Abstract
There is an ongoing need to develop strategic combinations of therapeutic agents to prevent type 1 diabetes (T1D) or to preserve islet β-cell mass in new-onset disease. Although clinical trials using candidate therapeutics are commonly based on preclinical studies, concern is growing regarding the reproducibility as well as the potential clinical translation of reported results using animal models of human disorders. In response, the National Institutes of Health Immune Tolerance Network and JDRF established a multicenter consortium of academic institutions designed to assess the efficacy and intergroup reproducibility of clinically applicable immunotherapies for reversing new-onset disease in the NOD mouse model of T1D. Predicated on prior studies, this consortium conducted coordinated, prospective studies, using joint standard operating procedures, fixed criteria for study entry, and common reagents, to optimize combined anti-CD3 treatment plus interleukin-1 (IL-1) blockade to reverse new-onset disease in NOD mice. We did not find that IL-1 blockade with anti–IL-1β monoclonal antibody or IL-1trap provided additional benefit for reversing new-onset disease compared with anti-CD3 treatment alone. These results demonstrate the value of larger, multicenter preclinical studies for vetting and prioritizing therapeutics for future clinical use.
Introduction
An ongoing objective for the treatment of type 1 diabetes (T1D) is to preserve residual islet β-cell survival and function after new-onset disease (1). Clinical trials are commonly based on results of testing candidate therapies in preclinical animal models of disease. However, there is an alarmingly growing concern regarding the reproducibility and clinical relevance of therapeutic agents tested in preclinical models (2–5), as exemplified by surprisingly low rates of reproducibility in animal models of neurologic diseases (2,6). Such discrepancies called for more scrutiny and rigor in the design, execution, and reporting of animal studies (4–6).
This important concern extends to preclinical studies intended to assess therapeutics for preventing T1D or preserving islet β-cell mass in recent-onset disease (3). Some therapies effective in NOD mice, such as anti-CD3 and anti-CD20, have translated to a degree of clinical benefit (7–12). However, other treatments, such as interleukin (IL)-2 plus rapamycin treatment (13), proved ineffective and possibly accelerated disease in human subjects (14). At present, it is uncertain whether such variability in clinical translation of results is due to intrinsic differences in disease mechanisms in NOD mice versus patients with T1D or may be related to the design and rigor of preclinical studies that are usually analogous to single-center pilot clinical trials.
To address these issues, the Immune Tolerance Network and JDRF assembled a preclinical consortium involving four participating academic institutions to evaluate whether rigorous design, execution, and reporting of animal studies leads to increased validation of results achieved in NOD mice. To attain this end, this multicenter consortium collaboratively assesses candidate combinational therapies for their relative efficacy in reversing new-onset disease in the NOD mouse model of T1D. Predicated on prior promising results (15), we set out to determine optimal conditions for using anti-CD3 plus IL-1 blockade to promote disease reversal. That is, in an attempt to guide potential clinical trials, this study formed the rationale for our consortium to refine clinically relevant protocols of combined anti-CD3 plus IL-1 blockade and to determine the efficacy, intersite reproducibility, and durability of combined treatment to reverse new-onset disease in NOD mice.
Research Design and Methods
Performance Sites
These studies were undertaken at the University of Florida, La Jolla Institute for Allergy and Immunology, University of Colorado Denver, and Yale University. Specific sites are blinded in data presented and are referred to as sites 1–4.
Mice
NOD/ShiLtJ mice were purchased from The Jackson Laboratory, except at performance site 1, where the NOD mice were bred in-house from the NOD/Bdc subline or were purchased from The Jackson Laboratory. All mice were housed under specific pathogen-free conditions and provided bedding and chow that was in standard use at each of the study sites.
