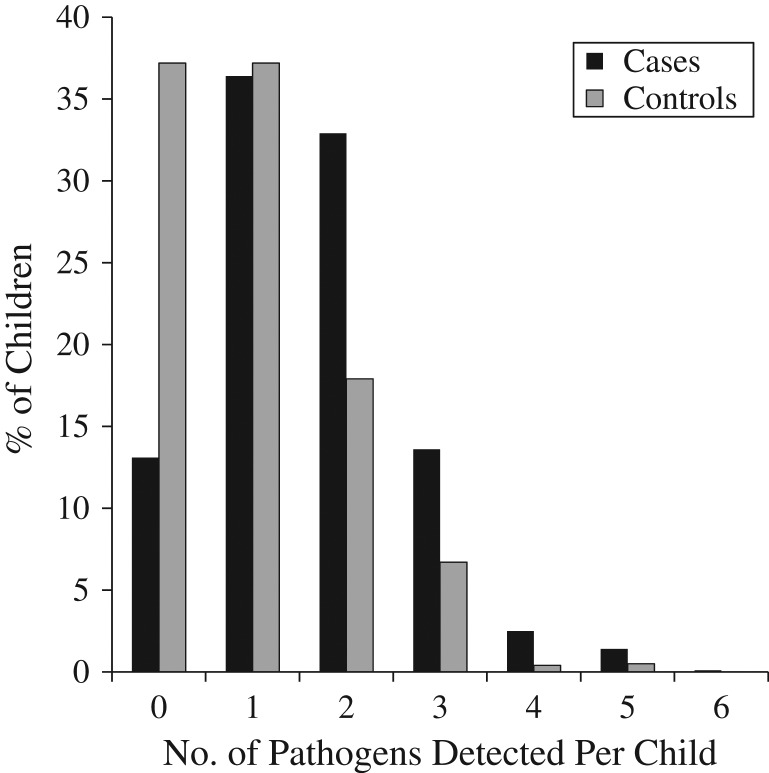
Among children with pathogens detected, 41.9% of cases 59.6% of controls had monoinfection. Coinfections (2 or more pathogens) were more prevalent in cases (58.1%, 365/628) than in controls (40.4%, 143/354) (OR = 2.03, 95% CI: 1.56, 2.64). Figure 1 shows number of pathogens detected per child for cases and controls.
Figure 1.
The number of enteric pathogens detected per child for cases with diarrhea and for controls, Dar es Salaam, Tanzania, 2010–2011. Number of pathogens detected ranged from 0 to 6 pathogens per child in cases and 0 to 5 pathogens per child in controls. The y-axis represents the percentage of children. In the regression analysis, the odds of having 2 or more pathogens were significantly higher among cases than among controls and were as follows: for 2 pathogens, odds ratio = 5.21 (95% confidence interval (CI): 3.72, 7.29); for 3 pathogens, odds ratio = 5.7 (95% CI: 3.65, 8.91); for 4 pathogens, odds ratio = 19.89 (95% CI: 4.52, 87.47), and for 5 pathogens, odds ratio = 7.37 (95% CI: 1.98, 27.38).
Pathogenicity across all ages of any infection, monoinfection, and coinfection
Odds ratios adjusted by age and sex for different pathogens are presented in Table 1 for the univariate and in Table 2 for the multivariate analysis. Web Table 2 shows the sample size for different infection categories in cases and controls. From the multivariate analysis, 8 pathogens; rotavirus, norovirus GII, Cryptosporidium, Giardia, typical EPEC, Shigella species/EIEC, and ST-ETEC were significantly associated with diarrhea in any infection (monoinfection and coinfections combined).
Table 2.
Odds Ratio Estimates for Diarrhea in Multivariate Analysis for Any Infection, Monoinfection, or Coinfections, Adjusted for Age and Sex, Dar es Salaam, Tanzania, 2010–2011a
| Pathogen | Any Infection | Monoinfection | Coinfections | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Rotavirus | 8.42 | 5.59, 12.47a | 3.59 | 1.93, 6.64a | 5.61 | 3.41, 9.25a |
| Norovirus GII | 4.83 | 3.10, 7.50a | 2.67 | 1.39, 5.12a | 2.66 | 1.46, 4.84a |
| Enteric adenovirus | 2.77 | 0.84, 9.13 | –b | –b | 2.41 | 0.60, 9.62 |
| Nonenteric adenovirus | 1.76 | 0.60, 5.11 | –b | –b | –b | –b |
| Cryptosporidium | 9.62 | 5.47, 16.90a | 4.73 | 1.80, 12.49a | 6.12 | 2.93, 12.79a |
| Giardia | 0.52 | 0.27, 0.97a | 0.07 | 0.02, 0.24a | 0.52 | 0.26, 1.04 |
| Typical EPEC | 2.77 | 1.23, 6.22a | 10.89 | 2.66, 44.57a | 1.11 | 0.45, 2.75 |
| Atypical EPEC | 0.92 | 0.65, 1.29 | 0.28 | 0.15, 0.55 | 0.83 | 0.54, 1.26 |
| ST-ETEC | 4.62 | 2.25, 9.49a | 1.69 | 0.38, 7.52 | 4.11 | 1.71, 9.88a |
| LT-ETEC | 0.89 | 0.54, 1.48 | 0.40 | 0.12, 1.31 | 0.75 | 0.44, 1.27 |
| EAEC | 1.23 | 0.93, 1.63 | 0.44 | 0.31, 0.65 | 1.01 | 0.69, 1.47 |
| Shigella species/EIEC | 6.61 | 3.05, 14.31a | 3.91 | 1.05, 14.55a | 4.05 | 1.74, 9.39a |
| Salmonella species | 4.18 | 0.99, 17.67 | –b | –b | –b | –b |
| Campylobacter jejuni | 1.02 | 0.58, 1.77 | 0.19 | 0.05, 0.76a | 1.01 | 0.55, 1.85 |
Abbreviations: CI, confidence interval; EAEC, enteroaggregative Escherichia coli; EIEC, enteroinvasive E. coli; EPEC, enteropathogenic E. coli; ETEC, enterotoxigenic E. coli; GII, genogroup II; LT, heat-labile toxin–producing; OR, odds ratio; ST, heat-stable toxin–producing.
