Abstract
Aims
Lack of financial incentive is a frequently cited barrier to alcohol screening in primary care. The Quality and Outcomes Framework (QOF) pay for performance scheme has reimbursed UK primary care practices for alcohol screening in people with schizophrenia since April 2011. This study aimed to determine the impact of financial incentives on alcohol screening by comparing rates of alcohol recording in people with versus those without schizophrenia between 2000 and 2013.
Methods
Cross-sectional and retrospective cohort study. Alcohol data were extracted from The Health Improvement Network (THIN) database of UK primary care records using (a) Read Codes for level of alcohol consumption, (b) continuous measures of drinking (e.g. units a week) and (c) Read Codes for types of screening test.
Results
A total of 14,860 individuals (54% (8068) men and 46% (6792) women) from 409 general practices aged 18–99 years with schizophrenia were identified during April 2011–March 2013. Of these, 11,585 (78%) had an alcohol record, of which 99% (8150/8257) of Read Codes for level of consumption were eligible for recompense in the QOF. There was an 839% increase in alcohol recording among people with schizophrenia over the 13-year period (rate ratio per annum increase 1.19 (95% CI 1.18–1.20)) compared with a 62% increase among people without a severe mental illness (rate ratio per annum increase 1.04 (95% CI 1.03–1.05)).
Conclusion
Financial incentives offered by the QOF appear to have a substantial impact on alcohol screening among people with schizophrenia in UK primary care.
Short summary
Alcohol screening among people with schizophrenia increased dramatically in primary health care following the introduction of the UK pay for performance incentive scheme (Quality and Outcomes Framework) for severe mental illness, with an 839% rise (>8-fold increase) compared with a 62% increase among people without a over the 13-year study period (2000–2013).
Introduction
Alcohol screening and brief intervention is an example of an efficacious intervention that has attempted, yet failed to be routinely delivered in primary health care across the world. There are three decades of research to support the use of alcohol screening and brief intervention in primary care, where brief intervention is an umbrella term that encompasses interventions that range from brief structured advice through to motivational interviewing. Alcohol screening and brief intervention is advocated by the WHO global strategy to reduce harmful use of alcohol (World Health Organisation, 2010), the WHO Comprehensive Mental Health Action Plan 2013–2020 (World Health Organisation, 2013) and by UK NICE Guidance (NICE Public Health Guidance 24, 2010). However, as few as 2% of risky drinkers are identified in UK primary care (Cheeta et al., 2008), and national survey data have shown that 6.5% of risky drinkers recall GP advice on their drinking compared with 50% of smokers who recall GP advice on smoking cessation (Brown et al., 2016). In addition, when alcohol screening does take place in primary care, it is rarely with WHO validated screening tools (Khadjesari et al., 2013). There is a vast literature on the barriers to implementing alcohol screening and brief intervention in primary care (Nilsen et al., 2006; Johnson et al., 2011), with lack of financial incentive one of the key obstacles (Adams et al., 1997; Babor et al., 2005).
Pay for performance schemes are widespread in the UK and USA, with financial incentives a recognized implementation/quality improvement strategy in health care systems (Powell et al., 2015). However, despite their widespread use, there is mixed evidence for their impact on quality of care, with few methodologically robust evaluations, such as interrupted time series or controlled before-and-after studies with contemporaneous control groups (Scott et al., 2011; Houle et al., 2012). The largest and most recent randomized controlled trial to explore the impact of implementation strategies for alcohol screening and brief interventions in 120 primary care practices in five European countries (Optimizing Delivery Health Care Interventions, ODHIN) found financial incentives combined with training and support to be most effective at improving rates of screening and brief intervention compared with (a) treatment as usual, (b) training and support, (c) financial reimbursement alone, (d) electronic screening and brief intervention, and combinations of these interventions (Keurhorst et al., 2013; Anderson et al., 2016). Moreover, financial incentives were found to be the most cost-effective of these implementation strategies, with incentive and delivery costs amounting to £110 million over 10 years, but leading to £250 million of savings to the NHS and saving 33,000 Quality-Adjusted Life-Years (Angus et al., 2014).
The Quality and Outcomes Framework (QOF), a pay for performance scheme, was introduced to primary care in the UK in 2004 to encourage best practice, currently in 19 clinical areas (including severe mental illness (SMI)) and 6 public health areas (British Medical Association. NHS England. NHS Employers, 2014). At present, there is no QOF incentive for universal alcohol screening of all registered patients, but there are QOF incentives for alcohol screening in specific subgroups of the population such as people with schizophrenia, where median prevalence of alcohol use disorders is 9.4% (interquartile range 4.6–19.0) and lifetime prevalence is 20.6% (interquartile range 12.0–34.5) (Koskinen et al., 2009). In April 2006, the QOF for SMI (including schizophrenia, bipolar affective disorder and other psychoses) was amended to provide additional incentives for health screening (Indicator MH9) (British Medical Association. NHS Employers, 2006), replaced in April 2011 with additional incentives for specific components of the health screen, including alcohol screening (Indicator MH11), Body Mass Index, blood pressure, cholesterol and blood glucose (British Medical Association. NHS Employers, 2011). A recent study based in one inner-city London Borough with 30 General Practices found that the introduction of a local version of QOF that incentivized alcohol screening and brief intervention for patients with SMI, cardiovascular disease, or risk or cardiovascular disease, increased recording of alcohol intake in patients with SMI from 0.65% (95% CI 0.49–0.81%) to 48.6% (95% CI 47.8–49.4%) (Hamilton et al., 2014). Interestingly, a small improvement in alcohol screening was also found in patients without an SMI, cardiovascular disease, or risk of cardiovascular disease from 0.32% (CI 0.29–0.36%) to 14.7% (CI 14.5–14.9%). This regional study provides some insights into the impact of financial incentives on alcohol consumption recording in primary care, but to-date there has been no investigation into their impact at a national level.
Aims
To determine the extent to which alcohol consumption is recorded in people with schizophrenia and other psychoses in primary care, whether recording varies by socio-demographics, registration status and geographic region, and to compare rates of alcohol consumption recording in people with and without schizophrenia between 2000 and 2013.
Objectives
To describe how alcohol consumption has been recorded in individuals with schizophrenia and other psychoses in primary care, e.g. use of Read Codes, quantity measures and screening tests.
To describe how the recording of alcohol consumption in individuals with schizophrenia and other psychoses varies by socio-demographic factors (age, sex and social deprivation), by registration status and by region (previous strategic health authority for England and country for Wales, Scotland and Northern Ireland).
