Abstract
Observational studies and randomized controlled trials of menopausal hormone therapy (HT) and chronic disease risk appear to have divergent results for cardiovascular disease. However, differences may be related to a modifying effect of age, time since menopause, and HT formulation. In the Nurses’ Health Study (NHS) (enrolling during 1980–1994 and following participants until 2002), we investigated associations between the use of oral conjugated equine estrogens (CEE) (0.625 mg/day) plus medroxyprogesterone acetate (MPA) (<10 mg/day) or oral CEE alone and cardiovascular disease, cancer, all-cause mortality, and other major endpoints among postmenopausal women, aged 50–79 years at HT initiation. Among women aged 50–59 years at HT initiation, associations of CEE alone or CEE+MPA with most clinical outcomes were highly concordant between NHS and Women's Health Initiative (WHI). However, for myocardial infarction, results for CEE+MPA were in the direction of risk elevation in WHI and in the direction of risk reduction in NHS. When examined according to years since menopause onset (<10 years) rather than age group, results were nonsignificant and concordant for both studies. Because few women in the NHS initiated HT after age 60 years, we did not examine associations in this group. Discrepancies between NHS and WHI could largely be attributed to differences in the age structure of the populations and age at HT initiation.
Keywords: cardiovascular disease, chronic disease, epidemiologic methods, hormone therapy, Nurses’ Health Study, randomized controlled trials, Women's Health Initiative
Findings from observational studies have suggested that menopausal hormone therapy (HT) use is associated with a lower risk of coronary heart disease (CHD), hip fracture, and all-cause mortality but with a higher risk of breast cancer, stroke, and pulmonary embolism (1–6). Randomized clinical trials of postmenopausal HT have reported similar results for many outcomes, but for CHD and all-cause mortality the findings appeared to be discrepant. For example, in the Women's Health Initiative (WHI) HT trials, neither oral conjugated equine estrogens (CEE) alone nor CEE combined with medroxyprogesterone acetate (MPA) was associated with reduced risk of CHD or all-cause mortality (7, 8).
Although observational studies are susceptible to confounding and other biases (9, 10), the discrepancy in findings may be rooted in biology and fundamental differences in study populations (9, 11–13). Two of the most important factors modifying the effects of HT on chronic disease risk are age and time since menopause at HT initiation (14). A large majority of women in randomized trials of HT were older and more distant from the onset of menopause than their counterparts in observational studies, which better reflect actual HT use patterns. In both the CEE and CEE+MPA trials of the WHI, the average age at randomization was 63 years. In the Nurses’ Health Study (NHS), women were 30–55 years of age at enrollment, and nearly 80% of HT users began use within 2 years of menopause onset, which occurred on average at age 50 years. The most recent WHI report found a significant trend of increasing risk of myocardial infarction (MI) (P-trend = 0.01) with increasing time since onset of menopause among women randomized to CEE+MPA compared with placebo. Likewise, in the CEE trial, there were age-related trends for colorectal cancer (P-trend = 0.02) and all-cause mortality (P-trend = 0.04), with more favorable results in younger women than in older women (15). Given the considerable differences in study populations in the NHS and WHI, the goals of the current study were to compare results of HT for major health outcomes in the NHS and WHI among women in comparable age groups at HT initiation and among women using similar formulations of HT.
METHODS
The Nurses’ Health Study cohort
The NHS began in 1976 as a long-term prospective investigation of risk factors for chronic disease among 121,701 female registered nurses, aged 30–55 years, from 11 US states. Every 2 years, participants returned a mailed, validated questionnaire that included detailed information on health status; lifestyle, behavioral, and reproductive factors; menopausal status; exogenous hormone use, including postmenopausal hormone therapy; other medications; and a wide range of other exposures and outcomes (16–18). Follow-up data were available for over 90% of the cohort.
Exposure measures
On each biennial questionnaire, women were asked about postmenopausal HT, including current use (within the last month), duration of use, type of hormones taken, route of delivery (oral, transdermal, vaginal), and dose.