a Confidence intervals that do not overlap the null value of OR = 1.
b Some calculations could not be made because of small sample size in the category.
Table 1.
Odds Ratios for Diarrhea in Univariate Analysis for Any Infection, Monoinfection, or Coinfections, Adjusted for Age and Sex, Dar es Salaam, Tanzania, 2010–2011
| Pathogen | Any Infection | Monoinfection | Coinfections | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Rotavirus | 5.45 | 3.82, 7.75a | 3.82 | 2.16, 6.75a | 5.27 | 3.44, 8.08a |
| Norovirus GII | 2.80 | 1.93, 4.08a | 2.75 | 1.49, 5.06a | 2.58 | 1.63, 4.08a |
| Enteric adenovirus | 1.60 | 0.59, 4.31 | –b | –b | 2.19 | 0.69, 6.96 |
| Nonenteric adenovirus | 1.42 | 0.55, 3.65 | 0.89 | 0.18, 4.52 | 1.7 | 0.54, 5.82 |
| Cryptosporidium | 5.91 | 3.49, 9.97a | 4.84 | 2.01, 11.61a | 5.95 | 3.13, 11.31a |
| Giardia | 0.57 | 0.33, 0.97a | 0.07 | 0.01, 0.56a | 0.82 | 0.46, 1.49 |
| Typical EPEC | 2.18 | 1.21, 3.92a | 3.81 | 1.08, 13.40a | 1.76 | 0.91, 3.44 |
| Atypical EPEC | 1.04 | 0.79, 1.39 | 0.45 | 0.27, 0.77 | 1.43 | 1.03, 1.98a |
| ST-ETEC | 3.32 | 1.78, 6.19a | 1.45 | 0.47, 4.48 | 4.39 | 2.05, 9.45a |
| LT-ETEC | 0.95 | 0.61, 1.48 | 0.43 | 0.14, 1.29 | 1.12 | 0.69, 1.81 |
| EAEC | 1.32 | 1.04, 1.66a | 0.39 | 0.27, 0.47a | 2.06 | 1.59, 2.69a |
| Shigella species/EIEC | 4.01 | 2.11, 7.62a | 3.92 | 1.10, 13.92a | 3.87 | 1.85, 8.10a |
| Salmonella species | 3.23 | 0.89, 11.74 | 0.52 | 0.08, 3.20 | –b | –b |
| Campylobacter jejuni | 1.23 | 0.73, 2.05 | 0.19 | 0.04, 0.92a | 1.71 | 0.96, 3.06 |
Abbreviations: CI, confidence interval; EAEC, enteroaggregative Escherichia coli; EIEC, enteroinvasive E. coli; EPEC, enteropathogenic E. coli; ETEC, enterotoxigenic E. coli; GII, genogroup II; LT, heat-labile toxin–producing; OR, odds ratio; ST, heat-stable toxin–producing.
a Confidence intervals that do not overlap the null value of OR = 1.
b Some calculations could not be made because of small sample size in the category.
After stratification for infection type (monoinfection vs. coinfection), only 4 pathogens (rotavirus, norovirus GII, Cryptosporidium, and Shigella species/EIEC) showed significant association with diarrhea both as monoinfection and in coinfections. Typical EPEC was associated with diarrhea only as a monoinfection, and the association disappeared when present in coinfections. Giardia monoinfection was negatively associated with diarrhea. On the other hand, ST-ETEC showed significant association with diarrhea only when present in coinfections and not when present as a monoinfection.
For pathogens showing a significant association with diarrhea, we found higher pathogenicity—higher odds ratio for causing diarrheal symptoms—when present in coinfections than in monoinfection.
In the univariate analysis, EAEC was significantly associated with diarrhea overall, while atypical EPEC and C. jejuni were negatively associated with diarrhea in monoinfection.
Pathogen-specific disease burden by attributable fraction
Across age groups, major contributors to diarrheal disease identified by AF, in decreasing order, were: rotavirus, Cryptosporidium, norovirus GII, Shigella species/EIEC, ST-ETEC, typical EPEC, and Salmonella species
An age-related pattern in the AF for diarrhea was observed for 2 pathogens. Norovirus GII presented with higher AF for infants aged ≤11 months than for children aged >11 months, while Shigella species/EIEC showed higher AF for children aged >11 months than for younger children. Values of AF for different pathogens are shown in Table 3.
Table 3.