To evaluate the impact of QOF incentives by comparing rates of alcohol consumption recording over time in individuals with versus without schizophrenia and other psychoses: April 2000–March 2004 (before the SMI QOF), April 2004–March 2006 (SMI QOF introduced), April 2006–March 2011 (addition of lifestyle screening to the SMI QOF indicators) and April 2011–March 2013 (addition of alcohol screening to the SMI QOF indicators).
Materials and Methods
Study design
Cross-sectional study and retrospective cohort study.
Data source
The Health Improvement Network (THIN, 2012) is a primary care database containing anonymized electronic patient records from over 500 general practices, covering ~6% of the UK population and broadly representative in terms of age, sex, deprivation and geographical distribution (Blak et al., 2011). Information is entered in primary care as free text or Read Codes, a hierarchical coding system standardized across all UK general practices (Chisholm, 1990; Dave and Petersen, 2009). THIN includes information about year of birth, gender and the Townsend deprivation index, which is a composite measure of social deprivation presented as quintiles (Townsend et al., 1988). Data from individual practices were included in this study if collected after the Acceptable Mortality Reporting (Maguire et al., 2009) and Acceptable Computer Usage dates (Horsfall et al., 2013); these are quality standards to ensure that data were acceptable for analyses. Acceptable mortality reporting is a validation of the mortality data recorded at the practice. Acceptable computer usage is measured by the number of clinical entries made on the computer systems. The scheme for THIN to obtain and provide anonymous patient data to researchers was approved by the National Health Service South East Multicenter Research Ethics Committee in 2002. The scientific approval for this study was obtained from the Scientific Review Committee of Cegedim Strategic Data (CSD) Medical Research (the THIN database providers) in January 2015 (15-005).
Participant eligibility
The SMI QOF relates to people with schizophrenia, bipolar affective disorder and other psychoses. Patients with bipolar disorder were excluded from these studies and explored by the research team in a separate analysis (Hardoon et al., 2016).
Cross-sectional study (Objectives 1 and 2): individuals aged between 18 and 99 years with a diagnosis of schizophrenia and other psychoses, registered with a THIN practice throughout April 2011–March 2013, were included in the cross-sectional study. Individuals with a diagnosis of schizophrenia and other psychoses were identified via relevant Read Codes in their electronic primary care health records, as used in a recent study of recording of SMI in primary care (Hardoon et al., 2013).
Retrospective cohort study (Objective 3): we expanded the study sample to include individuals registered for at least some part of the period April 2000–March 2013. A separate cohort of individuals without an SMI was randomly selected among those of the same gender, practice and age band (18–29, 30–49, 50–69 and 70–99 years). For every one individual with schizophrenia, six individuals without an SMI were selected in-line with a similar study of cardiovascular screening in individuals with SMI (Osborn et al., 2011).
Alcohol measures in THIN
Read Codes that reflect levels of drinking were selected. The codes were selected from those used in three recent studies of alcohol consumption recording in primary care (Khadjesari et al., 2013; Hamilton et al., 2014; O'Donnell, 2014). These Read Codes typically represent a drinking category, for example, moderate drinker 3–6 units a day, but also include codes that indicate drinking above limits; for example, harmful drinking.
-
Data on alcohol consumption as a continuous measure of the number of units consumed in a week were extracted.
The above measures were also used to summarize the proportion of people with schizophrenia drinking at harmful levels (defined as a Read Code indicative of heavy drinking/alcohol problem or a record of >35 units per week for women and >50 units per week men) (Prime Minister's Strategy Unit, 2004; Department of Health, 2008).
Read Codes that indicate the types of screening tool used were also included to determine how many people had completed a validated screening test. Results from validated screening tests are sometimes entered as free text and therefore not extractable for this study.
Analysis
To describe how alcohol consumption has been recorded in individuals with schizophrenia and other psychoses in primary care (Objective 1), we computed the numbers of individuals with schizophrenia, registered with a practice between April 2011 and March 2013, who had Read Codes for level of drinking and screening tests, and quantity measures for alcohol consumption during this period.
To describe how the recording of alcohol consumption in individuals with schizophrenia and other psychoses varies by socio-demographic factors, registration status and region (Objective 2), the proportions of men and women with schizophrenia with an alcohol record between April 2011 and March 2013 and relative ‘risk’ of recording of alcohol were presented by socio-demographic factors (age, sex and social deprivation), registration status and geographic region. Poisson regression was chosen here to enable computation of risk ratios as opposed to odds ratios to aid interpretation (Davies et al., 1998). Relative ‘risks’ were obtained from Poisson regression with robust standard errors to account for clustering of individuals within general practices. Random effects regression modelling, with individuals nested in general practices, was used to examine the extent of practice variation in alcohol recording (Cameron and Trivedi, 2013).
To evaluate the impact of QOF incentives in individuals with versus without schizophrenia and other psychoses (Objective 3), eligible individuals entered the retrospective cohort study at the latest of the following: registration, practice Acceptable Computer Usage and Acceptable Mortality Reporting date, 1 April 2000, 18th birthday, schizophrenia diagnosis date (or schizophrenia diagnosis date of matched individual with schizophrenia, among those without schizophrenia). Individuals left the study at the earliest of the following: date of death, when they leave the practice, the practice leaves THIN, 31 March 2013, or they reach 100 years of age. Rates of alcohol consumption recording were computed among those with and without schizophrenia during every two financial years follow-up period, between 1 April 2000 and 31 March 2013. Rates of alcohol consumption records in individuals with schizophrenia were compared with individuals without schizophrenia using Poisson regression, with robust standard errors to take into account potential clustering within practices. An interaction between schizophrenia status (yes or no) and calendar period (as a categorical variable, with categories for each two financial year period) was included to assess whether variations in recording among individuals with versus without schizophrenia have changed over time, in particular according to the four time periods described above. Poisson regression was chosen here as it enables proper treatment/accounting of the varying person-time each individual contributed to the analysis. Furthermore, Poisson regression was appropriate for this time-trend analysis given the limited number of time-points (six two-yearly periods, reflecting the reporting periods of QOF), which makes alternative time series analysis unsuitable. Age, gender, deprivation and UK region were included as covariates. All analyses were conducted in STATA version 13 (StataCorp., 2013).
Results
Recording of alcohol consumption in people with schizophrenia in primary care
In total, 14,860 individuals from 409 general practices aged 18–99 years with schizophrenia (or other psychoses) were identified throughout the period 1 April 2011–31 March 2013. There were 8068 men (54%) and 6792 (46%) women. The mean ages (standard deviation) at the start of the period were 40 years (19.4) and 49 years (19.6) for men and women, respectively. There was a median of 32 individuals (interquartile range 20–48) with a record of schizophrenia per general practice. Out of the 14,860 individuals, 881 (6%) were ‘newly registered’ at the start of the period (i.e. had registered with the practice in the year prior to the period). The proportion of people with schizophrenia drinking at higher risk/harmful levels was 21% (1658/8068) in men and 10% (655/6792) in women. There was considerable variation in recording levels between general practices, ranging from 42% to 100% (P < 0.001 for practice level variation from random effects regression modelling).
