Abstract
Air pollution has been linked to hypertension in the general population, but data on gestational hypertension (GH) are limited. We investigated criteria air pollutants and air toxics during the period before conception and in early gestation in relation to GH risk in the Consortium on Safe Labor/Air Quality and Reproductive Health Study (United States, 2002–2008). Modified Community Multi-scale Air Quality models estimated air pollution exposures for 6,074 singleton pregnancies in which GH was present and 199,980 normotensive pregnancies. Generalized estimating equations estimated relative risks per interquartile-range increment for pollutants and high exposure (≥75th percentile) for air toxics after adjustment for major risk factors. For an interquartile-range increment, GH risk was significantly increased by 18% for sulfur dioxide during the 3 months before conception and, during gestational weeks 1–20, 17% for nitrogen oxides, 10% for particulate matter with an aerodynamic diameter <2.5 μm, 7% for particulate matter with an aerodynamic diameter <10 μm, and 22% for sulfur dioxide. High exposures to several polycyclic aromatic hydrocarbons before conception and during the first trimester were significantly associated with 8%–20% higher risk of GH. Further, preconceptional exposures to several volatile organic compounds were significantly associated with 11%–19% higher risk. Our findings suggest that early exposures to criteria air pollutants, particularly from transport emissions, and high exposure to several air toxics before conception may increase GH risk.
Keywords: ambient air pollution, gestational hypertension, polycyclic aromatic hydrocarbons, volatile organic compounds
Hypertensive disorders are the second leading cause of maternal mortality, accounting for 14% of such deaths worldwide between 2003 and 2009 (1). Emerging data have linked air pollution to hypertensive disorders (2). In particular, pregnancy is characterized by profound hemodynamic changes in response to the needs of the developing fetus (3). Air pollution may induce systematic oxidative stress, vascular inflammation, and endothelial dysfunction (4, 5), which can potentially elevate blood pressure. Therefore, periconceptional exposure to air pollution may aggravate physiologic changes in the vascular system and predispose women to hypertensive complications during pregnancy (6).
As a leading cause of maternal morbidity and mortality (7) and a significant risk factor for future cardiovascular disease (8), hypertensive disorders during pregnancy comprise a wide spectrum of severity and phenotypes (9). Indeed, 2 recent meta-analyses suggest heterogeneity in the association between air pollution and various pregnancy hypertensive disorders (10, 11), among which gestational hypertension (GH) is common but has received less research attention. Specifically, given that only 3 (12–14) of 17 studies in one meta-analysis (10) reported specific data on GH, no synthesis could be derived for this outcome separately. Furthermore, several important methodological issues preclude firm conclusions from the previous data. First, 16 of the 17 studies included in the meta-analysis by Pedersen et al. (10) used birth certificate data, which often do not allow separation of GH from other subtypes of hypertensive disorders during pregnancy, as opposed to medical records and/or hospital discharge data. Second, all 3 studies on air pollution and GH as a separate outcome are single-city based (12–14), if not from a single hospital (12), which potentially limits exposure contrasts (15). In the few studies with a relatively large geographic coverage, none adjusted for location or used any other approaches to reduce residual confounding due to spatial factors (10). Further, data on more source-specific measures are lacking, such as elemental composition of the particulate matter and hazardous air toxics, including polycyclic aromatic hydrocarbons (PAHs) and volatile organic compounds (VOCs). Finally, given the potential for lag effects of air pollution (16), evaluation of the previously unexamined chronic preconceptional exposure is warranted.
In view of the inconsistencies of previous findings and to further address the remaining critical data gaps, we aimed to investigate the associations of maternal exposure to criteria air pollutants (carbon monoxide, nitrogen oxides (NOx), ozone, particulate matter with aerodynamic diameter <2.5 and <10 μm (PM2.5 and PM10), and sulfur dioxide (SO2)), PM2.5 constituents (elemental carbon, ammonium particles, nitrate particles, organic compounds, sulfite particles, dust particles), and air toxics (PAHs and VOCs) during preconception and early gestation with risk of GH, in a contemporary nationwide US cohort.
METHODS
Study population
The Consortium on Safe Labor (CSL) was a retrospective, nationwide cohort study of labor and delivery that included 12 clinical centers (19 hospitals, 15 nonoverlapping hospital referral regions) across the United States. As previously described in detail (17), data on maternal demographic characteristics, medical history, labor, delivery, and obstetric and neonatal outcomes of 228,438 births at ≥23 weeks of gestation were extracted from electronic medical records from 2002–2008. We excluded pregnancies with multiple gestation (n = 5,053) and missing air quality data (n = 10) or maternal age (n = 307) as well as those in which the women had chronic hypertension (n = 4,358) or superimposed preeclampsia (n = 1,889) and thus were not at risk for new-onset hypertension during pregnancy (9). We further excluded women with other hypertensive disorders during pregnancy (i.e., preeclampsia (n = 10,528) and eclampsia (n = 239)) to allow comparison of women with GH (n = 6,074) to a normotensive reference group (n = 199,980). This resulted in an analytical sample of 206,054 singleton pregnancies among 188,658 women, 92% of whom contributed 1 pregnancy during the study period. The study was approved by the institutional review boards of all participating institutions noted in the acknowledgements. All records were anonymized and individual patient consent was not required.
Outcome ascertainment
Women with GH, with an onset at or after week 20 of gestation by definition during the study period (2002–2008) (9), were identified from electronic medical records and/or the maternal discharge summary using International Classification of Diseases, Ninth Revision, diagnostic codes (642.30–642.34). Most likely, the medical record diagnoses were consistent with clinical practice of the time, although the precise date or clinical details of the diagnoses were not available. Further, although the use of electronic medical record data for ascertainment of GH in particular has not been validated, previous validation studies of the CSL data comparing systematically extracted electronic medical record data against manually abstracted chart data showed high validity (range, 91.9%–99.9%) for other perinatal outcomes, suggesting the reasonably accurate representation of electronic medical records for medical charts (17).