Adjusted Attributable Fraction for Pathogens Significantly Associated With Diarrhea, for All Ages and Age-Specific Strata, Dar es Salaam, Tanzania, 2010–2011
| Pathogen | All Ages | Age ≤11 Months | Age >11 Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | AF, % | 95% CI | Cases | Controls | AF, % | 95% CI | Cases | Controls | AF, % | 95% CI | |
| Rotavirus | 224 | 42 | 27.24 | 23.36, 30,93a | 163 | 28 | 27.91 | 22.89, 32.61a | 61 | 14 | 24.97 | 18.49, 30.94a |
| Cryptosporidium | 113 | 17 | 13.99 | 11.12, 16.77a | 81 | 10 | 14.37 | 10.79, 17.81a | 32 | 7 | 13.06 | 8.11, 17.74a |
| Norovirus GII | 129 | 40 | 14.12 | 10.80, 17.31a | 103 | 21 | 17.00 | 12.75, 21.04a | 26 | 19 | 7.38 | 2.01, 12.45a |
| Shigella species/EIEC | 53 | 12 | 6.11 | 3.99, 8.17a | 23 | 5 | 3.27 | 1.00, 5.56 | 30 | 7 | 12.10 | 7.32, 16.64a |
| ST-ETEC | 51 | 13 | 5.42 | 3.27, 7.51a | 30 | 7 | 4.31 | 1.80, 6.75a | 21 | 6 | 7.95 | 3.62, 12.07a |
| Typical EPEC | 43 | 16 | 3.66 | 1.15, 6.10a | 27 | 8 | 3.04 | −0.11, 6.09 | 16 | 8 | 490.00 | 0.62, 9.08 |
| Salmonella species | 11 | 3 | 1.15 | 0.12, 2.18 | 5 | 2 | 0.61 | −0.48, 1.69 | 6 | 1 | 2.20 | −0.41, 4.39 |
| Rotavirus | 224 | 42 | 27.24 | 23.36, 30,93a | 163 | 28 | 27.91 | 22.89, 32.61a | 61 | 14 | 24.97 | 18.49, 30.94a |
Abbreviations: AF, attributable fraction; CI, confidence interval; EIEC, enteroinvasive Escherichia coli; EPEC, enteropathogenic E. coli; ETEC, enterotoxigenic E. coli; GII, genogroup II; ST, heat-stable toxin–producing.
a Confidence intervals that do not overlap the null value of odds ratio = 1.
Biologic interaction between coinfecting pathogens associated with diarrhea in additive- and multiplicative-scale models
The values for RERI, AP, and multiplicative interaction for different coinfecting pathogens are presented in Table 4. Under the null hypothesis of no interaction, we would expect a RERI of zero and AP of zero in the additive model and a multiplicative interaction of 1. We found 2 significant positive interactions on the multiplicative model. These were for rotavirus and Giardia and for norovirus GII and EAEC. These 2 coinfections also showed values greater than additive effect (i.e., RERI > 1), but the test results for interactions were not statistically significant. Significant negative interaction was found between norovirus GII and typical EPEC in the multiplicative model but was not statistically significant in the additive model. Web Table 3 shows clinical characteristics of monoinfection and coinfection.
Table 4.
Assessment of the Biologic Interaction Between Coinfecting Pathogens Associated With Diarrhea Using Additive- or Multiplicative-Scale Models, Adjusted for Age and Sex, Dar es Salaam, Tanzania, 2010–2011
| Coinfection Category | Additive Model | Multiplicative Model | |||||
|---|---|---|---|---|---|---|---|
| OR | RERIa | 95% CI | APa | 95% CI | MIb | 95% CI | |
| Rotavirus (+), Giardia (−) | 3.79 | ||||||
| G. lamblia (+), rotavirus (−) | 0.078 | ||||||
| Rotavirus (+), Giardia (+) | 7.12 | 3.65 | −10.76, 19.24 | 59.55 | −0.29, 1.49 | 23.91 | 1.21, 470.14c |
| Rotavirus (+), norovirus GII (−) | 4.04 | ||||||
| Norovirus (+), rotavirus (−) | 3.04 | ||||||
| Rotavirus (+), norovirus GII (+) | 3.75 | −2.33 | −7.35, 2.69 | −62.0 | −2.54, 1.30 | 0.31 | 0.07, 1.19 |
| Rotavirus (+), Shigella species/EIEC (−) | 3.97 | ||||||
| Shigella species EIEC (+), rotavirus (−) | 4.23 | ||||||
| Rotavirus (+), Shigella species/EIEC (+) | 7.01 | −0.18 | −5.91, 15.53 | −2.69 | −2.32, 2.26 | 0.42 | 0.03–5.09 |
| Rotavirus (+), Campylobacter jejuni (−) | 3.87 | ||||||
| C. jejuni (+), rotavirus (−) | 0.21 | ||||||
| Rotavirus (+), C. jejuni (+) | 7.94 | 4.86 | −6.97, 16.71 | 61.21 | −0.01, 1.24 | 9.61 | 1.05, 87.63 |
| Cryptosporidium (+), norovirus GII (−) | 5.10 | ||||||
| Norovirus (+), Cryptosporidium (−) | 2.89 | ||||||
| Cryptosporidium (+), norovirus GII (+) | 2.43 | −4.56 | −10.75, 1.62 | −18.77 | −6.89, 3.14 | 0.16 | 0.02, 1.13 |
| Cryptosporidium (+), atypical EPEC (−) | 4.92 | ||||||
| Atypical EPEC (+), Cryptosporidium (−) | 0.93 | ||||||
| Cryptosporidium (+), atypical EPEC (+) | 10.02 | 5.18 | −9.90, 20.26 | 51.68 | −0.30, 1.33 | 2.21 | 0.39, 12.19 |
| Norovirus II (+), EAEC (−) | 2.64 | ||||||
| EAEC (+), norovirus GII (−) | 0.44 | ||||||
| Norovirus GII (+), EAEC (+) | 3.57 | 1.48 | −1.33, 4.30 | 41.60 | −0.17, 1.00 | 3.06 | 1.17, 7.98c |
| Norovirus II (+), typical EPEC (−) | 2.79 | ||||||
| Typical EPEC (+), norovirus GII (−) | 3.97 | ||||||
| Norovirus GII (+), typical EPEC (+) | 1.09 | −4.68 | −10.33, 0.96 | −43.03 | −14.99, 6.38 | 0.09 | 0.10, 0.95c |
Abbreviations: +, pathogen present; –, pathogen absent; AP, attributable proportion; CI, confidence interval; EAEC, enteroaggregative Escherichia coli; EIEC, enteroinvasive E. coli; EPEC, enteropathogenic E. coli; GII, genogroup II; LT, heat-labile toxin–producing; MI, multiplicative interaction; OR, odds ratio; ST, heat-stable toxin–producing; MI, multiplicative interaction; RERI, relative excess risk due to interaction.
