Abstract
Deep brain stimulation (DBS) is an effective (but non-curative) treatment for some of the motor symptoms and treatment complications associated with dopaminergic agents in Parkinson's disease (PD). DBS can be done relatively safely and is associated with quality of life gains. In most DBS centers, neuropsychological evaluations are performed routinely before surgery, and sometimes after surgery. The purpose of such evaluation is not to decide solely on its results whether or not to offer DBS to a given candidate, but to provide the patient and treatment team with the best available information to make reasonable risk-benefit assessments. This review provides information relevant to the questions often asked by patients and their carepartners, neurologists, and neurosurgeons about neuropsychological outcomes of DBS, including neuropsychological adverse event rates, magnitude of cognitive changes, outcomes after unilateral versus bilateral surgery directed at various targets, impact of mild cognitive impairment (MCI) on outcome, factors implicated in neurobehavioral outcomes, and safety of newer interventions or techniques such as asleep surgery and current steering.
Keywords: Neuropsychology, Parkinson's disease, Deep brain stimulation, Neuromodulation, Cognition, Mild cognitive impairment (MCI), Quality of life, Depression, Anxiety, Psychosis, Impulsiveness
Introduction
Deep brain stimulation (DBS) for Parkinson's disease (PD) in its most recent iteration has a short history of a couple of decades even though intermittent, chronic stimulation via externalized electrodes for movement disorders such as PD was used in the 1960s (Bechtereva, Bondartchuk, Smirnov, Meliutcheva, & Shandurina, 1975; Sem-Jacobsen, 1965) and the first report of a chronically, completely implanted system to treat intention tremor in multiple sclerosis in the 1970s was published in 1980 (Brice & McLellan, 1980). Benabid and colleagues in France studied thalamic stimulation for PD tremor in the 1980s and published outcomes of a DBS trial in the early 1990 s (Benabid et al., 1991), while the first large scale North American study reported its results in 1997 (Koller et al., 1997). In the US, DBS was approved by the Food and Drug Administration (FDA) to treat tremor in PD in 1997, to treat the symptoms of advanced PD in 2002, and to treat symptoms of persons with PD who have motor complications early in the disease (after a minimum of 4 years) in 2016.
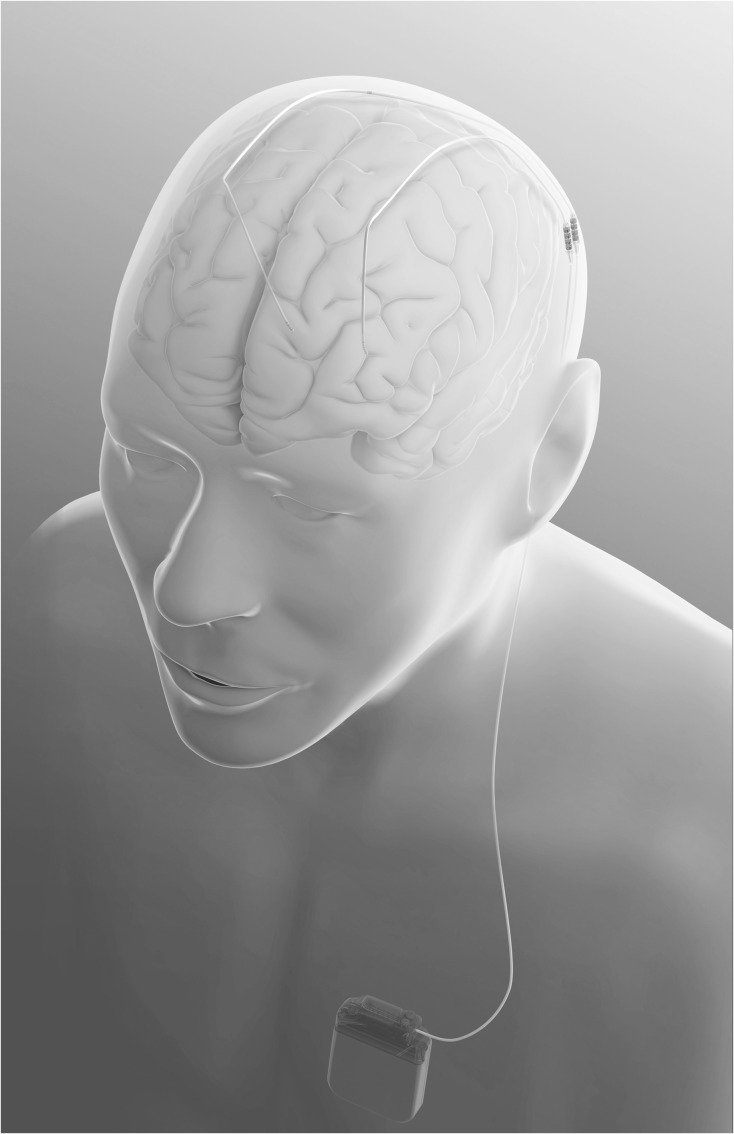
Modern DBS devices consist of an implantable pulse generator (akin to a pacemaker), usually implanted below the collarbone, that is connected via an extension running beneath the skin of the neck to the head where it connects to the leads which have multiple electrodes (contacts) and are implanted in the target structure (see Figs. 1 and 2). The three DBS targets for PD are the ventral intermediate nucleus of the thalamus (Vim) (though this procedure is rarely done anymore in PD because it alleviates only tremor), the internal segment of the globus pallidus (GPi), and the subthalamic nucleus (STN). There continues to be debate about the preferred target, and of relevance to neuropsychologists is the argument that GPi is safer than STN DBS from a neurobehavioral standpoint (though as shown later in this paper, the soundest empirical data fail to adequately support this contention).
Fig. 1.
Connection of DBS electrode and implantable pulse generator (reprinted with the permission of Medtronic, Inc., © 2017).
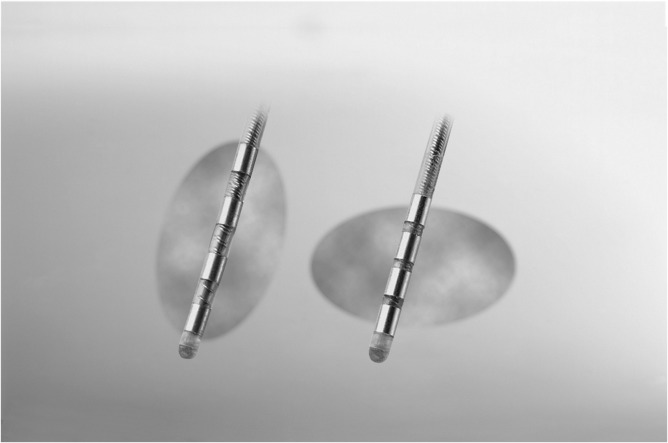
Fig. 2.
Examples of four-contact DBS leads with different spacing and electrical field shapes and sizes (reprinted with the permission of Medtronic, Inc., © 2017).
Several reviews and meta-analyses have been published about cognition (including specific aspects such as executive functions and verbal fluency) and, less often, emotional and behavioral, or other non-motor outcomes of DBS (Appleby, Duggan, Regenberg, & Rabins, 2007; Castrioto, Lhommee, Moro, & Krack, 2014; Combs et al., 2015; Elgebaly, Elfil, Attia, Magdy, & Negida, 2017; Hojlund, Petersen, Sridharan, & Ostergaard, 2017; Kasemsuk, Oyama, & Hattori, 2017; Kurtis, Rajah, Delgado, & Dafsari, 2017; Martínez-Martínez, Aguilar, & Acevedo-Triana, 2017; Parsons, Rogers, Braaten, Woods, & Tröster, 2006; Volkmann, Daniels, & Witt, 2010; Wang et al., 2016; Woods, Fields, & Tröster, 2002; Wyman-Chick, 2016; Xie, Meng, Xiao, Zhang, & Zhang, 2016). The goal of this paper is not to provide another exhaustive review of DBS neurobehavioral outcome studies, the neurophysiologic basis of DBS (DeLong & Wichmann, 2015), or cognitive neuropsychological studies addressing mechanisms underlying cognitive changes with DBS. Instead, this paper aims to extract and synthesize information useful in answering common questions that patients, their carepartners, and physicians tend to pose to the neuropsychologist regarding DBS in PD. Specifically, the paper seeks to address issues such as the rates of neurobehavioral adverse events reported in methodologically sound trials, the nature and frequency of the cognitive and neurobehavioral changes observed using tests and scales, the impact of unilateral versus bilateral DBS, the (differential) impact of subthalamic versus pallidal DBS, whether cognitive changes impact quality of life, and variables (including mild cognitive impairment or PD-MCI) occasionally linked to adverse outcomes.
Purposes of Neuropsychological Evaluation
Most centers performing DBS require patients to undergo neuropsychological evaluations prior to surgery and, less often, after surgery. The purpose of neuropsychological evaluation is not to decide solely on the basis of test results whether or not to offer a person DBS, but instead to provide the treatment team, the patient and their caregivers information to assess the risk-benefit ratio of the procedure within the patient's framework of desired outcomes, hopes, and expectations and their valuation or perceived importance to quality of life. Within this context, one purpose of pre-operative evaluation is to identify potential, relative cognitive and emotional contraindications to DBS, or to identify risks for adverse outcomes. Indeed, some centers report that they excluded from further consideration about a quarter of patients who had undergone screening. Of those patients excluded, about one-third were excluded for cognitive reasons (e.g., dementia, MCI suggestive of cortical dysfunction) and about a fifth for behavioral reasons (e.g., uncontrolled anxiety or depression), either alone or in combination with other factors (Abboud et al., 2014). These numbers are close to earlier reported ones suggesting that “of those excluded,” ~48% were not considered further due to cognitive or psychiatric reasons (Lopiano et al., 2002). Of all those already screened, fewer than 10% were excluded for cognitive reasons (Abboud et al., 2014), a number consonant with that reported by another study (Coleman, Kotagal, Patil, & Chou, 2014).
Other purposes for pre-operative evaluation are to help determine whether patients can cooperate with pre-, peri-, and post-operative demands (e.g., coping with stress, medication and appointment adherence, device operation, communication with healthcare providers). Evaluation can also assist with the identification of non-PD conditions compromising cognition (e.g., Alzheimer disease), evaluation of availability and quality of social support, documentation of ability to read and provide informed consent, and establishment of a baseline against which to evaluate post-operative function. The neuropsychological evaluation provides a rich opportunity to provide education regarding the potential cognitive and emotional AEs associated with DBS and potential strategies to limit the impact of cognitive and emotional problems on functioning and quality of life. The test battery should be sensitive to the neuropsychological alterations in PD, yet be relatively brief and acceptable to patients. Indeed, even batteries previously designed specifically for evaluation of surgical candidates with PD, such as the Core Assessment Program for Surgical Interventional Therapies in Parkinson's Disease (CAPSIT-PD) neuropsychological battery may not be tolerated well, especially by older and cognitively more impaired patients, and are in need of refinement (Pal et al., 2015).
Adverse Event Rates
Clinical trials typically require collection of data concerning adverse events (harms) (AEs) that occur during the course of the study and these enable an adjudication of risks relative to benefits of a therapy. Even once the FDA has approved a device (or drug), AEs can be reported by consumers and healthcare providers either to the FDA or the device/drug manufacturer. AEs are often classified according to their seriousness and/or severity. Serious AEs are typically those that result in any of the following: death, a life-threatening experience, new hospitalization or prolongation of existing hospitalization, or significant disability or incapacity. Thus, in the case of neurobehavioral AEs, serious AEs might include cognitive impairment resulting in incapacity or disability, depression requiring hospitalization, or suicide attempt. Severe AEs may or may not be serious. Moderate AEs have minimal impact on functioning or require treatment, while mild AEs have no functional significance and do not require treatment. In some trials, these latter two categories may be grouped together under the rubric of “non-serious AEs.” Note, however, there may be inconsistencies even across different reports of the same study whether AEs were serious or not (Deuschl et al., 2006; Witt et al., 2008). Furthermore, it appears that some studies report a separate severity rating (mild, moderate, and severe), but seemingly counterintuitively, non-serious events are sometimes described as severe. Assignment of an AE to a level of seriousness or severity may be quite subjective and differ from center to center in multi-center trials (Weaver et al., 2009). In the case of DBS, AEs are usually determined to be procedure (surgery)-related, device-related, stimulation related or unrelated to DBS (e.g., related to medication or disease progression). Studies using a separate control group (rather than a within-group control such as “on” and “off” stimulation) seek to address whether AE rates are similar in treatment and control groups and those studies using a “Best Medical Therapy” (BMT) control group are best positioned to inform about deficits potentially related to non-DBS factors such as disease progression and medications. Unfortunately, it is often not feasible from ethical and practical standpoints to keep persons in a BMT control group for extended periods (and persons willing or wanting to remain in such groups may be inherently different from those wishing ultimately to pursue DBS or other treatments, an issue that particularly confronts studies using control groups without random assignment to treatment groups).