Exposure assessment
The Air Quality and Reproductive Health Study developed detailed air quality assessment models in 2013 for CSL patient data, and we linked the medical record data described above to exposure assessments (18). Exposures to ambient air pollutants (criteria pollutants, PM2.5 constituents, and hazardous air toxics) were estimated using a modified version of Community Multi-scale Air Quality (CMAQ) model 4.7.1 with a 36-km horizontal resolution domain (18). Due to the anonymity of the CSL data, air pollutant exposures were based on the average pollutant concentrations in women's delivery hospital referral region (range, 415–312,644 km2) during each of the specified exposure windows (19). The CMAQ simulations were based on the meteorology data derived from the Weather Research and Forecasting model and emission data generated using the US Environmental Protection Agency National Emissions Inventory. Model results were weighted to reflect population density within the hospital referral region, discounting areas where women were unlikely to live and work. Hourly measurements of pollutant exposures were calculated over the entire US continent from 2001–2010 in the Air Quality and Reproductive Health study as previously described (20).
Despite the wide use of the CMAQ model in estimating regional air quality, potential biases in meteorology and emission inputs, uncertainties of other model components, and issues with spatial resolution can compromise the precision in estimation (20). Thus, we used an inverse distance weighted method to recalibrate the raw CMAQ estimations for criteria pollutants using observational air-quality monitor data retrieved from the US Environmental Protection Agency Air Quality System. This observation-fused technique led to significant improvement of the model performance and was demonstrated to best account for spatial variation in air pollutants and population density as compared with 4 alternative methods (20). Constituents of PM2.5, PAHs, and VOCs were based on raw CMAQ model output due to the lack of routinely monitored data on these pollutants. To address potentially critical timing of exposures, we assessed exposures across several a priori time windows: 3 months before conception (as a proxy of preconceptional chronic exposure), the first trimester (gestational weeks 1–13), and a 20-week average from weeks 1–20 (as a proxy of the average gestational exposure before diagnosis). Because GH is diagnosed after 20 weeks by definition, these exposure windows were selected to precede diagnosis. Gestational age in weeks was calculated from gestational age at delivery using the best obstetrical estimate as recorded in the medical record.
Covariates
A priori selected covariates were extracted from medical records: age at childbirth (continuous), race/ethnicity (white, black, Hispanic, Asian/Pacific Islander, other/unknown), marital status (married, unmarried, missing/unknown), insurance (private, public, self-pay/other), parity (nulliparous, multiparous), prepregnancy body mass index (calculated as weight (kg)/height (m)2; <18.5, 18.5–24.9, 25.0–29.9, ≥30.0, or unknown), preexisting chronic disease (any (diabetes mellitus, asthma, thyroid disease, or human immunodeficiency virus), none), smoking during pregnancy (yes, no), alcohol consumption during pregnancy (yes, no), and season of conception (spring, summer, fall, winter). Analyses were also adjusted for study site to account for both measured and unmeasured area-level indicators, including but not limited to socioeconomic status, case ascertainment, and sources of air pollution exposures. Given the small percentages of missing data on race/ethnicity (4.2%) and marital status (3.1%), missing/unknown categories were assigned for each. To reduce bias due to a relatively high proportion of missing data on prepregnancy body mass index (n = 68,322, 33%), we used multiple imputation based on all other covariates, exposures of interest, and GH status to create 10 complete datasets, and we combined the analytical results on each complete dataset using Rubin's rule (21).
Statistical analysis
Pregnancy was the unit of analysis in all statistical testing. Descriptive statistics for participant characteristics were presented as mean (standard deviation) for continuous variables and percentages for categorical variables. P values for comparing participant characteristics by status of GH were obtained from unadjusted generalized estimating equations, with robust standard errors to account for multiple pregnancies of the same woman during the study period. Distributions of exposures to criteria air pollutants and air toxics were presented as median and interquartile range (IQR) during each of the specified time windows.
Examination of the associations between criteria air pollutants in quartiles and risk of GH suggested a linear relationship. Therefore, criteria air pollutants and PM2.5 constituents were parameterized as per IQR in their original scale for ease of interpretation and comparability to previous data (10, 11). Generalized estimating equations with a log link function (22) were fitted to estimate relative risk and 95% confidence interval for GH per IQR increment of each criteria air pollutant and PM2.5 constituent during the specified exposure windows, adjusting for the covariates listed above. The robust sandwich standard errors were calculated to account for misspecified mean-variance relationship as well as clustering due to multiple births of the same woman during the study period. For hazardous air toxics (because PAHs and VOCs were generally observed at very low levels, and the exposures estimated by the CMAQ model were not fused with existing monitor data), we dichotomized the exposure to estimate risk associated with high exposure (≥75th percentile) rather than assuming a linear model. In addition to the fact that the CMAQ model accounts for biochemical reactions among air pollutants, effects of weather, and long-term sources of pollutants (18), multicollinearity among air pollutants derived from the same model remains a major methodological concern in multipollutant models. Therefore, criteria air pollutants and air toxics were fitted in the single-pollutant model separately during each exposure window. Post hoc multiple-comparison adjustment for P values was performed using the Benjamini-Hochberg false discovery rate–controlling method (23) within each exposure window for criteria air pollutants, PM2.5 constituents, PAHs, and VOCs. The false discovery rate–controlling method was designed to control the expected proportion of falsely rejected hypotheses, which has greater power than methods controlling the family-wise type I error rate, such as Bonferroni correction (23).
