Abstract
Data indicate that the prevalence of autism spectrum disorder (ASD) may be increasing and that it varies geographically. We investigated associations between residential location and ASD in the children of Nurses’ Health Study II (United States) participants in order to generate hypotheses about social and environmental factors related to etiology or diagnosis. Analyses included data on 13,507 children born during 1989–1999 (486 with ASD). We explored relationships between ASD and residential location both at birth and at age 6 years (i.e., closer to average age at diagnosis). Generalized additive models were used to predict ASD odds across the United States. Children born in New England were 50% more likely to be diagnosed with ASD compared with children born elsewhere in the United States. Patterns were not explained by geographic variation in maternal age, birth year, child's sex, community income, or prenatal exposure to hazardous air pollutants, indicating that spatial variation is not attributable to these factors. Using the residential address at age 6 years produced similar results; however, areas of significantly decreased ASD odds were observed in the Southeast, where children were half as likely to have ASD. These results may indicate that diagnostic factors are driving spatial patterns; however, we cannot rule out the possibility that other environmental factors are influencing distributions.
Keywords: autism spectrum disorder, geographic variation, Nurses’ Health Study II
The prevalence of autism spectrum disorder (ASD) has increased dramatically over the past 2 decades in the United States and in other parts of the world (1). Changes in diagnosis (e.g., changes in diagnostic criteria (2), increases in ASD awareness (2, 3), and availability of diagnostic resources (4, 5)) and exposure to environmental contaminants (6–17) have been suggested as contributors to increased prevalence, yet data on the association between ASD and many of these factors remain sparse.
Investigating geographic patterns of ASD prevalence may help identify diagnostic and environmental factors associated with prevalence. A growing number of spatially based analyses have used geographic variation in ASD to generate hypotheses about drivers of prevalence at local and state levels (4, 18–22). For example, Mazumdar et al. (4) reported that living in areas of California with greater diagnostic resources increased a child's odds of being diagnosed with ASD. Here we built on previous work and explore geographic differences in ASD risk across the United States. We used data from the Nurses’ Health Study II (NHSII) cohort, which includes nearly 35,000 children born from 1989 to 1999 (9). We investigated variation at 2 time periods, birth and age 6 years (i.e., closer to the age of diagnosis), to assess potential etiologic and diagnostic differences. We also investigated whether factors previously associated with ASD risk or diagnoses, such as air pollution or socioeconomic status, explain geographic differences.
METHODS
Study population
The NHSII is a cohort of 116,430 female nurses, established in 1989 and followed biennially to the present. The original cohort included nurses from 14 states, but over time nurses moved throughout the United States. Our sample included children born to nurses in all 48 contiguous states.
Nurses completed biennial questionnaires on their health as well as their children's health. In the 2005 and 2009 questionnaires, nurses were asked if any of their children had ever been diagnosed with ASD. Those mothers reporting a child with ASD on the 2005 questionnaire were mailed a follow-up questionnaire to confirm the child's diagnosis. We validated mothers’ reports of ASD by administering the Autism Diagnostic Interview–Revised (ADI-R) to a random subset of 50 children with ASD; 43 (86%) met full criteria for autistic disorder by this assessment; the rest missed by 1 point on 1 domain (n = 6) or met criteria on 1 domain and narrowly missed cut-offs for other domains (n = 1) (23).
A total of 586 children born 1989–1999 were reported to have ASD diagnoses. Of these, additional follow-up data were available for 262. Based on these data, we excluded 4 children for whom ASD was not confirmed by the mother, 2 reported to have genetic syndromes associated with ASD (Down syndrome and Rett syndrome), and 16 who were not reported to have ASD on the 2009 questionnaire. All remaining children that were reported to have ASD, but for whom we did not have follow-up questionnaire information (n = 324), were considered to have an ASD diagnosis. We additionally excluded 44 children who were members of multiple births or who had gestation <5 months and another 11 who were missing geocodable addresses in the year of birth (e.g., a post-office box or a rural route number) or because US Census information was not available for their census tract, leaving 509 children with ASD. Latitude and longitude data (i.e., geocodes), as well as complete data for covariates of interest, were available for 32,806 children without ASD (>95% of 34,455 children without ASD). Of the 33,315 total children eligible for inclusion in the birth address analyses, 33,242 also had geocodable addresses at age 6 years (99.8% of noncases and 100% of children with ASD).