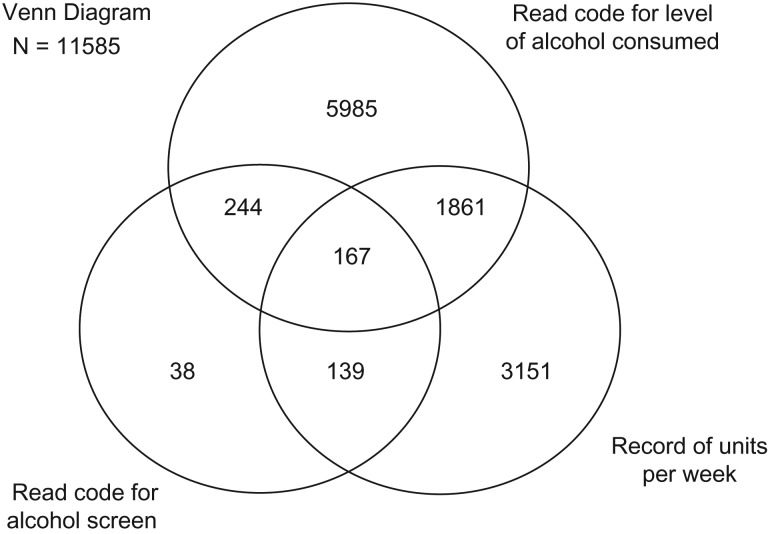
Out of the 14,860 individuals, 11,585 (78%) had a relevant alcohol consumption record during this period, with data recorded as a Read Code for units in a week (only) (5985, 52%), units of alcohol in a week (only) (3151, 27%), a Read Code for type of screening test used (only) (38, 0.33%) or >1 of these methods of recording (2411, 21%) (Fig. 1). Of the 8257 records comprising Read Codes for alcohol consumption, 8150 (99%) were codes found in the QOF for SMI business rules (QOF for SMI) (The NHS Information Centre—QOF Business Rules team, 2011). Appendices 1 and 2 list the Read Codes used in this study to indicate level of alcohol consumption and screening test administered, respectively.
Fig. 1.
Types of alcohol records among people with schizophrenia in April 2011–March 2013.
Recording of alcohol consumption in people with schizophrenia by socio-demographic factors, registration status and region
Over 80% (5435/6664) of individuals aged 50–79 years had an alcohol consumption record compared with 67% (650/978) of men and 72% (320/445) of women under the age of 30 (Table 1). The proportion of people with an alcohol consumption record in the least deprived quintile was 75% (1371/1840) compared with around 80% (3318/4136) in the most deprived quintile for both men and women (Table 1). There was also a statistically significant regional variation in alcohol consumption recording (overall P-values comparing all regions of P = 0.02 and P = 0.003 among men and women, respectively, indicative of some differences between regions). Recording rates appeared highest in London and lowest in the East Midlands (Table 1). There were no statistically significant differences in recording levels by registration status.
Table 1.
Proportions of men and women with a relevant alcohol record during the period April 2011–March 2013 and relative ‘risk’ of recording of alcohol by demographic group
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Records/N (%) | RR (95% CI) | P | Records/N (%) | RR (95% CI) | P | |
| All | 6247/8068 (77.4) | 5338/6792 (78.6) | ||||
| Age, years | ||||||
| 18–29 | 650/978 (66.5) | 0.85 (0.81–0.89) | 320/445 (71.9) | 0.92 (0.86–0.98) | ||
| 30–39 | 1278/1737 (73.6) | 0.94 (0.90–0.97) | 641/836 (76.7) | 0.98 (0.94–1.03) | ||
| 40–49 | 1655/2109 (78.5) | 1 | 1072/1380 (77.7) | 1 | ||
| 50–59 | 1243/1504 (82.6) | 1.06 (1.02–1.10) | 1155/1442 (80.1) | 1.03 (0.99–1.07) | ||
| 60–69 | 896/1089 (82.3) | 1.05 (1.02–1.09) | 1002/1251 (80.1) | 1.04 (0.99–1.08) | ||
| 70–79 | 406/489 (83.0) | 1.07 (1.02–1.12) | 733/889 (82.5) | 1.07 (1.02–1.11) | ||
| 80–89 | 112/151 (74.2) | 0.97 (0.88–1.06) | 363/476 (76.3) | 0.99 (0.93–1.05) | ||
| 90–99 | 7/11 (63.6) | 0.81 (0.51–1.27) | 52/73 (71.2) | 0.92 (0.8–1.06) | ||
| <0.001 | <0.001 | |||||
| Deprivation quintilea | ||||||
| 1 (least deprived) | 646/867 (74.5) | 0.94 (0.89–0.98) | 725/973 (74.5) | 0.93 (0.88–0.98) | ||
| 2 | 858/1121 (76.5) | 0.96 (0.92–1.00) | 849/1083 (78.4) | 0.98 (0.94–1.02) | ||
| 3 | 1157/1543 (75.0) | 0.94 (0.90–0.98) | 1094/1411 (77.5) | 0.97 (0.93–1.01) | ||
| 4 | 1605/2054 (78.1) | 0.98 (0.95–1.01) | 1333/1672 (79.7) | 0.99 (0.96–1.03) | ||
| 5 (most deprived) | 1981/2483 (79.8) | 1 | 1337/1653 (80.9) | 1 | ||
| 0.01 | 0.05 | |||||
| UK regionb | ||||||
| London | 874/1063 (82.2) | 1 | 753/897 (83.9) | 1 | ||
| East Midlands | 68/100 (68.0) | 0.83 (0.62–1.12) | 70/117 (59.8) | 0.72 (0.49–1.06) | ||
| East of England | 366/472 (77.5) | 0.95 (0.88–1.03) | 297/378 (78.6) | 0.95 (0.87–1.04) | ||
| West Midlands | 481/625 (77) | 0.94 (0.87–1.02) | 462/570 (81.1) | 0.97 (0.92–1.04) | ||
| North East | 150/190 (78.9) | 0.95 (0.88–1.04) | 124/152 (81.6) | 0.97 (0.89–1.05) | ||
| North West | 845/1084 (78.0) | 0.95 (0.89–1.01) | 681/863 (78.9) | 0.95 (0.89–1.00) | ||
| Yorkshire and Humber | 75/98 (76.5) | 0.92 (0.82–1.03) | 62/87 (71.3) | 0.85 (0.62–1.16) | ||
| Northern Ireland | 341/423 (80.6) | 0.98 (0.91–1.05) | 304/363 (83.7) | 1.00 (0.94–1.07) | ||
| Scotland | 988/1269 (77.9) | 0.93 (0.88–0.99) | 789/1002 (78.7) | 0.94 (0.89–0.99) | ||
| South Central | 675/869 (77.7) | 0.96 (0.90–1.02) | 637/801 (79.5) | 0.96 (0.92–1.01) | ||
| South East Coast | 432/595 (72.6) | 0.89 (0.83–0.95) | 389/528 (73.7) | 0.89 (0.83–0.95) | ||
| South West | 461/643 (71.7) | 0.87 (0.82–0.93) | 390/549 (71.0) | 0.86 (0.79–0.93) | ||
| Wales | 491/637 (77.1) | 0.94 (0.88–1.00) | 380/485 (78.4) | 0.94 (0.88–1.00) | ||
| 0.02 | 0.003 | |||||
| Registration status | ||||||
| Not newly registered | 5846/7531 (77.6) | 1 | 5072/6448 (78.7) | 1 | ||
| Newly registered | 401/537 (74.7) | 0.98 (0.93–1.03) | 266/344 (77.3) | 0.99 (0.93–1.06) | ||
| 0.4 | 0.9 | |||||
aDeprivation measured using Townsend score.