Outcome measures
To facilitate comparisons with the WHI HT trials (15), we considered the following major incident health outcomes: cardiovascular disease (total MI, stroke, pulmonary embolism, and cardiovascular deaths), cancer (total invasive cancer, invasive breast cancer, colorectal cancer, cancer deaths), type 2 diabetes mellitus, hip fracture, and all-cause mortality. Nurses (or next of kin for deceased participants) who reported these outcomes were asked for permission to have medical records reviewed by NHS investigators, who were blinded to the participant's risk-factor status.
Nonfatal MIs were confirmed using the World Health Organization criteria of typical symptoms plus either elevated enzymes or diagnostic ECG changes (19, 20). Infarctions that required hospital admission and were confirmed by interview or letter, but for which medical records were unobtainable, were included in the analysis as “probable.” Deaths were considered to be due to MI if the autopsy report showed evidence of fresh infarction or thrombus or if the medical records indicated EKG and/or enzyme changes characteristic of MI prior to death. Sudden cardiac deaths without evidence of MI were not included in our analyses.
Stroke was classified according to the National Survey of Stroke criteria (21) which require evidence of a neurological deficit with sudden or rapid onset that persisted for more than 24 hours or until death. We excluded silent strokes, subdural hematomas, and strokes due to infection or malignancy. Incident cases of pulmonary embolism were confirmed by medical records if a ventilation/perfusion lung scan was read by a radiologist as high probability for pulmonary embolism or if there was a filling defect on contrast-enhanced computed tomography of the pulmonary vasculature or a catheter-based pulmonary angiogram. Both “nonidiopathic” cases (associated with a history of surgery, major trauma, or malignancy) and “idiopathic” cases (without these predisposing risk factors) were included in our analyses.
For cancer outcomes, we considered all pathologically confirmed and probable cases of invasive cancer (except nonmelanoma skin cancer). For breast cancer, we included only confirmed cases with evidence of invasion on the pathology report. Cases of carcinoma in situ were not included in the analyses. Colorectal cancer and subsites were defined according to the International Classification of Diseases, Ninth Revision (22).
Women who reported a diagnosis of type 2 diabetes on the biennial main questionnaire were mailed a supplementary questionnaire regarding symptoms, diagnostic tests, and hypoglycemic therapy. Cases of type 2 diabetes were confirmed in accordance with the National Diabetes Data Group criteria (23). For cases identified after 1998, we applied the American Diabetes Association criteria (24). The present analysis includes only the cases confirmed by the supplementary questionnaire. The validity of the supplementary questionnaire has been established through medical record reviews, and diagnosis of type 2 diabetes was confirmed in more than 97% of the cases (25).
Information on hip fractures, with the date of occurrence and a description of the circumstances, was obtained from the biennial questionnaire. We excluded cases of fractures due to malignancy or major traumatic events (such as motor vehicle accidents or skiing).
Deaths were identified by notifications from next of kin, the US postal system, searches of the National Death Index, tumor registries, and death certificates obtained from state vital statistics departments. Deaths were classified according to the International Classification of Diseases, Eighth Revision (ICD-8), as cardiovascular deaths (ICD-8 codes 390–458) or cancer deaths (ICD-8 codes 140–207). Follow-up for deaths was >98% complete (26, 27). Our confirmation process for fatal and nonfatal events was similar to that used in WHI (7, 8).
Population for analysis
Women were included in the analyses as they became postmenopausal and reached the age of 50 years, the lower age limit for entry into the WHI HT trials. Women were classified as postmenopausal from the time of natural menopause or hysterectomy with bilateral oophorectomy. Women who underwent hysterectomy without bilateral oophorectomy were considered postmenopausal when they reached the age at which 90% of the NHS cohort went through menopause (54 years for smokers and 56 years for nonsmokers). A nurses’ report of age at menopause (28) and type of menopause (29) were found to be highly accurate in this cohort. Premenopausal women were not included in the analysis.