a Interaction if RERI is not 0.
b Interaction if MI is not 1.
c Confidence intervals that do not overlap the null value of OR = 1.
Risk factors for death for children admitted due to diarrhea
Among children admitted with diarrhea, 7.1% died. Table 5 shows risk factors for death in univariate and multivariate analysis. Persistent diarrhea and lack of breastfeeding were significantly associated with death in univariate analysis. Presence of blood in stool and severe dehydration were significantly associated with death in multivariate analysis.
Table 5.
Odds Ratios Estimating the Risk Factors for Death Among Children Admitted Due to Diarrhea, Dar es Salaam, Tanzania, 2010–2011
| Risk Factor | Total Number | Death | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|---|
| No. | % | OR | 95% CI | OR | 95% CI | ||
| Age group | |||||||
| >11 months | 223 | 14 | 6.3 | ||||
| ≤11 months | 505 | 37 | 7.3 | 1.2 | 0.634, 2.26 | 1.26 | 0.64, 2.48 |
| Presence of blood in stool | |||||||
| No | 693 | 45 | 6.5 | ||||
| Yes | 30 | 6 | 20.0 | 3.64 | 1.42, 9.37a | 3.12 | 1.14, 8.55a |
| Hydration status | |||||||
| None to moderate dehydration | 559 | 32 | 5.7 | ||||
| Severe dehydration | 164 | 19 | 11.6 | 1.48 | 0.71, 3.05 | 2.21 | 1.17, 4.17a |
| Type of diarrhea | |||||||
| Acute, watery | 644 | 41 | 6.4 | ||||
| Persistent | 79 | 10 | 12.7 | 2.2 | 1.05, 4.58a | 1.47 | 0.66, 3.30 |
| Underweightb | |||||||
| Normal | 307 | 23 | 7.5 | ||||
| Malnourished | 416 | 28 | 6.7 | 0.9 | 0.51, 1.59 | 0.64 | 0.32, 1.27 |
| Stuntingb | |||||||
| Normal | 223 | 11 | 4.9 | ||||
| Malnourished | 490 | 40 | 8.2 | 1.79 | 0.91, 3.57 | 1.98 | 0.88, 4.48 |
| Wastingb | |||||||
| Normal | 505 | 38 | 7.5 | ||||
| Malnourished | 218 | 13 | 5.9 | 0.78 | 0.41, 1.49 | 1.23 | 0.55, 2.74 |
| ST-ETEC | |||||||
| Present | 34 | 6 | 17.6 | 3.11 | 1.22, 7.94a | 2.03 | 0.79, 5.23 |
| Absent | 675 | 44 | 6.5 | ||||
| Cryptosporidium | |||||||
| Present | 60 | 8 | 13.3 | 2.36 | 1.04, 5.35a | 1.30 | 0.63, 2.69 |
| Absent | 649 | 42 | 6.5 | ||||
| C. jejuni | |||||||
| Present | 31 | 5 | 16.1 | 2.78 | 1.02, 7.62a | 0.53 | 0.12, 2.39 |
| Absent | 678 | 45 | 6.6 | ||||
| Breastfeedingc | |||||||
| Yes | 232 | 14 | 6.0 | ||||
| No | 52 | 9 | 17.3 | 3.26 | 1.33, 8.01a | –d | –d |
| HIV statusc | |||||||
| HIV negative | 78 | 7 | 9.0 | ||||
| HIV positive | 26 | 6 | 23.1 | 3.04 | 0.92, 10.08 | –d | –d |
Abbreviations: CI, confidence intervals; ETEC, enterotoxigenic E. coli; HIV, human immunodeficiency virus; OR, odds ratio; ST, heat-stable toxin–producing.
a Confidence intervals that do not overlap the null value of OR = 1.
b Defined by z score (length for age, weight for age, or weight for length).
c Breastfeeding status and HIV status were not included in multivariate analysis because of the small number of samples. Total sample sizes used are different from the rest of the table because breastfeeding status included only children aged 7–12 months, and HIV status included only the number of children tested for HIV.
d Some calculations could not be made because of small sample size in the category.
