Abstract
Placental abruption (early separation of the placenta) is associated with preterm birth and perinatal mortality, but associations with other neonatal morbidities remain understudied. We examined the association between abruption and newborn outcomes. We analyzed 223,341 singleton deliveries from the Consortium on Safe Labor study, a retrospective, multisite, observational study (2002–2008) of electronic medical records in the United States. Adjusted relative risks, incidence rate ratios, and 99% confidence intervals were estimated. Direct effects attributable to abruption were examined by conditioning on intermediates (preterm birth and small for gestational age) with sensitivity analyses. Incidence of abruption was 1.6% (n = 3,619). Abruption was associated with an elevated risk of newborn resuscitation (relative risk (RR) = 1.5, 99% confidence interval (CI): 1.5, 1.6), apnea (RR = 5.8, 99% CI: 5.1, 6.5), asphyxia (RR = 8.5, 99% CI: 5.7, 11.3), respiratory distress syndrome (RR = 6.5, 99% CI: 5.9, 7.1), neonatal intensive care unit admission (RR = 3.4, 99% CI: 3.2, 3.6), longer intensive care length of stay (incidence rate ratio = 2.0, 99% CI: 1.9, 2.2), stillbirth (RR = 6.3, 99% CI: 4.7, 7.9), and neonatal mortality (RR = 7.6, 99% CI: 5.2, 10.1). In sensitivity analyses, there was a direct effect of abruption associated with increased neonatal risks. These findings expand our knowledge of the association between abruption and perinatal and neonatal outcomes.
Keywords: abruption, apnea, neonatal morbidity, perinatal mortality, respiratory distress syndrome
Placental abruption, defined as the premature detachment of the placenta from the uterine wall, before birth and after 20 weeks’ gestation, occurs in 0.6%–1% of all pregnancies in the United States (1, 2). The disorder is characterized by placental dysfunction which, with progression, can result in a decrease in the surface area available for oxygen exchange and nutrient supply to the fetus (3, 4). Abruption is well-established as a risk factor for growth restriction (5–11), prematurity (6–9, 11–15), and perinatal mortality (1, 11–13, 16–20). However, other adverse neonatal outcomes associated with hypoxia and prematurity—such as asphyxia, respiratory distress syndrome, and apnea—remain understudied. Furthermore, for neonates who survive the delivery, little is known about the extent of medical interventions used, such as admission to the neonatal intensive care unit (NICU), NICU length of stay (LOS), or newborn resuscitation in the delivery room. Finally, it is unclear how much of the risk of neonatal morbidity associated with abruption is attributable to preterm birth or being small for gestational age (SGA).
We examined the association between placental abruption and neonatal interventions, morbidities, and perinatal mortality and conducted sensitivity analyses to examine the role of prematurity and SGA in these relationships.
METHODS
Study population
This study used data from the Consortium on Safe Labor study (Eunice Kennedy Shriver National Institute of Child Health and Human Development) (21, 22). This retrospective, observational study includes electronic medical record data from 12 clinical centers containing 19 hospitals. In total, data on 228,438 deliveries occurring from 2002–2008 were collected for the study, with 9.5% of women contributing more than 1 birth during the specified time period. All deliveries occurring at 23 weeks of gestation or later with the required electronic medical record data were included in the original study. We excluded multiple gestation pregnancies (n = 5,044), as they were likely to have a different risk profile for abruption (23), and women who were missing more than half of the specified covariates (n = 53), because the reliability of the imputed values would be questionable. After exclusions, 223,341 singleton delivery records remained for analysis. Analysis of resuscitation, NICU admission, respiratory distress syndrome, apnea, asphyxia, and neonatal death was restricted to live births (n = 222,047). Institutional review board approval was originally obtained from all participating institutions, and the present analysis received an exemption from the University of Maryland Institutional Review Board.
Abruption identification
A clinical diagnosis of placental abruption was abstracted from the prenatal history, labor, delivery, and discharge codes (International Classification of Diseases, Ninth Revision) portions of the electronic medical record. A patient was considered to have an abruption if there was a recorded diagnosis of antepartum or intrapartum abruption, abruption was recorded as the indication for cesarean delivery, or there was a discharge code for abruption.
Mediator and outcome definitions
For the mediation analysis described below, preterm birth was defined as delivery at <37 weeks of gestation, and SGA was defined as a birth weight below the tenth percentile for gestational age and fetal sex (24). Newborn resuscitation, NICU admission, NICU LOS, respiratory distress syndrome, apnea, asphyxia, antepartum or intrapartum stillbirth, and neonatal deaths (≤28 days of life) were all abstracted from electronic medical records (detailed description of methods previously published) (22). Because some overlap between outcomes is likely, a composite variable was also created to capture overall risk of having at least 1 of the specified outcomes.
Statistical analysis
Because approximately 10% of the women included in the study had more than 1 birth recorded during the study period, generalized estimating equation models were fitted to estimate the risk of all the outcomes of interest, while accounting for these instances of repeated observations. An exchangeable within-subject correlation structure was specified. A modified Poisson approach with a robust error variance estimator was fitted to estimate relative risk and 99% confidence intervals for all categorical neonatal outcomes (25). A negative binomial model was fitted to estimate incidence rate ratios and 99% confidence intervals for NICU LOS (26, 27). The incidence rate ratio for NICU LOS is interpreted as a relative increase in the rate (i.e., incidence rate ratio = 2.0 would be interpreted as the exposed group having a rate of NICU LOS that was twice as long as the unexposed group—2 days for every 1 day in the unexposed group). All models adjusted for maternal age, race/ethnicity, parity, prepregnancy body mass index, maternal comorbidities (chronic hypertension, gestational hypertension, preeclampsia, pregestational diabetes, and gestational diabetes), insurance, marital status, smoking, alcohol use, drug use, and study site. We imputed missing covariates using fully conditional specification, multiple imputation methods with SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina), using PROC MI and MIANALYZE. A total of 25 imputations were performed; the amount of missing data for the covariates that were imputed was as follows: maternal age (0.1%), race/ethnicity (4.1%), prepregnancy body mass index (33.6%), insurance status (10.4%), marital status (3.2%), and drug use (9.9%).