bUK region (former Strategic Health Authorities in England, plus Scotland, Wales and Northern Ireland).
RR, relative risk, estimated from Poisson regression, adjusting for the other factors considered and accounting for clustering of people within general practices.
Impact of the SMI QOF on alcohol consumption recording over time
Between April 2000 and April 2013, there were 34,440 individuals with schizophrenia or other psychoses and 226,984 individuals without an SMI identified as a matched cohort; a total of 261,424 individuals from 484 general practices. There were a median of 162 (interquartile range: 89–264) individuals with schizophrenia or other psychoses and 1167 (interquartile range: 624–1908) individuals without an SMI per general practice. Table 2 provides demographic characteristics of people with and without schizophrenia. Rates of alcohol recording increased rapidly over time among those with schizophrenia or other psychoses (Table 3 and Fig. 2). In contrast, there appears to be only a modest increase in recording rates among individuals without an SMI over time. Prior to April 2004, rates of alcohol recording among those individuals with schizophrenia or other psychoses were on par with or lower than those among individuals without an SMI (rate ratios of 0.89 and 0.99) (Table 3), but after April 2004, were higher, with the gap widening over time (rate ratio of 1.34 in April 2004–March 2006 to a rate ratio of 3.80 in the most recent time period).
Table 2.
Baseline demographic characteristics in people with and without schizophrenia 2000–2013
| People with schizophrenia | People without schizophrenia | |
|---|---|---|
| Total individuals | 34,440 | 226,984 |
| N (%) | N (%) | |
| Gender | ||
| Men | 18,540 (53.8) | 123,543 (54.4) |
| Women | 15,900 (46.2) | 103,441 (45.6) |
| Age, years | ||
| 18–29 | 6274 (18.2) | 43,151 (19.0) |
| 30–49 | 13,627 (39.6) | 89,457 (39.4) |
| 50–69 | 9299 (27.0) | 59,138 (26.1) |
| 70–99 | 5240 (15.2) | 35,238 (15.5) |
| Townsend deprivation quintile | ||
| 1 (least deprived) | 4427 (12.9) | 49,243 (21.7) |
| 2 | 5252 (15.3) | 46,676 (20.6) |
| 3 | 6872 (20.0) | 48,729 (21.5) |
| 4 | 8678 (25.2) | 45,989 (20.3) |
| 5 (most deprived) | 9211 (26.8) | 36,347 (16.0) |
| UK region | ||
| London | 4351 (12.6) | 27,908 (12.3) |
| East Midlands | 1353 (3.9) | 9060 (4.0) |
| East of England | 2380 (6.9) | 15,803 (7.0) |
| West Midlands | 2587 (7.5) | 17,201 (7.6) |
| North East | 1005 (2.9) | 6646 (2.9) |
| North West | 4083 (11.9) | 26,827 (11.8) |
| Yorkshire and Humber | 1429 (4.2) | 9147 (4.0) |
| Northern Ireland | 1221 (3.6) | 7769 (3.4) |
| Scotland | 4071 (11.8) | 26,231 (11.6) |
| South Central | 3902 (11.3) | 26,059 (11.5) |
| South East Coast | 2614 (7.6) | 17,430 (7.7) |
| South West | 3274 (9.5) | 22,381 (9.9) |
| Wales | 2170 (6.3) | 14,522 (6.4) |
Table 3.
Alcohol recording rates and adjusted rate ratios comparing people with and without schizophrenia within each time period
| Time period | Individuals with schizophrenia and psychoses | Individuals without schizophrenia and psychoses | Adjusted rate ratioa (95% CI)—cases vs. controls | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Total person-years | No. with alcohol record | Rate of alcohol recording per 1000 person-years (95% CI) | N | Total person-years | No. with alcohol record | Rate of alcohol recording per 1000 person-years (95% CI) | |||
| 1 April 2000–31 March 2002 | 9033 | 15,177 | 1241 | 81.8 (77.3–86.4) | 54,198 | 93,656 | 8339 | 89.0 (87.1–91.0) | 0.89 (0.84–0.96) | 0.001 |
| 1 April 2002–31 March 2004 | 12,303 | 19,650 | 3043 | 154.9 (149.5–160.5) | 78,869 | 129,750 | 19,675 | 151.6 (149.5–153.8) | 0.99 (0.95–1.03) | 0.6 |
| 1 April 2004–31 March 2006 | 15,617 | 22,436 | 5099 | 227.3 (221.1–233.6) | 106,136 | 169,080 | 27,571 | 163.1 (161.2–165.0) | 1.34 (1.28–1.41) | <0.001 |
| 1 April 2007–31 March 2009 | 18,128 | 25,313 | 7306 | 288.6 (282.1–295.3) | 136,277 | 219,761 | 34,050 | 154.9 (153.3–156.6) | 1.79 (1.69–1.89) | <0.001 |
| 1 April 2009–31 March 2011 | 19,229 | 24,265 | 9025 | 371.9 (364.3–379.7) | 151,126 | 234,391 | 40,034 | 170.8 (169.1–172.5) | 2.09 (1.96–2.22) | <0.001 |
| 1 April 2011–31 March 2013 | 19,545 | 18,701 | 13,515 | 722.7 (710.6–735.0) | 159,097 | 243,134 | 44,826 | 184.4 (182.7–186.1) | 3.80 (3.54–4.08) | <0.001 |
aAdjusted rate ratio comparing people with bipolar disorder to people without SMI, from Poisson regression adjusting for age, gender, deprivation, UK region and accounting for clustering of people in general practices.