Completion and return of questionnaires constituted implied consent. This work with the NHSII data was reviewed and approved by all relevant institutional review boards.
Spatial analyses
The primary goal of the spatial analysis was to determine whether the residential location at birth was associated with ASD. Mailing address at birth was obtained from the biennial NHSII questionnaire and was used as a proxy for residential location at birth. Children born from 1989 through 1990 were assigned the mailing address of their mother in 1989 (the first year of study). Children born in 1991 or 1992 were assigned their mother's mailing address in 1991, and births from 1993 through 1999 were assigned their mother's addresses, updated every other year. To determine the association with residential address more proximal to the time of diagnosis and ASD, we also investigated spatial patterns using the address at age 6 years. Address at age 6 years was chosen because children are generally diagnosed with ASD by age 6 years and because the actual age at the time of diagnosis was available only for a subset of cases.
We estimated ASD odds using a generalized additive model (also referred to as GAM), a form of nonparametric or semiparametric regression that can analyze binary outcome data while adjusting for covariates (24–26). Here, we modeled location using a bivariate smoothing of latitude and longitude (24, 25). We used locally weighted scatterplot smoothing, which adapts to changes in data density likely to occur in analyses of residential locations. The locally weighted scatterplot smoothing used data from nearby data points to predict the odds of ASD. The region or neighborhood from which data were drawn to predict prevalence was based on the percentage of data points in the neighborhood (the span size). Choice of span size is a trade-off between bias and variability. A larger span size includes more data and produces a flatter surface with low variability but increased bias, while a small span size results in high variability and comparatively low bias (26). We determined the optimal smoothing amount (i.e., optimal span size) by minimizing the Akaike information criterion (24–26).
To create a continuous surface of risk, we used an evenly spaced grid of points 12 km apart covering the continental United States. We restricted the grid in the northeast to where the data density was greater than 1 child per 100-km radius (27). At each point, we predicted the log odds of ASD and calculated odds ratios using the entire study area as the referent (i.e., odds at each point were divided by odds from a reduced model that omitted the latitude and longitude smoothing). Statistical analyses and mapping were conducted using R Package 3.3.0 (R Foundation for Statistical Computing, Vienna, Austria) and the MapGAM library (28).
Because our study cohort included siblings and census-tract covariates, including all the eligible data could induce spatial clustering due to familial (i.e., genetic) similarities in addition to geographically linked factors. To account for this, we sampled from our eligible cases and noncases 1 child per group using the sampcont function in the MapGAM library. Generalized additive models were then fitted to the independent, individual-level case-control data using inverse probability weighting (28). A total of 13,507 children (486 with ASD) were included in these analyses. The MapGAM library also allows for calculation of confidence intervals for the point estimates of the risk map. Geographic areas where the confidence interval excludes 1 are indicated on the point estimate map with black contour lines. The absence of such contours indicates that the odds ratios point estimates were not statistically significant (24). Maps of lower and upper confidence interval were also produced using the standard errors from the spatial model.
Covariates
We assessed spatial confounding by risk factors for ASD diagnosis by including each variable individually in a spatial model. An unadjusted model of location only provides an estimate of the underlying risk of ASD diagnosis (Web Figure 1, available at https://academic.oup.com/aje). Variables that did not change the underlying risk pattern by >10% were not included in the final models. For adjusted analyses of birth address and age-6 address, we considered and retained maternal age at child's birth (<35 years, ≥35 years), which has frequently been associated with increased ASD risk (29–31), and birth year to account for temporal trends in ASD diagnosis. We also adjusted for child's sex (male, female, missing), a strong predictor of ASD that changes the underlying risk (Web Figure 2) and is shown to vary spatially in this cohort (Web Figure 3). Sex of the child was missing for 3,326 (10.1%) children. To capture potential differences in the odds of ASD by socioeconomic factors, we adjusted analyses for the median household income of the census tract (assigned separately for each address and modeled categorically by quartile). In addition, we considered race, census-tract percentage with a college education, and educational attainment of the mother's parents for birth and age-6 analyses because they might be related to both etiology and likelihood of diagnostic services. For the birth analyses only, we considered birth weight, gestational diabetes, and preeclampsia, but these additional variables did not change the underlying risk pattern by >10% (see Web Figure 4, adjustment for gestational diabetes), nor did they vary spatially in similar patterns to ASD risk (see Web Figure 5, geographic distribution of gestational diabetes) and thus were not included as spatial confounders in final models.