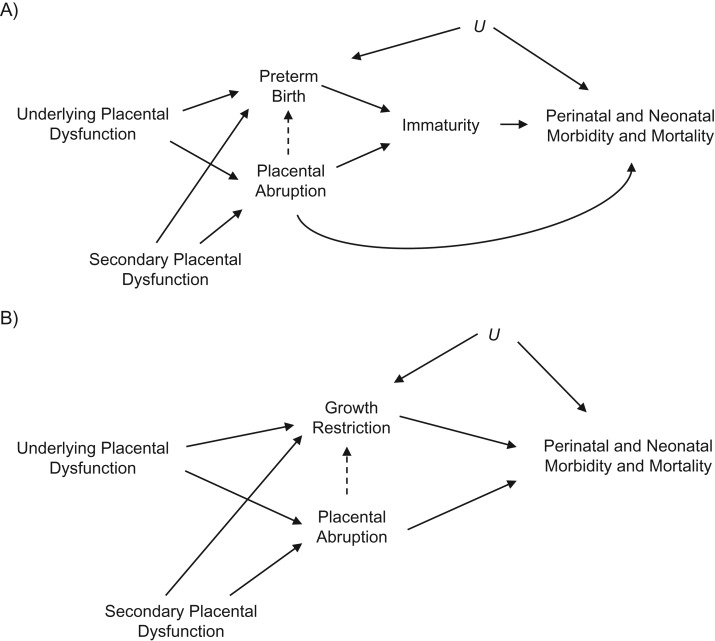
Preterm birth and fetal growth restriction are important risk factors for many poor neonatal outcomes (28–31); however, it is possible that these variables are on the causal pathway and serve as intermediates between abruption and the specified neonatal outcomes (Figure 1). Controlling for these types of variables can lead to inaccurate risk estimates if there is unmeasured confounding between the intermediate and the outcome, creating a collider bias. To estimate the direct effect of abruption independent of preterm birth and fetal growth restriction, we performed a sensitivity analysis conditioning on these intermediates, although using SGA as a proxy for fetal growth restriction (32). This approach was used to estimate direct effects, assess the potential impact of unmeasured confounding, and yield a range of bias-corrected risk estimates for individuals with preterm birth and SGA (32). For each variable, analyses are presented within each stratum, and sensitivity analyses were reported examining the impact of potential bias when the mediator was present (e.g., preterm/SGA). The analyses for this study were conducted using SAS software, version 9.4 (SAS Institute).
Figure 1.
Directed acyclic graphs illustrating theoretical relationships between placental abruption, preterm birth, growth restriction, and perinatal and neonatal morbidity and mortality. Underlying placental dysfunction due to inadequate trophoblast invasion, inflammation, and/or abnormal remodeling of the spiral arteries could lead to placental abruption, preterm birth, and growth restriction. Potential direct, indirect (dashed line), and independent relationships are shown for preterm birth (A) and growth restriction (B). Both scenarios could be affected by secondary placental dysfunction resulting from maternal factors, such as smoking or chronic hypertension, as well possible unmeasured confounders (U).
RESULTS
The incidence of placental abruption was 1.6% (n = 3,619 cases). Sample characteristics according to abruption group are presented in Table 1. Compared with women who had not had an abruption, women who had had an abruption were less likely to be white (46.6%), and more likely to be single (48.7%), be multiparous (64.3%), have public insurance (48.4%), and have a history of cesarean delivery if multiparous (26.2%). Neonates in pregnancies complicated by abruption weighed 695 grams less and were delivered 3 weeks earlier.
Table 1.
Maternal, Pregnancy, and Neonatal Sample Characteristics According to Placental Abruption Exposure, Consortium on Safe Labor Study, United States, 2002–2008
| No Abruption (n = 219,722) |
Abruption (n = 3,619) |
P | |||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Maternal age, yearsa | 28 (6) | 28 (6) | 0.89 | ||
| Maternal race/ethnicity | <0.001 | ||||
| White | 114,283 | 52.0 | 1,688 | 46.6 | |
| Black | 51,190 | 23.3 | 1,171 | 32.4 | |
| Hispanic | 39,573 | 18.0 | 583 | 16.1 | |
| Asian | 9,324 | 4.2 | 109 | 3.0 | |
| Multiple/other | 5,352 | 2.4 | 68 | 1.9 | |
| Prepregnancy BMIb,c | 24.3 (21.1–28.9) | 24.5 (21.1–29.3) | 0.07 | ||
| Comorbidity | |||||
| Chronic hypertension | 4,242 | 1.9 | 120 | 3.3 | <0.001 |
| Gestational hypertension | 5,991 | 2.7 | 87 | 2.4 | 0.24 |
| Preeclampsia/eclampsia | 12,282 | 5.6 | 395 | 10.9 | <0.001 |
| Pregestational diabetes | 3,205 | 1.5 | 104 | 2.9 | <0.001 |
| Gestational diabetes | 11,179 | 5.1 | 162 | 4.5 | 0.10 |
| Smoking during pregnancy | 14,457 | 6.6 | 468 | 12.9 | <0.001 |
| Alcohol use during pregnancy | 3,955 | 1.8 | 134 | 3.7 | <0.001 |
| Drug use during pregnancy | 4,614 | 2.1 | 266 | 7.4 | <0.001 |
| Insurance | <0.001 | ||||
| Private | 136,857 | 62.3 | 1,805 | 49.9 | |
| Public | 79,050 | 36.0 | 1,751 | 48.4 | |
| Self-pay/other | 3,815 | 1.7 | 63 | 1.7 | |
| Single marital status | 86,302 | 39.3 | 1,764 | 48.7 | <0.001 |
| Nulliparous | 87,722 | 39.9 | 1,293 | 35.7 | <0.001 |
| History of cesarean deliveryd | 30,879 | 23.4 | 610 | 26.2 | <0.001 |
| Cervical dilation at first exam, cmc | 3 (2–4) | 3 (1–4) | <0.001 | ||
| Induction of labor | 76,197 | 34.7 | 939 | 25.9 | <0.001 |
| Birth weight, gc | 3,295 (2,970–3,619) | 2,600 (1,710–3,185) | <0.001 | ||
| Birth weight <2,500 g | 17,151 | 7.8 | 1,663 | 45.9 | <0.001 |
| Small for gestational age | 24,187 | 11.0 | 565 | 15.6 | <0.001 |
| Gestational age, weeksc | 39 (38–40) | 36 (32–38) | <0.001 | ||
| Gestational age <37 weeks | 24,192 | 11.0 | 1,931 | 53.4 | <0.001 |
Abbreviation: BMI, body mass index.