Many AEs are symptoms (e.g., confusion). When reviewing the literature for neuropsychologically relevant AEs, several provisos should be borne in mind. First, clinicians tend to underestimate symptomatic AEs (suggesting that estimates may be conservative), and interrater reliability may be poor (Basch, 2014). In many trials, it remains unclear whether AEs were observed by clinicians, reported spontaneously by patients or carepartners, or elicited by questioning (standardized or not), or some combination thereof. Although some studies attempt to reduce such errors or bias by using standardized systems as found in the “Medical Dictionary for Regulatory Activities” (MedDRA) to code AEs, and/or including scripts to elicit information from patients (Follett et al., 2010), methods of ascertainment, causal attribution, and follow-up or treatment may differ across study centers and studies. A further point to remember is that AE rates tend to increase, especially in progressive conditions such as PD, as a function of length of follow-up. Importantly, AE rates reflect the proportion of cases in which a given AE occurs; they do not reflect the probability of “any” complications in a given patient because some patients have more than one AE while others have none (some studies present overall neurobehavioral complication rates or the total number of AEs). Although there is increasing interest in patient reported outcomes and patient reported outcome measures to counter potential clinician bias or error, the use of these measures in PD, especially in persons with cognitive impairment also introduces potential biases and inaccuracies. While persons with PD and their carepartners’ reports of memory dysfunction are related to objective test performance (Sitek, Soltan, Wieczorek, Robowski, & Slawek, 2011), both PD patients with MCI (PD-MCI) and their carers have difficulty identifying specific cognitive deficits as revealed by objective tests (Copeland, Lieberman, Oravivattanakul, & Tröster, 2016). Perhaps one of the most important issues confronting the clinician in discussing AE rates with DBS (or drug) treatment candidates is the extent to which the patient's demographic, disease, and (neuro)psychological characteristics resemble those of the clinical trial samples. Clinical trials often have stringent inclusion and exclusion criteria that are unlikely to be fully applied after device/drug approval in deciding whether or not a treatment is appropriate for that patient. That is, clinical trial samples are often more clinically homogenous than patients treated in clinical practice (Dal Pan, 2012). Consequently, clinical trial participants may have characteristics different from those of the person seeking routine clinical treatment and potentially experience AEs (and positive outcomes) at different rates and of different magnitude/severity.
Bearing in mind these limitations of AE reports, it appears that rates of serious, neuropsychologically relevant AEs are relatively rare. Overall, early uncontrolled studies showed that while serious cognitive AEs probably occur in no more than 1–2% of persons after STN DBS, mild to moderate changes (non-serious) are more common, occurring in 20% or more (Kleiner-Fisman et al., 2003; Rodriguez-Oroz et al., 2005). A meta-analysis of the early studies (most with small samples and no control group) did not list cognitive AEs among common adverse events, but did note the incidence of psychiatric AEs to be common (depression ~7%, mania ~2%, and miscellaneous psychiatric conditions [hypersexuality, hallucinations, psychosis, emotional lability] in ~4%) (Kleiner-Fisman et al., 2006).
When one considers only larger randomized trials that included a BMT control group (Table 1), only one study reported occurrence of serious cognitive AEs (confusion) related to surgery (in <3% of patients). Serious psychiatric AEs were also relatively rare: suicide and suicide attempts (<1.5%). As was true for early uncontrolled studies, non-serious AEs were more common: although two studies did not report non-serious cognitive AEs, three others reported confusion in ~5–11%, and general or unspecified cognitive decrements in 4–12%.
Table 1.
Adverse event rates reported in randomized, BMT-controlled clinical trials of DBS in PD (rates in intent to treat groups (percentage relative to subjects randomized) unless otherwise stated)
| Reference | Trial name or trial derivative/comment | Surgical target (bilateral) | Sample size randomized (intent to treat) | Length of follow- up (months) | Sample size at follow-up comparison | Serious cognitive AEs | Serious psychiatric AEs | Non-Serious Cognitive AEs | Non-Serious Psychiatric AEs |
|---|---|---|---|---|---|---|---|---|---|
| Deuschl et al. (2006) | German Parkinson Study Group, Neurostimulation Section | STN | 78 STN/78 BMT (total = 156) | 6 | 60 STN/60 BMT (paired comparisons) | NR | Suicide: STN 1.3%, BMT 0% Death in MVA during psychotic episode: STN 0%, BMT 1.3% | Post-operative confusion: STN 5.1%, Cognitive Disturbances: STN 3.8% BMT: 0% | Depression: STN 5.1%, BMT 0% Psychosis STN 5.1%, BMT 9.0% |
| Witt et al. (2008) | German Parkinson Study Group, Neurostimulation Section | STN | 78 STN/78 BMT (total = 156) | 6 | 60 STN/63 BMT (with available neuropsychological data) | NR | Suicide: STN 1.3%, BMT 0% Depression: STN 5.1%, BMT 0% Psychosis: STN 5.1%, BMT 9.0% Apathy: STN 1.3% BMT 0% Death in MVA during psychotic episode: STN 0%, BMT 1.3% | >2 SD decline in Mattis Dementia Rating scale (excluding verbal fluency): STN 5%, BMT 6% >2 SD decline in Mattis Dementia Rating Scale (including verbal fluency): STN 12%, BMT 6% | NR |
| Weaver et al. (2009) | CSP-468 | STN, GPi | 61 GPi/60 STN/134 BMT (total = 255) | 6 | 111 DBS and 119 BMT at 3 months; 108 DBS and 116 BMT at 6 months | NR | Psychiatric disorders: DBS 11 (unclear if patients or events) BMT 2 (events) | Moderate-severe confusional state (3 months): DBS 11% BMT <1% | NR |
| Williams et al. (2010) | PD SURG | STN, GPi | 183 DBS (174 STN DBS, 4 GPi DBS, 5 did not have surgery)/183 BMT | 12 | 162 DBS/153 BMT | Confusion: DBS 5 BMT 0 (unclear if patients or events) | Psychiatric AEs: DBS 6 (including 1 suicide attempt) BMT 1 (unclear if patients or events) | NR | NR |
| Schuepbach et al. (2013) | EARLYSTIM | STN | 124 DBS/127 BMT | 24 | Per protocol: 116 DBS/110 BMT | NR | Psychosis: DBS 0%, BMT 4.7% Anxiety: DBS 0%, BMT 1.6% Impulse control disorders: DBS 0.8%, BMT 3.9% Depression: DBS 4.8%, BMT 0.8% Suicidal ideation: DBS 0.8%, BMT 0% Suicide attempt: DBS 1.6%, BMT 1.6% | NR | Depression: DBS 16.9%, BMT 18.1% Impulse control disorder: DBS 14.5%, BMT 11.8% |
| Charles et al. (2014) | Early PD (no more than 4 years of treatment) | STN | 15 DBS/15 BMT | 24 | Per protocol: 13 DBS/14 BMT |
Note: E = adverse event; BMT = best medical therapy; DBS = deep brain stimulation; GPi = globus pallidus internus; MVA = motor vehicle accident; NR = not/none reported; SD = standard deviation; STN = subthalamic nucleus.
One other study (Okun et al., 2012) used what some (Castrioto et al., 2014) have described as a BMT control. That study, however, used a novel type of control group, namely a “delayed stimulation” group that had bilateral STN electrodes implanted but underwent medical therapy (n = 35) rather than stimulation (n = 101) for the first 3 months of the 12-month trial, allowing disentangling of the neuropsychological effects of surgery versus stimulation (Tröster, Jankovic, Tagliati, Peichel, & Okun, 2017). Given that the control group had surgery, AEs might be higher than in a true BMT only group. That study, however, reported low serious AE rates over 3 months: Confusion in 1% of the stimulation and 0% of the control group, and no serious psychiatric AEs (including depression) in either group.
To provide a balanced picture, it is helpful to also examine AEs in large trials that do not have control groups but involved random assignment to DBS of different targets (STN vs. GPi). One such study is the long-term (24 month) follow-up of the CSP-468 trial in which after 6 months of DBS versus BMT, all continuing patients had DBS. Of 299 patients randomized, 152 were assigned to bilateral GPi DBS (attrition 14 subjects) and 147 to STN DBS (attrition 24 subjects). Serious cognitive AEs included confusion (GPi 1.3%; STN 3.4%) and mental status change (GPi 2.6%; STN 0.7%) while serious psychiatric AEs included depression (GPi 2.6%; STN 0.7%) and suicidal depression (GPi 1.3%, STN 0.7%). Moderate to severe (but non-serious) AEs included confusion (GPi 19.7%; STN 22.4%). It is unclear if speech problems (GPi 28.3%; STN 34.7%) refer to dysarthria or dysphasia. Psychiatric AEs included depression (GPi 26.3%; STN 36.7%), which was the only neurobehavioral AE to trend toward a statistically significant difference in the frequency with which the event occurred in the GPi versus STN groups (p = .06). The seemingly high rate of non-serious depression may not be surprising, given that the trial had a 24 month follow-up and clinically relevant depression prevalence in PD is ~35%. It is important to note that the only 24-month BMT-controlled follow-up study (Schuepbach et al., 2013) found cognitive and psychiatric AE rates in many instances to be the same or higher in the BMT group, again indicating the probability that AEs in the longer term may reflect disease progression and medical or medication rather than DBS effects.
Another large trial (NSTAPS) randomized patients to GPi DBS (n = 65, attrition = 3) and STN DBS (n = 63). Unfortunately AEs over 12-month follow-up were not classified or reported by severity or seriousness, nor is it clear how the AEs were defined. Neurobehavioral AE rates did not differ between the two groups but the rate of “emotional lability” seems high: dysphasia (GPi 11%; STN 13%), delirium (GPi 22%; STN 24%), and “emotional lability” (GPi 72%, STN 84%).
In summary, as regards AEs collected via clinician or patient report, serious cognitive AEs are rare, especially over the first 3–6 months of treatment with STN or GPi DBS. The most commonly reported serious cognitive AE is confusion or delirium (<3%), while non-serious confusion may occur in up to 11% of patients. Though higher rates of confusion (20–24%) were reported at 24 months, it is likely that these higher rates reflect disease progression, medication and other medical conditions given the much lower rates over 6 months. From a psychiatric standpoint, the most common and consistently reported AE in the controlled and/or large trials is depression (as high as 37% over 24 months but lower over 6 months [~5% serious and 5% non-serious depression]). Other complications may include mania, psychosis, apathy, and suicide.
Cognitive and Emotional Change after DBS in Studies Using Neuropsychological Tests
In general, studies using formal neuropsychological instruments and behavioral rating scales report higher rates of cognitive decline after DBS than do studies using patient/clinician report. There are now many studies examining neurobehavioral outcomes after DBS: these studies generally compare the pre- and post-operative performance of patients on several neuropsychological tests. Because many studies are either not randomized and/or have small samples, and most use overlapping but not identical test batteries, the focus on this paper is on meta-analyses (characteristics in Table 2) that report on outcomes in cognitive and behavioral domains (Table 3) or individual tests (Table 4) supplemented by recent findings of either large RCTs (Odekerken et al., 2015; Rothlind et al., 2015) or well controlled studies not covered by the recent meta-analyses (Foki et al., 2017; Tramontana et al., 2015; Tröster et al., 2017).
Table 2.
Properties and characteristics of meta-analyses of the neuropsychological effects of DBS
| Reference | Number of Studies Included | Sample Size | Comments | Cognitive Domain (D) or Test (T) Effect Sizes/Mean Standardized Differences (MSD) Provided | Fixed or Random Effects Model(s) | STN SMD | GPi SMD | STN versus GPi Direct Comparison SMD | Unilateral versus Bilateral Comparison SMD |
|---|---|---|---|---|---|---|---|---|---|
| Parsons et al. (2006) | 28 | 612 | D | Random | Yes | No | No | No | |
| Combs et al. (2015) | 38 | 1622 | Includes mix of unilateral and bilateral DBS | D | Random | Yes | Yes | No | No |
| Wang et al. (2016) | 7 | 521 | Includes RCTs only; 7 studies from 4 trials; mix of unilateral and bilateral | D | Both depending on heterogeneity | No | No | Yes | No |
| Xie et al. (2016) | 10 | 797 | RCTs and non-randomized, controlled trials only | T | Both depending on heterogeneity | Yes | No | No | No |
| Elgebaly et al. (2017) | 4 | 345 | 4 RCT in meta-analysis, 7studies for qualitative analysis | T | Both depending on heterogeneity | No | No | Yes | Yes (subgroup analysis) |
| Martínez-Martínez et al. (2017) | 50 | Total not given; 69–246 per test | Executive function tests only; includes 1 GPi study | T | Both depending on heterogeneity | Yes (but includes 1 GPi) | No | No | No |
Note: GPi = globus pallidus internus; RCT = randomized controlled trial; SMD = standardized mean difference (effect size); STN = subthalamic nucleus.
