Abstract
Racial and ethnic disparities in the incidence of esophageal cancer have not been thoroughly characterized with quantitative health-disparity measures. Using data from 1992–2013 from 13 US cancer registries in the Surveillance, Epidemiology, and End Results database, we assessed such disparities according to histological type, based on a variety of disparity metrics. The age-standardized incidence rate of squamous cell carcinoma (SCC) was highest among black persons, while adenocarcinoma mainly affected white men. The rate of SCC decreased over time in all racial/ethnic groups, and this was most pronounced in black persons (by 5.7% per year among men and 5.0% among women). The adenocarcinoma rate rose among non-Hispanic whites and among black men. Racial/ethnic disparities in the incidence of total esophageal cancer decreased over time, which was due mainly to reduced disparities in SCC. The 2 absolute disparity measures—range difference and between-group variance—for adenocarcinoma rose by 3.2% and 6.8% per year, respectively, in men and by 1.8% and 5.3% per year, respectively, in women. This study demonstrates decreased racial/ethnic disparities in the incidence of esophageal SCC over time in the United States, while disparities increased in adenocarcinoma incidence as measured on the absolute scale.
Keywords: esophageal neoplasms, ethnic groups, incidence, population groups, United States
Esophageal cancer is the ninth most common type of malignancy and the sixth leading cause of cancer deaths globally (1, 2). It is among the most deadly malignancies, with an overall 5-year survival of less than 20% (3–5). The 2 main histological types of esophageal cancer, squamous cell carcinoma (SCC) and adenocarcinoma, differ greatly in incidence and etiology. The past 4 decades have witnessed a rapidly increasing incidence of adenocarcinoma in many Western societies, including North America, Europe, and Australia, while the incidence of SCC has declined in these areas (4, 5).
Marked racial and ethnic disparities have been noted in the incidence of esophageal cancer, and the patterns of disparity vary across histological types. In the United States, the incidence of SCC is lower in white persons than in other racial groups, while adenocarcinoma mainly affects white men (6–8). However, the temporal trends in the incidence of esophageal cancer across racial and ethnic groups have not been updated recently. Furthermore, assessing health disparity is complex, and the estimated magnitude of disparity is highly dependent on the disparity measures used; previous studies on the racial and ethnic disparities in esophageal cancer have measured only relative risk comparing groups (8, 9). Therefore, a more comprehensive assessment is warranted.
To characterize the racial and ethnic disparities in the incidence of esophageal cancer, we analyzed such disparities according to histological type over a period of 20 years based on data from 13 cancer registries in the United States, using a variety of metrics for measuring different aspects of disparity.
METHODS
Data sources
We used data from the Surveillance, Epidemiology, and End Results (SEER) database in the United States (10). Data were extracted from the November 2015 submission of the SEER 13 registries database, which included all incident cases of esophageal cancer from the 13 cancer registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, Utah, Los Angeles, San Jose–Monterey, Rural Georgia, and the Alaska Native Tumor Registry) since 1992. We extracted data on population estimates from the SEER program, which uses a slight modification of the annual population estimates from the US Census Bureau. The modification of population data, as recommended by the Epidemiology Program of the Hawaii Cancer Research Center, reduces the Census Bureau’s estimate of white population and increases the estimated Asian and Pacific Islander population in Hawaii (10).
Grouping of race and ethnicity
All patients were categorized into the following 5 racial/ethnic groups: Hispanic white, non-Hispanic white, black, Asian/Pacific Islander, and others. We did not separate blacks by ethnicity given the small total number (n = 36) of Hispanic black patients.
Measures of health disparity
We included the following 6 health disparity indicators: range difference, between-group variance, range ratio, index of disparity, mean log deviation, and Theil index. These indicators include measures on both absolute and relative scales, with and without weighting by population sizes (11, 12). The characteristics of these disparity measures are summarized in Appendix Table 1.
Disparity measures on the absolute scale
Range difference
The range difference (RD) is the absolute difference between the highest incidence rate (IR1) and the lowest incidence rate (IR2) (i.e., RD = IR1 − IR2), regardless of group sizes. In the absence of disparity, RD is equal to 0.
Between-group variance
The between-group variance (BGV) sums up all squared deviations from a population average of all racial/ethnic groups, which are weighted by population size. It is calculated as:
where and indicate the incidence rate and the population size of the jth group, respectively, is the average rate in the total population, and J is the number of groups.
Disparity measures on the relative scale
Range ratio
The range ratio (RR) is derived from the highest divided by the lowest incidence rate (i.e., RR = IR1/IR2). RR represents a summary measure of health disparity on the relative scale regardless of the sizes of racial/ethnic groups, and a value of 1 corresponds to no disparity.
Index of disparity
The index of disparity (IDisp) incorporates rates from all racial/ethnic groups. IDisp summarizes the average difference between the rates in all groups and a reference rate, which is calculated as:
where indicates the incidence rate in the jth group, is the rate in the reference population, which is the lowest rate in this study, and J is the number of racial/ethnic groups. If there is no disparity, IDisp has a value of 0.
Mean log deviation and Theil index
The mean log deviation (MLD) and the Theil index (T) summarize the disproportionality of the percentages of the total number of cancer cases from all groups as compared with the percentages of population size in the total population (expressed as a ratio on the natural logarithm scale). They are calculated as follows:
where and indicate the proportion of the total population and the ratio of the incidence rate relative to the population average rate in the jth group, respectively. Both are weighted by the population sizes of all racial/ethnic groups and are equal to 0 in the absence of disparity.
Statistical analysis
All analyses were performed separately for SCC (histological codes according to the International Classification of Diseases for Oncology, Third Edition (ICD-O-3): 8050–8078, 8083–8084) and adenocarcinoma (ICD-O-3 histological codes: 8140–8141, 8143–8145, 8190–8231, 8260–8263, 8310, 8401, 8480–8490, 8550–8551, 8570–8574, 8576) and combined.