Disease Monitoring and Definition
Female NOD mice, 10–26 weeks of age, were monitored for diabetes onset three times per week. Mice were entered into a predetermined treatment group on the second of two consecutive daily blood glucose value (BGV) readings ≥250 mg/dL (day 1 of study). A portable blood glucose monitor was used to monitor morning BGVs of treated mice twice per week and was determined from tail venous blood. The study was run for 60–62 days, at which point mice were killed. At study termination, mice were categorized as cured if their BGV reading was <250 mg/dL or diabetic if >250 mg/dL. Some animals were removed from the study and killed before day 60–62 if their body weight decreased below levels permitted by the local institutional animal care and use committees (IACUCs) or three consecutive maximum BGV readings as permitted by each site's IACUC.
Treatment With Antibodies and Fusion Proteins
Hamster anti-mouse CD3 145-2C11 monoclonal antibody (mAb) F(ab)2 fragments (BioXCell, West Lebanon, NH) or control hamster F(ab)2 fragments (BioXCell) were injected intraperitoneally (i.p.) for 5 consecutive days (5 µg/day). This dose was less than that used in previous studies of anti-CD3 mAb (15) to reduce potential toxicity and to enhance the identification of synergistic effects with anti–IL-1 agents. When indicated, anti–IL-1β mAb and isotype antibodies (a gift from Novartis) were given at a dosage of 75 µg/day i.p. Alternatively, the fusion protein IL-1trap (Rilonacept; a gift from Regeneron) was injected at 60 mg/kg i.p.
Study Trial Design and Enrollment
Two separate study trials were performed as follows:
Study 1: Anti-CD3 Plus Anti–IL-1β Antibody Treatment
To minimize the effects of subjective bias and to ensure a uniform population of treatment groups over time, each center enrolled animals sequentially in a revolving format into treatment arms. With the exception of group 2, recipients also received one LinBit sustained-release insulin pellet (LinShin Canada, Inc., Toronto, Ontario, Canada) implanted subcutaneously and were allocated to the following groups:
Group 1: Simultaneous anti-CD3 (5 µg/day) plus anti–IL-1β (75 µg/day) (day 1, 3, and 5)
Group 2: Simultaneous anti-CD3 (5 µg/day) plus anti–IL-1β (75 µg/day) (day 1, 3, and 5); no insulin
Group 3: Early (day 1, 3, and 5) anti-CD3 (5 µg/day) treatment, followed by delayed (day 5, 7, and 9) anti–IL-1β treatment (75 µg/day)
Group 4: Anti-CD3 (5 µg/day) plus early isotype control antibody (day 1, 3, and 5)
Group 5: Anti-CD3 (5 µg/day) (day 1, 3, and 5), followed by delayed isotype control antibody (day 5, 7, and 9)
Group 6: Simultaneous control F(ab)2 plus isotype control antibody (day 1, 3, and 5)
Group 7: Early control F(ab)2 (day 1, 3, and 5) plus delayed isotype control antibody (day 5, 7, and 9)
Group 8: Early anti–IL-1β alone (75 µg/day) (day 1, 3, and 5)
Group 9: Delayed anti–IL-1β alone (75 µg/day) (day 5, 7, and 9).
Study 2: Pilot Study of Anti-CD3 Plus IL-1trap (Rilonacept) Treatment
In a second abbreviated pilot study approach, center sites 1, 2, and 4 tested the efficacy of disease reversal using combined anti-CD3 treatment (5 µg/day) with IL-1 blockade by the fusion protein IL-1trap (60 mg/kg), without exogenous insulin therapy, according to the following treatment groups:
Group 1: Untreated diabetic control mice
Group 2: Early anti-CD3 alone
Group 3: Simultaneous anti-CD3 plus IL-1trap treatment
Group 4: Early anti-CD3 treatment with delayed IL-1trap treatment.
Animals were entered into treatment groups using the same enrollment scheme as described for study 1 above.
Statistics
The first protocol (anti-CD3 plus anti–IL-1β) was 80% powered to determine a 50% improvement in the reversal of diabetes in the combination versus the anti-CD3 mAb arms using anti–IL-1β mAb. A two-way ANOVA test was used for analyses of parameters at onset. Diabetes incidences were assessed using the log-rank test. Graphs were plotted and statistics calculated with GraphPad Prism 4 and 5 software.