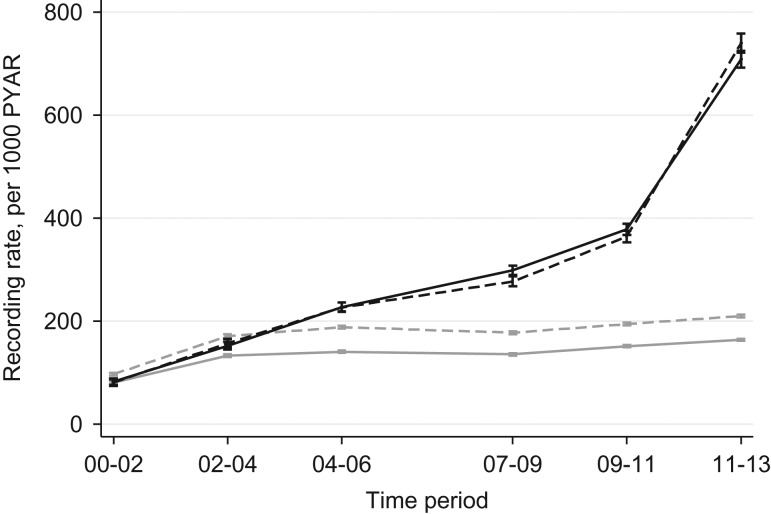
Fig. 2.
Graph of alcohol recording rates over time, people with and without schizophrenia. Solid black line, men with schizophrenia; dashed black line, women with schizophrenia; solid grey line, men without schizophrenia; dashed grey line, women without schizophrenia. Spikes with caps are 95% confidence interval.
Among individuals with schizophrenia or other psychoses, the alcohol recording rate ratio per annum increase in calendar time was 1.19 (95% CI 1.18–1.20), corresponding to a total percentage increase over the 13-year period of 839% (>8-fold increase). By contrast, among individuals without an SMI, the alcohol recording rate ratio per annum increase in calendar time was 1.04 (95% CI 1.03–1.05), corresponding to a total percentage increase over the 13-year period of 62%. The difference in the time trends in alcohol recording between individuals with and without schizophrenia was statistically significant (P < 0.001 for interaction between time period and patient group: with and without schizophrenia).
Discussion
The majority of individuals with schizophrenia and other psychoses included in this study had an alcohol consumption record (78%, 11,585/14,860). Around 21% (1658/8068) of men and 10% (655/6792) of women with schizophrenia were recorded as drinking at higher risk/harmful levels. Alcohol consumption recording varied statistically significantly by age, deprivation and region. Highest levels of recording were observed among those aged between 50 and 79 years, those with highest levels of deprivation and in London. Further analyses are needed to confirm differences between age categories, deprivation quintiles and regions to explore possible explanations for the differences. The recording of alcohol consumption increased dramatically following the introduction of the SMI QOF, with an 839% rise (>8-fold increase) over the 13-year period. The most substantial rise occurred after the introduction of the indicator for alcohol consumption recording in April 2011.
This study found recording of alcohol consumption in primary care to occur in 78% of individuals with schizophrenia and other psychoses (April 2011–March 2013), rising to 86% when individuals with exemption records were excluded. Perhaps unsurprisingly, 99% of Read Codes used to record alcohol consumption in this study were those provided in the SMI QOF business rules (The NHS Information Centre—QOF Business Rules team, 2011), thereby enabling financial reimbursement. Conversely, in a study of alcohol consumption recording in newly registered patients in UK primary care, the codes necessary for recompense by a different pay for performance scheme (i.e. the Directed Enhanced Service) were rarely used—9% (25,975/292,376) of patients completed a validated screening test, such as the AUDIT, AUDIT-C or the FAST (Khadjesari et al., 2013). The most plausible explanation for this finding is that the incentive offered by the Directed Enhanced Service was substantially lower than that offered by the QOF (£2.38 per patient vs. £133.76 per point (up to four QOF points) in 2012/2013, respectively), and therefore has less influence on practitioner behaviour.
The Directed Enhanced Service pay for performance scheme, which ran from April 2008 to April 2014, was an attempt to broaden the delivery of alcohol screening in primary care to a population level, by incentivizing alcohol screening among newly registered patients. A study of 16 primary care practices in North East England found better rates of alcohol screening and brief intervention recording in practices receiving Directed Enhanced Service incentives versus those not receiving incentives between 2010 and 2011 (3.73%, 95% CI: 3.65–3.89 vs. 0.05%, 95% CI: 0.03–0.08; P < 0.001) (O'Donnell, 2014). However, the national primary care data analysed in this study found no statistically significant differences in alcohol recording by registration status, again suggesting that the magnitude of the incentive reflects the degree of uptake of the activity. These findings are reinforced by a qualitative interview study with general practitioners, which found QOF incentives to be given priority above other incentive schemes because of their substantial contribution to practice income (O'Donnell, 2014). If alcohol screening is to be delivered at a population level, QOF incentives should be extended to all patient groups, especially in-light of their cost-effectiveness relative to other implementation strategies (Angus et al., 2014).
The QOF has been criticized for incentivizing recording practice, rather than quality of care, and is considered a move in the opposite direction from holistic, patient-centred practice that promotes self-management (Dixon et al., 2010). As a result, primary care practice is now seen as being task oriented, and where one-activity supersedes another, may result in detrimental effects on the quality of care for non-targeted patients. However, despite the failings of the QOF in this regard, it does appear to have an impact on alcohol consumption screening among patients with schizophrenia in this study. This is an important finding as these patients have high rates of harmful drinking compared with the general population: 21% men and 10% women with schizophrenia (2011–2013) vs. 1% men and 0.5% women who were newly registered with a UK general practice (2007–2009) (Khadjesari et al., 2013). It is important to note that the QOF indicator only provides recompense for the recording of alcohol consumption among patients with SMI and does not incentivize provision of treatment, leading to uncertainty over the extent to which patients with schizophrenia are supported in reducing their drinking. NICE guidance on the assessment and management of psychosis with substance misuse advocates the referral of patients identified in primary care to secondary care mental health services for assessment and further management (National Collaborating Centre for Mental Health (UK), 2011). Psychosocial treatment is advocated for patients drinking at harmful and dependent levels without SMI (NICE Clinical Guidance 115, 2011); however, there is no evidence that psychosocial treatment (including assertive community outreach, intensive case management, motivational interviewing, cognitive behavioural therapy, contingency management or social skills training) reduces alcohol consumption or improves mental health in people with SMI (Hunt et al., 2013), with most trials of poor methodological quality. Hunt et al.’s Cochrane review calls for robust research that uses validated outcome measures of clinical relevance to enable synthesis in future meta-analyses. Hunt et al. also recommend that future trials evaluate brief interventions due to their potential cost-effectiveness and ease of integration within mental health services (Hunt et al., 2013).