We calculated the sex-specific, age-standardized incidence rates by racial/ethnic group for each calendar year and for the entire observation period. The rates were calculated using the direct method with the US Standard Population in 2000 as the referent. We calculated absolute difference in each disparity measure from the beginning to the end of the observation period, and the 95% confidence interval for the change was calculated using the formula to estimate the standard error of the change based on the normal distribution assumption (11). To facilitate comparisons across different indices, we assessed the temporal changes in the disparity measures by the calculating the percent changes since 1992 in these measures for each calendar year. The percent change for RR was calculated for change in excess RR (i.e., RR − 1).
The joinpoint regression was used to identify any changing points of the trends in the annual age-standardized incidence rate and disparity measures over the study period. We estimated the annual percent change (APC) in the incidence rate and in disparity measures by fitting a least squares regression line to the natural logarithms of the dependent variable, using the calendar year as the regressor variable. A weighted average of APCs from the joinpoint models with weights equal to the length of the time segment, the average annual percent change, was estimated as a summary measure of the trend over the whole study period. The natural logarithm of RR was used as the dependent variable in joinpoint regression analysis for RR. Several disparity measures or the corresponding standard errors could not be estimated for some calendar years due to extremely low number of cases, and thus, we did not perform joinpoint regression analysis for these measures (i.e., relative disparity measures in women and for adenocarcinoma).
The data analysis was performed using SEER*Stat version 8.3.4 (10), Joinpoint Regression Program version 4.4.0.0 (12), and Health Disparities Calculator (HD*Calc) version 1.2.4 (13), all of which were developed by the US National Cancer Institute. All P values are 2-sided, and a P value less than 0.05 was considered statistically significant.
RESULTS
Incidence trends by racial/ethnic group
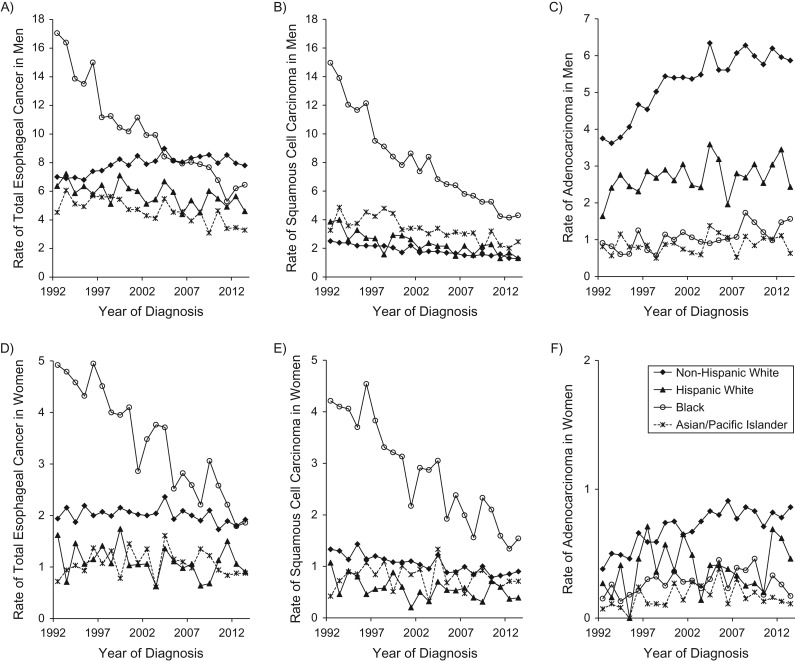
Among 35,932 patients diagnosed with esophageal cancer and recorded in the included registries during the study period (1992–2013), 26,992 (75%) were men and 8,940 (25%) were women. Table 1 presents the sex-specific, age-standardized incidence rates by histological tumor type for each racial/ethnic group in the beginning and ending calendar years and the entire observation period, and the average annual percent changes in the rates. Figure 1 shows the annual age-standardized incidence rates by sex and histological type in the 4 major racial/ethnic groups.
Table 1.
Age-Standardized Incidence Rate (1/100,000 Person-Years) of Esophageal Cancer by Histological Type, Sex, and Racial/Ethnic Group in the United States, 1992–2013
| Sex and Race/Ethnicity | 1992–2013 | 1992 | 2013 | AAPC | 95% CI | |||
|---|---|---|---|---|---|---|---|---|
| No. of Casesa | Rate | No. of Casesa | Rate | No. of Casesa | Rate | |||
| Total Esophageal Cancer | ||||||||
| Men | ||||||||
| Non-Hispanic white | 20,050 | 7.9 | 704 | 7.0 | 1,071 | 7.8 | 0.76 | 0.36, 1.16 |
| Hispanic white | 1,962 | 5.6 | 63 | 6.4 | 119 | 4.6 | −1.26 | −2.04, −0.47 |
| Black | 2,962 | 9.6 | 184 | 17 | 124 | 6.4 | −4.72 | −5.26, −4.19 |
| Asian/Pacific Islander | 1,752 | 4.5 | 51 | 4.5 | 88 | 3.3 | −2.28 | −3.15, −1.40 |
| Other specified | 197 | 5.6 | 5 | 6.1 | 15 | 5.3 | −2.51 | −5.54, 0.62 |
| Total | 26,992 | 7.5 | 1,008 | 7.5 | 1,425 | 6.8 | −0.34 | −0.66, −0.03 |
| Women | ||||||||
| Non-Hispanic white | 6,482 | 2.0 | 269 | 1.9 | 307 | 1.9 | −0.41 | −0.88, 0.05 |
| Hispanic white | 469 | 1.1 | 21 | 1.6 | 27 | 0.9 | −1.18 | −2.98, 0.64 |
| Black | 1,365 | 3.3 | 72 | 4.9 | 47 | 1.9 | −4.32 | −5.12, −3.50 |
| Asian/Pacific Islander | 524 | 1.1 | 8 | 0.7 | 31 | 0.9 | −0.71 | −2.47, 1.09 |
| Other specified | 72 | 1.7 | 0 | 0.0 | 4 | 1.2 | −0.02 | −0.07, 0.03 |
| Total | 8,940 | 1.9 | 370 | 2.1 | 418 | 1.6 | −1.30 | −1.74, −0.86 |
| Esophageal Squamous Cell Carcinoma | ||||||||
| Men | ||||||||