At least 1 pathogen could be detected in 40 of 51 children who died, but fatal outcome was not significantly more frequent among children with pathogens detected than among those without (40/531, 7.5% vs. 9/191, 4.7%). In univariate analysis, fatal outcome was associated with infection with ST-ETEC, Cryptosporidium, or C. jejuni. Infection with other pathogens was not associated with death. Death was not significantly more prevalent among children with coinfections than among children with monoinfection.
DISCUSSION
In the present study, rotavirus caused the highest burden of diarrheal disease in all age groups, concurring with findings from 2 large multicenter case-control studies conducted in developing countries (4, 5). In line with previous studies (4, 5, 27), norovirus GII, Cryptosporidium, ST-ETEC, and Shigella species/EIEC, were also important causes of severe diarrhea in children in the current study.
We observed differences in the burden of diarrheal disease attributed to norovirus GII and Shigella species/EIEC in different age groups. Norovirus GII caused significantly higher burden of disease in infants aged ≤11 months than in older children. This was also seen in the Global Enteric Multicenter Study and the MAL-ED study (4, 5).
In contrast to norovirus GII, Shigella species/EIEC caused a higher burden of disease in children older than 11 months than among younger children. In this study, older children had a higher prevalence of malnutrition than did younger children. Previous studies have reported an association of bacterial enteric pathogens with severe malnutrition. Malnutrition causes impaired gastric acid barrier and hypochlorhydria (28) and may thereby result in increased susceptibility to infection by bacteria (29–31). It has also been suggested that the prolonged small intestinal mucosal injury that commonly occurs in malnourished children leads to increased gut colonization by bacteria (32, 33).
Although the prevalence of pathogens was significantly lower in controls than in cases, nearly two-thirds of controls were carrying pathogens at detectable levels. Possible reasons for this finding include, first, prolonged excretion of some pathogens in stool after clinical recovery (34); and second, due to host factors, where susceptibility to infection and disease may be affected by the presence or absence of receptors or the expression of variant receptors. For example, volunteer studies show that some individuals are highly resistant to Norwalk virus, whereas persons of blood group O exhibit increased risk of developing clinical illness upon exposure (35). Third, asymptomatic carriage can result from immunity attained from previous exposure to the same pathogens or from maternal antibodies. Fourth, some pathogens may require interaction with a second pathogen to cause symptoms (e.g., Giardia and rotavirus) (9).
The high prevalence of coinfections in this study and others in developing countries implies that a multipathogenic etiology of diarrhea is common in these settings. In addition, there is variation in the reported prevalence of coinfection within developing countries and between developing and developed countries (4, 6–8, 36, 37). This could be due to variation in the number of pathogens tested for and methods used for detection. Differences between developing and developed countries could also reflect geographical variation or different levels of sanitation, because all these pathogens share the same mode of acquisition by the fecal-oral route. A high prevalence of coinfections both in cases and controls can represent both a consequence of and a predisposing factor for environmental enteropathy in developing countries (38).
We compared pathogenicity (which can be expressed as odds ratio, as described by Black et al. (23)) between monoinfection and coinfections for pathogens associated with diarrhea. Rotavirus, norovirus GII, Cryptosporidium, and Shigella species/EIC showed significant associations with diarrhea both when present as single pathogen and when present in coinfections. This finding concurs with the findings of other case-control studies that have compared monoinfection versus coinfection (39, 40). The significant associations with diarrhea mean these pathogens are capable of inducing diarrhea regardless of whether they occur as monoinfection or in coinfection.
Few studies have looked at biological interactions between coinfecting pathogens in diarrheal disease (9, 41, 42). In the present study, we analyzed different interactions of specific coinfecting pathogens, and we found 2 significant positive interactions, between rotavirus and Giardia and between norovirus GII and EAEC in the multiplicative model. Bhavnani et al. (9) found significant interactions between rotavirus and Giardia in both the additive and the multiplicative model. The lack of significant statistical interactions in the additive model in our study may be due to insufficient sample size. The mechanism might be through enhancement of adhesion or invasion or through up-regulation of specific receptors for these pathogens (9). Experimental studies conducted in animals have also demonstrated synergism between rotavirus and bacteria (43–45).
We found significant negative interaction between norovirus GII and typical EPEC. Similarly, a study in India (37) showed negative association for V. cholerae with Cryptosporidium. In another study, negative interactions between rotavirus and Shigella were reported (42). The biological mechanisms of these findings are not clear; however, the results suggest that 2 pathogens may interact antagonistically within the host by competing for receptors and resources. Understanding the negative interactions between pathogens is important when considering the need of specific interventions.
It is worth noting that pathogen detection does not translate to disease, and detection of more than 1 pathogen in stool samples is not sufficient to infer biological interactions. In addition, statistical interactions do not necessarily indicate biological interactions. However, our results together with findings from previous studies (9, 42) underscore the need for further research to explore possible biological mechanisms.