Ethics
These studies were performed on all sites upon approval of their respective IACUCs.
Standard Operating Procedures
A detailed version of all methods and standard operating procedures (SOPs) for these studies and all individual BGV data can be found at https://www.itntrialshare.org/.
Results
Study 1: Multicenter Study of Combining Anti-CD3 Treatment With Anti–IL-1β mAb Therapy
In this initial trial, this multicenter study combined anti–IL-1β mAb therapy at varied timing (simultaneous or delayed) relative to a fixed anti-CD3 treatment. We evaluated nine treatment groups that included placebo controls for anti-CD3 (control F[ab]2 fragments) and/or for anti–IL-1β (isotype control antibody). In addition, we determined whether exogenous insulin treatment early after disease onset affected the efficacy of disease reversal by simultaneous anti-CD3 plus anti–IL-1β mAb treatment (groups 1 and 2).
Anti-CD3 but Not Anti–IL-1β mAb Results in Significant Disease Reversal at All Sites
Because the age of onset or the severity of initial hyperglycemia could possibly contribute to variability in the ability to reverse new-onset disease, initial BGV and the age of new-onset disease were determined for enrolled animals. We found no significant difference in these initial disease characteristics between treatment groups or across centers (Supplementary Fig. 1). Thus, neither the baseline age nor the BGV of new-onset NOD mice were considered to bias study results.
Relative to isotype control-treated groups, anti-CD3 treatment combined with isotype control mAb led to significant early and sometimes sustained disease reversal in a proportion of animals at all sites (P < 0.05 for each site; Fig. 1). Interestingly, although low-dose anti-CD3 treatment resulted in significant disease reversal, this effect generally decayed over the 60-day observation period with a gradual reversion to hyperglycemia. This gradual decline in disease reversal is readily apparent when viewing the pooled composite data of anti-CD3–treated animals from all sites (Fig. 2B and Supplementary Fig. 2). In contrast, anti–IL-1β mAb treatment without anti-CD3 mAb did not result in significant disease reversal at any site (Supplementary Fig. 3). That is, although exogenous insulin therapy alone via an implanted insulin pellet resulted in transient reversion from hyperglycemia in most animals, neither early nor delayed anti–IL-1β mAb treatment without anti-CD3 led to prolonged disease reversal at any treatment site, consistent with previous results (15).
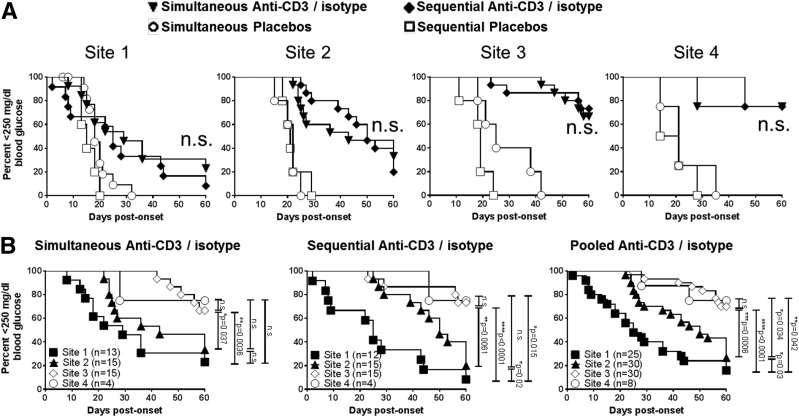
Figure 1.