| Non-Hispanic white | 4,590 | 1.8 | 249 | 2.5 | 180 | 1.3 | −2.88 | −3.30, −2.46 |
| Hispanic white | 747 | 2.2 | 37 | 3.9 | 37 | 1.3 | −3.80 | −5.06, −2.53 |
| Black | 2,370 | 7.6 | 163 | 15 | 86 | 4.3 | −5.71 | −6.18, −5.24 |
| Asian/Pacific Islander | 1,254 | 3.2 | 38 | 3.3 | 66 | 2.5 | −3.18 | −4.28, −2.07 |
| Other specified | 84 | 2.4 | 3 | 2.9 | 6 | 2.2 | −4.31 | −8.91, 0.53 |
| Total | 9,065 | 2.5 | 491 | 3.6 | 377 | 1.7 | −3.50 | −3.81, −3.19 |
| Women | ||||||||
| Non-Hispanic white | 3,323 | 1.0 | 184 | 1.3 | 142 | 0.9 | −2.21 | −2.81, −1.61 |
| Hispanic white | 238 | 0.5 | 15 | 1.1 | 12 | 0.4 | −2.66 | −4.76, −0.52 |
| Black | 1,116 | 2.7 | 62 | 4.2 | 39 | 1.5 | −4.98 | −5.90, −4.05 |
| Asian/Pacific Islander | 385 | 0.8 | 5 | 0.4 | 25 | 0.7 | −1.02 | −3.11, 1.12 |
| Other specified | 39 | 0.9 | 0 | 0.0 | 2 | 0.6 | −3.57 | −7.74, 0.79 |
| Total | 5,119 | 1.1 | 266 | 1.5 | 222 | 0.9 | −2.81 | −3.35, −2.26 |
| Esophageal Adenocarcinoma | ||||||||
| Men | ||||||||
| Non-Hispanic white | 13,586 | 5.3 | 381 | 3.7 | 804 | 5.9 | 2.14 | 1.52, 2.76 |
| Hispanic white | 991 | 2.7 | 20 | 1.6 | 62 | 2.4 | 0.98 | −0.18, 2.15 |
| Black | 336 | 1.1 | 10 | 0.9 | 28 | 1.6 | 2.98 | 1.43, 4.56 |
| Asian/Pacific Islander | 350 | 0.9 | 9 | 0.8 | 16 | 0.6 | 0.96 | −1.00, 2.97 |
| Other specified | 83 | 2.2 | 1 | 1.2 | 7 | 2.6 | −0.01 | −0.05,0.04 |
| Total | 15,388 | 4.2 | 421 | 3.1 | 923 | 4.4 | 1.68 | 1.08, 2.28 |
| Women | ||||||||
| Non-Hispanic white | 2,276 | 0.7 | 54 | 0.4 | 139 | 0.9 | 2.70 | 1.76, 3.64 |
| Hispanic white | 172 | 0.4 | 3 | 0.3 | 13 | 0.5 | 0.74 | −2.62, 4.22 |
| Black | 115 | 0.3 | 2 | 0.2 | 5 | 0.2 | 1.60 | −0.87, 4.13 |
| Asian/Pacific Islander | 84 | 0.2 | 1 | 0.1 | 4 | 0.1 | 2.14 | −1.20, 5.59 |
| Other specified | 26 | 0.6 | 0 | 0.0 | 2 | 0.6 | −4.34 | −9.10, −0.67 |
| Total | 2,680 | 0.6 | 60 | 0.3 | 163 | 0.6 | 2.01 | 1.08, 2.95 |
Abbreviations: AAPC, average annual percent change; CI, confidence interval.
a Numbers of cases in racial/ethnic groups do not always sum to the total due to unspecified race/ethnicity in some patients.
Figure 1.
Sex-specific, age-standardized incidence rates (1/100,000 person-years) of esophageal cancer by histological type and racial/ethnic group in the Surveillance, Epidemiology, and End Results 13 registries in the United States during 1992–2013. A) total esophageal cancer in men; B) squamous cell carcinoma in men; C) adenocarcinoma in men; D) total esophageal cancer in women; E) squamous cell carcinoma in women; F) adenocarcinoma in women.
The age-standardized incidence rate of total esophageal cancer among non-Hispanic white men increased slightly from 7.0 per 100,000 person-years in 1992 to 7.8 per 100,000 person-years in 2013 (APC = 0.76, 95% confidence interval (CI): 0.36, 1.16) but decreased over time among men of other racial/ethnic groups. The rate of total esophageal cancer in black men decreased on average by 4.72% (95% CI: 4.19, 5.26) per year from 1992 to 2013. The rate among black women dramatically decreased (APC = −4.32, 95% CI: −5.12, −3.50), but remained relatively stable in women of other racial/ethnic groups (Table 1).
The SCC rate decreased in all racial/ethnic groups regardless of sex (Table 1 and Figure 1). The decrease was more evident among black persons (in men, APC = −5.71; in women APC = −4.98 and) than in other groups. Still, the rates among black persons remained highest among those of all racial/ethnic groups throughout the study period.
The incidence rate of adenocarcinoma in men was highest in the non-Hispanic white group, followed by Hispanic whites (Table 1 and Figure 1C). The adenocarcinoma rates among men increased on average by 2.1% per year in non-Hispanic white and 3.0% per year in black persons, but they remained relatively stable in other racial/ethnic groups. Joinpoint regression showed that the increase of the adenocarcinoma rate in non-Hispanic white men was more rapid during 1992–1999 (APC = 5.72, 95% CI: 4.08, 7.39), while it slowed down from the year 2000 (APC = 0.78, 95% CI: 0.03, 1.55). The adenocarcinoma rates among women were lower than 1 per 100,000 person-years in all racial/ethnic groups. However, a rise in the rate of adenocarcinoma was observed among non-Hispanic white women (APC = 2.70, 95% CI: 1.76, 3.64).