Table 3.
Standardized or weighted mean differences (effect sizes) reported by meta-analyses of effects of DBS on cognition and emotion domains and Quality of Life
| Cognitive/emotion domain | Parsons et al. (2006) STN (change within group) | Combs et al. (2015) STN (change within group) | Combs et al. (2015) GPi (change within group) | Wang et al. (2016) STN versus GPi Difference (change between groups) |
|---|---|---|---|---|
| Global/cognitive screening | 0.04 | −0.24 | 0.23 | −4.30*# |
| Attention/concentration | 0.02 | −0.12 | −0.19 | −0.21* |
| Executive function | −0.08 | −0.13 | 0.00 | −0.12 |
| Psychomotor speed | 0.22 | −0.16 | −0.16 | |
| Verbal functions (memory) | −0.21 | |||
| Verbal fluency | −0.64 | −0.40 | −0.22 | −0.24* |
| Phonemic fluency | −0.51 | −0.36 | −0.19 | −2.93* |
| Semantic fluency | −0.73 | −0.48 | −0.24 | −1.55 |
| Visuoperceptual functions and visual memory | 0.06 | |||
| Learning and memory | −0.12 | −0.09 | −0.16* | |
| Language | 0.04 | 0.01 | 0.05 | |
| Visuoperceptual/spatial skills | −0.02 | 0.12 | ||
| Depression symptoms | 1.37 | |||
| Anxiety symptoms | −0.02 | |||
| Quality of life | −0.15 |
*STN greater decline than GPi; #based on Mattis Dementia Rating Scale (DRS) so high likelihood the result is heavily influenced by semantic fluency decline; GPi = globus pallidus internus; STN = subthalamic nucleus; – represents decline from pre-operative baseline or worse performance by STN than GPi.
Table 4.
Standardized or weighted mean differences (effect sizes) reported by meta-analyses of effects of DBS on specific neuropsychological tests
| Neuropsychological Test | Xie et al. (2016) STN (vs. control) (change between groups) | Martínez-Martínez et al. (2017) STN (but includes 1 GPi study) (change within group) | Elgebaly et al. (2017) STN versus GPi (direct comparison) (change between groups) | Elgebaly et al. (2017) Bilateral and Unilateral (indirect comparison) |
|---|---|---|---|---|
| Mini Mental State Exam | 0.06 | |||
| Mattis Dementia Rating Scale | −0.21* | |||
| Digit Span total | −0.05 | |||
| Digit Span forward | 0.08 | |||
| Digit Span backward | −0.14 | 0.19 | ||
| WAIS-R Digit Symbol | −0.16 | |||
| WAIS-R Arithmetic | 0.02 | |||
| Stroop Word reading | −0.21 | |||
| Stroop color naming | −0.31# | |||
| Stroop Color-Word | −0.20* | −0.21 | −0.16 | |
| Trailmaking Part A | 0.03 | −0.02 | −0.05 | |
| Trailmaking Part B | −0.39 | 0.05 | −0.14 | |
| Trailmaking A-B | −0.04 | |||
| Raven's Matrices | −0.15 | 0.06 | ||
| Wisconsin Card Sorting | 0.06@ | |||
| Verbal Fluency overall | −0.27 | |||
| Verbal Fluency—phonemic | −0.49* | −0.04/−0.05 | ||
| Verbal Fluency—semantic | −0.39* | −0.09/−0.29 | ||
| Boston Naming Test | 0.02 | −0.11 | ||
| Rey Auditory Verbal Learning Test—immediate recall | −2.06* | |||
| Rey Auditory Verbal Learning Test—delayed recall | −1.41* | |||
| Paired associate learning | −0.69 | |||
| Beck Depression Inventory | 0.15/0.36 |
*Significantly greater decline in STN DBS versus control; @different versions of WCST considering categories, errors and perseverations; #favors GPi versus STN DBS.
Meta-analyses can be helpful in detecting treatment effects when studies yield inconsistent findings, have relatively small samples, and/or effect sizes are small to moderate. Nonetheless, several points need be borne in mind when considering recent meta-analyses of DBS. First, they differ in the quality of studies included: even if greater weight is attached to higher quality or larger sample studies, weighting may not be sufficient to counter potential bias from large numbers of small sample or low quality studies. Although the majority of studies estimate effect sizes of a patient group's (e.g., STN DBS) neuropsychological changes relative to their own baseline, some combine outcomes from different surgical targets and/or uni- and bilateral DBS, or examine effect sizes relative to change in a control group or relative to DBS of another target (see Tables 2–4).
Several broad conclusions are warranted given the results of the meta-analyses. Generally, regardless of whether the target is GPi or STN, the changes within cognitive domains or specific tests are usually small and occasionally moderate. One meta-analysis of only controlled (albeit also non-randomized) studies, reported larger decrements in memory among STN DBS patient groups than in controls (Xie et al., 2016). This finding is consistent with those from reasonably sized, earlier controlled studies of STN DBS showing declines in not only verbal fluency but also attention and memory (Smeding et al., 2006; York et al., 2008). Even though STN DBS may be associated with small declines in executive function (Parsons et al., 2006), patients may subjectively perceive executive functions as improved after DBS (Pham et al., 2015). The most consistent and largest effect sizes (pre- to post-operative change) are reported for verbal fluency (semantic usually a little greater than phonemic). Thus, meta-analyses are consistent with large RCTs showing relatively small declines on a narrow range of tests, and the most robust effects of DBS on verbal fluency. Mild to moderate verbal fluency reductions occur in 25–50% of patients (Fields & Tröster, 2000; Funkiewiez et al., 2004) can persist 3–5 years (deficits may not be evident at every time point) (Contarino et al., 2007; Funkiewiez et al., 2004) and might even worsen between 5 and 8 years after surgery (Fasano et al., 2010). Importantly, the immediate post-operative verbal fluency decrements probably do not herald a poor long-term cognitive prognosis (more rapid or extensive cognitive decline) (Borden et al., 2014).
Psychiatric issues after DBS have been addressed in only one meta-analysis (Appleby et al., 2007) but its utility is limited as it does not report effect sizes and combines studies of DBS for a variety of conditions and anatomical targets. A useful recent review examines various psychiatric AEs referring to studies of various sample sizes and using various ascertainment methods (e.g., rating scales, self-report, etc.) (Castrioto et al., 2014). That analysis reports rates of various psychiatric issues in RCTs for BMT, STN DBS and GPi DBS. For depression, based on clinical symptoms, rates at 3–6 months ranged from 0% for BMT to 0–5% for STN DBS (no estimates at 6 months for GPi DBS). Rates of change were not reported for studies using depression scales, but STN, unlike BMT, uniformly involved group-wise reductions in symptom severity regardless of scale used. Suicidal ideation over 6–24 months ranged from 0.7 to 1.5% for STN DBS and 0–0.7% for GPi DBS. Completed suicides at 6–24 months ranged from 0 to 0.8% for BMT, 0–1.3% for STN DBS, and 0.7% for GPi DBS (at 24 months). Apathy was estimated to occur in 1.3–5% between 3 and 6 months for STN DBS. Psychosis may occur at higher rates in BMT (9%) compared to DBS (2.2–6%).
In addition to these meta-analyses and qualitative reviews, probably the two largest and well-designed RCT comparisons of STN and GPi (VA CSP-468 and NSTAPS) (Odekerken et al., 2013, 2015; Rothlind et al., 2015) and three other controlled studies of STN DBS have recently published detailed neuropsychological follow-up analyses (Foki et al., 2017; Tramontana et al., 2015; Tröster et al., 2017). The two STN and GPi comparisons are especially useful as they attempt to determine whether reliable changes occur by minimizing multiple comparisons and using multivariate comparisons and/or reliable change methods. The follow-up of the CSP-468 study (Rothlind et al., 2015) examined neuropsychological data from 117 BMT, 80 GPi DBS, and 84 STN DBS patients before and 6 months post-surgery. Because, there were minimal meaningful differences between STN and GPi DBS outcomes (STN performed better on one measure of learning and memory while GPi performed better on one measure of processing speed), the groups were combined for further analyses. Factor analysis disclosed that the tests administered covered five domains: processing speed, working memory, language, memory, and executive functions. Cognitive change at 6 months was determined on the basis of reliable change indices (RCI) derived from mean change in the BMT control group, and a decline in a domain was defined as reliable change having occurred on at least one-third of the measures used to evaluate the domain (one issue is that the number of measures/scores per domain varies). Declines in multiple domains were seen in 3% of BMT and 11% of DBS patients 6 months after DBS. Importantly, although those showing and not showing multiple domain cognitive declines both had significantly improved self-reported functioning and quality of life, the gain was smaller in the group with cognitive declines.
The initial report of the NSTAPS 12-month neurobehavioral outcomes among 62 GPi (3 of 65 subjects withdrew after randomization) and 63 STN DBS (Odekerken et al., 2013) used a rather broad composite based on loss of important relationship, loss of professional activity, decline per RCI on three or more neuropsychological tests, and diagnosis of anxiety, depression or psychosis for 3 months or more. Among the GPi DBS group, 58% had negative neurobehavioral composite scores (at least 1 of the listed adverse behavioral events), while 56% of the STN DBS group had at least one of the four negative neurobehavioral indicators within 12 months of surgery. Cognitive decline (per RCI on at least three measures) occurred in 27% of GPi and 35% of STN DBS (a non-significant difference). More specific 12-month neuropsychological test data (available for 58 GPi and 56 STN DBS patients) were published later (Odekerken et al., 2015). Despite having administered multiple tests, many with multiple scores, only Stroop task word reading and color naming (but not interference) and Trailmaking Part B declined more in the STN than GPi DBS group. Using the composite of changes on at least 3 of 12 tests per RCI, 29% of GPi and 39% of STN experienced declines (no significant group difference). Decline was associated with older age but not with quality of life changes.
Other recent trials also show circumscribed cognitive declines after STN DBS. A recent study of 18 STN DBS patients compared cognitive changes on the Neuropsychological Test Battery Vienna short-form over 12 months in that group against those changes in 25 PD undergoing BMT, 24 MCI (non-PD), and 12 healthy controls (Foki et al., 2017). Using RCI, 11% of the DBS group, but none of the BMT group showed phonemic verbal fluency declines. In a comparison of neuropsychological changes in 101 PD patients 3 months after STN DBS, and in 35 patients who had STN electrodes implanted but stimulation had not yet been activated, both groups showed decrements in semantic and switching verbal fluency (Tröster et al., 2017). Unique to the stimulation group was a decline in phonemic verbal fluency and on all parts of the Stroop task (suggesting these declines may be related to either stimulation or a combination lesion and stimulation effect). The stimulation group evidenced more frequent improvements in depressive symptomatology than the delayed activation control group.
One small, controlled trial is of importance because it examined the neuropsychological effects of bilateral STN DBS in 15 patients who were “early” in the course of the disease (treated with antiparkinsonian medication for 6 months—4 years) and in a group of 15 early PD patients undergoing BMT (Tramontana et al., 2015). The STN DBS group showed greater declines at 12 months in phonemic fluency, digit span, and on the Stroop task, and lesser gains on the Wisconsin Card Sorting Test (WCST perseverative errors) and some trials of the Paced Auditory Serial Addition test (PASAT). Overall, then, cognitive changes after DBS appear to be qualitatively similar in early and advanced PD.
Is Pallidal DBS Neuropsychologically Safer than Subthalamic DBS?
Some early studies raised the possibility that GPi may be associated with lesser neurobehavioral decrements (at least in circumscribed areas such as verbal fluency and processing speed) than STN DBS (Anderson, Burchiel, Hogarth, Favre, & Hammerstad, 2005; Follett et al., 2010; Okun et al., 2009) and greater quality of life improvement (Zahodne et al., 2009). It is likely that these findings lead some centers to select patients for STN versus GPi at least in part based on pre-operative cognitive deficit and perceived risk of exacerbation of deficit. Indeed, one study examined GPi DBS outcomes in 25 patients for whom STN DBS had been deemed unsuitable (primarily on cognitive grounds, such as Mattis DRS < 130/144 or executive function deficit, but also due to levodopa-resistant axial motor signs) (Bonenfant et al., 2017). No significant neuropsychological test score changes were observed 1 year after surgery, and declines at 3 years on the Stroop, Mattis, and Trailmaking tasks might well reflect disease progression. These findings are similar to those of a smaller study that found no neuropsychological changes 6 months after bilateral GPi DBS in patients in whom STN DBS was deemed to be contraindicated (Rouaud et al., 2010). Nonetheless, prior to concluding that GPi DBS is a safer alternative to STN DBS, a randomized trial (STN vs. GPi) would be necessary in patients with baseline cognitive characteristics similar to those in these two studies.
