Abstract
Objective
The primary aim of this study was to identify the independent and differential associations of obesity and hypertension with cognitive, physical, and directly observed functional abilities among middle age and older adults.
Method
Participants were 119 adults between the ages of 51 and 89, who underwent a cross-sectional assessment of cognitive, physical, functional and relevant health-related variables.
Results
Obesity predicted significantly poorer executive functions (β = −.301, t = −3.86, p < .001), mobility (β = .329, t = 3.59, p < .001), observed functional abilities (β = .220, t = 2.52, p = .013), and self-reported ability to perform activities of daily living that require physical capability (β = −.365, t = −4.23, p < .001). In contrast, hypertension was not independently associated with any of the outcome measures (ps > .05). Results from the path analysis revealed that executive functions mediated the association between obesity and poorer directly observed functional abilities. Additionally, obesity had a direct and indirect (through mobility) effect on self-reported basic activities of daily living.
Conclusions
These findings suggest a link between obesity, executive functions, and limitations in physical function and instrumental activities of daily living among middle age and older adults, however, longitudinal research is needed to further delineate the trajectory of these factors.
Keywords: Cardiovascular disease, Elderly/geriatrics/aging, Everyday functioning, Executive functions, Learning and memory, Endocrine
The risk of developing cardiovascular and metabolic disorders increases with age (Ervin, 2009). Among adults age 60 years and older, more than one-third have a body mass index (BMI) in the obese range (≥30 kg/m2; Mathus-Vliegen, 2012), and approximately two-thirds have hypertension (Guo, He, Zhang, & Walton, 2012). Hypertension and obesity have been identified as risk factors for cognitive decline and dementia (Elias, Elias, Sullivan, Wolf, & D'Agostino, 2003; Haring et al., 2015; Köhler et al., 2014). As the aging population continues to grow, examining modifiable factors that may contribute to cognitive, physical and functional decline is essential to developing interventions that will preserve and extend older adult's ability to function independently.
Across the adult lifespan, data indicate that overweight and obese individuals show greater cerebral white matter atrophy (Ronan et al., 2016), which may contribute to accelerated brain aging. Compared to normal and overweight individuals, obese individuals have been found to have smaller whole brain and gray matter volumes (Gunstad et al., 2008) and decreased white matter tract integrity (Stanek et al., 2011). Older age and obesity have also both been associated with decreased hippocampal volume and increased white matter hyperintensities (Jagust, Harvey, Mungas, & Haan, 2005). In addition, elevated BMI has been associated with neuronal abnormalities in the frontal lobe (Gazdzinski et al., 2010) and temporal lobe (Bolzenius et al., 2013). Even controlled hypertension has been associated with smaller prefrontal cortex, white matter volumes and increased white matter intensities (Raz, Rodrigue, & Acker, 2003). Neuroimaging research further suggests that such age-related decreases in white matter integrity may contribute to declines in executive functions, episodic memory, and speeded processing (for a review, see Gunning‐Dixon, Brickman, Cheng, & Alexopoulos, 2009).
Data evaluating the association between obesity and hypertension and memory abilities has been mixed. There is some evidence to suggest that among middle-aged and older adults higher BMI is associated with worse episodic memory (Cournot et al., 2006; Elias et al., 2003; Wright et al., 2015). Hypertension has also been shown to be related to both poorer memory performance at cross-sectional assessment (Elias et al., 2003) and to a faster rate of decline in memory abilities, even after controlling for BMI (Köhler et al., 2014). One study that did not find a direct association with cognition, did report an interactive effect of age and uncontrolled hypertension on verbal fluency and immediate word list recall (Brady, Spiro, & Gaziano, 2005). In contrast, other research has not shown a consistent link between memory abilities and hypertension or BMI (Brady et al., 2005; Dahle, Jacobs, & Raz, 2009; Dore, Elias, Robbins, Budge, & Elias, 2008; Hudak, Edwards, Athilingam, & McEvoy, 2013; Kesse-Guyot et al., 2015; Stanek et al., 2013).
In comparison to memory deficits, lower executive functioning abilities in context of cardiometabolic conditions is relatively more consistently reported. Longitudinal data from the Women's Health and Aging Study II suggests executive functioning deficits may precede decrements in memory skills among initially cognitively intact older women (Carlson, Xue, Zhou, & Fried, 2009). Indeed, researchers have reported that obesity is associated with worse executive functioning, but not verbal memory (Dore et al., 2008; Fergenbaum et al., 2009; Gunstad et al., 2007; Kesse-Guyot et al., 2015). Some studies have reported an interactive effect of age and BMI on executive functioning even after controlling for medical variables including hypertension, diabetes, and sleep apnea (Stanek et al., 2013), whereas others did not find such an effect (Gunstad et al., 2007). Although several studies have found an association between obesity and poorer executive abilities after controlling for hypertension (either statistically or through exclusion), few studies have simultaneously examined the effects of hypertension and obesity on cognitive performance.
In addition to cognition, hypertension and obesity have been found to impact physical functioning. Among middle age and older adults, hypertension and obesity independently predicted mobility difficulty, and co-morbid hypertension and obesity predicted greater mobility difficulty and ADL limitations (Oldridge, Stump, Nothwehr, & Clark, 2001). This is consistent with other research that has shown that compared to normal weight individuals, those who are either underweight or obese report impaired physical functioning and lower quality of life (Yan et al., 2004). Obesity has also been associated with greater physical mobility limitation, gross and fine motor limitations, as well increased ADL difficulties (An & Shi, 2015). There is also data to suggest that cognition may mediate the association between blood pressure and physical disability (Elias, Dore, Davey, Robbins, & Elias, 2010).
Currently, research examining the association between cardiometabolic disorders and functional status in middle age and older adults is relatively limited. A study that compared older adults with and without hypertension reported that hypertension was associated with worse processing speed, motor speed, and reaction time, but there were no between group differences in memory or on a performance-based timed IADL task (Hudak et al., 2013). Considering the body of prior work that has consistently linked memory and executive function deficits as well as physical disability to functional decline among older adults (Carlson et al., 1999; Farias et al., 2013; Grigsby, Kaye, Baxter, Shetterly, & Hamman, 1998; Royall, Palmer, Chiodo, & Polk, 2004; Schmitter-Edgecombe & Parsey, 2014), it follows that individuals with cardiometabolic factors may also show lower levels of everyday functional abilities. The aim of the current study was to identify the independent and differential effects of obesity and hypertension on cognitive, physical, and functional abilities in middle age and older adults. We hypothesized that: (1) obesity and hypertension would be associated with lower cognitive performance, physical performance, and functional abilities, and (2) cognitive and physical performance would moderate the association between cardiometabolic conditions and everyday physical and functional outcomes.