In order to simulate the entry criteria of the WHI HT trials, we excluded women with a diagnosis of cancer (except nonmelanoma skin cancer) at baseline (1980). For all outcomes (except cardiovascular outcomes), we included women with a history of cardiovascular disease. Women who initiated HT use prior to age 50 years and after age 79 years at time of entry into analysis were excluded. We also excluded premenopausal women and women with uncertain postmenopausal status (current smokers less than age 53 years or nonsmokers less than age 56 years). We distinguished smokers from nonsmokers because smoking causes earlier natural menopause. To further mimic the WHI, analyses were limited to users of 0.625 mg/day oral CEE alone or 0.625 mg/day oral CEE plus <10 mg/day of MPA (CEE+MPA). As was done in the WHI, we included only women who had had a hysterectomy in the analyses of CEE alone. Analyses of CEE+MPA users were restricted to women with an intact uterus.
Statistical analysis
The WHI HT trials categorized results by age at randomization (50–59 years, 60–69 years, and 70–79 years) and time since menopause (less than 10 years, 10 years to less than 20 years, 20 or more years). We included women in the analyses as they reached age 50 years, on a rolling basis, from 1980 to 1994. Person-time was calculated from the time they entered the analysis at age 50 years to the first diagnosis of an outcome, loss to follow-up, death, or 2002, when the WHI HT trials were stopped, whichever came first.
For all outcomes (except mortality endpoints), we used time-varying updates of HT use, and for each 2-year cycle women contributed person-time to the relevant HT category (never user (referent), current CEE user, current CEE+MPA user, missing). For mortality endpoints, to reduce the ability of a potentially lethal health condition to affect HT use (reverse causation due to prior disease diagnosis), we used baseline HT as our primary exposure. We used Cox proportional hazards regression models to estimate age- and multivariable-adjusted hazard ratios for the association between HT use and health outcomes. The regression models included age in years as the time scale, stratified by calendar time in 2-year intervals, and they allowed for possible interaction between calendar time and age in the baseline hazards to be accounted for nonparametrically. In multivariable analysis, we further adjusted for age at menopause, body mass index, smoking status, physical activity, alcohol intake, use of aspirin for at least 1 day per month, history of hypertension, history of high cholesterol, history of diabetes, parental history of MI before age 60, parental history of cancer, and duration of CEE and CEE+MPA use. Models with invasive breast cancer as an outcome additionally adjusted for height, parity, age at first birth, body mass index at age 18, and history of benign breast disease. We also adjusted for mammogram screening in the previous cycle, because those who had this imaging were more likely to be diagnosed with invasive breast cancer (30). Information on most covariates was updated biennially, including age, body mass index, smoking status, physical activity, alcohol intake, aspirin use, history of hypertension, history of high cholesterol, history of diabetes, duration of CEE or CEE+MPA use, parity, and mammogram in previous cycle.
The WHI HT trials evaluated the potential influence of age at HT initiation (50–59 years, 60–69 years, 70–79 years) and time since menopause (less than 10 years, 10 years to less than 20 years, 20 or more years) on the relationship between HT use and health outcomes (15). Because more than 85% of NHS HT users were of ages 50–59 years at HT initiation, and due to the uncertainty about timing of menopause onset in many women with hysterectomy, our primary analyses examined associations between HT use and chronic disease endpoints in this age group. In supplementary analyses, we additionally examined the potential modifying effect of time since onset of menopause (<10 years). We did not have statistical power to examine outcomes in NHS among women who were 60–69 years or 70–79 years at HT initiation or among women who were >10 years post menopause. We used figures to visually compare hazard ratios for the various clinical endpoints from our study with those in the intervention phase of the WHI (15) among women using the same HT formulation and in comparable age groups. To examine the consistency of results from the NHS and the WHI, we computed a P value derived from the Q test for heterogeneity proposed by DerSimonian and Laird (31). All statistical tests were 2-sided and performed using SAS, version 9.3, for UNIX (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Characteristics of participants at entry into analysis
At the time of entry into analysis, current users of HT were, in general, more likely to have a more favorable risk profile than were women who had never used hormones (Table 1). Compared with never users, HT users were less likely to be current smokers, were more physically active, and had a lower body mass index. However, they were more likely to have a history of elevated cholesterol and benign breast disease.