No changing point in the age-standardized incidence rate over time was identified from joinpoint regression, except for the adenocarcinoma rate in non-Hispanic white men.
Racial and ethnic disparities on the absolute scale
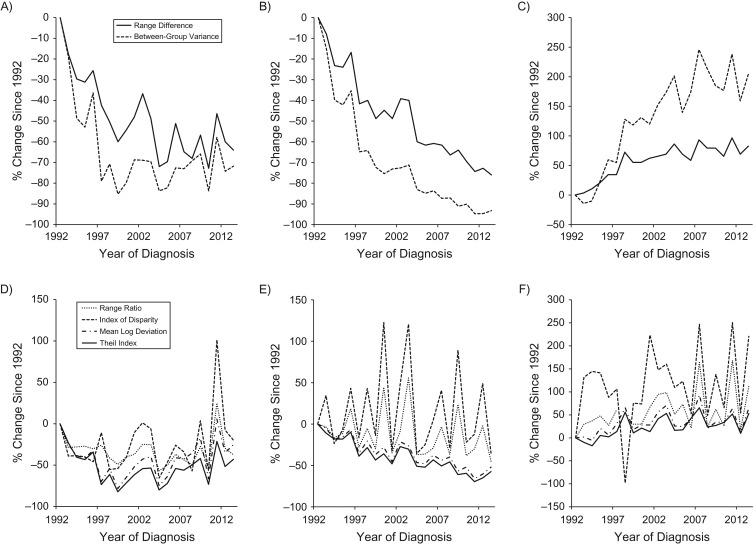
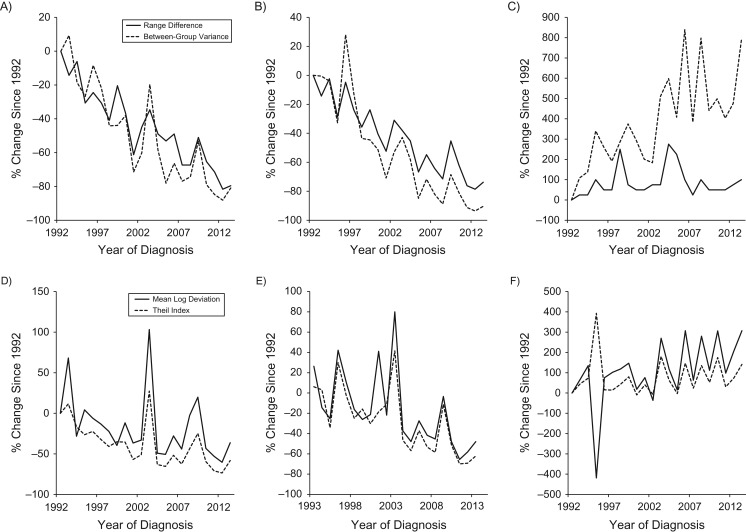
Table 2 presents the absolute and relative measures of racial and ethnic disparity in the incidence of esophageal cancer by sex and histological tumor type in the beginning and ending calendar years, and the absolute and percent changes in these measures during the study period. The percent changes since 1992 in disparity measures for each calendar year are displayed for men in Figure 2 and for women in Figure 3. The absolute disparity measures RD and BGV for the incidence of total esophageal cancer and SCC decreased over time in both sexes (Figures 2A and 3A). The RD for SCC decreased from 12.5 per 100,000 person-years to 3.0 per 100,000 person-years in men and from 4.2 per 100,000 person-years to 1.1 per 100,000 person-years in women during 1992–2013 (Table 2). The absolute disparity measures for adenocarcinoma increased over time (Figures 2C and 3C); the measures of RD and BGV increased by 83% and 205%, respectively, among men from 1992 to 2013 (Table 2 and Figure 2C).
Table 2.
Changes in Racial and Ethnic Disparities in the Incidence of Esophageal Cancer According to Sex and Histological Type in the United States, 1992–2013
| Sex and Disparity Measurea | 1992 | 2013 | Change, 1992–2013 | 95% CI for Change | % Change, 1992–2013 | ||
|---|---|---|---|---|---|---|---|
| Index | 95% CI | Index | 95% CI | ||||
| Total Esophageal Cancer | |||||||
| Men | |||||||
| Absolute scale | |||||||
| Range difference | 12.5 | 9.6, 15.4 | 4.5 | 3.6, 5.4 | −8.0 | −11.0, −5.0 | −64.0 |
| Between-group variance | 10.7 | 5.5, 15.9 | 3.0 | 1.9, 4.2 | −7.7 | −13.0, −2.4 | −71.8 |
| Relative scale | |||||||
| Range ratio | 3.8 | 2.7, 5.3 | 2.4 | 1.9, 3.0 | −1.4 | −2.8, 0.0 | −37.4 |
| Index of disparity | 102.8 | 31.2, 174.4 | 82.6 | 32.4, 132.8 | −20.2 | −107.7, 67.2 | −19.7 |
| Mean log deviation | 0.06 | 0.04, 0.09 | 0.05 | 0.03, 0.07 | −0.02 | −0.05, 0.02 | −25.2 |
| Theil index | 0.07 | 0.04, 0.10 | 0.04 | 0.02, 0.06 | −0.03 | −0.07, 0.00 | −42.8 |
| Women | |||||||
| Absolute scale | |||||||
| Range difference | 4.9 | 3.7, 6.1 | 1.0 | 0.6, 1.4 | −3.9 | −5.2, −2.6 | −79.6 |
| Between-group variance | 1.2 | 0.4, 2.0 | 0.2 | 0.1, 0.4 | −1.0 | −1.8, −0.2 | −81.0 |
| Relative scale | |||||||
| Mean log deviation | 0.09 | 0.06 | 0.01, 0.11 | −0.03 | −36.3 | ||
| Theil index | 0.13 | 0.05 | 0.01, 0.09 | −0.07 | −58.2 | ||
| Esophageal Squamous Cell Carcinoma | |||||||
| Men | |||||||
| Absolute scale | |||||||
| Range difference | 12.5 | 10.1, 14.9 | 3.0 | 2.0, 4.0 | −9.5 | −12.1, −6.9 | −76.0 |
| Between-group variance | 14.2 | 8.7, 19.7 | 1.0 | 0.4, 1.5 | −13.3 | −18.8, −7.8 | −93.3 |
| Relative scale | |||||||
| Range ratio | 6.0 | 4.8, 7.5 | 3.3 | 2.5, 4.3 | −2.7 | −4.3, −1.1 | −44.9 |
| Index of disparity | 151.0 | 91.9, 210.1 | 98.1 | 44.7, 151.5 | −52.9 | −132.6, 26.8 | −35.0 |
| Mean log deviation | 0.21 | 0.15, 0.27 | 0.10 | 0.05, 0.15 | −0.11 | −0.19, −0.03 | −51.9 |
| Theil index | 0.28 | 0.20, 0.36 | 0.12 | 0.06, 0.17 | −0.16 | −0.26, −0.06 | −57.5 |