Strengths and limitations
This is the first study to look at the impact of the SMI QOF on alcohol consumption recording among people with schizophrenia in a large nationally representative sample of individuals from general practices in the UK. Data on alcohol status in primary care rely on self-report in the absence of a gold standard approach to ascertaining alcohol intake. Self-report is the most widely used means of eliciting drinking behaviour in research with non-dependent drinkers, providing a valid, reliable and feasible approach when compared with biochemical markers, coverage of sales data and collateral reports (Midanik, 1988; Rehm, 1998; Connors and Maisto, 2003; Del Boca and Darkes, 2003). Finally, although we have data on whether an individual has completed a validated alcohol screening test, the results per se were not known, as they were recorded in the free text. Some of these results may be proxied as Read Codes or number of units consumed in a week. However, it is important to determine the extent to which primary care practitioners are using validated tests to record alcohol consumption.
Conclusion
Our findings suggest that the financial incentives provided by the alcohol indicator of the SMI QOF appear to have had a substantial impact on alcohol consumption recording among people with schizophrenia in primary care. Further research is needed to better inform the evidence base on interventions for patients with schizophrenia identified as drinking at higher risk levels.
Acknowledgements
Zarnie Khadjesari is currently a King's Improvement Science post-doctoral fellow at King's College London. King's Improvement Science is part of the Centre for Implementation Science at the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South London.
Appendix 1
Read Codes for level of alcohol consumed, and frequency of use among people with schizophrenia in April 2011–March 2013
| Read Code | Description | In QOF?a | N |
|---|---|---|---|
| 1361.00 | Teetotaller | Yes | 3603 |
| 1367.00 | Stopped drinking alcohol | Yes | 1690 |
| 1362.11 | Drinks rarely | Yes | 871 |
| 1362.12 | Drinks occasionally | Yes | 745 |
| 1362.00 | Trivial drinker: <1 u/day | Yes | 309 |
| 1361.11 | Nondrinker alcohol | Yes | 181 |
| 1363.00 | Light drinker: 1–2 u/day | Yes | 154 |
| 1364.00 | Moderate drinker: 3–6 u/day | Yes | 135 |
| 136L.00 | Alcohol intake within recommended sensible limits | Yes | 132 |
| E23.0.00 | Alcohol dependence syndrome | No | 59 |
| 1365.00 | Heavy drinker: 7–9 u/day | Yes | 51 |
| 136K.00 | Alcohol intake above recommended sensible limits | Yes | 48 |
| 1361.12 | Nondrinker alcohol | Yes | 43 |
| 1366.00 | Very heavy drinker: >9 u/day | Yes | 33 |
| 136R.00 | Binge drinker | Yes | 27 |
| E23.0.12 | Alcohol problem drinking | No | 25 |
| 136T.00 | Harmful alcohol use | Yes | 24 |
| 136J.00 | Social drinker | Yes | 19 |
| 136D.00 | Ex-heavy drinker: 7–9 u/day | Yes | 14 |
| 136S.00 | Hazardous alcohol use | Yes | 12 |
| 136C.00 | Ex-moderate drinker: 3–6 u/day | Yes | 10 |
| 136O.00 | Moderate drinker | Yes | 10 |
| 136P.00 | Heavy drinker | Yes | 10 |
| 136N.00 | Light drinker | Yes | 8 |
| 136B.00 | Ex-light drinker: 1–2 u/day | Yes | 6 |
| 136E.00 | Ex-very heavy drinker: >9 u/day | Yes | 6 |
| E23.0.11 | Alcoholism | No | 6 |
| 136A.00 | Ex-trivial drinker: <1 u/day | Yes | 5 |
| 136M.00 | Current nondrinker | No | 3 |
| 136W.00 | Alcohol misuse | No | 2 |
| 136d.00 | Lower risk drinking | Yes | 2 |
| 1462.00 | H/O: alcoholism | No | 2 |
| E231z00 | Chronic alcoholism NOS | No | 2 |
| E250.00 | Non-dependent alcohol abuse | No | 2 |
| 136Q.00 | Very heavy drinker | Yes | 1 |
| 136c.00 | Higher risk drinking | Yes | 1 |
| E230.00 | Acute alcoholic intoxication in alcoholism | No | 1 |
| E23z.00 | Alcohol dependence syndrome NOS | No | 1 |
| E250000 | Non-dependent alcohol abuse, unspecified | No | 1 |
| E250200 | Non-dependent alcohol abuse, episodic | No | 1 |
| Eu10100 | [X]Mental and behaviour disorder due to use of alcohol: harmful use | No | 1 |
| Eu10211 | [X]Alcohol addiction | No | 1 |
| 136Y.00 | Drinks in morning to get rid of hangover | No | 0 |
| 136a.00 | Increasing risk drinking | Yes | 0 |
| 136b.00 | Feels should cut down drinking | No | 0 |
| E230.11 | Alcohol dependence with acute alcoholic intoxication | No | 0 |
| E230000 | Acute alcoholic intoxication, unspecified, in alcoholism | No | 0 |
| E230100 | Continuous acute alcoholic intoxication in alcoholism | No | 0 |
| E230200 | Episodic acute alcoholic intoxication in alcoholism | No | 0 |
| E230300 | Acute alcoholic intoxication in remission, in alcoholism | No | 0 |
| E230z00 | Acute alcoholic intoxication in alcoholism NOS | No | 0 |
| E231.00 | Chronic alcoholism | No | 0 |
| E231.11 | Dipsomania | No | 0 |
| E231000 | Unspecified chronic alcoholism | No | 0 |
| E231100 | Continuous chronic alcoholism | No | 0 |
| E231200 | Episodic chronic alcoholism | No | 0 |
| E231300 | Chronic alcoholism in remission | No | 0 |
| E250100 | Non-dependent alcohol abuse, continuous | No | 0 |
| E250300 | Non-dependent alcohol abuse in remission | No | 0 |
| E250z00 | Non-dependent alcohol abuse NOS | No | 0 |
| Eu10200 | [X]Mental and behaviour disorder due to use alcohol: dependence syndrome | No | 0 |
| Eu10212 | [X]Chronic alcoholism | No | 0 |
| Eu10213 | [X]Dipsomania | No | 0 |
| ZV11300 | [V]Personal history of alcoholism | No | 0 |
aIn QOF, included in the list of codes eligible for recompense in the Quality and Outcomes Framework Pay for Performance Scheme for alcohol screening in people with SMI.