Table 1.
Characteristics of Nurses’ Health Study Participants at Time of Entry Into Analysis, United States, 1980–1994a
| Characteristic | Neverb (n = 6,136) | CEEc (n = 3,911) | Neverd (n = 34,622) | CEE+MPAe (n = 3,716) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | |
| Age at entry into analysis, years | 55.2 (2.4) | 55.5 (2.4) | 53.6 (2.6) | 53.4 (2.6) | ||||
| White | 96 | 97 | 97 | 98 | ||||
| Smoking | ||||||||
| Never | 46.1 | 49.3 | 42.4 | 42.9 | ||||
| Past | 28.9 | 33.3 | 30.6 | 42.5 | ||||
| Current | 25.1 | 17.3 | 27.1 | 14.6 | ||||
| Age at menopause, years | 49.7 (4.4) | 49.6 (3.9) | 50.6 (3.3) | 51.6 (2.7) | ||||
| Time since menopause onset, years | 5.7 (4.1) | 5.9 (4.5) | 3.2 (3.1) | 2.1 (2.0) | ||||
| Parity, no. | 3.3 (1.9) | 3.0 (1.7) | 3.1 (1.8) | 2.8 (1.4) | ||||
| Physical activity, MET-hours/week | 14.4 (21.5) | 16.2 (20.0) | 14.8 (21.0) | 18.9 (23.4) | ||||
| Height, inchesf | 64.3 (3.2) | 64.4 (3.5) | 64.4 (3.3) | 64.6 (2.4) | ||||
| Body mass indexg | 26.5 (5.2) | 25.1 (4.4) | 25.8 (5.1) | 25.0 (4.4) | ||||
| History of high blood pressure | 32 | 32 | 25 | 23 | ||||
| History of hypercholesterolemia | 19 | 24 | 15 | 34 | ||||
| History of type 2 diabetes mellitus | 7 | 4 | 4 | 2 | ||||
| History of benign breast disease | 31 | 38 | 29 | 50 | ||||
| Parental history of early MI | 20 | 20 | 18 | 19 | ||||
| Parental history of cancer | 14 | 12 | 13 | 10 | ||||
| Aspirin useh | 48 | 53 | 47 | 51 | ||||
Abbreviations: CEE, conjugated equine estrogens; MET, metabolic equivalents of task; MI, myocardial infarction; MPA, medroxyprogesterone acetate; SD, standard deviation.
a Women were enrolled as they became postmenopausal and were between the ages 50–79 years.
b Women who underwent a hysterectomy and never used hormone therapy.
c CEE use (0.625 mg/day) among women who underwent a hysterectomy.
d Women with an intact uterus who never used hormone therapy.
e CEE (0.625 mg/day) plus MPA (<10 mg/day) use among women with an intact uterus.
f To convert inches to centimeters, multiply by 2.54.
g Body mass index was calculated as weight (kg)/height (m)2.
h Defined as use of aspirin at least 1 day/month.