Method
Participants
Data for this study were derived from an ongoing cognitive aging project at Washington State University titled Smart Environment Technologies for Health Assessment and Assistance. The overall aims of the larger study are to develop more ecologically valid assessments of complex activities of daily living and use smart technologies to detect changes in cognitive and physical health. Study participants were recruited through advertisements, health and wellness fairs, physician referrals, and from prior studies in our laboratory. Exclusion criteria for the larger study were age under 50 years, unable to provide own informed consent, and non-fluent in English. Additional exclusion criteria for the current study included neurological (e.g., Parkinson's disease, multiple sclerosis, Tourette's, seizure disorder) or psychiatric conditions (e.g., Bipolar disorder), stroke, history of head injury with permanent brain lesion or dementia (American Psychiatric Association, 2000). The sample included 119 community-dwelling adults between the ages of 51 and 89 years old (M = 68.1, SD = 8.3). Basic demographic and health information were initially collected over the phone by trained research assistants. Further information regarding collection of relevant health information is provided subsequently. Participants were also screened with the Telephone Interview of Cognitive Status (TICS; Brandt & Folstein, 2003) to obtain an estimate of global cognitive functioning. The Institutional Review Board at Washington State University approved this research protocol and all participants provided informed consent. Participant characteristics including neuropsychological test standard scores are presented in Table 1.
Table 1.
Participant characteristics (n = 119)
| Mean (SD) or % | Range | |
|---|---|---|
| Age | 68.48 (8.32) | 51–89 |
| Education | 16.6 (2.61) | 9–20 |
| Sex (% women) | 74.8 | |
| Non-Hispanic White | 97.5 | |
| Wechsler test of adult reading | 114.43 (9.08) | 87–126 |
| Telephone interview for cognitive status | 35.06 (2.82) | 21–42 |
| PROMIS depression T-score | 49.07 | 38.20–71.70 |
| Neuropsychological test scores | ||
| MAS immed. prose scaled score | 9.82 (2.39) | |
| MAS delay prose scaled score | 9.67 (2.65) | |
| MAS delayed list scaled score | 9.75 (2.96) | |
| TMTB T-score | 52.41 (9.77) | |
| Design fluency 3 scaled score | 11.64 (2.29) | |
| CWIT 3 scaled score | 11.11 (2.75) | |
| Spatial span scaled score | 12.45 (2.66) | |
| Cardiometabolic variables | ||
| Obesity (%) | 40.3 | |
| Hypertension (%) | 58.0 | |
| Diabetes (%) | 16.0 | |
| Lipid-lowering medication use (%) | 35.3 | |
Notes: MAS = Memory Assessment Scale; TMT = Trail Making Test; Design Fluency Trial 3 (switching); CWIT = Color-Word Interference Test Trial 3 (inhibition).
Neuropsychological assessment
Neuropsychological tests for the current study were selected to represent verbal memory and executive functions. Given that research has shown that memory and executive abilities are sensitive to cardiometabolic dysregulation, tests to assess these two domains were selected for the current study. Details of the neuropsychological tests used in this study are presented subsequently.
Memory assessment scale
In the Memory Assessment Scale (MAS; Williams, 1991) list learning subtest the participant is read a list of 12 words up to six times in order to learn the list. Long delayed recall for the list is conducted approximately 20 min after the learning trial. For the MAS prose memory subtest, the participant is read a short story. The participant is then asked six yes/no question about the story. After a 20 min delay, the participant is again asked the same six questions about the story. The total number of correct answers yields the immediate and delayed prose memory scores, respectively. The MAS Delayed List Recall, Immediate Prose, and Delayed Prose subtests were used to assess verbal memory. The MAS has demonstrated ability to differentiate between normal cognition, mild cognitive impairment, and dementia (Mungas, Reed, & Kramer, 2003).
Delis-Kaplan Executive Functioning System subtests
The Delis-Kaplan Executive Functioning System (D-KEFS; Delis, Kaplan, & Kramer, 2001) Color-Word Interference Test-Inhibition (CWIT-I) trial is a variant of the Stroop procedure in which the participant is presented with a page with words written in different colored ink. The participant is instructed to say the color of the ink that the word is written in and not read the word. This subtest was used as a measure of inhibitory control and has shown to be sensitive to frontal lobe functioning and age-related decline in inhibition (Adólfsdóttir, Wollschlaeger, Wehling, & Lundervold, 2017; Adólfsdóttir et al., 2014). For the D-KEFS Design Fluency Trial 3 (switching), the participant is presented with a piece of paper with rows of boxes, each with several dots inside. The participant is instructed to draw a design in the boxes as quickly as possible, using four straight lines without repeating any designs. The total number of correct designs completed in 60 s was used as a measure of switching ability. This task has shown to be sensitive to frontal lobe lesions (Baldo, Shimamura, Delis, Kramer, & Kaplan, 2001).
Trail Making Test, Part B
In this task participants are instructed to connect 26 circles containing number and letters, in ascending order, alternating between numbers and letters (i.e., 1—A—2—B…). Total time to complete the task was used as a measure of inhibitory control and visual/spatial sequencing (Heaton, Miller, Taylor, & Grant, 2004; Strauss, Sherman, & Spreen, 2006).
Wechsler Memory Scale, Third Edition spatial span subtest
In this task, participants are instructed to touch blocks in either the same order (Trial 1) or reverse order (Trial 2) that the examiner touches. The total number of correct responses for both trials were used as a measure of visual working memory and sequencing (Wechsler, 1997).
Health assessment
The presence of hypertension was obtained through self-report of diagnosis or self-report of antihypertensive medication use. In addition, blood pressure measurements were obtained for 100 of the participants. Three separate measurements were taken in the seated position at 5 min intervals. The average of the three measurements were used to determine the presence or absence of hypertension based on measured systolic blood pressure greater than 139 mmHg or diastolic blood pressure greater than 89 mmHg for individuals who did not report a diagnosis of hypertension or antihypertensive medication use. Participant height and weight were measured by a trained research assistant. BMI was calculated with the following formula: weight (lb)/[height (in)]2 × 703 (World Health Organization, 2006). Participants with a BMI of 30 or higher were classified as obese. Information about other cardiometabolic variables that have been examined in relation to cognitive function were also collected. These variables were dyslipidemia and diabetes. Dyslipidemia was determined based on self-report of lipid-lowering medication use. The presence of diabetes was obtained through self-report of diagnosis and/or self-report of an antidiabetic medication.