To evaluate the robustness of the findings, we performed a series of sensitivity analyses. First, we stratified the analyses by parity and smoking, given the decrease in risk with higher parity and smoking (24). We also performed sensitivity analysis restricted to women who had complete data on prepregnancy body mass index (n = 137,732, 67%). Finally, to assess the impact of potential exposure misclassification due to the use of the hospital referral region as the geographic unit (given the anonymity of the CSL data), we performed simulation extrapolation procedures (25) on SO2 during the first trimester as an illustration of the potential exposure misclassification, assuming an additive measurement error rate of 10% or 20% within each site. The simulation extrapolation method was performed in 3 steps implemented in R package “simex”: 1) added known increments of measurement error to the data; 2) estimated the regression parameter of interest with the contaminated data; and 3) established a trend of the parameter estimates and extrapolated back to the case of no measurement error. All analyses were conducted using SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina), and R, version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Among 206,054 singleton deliveries, we identified 6,074 (3%) pregnancies in which GH was present. Compared with their normotensive counterparts, women with GH were slightly younger, more likely to be white or black, not married, nulliparous, and overweight or obese, as well as more likely to consume alcohol during pregnancy and to have preexisting chronic diseases (Table 1). Distributions of criteria air pollutants and air toxics by exposure window are available in Web Table 1 (available at https://academic.oup.com/aje).
Table 1.
Participant Characteristics by Gestational Hypertension Status (n = 206,054), Consortium on Safe Labor/Air Quality and Reproductive Health Study, United States, 2002–2008
| Characteristic | Normotensive (n = 199,980) | Gestational Hypertension (n = 6,074) | P Valuea | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Maternal age, yearsb | 27.5 (6.1) | 26.9 (6.2) | <0.001 | ||
| Race/ethnicity | <0.001 | ||||
| White | 100,541 | 50.3 | 3,386 | 55.7 | |
| Black | 42,519 | 21.3 | 1,567 | 25.8 | |
| Hispanic | 35,187 | 17.6 | 784 | 12.9 | |
| Asian/Pacific Islander | 13,251 | 6.6 | 189 | 3.1 | |
| Other/unknown | 8,482 | 4.2 | 148 | 2.4 | |
| Marital status | <0.001 | ||||
| Married | 119,102 | 59.6 | 3,425 | 56.4 | |
| Not married | 74,535 | 37.3 | 2,538 | 41.8 | |
| Missing/unknown | 6,343 | 3.2 | 111 | 1.8 | |
| Insurance | <0.001 | ||||
| Private | 112,640 | 56.3 | 3,460 | 57.0 | |
| Public | 66,059 | 33.0 | 2,298 | 37.8 | |
| Self-pay/other | 21,281 | 10.6 | 316 | 5.2 | |
| Nulliparous | 77,293 | 38.7 | 3,411 | 56.2 | <0.001 |
| Prepregnancy BMIb,c | 25.0 (5.9) | 28.2 (7.2) | <0.001 | ||
| <18.5 | 7,579 | 3.8 | 117 | 1.9 | |
| 18.5–24.9 | 73,959 | 37.0 | 1,497 | 24.6 | |
| 25–29.9 | 29,763 | 14.9 | 1,064 | 17.5 | |
| ≥30.0 | 22,414 | 11.2 | 1,339 | 22.0 | |
| Unknown | 66,265 | 33.1 | 2,057 | 33.9 | |
| Smoking during pregnancy | 13,364 | 6.7 | 386 | 6.4 | 0.3 |
| Alcohol consumption during pregnancy | 3,634 | 1.8 | 140 | 2.3 | 0.006 |
| Preexisting chronic disease | |||||
| Pregestational diabetes | 2,218 | 1.1 | 124 | 2.0 | <0.001 |
| Asthma | 14,789 | 7.4 | 557 | 9.2 | <0.001 |
| Thyroid disease | 5,700 | 2.9 | 221 | 3.6 | <0.001 |
| Human immunodeficiency virus | 775 | 0.4 | 15 | 0.2 | <0.001 |
| Season of conception | 0.2 | ||||
| Spring (March–May) | 47,140 | 23.6 | 1,493 | 24.6 | |
| Summer (June–August) | 51,574 | 25.8 | 1,596 | 26.3 | |
| Fall (September–November) | 55,297 | 27.7 | 1,546 | 25.5 | |
| Winter (December–February) | 45,969 | 23.0 | 1,439 | 23.7 | |
Abbreviation: BMI, body mass index.
aP values were obtained by generalized estimating equations, accounting for multiple pregnancies of the same woman during the study period.
b Mean values (standard deviation).
c BMI was calculated as weight (kg)/height (m)2.
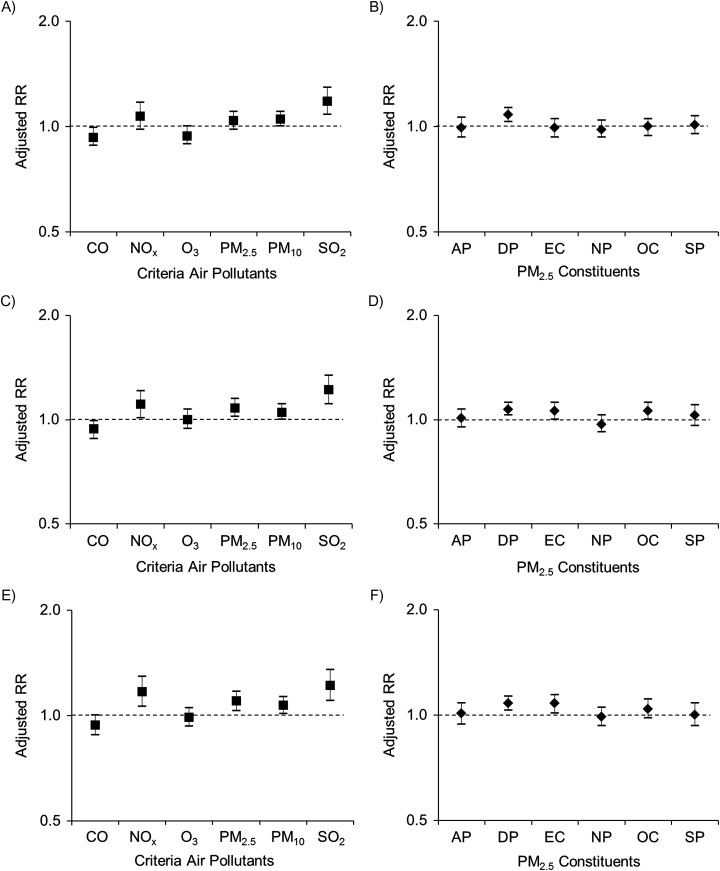
Risk of GH per IQR increase in criteria air pollutant was significantly increased by 18% (95% confidence interval (CI): 8, 29) for exposure to SO2 during the 3 months before conception and, during the first trimester, 11% (95% CI: 1, 21) for NOx, 8% (95% CI: 2, 15) for PM2.5, and 22% (95% CI: 11, 34) for SO2, after adjustment for covariates (all false discovery rate–adjusted P values were <0.05) (Figure 1; see Web Table 2 for point estimates). Results for criteria pollutants during gestational weeks 1–20, as a proxy of the average gestational exposure before diagnosis, were similar to those for trimester 1 (weeks 1–13). Among the constituents of PM2.5, an IQR increment in dust particles was significantly associated with a 7%–8% higher risk of GH consistently across all exposure windows (Figure 1; all false discovery rate–adjusted P values were <0.05, Web Table 2). Moreover, an IQR increment in elemental carbon exposure across gestational weeks 1–20 was significantly associated with an 8% higher risk of GH.