We investigated further potential spatial confounding by perinatal exposure to hazardous air pollutants (HAPs), which has been associated with the odds of ASD in this population previously; methods for assigning HAP exposure have been discussed previously (9). Briefly, data were obtained from the US EPA National Air Toxics Assessments in 1990, 1996, 1999, and 2002 (32). Air pollution concentrations were linked to nurses’ residential census tract at the time of the child's birth. We focused on diesel, lead, manganese, cadmium, and a summary measure of metals (lead, arsenic, cadmium, manganese, nickel, mercury, chromium, antimony) as potential spatial confounders (modeled categorically by tertile), because these were associated with ASD in previous analyses (9). Because HAPs exposures are correlated, we entered each compound into a separate model.
Sensitivity analyses
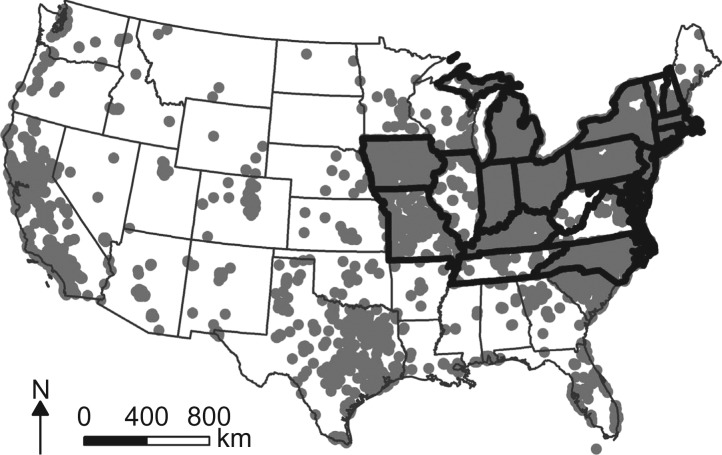
Although data were available for participants living in nearly every state, the original study design included participants geographically concentrated in the Northeast United States. Because of potential unreliability of estimates in areas of low data density, we performed a restricted analysis that included only children born in the 22 spatially contiguous states in the northeast quadrant of the United States (see Figure 1). In these states, there were 389 children with ASD and 25,036 children without ASD eligible for our spatial case-control analysis.
Figure 1.
Residential location at the time of birth for 33,315 children born to women participating in Nurses’ Health Study II, United States, 1989–1999. Thick borders indicate states that were included in the Northeast analyses.
RESULTS
Selected characteristics of our study population are displayed in Table 1. The stratified sample is comparable to the eligible population, although slightly less likely to be male. The geographic distribution of birth locations for each participant is displayed in Figure 1. To preserve confidentiality, the figure was created by randomly placing residences within a small grid that includes the actual location; however, actual locations were used in analyses.
Table 1.
Selected Characteristics According to Diagnosis Status (Autism Spectrum Disorder) of Children Born to Women Participating in Nurses’ Health Study II, United States, 1989–1999
| Characteristic | Eligible Participants | Analytical Sample | ||||||
|---|---|---|---|---|---|---|---|---|
| Cases (n = 509) | Noncases (n = 32,806) | Cases (n = 486) | Controls (n = 12,021) | |||||
| No. of Children | % | No. of Children | % | No. of Children | % | No. of Children | % | |
| Male sex | 384 | 81.0 | 15,007 | 50.84 | 367 | 75.51 | 5,877 | 45.13 |
| Census-tract income quartile, $a | ||||||||
| Quartile 1: ≤52,316 | 108 | 21.2 | 8,221 | 25.1 | 92 | 18.9 | 3,285 | 25.2 |
| Quartile 2: 52,317–66,042 | 108 | 21.2 | 8,221 | 25.1 | 103 | 21.2 | 3,275 | 25.2 |
| Quartile 3: 66,043–82,955 | 148 | 29.1 | 8,183 | 24.9 | 139 | 28.6 | 3,240 | 24.9 |
| Quartile 4: >82,955 | 145 | 28.5 | 8,181 | 24.9 | 152 | 31.3 | 3,221 | 24.7 |
| Maternal age at child's birth, years | ||||||||
| <35 | 295 | 58.0 | 22,754 | 69.4 | 277 | 57 | 8,664 | 66.5 |
| ≥35 | 214 | 42.0 | 10,052 | 30.6 | 209 | 43 | 4,357 | 33.5 |
| Birth year | ||||||||
| 1989–1990 | 133 | 26.1 | 10,838 | 33.0 | 129 | 26.5 | 4,975 | 38.2 |
| 1991–1992 | 125 | 24.6 | 8,582 | 26.2 | 115 | 23.7 | 3,358 | 25.8 |
| 1993–1994 | 96 | 18.9 | 6,125 | 18.7 | 94 | 19.3 | 2,106 | 16.2 |
| 1995–1996 | 84 | 16.5 | 3,997 | 12.2 | 80 | 16.5 | 1,347 | 10.3 |
| 1997–1999 | 71 | 13.9 | 3,264 | 9.9 | 68 | 14.0 | 1,235 | 9.5 |
a Median income of birth-address census tract.