a Data are given as mean values (standard deviations).
b BMI was calculated as weight (kg)/height (m)2.
c Data are given as median values (interquartile ranges).
d Among multiparous women only.
Descriptive statistics and results from adjusted regression analyses of the neonatal outcomes appear in Table 2. Overall, abruption was associated with a 1.9-fold risk of having at least 1 poor outcome, although risk estimates varied considerably according to outcome. Compared with neonates in pregnancies not complicated by abruption, the neonates in pregnancies complicated by abruption were more likely to need resuscitation in the delivery room (relative risk = 1.5, 99% confidence interval (CI): 1.5, 1.6) and to be admitted to the NICU (relative risk = 3.4, 99% CI: 3.2, 3.6). Among the neonates admitted to the NICU, abruption was also associated with a LOS nearly twice as long (incidence rate ratio = 2.0, 99% CI: 1.9, 2.2). Abruption was also associated with a 6.5-fold elevated risk of respiratory distress syndrome, 5.8-fold risk of apnea, 8.5-fold risk of asphyxia, 6.3-fold risk of stillbirth, and a 7.6-fold risk of neonatal mortality.
Table 2.
Adjusted Relative Risk for Neonatal Outcomes According to Presence or Absence of Placental Abruption, Consortium on Safe Labor Study, 2002–2008
| Neonatal Outcome | No Abruption (n = 219,722) |
Abruption (n = 3,619) |
RR | 99% CIa | ||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Composite perinatal-neonatal outcomeb | 1.9 | 1.8, 2.0 | ||||
| No | 152,234 | 69.3 | 1,195 | 33.0 | ||
| Yes | 67,488 | 30.7 | 2,424 | 67.0 | ||
| Newborn resuscitationc | 1.5 | 1.5, 1.6 | ||||
| No | 168,614 | 77.1 | 2,084 | 59.8 | ||
| Yes | 49,948 | 22.9 | 1,401 | 40.2 | ||
| NICU admissionc | 3.4 | 3.2, 3.6 | ||||
| No | 193,186 | 88.4 | 1,796 | 51.5 | ||
| Yes | 25,376 | 11.6 | 1,689 | 48.5 | ||
| NICU LOS, daysc,d | 7 (3–17) | 20 (7–48) | 2.0e | 1.9, 2.2e | ||
| Respiratory distress syndromec | 6.5 | 5.9, 7.1 | ||||
| No | 212,145 | 97.1 | 2,599 | 74.6 | ||
| Yes | 6,417 | 2.9 | 886 | 25.4 | ||
| Apneac,f | 5.8 | 5.1, 6.5 | ||||
| No | 193,982 | 97.9 | 2,854 | 84.9 | ||
| Yes | 4,180 | 2.1 | 507 | 15.1 | ||
| Asphyxiac | 8.5 | 5.7, 11.3 | ||||
| No | 218,051 | 99.8 | 3,408 | 97.8 | ||
| Yes | 511 | 0.2 | 77 | 2.2 | ||
| Antepartum or intrapartum stillbirth | 6.3 | 4.7, 7.9 | ||||
| No | 218,562 | 99.5 | 3,485 | 96.3 | ||
| Yes | 1,160 | 0.5 | 134 | 3.7 | ||
| Neonatal mortality ≤28 daysc | 7.6 | 5.2, 10.1 | ||||
| No | 217,932 | 99.7 | 3,395 | 97.4 | ||
| Yes | 630 | 0.3 | 90 | 2.6 | ||
Abbreviations: CI, confidence interval; LOS, length of stay; NICU, neonatal intensive care unit; RR, relative risk.
a Relative risk estimated with modified Poisson model adjusting for maternal age, race/ethnicity, parity, prepregnancy body mass index, maternal comorbidities (chronic hypertension, gestational hypertension, preeclampsia, pregestational diabetes, and gestational diabetes), insurance, marital status, smoking status, alcohol use, drug use, and study site. All models were significant at P < 0.001.
b Composite outcome included resuscitation, NICU admission, respiratory distress syndrome, apnea, asphyxia, stillbirth, and neonatal mortality.
c Excluding antepartum and intrapartum stillbirths.
d Data are given as median values (interquartile ranges) among those admitted to NICU; excludes sites 4 and 8, which did not report NICU LOS.
e Incidence rate ratio estimated with negative binomial model.
f Excluding site 6, which did not report neonatal apnea.
To determine whether there was a direct effect attributed to abruption, beyond the risks associated with preterm birth and SGA as a proxy for fetal growth restriction, we conditioned on these intermediates with sensitivity analyses (32). For each variable, we have presented descriptive statistics and adjusted regression analyses of the neonatal outcomes within each stratum (Tables 3–4). Overall, when examining the stratified estimates, we did not observe any illogical protective effects associated with risk exposure, but there were some differences in magnitude of association. However, application of the correction factor resulted in only slight shifts in magnitude of association (Web Tables 1 and 2, available at https://academic.oup.com/aje), which would suggest the absence of substantial collider bias. The risk estimates within the term and non-SGA strata represent the natural direct effects for abruption and risk of neonatal morbidity. With the exception of NICU LOS among term deliveries, all other outcomes remained significant among the respective strata: term and preterm, SGA and non-SGA.