Figure 1.
Adjusted relative risks (RRs) for gestational hypertension associated with an interquartile-range increment of exposure to criteria air pollutants and constituents of particulate matter with aerodynamic diameter ≤2.5 μm (PM2.5) during 3 months before conception (A, B), gestational weeks 1–13 (C, D), and gestational weeks 1–20 (E, F), Consortium on Safe Labor/Air Quality and Reproductive Health Study, United States, 2002–2008. The models adjusted for study site, maternal age, race/ethnicity, marital status, insurance, parity, prepregnancy body mass index, preexisting chronic disease, smoking during pregnancy, alcohol consumption during pregnancy, and season of conception. AP, ammonium particles; CO, carbon monoxide; DP, dust particles; EC, elemental carbon; NOx, nitrogen oxides; NP, nitrate particles; O3, ozone; OC, organic compounds; PM10, particulate matter with aerodynamic diameter ≤10 μm; SO2, sulfur dioxide; SP, sulfite particles. Bars, 95% confidence intervals.
After post hoc false discovery rate adjustment, higher exposures (≥75th percentile) to several PAH exposures during the 3 months before conception were positively associated with risk of GH, with a higher risk of 11% for benzo[a]-pyrene (95% CI: 3, 18), fluoranthene (95% CI: 3, 21), and ideno[1,2,3-Cd]pyrene (95% CI: 4, 19) and 20% (95% CI: 9, 32) for naphthalene (Table 2). A higher risk of GH, ranging from 8%–13%, was also observed in association with exposures ≥75th percentile to benzo[a]pyrene, ideno[1,2,3-cd]pyrene, and naphthalene during the first trimester, with slightly smaller point estimates compared with the period before conception. As for VOCs, a higher risk of GH was observed for higher exposures to cyclohexane, ethylbenzene, m-xylene, n-hexane, o-xylene, sesquiterpene, and toluene during the 3 months before conception (all false discovery rate–adjusted P values were <0.05, Table 3). The positive associations with o-xylene and toluene from weeks 1–20 did not persist after false discovery rate adjustment.
Table 2.
Adjusted Relative Risks for Gestational Hypertension Associated With High Exposure to Polycyclic Aromatic Hydrocarbons According to Exposure Window (n = 206,054)a, Consortium on Safe Labor/Air Quality and Reproductive Health Study, United States, 2002–2008
| Polycyclic Aromatic Hydrocarbon | 3 Months Before Conception | Trimester 1b | Gestational Weeks 1–20 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | P Valuec | RR | 95% CI | P Valuec | RR | 95% CI | P Valuec | |
| Acenaphthene | 1.05 | 0.97, 1.14 | 0.390 | 1.02 | 0.94, 1.12 | 0.883 | 0.96 | 0.88, 1.05 | 0.783 |
| Acenaphthylene | 1.01 | 0.93, 1.10 | 0.811 | 1.00 | 0.92, 1.10 | 0.933 | 0.99 | 0.91, 1.08 | 0.832 |
| Anthracene | 0.98 | 0.91, 1.05 | 0.710 | 1.02 | 0.94, 1.10 | 0.883 | 1.02 | 0.95, 1.10 | 0.783 |
| Benzo[a]anthracene | 0.99 | 0.92, 1.06 | 0.815 | 1.05 | 0.97, 1.13 | 0.600 | 1.04 | 0.96, 1.12 | 0.783 |
| Benzo[a]pyrene | 1.11 | 1.03, 1.18 | 0.012 | 1.08 | 1.02, 1.16 | 0.048 | 1.09 | 1.02, 1.16 | 0.102 |
| Chrysene | 1.06 | 0.99, 1.14 | 0.183 | 1.00 | 0.93, 1.07 | 0.933 | 0.99 | 0.92, 1.06 | 0.783 |
| Fluoranthene | 1.11 | 1.03, 1.21 | 0.030 | 1.05 | 0.96, 1.14 | 0.679 | 1.03 | 0.94, 1.12 | 0.783 |
| Fluorene | 1.04 | 0.96, 1.12 | 0.460 | 1.02 | 0.94, 1.11 | 0.883 | 1.02 | 0.94, 1.11 | 0.783 |
| Ideno[1,2,3-Cd]pyrene | 1.11 | 1.04, 1.19 | 0.012 | 1.09 | 1.03, 1.16 | 0.048 | 1.08 | 1.01, 1.15 | 0.102 |
| Naphthalene | 1.20 | 1.09, 1.32 | 0.001 | 1.13 | 1.03, 1.25 | 0.048 | 1.07 | 0.96, 1.19 | 0.783 |
| Phenanthrene | 1.07 | 1.00, 1.15 | 0.120 | 0.98 | 0.91, 1.05 | 0.882 | 0.98 | 0.91, 1.05 | 0.783 |
| Pyrene | 1.10 | 1.01, 1.20 | 0.072 | 1.01 | 0.92, 1.11 | 0.932 | 1.04 | 0.94, 1.15 | 0.783 |
Abbreviations: CI, confidence interval; RR, relative risk.