Functional outcome variables
Self-report
Patient Reported Outcome Measurement Information System-Physical Function 10a
This is a 10-item self-report questionnaire designed to assess the extent in which an individual's health limits their ability to engage in everyday activities that require physical capability (Cella et al., 2010). Items are rated on a Likert scale ranging from a rating of 1 (“unable to do”) to 5 (“without any difficulty”). A total raw score was created by summing all of the individual items for each participant. The total raw score was then converted to a T-score based on standard procedures (http://www.assessmentcenter.net/Manuals.aspx). Higher T-scores indicate better physical functioning. On the Patient Reported Outcome Measurement Information System (PROMIS) Physical Function measure, 2.5% of participants scored 1.5 standard deviations below the mean.
Direct observation
Six activities
Participants completed a series of six activities of daily living within a campus smart apartment. These activities included sweeping and dusting the kitchen and living room, washing hands in kitchen sink, filling a medication dispenser, watering house plants in kitchen and living room, washing countertops in kitchen, and preparing a cup of soup and glass of water. Prior to performing each activity, experimenters provided brief verbal instructions about the task. Using our real time annotation system, trained research assistants tagged the time for each activity and each activity task step as well as coded task errors. Occurrences of the following error types were coded for each of the six activities (see Table 2): omission errors (task steps not completed), substitution errors (alternate objects or locations for action substituted), inefficient actions (corrected omission and substitution errors, searching behaviors, related but unneeded task related behaviors, perseverations) and irrelevant actions (non-task related behaviors, wandering). Video recordings of participants’ performances were then reviewed by an independent rater and recoded when necessary for accuracy. These six activities are a modified variant of a previously used set of tasks that have shown to be related to cognitive and functional abilities among older adults (Schmitter-Edgecombe & Parsey, 2014; Schmitter-Edgecombe, Parsey & Cook, 2011). Subtask accuracy scores were derived for each of the six activities based on a percentage of the number and types of errors committed during completion of each activity (see Table 2). The six activities total score represents the sum of the six subtask accuracy scores (range 6–30), with a higher score indicating poorer task accuracy. On this functional measure, 7.7% of participants performed greater than or equal to 1.5 standard deviations below the mean.
Table 2.
Coding schema for error types and overall accuracy score for six activities
| An overall score was derived for each activity based on the occurrence of errors. The eight scores were then summed for a direct observation total accuracy score. |
| Omission errors: Coded when a step necessary for accurate task completion is not performed (e.g., failure to watering can for plant watering task). |
| Substitution errors: Coded when an alternate object, or a correct object but an incorrect gesture, is used and disrupts accurate completion of the activity (e.g., dusting the kitchen instead of the living room). |
| Inefficient actions: Coded when an action that slows down or compromises the efficiency of task completion is performed (e.g., making multiple trips to fill up the watering can, searching in multiple locations, perseverating on an activity). Inefficient actions were also coded when omission or substitution errors were corrected, or when unnecessary task related activities were completed (e.g., drying off of watering can). |
| Irrelevant actions: Coded when an action that is unrelated to the activity, and completely unnecessary for activity completion, is performed (e.g., wandering, opens refrigerator when completing the washing countertops task). |
Subtask activity score (derived for each of the 6 tasks)
|
Timed up and go
For this test, participants are instructed to begin in a seated position in a chair, then rise from the chair, walk 10 feet, turn around, walk back to the chair and sit back down. The score is the total time in seconds to complete the task. This task was included to assess the extent to which cardiometabolic variables contribute to mobility, and the way in which mobility may moderate the association with activities of daily living that require physical capacity. Slower TUG speed, particularly ≥12 s, is associated with an increased fall risk in older adults (see Lusardi et al., 2016 for a meta-analysis and systematic review). In the current study, 11% of participants completed this task in greater than or equal to 12 s.
Additional measures
PROMIS emotional distress-depression 8-item
This is an 8-item self-report questionnaire used to assess the frequency (1 = Never to 5 = Always) of depressive symptoms (e.g., “I felt depressed”) in the past 7 days (Cella et al., 2010). Individual items are summed to create a total score which was then converted to a T-score using standard procedures (http://www.assessmentcenter.net/Manuals.aspx). Higher scores indicate a greater frequency of depressive symptoms.
Statistical analyses
Prior to analysis all continuous data were examined for normality. Non-normally distributed data, including Depression T-scores, MAS delayed list recall, CWIT 3, and Trails B were log transformed to improve distribution normality. To reduce the number of dependent variables, a principal components analysis (PCA) with a varimax rotation was conducted with all seven cognitive measures. All health variables (hypertension, obesity, diabetes, and lipid-lowering medication) were dichotomously coded (0 = No, 1 = Yes). Bivariate correlations were completed to examine relations between predictor and outcome variables.
Hierarchical regression analyses were conducted to determine the independent and differential effects of obesity and hypertension on cognitive, physical and functional outcome measures. These analyses were also used to identify the unique contribution of relevant covariates on outcome measures. Age, sex, education, diabetes, cholesterol medication use, and depression were entered in the first block of the regression analyses as covariates. Obesity and hypertension were entered into the second block as predictors. Multicollinearity was inspected and was acceptable for all regression models (VIF < 2). Missing data reduced the number of cases included in regression analyses with the Six Activities task and TUG (n = 116–118).
Path analysis was conducted to examine the direct and indirect (through cognition and TUG) effects of cardiometabolic variables on functional outcomes. Based on theory and previous research, variables for the path analyses were classified into primary (cardiometabolic and control variables), intermediate (cognitive variables and TUG), and everyday physical and functional outcomes (PROMIS Physical Functioning and Six Activities Task). Bias-corrected bootstrapping (2,000 samples) with a 90% confidence interval was used to determine indirect effects. Using this method, indirect effects cannot be estimated with missing data. Three participants were missing Six Activities Task data due to administrative error (n = 2) or participant fatigue (n = 1) and one participant was missing TUG data due to administrative error. Therefore, path models were run with 115 participants. Model fit was evaluated using multiple indices recommended by Hu and Bentler (1999) including (1) the comparative fit index (CFI), (2) the root mean square error of approximation (RMSEA), (3) the normed fit index (NFI), and (4) chi-squared test for lack of fit. An alpha level of p < .05 was set for all analyses.