Table 3.
Adjusted Relative Risk for Neonatal Outcomes According to Presence or Absence of Placental Abruption, Conditioned on Gestational Age at Delivery, Consortium on Safe Labor Study, 2002–2008a
| Neonatal Outcome | Term, ≥37 Weeks (n = 197,218) | Preterm, <37 Weeks (n = 26,123) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Abruption | Abruption | RR | 99% CIb | No Abruption | Abruption | RR | 99% CIb | |||||
| No. | % | No. | % | No. | % | No. | % | |||||
| Composite perinatal-neonatal outcomec | 1.4 | 1.3, 1.5 | 1.3 | 1.3, 1.4 | ||||||||
| No | 143,412 | 73.4 | 939 | 55.6 | 8,822 | 36.5 | 256 | 13.3 | ||||
| Yes | 52,118 | 26.6 | 749 | 44.4 | 15,370 | 63.5 | 1,675 | 86.7 | ||||
| Newborn resuscitationd | 1.3 | 1.3, 1.4 | 1.3 | 1.2, 1.4 | ||||||||
| No | 152,757 | 78.3 | 1,070 | 64.5 | 15,857 | 67.6 | 1,014 | 55.5 | ||||
| Yes | 42,349 | 21.7 | 589 | 35.5 | 7,599 | 32.4 | 812 | 44.5 | ||||
| NICU admissiond | 1.9 | 1.6, 2.2 | 1.5 | 1.5, 1.6 | ||||||||
| No | 181,549 | 93.0 | 1,412 | 85.1 | 11,637 | 49.6 | 384 | 21.0 | ||||
| Yes | 13,557 | 7.0 | 247 | 14.9 | 11,819 | 50.4 | 1,442 | 79.0 | ||||
| NICU LOS, daysd,e | 3 (2–7) | 4 (2–7) | 1.0f | 0.7, 1.2f | 15 (6–34) | 26 (10–52) | 1.4f | 1.3, 1.5f | ||||
| Respiratory distress syndromed | 3.3 | 2.0, 4.6 | 2.1 | 1.9, 2.2 | ||||||||
| No | 193,886 | 99.4 | 1,615 | 97.4 | 18,259 | 77.8 | 984 | 53.9 | ||||
| Yes | 1,220 | 0.6 | 44 | 2.6 | 5,197 | 22.2 | 842 | 46.1 | ||||
| Apnead,g | 2.6 | 1.2, 4.1 | 1.9 | 1.7, 2.1 | ||||||||
| No | 175,089 | 99.5 | 1,578 | 98.5 | 18,893 | 85.0 | 1,276 | 72.5 | ||||
| Yes | 850 | 0.5 | 24 | 1.5 | 3,330 | 15.0 | 483 | 27.5 | ||||
| Asphyxiad | 7.3 | 2.8, 11.9 | 3.7 | 2.2, 5.1 | ||||||||
| No | 194,805 | 99.9 | 1,639 | 98.8 | 23,246 | 99.1 | 1,769 | 96.9 | ||||
| Yes | 301 | 0.1 | 20 | 1.2 | 210 | 0.9 | 57 | 3.1 | ||||
| Antepartum or intrapartum stillbirth | 9.4 | 4.4, 14.3 | 1.7 | 1.3, 2.2 | ||||||||
| No | 195,106 | 99.8 | 1,659 | 98.3 | 23,456 | 97.0 | 1,826 | 94.6 | ||||
| Yes | 424 | 0.2 | 29 | 1.7 | 736 | 3.0 | 105 | 5.4 | ||||
| Neonatal mortality (≤28 days)d | –h | –h | 2.4 | 1.6, 3.1 | ||||||||
| No | 194,968 | 99.9 | 1,655 | 99.8 | 22,964 | 97.9 | 1,740 | 95.3 | ||||
| Yes | 138 | 0.1 | 4 | 0.2 | 492 | 2.1 | 86 | 4.7 | ||||
Abbreviations: CI, confidence interval; LOS, length of stay; NICU, neonatal intensive care unit; RR, relative risk.
a The stratified risk estimates did not show evidence for potential collider bias, and therefore uncorrected estimates were reported here. Corrected estimates for the preterm stratum are available in Web Table 1.
b Relative risk estimated with modified Poisson model adjusting for maternal age, race/ethnicity, parity, prepregnancy body mass index, maternal comorbidities (chronic hypertension, gestational hypertension, preeclampsia, pregestational diabetes, and gestational diabetes), insurance, marital status, smoking status, alcohol use, drug use, and study site. All comparisons significant at P < 0.001, except NICU LOS in the term group.
c Composite outcome included resuscitation, NICU admission, respiratory distress syndrome, apnea, asphyxia, stillbirths, and neonatal mortality.
d Excluding antepartum and intrapartum stillbirths.
e Data are given as median values (interquartile ranges) among those admitted to NICU; excludes sites 4 and 8, which did not report NICU LOS.
f Incidence rate ratio estimated with negative binomial model.
g Excluding site 6, which did not report neonatal apnea.
h Too few neonatal deaths among abruption group for model convergence.
Table 4.