In the present study, we did not find significant differences in clinical severity of diarrhea (i.e., presence of dehydration) among children with single infections compared to children with coinfections overall. Nor was this seen for specific coinfections, which showed biological interactions (data not shown). This may suggest that biological interactions may enhance pathogenicity by increasing the risk for these pathogens to cause diarrhea but without measurably affecting the clinical severity of diarrhea. The clinical presentation of diarrhea (mild or severe) is likely also to depend on other factors, such as the immune status of the host, and not merely the presence of multiple pathogens—bearing in mind that high prevalences of coinfections are also observed among controls that do not have any diarrhea symptoms. This nonmodulation of clinical severity in coinfections was also reported in studies conducted in Israel and China (13, 41). Bilenko et al. (13) did not find any increase in severity between children infected with Giardia alone and those with different coinfections. In contrast, other studies conducted in Europe have reported increase in clinical severity of diarrhea in children with coinfections (6, 7, 46). An important note is that these studies, which reported increased severity of diarrhea in coinfections, did not analyze for the presence of biological interactions, whereas Bhavnani et al. (9), who reported biological interactions, did not look for clinical severity of diarrhea. Previous studies in animals have showed both increased pathogenicity and increased severity of diarrhea with coinfection (47–51). The present study is among very few case-control studies to do comprehensive analysis of coinfection in diarrhea, analyzing both possible biological interactions and clinical severity.
The majority of deaths due to diarrhea occur in just 15 countries, of which Tanzania is one (3). In the present study, 7% of children admitted due to diarrhea died. Most of them were young infants. We found presence of blood in stool, severe dehydration, and lack of breastfeeding to be risk factors associated with the deaths of these children. Lack of breastfeeding has previously been reported to increase risk of death from diarrhea (52, 53). Furthermore, the case fatality rate was high in children infected with Cryptosporidium, ST-ETEC, and C. jejuni in univariate analysis. ST-ETEC was also associated with fatal outcome in the Global Enteric Multicenter Study (4). The present study included hospitalized children, who are likely to have more severe disease than community-based studies. Therefore, it is likely that pathogens causing mild diarrhea were missed.
Most of the coinfection subgroups were small; this made statistical comparison of monoinfections and coinfections difficult, especially for assessing biological interactions in coinfections. This is probably the reason why most of the 95% confidence intervals included 0 or 1 in both interaction models, which indicates that these interactions are not statistically significant. Another limitation of this study is that it was an unmatched case-control study. However, we had a sufficient number of cases and controls for analyses. Some pathogens were not tested for in this study (such as enterohemorrhagic E. coli, Aeromonas, and helminthes), but previous studies in the same setting have shown absence or low prevalence of these pathogens. We focused on the 17 most frequent and predominant pathogens associated with diarrhea.
In conclusion, our results showed that the pathogenicity of each organism appears to be enhanced by some coinfections and weakened by others. Coinfections may enhance pathogenicity by causing diarrhea symptoms but without increasing diarrhea severity. Rotavirus vaccination is likely to reduce the burden of diarrhea, because rotavirus showed the highest AF across all age groups, and its pathogenicity was high whether it occurred as a single pathogen or in coinfections.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Clinical Science, Faculty of Medicine, University of Bergen, Bergen, Norway (Sabrina J. Moyo, Bjørn Blomberg, Kurt Hanevik, Nina Langeland); Department of Microbiology, Haukeland University Hospital, Bergen, Norway (Øyvind Kommedal); National Centre for Tropical Infectious Diseases, Haukeland University Hospital, Bergen, Norway (Bjørn Blomberg, Marit Gjerde Tellevik, Kurt Hanevik, Nina Langeland); and Department of Microbiology and Immunology, School of Medicine, Muhimbili University of Health and Allied Sciences (MUHAS), Dar es Salaam, Tanzania (Sabrina J. Moyo, Samuel Y. Maselle).
Financial support was provided by the University of Bergen, Bergen, Norway.
We thank the staff at Muhimbili National Hospital and Muhimbili University of Allied Sciences for technical assistance during data collection. We thank Steinar Sørnes of the Department of Clinical Science, University of Bergen, Bergen, Norway, for technical assistance. Further, we thank the Department of Clinical Microbiology, Haukeland University Hospital, Bergen, Norway, and Norwegian Institute of Public Health, Oslo, Norway, for providing bacteria used as positive controls for PCR.
Conflict of interest: none declared.