The meta-analyses discussed already either found no significant differences between STN and GPi DBS (Elgebaly et al., 2017) or small (except verbal fluency) differences favoring GPi over STN (Wang et al., 2016). Although one meta-analysis reviewed outcomes for both STN and GPi DBS and inferred that fewer cognitive declines occurred in GPi than STN DBS, that study did not provide a direct comparison of outcomes associated with the two targets and the study's authors note that the GPi effect size estimates were based on a small number of studies (Combs et al., 2015). Given these considerations, and the small effects that both interventions had on cognition, it is likely that the differences in neuropsychological outcome between DBS of the two targets are of limited clinical significance. Furthermore, the two largest randomized studies comparing STN and GPi DBS have failed to establish significant neurobehavioral outcome differences between the two treatments (Odekerken et al., 2013, 2015; Rothlind et al., 2015), as did a smaller retrospective study using the MMSE and Cambridge Examination for Mental Disorders Cognitive subscale (Volkmann et al., 2001). In summary, there currently is no compelling empirical evidence that one DBS target represents a significantly safer cognitive alternative than the other.
A recent detailed examination of psychiatric outcomes in the NSTAPS trial, albeit limited by subject attrition and range of evaluation, also failed to observe significantly different outcomes between STN and GPi groups 3 years after surgery (Boel, Odekerken, Geurtsen, et al. 2016; Boel, Odekerken, Schmand, et al. 2016). One meta-analysis examining changes in Beck Depression Inventory (BDI) scores, however, concluded that GPi DBS lead to a greater reduction in depression symptoms than STN DBS (Liu et al., 2014). It is important to note that changes in symptom severity do not necessarily imply changes in caseness or diagnostic group (i.e., depressed vs. not) (Burn & Tröster, 2004).
Is Unilateral DBS Safer than Bilateral DBS?
No direct, randomized comparisons of neuropsychological outcomes after unilateral as opposed to bilateral DBS are available. The only meta-analysis addressing this issue did so indirectly, examined only verbal fluency and a depression rating scale score, and observed no significant differences (Elgebaly et al., 2017). A small, non-randomized study found that unilateral left hemisphere STN DBS (n = 6) was associated with lesser declines in verbal fluency than was bilateral STN DBS (n = 10) (Sjoberg et al., 2012), but this finding is difficult to interpret given somewhat longer disease duration and greater baseline executive dysfunction in the bilateral treatment group.
An alternative approach to comparing separate groups of patients undergoing unilateral versus bilateral surgery involves examination of patients undergoing staged bilateral DBS procedures. Rothlind and colleagues undertook a study of randomized, staged GPi and STN DBS in 42 patients and observed that minimal cognitive decline ensued from the second operation. However, semantic verbal fluency (naming items from a category within a time limit) declined after left DBS, regardless of whether the left (presumably language-dominant hemisphere) was operated upon first or second. Phonemic verbal fluency (retrieval of words beginning with a given letter) declined only after left DBS but no significant effect of second surgery was noted (Rothlind, Cockshott, Starr, & Marks, 2007). This finding is reminiscent of the observation of declines on a card sorting task demanding of conceptualization and cognitive flexibility after left but not right unilateral STN DBS (Lueken, Schwarz, Hertel, Schweiger, & Wittling, 2008). In a smaller study (n = 6), following up some of the patients having undergone unilateral pallidal DBS (Tröster et al., 1997), none of the patients experienced significant cognitive declines 3 months after the second surgery relative to pre-surgical baseline, and, indeed, delayed recall was improved (perhaps related to a test practice effect) (Fields, Tröster, Wilkinson, Pahwa, & Koller, 1999).
Another tack is to examine cognitive performance with one or both electrodes turned on or off. Although this approach allows one to examine effects of stimulation per se, the approach fails to account for pre-operative performance and potential microlesion effects. It is possible that the impact of unilateral DBS on working memory, for example, is greater in the more affected hemisphere of the brain than in the less affected hemisphere, and that unilateral DBS of the more affected hemisphere may be equivalent in effect (at least on working memory) to bilateral stimulation (Hershey et al., 2008). Overall then, whether DBS is done on the language dominant or more affected hemisphere may be more important a determinant of DBS's impact on cognition than whether DBS is applied unilaterally or bilaterally.
Does MCI or Dementia Impact Cognitive Outcomes?
DBS is not currently indicated for persons with dementia. Although systematic studies of DBS in Parkinson's disease with dementia (PDD) are lacking for obvious practical and ethical reasons, case studies raise concern that persons with marked cognitive impairment may become more impaired and lose functional independence after DBS (Hariz et al., 2000). Furthermore, there is concern whether a person with dementia or significant cognitive impairment can give truly informed consent (e.g., understand positive and negative treatment effects and treatment alternatives) and cooperate with a complex and arduous pre-, peri-, and post-operative evaluation and treatment protocol (Massano & Garrett, 2012). Additionally, neuropsychological evaluation may highlight potential non-PD causes of dementia. There may, however, be circumstances wherein DBS is considered in a person with PDD on humanitarian grounds (see Kubu et al., in this issue). Furthermore, a case of nucleus basalis of Meynert (nbM) stimulation in conjunction with STN DBS has been reported, and stimulation of the nbM, a source of major cholinergic cortical input, may improve aspects of cognition (Freund et al., 2009).
What is less clear is what bearing MCI might have on DBS outcome. There is little empirical literature available and only some studies have utilized the International Parkinson and Movement Disorders Society (MDS) criteria for PD-MCI (Litvan et al., 2012). One retrospective study employing the MDS criteria Level II (most detailed assessment), did not examine cognitive outcomes. In that study, 60% of 130 patients had multiple-domain MCI and 21% had single domain MCI prior to surgery. The presence of MCI did not impact bilateral STN DBS outcome in that study. However, patients with pre-operative attention impairments (per Digit Span and Letter Number Sequencing) were significantly more likely to have hospital stays of 3 or more days (39% vs. 12%), and tended to more often have post-operative confusion (11% vs. 3%) (Abboud et al., 2015). Another retrospective study of 103 bilateral STN DBS patients found that 63% of the patients had MCI at baseline using MDS Level I assessment and criteria (Kim et al., 2014). Although the authors of the study reported proportions of amnestic and non-amnestic MCI, this subtyping is likely unreliable because, per MDS criteria, subtyping is only possible after Level II assessment. Patients were followed for up to 7 years (mean 42 months). Although annual rate of decline on the MMSE was small (0.4 ± 1.7), and not associated with MCI diagnosis, a faster rate of decline was associated with poorer baseline scores on test of attention and executive function (Stroop, Trailmaking) and visuoconstruction (Rey Complex Figure). During follow-up, 10 of the 103 patients developed dementia and the probability of developing dementia was significantly greater among those with than without MCI, regardless whether amnestic or non-amnestic MCI. It is likely then, given similar rates of MMSE decline in those with than without MCI at baseline, that the higher probability of dementia in the MCI-diagnosed reflects natural history of PD rather than an effect of DBS. A similar conclusion was drawn by another retrospective study using MDS Level I assessment (Merola et al., 2014). Of 184 patients, 23% had MCI prior to surgery. Patients were evaluated at 1, 3, 5, and >5 years. Although dementia affected those with MCI sooner (median 6 years) than those without MCI (median 11 years), no cases of dementia were observed early after DBS suggesting that the more precocious development of dementia in the MCI group might reflect the natural history of the disease. Indeed, the presence MCI in PD has been shown to significantly increase the hazard of dementia even once one accounts for factors such as age, education, gender, disease severity, and depression symptoms (Hoogland et al., 2017). Overall then, the literature suggests that while specific cognitive impairments may be associated with a variety of poorer outcomes (confusion, length of hospitalization after DBS, faster rate of cognitive decline), MCI as a diagnostic entity has not been associated with poorer outcome or dementia risk other than that expected on the basis of the natural disease course. The latter suggestion is consistent with the observation that the incidence of dementia within 3 years after STN DBS is similar to that in medically treated patients (Aybek et al., 2007).
Several issues remain unaddressed by current literature. Unfortunately, no studies yet address cognitive outcome quantitatively among those with and without MCI prior to DBS, so it remains possible that those with MCI have a poorer cognitive outcome even if the decline does not warrant a diagnosis of dementia. Certainly, it appears that the rate of MCI is higher after than before STN DBS: a study not using MDS criteria found that MCI increased from 47% prior to surgery to 63% an average of 9 months after surgery among 30 STN DBS patients (Yaguez et al., 2014). Given the rarity of changes exceeding RCIs over 18 months in PD patients without dementia (Tröster, Woods, & Morgan, 2007), such an increase in MCI over just 9 months is noteworthy. Additionally, it remains unknown whether bilateral and unilateral DBS have a different impact in persons with MCI. Furthermore, because MCI represents a continuum of severity of functionally non-incapacitating cognitive impairment, it remains to be shown whether those with more “severe” MCI might be at greater risk of being propelled into the dementia diagnostic category after DBS than those with “milder” MCI.
Exploring New Boundaries: Role of Patient and Caregiver Expectations in Outcome Satisfaction
It is well-known that patient expectations can influence treatment outcome. Effects that have been described include: a therapeutic benefit related to expectation of benefit in a given therapeutic context (placebo), reduction of therapeutic benefit in an active treatment group in the presence of a placebo control group (lessebo effect), and occurrence of adverse events in a placebo group (nocebo effect) (Mestre, Lang, & Okun, 2016). These, as well as motor or neuropsychological microlesion effects related to electrode implantation in the absence of stimulation (Okun et al., 2012) can impact patient perception and satisfaction with outcome. What is equally important are patient expectations prior to surgery (Maier et al., 2013), specifically whether these are realistic with regard to outcome that might reasonably be expected (e.g., magnitude and duration of effect as well as symptoms impacted by treatment, occurrence of adverse events).
In an elegant study, patients were asked before surgery to rate currently perceived QoL covering several functional domains (PDQ-39) and to indicate on the same scale how they expect to see themselves after surgery. QoL improved significantly 6 months after surgery but there was a marked discrepancy between expected and actual (much smaller) change. Nonetheless most patients rated themselves as satisfied and having their expectations fulfilled on visual analog scales. Importantly, satisfaction was highly correlated (r = 0.91) with fulfillment of expectations but not with actual PDQ-39 changes, raising the possibility that at least some patients may be satisfied with surgical outcomes despite lesser QoL gains. However, another study's results question this possibility. In a study, using semi-structured interviews with 28 patients 12 months after STN DBS, dissatisfaction rate was similar to that in the previously described study (25% were disappointed by DBS outcome, 32% reported a “mixed” outcome, and 43% reported a satisfactory outcome) (Maier et al., 2016). QoL improved only in the mixed and satisfied groups, and post-operative dissatisfaction was predicted by pre-operative apathy and axial symptoms.
Pre-operative teaching or education programs for patients and caregivers may allow many of them to feel fully prepared for surgery (Lanier-Bohan & Heath, 2016) and pre-operative psychoeducation can enhance social adjustment, coping, and reduce anxiety and depression symptoms up to a year after STN DBS (Flores Alves Dos Santos et al., 2017). Also, important to address are a patient's caregiver's expectations. These have received little empirical attention, although exploratory studies suggest that even effective DBS may neither worsen nor improve caregiver burden (Soileau, Persad, Taylor, Patil, & Chou, 2014) or strain (Oyama et al., 2014). Caregiver age and depression (Lewis et al., 2015) may predispose to negative caregiver outcomes after DBS as may patient depression, apathy and disinhibition (Lewis et al., 2015; Soileau et al., 2014). Even though caregiver burden may not change, caregiver QoL gains have been observed up to 2 years after STN DBS (Lezcano et al., 2004).
Do Neurobehavioral Changes Impact Quality of Life?
The two largest randomized STN/GPi DBS trials reached somewhat different conclusions about the impact of cognitive decline on QoL after DBS. Whereas those experiencing multivariate cognitive decline showed similar QoL change than those not experiencing such a decline in the NSTAPS trial (Odekerken et al., 2015), the persons with cognitive decline in the CSP-468 study showed lesser QoL gains than non-decliners (Rothlind et al., 2015). A few studies have shown that dissatisfaction relevant to cognition and behavior may occur in only select domains of QoL after DBS, e.g., communication (Drapier et al., 2005; Erola et al., 2005), and one trial (Tröster et al., 2017) has associated satisfaction with communication (assessed with the PDQ-39) to phonemic verbal fluency performance 12 months after surgery. Despite the suggestion that neuropsychological changes may have limited impact on QoL, neuropsychological factors such as pre-operative overall level of cognitive impairment (per Mattis DRS total score) (Witt et al., 2011) and changes in depression symptom severity (Tröster et al., 2003) can impact QoL after surgery. Although patients use a wide variety of coping strategies to deal with the stressor of upcoming surgery (Croyle et al., 2003), coping styles can limit QoL gains after DBS despite good motor outcomes (Soulas, Sultan, Gurruchaga, Palfi, & Fenelon, 2011).
Does Neuropsychological Outcome Differ Between DBS Surgery Done Awake with MER or Under General Anesthesia Under Direct Anatomical Targeting?