Appendix 2
Read Codes for screening and frequency of use among people with schizophrenia in April 2011–March 2013
| Read Code | Description | N |
|---|---|---|
| 9k17.00 | Alcohol screen: AUDIT C completed | 263 |
| 9k16.00 | Alcohol screen: fast alcohol screening test completed | 117 |
| 68S.0.00 | Alcohol consumption screen | 67 |
| 9k13.00 | Alcohol questionnaire completed | 36 |
| 9k15.00 | Alcohol screen: AUDIT completed | 35 |
| 38D3.00 | Alcohol use disorders identification test | 28 |
| 6892.00 | Alcohol consumption screen | 16 |
| 388u.00 | Fast alcohol screening test | 12 |
| 38D4.00 | Alcohol use disorder identifications test consumption questionnaire | 5 |
| ZR1F.11 | AUDIT: alcohol use disorders identification test | 4 |
| ZR1F.00 | Alcohol use disorders identification test | 2 |
| ZRLfD00 | Health of the Nation Outcome Scale Item 3 | 2 |
| 38Dz.00 | Severity of alcohol dependence questionnaire | 1 |
| 388j.00 | CAGE questionnaire | 0 |
| 38D2.00 | Single alcohol screening questionnaire | 0 |
| 38D5.00 | Alcohol use disorder identification test Piccinelli consumption questionnaire | 0 |
| 38Df.00 | Five-shot questionnaire on heavy drinking | 0 |
| 38Dz.11 | SADQ: severity of alcohol dependence questionnaire | 0 |
| 9k18.00 | Alcohol screen: AUDIT PC completed | 0 |
| ZR1E.00 | Alcohol dependence scale | 0 |
| ZR1E.11 | ADS: alcohol dependence scale | 0 |
| ZR1G.00 | Alcohol use inventory | 0 |
| ZR31.00 | CAGE questionnaire | 0 |
| ZR3f.00 | Comprehensive drinker profile | 0 |
| ZR3f.11 | CDP: comprehensive drinker profile | 0 |
| ZRBJ.00 | Drinking problem scale | 0 |
| ZRBJ.11 | DPS: drinking problem scale | 0 |
| ZRLfD11 | HoNOS Item 3 | 0 |
| ZRLfD12 | HoNOS Item 3: alcohol/drug problem | 0 |
| ZRR.0.00 | Inventory of drinking situations | 0 |
| ZRVK.00 | Last 6 months of drinking questionnaire | 0 |
| ZRa1.00 | Michigan alcoholism screening test | 0 |
| ZRa1.11 | MAST: Michigan alcoholism screening test | 0 |
| ZRa1100 | Brief Michigan alcoholism screening test | 0 |
| ZRa1111 | BMAST: brief Michigan alcoholism screening test | 0 |
| ZRa1200 | Short Michigan alcoholism screening test | 0 |
| ZRa1211 | SMAST: short Michigan alcoholism screening test | 0 |
| ZRaU.00 | Munich alcoholism test | 0 |
| ZRaU.11 | MALT: Munich alcoholism test | 0 |
| ZRk6.00 | Severity of alcohol dependence questionnaire | 0 |
| ZRk6.11 | SADQ: severity of alcohol dependence questionnaire | 0 |
| ZRk9.00 | Short alcohol dependence data | 0 |
| ZRk9.11 | SADD: short alcohol dependence data | 0 |
| ZV79100 | [V]Screening for alcoholism | 0 |
Author contributions
I.N., I.P. and Z.K. conceived the study. Z.K. prepared the protocol and all authors contributed to the study design and edited the protocol. S.L.H. extracted and cleaned the data, undertook the statistical analysis. Z.K. wrote the first draft of the manuscript. All authors contributed to, and have approved, the final manuscript.
Funding
This research was carried out independently of the funders. The views expressed are those of the authors and not necessarily those of the funders. Sarah Hardoon is supported by a National Institute for Health Research School for Primary Care Research post-doctoral launching award.
Conflict of interest statement
None declared.
References
- Adams PJ, Powell A, McCormick R, et al. (1997) Incentives for general practitioners to provide brief interventions for alcohol problems. N Z Med J 110:291–4. [PubMed] [Google Scholar]
- Anderson P, Bendtsen P, Spak F, et al. (2016) Improving the delivery of brief interventions for heavy drinking in primary health care: outcome results of the ODHIN five country cluster randomized factorial trial. Addiction. (E-pub ahead of print) doi: 10.1111/add.13476. [DOI] [PubMed] [Google Scholar]
- Angus C, Li J, Parrott S, et al. (2014) Optimizing Delivery of Health Care. Interventions (ODHIN) Cost-Effectiveness—Analysis of the WP5 Trial. Addendum to Deliverable D3.1, Work Package 3.
- Babor TE, Higgins-Biddle J, Dauser D, et al. (2005) Alcohol screening and brief intervention in primary care settings: implementation models and predictors. J Stud Alcohol 66:361–8. [DOI] [PubMed] [Google Scholar]
- Blak BT, Thompson M, Dattani H, et al. (2011) Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care 19:251–5. [DOI] [PubMed] [Google Scholar]
- British Medical Association. NHS Employers (2006) Revisions to the GMS Contract 2006/07: Delivering Investment in General Practice. London: NHS Employers. [Google Scholar]
- British Medical Association. NHS Employers (2011) Quality and Outcomes Framework Guidance for GMS Contract 2011/12: Delivering Investment in General Practice. London: NHS Employers. [Google Scholar]
- British Medical Association. NHS England. NHS Employers (2014) 2014/15 General Medical Services (GMS) Contract Quality and Outcomes Framework (QOF) Guidance for GMS Contract 2014/15. NHS Gateway Reference 01264: NHS Employers.
- Brown J, West R, Angus C, et al. (2016) Comparison of brief interventions in primary care on smoking and excessive alcohol consumption: a population survey in England. Br J Gen Pract 66:e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron CA, Trivedi PK (2013) Regression Analysis of Count Data. New York: Cambridge University Press. [Google Scholar]
- Cheeta S, Drummond C, Oyefeso A, et al. (2008) Low identification of alcohol use disorders in general practice in England. Addiction 103:766–73. [DOI] [PubMed] [Google Scholar]
- Chisholm J. (1990) The read clinical classification. BMJ 300:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors GJ, Maisto SA (2003) Drinking reports from collateral individuals. Addiction 98:21–9. [DOI] [PubMed] [Google Scholar]
- Dave S, Petersen I (2009) Creating medical and drug code lists to identify cases in primary care databases. Pharmacoepidemiol Drug Saf 18:704–7. [DOI] [PubMed] [Google Scholar]
- Davies HT, Crombie IK, Tavakoli M (1998) When can odds ratios mislead. BMJ 316:989–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Boca FK, Darkes J (2003) The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction 98:1–12. [DOI] [PubMed] [Google Scholar]
- Department of Health (2008) The Cost of Alcohol Harm to the NHS in England. An Update to the Cabinet Office (2003) Study. London: Department of Health. [Google Scholar]
- Dixon A, Khachatryan A, Wallace A, et al. (2010) The Quality and Outcomes Framework (QOF): does it reduce health inequalities? Final report: NIHR Service Delivery and Organisation Programme.