Intrasite consistency (A) but intersite variability (B) of diabetes incidence with anti-CD3 treatment with simultaneous or sequential administration of isotype control antibody. A: Diabetes incidence in mice treated with anti-CD3 F(ab)2 (5 µg/day) plus isotype control mAb (control for anti–IL-1β) plus adjunct insulin treatment. Control mAb is given simultaneously with anti-CD3 (n = 13 for site 1, n = 15 for site 2, n = 15 for site 3, and n = 4 for site 4) or is sequential (n = 12 for site 1, n = 15 for site 2, n = 15 for site 3, and n = 4 for site 4). B: Variation in diabetes incidence for mice treated with anti-CD3 (5 µg/day) plus isotype control mAb treatment across the four research sites: site 1, site 2, site 3, and site 4. Results from site 2 are significantly different from those of site 3 (P = 0.0006) and site 4 (P = 0.034). Results from sites 3 and 4 do not differ from each other (P = not significant [n.s.]). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
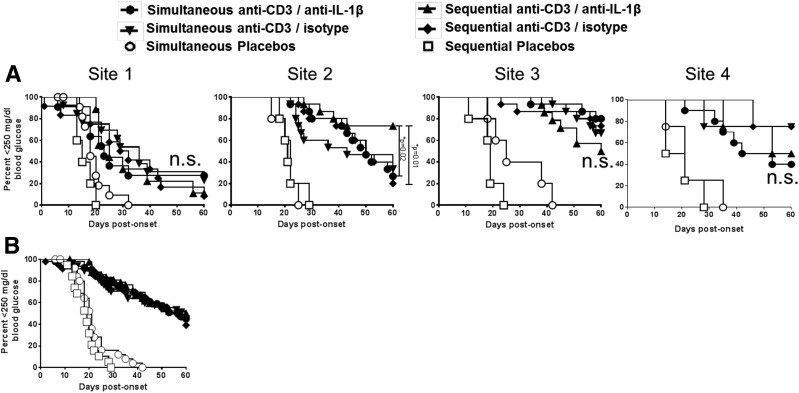
Figure 2.
Addition of anti–IL-1β mAb treatment does little to improve the effects of anti-CD3 therapy. A: New-onset NOD mice received simultaneous (n = 11 for site 1, n = 15 for site 2, n = 15 for site 3, and n = 10 for site 4) or sequential (n = 9 for site 1, n = 15 for site 2, n = 14 for site 3, and n = 4 for site 4) anti–IL-1β mAb (75 µg/day) relative to anti-CD3 therapy (5 µg/day). B: Pooled diabetes incidence with simultaneous or sequential anti–IL-1β mAb relative to anti-CD3 therapy for all four sites. At sites 1, 3, and 4 combined treatment did not differ in rates of disease reversal from animal treatment combined, simultaneous, or sequential anti-CD3 plus isotype control mAb (P = not significant [n.s.]). At site 2, anti-CD3 plus sequential anti–IL-1β mAb treatment showed significantly improved disease reversal relative to simultaneous anti-CD3/anti–IL-1β mAb (P = 0.02) or to sequential anti-CD3/isotype control mAb (P = 0.01). *P < 0.05.
Intrasite Consistency but Intersite Variability of Anti-CD3 Treatment With Simultaneous or Sequential Administration of Isotype Antibody
Despite considerable effort to generate study uniformity, we nevertheless saw surprising variability in treatment outcomes among the centers (Fig. 2). For example, although all sites demonstrated a clear benefit of anti-CD3 treatment for achieving disease reversal (P < 0.05 for all sites), the actual frequency and duration of successful reversion to euglycemia was greater in sites 2 and 3 relative to site 1 (Fig. 2), regardless of the timing of isotype control antibodies (P < 0.03 in all cases).
IL-1β Blockade by Anti–IL-1β mAb Does Not Improve the Efficacy of Anti-CD3 Treatment
Importantly, despite differences in the rate of disease reversal induced by anti-CD3 found between treatment sites, three of the four participating centers failed to detect significant improvement in disease reversal using combined anti-CD3 plus anti–IL-1β mAb therapy relative to anti-CD3 treatment alone (Fig. 2A). At sites 1, 3, and 4 neither simultaneous nor delayed addition of anti–IL-1β mAb improved rates of disease reversal from relative to anti-CD3 plus isotype control mAb (P = NS). At site 2, however, anti-CD3 plus sequential anti–IL-1β mAb treatment showed significantly improved disease reversal relative to simultaneous anti-CD3 plus anti–IL-1β mAb (P = 0.02) or to sequential anti-CD3/isotype control mAb (P = 0.01). Nevertheless, results of composite data pooled from all centers indicate a clear distinction in the rate of disease reversal based only on the presence or absence of anti-CD3 treatment, whether or not anti–IL-1β mAb is added (Supplementary Fig. 2).