Adjusted Relative Risk for Neonatal Outcomes According to Presence or Absence of Placental Abruption, Conditioned on Birth Weight for Gestational Age and Fetal Sex, Consortium on Safe Labor Study, 2002–2008a
| Neonatal Outcome | Not Small for Gestational Age and Fetal Sex (n = 198,589) | Small for Gestational Age and Fetal Sex (n = 24,752) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Abruption | Abruption | RR | 99% CIb | No Abruption | Abruption | RR | 99% CIb | |||||
| No. | % | No. | % | No. | % | No. | % | |||||
| Composite perinatal-neonatal outcomec | 1.9 | 1.9, 2.0 | 1.6 | 1.5, 1.8 | ||||||||
| No | 136,076 | 69.6 | 1,007 | 33.0 | 16,158 | 66.8 | 188 | 33.3 | ||||
| Yes | 59,459 | 30.4 | 2,047 | 67.0 | 8,029 | 33.2 | 377 | 66.7 | ||||
| Newborn resuscitationd | 1.6 | 1.5, 1.6 | 1.5 | 1.3, 1.7 | ||||||||
| No | 149,709 | 76.9 | 1,770 | 59.8 | 18,905 | 79.3 | 314 | 59.8 | ||||
| Yes | 45,024 | 23.1 | 1,190 | 40.2 | 4,924 | 20.7 | 211 | 40.2 | ||||
| NICU admissiond | 3.7 | 3.5, 3.9 | 2.1 | 1.8, 2.4 | ||||||||
| No | 173,484 | 89.1 | 1,518 | 51.3 | 19,702 | 82.7 | 278 | 53.0 | ||||
| Yes | 21,249 | 10.9 | 1,442 | 48.7 | 4,127 | 17.3 | 247 | 47.1 | ||||
| NICU LOS, daysd,e | 6 (3–17) | 22 (7–49) | 2.1f | 2.0, 2.3f | 7 (3–20) | 14 (5–37) | 1.4f | 1.1, 1.7f | ||||
| Respiratory distress syndromed | 7.2 | 6.5, 7.9 | 3.5 | 2.7, 4.4 | ||||||||
| No | 189,256 | 97.2 | 2,184 | 73.8 | 22,889 | 96.1 | 415 | 79.0 | ||||
| Yes | 5,477 | 2.8 | 776 | 26.2 | 940 | 3.9 | 110 | 21.0 | ||||
| Apnead,g | 6.7 | 5.8, 7.6 | 2.5 | 1.6, 3.3 | ||||||||
| No | 173,944 | 98.0 | 2,404 | 84.2 | 20,038 | 96.7 | 450 | 89.1 | ||||
| Yes | 3,487 | 2.0 | 452 | 15.8 | 693 | 3.3 | 55 | 10.9 | ||||
| Asphyxiad | 8.9 | 5.6, 12.1 | –h | –h | ||||||||
| No | 194,302 | 99.8 | 2,896 | 97.8 | 23,749 | 99.7 | 512 | 97.5 | ||||
| Yes | 431 | 0.2 | 64 | 2.2 | 80 | 0.3 | 13 | 2.5 | ||||
| Antepartum or intrapartum stillbirth | 7.1 | 5.0, 9.2 | 4.4 | 2.5, 6.3 | ||||||||
| No | 194,733 | 99.6 | 2,960 | 96.9 | 23,829 | 98.5 | 525 | 92.9 | ||||
| Yes | 802 | 0.4 | 94 | 3.1 | 358 | 1.5 | 40 | 7.1 | ||||
| Neonatal mortality (≤28 days)d | 8.8 | 5.5, 12.2 | 4.6 | 1.8, 7.4 | ||||||||
| No | 194,292 | 99.8 | 2,892 | 97.7 | 23,640 | 99.2 | 503 | 95.8 | ||||
| Yes | 441 | 0.2 | 68 | 2.3 | 189 | 0.8 | 22 | 4.2 | ||||
Abbreviations: CI, confidence interval; LOS, length of stay; NICU, neonatal intensive care unit; RR, relative risk.
a The stratified risk estimates did not show evidence for potential collider bias, and therefore uncorrected estimates were reported here. Corrected estimates for the low birth weight stratum are available in Web Table 2.
b Relative risk estimated with modified Poisson model adjusting for maternal age, race/ethnicity, parity, prepregnancy body mass index, maternal comorbidities (chronic hypertension, gestational hypertension, preeclampsia, pregestational diabetes, and gestational diabetes), insurance, marital status, smoking status, alcohol use, drug use, and study site. All comparisons significant at P < 0.001.
c Composite outcome included resuscitation, NICU admission, respiratory distress syndrome, apnea, asphyxia, stillbirths and neonatal mortality.
d Excluding antepartum and intrapartum stillbirths.
e Data are given as median values (interquartile ranges) among those admitted to NICU; excludes sites 4 and 8, which did not report NICU LOS.
f Incidence rate ratio estimated with negative binomial model.
g Excluding site 6, which did not report neonatal apnea.
h Model for risk of asphyxia in low–birth weight neonates would not converge due to small sample sizes at some of the individual study sites.
DISCUSSION
Among a large, population-based sample of US women, we found an association between placental abruption and elevated risk of neonatal asphyxia, respiratory distress syndrome, and perinatal mortality beyond what can be attributed to preterm birth or SGA. Additionally, abruption was associated with a greater need for neonatal resuscitation in the delivery room, NICU admission, and longer NICU LOS. Finally, abruption was also associated with elevated risk of apnea. Of particular note, the estimated risk of all neonatal outcomes remained elevated in the term group and the non-SGA group, which suggests that placental abruption had a direct negative effect on neonatal health and that there were additional risks for the neonate, such as apnea, that have not, to our knowledge, been previously documented.
Strengths and limitations of this study
Among the strengths of this study is that it was based on a large, demographically diverse, population-based, contemporary cohort with detailed clinical information that enabled adjustment for multiple confounders. Our study expanded knowledge of the occurrence of neonatal outcomes associated with abruption in multiple ways. Our sample, which included 3,619 cases of abruption, allowed estimation of infrequently reported outcomes, including neonatal delivery-room resuscitation, NICU admission, and LOS, as well as asphyxia, apnea, and respiratory distress syndrome. Furthermore, we examined the direct effects of abruption, which furthers understanding of the associated risk beyond the mediators of preterm delivery and SGA.