| Women | |||||||
| Absolute scale | |||||||
| Range difference | 4.2 | 3.2, 5.2 | 1.1 | 0.5, 1.7 | −3.1 | −4.3, −1.9 | −73.8 |
| Between-group variance | 1.0 | 0.4, 1.6 | 0.1 | 0.0, 0.2 | −0.9 | −1.5, −0.3 | −90.4 |
| Relative scale | |||||||
| Mean log deviation | 0.14 | 0.07 | 0.01, 0.14 | −0.07 | −48.0 | ||
| Theil index | 0.18 | 0.07 | 0.01, 0.13 | −0.11 | −62.1 | ||
| Esophageal Adenocarcinoma | |||||||
| Men | |||||||
| Absolute scale | |||||||
| Range difference | 2.9 | 2.2, 3.6 | 5.3 | 4.7, 5.9 | 2.4 | 1.5, 3.3 | 82.8 |
| Between-group variance | 1.5 | 0.9, 2.2 | 4.7 | 3.6, 5.7 | 3.1 | 1.9, 4.3 | 204.6 |
| Relative scale | |||||||
| Range ratio | 4.6 | 2.2, 9.7 | 9.8 | 5.1, 19 | 5.2 | −2.1, 12.5 | 112.6 |
| Index of disparity | 131.3 | −56.8, 319.3 | 420.8 | 66.8, 774.8 | 289.6 | −111.3, 690.5 | 220.6 |
| Mean log deviation | 0.15 | 0.07, 0.23 | 0.25 | 0.16, 0.33 | 0.10 | −0.02, 0.21 | 64.5 |
| Theil index | 0.12 | 0.06, 0.17 | 0.18 | 0.14, 0.22 | 0.06 | −0.01, 0.13 | 52.9 |
| Women | |||||||
| Absolute scale | |||||||
| Range difference | 0.4 | 0.2, 0.6 | 0.8 | 0.5, 1.1 | 0.4 | 0.1, 0.7 | 100.0 |
| Between-group variance | 0.01 | −0.02, 0.04 | 0.10 | 0.03, 0.17 | 0.09 | 0.02, 0.17 | 787.6 |
| Relative scale | |||||||
| Mean log deviation | 0.06 | 0.24 | −0.01, 0.50 | 0.18 | 306.7 | ||
| Theil index | 0.07 | 0.17 | 0.06, 0.28 | 0.10 | 140.9 | ||
Abbreviation: CI, confidence interval.
a Range ratio and index of disparity are not presented for women due to multiple missing values for several calendar years and lack of precision in these measures.
Figure 2.
Percent changes since 1992 in the racial and ethnical disparities in the incidence of esophageal cancer by histological type in men in the Surveillance, Epidemiology, and End Results 13 registries in the United States during 1992–2013. A) absolute measures for total esophageal cancer; B) absolute measures for squamous cell carcinoma; C) absolute measures for adenocarcinoma; D) relative measures for total esophageal cancer; E) relative measures for squamous cell carcinoma; F) relative measures for adenocarcinoma.
Figure 3.
Percent changes since 1992 in the racial and ethnical disparities in the incidence of esophageal cancer by histological type in women in the Surveillance, Epidemiology, and End Results 13 registries in the United States during 1992–2013. A) absolute measures for total esophageal cancer; B) absolute measures for squamous cell carcinoma; C) absolute measures for adenocarcinoma; D) relative measures for total esophageal cancer; E) relative measures for squamous cell carcinoma; F) relative measures for adenocarcinoma.
Racial and ethnic disparities on the relative scale
All relative disparity measures in total esophageal cancer incidence decreased in both sexes from 1992 to 2013, though the absolute changes were not statistically significant (Table 2). Relative disparity measures for SCC incidence decreased over time in both sexes (Table 2, Figures 2E and 3E), while the relative disparity measures for adenocarcinoma incidence remained relatively stable during the study period (Table 2, Figures 2F and 3F). The relative disparity measures RR and IDisp are not presented for women due to multiple missing values for several calendar years and lack of precision in these measures.