Effect of Insulin Therapy on Disease Reversal
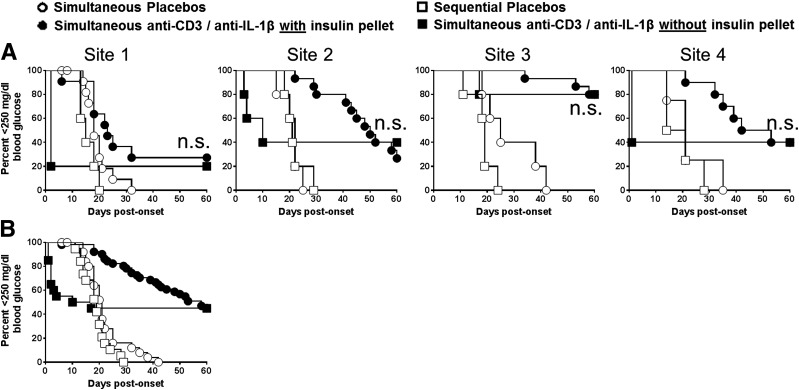
Another important issue is whether exogenous insulin therapy given to relieve potential hyperglycemic-related islet injury (16) improved the short- or longer-term efficacy of immunotherapies. This was tested in animals receiving simultaneous anti-CD3 and anti–IL-β mAb, with or without insulin therapy (groups 1 and 2). Early exogenous insulin treatment greatly improved the initial success of achieving disease reversal at sites 1, 2, and 4 (Fig. 3A). However, this benefit of insulin treatment waned over time. That is, none of the performing sites observed differences in long-term (60 days) disease reversal, with or without early insulin treatment (Fig. 3A), as was also reflected in the pooled data from all sites (Fig. 3B and Supplementary Fig. 2).
Figure 3.
Use of insulin pellets does not improve outcomes with anti-CD3 plus anti–IL-1β treatment. Adjunct insulin therapy results in improved early but not long-term disease reversal with simultaneous anti-CD3 (5 µg/day)/anti–IL-1β mAb (75 µg/day) therapy. A: Diabetes incidence for mice that did and did not receive an insulin pellet (n = 5 for all sites). Results from sites 1–4 show that early disease reversal was significantly improved at sites 1, 2, and 4 (P < 0.05) but that longer-term disease reversal (≥60 days) was not different at any site (P = not significant [n.s.]). B: Pooled composite data from sites 1–4 show significant initial (P > 0.002) but not late (≥60 days; P = n.s.) disease reversal using adjunct insulin treatment.