Results
Neurocognitive domains
Results from the PCA along with means and standard deviations for the cognitive tests are presented in Table 3. The Kaiser–Meyer–Olkin measure of sampling adequacy was .72 (greater than .5 is recommended; Kaiser, 1974), which indicates that the sample size is adequate for PCA. Bartlett's test of sphericity was significant, χ2 (21) = 329.04, p < .001, which indicates that between item correlations are sufficient for PCA. Components with an eigenvalue greater than or equal to 1 were extracted (Kaiser, 1960). The PCA yielded two factors with eigenvalues of 1 or greater accounting for 43.8% and 19.6% of the total variance, respectively. The first component (episodic memory) comprised the MAS Prose Immediate and Delayed number of questions correctly answered and the MAS Delayed List Recall. D-KEFS Color-Word Interference Test-Inhibition, Spatial Span, Trails B, and Design Fluency Trial 3 loaded on the second component (executive functions). These two components were used in subsequent analyses.
Table 3.
Principal components analysis of cognitive measures with a Varimax rotation (n = 119)
| Mean | SD | Memory | Executive | |
|---|---|---|---|---|
| MAS immed. prose | 6.46 | 1.38 | .946 | .042 |
| MAS delay prose | 6.30 | 1.46 | .937 | .123 |
| MAS delayed list | 10.97 | 1.56 | .563 | .360 |
| TMTB | 76.94 | 34.34 | .184 | .783 |
| Design fluency 3 | 7.39 | 2.23 | .121 | .708 |
| CWIT 3 | 64.42 | 18.69 | .381 | .694 |
| Spatial span | 15.96 | 2.89 | −.014 | .640 |
| Eigenvalue | 3.06 | 1.37 | ||
| % of variance | 43.79 | 19.61 | ||
Notes: MAS = Memory Assessment Scale; TMT = Trail Making Test; Design Fluency Trial 3 (switching); CWIT = Color-Word Interference Test Trial 3 (inhibition); Component loadings greater than .4 are bolded.
Preliminary analyses
Correlations between cardiometabolic predictors, neurocognitive composite scores, physical measures and functional outcome are presented in Table 4. Regarding the associations between cardiometabolic predictors of interest and control variables, obesity correlated with age (r = −.203, p = .027) and hypertension correlated with lipid-lowering medication use (r = .201, p = .028). None of the other control variables, including depression, were associated with obesity or hypertension (p’s > .05).
Table 4.
Bivariate correlations between cardiometabolic and outcome variables
| Obesity | HTN | Memory | Executive | Physical T | TUG | |
|---|---|---|---|---|---|---|
| HTN | .179 | – | ||||
| Memory | .139 | .034 | – | |||
| Executive | −.210* | −.161 | .000 | – | ||
| Physical T | −.365*** | −.055 | −.047 | .246** | – | |
| TUGa | .253 | −.002 | .027 | −.322*** | −.402*** | – |
| Six activitiesb | .173 | .054 | −.224* | −.373*** | −.076 | .206* |
Notes: *p < .05, **p < .01, ***p < .001. HTN = Hypertension; TUG = Timed Up and Go.
an = 118.
bn = 116.
Mediators
After controlling for age, sex, education, diabetes, lipid-lowering medication use, and depression, results from the regression model predicting executive function revealed a significant main effect of the predictors on executive function [∆R2 = .091; ∆F(2, 110) = 8.31; p < .001] accounting for 9.1% of additional variance (Table 5). Obesity emerged as the only significant predictor of executive function (β = −.301, t = −3.86, p < .001). Among the covariates, age was the only variable that was a significant predictor of executive function (β = −.515, t = −6.06, p < .001). There was no main effect of the cardiometabolic variables on the memory composite [∆R2 = .018; ∆F (2, 110) = 1.06; p = .350]. Moreover, none of the covariates accounted for a significant amount of the variance in memory performance (ps > .05).
Table 5.
Standardized coefficients for regression analyses
| Predictor | Memory | Executive | Physical | TUGa | SATb |
|---|---|---|---|---|---|
| β | β | β | β | β | |
| Step 1 | |||||
| Age | −.052 | −.515*** | −.173 | .248* | .164 |
| Sex | −.186 | −.131 | .124 | .042 | .406*** |
| Education | −.034 | .077 | .087 | −.081 | .006 |
| Depression | −.048 | −.151 | −.271** | .181 | .211* |
| Diabetes | −.018 | −.003 | −.154 | −.125 | −.097 |
| Dyslipidemia | −.067 | −.106 | −.026 | .029 | .064 |
| ∆R2 | .049 | .314 | .146 | .100 | .225 |
| F for ∆R2 | .953 | 8.54 | 3.192** | 2.062 | 5.264*** |
| Step 2 | |||||
| Age | −.031 | −.565*** | −.245** | . 323** | .201* |
| Sex | −.183 | −.136 | .128 | .036 | .408*** |
| Education | −.027 | .060 | .058 | −.049 | .024 |
| Depression | −.057 | −.133 | −.255** | .156 | .200* |
| Diabetes | −.032 | .028 | −.127 | −.138 | −.125 |
| Dyslipidemia | −.080 | −.080 | −.015 | .013 | .048 |
| Obesity | .132 | −.300*** | −.365*** | .329*** | .220* |
| HTN | .025 | −.040 | .064 | −.085 | .025 |
| ∆R2 | .018 | .090 | .120 | .096 | .047 |
| F for ∆R2 | 1.060 | 8.304*** | 8.966*** | 6.492** | 3.464* |
Notes: *p < .05, **p < .01, ***p < .001. an = 118, bn = 116. HTN = Hypertension; TUG = Timed Up and Go.
After adjusting for covariates, results from the regression model predicting TUG performance revealed a significant main effect of the cardiometabolic predictors [∆R2 = .096; ∆F (2, 119) = 6.49; p = .002]. Obesity was the only significant predictor of TUG performance (β = .329, t = 3.59, p < .001). Age was the only covariate with significant relation to TUG performance.