Risk for major cardiovascular outcomes
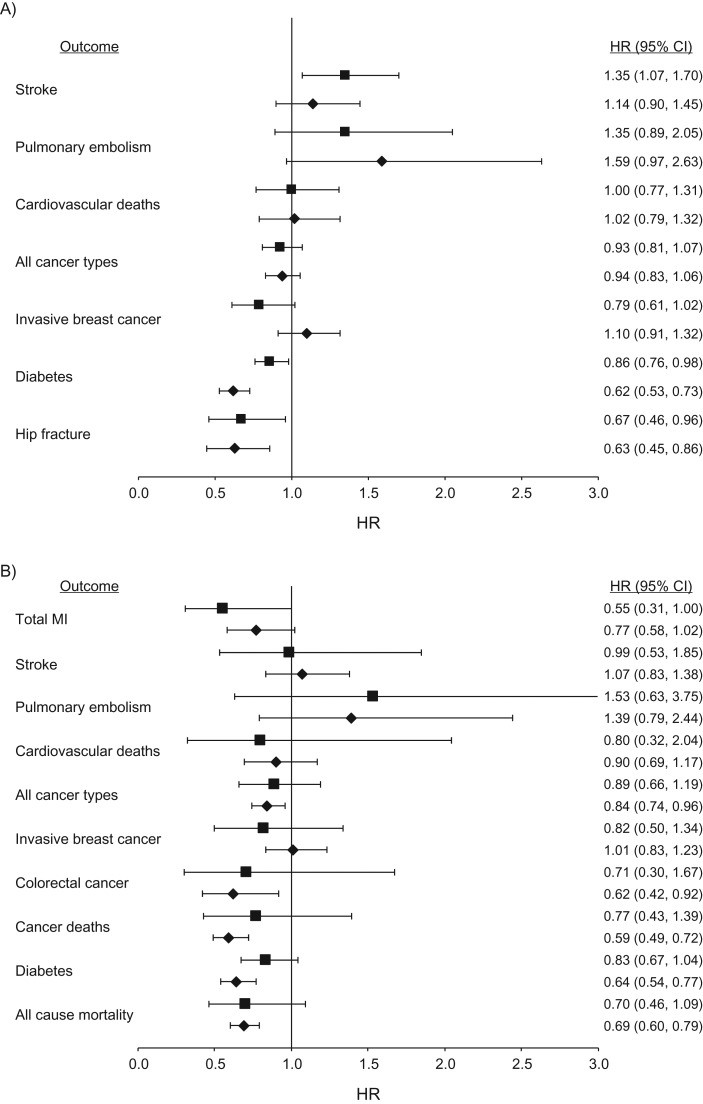
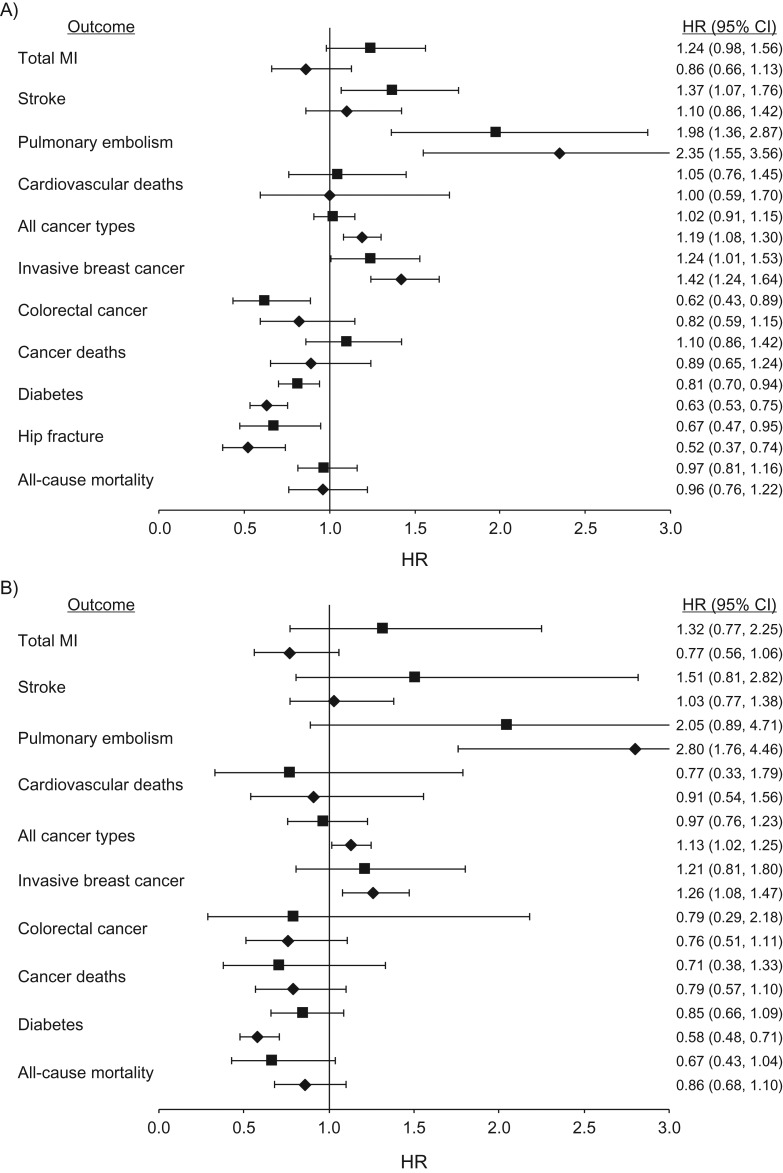
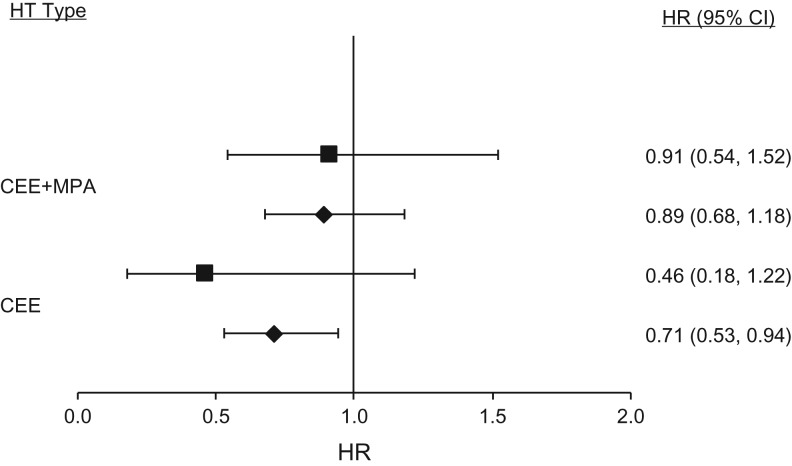
In both primary analyses restricted to women 50–59 years of age and in the overall cohort (ages 50–79 years), results for HT use (CEE alone or with MPA) were congruent between NHS and WHI for all cardiovascular outcomes except total MI (Table 2, Figures 1 and 2). Among current users of oral CEE alone or with MPA, compared with women who never used hormones, we documented a pattern of higher risk for total stroke and pulmonary embolism, and we found neutral results for cardiovascular deaths, similar to the WHI findings. In the age group 50–79 years, use of CEE alone was associated with a lower risk of total MI in the NHS (hazard ratio (HR) = 0.73, 95% confidence interval (CI): 0.57, 0.95), but results were neutral in the WHI (HR = 0.97, 95% CI: 0.79, 1.21) (Table 2). Current use of CEE+MPA was inversely associated with total MI in the NHS (HR = 0.86, 95% CI: 0.66, 1.13) and positively associated in the WHI (HR = 1.24, 95% CI: 0.98, 1.56) (Table 2). These discrepancies could largely be attributed to differences in age at HT initiation and time since onset of menopause. When results were stratified by time since menopause (<10 years) rather than by age group (50–59 years)—because time since menopause onset can be a better marker of atherosclerotic risk—hazard ratios were neutral and concordant in both studies (NHS, HR = 0.89, 95% CI: 0.68, 1.18; WHI, HR = 0.91, 95% CI: 0.54, 1.52; P-heterogeneity = 0.94) (Table 3 and Figure 3).
Table 2.