Targeting using microelectrode recording (MER) and/or test stimulation with the patient awake during the surgical procedure remains the gold standard for DBS lead placement. One study reported that 40% of patients have significant pain during the awake procedure (especially during stereotactic frame placement and burr hole drilling) and that many, due to the lengthy surgery, suffer from “off” state motor symptoms and dysphoria (Mulroy, Robertson, Macdonald, Bok, & Simpson, 2017). Some surgical centers have begun to perform DBS surgery under general anesthesia, either with MER (Fluchere et al., 2014) or without MER, using intraoperative scanning (Mirzadeh et al., 2016). Although randomized trials remain needed, preliminary data suggest that similar motor outcomes can be achieved with the two methods (Chen, Mirzadeh, & Ponce, 2017). Data are lacking regarding neuropsychological outcomes after “awake” DBS and only one study with a small sample (n = 12) has used a limited test battery (Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) and four subtests from the Delis-Kaplan Executive Function Scale (D-KEFS)) to evaluate changes ~18 months after STN or GPi DBS (Sidiropoulos et al., 2016). A significant decline was seen on only one subtest of working memory and psychomotor speed (RBANS Coding). Given lack of data, it is too early to tell whether “asleep” DBS achieves the same motor outcomes with comparable or lesser neuropsychological morbidity than “awake” DBS.
Do Newer Electrodes Allowing Current Steering Offer Greater Neurobehavioral Safety?
It has been shown that selection of stimulation parameters via computational modeling so as to minimize current spread to non-desired areas of a stimulation target can reduce the cognitive impact of stimulation (Frankemolle et al., 2010). Consequently, the advent of new leads with electrodes that permit current steering (i.e., shaping and sizing of the electrical field by selecting how much and in which direction current flows from a given contact) holds promise that adverse events and side effects of DBS can be reduced while maximizing benefit. It is too early still to draw conclusions about the effectiveness of current steering in improving cognitive outcomes. However, an open-label trial (VANTAGE) investigating bilateral STN DBS in 40 patients found the investigational device to be effective in controlling motor symptoms, and non-serious neurobehavioral adverse events related to stimulation (confusional state in 1, speech disorder in 2, apathy in 3 patients) to be quite rare (Timmermann et al., 2015). Detailed neuropsychological data were not presented.
The Role of Cognitive Screening Tests and Computerized Testing
Cognitive screening is less time consuming and costly than a full neuropsychological evaluation and the question thus arises whether screening is sufficient for pre- and post-operative evaluation. While screening instruments such as the MOCA and DRS adequately detect dementia in PD (Lucza et al., 2015) and can be used to exclude persons from further consideration (with the proviso that DRS scores may fall below cutoff not due to dementia but due to poor verbal fluency given the heavy weighting [20/144 points] of the DRS toward this domain), it is likely that those “passing” screening should undergo full neuropsychological evaluation. The most compelling argument for this conclusion is that MCI is common in PD (Litvan et al., 2011) and among DBS candidates (Abboud et al., 2015; Yaguez et al., 2014), yet instruments such as the MMSE and MOCA may have approximately only 65% accuracy in MCI detection in PD (Chou, Lenhart, Koeppe, & Bohnen, 2014; Wyman-Chick, Martin, Barrett, Manning, & Sperling, 2017), and even the Mattis DRS, at scores 137–138 that optimally balance test sensitivity and specificity, may fail to exceed sensitivity of mid-70% in detecting cognitive impairment (Pirogovsky et al., 2013; Villeneuve et al., 2011). As regards the use of screening measures as outcome measures, it is likely that they are insufficient to accurately capture cognitive outcome. While the Mattis DRS appears sensitive to verbal fluency declines after DBS (Witt et al., 2008), the instrument may neither reveal cognitive changes nor predict motor outcomes, medication reduction, or quality of life after DBS (Floden, Busch, Cooper, Kubu, & Machado, 2015).
There is currently insufficient empirical evidence to support the use of computerized testing as a standalone assessment in the evaluation of DBS patients, although an argument could be made that, similar to screening tests, computerized measures [evaluated for quality using proposed criteria (Bauer et al., 2012)] might represent an efficient means of determining which candidates to exclude from further assessment. One study in PD found that the Neurotrax computerized battery, while identifying executive deficits, lacks sensitivity to overall cognitive deficits in PD (Hanna-Pladdy et al., 2010). Additionally, a computerized screening for DBS incorporating a cognitive assessment from Neurotrax did not provide advantage over a shorter pencil-and-paper questionnaire (Oyama et al., 2012). The Automated Neuropsychological Assessment Metrics (ANAM) battery evaluates a narrower range of functions than pencil-and-paper test batteries and may less well discriminate patients with PD from healthy individuals (Hawkins et al., 2012).
Factors Associated with Neuropsychological Outcomes of DBS
Probably one of the first studies to examine potential predictors of neuropsychological outcome after DBS found that higher age, poorer response to levodopa, and attention impairment (considered in tandem) were associated with poorer cognitive outcomes (Smeding, Speelman, Huizenga, Schuurman, & Schmand, 2011). Unfortunately, subsequent studies, albeit using slightly different tests and samples with slightly different baseline characteristics, have not replicated this finding (Odekerken et al., 2015; Tröster et al., 2017). Furthermore, demographic and disease variables that have occasionally been identified as associated with poorer outcomes, such as baseline dopaminergic medication, severity of motor deficit, disease duration, and age, were not related to outcome in any cognitive domain in a meta-analysis (Combs et al., 2015). Nonetheless it is likely that baseline deficits in attention, executive functions, memory and overall level of cognitive impairment are associated with poorer neuropsychological outcome. Table 5 presents some of the cognitive, demographic, disease, anatomic, and stimulation variables that have been associated with neuropsychological outcomes. It is emphasized that most of these factors have not emerged consistently as predictors or associates of neuropsychological outcome. Multiple problems, including small samples, differences in sample characteristics, length of follow-up and varying evaluation methods likely hinder replication. Furthermore, over time, screening and selection criteria may have widened or narrowed the range of acceptable pre-operative characteristics and surgical variability such that restricted ranges may hinder identification of reliable predictors. Nonetheless, Table 5 alerts readers to potential variables that should be attended to because it is likely that a greater presence of negative predictors might foretell a negative neuropsychological outcome. Future research will need to identify risk, for example via odds ratios, in large cohorts. Furthermore, combination of neuropsychological findings with potential biomarkers of suboptimal motor outcome (e.g., reduced frontal cortical thickness (Muthuraman et al., 2017)) may facilitate candidate selection and facilitate provision of accurate prognostic information to patients, caregivers, and their physicians.
Table 5.
Neuropsychological, demographic, disease, anatomical, and stimulation variables associated with DBS neuropsychological and functional outcome and sample references
| Neuropsychological | Demographic | Disease | Anatomical | Surgical/stimulation |
|---|---|---|---|---|
| Overall level of cognitive impairment Witt et al. (2011) | Age Hrabovsky et al. (2017); Smeding et al. (2011) | Motor symptom severity Merola et al. (2014) | White matter lesion burden Blume et al. (2017) | Ventral and anterior stimulation Tsai et al. (2007) |
| Attention Abboud et al. (2015); Smeding et al. (2011) | Axial symptom severity Daniels et al. (2010); Fukaya et al. (2017) | Thalamic and hippocampal volumes Geevarghese et al. (2016) | Stimulation amplitude and pulse width Schoenberg, Mash, Bharucha, Francel, and Scott (2008) | |
| Executive dysfunction Kim et al. (2014); Pilitsis et al. (2005) | Age at disease onset Fukaya et al. (2017) | Greater intermammillary distance (third ventricular size; indirect measure of atrophy) Hrabovsky et al. (2017) | Anterior lead implant trajectory Hrabovsky et al. (2017) | |
| Intelligence Yaguez et al. (2014) | Baseline dopaminergic medication dosage Daniels et al. (2010); Kim et al. (2014) | Stimulation frequency Francel et al. (2004) | ||
| List learning Yaguez et al. (2014) | Baseline response to dopaminergic medication Smeding et al. (2011) | Electrode trajectory through caudate Isler, Albi, Schaper, Temel, and Duits (2016); Witt et al. (2013) | ||
| Apathy Drapier et al. (2005) | Electrode placed through posterior-lateral quadrant of frontal lobe York, Wilde, Simpson, and Jankovic (2009) | |||
| Hallucinations Blume et al. (2017) | ||||
| Anxiety Schoenberg et al. (2008) | ||||
| Visuospatial impairment Abboud et al. (2015) |
Conclusions
DBS provides for significant QoL gains in persons with PD and can be achieved in a generally neuropsychologically safe manner both in advanced and early disease. Nonetheless, within 6 months of surgery, a minority (~10–15% of patients) may experience reliable declines on multiple neuropsychological measures. These changes do not preclude but attenuate QoL gains, although the QoL impact of neuropsychological changes after DBS remains a matter of debate. Serious cognitive adverse events occur in <1–2% of patients. The most common cognitive change is a reduction in verbal fluency, while the commonest emotional change is depression. Empirical data do not provide compelling evidence that GPi DBS is neuropsychologically safer than STN DBS, and side of surgery (language dominant hemisphere or hemisphere most affected by disease) may be as or more important determinants of cognitive outcome than whether surgery is unilateral or bilateral.
MCI as a construct may not impact cognitive outcome, but impairments in specific domains may. Attention, executive functions, visuospatial impairment, and memory impairments prior to DBS have all been implicated in cognitive decrements after DBS, albeit inconsistently so. Whether advances such as surgery under general anesthesia using direct targeting via intraoperative neuroimaging, and use of devices permitting current shaping and steering, will enhance neuropsychological safety of DBS remains an open question. Given prevalence and prognosis of MCI and neuropsychiatric disturbances in PD's disease course, pre-operative neuropsychological evaluation is deemed essential. Such evaluation should also attend to patient and carepartner expectations and coping that are important in patient satisfaction and QoL outcomes. Additionally, pre-operative psychoeducation tends to enhance patient satisfaction. Paper-and-pencil and computerized screening tests probably are best used in selecting patients for further neuropsychological evaluation, but such techniques, given suboptimal sensitivity, should not serve as the sole measures of cognitive outcome until better empirical support emerges. Future studies are needed to identify reliable predictors of neuropsychological outcome and it is suggested that trials, in addition to reporting group outcome, routinely report individual outcomes and measures of risk (such as odds ratios) when examining pre- and peri-operative variables in neuropsychological outcome. Given the apparent relative neuropsychological safety of DBS for PD, the time may be ripe, as was suggested almost a decade ago (Tröster, 2008), to re-evaluate current neuropsychological inclusion/exclusion criteria and to empirically evaluate the impact of more relaxed neurobehavioral criteria on DBS outcome. Research regarding revised neuropsychological criteria would reveal the safety of making DBS potentially available to persons with PD with a broader range of neurobehavioral characteristics.
Conflict of Interest
None declared.