Functional outcomes
After controlling for relevant covariates, there was a significant main effect of predictors on the Six Activities Task [∆R2 = .047; ∆F (2, 107) = 3.46; p = .035], with obesity emerging as the only significant predictor (β = .220, t = 2.52, p = .013). Of the covariates included in the model, age, sex, and depression independently accounted for a significant amount of the variance in Six Activities Task performance.
The regression model predicting self-reported limitations in activities of daily living that require physical capability revealed a significant main effect, after controlling for covariates [∆R2 = .12; ∆F (2, 110) = 8.97; p < .001]. Obesity (β = −.365, t = −4.23, p < .001) was the only significant predictor of greater limitation in physical functioning. Age and depression were the only covariates that accounted for a significant amount of variance in Physical T-scores.
Path analysis
Path analyses were conducted to examine the direct and indirect effects of cardiometabolic conditions on functional outcomes. Given that hypertension did not correlate with any of the outcome measures, and memory was not associated with any of the functional outcomes, both variables were excluded from the path analyses. The initial (baseline; Model 0) path model was specified to examine all the direct effects of obesity and age on executive functions, Six Activities Task, PROMIS Physical Functioning, and TUG. All paths were significant (p < .05), with the exception of the direct path from age to self-reported physical functioning (p > .05). As expected, the model was a poor fit for the data (see Table 6 for fit indices).
Table 6.
Fit indices for the path models
| Model | χ2 | df | p | CFI | TLI | RMSEA |
|---|---|---|---|---|---|---|
| 0 | 24.2 | 6 | <.001 | .838 | .595 | .163 |
| 1 | 4.5 | 4 | .342 | .996 | .983 | .033 |
| 2 | 6.23 | 7 | .513 | 1.000 | 1.015 | <.001 |
Notes: CFI = comparative fit index; TLI = Tucker-Lweis index; RMSEA = root mean square error of approximation.
Next, based on theory and statistical considerations, the model was modified. Consistent with our hypotheses, executive function and TUG were specified as moderators of the Six Activities Task score and self-reported everyday physical functioning, respectively (Model 1). These modifications improved the fit of the model. Of note, after specifying a direct path from the executive functioning composite to Six Activities Task, there was no longer a significant direct effect of obesity on Six Activities.
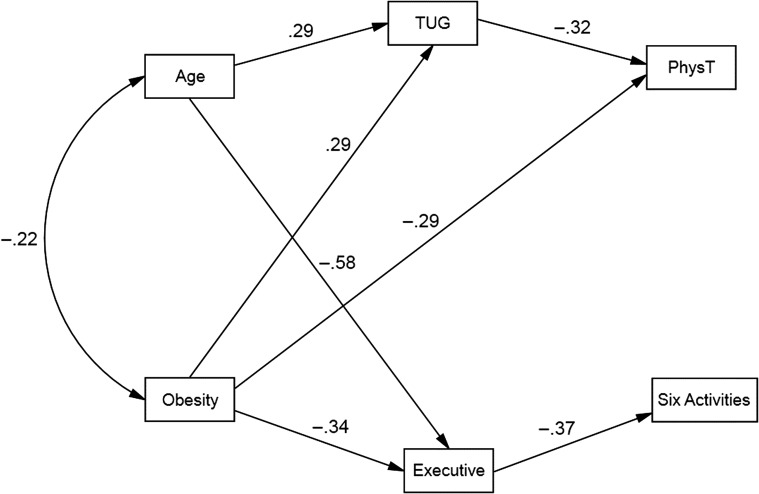
Finally, in order to identify the most parsimonious model (Model 2) that provided a good fit for the data, nonsignificant paths were removed one-by-one beginning with the direct path from age to self-reported physical function and the direct path from obesity to Six Activities Task performance. The most parsimonious model also provided the best fit for the data. In this model (Fig. 1), obesity had a direct effect and indirect effect (through TUG) on lower self-reported physical functioning; whereas, the effect of obesity on Six Activities performance was fully mediated by executive functions. Obesity was directly related to lower executive functioning and exerted an indirect effect (through executive function) on Six Activities Task performance. Age had a significant direct effect on TUG performance and executive functioning, such that older age was associated with slower TUG and poorer executive functions, even after controlling for the effects of obesity. Age also had an indirect effect (through TUG) on self-reported physical functioning.
Fig. 1.
Final path model of relations between obesity, executive functions, mobility, and everyday physical function and directly observed instrumental activities of daily living. All paths shown are significant (p < .05).
Discussion
The results from this study indicate that obesity is associated with lower executive functions, physical ability, and directly observed performance on instrumental activities of daily living (IADL) as measured by the Six Activities task. In particular, executive function fully mediated the relation between obesity and IADL performance. In contrast, obesity exhibited a direct and indirect (through TUG) effect on self-reported everyday physical functioning. These findings implicate obesity as one potential mechanism underlying the link between executive dysfunction and functional limitations among middle age and older adults.
In the current study, both age and obesity had a direct effect on executive functions. These findings are consistent with previous reports demonstrating that elevated BMI was associated with poorer executive functions (Gunstad et al., 2007). It also extends previous research that has shown an interactive effect between age and obesity (Stanek et al., 2013), suggesting that obesity in older age may be particularly detrimental to higher order cognitive abilities. Indeed, evidence suggests that obesity may accelerate neurodegeneration associated with brain aging (Ronan et al., 2016). These findings are also in line with studies that have reported higher BMI to be associated with neuronal abnormalities and reduced cortical thickness in frontal brain regions responsible for higher order cognitive abilities and inhibitory control (Gazdzinski et al., 2010; Lavagnino et al., 2016).
Inflammatory processes have been proposed to at least partially explain relations between obesity, cognitive dysfunction, and functional limitations. One explanation is that obesity increases oxidative stress and proinflammatory cytokines through adipose tissue. Systemic inflammation has been associated with brain atrophy and may potentiate age-related cognitive decline (Jefferson et al., 2007). Chronic systemic inflammation, particularly IL-6, has been associated with increased risk of cognitive impairment (Wichmann et al., 2014). The presence of multiple chronic medical conditions has also been associated with inflammation and functional limitations among middle-aged and older adults and there is evidence that inflammation may partially mediate the association between multiple medical conditions and functional abilities (Friedman, Christ, & Mroczek, 2015).