Association Between Hormone Therapy Use and Health Outcomes Among Postmenopausal Women in the Nurses’ Health Study (1980–2002) and in the Intervention Phase of the Women's Health Initiative Hormone Therapy Trials (15), United States
| Outcome, Age Group, and Study | Never/Placeboa | Current CEEb | HR | 95% CI | Never/Placeboc | Current CEE+MPAd | HR | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cases | Person-Years | No. of Cases | Person-Years | No. of Cases | Person Years | No. of Cases | Person-Years | |||||
| Total myocardial infarction | ||||||||||||
| 50–79 yearse | ||||||||||||
| NHSf | 179 | 73,875 | 127 | 78,895 | 0.73 | 0.57, 0.95 | 618 | 3,65,983 | 86 | 77,699 | 0.86 | 0.66, 1.13 |
| WHI | 173 | 38,444 | 164 | 37,273 | 0.97 | 0.79, 1.21 | 159 | 54,828 | 196 | 56,000 | 1.24 | 0.98, 1.56 |
| P-heterogeneityg | 0.10 | 0.07 | ||||||||||
| 50–59 yearsh | ||||||||||||
| NHSf | 143 | 57,740 | 109 | 70,492 | 0.77 | 0.58, 1.02 | 527 | 3,02,138 | 65 | 68,196 | 0.77 | 0.56, 1.06 |
| WHI | 31 | 12,400 | 17 | 12,143 | 0.55 | 0.31, 1.00 | 57 | 38,000 | 75 | 39,474 | 1.32 | 0.77, 2.25 |
| P-heterogeneityg | 0.14 | 0.19 | ||||||||||
| Stroke | ||||||||||||
| 50–79 yearse | ||||||||||||
| NHSf | 150 | 74,407 | 192 | 78,864 | 1.14 | 0.90, 1.45 | 523 | 3,67,487 | 113 | 78,047 | 1.10 | 0.86, 1.42 |
| WHI | 130 | 38,235 | 169 | 37,556 | 1.35 | 1.07, 1.70 | 109 | 45,417 | 159 | 48,182 | 1.37 | 1.07, 1.76 |
| P-heterogeneityg | 0.40 | 0.32 | ||||||||||
| 50–59 yearsh | ||||||||||||
| NHSf | 139 | 58,146 | 165 | 70,349 | 1.07 | 0.83, 1.38 | 475 | 3,03,413 | 87 | 68,447 | 1.03 | 0.77, 1.38 |
| WHI | 21 | 12,353 | 19 | 11,875 | 0.99 | 0.53, 1.85 | 16 | 16,000 | 26 | 17,333 | 1.51 | 0.81, 2.82 |
| P-heterogeneityg | 0.84 | 0.47 | ||||||||||
| Pulmonary embolism | ||||||||||||
| 50–79 yearse | ||||||||||||
| NHSf | 31 | 74,835 | 50 | 79,557 | 1.59 | 0.97, 2.63 | 115 | 3,69,427 | 54 | 78,363 | 2.35 | 1.55, 3.56 |
| WHI | 39 | 39,000 | 52 | 37,143 | 1.35 | 0.89, 2.05 | 41 | 45,556 | 87 | 48,333 | 1.98 | 1.36, 2.87 |
| P-heterogeneityg | 0.67 | 0.70 | ||||||||||
| 50–59 yearsh | ||||||||||||
| NHSf | 25 | 58,498 | 39 | 70,955 | 1.39 | 0.79, 2.44 | 96 | 3,05,194 | 49 | 68,689 | 2.80 | 1.76, 4.46 |
| WHI | 8 | 13,333 | 12 | 12,000 | 1.53 | 0.63, 3.75 | 8 | 16,000 | 18 | 16,364 | 2.05 | 0.89, 4.71 |
| P-heterogeneityg | 0.91 | 0.76 | ||||||||||
| Cardiovascular deathsi | ||||||||||||