References
- Abboud H., Floden D., Thompson N. R., Genc G., Oravivattanakul S., Alsallom F., et al. (2015). Impact of mild cognitive impairment on outcome following deep brain stimulation surgery for Parkinson's disease. Parkinsonism & Related Disorders, 21, 249–253. doi:10.1016/j.parkreldis.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Abboud H., Mehanna R., Machado A., Ahmed A., Gostkowski M., Cooper S., et al. (2014). Comprehensive, multidisciplinary deep brain stimulation screenig for Parkinson patients: no room for “short cuts”. Movement Disorders: Clinical Practice, 1 (4), 336–341. doi:10.1002/mdc3.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V. C., Burchiel K. J., Hogarth P., Favre J., & Hammerstad J. P. (2005). Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Archives of Neurology, 62, 554–560. [DOI] [PubMed] [Google Scholar]
- Appleby B. S., Duggan P. S., Regenberg A., & Rabins P. V. (2007). Psychiatric and neuropsychiatric adverse events associated with deep brain stimulation: a meta-analysis of ten years’ experience. Movement Disorders, 22, 1722–1728. doi:10.1002/mds.21551. [DOI] [PubMed] [Google Scholar]
- Aybek S., Gronchi-Perrin A., Berney A., Chiuve S. C., Villemure J. G., Burkhard P. R., et al. (2007). Long-term cognitive profile and incidence of dementia after STN-DBS in Parkinson's disease. Movement Disorders, 22, 974–981. [DOI] [PubMed] [Google Scholar]
- Basch E. (2014). New frontiers in patient-reported outcomes: adverse event reporting, comparative effectiveness, and quality assessment. Annual Review of Medicine, 65, 307–317. doi:10.1146/annurev-med-010713-141500. [DOI] [PubMed] [Google Scholar]
- Bauer R. M., Iverson G. L., Cernich A. N., Binder L. M., Ruff R. M., & Naugle R. I. (2012). Computerized neuropsychological assessment devices: joint position paper of the American Academy of Clinical Neuropsychology and the National Academy of Neuropsychology. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 27, 362–373. doi:10.1093/arclin/acs027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtereva N. P., Bondartchuk A. N., Smirnov V. M., Meliutcheva L. A., & Shandurina A. N. (1975). Method of electrostimulation of the deep brain structures in treatment of some chronic diseases. Confinia Neurologica, 37, 136–140. [DOI] [PubMed] [Google Scholar]
- Benabid A. L., Pollak P., Gervason C., Hoffmann D., Gao D. M., Hommel M., et al. (1991). Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet, 337, 403–406. [DOI] [PubMed] [Google Scholar]
- Blume J., Lange M., Rothenfusser E., Doenitz C., Bogdahn U., Brawanski A., et al. (2017). The impact of white matter lesions on the cognitive outcome of subthalamic nucleus deep brain stimulation in Parkinson's disease. Clinical Neurology and Neurosurgery, 159, 87–92. doi:10.1016/j.clineuro.2017.05.023. [DOI] [PubMed] [Google Scholar]
- Boel J. A., Odekerken V. J., Geurtsen G. J., Schmand B. A., Cath D. C., & Figee M., group, Nstaps study. (2016). Psychiatric and social outcome after deep brain stimulation for advanced Parkinson's disease. Movement Disorders, 31, 409–413. doi:10.1002/mds.26468. [DOI] [PubMed] [Google Scholar]
- Boel J. A., Odekerken V. J., Schmand B. A., Geurtsen G. J., Cath D. C., & Figee M., group, Nstaps study. (2016). Cognitive and psychiatric outcome 3 years after globus pallidus pars interna or subthalamic nucleus deep brain stimulation for Parkinson's disease. Parkinsonism & Related Disorders, 33, 90–95. doi:10.1016/j.parkreldis.2016.09.018. [DOI] [PubMed] [Google Scholar]
- Bonenfant J., Drapier S., Houvenaghel J. F., Naudet F., Haegelen C., Sauleau P., et al. (2017). Pallidal stimulation in Parkinson's patients with contraindications to subthalamic target: A 3 years follow-up. Parkinsonism & Related Disorders, 34, 20–25. doi:10.1016/j.parkreldis.2016.10.007. [DOI] [PubMed] [Google Scholar]
- Borden A., Wallon D., Lefaucheur R., Derrey S., Fetter D., Verin M., et al. (2014). Does early verbal fluency decline after STN implantation predict long-term cognitive outcome after STN-DBS in Parkinson's disease? Journal of the Neurological Sciences, 346, 299–302. doi:10.1016/j.jns.2014.07.063. [DOI] [PubMed] [Google Scholar]
- Brice J., & McLellan L. (1980). Suppression of intention tremor by contingent deep-brain stimulation. Lancet, 1, 1221–1222. [DOI] [PubMed] [Google Scholar]
- Burn D. J., & Tröster A. I. (2004). Neuropsychiatric complications of medical and surgical therapies for Parkinson's disease. Journal of Geriatric Psychiatry and Neurology, 17, 172–180. [DOI] [PubMed] [Google Scholar]
- Castrioto A., Lhommee E., Moro E., & Krack P. (2014). Mood and behavioural effects of subthalamic stimulation in Parkinson's disease. Lancet Neurology, 13, 287–305. doi:10.1016/S1474-4422(13)70294-1. [DOI] [PubMed] [Google Scholar]
- Charles D., Konrad P. E., Neimat J. S., Molinari A. L., Tramontana M. G., Finder S. G., et al. (2014). Subthalamic nucleus deep brain stimulation in early stage Parkinson's disease. Parkinsonism & Related Disorders, 20, 731–737. doi:10.1016/j.parkreldis.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Mirzadeh Z., & Ponce F. A. (2017). “Asleep” deep brain stimulation surgery: a critical review of the literature. World Neurosurgery. doi:10.1016/j.wneu.2017.05.042. [DOI] [PubMed] [Google Scholar]
- Chou K. L., Lenhart A., Koeppe R. A., & Bohnen N. I. (2014). Abnormal MoCA and normal range MMSE scores in Parkinson disease without dementia: cognitive and neurochemical correlates. Parkinsonism & Related Disorders, 20, 1076–1080. doi:10.1016/j.parkreldis.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. R., Kotagal V., Patil P. G., & Chou K. L. (2014). Validity and efficacy of screening algorithms for assessing deep brain stimulation candidacy in Parkinson disease. Mov Disord Clin Pract, 1, 342–347. doi:10.1002/mdc3.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs H. L., Folley B. S., Berry D. T., Segerstrom S. C., Han D. Y., Anderson-Mooney A. J., et al. (2015). Cognition and depression following deep brain stimulation of the subthalamic nucleus and globus pallidus pars internus in Parkinson's disease: a meta-analysis. Neuropsychology Review, 25, 439–454. doi:10.1007/s11065-015-9302-0. [DOI] [PubMed] [Google Scholar]
- Contarino M. F., Daniele A., Sibilia A. H., Romito L. M., Bentivoglio A. R., Gainotti G., et al. (2007). Cognitive outcome 5 years after bilateral chronic stimulation of subthalamic nucleus in patients with Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry, 78, 248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J. N., Lieberman A., Oravivattanakul S., & Tröster A. I. (2016). Accuracy of Patient and Care Partner Identification of Cognitive Impairments in Parkinson's Disease-Mild Cognitive Impairment. Movement Disorders, 31, 693–698. doi:10.1002/mds.26619. [DOI] [PubMed] [Google Scholar]
- Croyle K. L., Tröster A. I., Fields J. A., Straits-Tröster K. A., Lyons K. E., & Pahwa R. (2003). Presurgical coping, depression, and quality of life in persons with Parkinson's disease. Journal of Clinical Psychology in Medical Settings, 10, 101–107. [Google Scholar]
- Dal Pan G. J. (2012). Clincial approaches to post-marketing drug safety assessment In Ravina B., Cummings J., McDermott M., & Poole R. (Eds.), Clinical Trials in Neurology (pp. 160–172). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Daniels C., Krack P., Volkmann J., Pinsker M. O., Krause M., Tronnier V., et al. (2010). Risk factors for executive dysfunction after subthalamic nucleus stimulation in Parkinson's disease. Movement Disorders, 25, 1583–1589. doi:10.1002/mds.23078. [DOI] [PubMed] [Google Scholar]
- DeLong M. R., & Wichmann T. (2015). Basal Ganglia Circuits as Targets for Neuromodulation in Parkinson Disease. JAMA Neurology, 72, 1354–1360. doi:10.1001/jamaneurol.2015.2397. [DOI] [PubMed] [Google Scholar]
- Deuschl G., Schade-Brittinger C., Krack P., Volkmann J., Schafer H., & Botzel K., German Parkinson Study Group, Neurostimulation Section. (2006). A randomized trial of deep-brain stimulation for Parkinson's disease. New England Journal of Medicine, 355, 896–908. doi:10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- Drapier S., Raoul S., Drapier D., Leray E., Lallement F., Rivier I., et al. (2005). Only physical aspects of quality of life are significantly improved by bilateral subthalamic stimulation in Parkinson's disease. Journal of Neurology, 252, 583–588. [DOI] [PubMed] [Google Scholar]
- Elgebaly A., Elfil M., Attia A., Magdy M., & Negida A. (2017). Neuropsychological performance changes following subthalamic versus pallidal deep brain stimulation in Parkinson's disease: a systematic review and metaanalysis. CNS Spectrums, 1–14. doi:10.1017/S1092852917000062. [DOI] [PubMed] [Google Scholar]
- Erola T., Karinen P., Heikkinen E., Tuominen J., Haapaniemi T., Koivukangas J., et al. (2005). Bilateral subthalamic nucleus stimulation improves health-related quality of life in Parkinsonian patients. Parkinsonism & Related Disorders, 11, 89–94. [DOI] [PubMed] [Google Scholar]
- Fasano A., Romito L. M., Daniele A., Piano C., Zinno M., Bentivoglio A. R., et al. (2010). Motor and cognitive outcome in patients with Parkinson's disease 8 years after subthalamic implants. Brain, 133, 2664–2676. doi:10.1093/brain/awq221. [DOI] [PubMed] [Google Scholar]
- Fields J. A., & Tröster A. I. (2000). Cognitive outcomes after deep brain stimulation for Parkinson's disease: a review of initial studies and recommendations for future research. Brain and Cognition, 42, 268–293. [DOI] [PubMed] [Google Scholar]
- Fields J. A., Tröster A. I., Wilkinson S. B., Pahwa R., & Koller W. C. (1999). Cognitive outcome following staged bilateral pallidal stimulation for the treatment of Parkinson's disease. Clinical Neurology and Neurosurgery, 101, 182–188. [DOI] [PubMed] [Google Scholar]
- Floden D., Busch R. M., Cooper S. E., Kubu C. S., & Machado A. G. (2015). Global cognitive scores do not predict outcome after subthalamic nucleus deep brain stimulation. Movement Disorders, 30, 1279–1283. doi:10.1002/mds.26292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores Alves Dos Santos J., Tezenas du Montcel S., Gargiulo M., Behar C., Montel S., Hergueta T., et al. (2017). Tackling psychosocial maladjustment in Parkinson's disease patients following subthalamic deep-brain stimulation: a randomised clinical trial. PloS One, 12, e0174512 doi:10.1371/journal.pone.0174512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluchere F., Witjas T., Eusebio A., Bruder N., Giorgi R., Leveque M., et al. (2014). Controlled general anaesthesia for subthalamic nucleus stimulation in Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry, 85, 1167–1173. doi:10.1136/jnnp-2013-305323. [DOI] [PubMed] [Google Scholar]
- Foki T., Hitzl D., Pirker W., Novak K., Pusswald G., Auff E., et al. (2017). Assessment of individual cognitive changes after deep brain stimulation surgery in Parkinson's disease using the Neuropsychological Test Battery Vienna short version. Wiener Klinische Wochenschrift. doi:10.1007/s00508-017-1169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett K. A., Weaver F. M., Stern M., Hur K., Harris C. L., Luo P., et al. (2010). Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. The New England journal of medicine, 362, 2077–2091. doi:10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- Francel P., Ryder K., Wetmore J., Stevens A., Bharucha K., Beatty W. W., et al. (2004). Deep brain stimulation for Parkinson's disease: association between stimulation parameters and cognitive performance. Stereotactic and Functional Neurosurgery, 82, 191–193. [DOI] [PubMed] [Google Scholar]
- Frankemolle A. M., Wu J., Noecker A. M., Voelcker-Rehage C., Ho J. C., Vitek J. L., et al. (2010). Reversing cognitive-motor impairments in Parkinson's disease patients using a computational modelling approach to deep brain stimulation programming. Brain, 133, 746–761. doi:10.1093/brain/awp315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund H. J., Kuhn J., Lenartz D., Mai J. K., Schnell T., Klosterkoetter J., et al. (2009). Cognitive functions in a patient with Parkinson-dementia syndrome undergoing deep brain stimulation. Archives of Neurology, 66, 781–785. doi:66/6/781[pii]: 10.1001/archneurol.2009.102. [DOI] [PubMed] [Google Scholar]