One of the more unique findings from the current study is that executive functions fully mediated the relation between obesity and directly observed IADL performances. This finding extends previous research that has found that higher BMI is associated with lower cognitive performance and functional abilities. However, to our knowledge, this is the first study to demonstrate a statistical connection between these three variables. The lack of association of TUG performance with directly observed IADLs suggests that the observed functional limitations were not due to mobility difficulties.
In the path model, obesity had a direct effect on both TUG performance and self-reported everyday activities of daily living that require physical capability. Obesity also had an indirect effect, through TUG, on these everyday activities requiring physical capability (e.g., climbing stairs, carrying groceries, dressing, bathing, etc.). This is consistent with previous research that found that adiposity is associated with poorer physical performance among older adults with metabolic syndrome (Beavers et al., 2013) and obesity is associated with declines in physical mobility and ability to perform activities of daily living (An & Shi, 2015). It is possible that obesity-related physical and functional limitations among otherwise healthy adults leads to increasing levels of inactivity which then compounds the effect of age-related decline. This is particularly relevant for older adults given the association between TUG performance, increased fall risk, and subsequent disability. More longitudinal research is needed to identify the extent to which obesity contributes to physical and functional decline over time.
The hypothesis that cardiometabolic conditions would be associated with verbal memory ability was not supported. In addition, hypertension was not significantly related to any of the cognitive, physical, or functional outcomes. This finding differs from some previous research that has found elevated BMI and hypertension to be associated with poorer verbal memory abilities (Cournot et al., 2006; Elias et al., 2003; Wright et al., 2015). It is possible that the discrepant findings are at least in part due to differences in sample characteristics. In two of the studies, poorer verbal memory performance was only observed for men, not women (Elias et al., 2003; Wright et al., 2015). If there is indeed a sex difference in the association between cardiometabolic factors and verbal memory abilities, it is possible that there was not an adequate number of men in the current sample to detect such an effect. Previous research has shown performance differences on the MAS between normal, mild cognitive impairment, and dementia as well as dementia subtypes (i.e., Alzheimer's disease vs. vascular dementia) and association with cortical atrophy (Lafosse et al., 1997; Mungas et al., 2003). Nevertheless, the MAS is less commonly used than other memory measures and may account for the disparate findings in the current study as compared to previous research that has demonstrated that elevated BMI and hypertension are associated with lower verbal memory abilities. Other prior research, however, has failed to find an association of BMI and hypertension with verbal memory abilities and is consistent with the current study findings (Brady et al., 2005; Dahle et al., 2009; Dore et al., 2008; Hudak et al., 2013; Kesse-Guyot et al., 2015; Stanek et al., 2013).
With regard to limitations, several factors should be considered in the interpretation of the results from the current study. First, the participants in the current study were generally healthy predominantly well-educated non-Hispanic white women. Therefore, the results may not necessarily generalize to more diverse samples or clinical populations. Other factors associated with obesity, such as smoking, substance abuse and sleep apnea were not assessed in this study and may contribute to cognitive and functional abilities. Moreover, even though the results delineate an association between obesity, cognition, and functional outcomes, causal mechanisms cannot be determined through this type of cross-sectional research. More longitudinal research is needed to provide a better mechanistic understanding of proportional changes in these factors over time. It has been proposed that inhibitory control contributes to overeating and subsequent obesity, which suggests that poorer executive abilities may precede incident obesity. However, there is also evidence that intentional weight loss through calorie restriction in obese older adults with MCI is associated with incremental improvement in executive functions, memory, language, and global cognition (Horie et al., 2016). Improvement in memory and executive abilities have also been reported in adults after bariatric surgery (for a review see Handley, Williams, Caplin, Stephens, & Barry, 2016). These studies suggest that obesity contributes to cognitive limitations and offer preliminary evidence that weight loss among obese adults may improve cognitive functions.
The way in which health variables were defined in the current study must also be considered. With the exception of BMI and hypertension, the other health variables (i.e., diabetes, dyslipidemia) were defined based only on self-report of a diagnosis and/or medication for the condition of interest. Furthermore, hypertension was defined based on self-report of a diagnosis, and/or antihypertensive use, and/or measured systolic blood pressure greater than 139 mmHg or diastolic blood pressure greater than 89 mmHg. This classified treated and possibly untreated/uncontrolled hypertension together. Additionally, we defined obesity as a BMI of 30 or greater. However, several other methods have been developed to measure adiposity including waist circumference, waist/hip ratio, as well as comparing amount of lean muscle mass to fat mass. Future research should include multiple measures of adiposity in order to clarify associations with cognitive and functional abilities, as this may partially account for discrepant findings across studies.
The neurocognitive measures used in the current study were limited to the domains of verbal memory and executive functions. Tests to assess these constructs were selected based on findings from previous research that has investigated cardiometabolic conditions and cognition, but it is certainly possible that other areas of ability are affected. However, the size of the current sample precluded inclusion of tests to measure additional aspects of cognitive ability and used PCA to define cognitive domains. More research is needed with larger samples and a wider variety of neuropsychological tests to assess other domains (e.g., information processing speed, language, and visual/spatial skills).
Taken together the results of this study indicate separate pathways for limitations in everyday activities of daily living that require physical functions and IADLs among obese adults. In one pathway, obesity was associated with lower executive functions and poorer instrumental IADL performance. In the other pathway, obesity was related to decreased mobility and less cognitively demanding daily activities that require physical capability. Further exploration of factors that contribute to lower cognitive and functional abilities in generally healthy adults is important to understanding the trajectory of impairment and disability across the lifespan.
Funding
This work was supported by grants from the National Institute of Biomedical Imaging and Bioengineering [R01 EB009675].
Conflict of Interest
None declared.
Acknowledgements
We thank our study participants for their participation in our research studies. We also thank members of our research lab for their assistance in data collection.