Study 2: Pilot Study of Anti-CD3 Plus IL-1trap (Rilonacept) Treatment
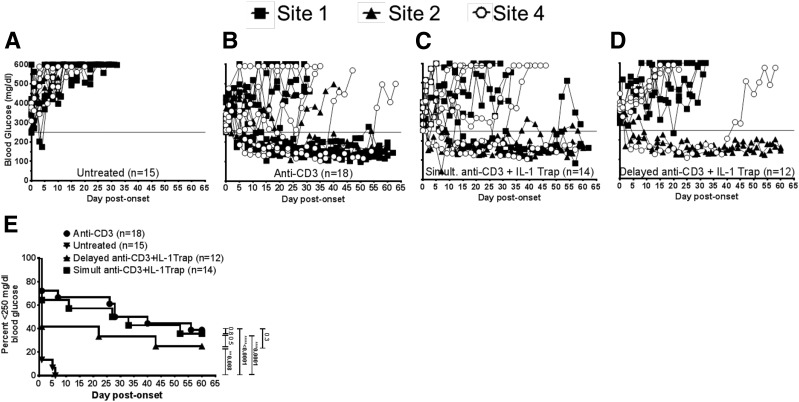
Given the unexpected inability of anti–IL-1β mAb therapy to improve the efficacy of anti-CD3 treatment, a second trial was conducted to determine if a different approach to IL-1 blockade would improve disease reversal. In this case, we used the IL-1 receptor fusion protein IL-1trap (17) as an agent to inhibit IL-1 in vivo in new-onset NOD mice. However, in this second trial, we opted to perform a more targeted pilot study approach in which we focused on the key question of whether the addition of simultaneous or delayed IL-1trap treatment to anti-CD3 therapy would improve the rate of disease reversal in new-onset NOD mice relative to anti-CD3 treatment alone. Three sites (1, 2, and 4) performed this second trial (Fig. 4). Results from this pilot study indicated that neither simultaneous (Fig. 4C) nor delayed (Fig. 4D) IL-1trap treatment improved the rate of disease reversal relative to anti-CD3 alone at any site (Fig. 4B). Although all treatment groups showed significant disease reversal relative to untreated control animals (P < 0.04 for all groups), neither simultaneous nor delayed IL-1trap treatment improved disease reversal relative to anti-CD3 alone (P = NS). Again, although results showed intersite variability, composite results clearly indicated that IL-1trap did not improve disease reversal relative to anti-CD3 treatment alone (Fig. 4E).
Figure 4.
Combination of anti-CD3 and IL-1trap is not more efficacious than anti-CD3 alone for diabetes reversal. Results of pilot study using combined anti-CD3 therapy (5 µg/day) plus IL-1trap (60 mg/kg) in reversing new-onset disease in NOD mice. Individual animal BGV readings are shown from sites 1, 2, and 4 (sample size for each group is small and does not lend itself to survival curves). A: Untreated new-onset NOD mice (n = 15). B: Anti-CD3 alone (n = 18). C: Simultaneous anti-CD3 plus IL-1trap treatment (n = 14). D: Anti-CD3 plus delayed IL-1trap treatment (n = 12). E: Pooled data show the frequency of disease reversal in untreated, anti-CD3 alone, simultaneous anti-CD3 plus IL-1trap treatment, and anti-CD3 plus delayed IL-1trap treatment. **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
We established a multicenter, preclinical consortium to test the efficacy of and intersite reproducibility of candidate combinations of therapeutic agents for their ability to reverse new-onset disease in the NOD mouse model of T1D. Importantly, this group used joint SOP methodologies and shared therapeutic agents and oversight to perform robust, prospective animal studies (18). A key translational goal of this program is to scrutinize clinically applicable therapeutic agents in the NOD model to guide clinical trial design for the preservation of islet β-cell mass in patients with new-onset T1D. Our initial goal was to determine if the timing of IL-1 blockade relative to anti-CD3 therapy had an effect on the efficacy of reversing disease with this combined therapeutic approach. This issue has direct practical relevance for guiding clinical trials that might be performed using a combination of agents. For example, studies in NOD mice indicate that adjunct rapamycin treatment can impair (19) or improve (20) the efficacy of anti-CD3 treatment, depending on the timing of rapamycin treatment relative to anti-CD3 therapy. Although significant reversal of disease occurred with anti-CD3 mAb at all sites, we found limited improvement with additional anti–IL-1β mAb treatment, with only one treatment group at one site suggesting efficacy for this combination. This latter result is significant and illustrates the potential bias toward positive results that has been a significant concern raised in a number of commentaries regarding smaller, single-center small-animal studies (2,4,6). Interestingly, site 2 found that delayed (but not simultaneous) anti–IL-1β mAb could improve disease reversal; however, this was not observed at other sites (Supplementary Fig. 1). Thus, one subgroup suggesting a positive effect of adjunct anti–IL-1β with anti-CD3 was tempered by the results from a larger sample size and multiple performance sites.