- Fukaya C., Watanabe M., Kobayashi K., Oshima H., Yoshino A., & Yamamoto T. (2017). Predictive factors for long-term outcome of subthalamic nucleus deep brain stimulation for parkinson's disease. Neurologia Medico-Chirurgica, 57, 166–171. doi:10.2176/nmc.oa.2016-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkiewiez A., Ardouin C., Caputo E., Krack P., Fraix V., Klinger H., et al. (2004). Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry, 75, 834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geevarghese R., Lumsden D. E., Costello A., Hulse N., Ayis S., Samuel M., et al. (2016). Verbal Memory decline following DBS for Parkinson's disease: structural volumetric MRI relationships. PloS One, 11, e0160583 doi:10.1371/journal.pone.0160583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna-Pladdy B., Enslein A., Fray M., Gajewski B. J., Pahwa R., & Lyons K. E. (2010). Utility of the NeuroTrax computerized battery for cognitive screening in Parkinson's disease: comparison with the MMSE and the MoCA. International Journal of Neuroscience, 120, 538–543. doi:10.3109/00207454.2010.496539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariz M. I., Johansson F., Shamsgovara P., Johansson E., Hariz G. M., & Fagerlund M. (2000). Bilateral subthalamic nucleus stimulation in a parkinsonian patient with preoperative deficits in speech and cognition: persistent improvement in mobility but increased dependency: a case study. Movement Disorders, 15, 136–139. [DOI] [PubMed] [Google Scholar]
- Hawkins K. A., Jennings D., Vincent A. S., Gilliland K., West A., & Marek K. (2012). Traditional neuropsychological correlates and reliability of the automated neuropsychological assessment metrics-4 battery for Parkinson's disease. Parkinsonism & Related Disorders, 18, 864–870. doi:10.1016/j.parkreldis.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Hershey T., Wu J., Weaver P. M., Perantie D. C., Karimi M., Tabbal S. D., et al. (2008). Unilateral vs. bilateral STN DBS effects on working memory and motor function in Parkinson disease. Experimental Neurology, 210, 402–408. doi: S0014-4886(07)00427-X [pii]: 10.1016/j.expneurol.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojlund A., Petersen M. V., Sridharan K. S., & Ostergaard K. (2017). Worsening of Verbal Fluency After Deep Brain Stimulation in Parkinson's Disease: A Focused Review. Computational and Structural Biotechnology Journal, 15, 68–74. doi:10.1016/j.csbj.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogland J., Boel J. A., de Bie R. M. A., Geskus R. B., Schmand B. A., Dalrymple-Alford J. C., et al. Study Group “Validation of Mild Cognitive Impairment in Parkinson. (2017). Mild cognitive impairment as a risk factor for Parkinson's disease dementia. Movement Disorders, 32, 1056–1065. doi:10.1002/mds.27002. [DOI] [PubMed] [Google Scholar]
- Hrabovsky D., Balaz M., Rab M., Feitova V., Hummelova Z., Novak Z., et al. (2017). Factors responsible for early postoperative mental alterations after bilateral implantation of subthalamic electrodes. British Journal of Neurosurgery, 31, 212–216. doi:10.1080/02688697.2016.1226256. [DOI] [PubMed] [Google Scholar]
- Isler C., Albi A., Schaper F. L., Temel Y., & Duits A. (2016). Neuropsychological Outcome in Subthalamic Nucleus Stimulation Surgeries with Electrodes Passing through the Caudate Nucleus. Stereotactic and Functional Neurosurgery, 94, 413–420. doi:10.1159/000453278. [DOI] [PubMed] [Google Scholar]
- Kasemsuk C., Oyama G., & Hattori N. (2017). Management of impulse control disorders with deep brain stimulation: A double-edged sword. Journal of the Neurological Sciences, 374, 63–68. doi:10.1016/j.jns.2017.01.019. [DOI] [PubMed] [Google Scholar]
- Kim H. J., Jeon B. S., Paek S. H., Lee K. M., Kim J. Y., Lee J. Y., et al. (2014). Long-term cognitive outcome of bilateral subthalamic deep brain stimulation in Parkinson's disease. Journal of Neurology, 261, 1090–1096. doi:10.1007/s00415-014-7321-z. [DOI] [PubMed] [Google Scholar]
- Kleiner-Fisman G., Fisman D. N., Sime E., Saint-Cyr J. A., Lozano A. M., & Lang A. E. (2003). Long-term follow up of bilateral deep brain stimulation of the subthalamic nucleus in patients with advanced Parkinson disease. Journal of Neurosurgery, 99, 489–495. [DOI] [PubMed] [Google Scholar]
- Kleiner-Fisman G., Herzog J., Fisman D. N., Tamma F., Lyons K. E., Pahwa R., et al. (2006). Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Movement Disorders, 21 (Suppl. 14), S290–S304. [DOI] [PubMed] [Google Scholar]
- Koller W., Pahwa R., Busenbark K., Hubble J., Wilkinson S., Lang A., et al. (1997). High-frequency unilateral thalamic stimulation in the treatment of essential and parkinsonian tremor. Annals of Neurology, 42, 292–299. [DOI] [PubMed] [Google Scholar]
- Kurtis M. M., Rajah T., Delgado L. F., & Dafsari H. S. (2017). The effect of deep brain stimulation on the non-motor symptoms of Parkinson's disease: a critical review of the current evidence. npj Parkinson's Disease, 3, 16024 doi:10.1038/npjparkd.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier-Bohan E. M., & Heath S. L. (2016). Patient and caregiver perspectives of preoperative teaching for deep brain stimulation surgery. Journal of Neuroscience Nursing, 48, 247–255. doi:10.1097/JNN.0000000000000221. [DOI] [PubMed] [Google Scholar]
- Lewis C. J., Maier F., Horstkotter N., Eggers C., Visser-Vandewalle V., Moro E., et al. (2015). The impact of subthalamic deep brain stimulation on caregivers of Parkinson's disease patients: an exploratory study. Journal of Neurology, 262, 337–345. doi:10.1007/s00415-014-7571-9. [DOI] [PubMed] [Google Scholar]
- Lezcano E., Gomez-Esteban J. C., Zarranz J. J., Lambarri I., Madoz P., Bilbao G., et al. (2004). Improvement in quality of life in patients with advanced Parkinson's disease following bilateral deep-brain stimulation in subthalamic nucleus. European Journal of Neurology, 11, 451–454. [DOI] [PubMed] [Google Scholar]
- Litvan I., Aarsland D., Adler C. H., Goldman J. G., Kulisevsky J., Mollenhauer B., et al. (2011). MDS task force on mild cognitive impairment in Parkinson's disease: Critical review of PD-MCI. Movement disorders: official journal of the Movement Disorder Society. doi:10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I., Goldman J. G., Tröster A. I., Schmand B. A., Weintraub D., Petersen R. C., et al. (2012). Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Movement Disorders, 27, 349–356. doi:10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Li W., Tan C., Liu X., Wang X., Gui Y., et al. (2014). Meta-analysis comparing deep brain stimulation of the globus pallidus and subthalamic nucleus to treat advanced Parkinson disease. Journal of Neurosurgery, 121, 709–718. doi:10.3171/2014.4.JNS131711. [DOI] [PubMed] [Google Scholar]
- Lopiano L., Rizzone M., Bergamasco B., Tavella A., Torre E., Perozzo P., et al. (2002). Deep brain stimulation of the subthalamic nucleus in PD: an analysis of the exclusion causes. Journal of the Neurological Sciences, 195, 167–170. [DOI] [PubMed] [Google Scholar]
- Lucza T., Karadi K., Kallai J., Weintraut R., Janszky J., Makkos A., et al. (2015). Screening Mild and Major Neurocognitive Disorders in Parkinson's Disease. Behavioural Neurology, 2015, 983606 doi:10.1155/2015/983606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueken U., Schwarz M., Hertel F., Schweiger E., & Wittling W. (2008). Impaired performance on the Wisconsin Card Sorting Test under left- when compared to right-sided deep brain stimulation of the subthalamic nucleus in patients with Parkinson's disease. Journal of Neurology, 255, 1940–1948. doi:10.1007/s00415-009-0040-1. [DOI] [PubMed] [Google Scholar]
- Maier F., Lewis C. J., Horstkoetter N., Eggers C., Dembek T. A., Visser-Vandewalle V., et al. (2016). Subjective perceived outcome of subthalamic deep brain stimulation in Parkinson's disease one year after surgery. Parkinsonism & Related Disorders, 24, 41–47. doi:10.1016/j.parkreldis.2016.01.019. [DOI] [PubMed] [Google Scholar]
- Maier F., Lewis C. J., Horstkoetter N., Eggers C., Kalbe E., Maarouf M., et al. (2013). Patients’ expectations of deep brain stimulation, and subjective perceived outcome related to clinical measures in Parkinson's disease: a mixed-method approach. Journal of Neurology, Neurosurgery, and Psychiatry, 84, 1273–1281. doi:10.1136/jnnp-2012-303670. [DOI] [PubMed] [Google Scholar]
- Martínez-Martínez A. M., Aguilar O. M., & Acevedo-Triana C. A. (2017). Meta-analysis of the relationship between deep brain stimulation in patients with Parkinson's disease and performance in evaluation tests for executive brain functions. Parkinson's Disease, 2017, 9641392 doi:10.1155/2017/9641392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massano J., & Garrett C. (2012). Deep brain stimulation and cognitive decline in Parkinson's disease: a clinical review. Frontiers in Neurology, 3, 66 doi:10.3389/fneur.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merola A., Rizzi L., Artusi C. A., Zibetti M., Rizzone M. G., Romagnolo A., et al. (2014). Subthalamic deep brain stimulation: clinical and neuropsychological outcomes in mild cognitive impaired parkinsonian patients. Journal of Neurology, 261, 1745–1751. doi:10.1007/s00415-014-7414-8. [DOI] [PubMed] [Google Scholar]
- Mestre T. A., Lang A. E., & Okun M. S. (2016). Factors influencing the outcome of deep brain stimulation: Placebo, nocebo, lessebo, and lesion effects. Movement Disorders, 31, 290–296. doi:10.1002/mds.26500. [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z., Chapple K., Lambert M., Evidente V. G., Mahant P., Ospina M. C., et al. (2016). Parkinson's disease outcomes after intraoperative CT-guided “asleep” deep brain stimulation in the globus pallidus internus. Journal of Neurosurgery, 124, 902–907. doi:10.3171/2015.4.JNS1550. [DOI] [PubMed] [Google Scholar]
- Mulroy E., Robertson N., Macdonald L., Bok A., & Simpson M. (2017). Patients’ perioperative experience of awake deep brain stimulation for Parkinson's disease. World Neurosurgery. doi:10.1016/j.wneu.2017.05.132. [DOI] [PubMed] [Google Scholar]
- Muthuraman M., Deuschl G., Koirala N., Riedel C., Volkmann J., & Groppa S. (2017). Effects of DBS in parkinsonian patients depend on the structural integrity of frontal cortex. Scientific Reports, 7, 43571 doi:10.1038/srep43571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odekerken V. J., Boel J. A., Geurtsen G. J., Schmand B. A., Dekker I. P., & de Haan R. J., Group, Nstaps Study. (2015). Neuropsychological outcome after deep brain stimulation for Parkinson disease. Neurology, 84, 1355–1361. doi:10.1212/WNL.0000000000001419. [DOI] [PubMed] [Google Scholar]
- Odekerken V. J., van Laar T., Staal M. J., Mosch A., Hoffmann C. F., & Nijssen P. C. (2013). Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): a randomised controlled trial. Lancet Neurology, 12, 37–44. doi:10.1016/S1474-4422(12)70264-8. [DOI] [PubMed] [Google Scholar]
- Okun M. S., Fernandez H. H., Wu S. S., Kirsch-Darrow L., Bowers D., & Bova F. (2009). Cognition and mood in Parkinson's disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Annals of Neurology, 65, 586–595. doi:10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun M. S., Gallo B. V., Mandybur G., Jagid J., Foote K. D., Revilla F. J., et al. (2012). Subthalamic deep brain stimulation with a constant-current device in Parkinson's disease: an open-label randomised controlled trial. Lancet Neurology, 11, 140–149. doi:10.1016/S1474-4422(11)70308-8. [DOI] [PubMed] [Google Scholar]
- Oyama G., Okun M. S., Schmidt P., Tröster A. I., Nutt J., & Go C. L., Investigators, Npf-Qii. (2014). Deep brain stimulation may improve quality of life in people with Parkinson's disease without affecting caregiver burden. Neuromodulation, 17, 126–132. doi:10.1111/ner.12097. [DOI] [PubMed] [Google Scholar]
- Oyama G., Rodriguez R. L., Jones J. D., Swartz C., Merritt S., Unger R., et al. (2012). Selection of deep brain stimulation candidates in private neurology practices: referral may be simpler than a computerized triage system. Neuromodulation, 15, 246–250; discussion 250doi:10.1111/j.1525-1403.2012.00437.x. [DOI] [PubMed] [Google Scholar]
- Pal G. D., Persinger V., Bernard B., Ouyang B., Goetz C. G., & Metman L. V. (2015). The Core Assessment Program for Surgical Interventional Therapies in Parkinson's Disease (CAPSIT‐PD): Tolerability of Preoperative Neuropsychological Testing for Deep Brain Stimulation in Parkinson's Disease. Movement Disorders Clinical Practice, 2, 379–383. doi:10.1002/mdc3.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T. D., Rogers S. A., Braaten A. J., Woods S. P., & Tröster A. I. (2006). Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson's disease: a meta-analysis. Lancet Neurology, 5, 578–588. [DOI] [PubMed] [Google Scholar]