References
- Adólfsdóttir S., Haász J., Wehling E., Ystad M., Lundervold A., & Lundervold A. J. (2014). Salient measures of inhibition and switching are associated with frontal lobe gray matter volume in healthy middle-aged and older adults. Neuropsychology, 28, 859. [DOI] [PubMed] [Google Scholar]
- Adólfsdóttir S., Wollschlaeger D., Wehling E., & Lundervold A. J. (2017). Inhibition and switching in healthy aging: a longitudinal study. Journal of the International Neuropsychological Society, 23, 90–97. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision (75). Washington, DC: American Psychiatric Association. [Google Scholar]
- An R., & Shi Y. (2015). Body weight status and onset of functional limitations in US middle-aged and older adults. Disability and Health Journal, 8, 336–344. [DOI] [PubMed] [Google Scholar]
- Baldo J. V., Shimamura A. P., Delis D. C., Kramer J., & Kaplan E. (2001). Verbal and design fluency in patients with frontal lobe lesions. Journal of the International Neuropsychological Society, 7, 586–596. [DOI] [PubMed] [Google Scholar]
- Beavers K. M., Hsu F. C., Houston D. K., Beavers D. P., Harris T. B., Hue T. F., et al. (2013). The role of metabolic syndrome, adiposity, and inflammation in physical performance in the Health ABC Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 68, 617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzenius J. D., Laidlaw D. H., Cabeen R. P., Conturo T. E., McMichael A. R., Lane E. M., et al. (2013). Impact of body mass index on neuronal fiber bundle lengths among healthy older adults. Brain Imaging and Behavior, 7, 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J., & Folstein M. (2003). Telephone Interview for Cognitive Status. Lutz, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Brady C. B., Spiro III A., & Gaziano J. M. (2005). Effects of age and hypertension status on cognition: the Veterans Affairs Normative Aging Study. Neuropsychology, 19, 770. [DOI] [PubMed] [Google Scholar]
- Carlson M. C., Fried L. P., Xue Q. L., Bandeen-Roche K., Zeger S. L., & Brandt J. (1999). Association between executive attention and physical functional performance in community-dwelling older women. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 54, S262–S270. [DOI] [PubMed] [Google Scholar]
- Carlson M. C., Xue Q. L., Zhou J., & Fried L. P. (2009). Executive decline and dysfunction precedes declines in memory: the Women's Health and Aging Study II. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 64A, 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D., Riley W., Stone A., Rothrock N., Reeve B., Yount S., et al. (2010). The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of clinical epidemiology, 63, 1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournot M., Marquie J. C., Ansiau D., Martinaud C., Fonds H., Ferrieres J., et al. (2006). Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology, 67, 1208–1214. [DOI] [PubMed] [Google Scholar]
- Dahle C. L., Jacobs B. S., & Raz N. (2009). Aging, vascular risk, and cognition: blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychology and Aging, 24, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D. C., Kaplan E., & Kramer J. H. (2001). Delis-Kaplan Executive Function System: Examiner's manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Dore G. A., Elias M. F., Robbins M. A., Budge M. M., & Elias P. K. (2008). Relation between central adiposity and cognitive function in the Maine–Syracuse Study: attenuation by physical activity. Annals of Behavioral Medicine, 35, 341–350. [DOI] [PubMed] [Google Scholar]
- Elias M. F., Dore G. A., Davey A., Robbins M. A., & Elias P. K. (2010). From blood pressure to physical disability. Hypertension, 55, 1360–1365. [DOI] [PubMed] [Google Scholar]
- Elias M. F., Elias P. K., Sullivan L. M., Wolf P. A., & D'Agostino R. B. (2003). Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. International Journal of Obesity, 27, 260–268. [DOI] [PubMed] [Google Scholar]
- Ervin R. B. (2009). Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States. National Health Statistics Reports, 13, 1–8. [PubMed] [Google Scholar]
- Farias S. T., Park L. Q., Harvey D. J., Simon C., Reed B. R., Carmichael O., et al. (2013). Everyday cognition in older adults: associations with neuropsychological performance and structural brain imaging. Journal of the International Neuropsychological Society, 19, 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergenbaum J. H., Bruce S., Lou W., Hanley A. J., Greenwood C., & Young T. K. (2009). Obesity and lowered cognitive performance in a Canadian First Nations population. Obesity, 17, 1957–1963. [DOI] [PubMed] [Google Scholar]
- Friedman E. M., Christ S. L., & Mroczek D. K. (2015). Inflammation partially mediates the association of multimorbidity and functional limitations in a national sample of middle-aged and older adults: The MIDUS Study. Journal of Aging and Health, 27, 843–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S., Millin R., Kaiser L. G., Durazzo T. C., Mueller S. G., Weiner M. W., et al. (2010). BMI and neuronal integrity in healthy, cognitively normal elderly: a proton magnetic resonance spectroscopy study. Obesity, 18, 743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigsby J., Kaye K., Baxter J., Shetterly S. M., & Hamman R. F. (1998). Executive cognitive abilities and functional status among community‐dwelling older persons in the San Luis Valley Health and Aging Study. Journal of the American Geriatrics Society, 46, 590–596. [DOI] [PubMed] [Google Scholar]
- Gunning‐Dixon F. M., Brickman A. M., Cheng J. C., & Alexopoulos G. S. (2009). Aging of cerebral white matter: a review of MRI findings. International Journal of Geriatric Psychiatry, 24, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstad J., Paul R. H., Cohen R. A., Tate D. F., Spitznagel M. B., Grieve S., & Gordon E. (2008). Relationship between body mass index and brain volume in healthy adults. International Journal of Neuroscience, 118, 1582–1593. [DOI] [PubMed] [Google Scholar]
- Gunstad J., Lhotsky A., Wendell C. R., Ferrucci L., & Zonderman A. B. (2010). Longitudinal examination of obesity and cognitive function: results from the Baltimore longitudinal study of aging. Neuroepidemiology, 34, 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstad J., Paul R. H., Cohen R. A., Tate D. F., Spitznagel M. B., & Gordon E. (2007). Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Comprehensive Psychiatry, 48, 57–61. [DOI] [PubMed] [Google Scholar]
- Guo F., He D., Zhang W., & Walton R. G. (2012). Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. Journal of the American College of Cardiology, 60, 599–606. [DOI] [PubMed] [Google Scholar]
- Handley J. D., Williams D. M., Caplin S., Stephens J. W., & Barry J. (2016). Changes in cognitive function following bariatric surgery: a systematic review. Obesity Surgery, 26, 2530–2537. [DOI] [PubMed] [Google Scholar]