a Adjusted for study site, maternal age, race/ethnicity, marital status, insurance, parity, prepregnancy body mass index, preexisting chronic disease, smoking during pregnancy, alcohol consumption during pregnancy, and season of conception. Levels of air toxics were dichotomized at the 75th percentile, and high exposure (≥75th percentile) was compared with the rest of the distribution.
b Trimester 1 refers to gestational weeks 1–13.
cP values were false discovery rate–adjusted within each exposure window.
Table 3.
Adjusted Relative Risks for Gestational Hypertension Associated With High Exposure to Volatile Organic Compounds According to Exposure Window (n = 206,054)a, Consortium on Safe Labor/Air Quality and Reproductive Health Study, United States, 2002–2008
| Volatile Organic Compound | 3 Months Before Conception | Trimester 1b | Gestational Weeks 1–20 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | P Valuec | RR | 95% CI | P Valuec | RR | 95% CI | P Valuec | |
| Benzene | 1.06 | 0.94, 1.20 | 0.452 | 1.07 | 0.95, 1.21 | 0.596 | 1.08 | 0.95, 1.23 | 0.514 |
| 1,3-butadiene | 0.98 | 0.91, 1.06 | 0.813 | 0.99 | 0.91, 1.07 | 0.878 | 1.03 | 0.94, 1.12 | 0.746 |
| Cyclohexane | 1.12 | 1.02, 1.23 | 0.042 | 0.99 | 0.90, 1.09 | 0.878 | 1.00 | 0.90, 1.10 | 0.945 |
| Ethylbenzene | 1.18 | 1.03, 1.35 | 0.042 | 1.09 | 0.94, 1.25 | 0.596 | 1.07 | 0.93, 1.24 | 0.625 |
| Methyl-tertiary butyl ether | 1.06 | 0.98, 1.13 | 0.218 | 1.03 | 0.96, 1.11 | 0.691 | 1.06 | 0.99, 1.15 | 0.228 |
| Methyl ethyl ketone | 0.99 | 0.91, 1.08 | 0.912 | 0.98 | 0.90, 1.08 | 0.878 | 0.96 | 0.88, 1.06 | 0.627 |
| M-xylene | 1.19 | 1.04, 1.36 | 0.042 | 1.03 | 0.89, 1.20 | 0.878 | 1.09 | 0.94, 1.27 | 0.514 |
| N-hexane | 1.16 | 1.05, 1.29 | 0.028 | 1.06 | 0.95, 1.18 | 0.596 | 1.03 | 0.92, 1.17 | 0.746 |
| O-xylene | 1.19 | 1.04, 1.36 | 0.042 | 1.23 | 1.07, 1.41 | 0.056 | 1.17 | 1.01, 1.35 | 0.201 |
| P-xylene | 1.13 | 1.00, 1.26 | 0.081 | 1.01 | 0.88, 1.16 | 0.894 | 1.02 | 0.88, 1.19 | 0.889 |
| Propene | 1.01 | 0.94, 1.08 | 0.912 | 1.02 | 0.95, 1.10 | 0.828 | 1.00 | 0.92, 1.07 | 0.945 |
| Sesquiterpene | 1.11 | 1.04, 1.20 | 0.028 | 1.07 | 0.99, 1.15 | 0.397 | 1.08 | 1.00, 1.16 | 0.201 |
| Styrene | 1.00 | 0.93, 1.08 | 0.912 | 1.07 | 0.98, 1.15 | 0.413 | 1.07 | 0.99, 1.16 | 0.288 |
| Toluene | 1.17 | 1.03, 1.33 | 0.042 | 1.20 | 1.04, 1.38 | 0.084 | 1.17 | 1.01, 1.35 | 0.201 |
Abbreviations: CI, confidence interval; RR, relative risk.
a Adjusted for study site, maternal age, race/ethnicity, marital status, insurance, parity, prepregnancy body mass index, preexisting chronic disease, smoking during pregnancy, alcohol consumption during pregnancy, and season of conception. Levels of air toxics were dichotomized at the 75th percentile, and high exposure (≥75th percentile) was compared with the rest of the distribution.
b Trimester 1 refers to gestational weeks 1–13.
cP values were false discovery rate–adjusted within each exposure window.
We observed similar results in the sensitivity analysis restricted to multiparous women (n = 125,350, 61%), whereas models restricted to nulliparous women did not converge due to the smaller sample size (data not shown). Likewise, results were robust among nonsmokers during pregnancy (n = 192,304, 93%). Findings were also similar when we restricted our analysis to women whose prepregnancy body mass index was not missing (data not shown). When we examined the potential impact of 10% or 20% exposure misclassification using simulation extrapolation procedures, we found similar results for SO2 exposure during the first trimester in association with risk of GH (Table 4 and Web Figure 1).
Table 4.
Naive and Simulation Extrapolation Estimation of the Association Between Ambient SO2 Exposure During the First Trimester With Risk Of Gestational Hypertension (n = 206,054), Consortium on Safe Labor/Air Quality and Reproductive Health Study, United States, 2002–2008
| Estimation Method | Adjusted RRa | 95% CI |
|---|---|---|
| Naive estimationb | 1.22 | 1.14, 1.31 |
| Simulation extrapolation estimation (10% measurement error) | 1.26 | 1.16, 1.37 |
| Simulation extrapolation estimation (20% measurement error) | 1.28 | 1.18, 1.40 |
Abbreviations: CI, confidence interval; RR, relative risk.
a Adjusted for study site, maternal age, race/ethnicity, marital status, insurance, parity, prepregnancy body mass index, preexisting chronic disease, smoking during pregnancy, alcohol consumption during pregnancy, and season of conception.
b The naive estimation was based on the same Poisson model reported in Web Table 2, except that we did not account for multiple pregnancies of the same woman, because the simex function in R does not allow clustering. Because most participants contributed only 1 pregnancy (92%), we would expect the inference from Poisson regression to be very close to that from generalized estimating equations (adjusted RR = 1.22, 95% CI: 1.11, 1.34; Web Table 2).