- Pham U. H., Andersson S., Toft M., Pripp A. H., Konglund A. E., Dietrichs E., et al. (2015). Self-Reported Executive Functioning in Everyday Life in Parkinson's Disease after Three Months of Subthalamic Deep Brain Stimulation. Parkinson's Disease, 2015, 461453 doi:10.1155/2015/461453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilitsis J. G., Rezai A. R., Boulis N. M., Henderson J. M., Busch R. M., & Kubu C. S. (2005). A preliminary study of transient confusional states following bilateral subthalamic stimulation for Parkinson's disease. Stereotactic and Functional Neurosurgery, 83, 67–70. [DOI] [PubMed] [Google Scholar]
- Pirogovsky E., Martinez-Hannon M., Schiehser D. M., Lessig S. L., Song D. D., Litvan I., et al. (2013). Predictors of performance-based measures of instrumental activities of daily living in nondemented patients with Parkinson's disease. Journal of Clinical and Experimental Neuropsychology, 35, 926–933. doi:10.1080/13803395.2013.838940. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz M. C., Obeso J. A., Lang A. E., Houeto J. L., Pollak P., Rehncrona S., et al. (2005). Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain, 128, 2240–2249. doi:10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- Rothlind J. C., Cockshott R. W., Starr P. A., & Marks W. J. (2007). Neuropsychological performance following staged bilateral pallidal or subthalamic nucleus deep brain stimulation for Parkinson's disease. Journal of the International Neuropsychological Society, 13, 68–79. [DOI] [PubMed] [Google Scholar]
- Rothlind J. C., York M. K., Carlson K., Luo P., Marks W. J. Jr., & Weaver F. M., Group, C. S. P. Study. (2015). Neuropsychological changes following deep brain stimulation surgery for Parkinson's disease: comparisons of treatment at pallidal and subthalamic targets versus best medical therapy. Journal of Neurology, Neurosurgery and Psychiatry, 86, 622–629. doi:10.1136/jnnp-2014-308119. [DOI] [PubMed] [Google Scholar]
- Rouaud T., Dondaine T., Drapier S., Haegelen C., Lallement F., Peron J., et al. (2010). Pallidal stimulation in advanced Parkinson's patients with contraindications for subthalamic stimulation. Movement Disorders, 25, 1839–1846. doi:10.1002/mds.23171. [DOI] [PubMed] [Google Scholar]
- Schoenberg M. R., Mash K. M., Bharucha K. J., Francel P. C., & Scott J. G. (2008). Deep brain stimulation parameters associated with neuropsychological changes in subthalamic nucleus stimulation for refractory Parkinson's disease. Stereotactic and Functional Neurosurgery, 86, 337–344. doi:000163554 [pii]: 10.1159/000163554. [DOI] [PubMed] [Google Scholar]
- Schuepbach W. M., Rau J., Knudsen K., Volkmann J., Krack P., Timmermann L., et al. (2013). Neurostimulation for Parkinson's disease with early motor complications. New England Journal of Medicine, 368, 610–622. doi:10.1056/NEJMoa1205158. [DOI] [PubMed] [Google Scholar]
- Sem-Jacobsen C. W. (1965). Depth electrographic stimulation and treatment of patients with Parkinson's disease including neurosurgical technique. Acta Neurologica Scandinavica. Supplementum, 13, 365–377. [DOI] [PubMed] [Google Scholar]
- Sidiropoulos C., Rammo R., Merker B., Mahajan A., LeWitt P., Kaminski P., et al. (2016). Intraoperative MRI for deep brain stimulation lead placement in Parkinson's disease: 1 year motor and neuropsychological outcomes. Journal of Neurology, 263, 1226–1231. doi:10.1007/s00415-016-8125-0. [DOI] [PubMed] [Google Scholar]
- Sitek E. J., Soltan W., Wieczorek D., Robowski P., & Slawek J. (2011). Self-awareness of memory function in Parkinson's disease in relation to mood and symptom severity. Aging and Mental Health, 15, 150–156. doi:10.1080/13607863.2010.508773. [DOI] [PubMed] [Google Scholar]
- Sjoberg R. L., Lidman E., Haggstrom B., Hariz M. I., Linder J., Fredricks A., et al. (2012). Verbal fluency in patients receiving bilateral versus left-sided deep brain stimulation of the subthalamic nucleus for Parkinson's disease. Journal of the International Neuropsychological Society, 18, 606–611. doi:10.1017/S1355617711001925. [DOI] [PubMed] [Google Scholar]
- Smeding H. M., Speelman J. D., Huizenga H. M., Schuurman P. R., & Schmand B. (2011). Predictors of cognitive and psychosocial outcome after STN DBS in Parkinson's Disease. Journal of Neurology, Neurosurgery, and Psychiatry, 82, 754–760. doi:10.1136/jnnp.2007.140012. [DOI] [PubMed] [Google Scholar]
- Smeding H. M., Speelman J. D., Koning-Haanstra M., Schuurman P. R., Nijssen P., van Laar T., et al. (2006). Neuropsychological effects of bilateral STN stimulation in Parkinson disease: a controlled study. Neurology, 66, 1830–1836. [DOI] [PubMed] [Google Scholar]
- Soileau M. J., Persad C., Taylor J., Patil P. G., & Chou K. L. (2014). Caregiver burden in patients with Parkinson disease undergoing deep brain stimulation: an exploratory analysis. Journal of Parkinson's Disease, 4, 517–521. doi:10.3233/JPD-140380. [DOI] [PubMed] [Google Scholar]
- Soulas T., Sultan S., Gurruchaga J. M., Palfi S., & Fenelon G. (2011). Depression and coping as predictors of change after deep brain stimulation in Parkinson's disease. World Neurosurgery, 75, 525–532. doi:10.1016/j.wneu.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Timmermann L., Jain R., Chen L., Maarouf M., Barbe M. T., Allert N., et al. (2015). Multiple-source current steering in subthalamic nucleus deep brain stimulation for Parkinson's disease (the VANTAGE study): a non-randomised, prospective, multicentre, open-label study. Lancet Neurology, 14, 693–701. doi:10.1016/S1474-4422(15)00087-3. [DOI] [PubMed] [Google Scholar]
- Tramontana M. G., Molinari A. L., Konrad P. E., Davis T. L., Wylie S. A., Neimat J. S., et al. (2015). Neuropsychological effects of deep brain stimulation in subjects with early stage Parkinson's disease in a randomized clinical trial. Journal of Parkinson's Disease, 5, 151–163. doi:10.3233/JPD-140448. [DOI] [PubMed] [Google Scholar]
- Tröster A. I. (2008). Short-term cognitive and affective outcomes after subthalamic deep brain stimulation for Parkinson's disease. Lancet Neurology, 7, 565–567. doi: S1474-4422(08)70115-7 [pii]: 10.1016/S1474-4422(08)70115-7. [DOI] [PubMed] [Google Scholar]
- Tröster A. I., Fields J. A., Wilkinson S. B., Pahwa R., Miyawaki E., Lyons K. E., et al. (1997). Unilateral pallidal stimulation for Parkinson's disease: neurobehavioral functioning before and 3 months after electrode implantation. Neurology, 49, 1078–1083. [DOI] [PubMed] [Google Scholar]
- Tröster A. I., Fields J. A., Wilkinson S., Pahwa R., Koller W. C., & Lyons K. E. (2003). Effect of motor improvement on quality of life following subthalamic stimulation is mediated by changes in depressive symptomatology. Stereotactic and Functional Neurosurgery, 80, 43–47. [DOI] [PubMed] [Google Scholar]
- Tröster A. I., Jankovic J., Tagliati M., Peichel D., & Okun M. S. (2017). Neuropsychological outcomes from constant current deep brain stimulation for Parkinson's disease. Movement Disorders, 32, 433–440. doi:10.1002/mds.26827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tröster A. I., Woods S. P., & Morgan E. E. (2007). Assessing cognitive change in Parkinson's disease: development of practice effect-corrected reliable change indices. Archives of Clinical Neuropsychology, 22, 711–718. [DOI] [PubMed] [Google Scholar]
- Tsai S. T., Lin S. H., Lin S. Z., Chen J. Y., Lee C. W., & Chen S. Y. (2007). Neuropsychological effects after chronic subthalamic stimulation and the topography of the nucleus in Parkinson's disease. Neurosurgery, 61, E1024–E1029; discussionE1029-1030doi:10.1227/01.neu.0000303198.95296.6f: 00006123-200711000-00016 [pii]. [DOI] [PubMed] [Google Scholar]
- Villeneuve S., Rodrigues-Brazete J., Joncas S., Postuma R. B., Latreille V., & Gagnon J. F. (2011). Validity of the Mattis Dementia Rating Scale to detect mild cognitive impairment in Parkinson's disease and REM sleep behavior disorder. Dementia and Geriatric Cognitive Disorders, 31, 210–217. doi:10.1159/000326212. [DOI] [PubMed] [Google Scholar]
- Volkmann J., Allert N., Voges J., Weiss P. H., Freund H.-J., & Sturm V. (2001). Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. Neurology, 56, 548–551. [DOI] [PubMed] [Google Scholar]
- Volkmann J., Daniels C., & Witt K. (2010). Neuropsychiatric effects of subthalamic neurostimulation in Parkinson disease. Nature Reviews: Neurology, 6, 487–498. doi:10.1038/nrneurol.2010.111. [DOI] [PubMed] [Google Scholar]
- Wang J. W., Zhang Y. Q., Zhang X. H., Wang Y. P., Li J. P., & Li Y. J. (2016). Cognitive and Psychiatric Effects of STN versus GPi Deep Brain Stimulation in Parkinson's Disease: A Meta-Analysis of Randomized Controlled Trials. PloS One, 11, e0156721 doi:10.1371/journal.pone.0156721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver F. M., Follett K., Stern M., Hur K., Harris C., Marks W. J. Jr., et al. (2009). Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. Journal of the American Medical Association, 301, 63–73. doi:301/1/63 [pii]: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A., Gill S., Varma T., Jenkinson C., Quinn N., Mitchell R., et al. (2010). Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson's disease (PD SURG trial): a randomised, open-label trial. Lancet Neurology, 9, 581–591. doi:10.1016/S1474-4422(10)70093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt K., Daniels C., Krack P., Volkmann J., Pinsker M. O., Kloss M., et al. (2011). Negative impact of borderline global cognitive scores on quality of life after subthalamic nucleus stimulation in Parkinson's disease. Journal of the Neurological Sciences, 310, 261–266. doi:10.1016/j.jns.2011.06.028. [DOI] [PubMed] [Google Scholar]
- Witt K., Daniels C., Reiff J., Krack P., Volkmann J., Pinsker M. O., et al. (2008). Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson's disease: a randomised, multicentre study. Lancet Neurology, 7, 605–614. doi: S1474-4422(08)70114-5 [pii]: 10.1016/S1474-4422(08)70114-5. [DOI] [PubMed] [Google Scholar]
- Witt K., Granert O., Daniels C., Volkmann J., Falk D., van Eimeren T., et al. (2013). Relation of lead trajectory and electrode position to neuropsychological outcomes of subthalamic neurostimulation in Parkinson's disease: results from a randomized trial. Brain, 136, 2109–2119. doi:10.1093/brain/awt151. [DOI] [PubMed] [Google Scholar]
- Woods S. P., Fields J. A., & Tröster A. I. (2002). Neuropsychological sequelae of subthalamic nucleus deep brain stimulation in Parkinson's disease: a critical review. Neuropsychology Review, 12, 111–126. [DOI] [PubMed] [Google Scholar]
- Wyman-Chick K. A. (2016). Verbal fluency in Parkinson's patients with and without bilateral deep brain stimulation of the subthalamic nucleus: a meta-analysis. Journal of the International Neuropsychological Society, 22, 478–485. doi:10.1017/S1355617716000035. [DOI] [PubMed] [Google Scholar]
- Wyman-Chick K. A., Martin P. K., Barrett M. J., Manning C. A., & Sperling S. A. (2017). Diagnostic accuracy and confidence in the clinical detection of cognitive impairment in early-stage parkinson disease. Journal of Geriatric Psychiatry and Neurology, 30, 178–183. doi:10.1177/0891988717701001. [DOI] [PubMed] [Google Scholar]
- Xie Y., Meng X., Xiao J., Zhang J., & Zhang J. (2016). Cognitive changes following bilateral deep brain stimulation of subthalamic nucleus in Parkinson's disease: a meta-analysis. BioMed Research International, 2016, 3596415 doi:10.1155/2016/3596415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguez L., Costello A., Moriarty J., Hulse N., Selway R., Clough C., et al. (2014). Cognitive predictors of cognitive change following bilateral subthalamic nucleus deep brain stimulation in Parkinson's disease. Journal of Clinical Neuroscience, 21, 445–450. doi:10.1016/j.jocn.2013.06.005. [DOI] [PubMed] [Google Scholar]
- York M. K., Dulay M., Macias A., Levin H. S., Grossman R., Simpson R., et al. (2008). Cognitive declines following bilateral subthalamic nucleus deep brain stimulation for the treatment of Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry, 79, 789–795. doi: jnnp.2007.118786 [pii]: 10.1136/jnnp.2007.118786. [DOI] [PubMed] [Google Scholar]
- York M. K., Wilde E. A., Simpson R., & Jankovic J. (2009). Relationship between neuropsychological outcome and DBS surgical trajectory and electrode location. Journal of the Neurological Sciences, 287, 159–171. doi:10.1016/j.jns.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne L. B., Okun M. S., Foote K. D., Fernandez H. H., Rodriguez R. L., Wu S. S., et al. (2009). Greater improvement in quality of life following unilateral deep brain stimulation surgery in the globus pallidus as compared to the subthalamic nucleus. Journal of Neurology, 256, 1321–1329. doi:10.1007/s00415-009-5121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]