Several noteworthy features of the study design differ from practices used in most preclinical studies in this model. We designed the study to involve robust sample sizes and multiple sites that used the same reagent lots and SOPs for designation of diabetes, drug dosing, and an animal enrollment scheme designed to ensure unbiased allotments to study groups. In total, data from these studies did not independently support the use of IL-1 blockade as an adjunct therapy to improve the efficacy of disease reversal achieved by anti-CD3. We must emphasize that although we used the same reagents used in a prior study combining anti-CD3 and anti–IL-1β in new-onset NOD mice (15), this study was not designed to directly replicate these earlier results. Rather, we intentionally reduced anti-CD3 dosing to alleviate potential dose-related toxicity and to increase the likelihood of achieving synergism with additional IL-1 blockade. We also varied the timing of anti–IL-1β mAb relative to anti-CD3 treatment as a means of potentially guiding the development of future clinical studies. Importantly, if subtle differences in the treatment regimens using the same agents between studies can have a major effect on disease reversal in the NOD mouse model, this issue should arguably create pause in proceeding with clinical trials without further preclinical testing.
Finally, although anti-CD3 mAb alone consistently showed efficacy at all sites, there was nevertheless a substantial variability in the rate of successful disease reversal between sites, with or without IL-1 blockade. This was not due to any detectable differences among the sites in the initial age or degree of hyperglycemia in new-onset NOD mice (Supplementary Fig. 1). At present, we cannot identify specific factors that influenced intersite variability. However, some potential differences among the sites may include subtle differences between NOD sublines (e.g., between the NOD/ShiLtJ and NOD/Bdc lines) and housing or diet conditions with a subsequent effect on disease pathogenesis, such as potential differences in the NOD colony microbiome (21,22). Similar variability in outcomes has been observed in other animal models of human disease (23). However, despite some intersite variability, we did not note significant differences in outcome trends among the centers. That is, anti-CD3 alone was significantly effective at reversing disease at all sites (albeit to differing degrees), and adjunct IL-1 blockade provided little or no additional benefit at any site. In our view, the variability in results found among the sites enhances the rationale for this type of multicenter, preclinical approach to testing therapeutics in NOD mice. We would assert that validating results among sites enhances the confidence in a specific study in NOD mice and greatly lessens the likelihood of a false-positive (or -negative) outcome based on a small, single-center study.
Supplementary Material
Article Information
Acknowledgments. The authors thank Dr. Hermann Gram (Novartis) and Dr. Desper Gromada (Regeneron) for kindly providing anti–IL-1β mAb and IL-1trap reagents, respectively. We also thank Dr. Richard Insel (JDRF) for careful review of the manuscript and Dr. Katarzyna Bourcier (NIAID), Dr. James McNamara (NIAID), Dr. Julia Greenstein (JDRF), and Barbara Droker (BRI) for helpful discussion.
Funding. Research reported in this article was performed as a project of the ITN-JDRF Type 1 Diabetes Preclinical Consortium supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number UM1AI109565 and JDRF grant 9-2012-22 and the National Institute of Diabetes and Digestive and Kidney Diseases.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.G.G., M.A.A., K.C.H., and M.v.H. designed studies and prepared and reviewed the manuscript. P.P.P. performed studies, prepared data, and prepared the manuscript. T.K. researched and prepared data and reviewed and edited the manuscript. C.H.W. designed studies and prepared data and reviewed and edited the manuscript. S.D., A.P., Y.M., and A.B.H. researched and prepared data. L.S. was involved in project management and data analysis. P.B. organized data and reviewed and edited the manuscript. T.S. designed studies and contributed to discussion. M.R.E. designed studies and reviewed the manuscript. G.T.N. designed studies and reviewed and edited the manuscript. R.G.G., M.A.A., K.C.H., and M.v.H. are joint guarantors of this study and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-0492/-/DC1.
See accompanying article, p. 1161.