The association between abruption and delivery-room resuscitation, NICU admission, and longer LOS in the NICU is in accord with extant research; however, the few previous reports are based on samples with fewer than 250 cases and primarily from single sites (9, 19, 33–35). A major strength of our study was the ability to report the incidence of rare outcomes in a large, multisite US cohort. Furthermore, confirmation of a previous report of elevated risk of respiratory distress syndrome among term deliveries (36), coupled with the novel finding of a 5.8-fold increased risk of apnea, suggests that abruption may be associated with a negative impact on the neonate that is not well recognized. Neonatal respiratory distress syndrome and neonatal apnea are both primarily associated with underdevelopment due to preterm birth (37, 38). In pregnancies complicated by abruption, it is possible that neonates experience chronic hypoxia that leads to underdevelopment even in the absence of prematurity. Hypoxia may also explain the elevated risk of poor outcomes even among term neonates. Early delivery may shorten the duration of hypoxic conditions among preterm neonates and thereby allow better outcomes. It is also possible that abruptions associated with term delivery are distinct disorders from abruptions that trigger preterm delivery.
Analytically, it is important to acknowledge that our choice of method to examine “direct effects” attributed to abruption, by performing stratified analyses, assumed that there was no interaction between gestational age and abruption or SGA and abruption. Our choice of methodology also assumed that there was no uncontrolled confounding of the mediator-outcome association. To address this second issue, we performed sensitivity analyses, which suggested low likelihood of significant confounding effects. However, that possibility cannot be ruled out.
Another limitation of our study was that gestational age at diagnosis of antepartum abruption was not available, so we were unable to determine elapsed time between the abruption and delivery. Gestational timing of the abruption is likely an important determinant of the impact on the neonate. The severity of the abruption (either through percentage detachment or pathological examination) was also unavailable to us. This information could help elucidate the relationship between abruption, growth restriction, and the course of pregnancy. However, these limitations are not unique to our study. A related issue was our use of SGA as a proxy for intrauterine growth restriction; the former is a classification based on failure to achieve a specific weight for a particular gestational age and fetal sex, whereas the latter is defined as a failure to reach growth potential or reduced growth velocity (the specific definition of which has continued to be debated) (39, 40). Although they are highly related, these conditions are not identical and do not always overlap. Some neonates classified as SGA may simply be constitutionally small rather than growth restricted. Although intrauterine growth restriction would have been the preferable variable, the Consortium on Safe Labor study does not include sufficient detail to determine it, and thus we used SGA as a proxy. It is also important to note that severe cases of abruption are associated with an elevated incidence of stillbirth (41, 42), and so a fetus must have first survived the gestation and delivery to be at risk of neonatal morbidities. A complete understanding of the neonatal morbidity associated with abruption must be viewed in the context of elevated perinatal mortality.
Delivery mode is another important variable that was ultimately excluded from the analyses presented here for several reasons. It is reasonable to suspect that, in the setting of abruption, vaginal delivery may lead to prolonged exposure to acute hypoxic conditions for the neonate, which could increase risk of apnea and asphyxia as well as admission to NICU and longer LOS—and potentially even death. Abruption is also associated with significant maternal morbidity (primarily in the form of blood loss) (9, 11, 43), so it would be reasonable to conclude that cesarean delivery may be advisable to limit maternal blood loss as well as neonatal exposure to hypoxia. However, cesarean delivery is a major surgical procedure that presents risks both in the short term and in subsequent pregnancies (44). Thus, it is difficult to weigh whether vaginal or cesarean delivery is the best option when considering both neonatal and maternal health in the index pregnancy as well as the health of potential future pregnancies. Analytically, mode of delivery is on the causal pathway and may mediate the association between abruption and neonatal (and maternal) outcomes and, as such, would be a candidate for the type of analysis we performed with preterm birth and being SGA. However, the analysis is also likely subject to substantial confounding by indication, because the most severe cases of abruption are probably more likely to be delivered by cesarean than vaginally, and vice versa. The alternative of including mode of delivery as a covariate is also problematic, because it could lead to biased estimates.
Finally, there is known error in the classification of cases of abruption. Among our sample, abruption was classified based on 2 sources of information—electronic medical records and discharge codes—that are both subject to error, although the direction is unclear. In some studies, abruption is classified only in the presence of certain maternal symptoms (such as vaginal bleeding, abdominal pain, or both) or fetal signs (such as low birth weight or perinatal mortality). However, because there is a spectrum of abruption cases, excluding women that did not present with vaginal bleeding could have resulted in the exclusion of 30%–50% of cases exhibiting concealed abruption, which is typically associated with worse perinatal outcomes (45, 46). Likewise, limiting cases only to low–birth weight outcomes might have excluded acute cases that occurred toward the end of pregnancy. These exclusions could plausibly bias morbidity estimates in either direction, depending on the criteria applied. We did not apply any further criteria for the diagnosis of abruption, which may have resulted in the inclusion of milder cases of abruption, which likely led to an underestimation of associated risk. However, these methodological quandaries are present in most studies of abruption.
Comparison with other studies
To our knowledge, this is the first study to examine the association between abruption and risk of neonatal apnea. It is also the largest study to examine the association between abruption and respiratory distress syndrome, as well as the need for medical intervention. Previous reports of the association between abruption and need for medical intervention are limited by small sample sizes or focused on specific high-risk subpopulations, such as neonates requiring positive pressure ventilation at birth (34) or pregnancies complicated specifically by chronic abruption-oligohydramnios sequence (35). Furthermore, in contrast to the majority of existing studies of abruption, which have controlled for gestational age and birth weight in the analyses, we also estimated risk of poor outcomes conditioned on preterm birth and SGA. This analysis yielded valuable information about the direct effects of abruption on neonatal outcomes that were not attributable to the association with preterm birth or being SGA. Our results also confirmed the previous reports of an association between abruption and elevated risk of respiratory distress syndrome, among both preterm and term neonates (36).
Implications and conclusion
Our findings suggest that placental abruption is associated with an elevated risk of need for neonatal delivery-room resuscitation, NICU admission, and longer NICU LOS. Additionally, our finding of elevated risk of both respiratory distress syndrome and apnea among both preterm and term neonates suggests that abruption may be associated with physiologic underdevelopment, which has not been previously recognized. Together, our results suggest that neonates in pregnancies complicated by abruption are vulnerable beyond the immediate perinatal time frame. The discovery of the elevated risk of neonatal apnea may also be key to understanding the mechanism behind the association between abruption and sudden infant death syndrome (47–49).