Residential location and ASD in the United States
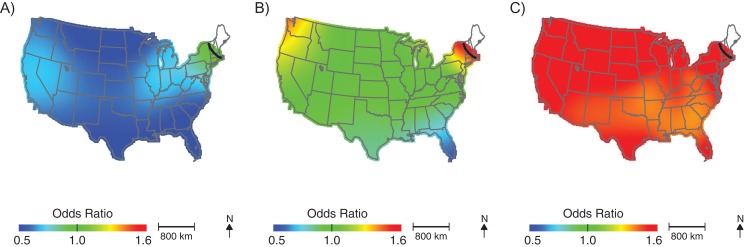
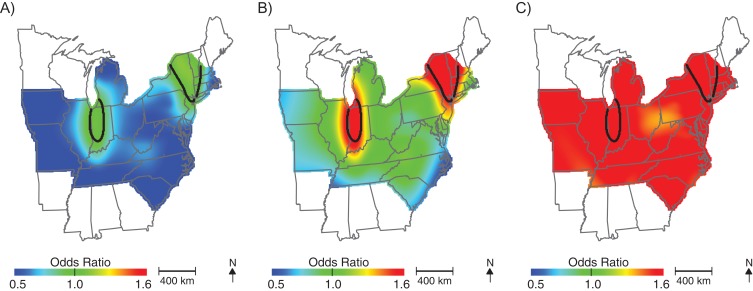
After adjusting for the child's sex, maternal age, birth year, and median census-tract income, we observed elevated ASD odds among children born in New England (Figure 2; Table 2; optimal span size = 0.70). Children born in this area were approximately 1.5 times more likely to be diagnosed with ASD than were children born in the study area as a whole. Conversely, children born in the southeast United States had lower odds of being diagnosed with ASD, although differences were not statistically significant. Unadjusted analyses produced very similar maps using the same span = 0.70, suggesting that spatial confounding by child's sex, maternal age, and median census-tract income are not driving geographic patterns in ASD odds in the northeast (Figure 3). Results were materially unchanged when we adjusted for perinatal exposure to HAPs (i.e., lead, diesel, manganese, cadmium, and a summary measure of 8 metals; results not shown).
Figure 2.
Geographic distribution of the risk of diagnosis with autism spectrum disorder at birth addresses across the continental United States for children born to women participating in Nurses’ Health Study II, 1989–1999. The figure shows lower confidence estimates (A), point estimates (B), and upper confidence estimates (C), adjusted for child's sex, mother's age at child's birth, birth year, and census-tract median income, using optimal span size of 0.70. Black contour bands indicate statistically significant areas of increased or decreased risk.
Table 2.
Summary Information for Adjusteda Spatial Models in Each Analysis Observed Across the Study Area Among Children Born to Women Participating in Nurses’ Health Study II, United States, 1989–1999
| Analysis | No. of Cases | No. of Controls | Optimal Span | OR Range |
|---|---|---|---|---|
| Birth address | ||||
| Continental United States | 486 | 13,021 | 0.70 | 0.55–1.63 |
| Northeastern United States only | 375 | 9,755 | 0.35 | 0.46–3.03 |
| Address at age 6 years | ||||
| Continental United States | 486 | 12,983 | 0.65 | 0.23–1.69 |
| Northeastern United States only | 363 | 9,164 | 0.35 | 0.51–1.69 |
Abbreviation: OR, odds ratio.
a Adjusted for mother's age, child's sex, birth year, and census-tract median income.