Joinpoint regression on racial and ethnic disparity measures
Results from joinpoint regression on the racial and ethnic disparity measures by sex and histological type are presented in Table 3. The RD for total esophageal cancer in men decreased by 7.6% per year from 1992 to 2004 and remained relatively stable afterwards, resulting in an estimated average annual percent change of −3.9 (95% CI: −6.5, −1.2) during the entire study period. The BGV for total esophageal cancer in men decreased during 1992–1999 (APC = −18.7, 95% CI: −28.1, −8.0) and remained relatively stable after 1999. The relative disparity measures T and MLD for total esophageal cancer in men decreased from 1992 to 1999, but they increased on average by 5.6% and 6.3% per year, respectively, since 1999. All disparity measures for SCC decreased at a seemingly constant rate in both sexes during the study period. The absolute disparity measures RD and BGV for adenocarcinoma increased on average by 3.2% and 6.8% per year, respectively, in men and by 1.8% and 5.3% per year, respectively, in women from 1992 to 2013. The increases in RD and BGV for the adenocarcinoma rate in men were more rapid during the 1990s than in later periods.
Table 3.
Joinpoint Regression on Racial and Ethnic Disparity Measures of Esophageal Cancer Incidence by Sex and Histological Type in the United States, 1992–2013
| Scale and Disparity Measurea | Total Esophageal Cancer | Squamous Cell Carcinoma | Adenocarcinoma | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Calendar Period | APC | 95% CI | Calendar Period | APC | 95% CI | Calendar Period | APC | 95% CI | |
| Men | |||||||||
| Absolute scale | |||||||||
| Range difference | 1992–2004 | −7.6 | −11.0, −4.2 | 1992–2013 | −6.1 | −6.8, −5.5 | 1992–1998 | 9.8 | 4.1, 15.9 |
| Range difference | 2004–2013 | 1.4 | −3.3, 6.3 | 1998–2013 | 0.7 | −0.0, 1.4 | |||
| AAPC, range difference | 1992–2013 | −3.9 | −6.5, −1.2 | AAPC | 3.2 | 1.7, 4.8 | |||
| Between-group variance | 1992–1999 | −18.7 | −28.1, −8.0 | 1992–2013 | −12.2 | −13.3, −11.1 | 1992–1999 | 17.1 | 9.8, 24.8 |
| Between-group variance | 1999–2013 | 2.4 | −1.4, 6.3 | 1999–2013 | 2.0 | 0.4, 3.7 | |||
| AAPC, between-group variance | 1992–2013 | −5.2 | −0.3, −0.8 | AAPC | 6.8 | 4.5, 9.2 | |||
| Relative scale | |||||||||
| Range ratio | 1992–2013 | −1.6 | −3.2, −0.1 | 1992–2013 | −1.7 | −2.2, −1.3 | |||
| Index of disparity | 1992–2013 | 0.9 | −1.4, 3.2 | 1992–2013 | −1.8 | −3.8, 0.2 | |||
| Theil index | 1992–1999 | −15.7 | −24.9, −5.3 | 1992–2013 | −4.3 | −5.3, −3.4 | |||
| Theil index | 1999–2013 | 5.6 | 1.5, 9.8 | ||||||
| AAPC, Theil index | 1992–2013 | −2.1 | −6.2, 2.3 | ||||||
| Mean log deviation | 1992–1999 | −12.8 | −22.1, −2.2 | 1992–2013 | −3.8 | −4.7, −2.9 | |||
| Mean log deviation | 1999–2013 | 6.3 | 2.0, 10.8 | ||||||
| AAPC, mean log derivation | 1992–2013 | −0.5 | −4.7, 4.0 | ||||||
| Women | |||||||||
| Absolute scale | |||||||||
| Range difference | 1992–2013 | −6.3 | −7.5, −5.0 | 1992–2013 | −5.4 | −6.7, −4.1 | 1992–2013 | 1.8 | 0.6, 3.1 |
| Between-group variance | 1992–2013 | −8.0 | −10.2, −5.8 | 1992–2013 | −10.7 | −12.9, −8.3 | 1992–2013 | 5.3 | 2.8, 7.8 |
Abbreviations: AAPC, average annual percent change; APC, annual percent change; CI, confidence interval.
a Analysis was not performed for relative disparity measures in women and for esophageal adenocarcinoma because these measures or their standard errors could not be estimated for some calendar years.
DISCUSSION
On the basis of a variety of disparity metrics, we evaluated disparities in the incidence of esophageal cancer across racial/ethnic groups during a period of over 20 years from 1992 to 2013 in the United States. The incidence rate of SCC decreased in all racial/ethnic groups, but the decrease was more rapid among black persons than in the other racial/ethnic groups. An increasing incidence of adenocarcinoma was instead observed among non-Hispanic whites and black men. The racial and ethnic disparities in the incidence of total esophageal cancer decreased over time for most disparity indicators, which was driven mainly by the reduced disparities in SCC incidence. However, the absolute-scale metrics showed increasing racial and ethnic disparities in adenocarcinoma incidence.
The substantially higher incidence of SCC among black persons compared with other racial/ethnic groups observed in the present study is consistent with earlier reports (6–9). The higher risk in black men might be explained partly by a historically higher prevalence of tobacco smoking relative to other racial/ethnic groups (14, 15). However, the disparities in SCC rates among women across racial/ethnic groups do not correspond with historic differences in smoking prevalence. The SCC rate among women has been the highest in blacks followed by lower, similar rates for the other racial/ethnic groups. Inconsistently, there is no marked difference between black and white women in smoking prevalence, although the smoking prevalence of Asian women in the United States has been much lower than that of other racial/ethnic groups (14). The decreasing incidence of SCC in all groups parallels declining smoking prevalence (14, 16). Although the magnitude of decline in SCC rates and smoking prevalence by racial/ethnic group did not correlate exactly with one another, the more rapidly decreased smoking prevalence among black persons compared with white persons was in line with the steeper decline in the incidence of esophageal SCC among black persons observed in this study (17). The prevalence of heavy alcohol consumption is highest among white persons, followed by lower similar prevalence for all other racial/ethnic groups in the United States (16), which is inconsistent with the SCC rates. The observed racial and ethnic disparities in SCC rates and the temporal tends in such disparities might also be explained by variations in other risk factors (e.g., dietary factors) or interactions between risk factors. The relatively high SCC rates in Asian Americans might be explained partly by exposures related to their traditional lifestyle (e.g., high consumption of processed meat and drinking high-temperature beverages) (18, 19). Because divergent trends were observed between the 2 histological types, the differential trends in the incidence rate across racial/ethnic groups are less likely to be explained by any difference in case detection across these groups, which would have influenced both histological types in an equal manner.