- Haring B., Wu C., Coker L. H., Seth A., Snetselaar L., Manson J. E., et al. (2015). Hypertension, dietary sodium, and cognitive decline: results from the women's health initiative memory study. American Journal of Hypertension, 29, 202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R. K., Miller S. W., Taylor M. J., & Grant I. (2004). Revised comprehensive norms for an expanded Halstead-Reitan Battery: demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Horie N. C., Serrao V. T., Simon S. S., Gascon M. R. P., Dos Santos A. X., Zambone M. A., et al. (2016). Cognitive effects of intentional weight loss in elderly obese individuals with mild cognitive impairment. The Journal of Clinical Endocrinology & Metabolism, 101, 1104–1112. [DOI] [PubMed] [Google Scholar]
- Hu L. T., & Bentler P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling: a Multidisciplinary Journal, 6, 1–55. [Google Scholar]
- Hudak E. M., Edwards J. D., Athilingam P., & McEvoy C. L. (2013). A comparison of cognitive and everyday functional performance among older adults with and without hypertension. Clinical Gerontologist, 36, 113–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W., Harvey D., Mungas D., & Haan M. (2005). Central obesity and the aging brain. Archives of Neurology, 62, 1545–1548. [DOI] [PubMed] [Google Scholar]
- Jefferson A. L., Massaro J. M., Wolf P. A., Seshadri S., Au R., Vasan R. S., et al. (2007). Inflammatory biomarkers are associated with total brain volume The Framingham Heart Study. Neurology, 68, 1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser H. F. (1960). The application of electronic computers to factor analysis. Educational and Psychological Measurement, 20, 141–151. [Google Scholar]
- Kaiser H. F. (1974). An index of factor simplicity. Psychometrika, 39, 31–36. [Google Scholar]
- Kesse-Guyot E., Julia C., Andreeva V., Fezeu L., Hercberg S., & Galan P. (2015). Evidence of a cumulative effect of cardiometabolic disorders at midlife and subsequent cognitive function. Age and Ageing, 44, 648–654. [DOI] [PubMed] [Google Scholar]
- Köhler S., Baars M. A., Spauwen P., Schievink S., Verhey F. R., & van Boxtel M. J. (2014). Temporal evolution of cognitive changes in incident hypertension novelty and significance. Hypertension, 63, 245–251. [DOI] [PubMed] [Google Scholar]
- Lafosse J. M., Reed B. R., Mungas D., Sterling S. B., Wahbeh H., & Jagust W. J. (1997). Fluency and memory differences between ischemic vascular dementia and Alzheimer's disease. Neuropsychology, 11, 514. [DOI] [PubMed] [Google Scholar]
- Lavagnino L., Mwangi B., Bauer I. E., Cao B., Selvaraj S., Prossin A., et al. (2016). Reduced inhibitory control mediates the relationship between cortical thickness in the right superior frontal gyrus and body mass index. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 41, 2275–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusardi M. M., Fritz S., Middleton A., Allison L., Wingood M., Phillips E., et al. (2016). Determining risk of falls in community dwelling older adults: a systematic review and meta-analysis using posttest probability. Journal of Geriatric Physical Therapy, 40, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathus-Vliegen E. M. (2012). Obesity and the elderly. Journal of Clinical Gastroenterology, 46, 533–544. [DOI] [PubMed] [Google Scholar]
- Mungas D., Reed B. R., & Kramer J. H. (2003). Psychometrically matched measures of global cognition, memory, and executive function for assessment of cognitive decline in older persons. Neuropsychology, 17, 380. [DOI] [PubMed] [Google Scholar]
- Oldridge N. B., Stump T. E., Nothwehr F. K., & Clark D. O. (2001). Prevalence and outcomes of comorbid metabolic and cardiovascular conditions in middle-and older-age adults. Journal of Clinical Epidemiology, 54, 928–934. [DOI] [PubMed] [Google Scholar]
- Raz N., Rodrigue K. M., & Acker J. D. (2003). Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behavioral Neuroscience, 117, 1169. [DOI] [PubMed] [Google Scholar]
- Ronan L., Alexander-Bloch A. F., Wagstyl K., Farooqi S., Brayne C., Tyler L. K., et al. (2016). Obesity associated with increased brain age from midlife. Neurobiology of Aging, 47, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royall D. R., Palmer R., Chiodo L. K., & Polk M. J. (2004). Declining executive control in normal aging predicts change in functional status: the Freedom House Study. Journal of the American Geriatrics Society, 52, 346–352. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M., Parsey C., & Cook D. J. (2011). Cognitive correlates of functional performance in older adults: comparison of self-report, direct observation, and performance-based measures. Journal of the International Neuropsychological Society, 17, 853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M., & Parsey C. M. (2014). Assessment of functional change and cognitive correlates in the progression from healthy cognitive aging to dementia. Neuropsychology, 28, 881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek K. M., Grieve S. M., Brickman A. M., Korgaonkar M. S., Paul R. H., Cohen R. A., et al. (2011). Obesity is associated with reduced white matter integrity in otherwise healthy adults. Obesity, 19, 500–504. [DOI] [PubMed] [Google Scholar]
- Stanek K. M., Strain G., Devlin M., Cohen R., Paul R., Crosby R. D., et al. (2013). Body mass index and neurocognitive functioning across the adult lifespan. Neuropsychology, 27, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E., Sherman E. M. S., & Spreen O. (2006). A compendium of neuropsychological tests: administration, norms, and commentary (3rd ed.). Oxford: Oxford University Press. [Google Scholar]
- Wichmann M. A., Cruickshanks K. J., Carlsson C. M., Chappell R., Fischer M. E., Klein B. E., et al. (2014). Long‐term systemic inflammation and cognitive impairment in a population‐based cohort. Journal of the American Geriatrics Society, 62, 1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. M. (1991). Memory Assessment Scales professional manual. Odessa, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Wechsler D. (1997). Wechsler memory scale (WMS-III). San Antonio, TX: Psychological Corporation. [Google Scholar]
- World Health Organization (2006). Global database on body mass index. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- Wright R. S., Cole A. P., Ali M. K., Skinner J., Whitfield K. E., & Mwendwa D. T. (2015). Examining the influence of measures of adiposity on cognitive function in middle age and older African Americans. Archives of Clinical Neuropsychology, 31, 23–28. [DOI] [PubMed] [Google Scholar]
- Yan L. L., Daviglus M. L., Liu K., Pirzada A., Garside D. B., Schiffer L., et al. (2004). BMI and health‐related quality of life in adults 65 years and older. Obesity Research, 12, 69–76. [DOI] [PubMed] [Google Scholar]