DISCUSSION
In this large retrospective cohort, we observed that exposures to NOx, PM2.5, and SO2 during the first trimester, and NOx, PM2.5, PM10, and SO2 during gestational weeks 1–20 were associated with higher risk of GH. Among PM2.5 constituents, an IQR increment of exposure to dust particles was associated with a 7%–8% higher risk of GH during both the 3 months before conception and weeks 1–20 of gestation. Elemental carbon was only positively and significantly related to the risk during the gestational window from weeks 1–20. PAHs, benzo-[a]pyrene and ideno[1,2,3-cd]pyrene, were associated with an 8%–11% higher risk across all time periods. Further, higher exposures (≥75th percentile) to several VOCs were associated with an 11%–19% higher risk only during the 3 months before conception, suggesting a potential chronic pathway as opposed to an acute one during early gestation.
Given the heterogeneity in severity and phenotype of hypertensive disorders during pregnancy (9) and inconsistent findings on these disorders as a combined outcome (10, 11), investigation of the association of air pollution with GH as a separate outcome is warranted (10, 26). Indeed, similar to findings by Savitz et al. (26), we previously demonstrated overall null associations between exposures to air pollutants during early gestation and risk of preeclampsia among nonasthmatic women (27) as opposed to findings on GH in the current study. However, among the 17 studies included in a recent meta-analysis on air pollution in relation to pregnancy-induced hypertensive disorders (10), only 3 treated GH separately (12–14). Specifically, van den Hooven et al. (13) reported a 72% increased risk of GH (95% CI: 1.12, 2.63) for whole-gestation PM10 exposure (per 10-μg/m3 increase) in Rotterdam, Netherlands, whereas Lee et al. (12) observed an 11% increased risk (95% CI: 1.00, 1.23) for first-trimester PM2.5 exposure (per IQR increase) but not for PM10 in Pittsburgh, Pennsylvania. These findings are generally in line with ours, although the point estimate reported in Netherlands was greater (13), which might be partly explained by the higher levels of PM10 exposure than those in our study (whole-gestation mean = 30.7 (standard deviation, 3.9) μg/m3 vs. 22.1 (standard deviation, 4.4) μg/m3). In a population-based study in New York, New York, despite the positive associations between first- or second-trimester exposures to PM2.5 and NO2 and risk of GH, associations were attenuated toward the null after further adjustment for delivery hospital (26). As discussed by Savitz et al. (26), a relatively large geographic coverage might address the issue of small exposure contrasts, whereas the uncertain validity of adjusting for location-related factors should be recognized, which may partially adjust out the influence of spatial variation. Nonetheless, our findings remain robust even after adjustment for study site and a simulation to consider potential exposure misclassification.
Our novel findings of positive associations between GH risk and SO2 exposures both before conception and during early gestation address a data gap on this criteria pollutant. In contrast, the positive associations of GH with NOx and particulate matter were only significant during the early gestation window. Collectively, these data suggest that the associations were more pronounced during early gestation for air pollutants that are primarily from transport emissions (i.e., NOx and particulate matter), whereas SO2, a pollutant released mainly during fossil fuel combustion, might have a more chronic and sustained pathway.
Despite the lack of previous epidemiologic data on PM2.5 constituents and risk of GH, our novel findings on positive associations of dust particles and elemental carbon with increased risk of GH are biologically plausible. Animal studies suggest that acute cardiovascular dysregulation in response to PM2.5 exposure is driven mostly by elemental carbon and its fractions (28). In addition, concentrated dust-storm particles induce adverse cardiovascular effects on spontaneously hypertensive rats, suggesting its toxic nature (29). Indeed, certain particle constituents may reach the systemic circulation via inhalation, stimulating endothelial dysfunction and vasomotor imbalance via vascular inflammation and oxidative stress (30–32).
Notably, we observed novel, positive associations of GH with air toxics, particularly VOCs before conception. Consistent with our findings, available yet limited data suggest that total VOC exposure is significantly associated with higher levels of blood pressure among overweight/obese individuals (33). In particular, we observed a 17% higher risk of GH associated with preconceptional exposure to toluene, which originates mostly from gasoline/solvent usage, automobile emissions, and paint/varnish/adhesive evaporation (34). Similarly, toluene has been linked to higher blood pressure among synthetic leather workers and drivers (35, 36). In addition, mixed (m-, o-, and p-) xylenes, commonly used industrial products via methylation of toluene and benzene (37), have been linked to adverse reproductive outcomes including fetal resorption, anomalies, and decreased fetal weight in animals (38, 39). Taken together, despite the relatively low exposure levels, the ubiquitous and involuntary nature of these underappreciated exposures to ambient air toxics warrants further investigation in relation to reproductive outcomes. Further, regarding the observed pollutant- and toxic-specific associations, in addition to the limited mechanistic data on particular exposures in relation to the disease outcome as discussed above, these novel findings suggest areas for future investigation to elucidate potential mechanisms beyond overall oxidative stress and inflammatory effects (40).
There are several notable strengths of this study. In this national cohort with both temporal and spatial dispersion, we were able to examine the associations of criteria pollutants and source-specific exposures (PM2.5 constituents, PAHs, and VOCs) within specific exposure windows. The ascertainment of outcome and other detailed clinical factors as covariates was based on medical records and hospital discharge data, which may be unavailable or unreliably reported in investigations that use birth certificates or insurance data (41). Further, we accounted for location-related factors by adjusting for study site, which is a common limitation in previous studies as recognized by Pedersen et al. (10) in a recent meta-analysis.