Obesity and gastroesophageal reflux disease are the main established risk factors for esophageal adenocarcinoma (4, 5). While black persons have a higher prevalence of obesity than white persons (16, 20, 21), blacks have lower visceral fat than whites (22, 23). Additionally, no studies to date have specifically determined the relationship between obesity and esophageal adenocarcinoma among black persons. Abdominal obesity in particular has been suggested as an important risk factor for adenocarcinoma (24). The observed racial and ethnic disparities in adenocarcinoma incidence are consistent with the higher prevalence of abdominal obesity in white men than in other racial/ethnic groups (25, 26). A higher prevalence of reflux in whites than in blacks and other racial/ethnic groups in the Unites States has been reported (27, 28), and that might contribute to the observed disparities in adenocarcinoma incidence. The increasing prevalence of obesity and reflux parallels the increasing adenocarcinoma incidence in white men and women and in black men (16, 21, 27). However, it has been suggested that the rise of esophageal adenocarcinoma incidence in the United States started in the late 1960s, which was a decade earlier than the rise of obesity (29). How environmental exposures other than obesity have contributed to the changing epidemiology of esophageal adenocarcinoma remains to be further investigated.
Heath disparities are conceptually complex, and it is recommended that a variety of disparity indices, taking different relevant issues into account, be used to assess them (11, 12, 30). Particularly, disparity should be measured on both relative and absolute scales. Furthermore, the size of the groups being compared should be incorporated into disparity measures to account for inevitable demographic changes over time. The present study is, to our knowledge, the first to explore the racial and ethnic disparities in the population-based incidence of esophageal cancer with multiple disparity indices. This approach provides a more comprehensive characterization of such disparities than have previous studies. We found that all disparity measures for SCC decreased over time, although with varying magnitudes of change. In terms of percent change, the absolute disparity measures RD and BGV decreased more rapidly than other measures for SCC rate. There were also noteworthy increased racial and ethnic disparities in adenocarcinoma rate, particularly when using the absolute disparity measures. Therefore, the results indicate a significant narrowing of the absolute gap in the incidence of SCC across racial/ethnic groups and an enlarging racial/ethnic gap in adenocarcinoma incidence. The population-weighted measures (BGV, T, and MLD) count all individuals equally and incorporate changes in the distribution of racial/ethnic groups. On the other hand, the unweighted measures (RD, RR, and IDisp) treat all racial/ethnic groups equally irrespective of their population sizes. In this study, changes in population-weighted disparity measures over time were generally greater than those in unweighted disparity measures, which might be explained by the fact that the changes in incidence rates were generally higher in the predominant racial/ethnic groups (i.e., blacks and non-Hispanic whites) than in other racial/ethnic groups. However, interpretation of the results should be made based on both population-weighted and unweighted measures. Larger social groups should not overshadow the magnitude of the health disparities or the importance of health status among smaller groups. Meanwhile, the greater disease burden, as indicated by higher absolute number of individuals experiencing such burden in larger groups, might be equally important in evaluating health disparity (31).
This study has some limitations. We included only the 13 cancer registries for which data are available with expanded categories of race and ethnicity since 1992, and the results might not be representative of the entire population of the United States. Due to the limited incidence of esophageal cancer in the United States, some estimates of the disparities and changes over time, particularly those for adenocarcinoma rates among women, had low precision. Furthermore, due to the limited statistical power and racial/ethnic classification, we were able to evaluate disparities only in broad racial and ethnic groups. Thus, we could not explore disparities across more detailed subgroups (e.g., those differentiated by national origin). In addition, some level of misclassification of race and ethnicity cannot be ruled out, and the categorization of race/ethnicity did not account for mixed heritages. Finally, we were unable to directly investigate the disparities in known risk factors because information on such factors is not available in the SEER database.
Reducing health disparities is a major public health aim. Achieving health equity, eliminating disparities, and improving the health of all groups has been set as one of the overarching goals in Healthy People 2020 in the United States (32). With specific regard to esophageal cancer, this study reveals an encouraging decrease in the disparities in SCC rates across racial/ethnic groups, but there appears to be increasing racial/ethnic disparities in adenocarcinoma rates. It is important to continue monitoring the racial and ethnic disparities in esophageal cancer and to try to prevent this cancer through health-promotion activities among all racial/ethnic groups. In addition, the observed racial and ethnic disparities and their trends cannot be completely explained by known risk factors for esophageal cancer. Thus, there remains a need for more etiological studies of this cancer.
In summary, with a panel of metrics measuring different aspects of health disparities, this study provides a broad assessment on the racial and ethnic disparities in the incidence of esophageal cancer over a 20-year period in the United States. The incidence of esophageal SCC has decreased in all racial/ethnic groups, and the disparity across groups has decreased over time. The results demonstrate an increasing incidence of adenocarcinoma among non-Hispanic whites and black men and a seemingly increasing disparity of adenocarcinoma incidence. More etiological studies are warranted to better understand the underlying mechanisms for the racial and ethnic disparities in the incidence of esophageal cancer, which could provide information about how to eliminate such disparities in the population.
ACKNOWLEDGMENTS
Author affiliations: Upper Gastrointestinal Surgery, Department of Molecular Medicine and Surgery, Karolinska Institutet, Karolinska University Hospital, Stockholm, Sweden (Shao-Hua Xie, Sirus Rabbani, Jesper Lagergren); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland, the United States (Jessica L. Petrick, Michael B. Cook); and Division of Cancer Studies, King’s College London, London, United Kingdom (Jesper Lagergren).
This work was supported by the Swedish Research Council (grant 521-2014-2536), the Swedish Cancer Society (grant CAN 2015/460), and the Intramural Program of the US National Institutes of Health.