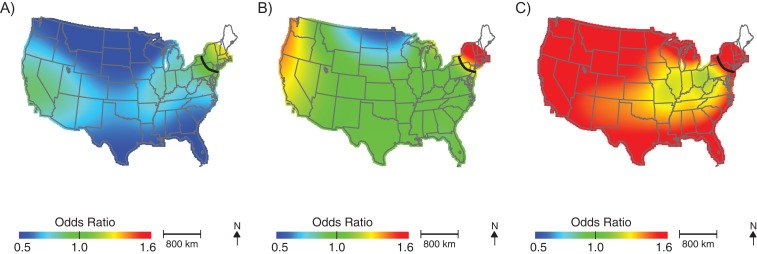
Figure 3.
Geographic distribution of the risk of diagnosis with autism spectrum disorder at birth addresses across the continental United States for children born to women participating in Nurses’ Health Study II, 1989–1999. The figure shows lower confidence estimates (A), point estimates (B), and upper confidence estimates (C), unadjusted, using span size of 0.70. Black contour bands indicate statistically significant areas of increased or decreased risk.
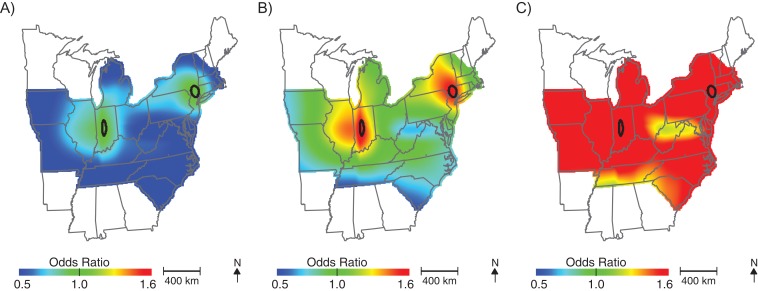
More than half of the children in our cohort (51.6%) moved between birth and age 6 years. Most families (66.6%) moved <25 km; however, approximately 22.6% of families moved >100 km between birth and age 6 years. The percentage of families that moved was similar among cases and noncases (49.9% and 51.6% moved, respectively); however, cases tended to move slightly further than controls (median move = 9.8 km for noncases and 13.1 km for children with ASD). Analyses using addresses at age 6 years produced similar geographic patterns to those using birth addresses (Figure 4, Table 2, optimal span size = 0.65); children living in New England at age 6 years had elevated odds of ASD diagnosis. However, we also observed significantly lower odds of being diagnosed with ASD for children living in the southeast United States at 6 years of age; children in these areas were approximately half as likely to be diagnosed with ASD compared with the study population as a whole. The odds of ASD also appeared elevated in the Pacific Northwest but were not statistically significant. As in the birth address analyses, adjusting for potential spatial confounding by demographic characteristics and perinatal exposure to HAPs did not meaningfully change the results (not shown).
Figure 4.
Geographic distribution of the risk of diagnosis with autism spectrum disorder at residential addresses when the child was 6 years of age, across the continental United States for children born to women participating in Nurses’ Health Study II, 1989–1999. The figure shows lower confidence estimates (A), point estimates (B), and upper confidence estimates (C), adjusted for child's sex, mother's age at child's birth, birth year, and census-tract median income, using optimal span size of 0.65. Black contour bands indicate statistically significant areas of increased or decreased risk.
Sensitivity analyses
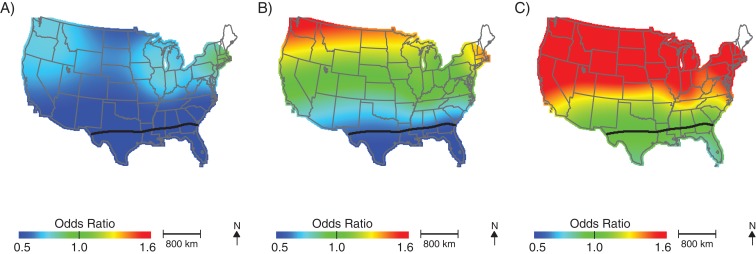
Because of the potential unreliability of estimates in areas of low data density, we performed a restricted analysis that included only children born in the northeastern quadrant of the United States. Spatial patterns were similar to those of the United States as a whole. However, analyses suggested greater spatial variation in these states, as evidenced by the smaller optimal span size of 0.35 and the increased range of predicted odds ratios. As in the countrywide analysis, odds of ASD were significantly elevated for children born in New England (Figure 5; Table 2, optimal span size = 0.35), and the restricted analysis further revealed elevated odds of ASD for children born in portions of Indiana. Again, patterns using age-6 addresses were very similar (Figure 6; Table 2, optimal span size = 0.35).