REFERENCES
- 1. Liu L, Oza S, Hogan D, et al. . Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–440. [DOI] [PubMed] [Google Scholar]
- 2. Black RE, Cousens S, Johnson HL, et al. . Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–1987. [DOI] [PubMed] [Google Scholar]
- 3. Walker CL, Rudan I, Liu L, et al. . Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kotloff KL, Nataro JP, Blackwelder WC, et al. . Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–222. [DOI] [PubMed] [Google Scholar]
- 5. Platts-Mills JA, Babji S, Bodhidatta L, et al. . Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health. 2015;3(9):e564–e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Román E, Wilhelmi I, Colomina J, et al. . Acute viral gastroenteritis: proportion and clinical relevance of multiple infections in Spanish children. J Med Microbiol. 2003;52(Pt 5):435–440. [DOI] [PubMed] [Google Scholar]
- 7. Valentini D, Vittucci AC, Grandin A, et al. . Coinfection in acute gastroenteritis predicts a more severe clinical course in children. Eur J Clin Microbiol Infect Dis. 2013;32(7):909–915. [DOI] [PubMed] [Google Scholar]
- 8. Levidiotou S, Gartzonika C, Papaventsis D, et al. . Viral agents of acute gastroenteritis in hospitalized children in Greece. Clin Microbiol Infect. 2009;15(6):596–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhavnani D, Goldstick JE, Cevallos W, et al. . Synergistic effects between rotavirus and coinfecting pathogens on diarrheal disease: evidence from a community-based study in northwestern Ecuador. Am J Epidemiol. 2012;176(5):387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grimprel E, Rodrigo C, Desselberger U. Rotavirus disease: impact of coinfections. Pediatr Infect Dis J. 2008;27(1):S3–S10. [DOI] [PubMed] [Google Scholar]
- 11. Nimri LF, Elnasser Z, Batchoun R. Polymicrobial infections in children with diarrhoea in a rural area of Jordan. FEMS Immunol Med Microbiol. 2004;42(2):255–259. [DOI] [PubMed] [Google Scholar]
- 12. Souza EC, Martinez MB, Taddei CR, et al. . [Etiologic profile of acute diarrhea in children in São Paulo]. J Pediatr (Rio J). 2002;78(1):31–38. [PubMed] [Google Scholar]
- 13. Bilenko N, Levy A, Dagan R, et al. . Does co-infection with Giardia lamblia modulate the clinical characteristics of enteric infections in young children? Eur J Epidemiol. 2004;19(9):877–883. [DOI] [PubMed] [Google Scholar]
- 14. Uhnoo I, Olding-Stenkvist E, Kreuger A. Clinical features of acute gastroenteritis associated with rotavirus, enteric adenoviruses, and bacteria. Arch Dis Child. 1986;61(8):732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Unicomb LE, Faruque SM, Malek MA, et al. . Demonstration of a lack of synergistic effect of rotavirus with other diarrheal pathogens on severity of diarrhea in children. J Clin Microbiol. 1996;34(5):1340–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moyo S, Hanevik K, Blomberg B, et al. . Genetic diversity of norovirus in hospitalised diarrhoeic children and asymptomatic controls in Dar es Salaam, Tanzania. Infect Genet Evol. 2014;26:340–347. [DOI] [PubMed] [Google Scholar]
- 17. Moyo SJ, Blomberg B, Hanevik K, et al. . Genetic diversity of circulating rotavirus strains in Tanzania prior to the introduction of vaccination. PLoS One. 2014;9(5):e97562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moyo SJ, Hanevik K, Blomberg B, et al. . Prevalence and molecular characterisation of human adenovirus in diarrhoeic children in Tanzania; a case control study. BMC Infect Dis. 2014;14:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tellevik MG, Moyo SJ, Blomberg B, et al. . Prevalence of Cryptosporidium parvum/hominis, Entamoeba histolytica and Giardia lamblia among young children with and without diarrhea in Dar es Salaam, Tanzania. PLoS Negl Trop Dis. 2015;9(10):e0004125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Antikainen J, Kantele A, Pakkanen SH, et al. . A quantitative polymerase chain reaction assay for rapid detection of 9 pathogens directly from stools of travelers with diarrhea. Clin Gastroenterol Hepatol. 2013;11(10):1300.e3–1307.e3. [DOI] [PubMed] [Google Scholar]
- 21. Iida M, Yamazaki M, Yatsuyanagi J, et al. . Typing of bfpA genes of enteropathogenic Escherichia coli isolated in Thailand and Japan by heteroduplex mobility assay. Microbiol Immunol. 2006;50(9):713–717. [DOI] [PubMed] [Google Scholar]
- 22. Elfving K, Andersson M, Msellem MI, et al. . Real-time PCR threshold cycle cutoffs help to identify agents causing acute childhood diarrhea in Zanzibar. J Clin Microbiol. 2014;52(3):916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Black RE. Epidemiology of diarrhoeal disease: implications for control by vaccines. Vaccine. 1993;11(2):100–106. [DOI] [PubMed] [Google Scholar]
- 24. Blackwelder WC, Biswas K, Wu Y, et al. . Statistical methods in the Global Enteric Multicenter Study (GEMS). Clin Infect Dis. 2012;55(suppl 4):S246–S253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics. 1993;49(3):865–872. [PubMed] [Google Scholar]
- 26. Vanderweele TJ. Invited commentary: assessing mechanistic interaction between coinfecting pathogens for diarrheal disease. Am J Epidemiol. 2012;176(5):396–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Valentiner-Branth P, Steinsland H, Fischer TK, et al. . Cohort study of Guinean children: incidence, pathogenicity, conferred protection, and attributable risk for enteropathogens during the first 2 years of life. J Clin Microbiol. 2003;41(9):4238–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gilman RH, Partanen R, Brown KH, et al. . Decreased gastric acid secretion and bacterial colonization of the stomach in severely malnourished Bangladeshi children. Gastroenterology. 1988;94(6):1308–1314. [DOI] [PubMed] [Google Scholar]