Our findings also point toward the need for changes in the way information about placental disorders is clinically collected and documented. There is a notable paucity of the detailed information about the timing and nature of placental events during pregnancy that is vital for understanding both the epidemiology of the diseases as well as its likely impact on both the mother and the neonate. Likewise, assessment of placental health and functioning during pregnancy is an area in need of further development, as reflected in the establishment of the National Institute of Health’s Human Placenta Project. The invention of new, noninvasive surveillance methods for the placenta and implementing routine, detailed documentation of placental disorders are vital first steps toward furthering our understanding of placental function and disease.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Epidemiology Branch, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland (Katheryne L. Downes, Katherine L. Grantz); Maternal and Child Health Program, Department of Family Science, School of Public Health, University of Maryland, College Park, Maryland (Katheryne L. Downes, Edmond D. Shenassa); Maternal and Child Health Research Center, Department of Obstetrics and Gynecology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania (Katheryne L. Downes); Department of Epidemiology and Biostatistics, School of Public Health, University of Maryland, College Park, Maryland (Edmond D. Shenassa); Department of Epidemiology and Biostatistics, School of Medicine, University of Maryland, Baltimore, Maryland (Edmond D. Shenassa); and Epidemiology Department, School of Public Health, Brown University, Providence, Rhode Island (Edmond D. Shenassa).
This work was supported by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development. The Consortium on Safe Labor was funded by the Intramural Research Program of the National Institute of Child Health and Human Development (contract HHSN267200603425C).
This research was presented at the Society for Pediatric and Perinatal Epidemiologic Research 28th Annual Meeting, June 15–16, 2015, Denver, Colorado.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. K.L.G. is an employee of the US government.
Conflict of interest: none declared.
Abbreviations
- CI
confidence interval
- LOS
length of stay
- NICU
neonatal intensive care unit
- SGA
small for gestational age
REFERENCES
- 1. Tikkanen M. Placental abruption: epidemiology, risk factors and consequences. Acta Obstet Gynecol Scand. 2011;90(2):140–149. [DOI] [PubMed] [Google Scholar]
- 2. Drake AJ, Liu L. Intergenerational transmission of programmed effects: public health consequences. Trends Endocrinol Metab. 2010;21(4):206–213. [DOI] [PubMed] [Google Scholar]
- 3. Ananth CV. Ischemic placental disease: a unifying concept for preeclampsia, intrauterine growth restriction, and placental abruption. Semin Perinatol. 2014;38(3):131–132. [DOI] [PubMed] [Google Scholar]
- 4. Parker SE, Werler MM. Epidemiology of ischemic placental disease: a focus on preterm gestations. Semin Perinatol. 2014;38(3):133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ticconi C, Arpino C, Longo B, et al. Prevalence and risk factors for low birth weight in Northern Zimbabwe. Int J Gynaecol Obstet. 2005;88(2):146–147. [DOI] [PubMed] [Google Scholar]
- 6. Ananth CV, Smulian JC, Srinivas N, et al. Risk of infant mortality among twins in relation to placental abruption: contributions of preterm birth and restricted fetal growth. Twin Res Hum Genet. 2005;8(5):524–531. [DOI] [PubMed] [Google Scholar]
- 7. Ananth CV, Getahun D, Peltier MR, et al. Placental abruption in term and preterm gestations: evidence for heterogeneity in clinical pathways. Obstet Gynecol. 2006;107(4):785–792. [DOI] [PubMed] [Google Scholar]
- 8. Tikkanen M, Riihimäki O, Gissler M, et al. Decreasing incidence of placental abruption in Finland during 1980–2005. Acta Obstet Gynecol Scand. 2012;91(9):1046–1052. [DOI] [PubMed] [Google Scholar]
- 9. Boisramé T, Sananès N, Fritz G, et al. Placental abruption: risk factors, management and maternal-fetal prognosis. Cohort study over 10 years. Eur J Obstet Gynecol Reprod Biol. 2014;179:100–104. [DOI] [PubMed] [Google Scholar]
- 10. Nath CA, Ananth CV, DeMarco C, et al. Low birthweight in relation to placental abruption and maternal thrombophilia status. Am J Obstet Gynecol. 2008;198(3):293.e1–293.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pariente G, Wiznitzer A, Sergienko R, et al. Placental abruption: critical analysis of risk factors and perinatal outcomes. J Matern Fetal Neonatal Med. 2011;24(5):698–702. [DOI] [PubMed] [Google Scholar]
- 12. Ananth CV, Vintzileos AM. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol. 2006;195(6):1557–1563. [DOI] [PubMed] [Google Scholar]
- 13. Ananth CV, VanderWeele TJ. Placental abruption and perinatal mortality with preterm delivery as a mediator: disentangling direct and indirect effects. Am J Epidemiol. 2011;174(1):99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Räisänen S, Gissler M, Saari J, et al. Contribution of risk factors to extremely, very and moderately preterm births—register-based analysis of 1,390,742 singleton births. PLoS One. 2013;8(4):e60660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ofori BD, Le Tiec M, Bérard A. Risk factors associated with preterm birth according to gestational age at birth. Pharmacoepidemiol Drug Saf. 2008;17(6):556–564. [DOI] [PubMed] [Google Scholar]
- 16. Ananth CV, Berkowitz GS, Savitz DA, et al. Placental abruption and adverse perinatal outcomes. JAMA. 1999;282(17):1646–1651. [DOI] [PubMed] [Google Scholar]
- 17. Salihu HM, Bekan B, Aliyu MH, et al. Perinatal mortality associated with abruptio placenta in singletons and multiples. Am J Obstet Gynecol. 2005;193(1):198–203. [DOI] [PubMed] [Google Scholar]