Figure 5.
Geographic distribution of the risk of diagnosis with autism spectrum disorder at the birth address in the northeastern United States for children born to women participating in Nurses’ Health Study II, 1989–1999. The figure shows lower confidence estimates (A), point estimates (B), and upper confidence estimates (C), adjusted for child's sex, mother's age at child's birth, birth year, and census-tract median income, using optimal span size of 0.35. Black contour bands indicate statistically significant areas of increased or decreased risk.
Figure 6.
Geographic distribution of the risk of diagnosis with autism spectrum disorder at the age-6-years address in the northeastern United States for children born to women participating in Nurses’ Health Study II, 1989–1999. The figure shows lower confidence estimates (A), point estimates (B), and upper confidence estimates (C), adjusted for child's sex, mother's age at child's birth, birth year, and census-tract median income, using optimal span size of 0.35. Black contour bands indicate statistically significant areas of increased or decreased risk.
DISCUSSION
Our analyses suggest that children of participants in NHSII have different odds of ASD diagnosis depending on where in the United States they live at the time of birth or at age 6 years. Children born in New England and Indiana had increased odds of ASD diagnosis, while living in the central and the southern United States was associated with lower odds of diagnosis. To our knowledge, our analyses are the first to investigate large-scale geographic differences while controlling for spatial confounding by individual-level factors (e.g., maternal age and child sex) and considering socioeconomic status and environmental exposures (i.e., HAPs). Although direct comparison is difficult due to differences in case ascertainment across studies and sites, our results are consistent with data from the Autism and Developmental Disability Monitoring Network (ADDM) sites, which reported lower ASD prevalence at its Alabama site and the highest prevalence at a New Jersey site (1).
Spatial variation in ASD appeared to be independent of several established risk and diagnostic factors (e.g., child's sex, year of birth, maternal age, census-tract median income), suggesting that these factors are not driving the patterns we observed and that other geographically distributed causal or diagnostic factors may be associated with ASD odds. Differences in perinatal exposure to air pollution provide a possible explanation for geographic variability, particularly as a growing number of studies have linked exposure to increased ASD risk (7–10, 15–17). However, in our data, adjusting for perinatal HAP exposure did not meaningfully change the spatial patterns. We cannot rule out potential measurement error in HAP models; however, these HAPs were found to be associated with ASD in the NHSII previously (9). It is possible that other types of geographically distributed exposure, such as particulate matter (15, 16), pesticides (12) or vitamin D (33), may contribute to patterns observed here, as these have been previously related to ASD or other relevant health outcomes such as ADHD. Although particulate matter–exposure data were available for a subset of our study population (n = 1,767), we did not have sufficient data to evaluate the role of this exposure on spatial patterns.
Spatial differences in social and political factors may also explain the geographic differences we observed (4). Although the diagnosis of ASD is ideally based on established Diagnostic and Statistical Manual of Mental Disorders criteria and made by a qualified provider, policies guiding the diagnosis of ASD and available diagnostic resources are likely to vary geographically and may have influenced the observed pattern of occurrence. For example, children may be classified as having ASD for the purposes of receiving special education services by schools (34). The criteria used for a school classification may vary considerably from state to state, and even within a state, across public school systems (34). In general, states where we observed higher prevalence have less restrictive criteria for ASD classification in school (34). Interestingly, we see elevated odds of ASD in Indiana, which was the first state to require insurance coverage of ASD services (Indiana Code § 27-8-14.2-1). Although we are unable to investigate the role of this provision in our cohort, the availability of insurance-covered ASD services in Indiana may contribute to observed elevated odds. It is important to recognize that our work was aimed at assessing large-scale geographic variation in ASD across the country. As such, we did not have the spatial resolution to assess small areas of increased risk. For example, it is unlikely that our analyses would have identified any specific communities with elevated ASD risk. Instead, we identify regions of increased or decreased risk. Previous research has shown that smaller-scale variation is likely (4, 19). For example, we previously reported geographic variation in surveillance-recognized ASD in North Carolina (19) and Mazumdar et al. (4) reported neighborhood-specific variation in ASD risk.