- Hamilton FL, Laverty AA, Gluvajic D, et al. (2014) Effect of financial incentives on delivery of alcohol screening and brief intervention (ASBI) in primary care: longitudinal study. J Public Health 36:450–9. [DOI] [PubMed] [Google Scholar]
- Hardoon S, Hayes JF, Blackburn R, et al. (2013) Recording of severe mental illness in United Kingdom primary care, 2000-2010. PLoS One 8:e82365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardoon SL, Khadjesari Z, Nazareth I, et al. (2016) Monitoring of alcohol consumption in primary care among adults with bipolar disorder: a cross-sectional and retrospective cohort study. J Affect Disord 198:83–7. [DOI] [PubMed] [Google Scholar]
- Horsfall L, Walters K, Petersen I (2013) Identifying periods of acceptable computer usage in primary care research databases. Pharmacoepidemiol Drug Saf 22:64–9. [DOI] [PubMed] [Google Scholar]
- Houle SK, McAlister FA, Jackevicius CA, et al. (2012) Does performance-based remuneration for individual health care practitioners affect patient care? A systematic review. Ann Intern Med 157:889–99. [DOI] [PubMed] [Google Scholar]
- Hunt GE, Siegfried N, Morley K, et al. (2013) Psychosocial interventions for people with both severe mental illness and substance misuse. Cochrane Database Syst Rev, Issue 10. Art. No.: CD001088. doi: 10.1002/14651858.CD001088.pub3. [DOI] [PubMed] [Google Scholar]
- Johnson M, Jackson R, Guillaume L, et al. (2011) Barriers and facilitators to implementing screening and brief intervention for alcohol misuse: a systematic review of qualitative evidence. J Public Health 33:412–21. [DOI] [PubMed] [Google Scholar]
- Keurhorst MN, Anderson P, Spak F, et al. (2013) Implementing training and support, financial reimbursement, and referral to an internet-based brief advice program to improve the early identification of hazardous and harmful alcohol consumption in primary care (ODHIN): study protocol for a cluster randomized factorial trial. Implement Sci 8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadjesari Z, Marston L, Petersen I, et al. (2013) Alcohol consumption screening of newly-registered patients in primary care: a cross-sectional analysis. Br J Gen Pract 63:e706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen J, Lohonen J, Koponen H, et al. (2009) Prevalence of alcohol use disorders in schizophrenia--a systematic review and meta-analysis. Acta Psychiatr Scand 120:85–96. [DOI] [PubMed] [Google Scholar]
- Maguire A, Blak BT, Thompson M (2009) The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Saf 18:76–83. [DOI] [PubMed] [Google Scholar]
- Midanik LT. (1988) Validity of self-reported alcohol use: a literature review and assessment. Br J Addict 83:1019–30. [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Mental Health (UK) (2011) Psychosis with Coexisting Substance Misuse: Assessment and Management in Adults and Young People. Leicester (UK): British Psychological Society; NICE Clinical Guidelines, No. 120. [PubMed] [Google Scholar]
- NICE Clinical Guidance 115 (2011) Alcohol-Use Disorders: Diagnosis, Assessment and Management of Harmful Drinking and Alcohol Dependence. Manchester: National Institute for Health and Clinical Excellence. [PubMed] [Google Scholar]
- NICE Public Health Guidance 24 (2010) Alcohol-Use Disorders: Preventing The Development of Hazardous and Harmful Drinking. London: National Institute for Health and Clinical Excellence. [Google Scholar]
- Nilsen P, Aalto M, Bendtsen P, et al. (2006) Effectiveness of strategies to implement brief alcohol intervention in primary healthcare: a systematic review. Scand J Prim Health Care 24:5–15. [DOI] [PubMed] [Google Scholar]
- O'Donnell A. (2014) A mixed-methods investigation of the extent to which routinely collected information can help evaluate the implementation of screening and brief alcohol interventions in primary health care Institute of Health and Society, Faculty of Medical Sciences. UK: Newcastle University, 352 theses.ncl.ac.Vol. PhD. [Google Scholar]
- Osborn DP, Baio G, Walters K, et al. (2011) Inequalities in the provision of cardiovascular screening to people with severe mental illnesses in primary care: cohort study in the United Kingdom THIN Primary Care Database 2000-2007. Schizophr Res 129:104–10. [DOI] [PubMed] [Google Scholar]
- Powell BJ, Beidas RS, Lewis CC, et al. (2015) Methods to improve the selection and tailoring of implementation strategies. J Behav Health Serv Res. (E-pub ahead of print) doi: 10.1007/s11414-015-9475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prime Minister's Strategy Unit (2004) Alcohol Harm Reduction Strategy for England. London: Cabinet Office. [Google Scholar]
- Rehm J. (1998) Measuring quantity, frequency, and volume of drinking. Alcohol Clin Exp Res 22:4S–14S. [DOI] [PubMed] [Google Scholar]
- Scott A, Sivey P, Ait Ouakrim D, et al. (2011) The effect of financial incentives on the quality of health care provided by primary care physicians. Cochrane Database Syst Rev, Issue 9. Art. No.: CD008451. doi: 10.1002/14651858.CD008451.pub2. [DOI] [PubMed] [Google Scholar]
- StataCorp (2013) Stata Statistical Software: Release 13. College Station, TX: StataCorp LP. [Google Scholar]
- The Health Improvement Network (2012). In http://csdmruk.cegedim.com/s (ed).
- The NHS Information Centre—QOF Business Rules team (2011) New GMS Contract QOF Implementation. Dataset and Business Rules—Mental Health Indicator Set. Department of Health; http://www.pcc-cic.org.uk/sites/default/files/articles/attachments/mental_health_ruleset_v21.0.pdf. [Google Scholar]
- Townsend P, Phillimore P, Beattie A (1988) Health and Deprivation: Inequality and the North. London: Croom Helm. [Google Scholar]
- World Health Organisation (2010) Global Strategy to Reduce The Harmful Use of Alcohol. Italy: World Health Organisation. [Google Scholar]
- World Health Organisation (2013) Comprehensive Mental Health Action Plan 2013–2020. Geneva: World Health Organisation. [Google Scholar]