- 29. Cook GC. Infective gastroenteritis and its relationship to reduced gastric acidity. Scand J Gastroenterol Suppl. 1985;111:17–23. [DOI] [PubMed] [Google Scholar]
- 30. DuPont HL, Hornick RB, Snyder MJ, et al. . Immunity in shigellosis. I. Response of man to attenuated strains of Shigella. J Infect Dis. 1972;125(1):5–11. [DOI] [PubMed] [Google Scholar]
- 31. Giannella RA, Broitman SA, Zamcheck N. Influence of gastric acidity on bacterial and parasitic enteric infections. A perspective. Ann Intern Med. 1973;78(2):271–276. [DOI] [PubMed] [Google Scholar]
- 32. Rossi TM, Lebenthal E, Nord KS, et al. . Extent and duration of small intestinal mucosal injury in intractable diarrhea of infancy. Pediatrics. 1980;66(5):730–735. [PubMed] [Google Scholar]
- 33. Rossi TM, Lee PC, Young CM, et al. . Effect of nutritional rehabilitation on the development of intestinal brush border disaccharidases of postnatally malnourished weanling rats. Pediatr Res. 1986;20(8):793–797. [DOI] [PubMed] [Google Scholar]
- 34. Aoki Y, Suto A, Mizuta K, et al. . Duration of norovirus excretion and the longitudinal course of viral load in norovirus-infected elderly patients. J Hosp Infect. 2010;75(1):42–46. [DOI] [PubMed] [Google Scholar]
- 35. Hutson AM, Atmar RL, Graham DY, et al. . Norwalk virus infection and disease is associated with ABO histo-blood group type. J Infect Dis. 2002;185(9):1335–1337. [DOI] [PubMed] [Google Scholar]
- 36. Bodhidatta L, McDaniel P, Sornsakrin S, et al. . Case-control study of diarrheal disease etiology in a remote rural area in Western Thailand. Am J Trop Med Hyg. 2010;83(5):1106–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lindsay B, Ramamurthy T, Sen Gupta S, et al. . Diarrheagenic pathogens in polymicrobial infections. Emerg Infect Dis. 2011;17(4):606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McKay S, Gaudier E, Campbell DI, et al. . Environmental enteropathy: new targets for nutritional interventions. Int Health. 2010;2(3):172–180. [DOI] [PubMed] [Google Scholar]
- 39. Black RE, Lopez de Romaña G, Brown KH, et al. . Incidence and etiology of infantile diarrhea and major routes of transmission in Huascar, Peru. Am J Epidemiol. 1989;129(4):785–799. [DOI] [PubMed] [Google Scholar]
- 40. Ochoa TJ, Ecker L, Barletta F, et al. . Age-related susceptibility to infection with diarrheagenic Escherichia coli among infants from Periurban areas in Lima, Peru. Clin Infect Dis. 2009;49(11):1694–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li LL, Liu N, Humphries EM, et al. . Aetiology of diarrhoeal disease and evaluation of viral-bacterial coinfection in children under 5 years old in China: a matched case-control study. Clin Microbiol Infect. 2016;22(4):381.e9–381.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lindsay B, Oundo J, Hossain MA, et al. . Microbiota that affect risk for shigellosis in children in low-income countries. Emerg Infect Dis. 2015;21(2):242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bukholm G. Human rotavirus infection enhances invasiveness of enterobacteria in MA-104 cells. APMIS. 1988;96(12):1118–1124. [DOI] [PubMed] [Google Scholar]
- 44. Di Biase AM, Petrone G, Conte MP, et al. . Infection of human enterocyte-like cells with rotavirus enhances invasiveness of Yersinia enterocolitica and Y. pseudotuberculosis. J Med Microbiol. 2000;49(10):897–904. [DOI] [PubMed] [Google Scholar]
- 45. Superti F, Petrone G, Pisani S, et al. . Superinfection by Listeria monocytogenes of cultured human enterocyte-like cells infected with poliovirus or rotavirus. Med Microbiol Immunol. 1996;185(3):131–137. [DOI] [PubMed] [Google Scholar]
- 46. Medici MC, Tummolo F, Albonetti V, et al. . Molecular detection and epidemiology of astrovirus, bocavirus, and sapovirus in Italian children admitted to hospital with acute gastroenteritis, 2008–2009. J Med Virol. 2012;84(4):643–650. [DOI] [PubMed] [Google Scholar]
- 47. Newsome PM, Coney KA. Synergistic rotavirus and Escherichia coli diarrheal infection of mice. Infect Immun. 1985;47(2):573–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thouless ME, DiGiacomo RF, Deeb BJ. The effect of combined rotavirus and Escherichia coli infections in rabbits. Lab Anim Sci. 1996;46(4):381–385. [PubMed] [Google Scholar]
- 49. Tzipori S, Chandler D, Makin T, et al. . Escherichia coli and rotavirus infections in four-week-old gnotobiotic piglets fed milk or dry food. Aust Vet J. 1980;56(6):279–284. [DOI] [PubMed] [Google Scholar]
- 50. Tzipori SR, Makin TJ, Smith ML, et al. . Clinical manifestations of diarrhea in calves infected with rotavirus and enterotoxigenic Escherichia coli. J Clin Microbiol. 1981;13(6):1011–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wray C, Dawson M, Afshar A, et al. . Experimental Escherichia coli and rotavirus infection in lambs. Res Vet Sci. 1981;30(3):379–381. [PubMed] [Google Scholar]
- 52. Arifeen S, Black RE, Antelman G, et al. . Exclusive breastfeeding reduces acute respiratory infection and diarrhea deaths among infants in Dhaka slums. Pediatrics. 2001;108(4):E67. [DOI] [PubMed] [Google Scholar]
- 53. Lamberti LM, Fischer Walker CL, Noiman A, et al. . Breastfeeding and the risk for diarrhea morbidity and mortality. BMC Public Health. 2011;11(suppl 3):S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.