These spatial analyses have some potential limitations. Although data were available for children born throughout the United States, they were sparse in some regions (e.g., the Pacific Northwest). While generalized additive models may exhibit biased behavior at the edges of the data, our past work with synthetic data suggested little to no bias when locally weighted scatterplot smoothing is used (24, 25). Nonetheless, we are cautious in interpreting data in these regions. In the present analyses, we identified areas with statistically significant risk using confidence maps produced from standard errors, with areas that exclude odds ratios of 1, delineated by black contour lines. Although we controlled for several individual-level confounders, it is possible that observed patterns are the result of residual confounding. Our study population is relatively homogeneous, which may minimize the likelihood of confounding by demographic variables (e.g., maternal education and race); however, this may also limit generalizability to other populations. For example, 95% of women in our study population were white. It is not clear whether our findings would reflect spatial patterns among other racial groups. In addition, patterns may be more pronounced for groups that have greater obstacles to obtaining care than children of nurses. Our analyses are also limited by our reliance on maternal report of ASD diagnosis. In our past work, we conducted an assessment of the reliability of maternal ASD report and found good agreement between Autism Diagnostic Interview–Revised results and maternal report—as might be expected given that all of our mothers are nurses. However, we did not independently evaluate all of the children included in analyses, and some outcome misclassification is likely. Additionally, we did not have access to the address at the exact time of birth or diagnosis which could have resulted in some misclassification of exposure.
Although spatial assessments often focus on areas of increased risk, for conditions like ASD, which have variable diagnostic criteria, areas of decreased odds may be of greater public heath interest. It is possible that ASD is underdiagnosed in these areas. Identifying areas with decreased ASD prevalence may be helpful in recognizing regions that would benefit from additional ASD services. Areas of significant increased and decreased ASD risk are important for directing future research but should not be considered causal without further information.
Using the MapGAM library in R (R Foundation for Statistical Computing), we generated maps of maternally-reported ASD risk. We identified statistically significant areas of increased risk in the northeastern United States and Indiana, suggesting that children living in these regions may be at greater risk of ASD diagnosis than children living in other areas of the country. Conversely, children living in the South at age 6 years are significantly less likely to be diagnosed with ASD. Our results indicate that maternal age, child's sex, census-tract median income, and prenatal exposure to HAPs do not account for spatial patterns. Variation may be explained by spatial differences in ASD diagnosis or other uncontrolled environmental exposures. Further research is needed to understand geographic differences, both in terms of identifying risk factors and providing ASD services.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Environmental Sciences and Policy Division, Nicholas School of the Environment, Duke University, Durham, North Carolina (Kate Hoffman); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Marc G. Weisskopf, Francine Laden); Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Marc G. Weisskopf, Francine Laden, Raanan Raz, Jaime E. Hart); Department of Social and Behavioral Sciences, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (Andrea L. Roberts); Braun School of Public Health and Community Medicine, Hebrew University of Jerusalem, Jerusalem, Israel (Raanan Raz); Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Francine Laden, Jaime E. Hart); Modifiable Risk Factors Program, AJ Drexel Autism Institute, Drexel University, Philadelphia, Pennsylvania (Kristen Lyall); Department of Reading Education and Special Education, College of Education, Appalachian State University, Boone, North Carolina (Elin M. Hoffman); and Program in Public Health, College of Health Sciences, University of California Irvine, Irvine, California (Verónica M. Vieira).
This work was supported by the National Institute of Environmental Health Sciences (grants P42 ES007381 and P30 ES000002) and the National Cancer Institute (grant UM1 CA176726).
The contents of this work are solely the responsibility of the authors and do not necessarily represent the views of the National Institutes of Health.
Conflict of interest: none declared.