Conflict of interest: none declared.
Abbreviations
- APC
annual percent change
- BGV
between-group variance
- CI
confidence interval
- IDisp
index of disparity
- MLD
mean log deviation
- RD
range difference
- RR
range ratio
- SEER
Surveillance, Epidemiology, and End Results
- SCC
squamous cell carcinoma
- T
Theil index
Appendix
Appendix Table 1.
Summary of Characteristics of Health Disparity Measures
| Disparity Measure | Reference Rate | Incorporating Rates of all Groups | Population-Weighted |
|---|---|---|---|
| Absolute measures | |||
| Range difference | Lowest | No | No |
| Between-group variance | Average | Yes | Yes |
| Relative measures | |||
| Range ratio | Lowest | No | No |
| Index of disparity | Lowest | Yes | No |
| Mean log deviation | Average | Yes | Yes |
| Theil index | Average | Yes | Yes |
REFERENCES
- 1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 Lyon, France: International Agency for Research on Cancer, 2013. [Google Scholar]
- 2. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5—a population-based study. Lancet Oncol. 2014;15(1):23–34. [DOI] [PubMed] [Google Scholar]
- 4. Lagergren J, Lagergren P. Recent developments in esophageal adenocarcinoma. CA Cancer J Clin. 2013;63(4):232–248. [DOI] [PubMed] [Google Scholar]
- 5. Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371(26):2499–2509. [DOI] [PubMed] [Google Scholar]
- 6. Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977–2005. Br J Cancer. 2009;101(5):855–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83(10):2049–2053. [PubMed] [Google Scholar]
- 8. González L, Magno P, Ortiz AP, et al. Esophageal cancer incidence rates by histological type and overall: Puerto Rico versus the United States Surveillance, Epidemiology, and End Results population, 1992–2005. Cancer Epidemiol. 2013;37(1):5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baquet CR, Commiskey P, Mack K, et al. Esophageal cancer epidemiology in blacks and whites: racial and gender disparities in incidence, mortality, survival rates and histology. J Natl Med Assoc. 2005;97(11):1471–1478. [PMC free article] [PubMed] [Google Scholar]
- 10. Surveillance Research Program, National Cancer Institute SEER*Stat software, version 8.3.4 https://seer.cancer.gov/seerstat. Last Updated March 23, 2017. Accessed October 26, 2017.
- 11. Harper S, Lynch J, Meersman SC, et al. An overview of methods for monitoring social disparities in cancer with an example using trends in lung cancer incidence by area-socioeconomic position and race-ethnicity, 1992–2004. Am J Epidemiol. 2008;167(8):889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Breen N, Scott S, Percy-Laurry A, et al. Health disparities calculator: a methodologically rigorous tool for analyzing inequalities in population health. Am J Public Health. 2014;104(9):1589–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 14. Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults—United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–1240. [DOI] [PubMed] [Google Scholar]
- 15. Trinidad DR, Perez-Stable EJ, White MM, et al. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health. 2011;101(4):699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clarke TC, Ward BW, Freeman G, et al. Early Release of Selected Estimates Based on Data From the January-March 2016 National Health Interview Survey. National Center for Health Statistics; 2016. http://www.cdc.gov/nchs/nhis.htm. Accessed December 20, 2016. [Google Scholar]
- 17. Office on Smoking and Health The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014 Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 2014. [Google Scholar]
- 18. Qu X, Ben Q, Jiang Y. Consumption of red and processed meat and risk for esophageal squamous cell carcinoma based on a meta-analysis. Ann Epidemiol. 2013;23(12):762.e1–770.e1. [DOI] [PubMed] [Google Scholar]
- 19. Islami F, Boffetta P, Ren JS, et al. High-temperature beverages and foods and esophageal cancer risk—a systematic review. Int J Cancer. 2009;125(3):491–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005–2014. JAMA. 2016;315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. [DOI] [PubMed] [Google Scholar]
- 22. Carroll JF, Chiapa AL, Rodriquez M, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring). 2008;16(3):600–607. [DOI] [PubMed] [Google Scholar]
- 23. Camhi SM, Bray GA, Bouchard C, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring). 2011;19(2):402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh S, Sharma AN, Murad MH, et al. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11(11):1399.e7–1412.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Okosun IS, Choi ST, Boltri JM, et al. Trends of abdominal adiposity in white, black, and Mexican-American adults, 1988–2000. Obes Res. 2003;11(8):1010–1017. [DOI] [PubMed] [Google Scholar]
- 26. Wen M, Kowaleski-Jones L, Fan JX. Ethnic-immigrant disparities in total and abdominal obesity in the US. Am J Health Behav. 2013;37(6):807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petrick JL, Nguyen T, Cook MB. Temporal trends of esophageal disorders by age in the Cerner Health Facts database. Ann Epidemiol. 2016;26(2):151–154.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. El-Serag HB, Petersen NJ, Carter J, et al. Gastroesophageal reflux among different racial groups in the United States. Gastroenterology. 2004;126(7):1692–1699. [DOI] [PubMed] [Google Scholar]
- 29. Abrams JA, Sharaiha RZ, Gonsalves L, et al. Dating the rise of esophageal adenocarcinoma: analysis of Connecticut Tumor Registry data, 1940–2007. Cancer Epidemiol Biomarkers Prev. 2011;20(1):183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harper S, Lynch J. Selected Comparisons of Measures of Health Disparities: A Review Using Databases Relevant to Healthy People 2010 Cancer-Related Objectives Bethesda, MD: National Cancer Institute; 2007. (NCI Cancer Surveillance Monograph Series, Number 7) (NIH Pub. No. 07-6281). [Google Scholar]
- 31. Kindig D. Population health equity: rate and burden, race and class. JAMA. 2017;317(5):467–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion Healthy People 2020. Washington, DC: https://www.healthypeople.gov/2020/about/foundation-health-measures/Disparities. Accessed December 20, 2016. [Google Scholar]