References
- 1.Matthews JB, Staeva TP, Bernstein PL, Peakman M, von Herrath M; ITN-JDRF Type 1 Diabetes Combination Therapy Assessment Group . Developing combination immunotherapies for type 1 diabetes: recommendations from the ITN-JDRF Type 1 Diabetes Combination Therapy Assessment Group. Clin Exp Immunol 2010;160:176–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jucker M. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat Med 2010;16:1210–1214 [DOI] [PubMed] [Google Scholar]
- 3.Atkinson MA. Evaluating preclinical efficacy. Sci Transl Med 2011;3:96cm22. [DOI] [PubMed] [Google Scholar]
- 4.Landis SC, Amara SG, Asadullah K, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 2012;490:187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peers IS, South MC, Ceuppens PR, Bright JD, Pilling E. Can you trust your animal study data? Nat Rev Drug Discov 2014;13:560. [DOI] [PubMed] [Google Scholar]
- 6.Steward O, Popovich PG, Dietrich WD, Kleitman N. Replication and reproducibility in spinal cord injury research. Exp Neurol 2012;233:597–605 [DOI] [PubMed] [Google Scholar]
- 7.Herold KC, Bluestone JA, Montag AG, et al. Prevention of autoimmune diabetes with nonactivating anti-CD3 monoclonal antibody. Diabetes 1992;41:385–391 [DOI] [PubMed] [Google Scholar]
- 8.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A 1994;91:123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu CY, Rodriguez-Pinto D, Du W, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest 2007;117:3857–3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 [DOI] [PubMed] [Google Scholar]
- 11.Herold KC, Gitelman SE, Ehlers MR, et al. ; AbATE Study Team . Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 2013;62:3766–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Type 1 Diabetes TrialNet Anti-CD20 Study Group . Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 2009;361:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabinovitch A, Suarez-Pinzon WL, Shapiro AM, Rajotte RV, Power R. Combination therapy with sirolimus and interleukin-2 prevents spontaneous and recurrent autoimmune diabetes in NOD mice. Diabetes 2002;51:638–645 [DOI] [PubMed] [Google Scholar]
- 14.Long SA, Rieck M, Sanda S, et al. Diabetes TrialNet and the Immune Tolerance Network . Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes 2012;61:2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ablamunits V, Henegariu O, Hansen JB, et al. Synergistic reversal of type 1 diabetes in NOD mice with anti-CD3 and interleukin-1 blockade: evidence of improved immune regulation. Diabetes 2012;61:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atkinson MA, Maclaren NK, Luchetta R. Insulitis and diabetes in NOD mice reduced by prophylactic insulin therapy. Diabetes 1990;39:933–937 [DOI] [PubMed] [Google Scholar]
- 17.Economides AN, Carpenter LR, Rudge JS, et al. Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nat Med 2003;9:47–52 [DOI] [PubMed] [Google Scholar]
- 18.Grant CW, Moran-Paul CM, Duclos SK, Guberski DL, Arreaza-Rubín G, Spain LM. Testing agents for prevention or reversal of type 1 diabetes in rodents. PLoS One 2013;8:e7w989 [DOI] [PMC free article] [PubMed]
- 19.Valle A, Jofra T, Stabilini A, Atkinson M, Roncarolo MG, Battaglia M. Rapamycin prevents and breaks the anti-CD3-induced tolerance in NOD mice. Diabetes 2009;58:875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perl S, Perlman J, Weitzel RP, Phang O, Hsieh MM, Tisdale J. Addition of rapamycin to anti-CD3 antibody improves long-term glycaemia control in diabetic NOD mice. PLoS One 2013;8:e67189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen L, Ley RE, Volchkov PY, et al. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature 2008;455:1109–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkinson MA, Chervonsky A. Does the gut microbiota have a role in type 1 diabetes? Early evidence from humans and animal models of the disease. Diabetologia 2012;55:2868–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker D, Amor S. Experimental autoimmune encephalomyelitis is a good model of multiple sclerosis if used wisely. Mult Scler Relat Disord 2014;3:555–564 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.