REFERENCES
- 1. Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63(2):1–21. [PubMed] [Google Scholar]
- 2. King M, Bearman P. Diagnostic change and the increased prevalence of autism. Int J Epidemiol. 2009;38(5):1224–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu KY, King M, Bearman PS. Social influence and the autism epidemic. AJS. 2010;115(5):1387–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mazumdar S, Winter A, Liu KY, et al. Spatial clusters of autism births and diagnoses point to contextual drivers of increased prevalence. Soc Sci Med. 2013;95:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalkbrenner AE, Daniels JL, Emch M, et al. Geographic access to health services and diagnosis with an autism spectrum disorder. Ann Epidemiol. 2011;21(4):304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Newschaffer CJ, Croen LA, Daniels J, et al. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 2007;28:235–258. [DOI] [PubMed] [Google Scholar]
- 7. Volk HE, Kerin T, Lurmann F, et al. Autism spectrum disorder: interaction of air pollution with the MET receptor tyrosine kinase gene. Epidemiology. 2014;25(1):44–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalkbrenner AE, Daniels JL, Chen JC, et al. Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8. Epidemiology. 2010;21(5):631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roberts AL, Lyall K, Hart JE, et al. Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ Health Perspect. 2013;121(8):978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Windham GC, Zhang L, Gunier R, et al. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco Bay Area. Environ Health Perspect. 2006;114(9):1438–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roberts EM, English PB, Grether JK, et al. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect. 2007;115(10):1482–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eskenazi B, Marks AR, Bradman A, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115(5):792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shelton JF, Geraghty EM, Tancredi DJ, et al. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Perspect. 2014;122(10):1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rasalam AD, Hailey H, Williams JH, et al. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev Med Child Neurol. 2005;47(8):551–555. [DOI] [PubMed] [Google Scholar]
- 15. Kalkbrenner AE, Windham GC, Serre ML, et al. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology. 2015;26(1):30–42. [DOI] [PubMed] [Google Scholar]
- 16. Raz R, Roberts AL, Lyall K, et al. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case-control analysis within the Nurses’ Health Study II Cohort. Environ Health Perspect. 2015;123(3):264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weisskopf MG, Kioumourtzoglou MA, Roberts AL. Air pollution and autism spectrum disorders: causal or confounded. Curr Environ Health Rep. 2015;2(4):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bakian AV, Bilder DA, Coon H, et al. Spatial relative risk patterns of autism spectrum disorders in Utah. J Autism Dev Disord. 2015;45(4):988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoffman K, Kalkbrenner AE, Vieira VM, et al. The spatial distribution of known predictors of autism spectrum disorders impacts geographic variability in prevalence in central North Carolina. Environ Health. 2012;11:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoffman K, Vieira VM, Daniels JL. Brief report: diminishing geographic variability in autism spectrum disorders over time. J Autism Dev Disord. 2014;44(3):712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mazumdar S, King M, Liu KY, et al. The spatial structure of autism in California, 1993–2001. Health Place. 2010;16(3):539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Meter K, Christiansen L, Delwiche L, et al. Geographic distribution of autism in California: a retrospective birth cohort analysis. Autism Res. 2010;3(1):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lyall K, Pauls DL, Spiegelman D, et al. Fertility therapies, infertility and autism spectrum disorders in the Nurses’ Health Study II. Paediatr Perinat Epidemiol. 2012;26(4):361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bai L. Adaptive Statistical Methods for the Analysis of Sequential and Spatial Observation Data [dissertation]. Irvine, CA: University of California, Irvine; 2016.
- 25. Vieira V, Webster T, Weinberg J, et al. Spatial analysis of lung, colorectal, and breast cancer on Cape Cod: an application of generalized additive models to case-control data. Environ Health. 2005;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hastie T, Tibshirani R. Generalized Additive Models. New York, NY: Chapman and Hall; 1990. [Google Scholar]
- 27. Vieira V, Hart JE, Webster TF, et al. Association between residences in US northern latitudes and rheumatoid arthritis: a spatial analysis of the Nurses’ Health Study. Environ Health Perspect. 2010;118(7):957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bai L, Bartell SM, Bliss RL, et al. MapGAM: Mapping Smoothed Odds Ratios from Individual-Level Data, R package version 1.0 2016. https://CRAN.R-project.org/package=MapGAM.
- 29. Idring S, Magnusson C, Lundberg M, et al. Parental age and the risk of autism spectrum disorders: findings from a Swedish population-based cohort. Int J Epidemiol. 2014;43(1):107–115. [DOI] [PubMed] [Google Scholar]
- 30. Durkin MS, Maenner MJ, Newschaffer CJ, et al. Advanced parental age and the risk of autism spectrum disorder. Am J Epidemiol. 2008;168(11):1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shelton JF, Tancredi DJ, Hertz-Picciotto I. Independent and dependent contributions of advanced maternal and paternal ages to autism risk. Autism Res. 2010;3(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. US Environmental Protection Agency Technology Transfer Network Air Toxics: National Air Toxics Assessments. 2011. http://www.epa.gov/nata/. Accessed February 28, 2011.
- 33. Fernell E, Bejerot S, Westerlund J, et al. Autism spectrum disorder and low vitamin D at birth: a sibling control study. Mol Autism. 2015;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. MacFarlane J, Kanaya T. What does it mean to be autistic? Inter-state variation in special education criteria for autism services. J Child Fam Stud. 2009;18:662–669. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.