Several limitations of the study should be noted. First, exposure misclassification cannot be ruled out; we used hospital referral region as the geographic unit due to the anonymity of the CSL data. Women may move during the exposure window of interest, although this should be nondifferential by case status. Maternal residential mobility during pregnancy tends to involve short-distance moves with a median of <10 km (42), which would likely leave most women in the same hospital referral region. Further, the simulation extrapolation demonstrated the robustness of the results for SO2 exposure during the first trimester with a small to moderate measurement error rate. Second, because the dates of diagnoses were not available from the medical records, we were unable to evaluate potential competing risks of other hypertensive disorders of pregnancy and, as such, focus on women who were normotensive throughout pregnancy in comparison with those who had GH at delivery. Because GH occurs after 20 weeks of gestation by definition, we selected exposure windows before week 20 to ensure that the exposure always preceded the outcome. Furthermore, although the etiology of GH remains to be confirmed, this pregnancy complication is believed to begin in early pregnancy, when important events including placentation and organogenesis occur. Third, given that our cohort was compiled during 2002–2008, more recent data applying the new definition of hypertensive disorders during pregnancy by the American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy (43) are warranted to confirm our findings. In particular, the impact of classifying new-onset hypertension without proteinuria, but with new onset of other symptoms, as preeclampsia (which could have been diagnosed as GH by the old definition) is yet to be assessed. Finally, we could not completely rule out the possibility of findings due to chance given the potential multiplicity issue, despite the false discovery rate adjustment. However, the number of significant associations with a consistent direction may suggest a pattern of findings beyond chance.
In conclusion, our findings based on a large, contemporary nationwide cohort suggest that early pregnancy is a critical exposure window particularly for transport emission–related pollutants (NOx, PM2.5, and PM10) in relation to risk of GH. In addition, SO2 exposure both before conception and during early gestation illustrated a consistent pattern of 18%–22% higher risk of GH. Further, several air toxics, particularly greater levels of several VOCs and some PAHs during the 3 months before conception, were significantly associated with higher risk of GH. The potential chronic impact of these air toxics associated with preconceptional exposures on reproductive outcomes warrants further investigation.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of Research, Kaiser Permanente Northern California, Oakland, California (Yeyi Zhu); Epidemiology Branch, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland (Yeyi Zhu, Cuilin Zhang, Sandie Ha, Sung Soo Kim, Pauline Mendola); Biostatistics and Bioinformatics Branch, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland (Danping Liu); and Department of Global and Community Health, George Mason University, Fairfax, Virginia (Anna Pollack).
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (Contract HHSN267200603425C, Consortium on Safe Labor; Contract HHSN275200800002I; and Task Order HHSN27500008, Air Quality and Reproductive Health).
We thank all the participating study centers involved in the Consortium on Safe Labor: Baystate Medical Center, Springfield, Massachusetts; Cedars-Sinai Medical Center Burnes Allen Research Center, Los Angeles, California; Christiana Care Health System, Newark, Delaware; Georgetown University Hospital, MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, Indiana; Intermountain Healthcare and the University of Utah, Salt Lake City, Utah; Maimonides Medical Center, New York, New York; MetroHealth Medical Center, Cleveland, Ohio; Summa Health System, Akron City Hospital, Akron, Ohio; University of Illinois at Chicago, Chicago, Illinois; University of Miami, Miami, Florida; and University of Texas Health Science Center at Houston, Houston, Texas. We also thank the EMMES Corporation, Rockville, Maryland, which provided data coordination, and the Texas A&M Supercomputing Facility and the Texas Advanced Computing Center, which provided computing resources essential to completing the air quality simulations for exposure estimations in this study.
Conflict of interest: none declared.
REFERENCES
- 1. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323–e333. [DOI] [PubMed] [Google Scholar]
- 2. Giorgini P, Di Giosia P, Grassi D, et al. Air pollution exposure and blood pressure: an updated review of the literature. Curr Pharm Des. 2015;22(1):28–51. [DOI] [PubMed] [Google Scholar]
- 3. Yoder SR, Thornburg LL, Bisognano JD. Hypertension in pregnancy and women of childbearing age. Am J Med. 2009;122(10):890–895. [DOI] [PubMed] [Google Scholar]
- 4. Brook RD, Rajagopalan S, Pope CA 3rd, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. [DOI] [PubMed] [Google Scholar]
- 5. Shah AS, Langrish JP, Nair H, et al. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet. 2013;382(9897):1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slama R, Darrow L, Parker J, et al. Meeting report: atmospheric pollution and human reproduction. Environ Health Perspect. 2008;116(6):791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. ACOG technical bulletin. Hypertension in pregnancy. Number 219–January 1996 (replaces no. 91, February 1986). Committee on Technical Bulletins of the American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 1996;53(2):175–183. [PubMed] [Google Scholar]
- 8. Freibert SM, Mannino DM, Bush H, et al. The association of adverse pregnancy events and cardiovascular disease in women 50 years of age and older. J Womens Health (Larchmt). 2011;20(2):287–293. [DOI] [PubMed] [Google Scholar]
- 9. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22. [PubMed] [Google Scholar]
- 10. Pedersen M, Stayner L, Slama R, et al. Ambient air pollution and pregnancy-induced hypertensive disorders: a systematic review and meta-analysis. Hypertension. 2014;64(3):494–500. [DOI] [PubMed] [Google Scholar]