- 18. Tikkanen M, Luukkaala T, Gissler M, et al. Decreasing perinatal mortality in placental abruption. Acta Obstet Gynecol Scand. 2013;92(3):298–305. [DOI] [PubMed] [Google Scholar]
- 19. Macheku G, Philemon RN, Oneko O, et al. Frequency, risk factors and feto-maternal outcomes of abruptio placentae in northern Tanzania: a registry-based retrospective cohort study. BMC Pregnancy Childbirth. 2015;15:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tikkanen M, Nuutila M, Hiilesmaa V, et al. Clinical presentation and risk factors of placental abruption. Acta Obstet Gynecol Scand. 2006;85(6):700–705. [DOI] [PubMed] [Google Scholar]
- 21. Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116(6):1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang J, Troendle J, Reddy UM, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203(4):326.e1–326.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ananth CV, Smulian JC, Demissie K, et al. Placental abruption among singleton and twin births in the United States: risk factor profiles. Am J Epidemiol. 2001;153(8):771–778. [DOI] [PubMed] [Google Scholar]
- 24. Männistö T, Mendola P, Reddy U, et al. Neonatal outcomes and birth weight in pregnancies complicated by maternal thyroid disease. Am J Epidemiol. 2013;178(5):731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 26. Austin PC, Rothwell DM, Tu JV. A comparison of statistical modeling strategies for analyzing length of stay after CABG surgery. Health Serv Outcomes Res Methodol. 2002;3(2):107–133. [Google Scholar]
- 27. Allison PD. Logistic Regression Using SAS: Theory and Application. Cary, NC: SAS Institute Inc.; 2012:265–290. [Google Scholar]
- 28. Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Y, Li G, Zou L, et al. An epidemiological survey on low birth wieght infants in China and analysis of outcomes of full-term low birth weight infants. BMC Pregnancy Childbirth. 2013;13:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fanaroff AA, Stoll BJ, Wright LL, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196(2):147.e1–147.e8. [DOI] [PubMed] [Google Scholar]
- 31. Kamath BD, Todd JK, Glazner JE, et al. Neonatal outcomes after elective cesarean delivery. Obstet Gynecol. 2009;113(6):1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. VanderWeele TJ, Mumford SL, Schisterman EF. Conditioning on intermediates in perinatal epidemiology. Epidemiology. 2012;23(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hasegawa J, Nakamura M, Hamada S, et al. Capable of identifying risk factors for placental abruption. J Matern Fetal Neonatal Med. 2014;27(1):52–56. [DOI] [PubMed] [Google Scholar]
- 34. Akinloye O, O’Connell C, Allen AC, et al. Post-resuscitation care for neonates receiving positive pressure ventilation at birth. Pediatrics. 2014;134(4):e1057–e1062. [DOI] [PubMed] [Google Scholar]
- 35. Kobayashi A, Minami S, Tanizaki Y, et al. Adverse perinatal and neonatal outcomes in patients with chronic abruption-oligohydramnios sequence. J Obstet Gynaecol Res. 2014;40(6):1618–1624. [DOI] [PubMed] [Google Scholar]
- 36. Gouyon JB, Ribakovsky C, Ferdynus C, et al. Severe respiratory disorders in term neonates. Paediatr Perinat Epidemiol. 2008;22(1):22–30. [DOI] [PubMed] [Google Scholar]
- 37. Whitsett JA, Weaver TE. Alveolar development and disease. Am J Respir Cell Mol Biol. 2015;53(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abu-Shaweesh JM, Martin RJ. Neonatal apnea: what’s new? Pediatr Pulmonol. 2008;43(10):937–944. [DOI] [PubMed] [Google Scholar]
- 39. Goldenberg RL, Cliver SP. Small for gestational age and intrauterine growth restriction: definitions and standards. Clin Obstet Gynecol. 1997;40(4):704–714. [DOI] [PubMed] [Google Scholar]
- 40. Tamblyn JA, Morris KR. Small for gestational age and intrauterine growth restriction In: Luesley DM, Kilby MD, eds. Obstetrics & Gyneaecology: An Evidence-Based Text for MRCOG. Boca Raton, FL: CRC Press; 2016:281–291. [Google Scholar]
- 41. Getahun D, Ananth CV, Kinzler WL. Risk factors for antepartum and intrapartum stillbirth: a population-based study. Am J Obstet Gynecol. 2007;196(6):499–507. [DOI] [PubMed] [Google Scholar]
- 42. Flenady V, Koopmans L, Middleton P, et al. Major risk factors for stillbirths in high-income countries: a sytematic review and meta-analysis. Lancet. 2011;377(9774):1331–1340. [DOI] [PubMed] [Google Scholar]
- 43. Räisänen S, Gissler M, Kramer MR, et al. Influence of delivery characteristics and socioeconomic status on giving birth by caesarean section—a cross sectional study during 2000–2010 in Finland. BMC Pregnancy Childbirth. 2014;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spong CY, Berghella V, Wenstrom KD, et al. Preventing the first cesarean delivery: summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstet Gynecol. 2012;120(5):1181–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kasai M, Aoki S, Ogawa M, et al. Prediction of perinatal outcomes based on primary symptoms in women with placental abruption. J Obstet Gynaecol Res. 2015;41(6):850–856. [DOI] [PubMed] [Google Scholar]
- 46. Chang YL, Chang SD, Cheng PJ. Perinatal outcome in patients with placental abruption with and without antepartum hemorrhage. Int J Gynecol Obstet. 2001;75(2):193–194. [DOI] [PubMed] [Google Scholar]
- 47. Getahun D, Amre D, Rhoads GG, et al. Maternal and obstetric risk factors for sudden infant death syndrome in the United States. Obstet Gynecol. 2004;103(4):646–652. [DOI] [PubMed] [Google Scholar]
- 48. Li DK, Wi S. Maternal placental abnormality and the risk of sudden infant death syndrome. Am J Epidemiol. 1999;149(7):608–611. [DOI] [PubMed] [Google Scholar]
- 49. Guntheroth WG, Spiers PS. The triple risk hypotheses in sudden infant death syndrome. Pediatrics. 2002;110(5):e64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.