- 11. Hu H, Ha S, Roth J, et al. Ambient air pollution and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Atmos Environ (1994). 2014;97:336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee PC, Roberts JM, Catov JM, et al. First trimester exposure to ambient air pollution, pregnancy complications and adverse birth outcomes in Allegheny County, PA. Matern Child Health J. 2013;17(3):545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van den Hooven EH, de Kluizenaar Y, Pierik FH, et al. Air pollution, blood pressure, and the risk of hypertensive complications during pregnancy: the Generation R Study. Hypertension. 2011;57(3):406–412. [DOI] [PubMed] [Google Scholar]
- 14. Vigeh M, Yunesian M, Shariat M, et al. Environmental carbon monoxide related to pregnancy hypertension. Women Health. 2011;51(8):724–738. [DOI] [PubMed] [Google Scholar]
- 15. Pedersen M, Siroux V, Pin I, et al. Does consideration of larger study areas yield more accurate estimates of air pollution health effects? An illustration of the bias-variance trade-off in air pollution epidemiology. Environ Int. 2013;60:23–30. [DOI] [PubMed] [Google Scholar]
- 16. van den Hooven EH, de Kluizenaar Y, Pierik FH, et al. Chronic air pollution exposure during pregnancy and maternal and fetal C-reactive protein levels: the Generation R Study. Environ Health Perspect. 2012;120(5):746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang J, Troendle J, Reddy UM, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203(4):326.e1–326.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Foley KM, Roselle SJ, Appel KW, et al. Incremental testing of the Community Multiscale Air Quality (CMAQ) modeling system version 4.7. Geosci Model Dev. 2010;3:205–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The Dartmouth Institute for Health Policy and Clinical Practice The Dartmouth Atlas of Health Care. Lebanon, NH: The Trustees of Dartmouth College; 2013. [Google Scholar]
- 20. Chen G, Li JY, Ying Q, et al. Evaluation of observation-fused regional air quality model results for population air pollution exposure estimation. Sci Total Environ. 2014;485–486:563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10(4):585–598. [DOI] [PubMed] [Google Scholar]
- 22. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 23. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol. 1995;57(1):289–300. [Google Scholar]
- 24. Savitz DA, Danilack VA, Engel SM, et al. Descriptive epidemiology of chronic hypertension, gestational hypertension, and preeclampsia in New York State, 1995–2004. Matern Child Health J. 2014;18(4):829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cook JR, Stefanski LA. Simulation-extrapolation estimation in parametric measurement error models. J Am Stat Assoc. 1994;89(428):1314–1328. [Google Scholar]
- 26. Savitz DA, Elston B, Bobb JF, et al. Ambient fine particulate matter, nitrogen dioxide, and hypertensive disorders of pregnancy in New York City. Epidemiology. 2015;26(5):748–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mendola P, Wallace M, Liu D, et al. Air pollution exposure and preeclampsia among US women with and without asthma. Environ Res. 2016;148:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wagner JG, Kamal AS, Morishita M, et al. PM2.5-induced cardiovascular dysregulation in rats is associated with elemental carbon and temperature-resolved carbon subfractions. Part Fibre Toxicol. 2014;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chang CC, Hwang JS, Chan CC, et al. Effects of concentrated ambient particles on heart rate, blood pressure, and cardiac contractility in spontaneously hypertensive rats during a dust storm event. Inhal Toxicol. 2007;19(11):973–978. [DOI] [PubMed] [Google Scholar]
- 30. Brook RD, Rajagopalan S. Particulate matter, air pollution, and blood pressure. J Am Soc Hypertens. 2009;3(5):332–350. [DOI] [PubMed] [Google Scholar]
- 31. Ghelfi E, Rhoden CR, Wellenius GA, et al. Cardiac oxidative stress and electrophysiological changes in rats exposed to concentrated ambient particles are mediated by TRP-dependent pulmonary reflexes. Toxicol Sci. 2008;102(2):328–336. [DOI] [PubMed] [Google Scholar]
- 32. Sun Q, Wang A, Jin X, et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294(23):3003–3010. [DOI] [PubMed] [Google Scholar]
- 33. Jung CC, Su HJ, Liang HH. Association between indoor air pollutant exposure and blood pressure and heart rate in subjects according to body mass index. Sci Total Environ. 2016;539:271–276. [DOI] [PubMed] [Google Scholar]
- 34. Agency for Toxic Substances and Disease Registry Toxicological Profile for Toluene. Atlanta, GA: US Department of Health and Human Services; 2000. [Google Scholar]
- 35. Capron B, Logan BK. Toluene-impaired drivers: behavioral observations, impairment assessment, and toxicological findings. J Forensic Sci. 2009;54(2):486–489. [DOI] [PubMed] [Google Scholar]
- 36. Chang TY, Wang VS, Hwang BF, et al. Effects of co-exposure to noise and mixture of organic solvents on blood pressure. J Occup Health. 2009;51(4):332–339. [DOI] [PubMed] [Google Scholar]
- 37. Agency for Toxic Substances and Disease Registry Toxicological Profile for Xylenes (Update). Altanta, GA: US Department of Health and Human Services; 1995. [Google Scholar]
- 38. National Toxicology Information Program Xylenes CASRN: 1330-20-7 (Hazardous Substances Data Bank). Bethesda, MD: National Library of Medicine; 2010. https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/~5726BP:1. Accessed September 7, 2016.
- 39. Calabrese EJ, Kenyon EM. Air Toxics and Risk Assessment. Chelsea, MI: Lewis Publishers; 1991. [Google Scholar]
- 40. Munzel T, Sorensen M, Gori T, et al. Environmental stressors and cardio-metabolic disease: part I—epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J. 2017;38(8):550–556. [DOI] [PubMed] [Google Scholar]
- 41. Lydon-Rochelle MT, Holt VL, Cardenas V, et al. The reporting of pre-existing maternal medical conditions and complications of pregnancy on birth certificates and in hospital discharge data. Am J Obstet Gynecol. 2005;193(1):125–134. [DOI] [PubMed] [Google Scholar]
- 42. Bell ML, Belanger K. Review of research on residential mobility during pregnancy: consequences for assessment of prenatal environmental exposures. J Expo Sci Environ Epidemiol. 2012;22(5):429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.