| 50–79 yearse | ||||||||||||
| NHSf | 339 | 1,29,625 | 142 | 66,545 | 1.02 | 0.79, 1.32 | 665 | 5,17,726 | 17 | 37,834 | 1.00 | 0.59, 1.70 |
| WHI | 112 | 38,621 | 109 | 37,586 | 1.00 | 0.77, 1.31 | 70 | 46,667 | 79 | 49,735 | 1.05 | 0.76, 1.45 |
| P-heterogeneityg | 0.92 | 0.88 | ||||||||||
| 50–59 yearsh | ||||||||||||
| NHSf | 263 | 90,188 | 140 | 65,987 | 0.90 | 0.69, 1.17 | 595 | 4,08,752 | 17 | 37,495 | 0.91 | 0.54, 1.56 |
| WHI | 10 | 12,500 | 8 | 11,429 | 0.80 | 0.32, 2.04 | 35 | 43,750 | 31 | 51,667 | 0.77 | 0.33, 1.79 |
| P-heterogeneityg | 0.80 | 0.71 | ||||||||||
| All cancer typesj | ||||||||||||
| 50–79 yearse | ||||||||||||
| NHSf | 636 | 67,151 | 734 | 76,440 | 0.94 | 0.83, 1.06 | 3,133 | 3,54,790 | 905 | 76,381 | 1.19 | 1.08, 1.30 |
| WHI | 438 | 37,436 | 399 | 36,606 | 0.93 | 0.81, 1.07 | 543 | 43,790 | 598 | 47,087 | 1.02 | 0.91, 1.15 |
| P-heterogeneityg | 0.95 | 0.05 | ||||||||||
| 50–59 yearsh | ||||||||||||
| NHSf | 581 | 51,540 | 655 | 68,295 | 0.84 | 0.74, 0.96 | 2,856 | 2,91,276 | 776 | 67,022 | 1.13 | 1.02, 1.25 |
| WHI | 96 | 12,152 | 85 | 11,972 | 0.89 | 0.66, 1.19 | 321 | 38,214 | 361 | 43,494 | 0.97 | 0.76, 1.23 |
| P-heterogeneityg | 0.72 | 0.24 | ||||||||||
| Invasive breast cancer | ||||||||||||
| 50–79 yearse | ||||||||||||
| NHSk | 248 | 72,795 | 360 | 79,774 | 1.10 | 0.91, 1.32 | 1,170 | 3,61,932 | 454 | 78,141 | 1.42 | 1.24, 1.64 |
| WHI | 135 | 38,571 | 104 | 37,143 | 0.79 | 0.61, 1.02 | 155 | 44,286 | 206 | 47,907 | 1.24 | 1.01, 1.53 |
| P-heterogeneityg | 0.02 | 0.36 | ||||||||||
| 50–59 yearsh | ||||||||||||
| NHSk | 218 | 56,486 | 328 | 71,142 | 1.01 | 0.83, 1.23 | 1,091 | 2,97,525 | 390 | 68,505 | 1.26 | 1.08, 1.47 |
| WHI | 36 | 12,414 | 29 | 12,083 | 0.82 | 0.50, 1.34 | 42 | 15,556 | 55 | 16,667 | 1.21 | 0.81, 1.80 |
| P-heterogeneityg | 0.38 | 0.87 | ||||||||||
| Colorectal cancer | ||||||||||||
| 50–79 yearse | ||||||||||||
| NHSf | 95 | 75,461 | 58 | 79,886 | 0.67 | 0.47, 0.96 | 347 | 3,71,244 | 61 | 78,345 | 0.82 | 0.59, 1.15 |
| WHI | 58 | 38,667 | 65 | 38,235 | 1.15 | 0.81, 1.64 | 75 | 44,118 | 50 | 50,000 | 0.62 | 0.43, 0.89 |
| P-heterogeneityg | 0.05 | 0.18 | ||||||||||
| 50–59 yearsh | ||||||||||||
| NHSf | 86 | 59,070 | 50 | 71,309 | 0.62 | 0.42, 0.92 | 313 | 3,06,626 | 49 | 68,700 | 0.76 | 0.51, 1.11 |
| WHI | 13 | 13,000 | 9 | 12,857 | 0.71 | 0.30, 1.67 | 8 | 16,000 | 7 | 17,500 | 0.79 | 0.29, 2.18 |
| P-heterogeneityg | 0.74 | 0.93 | ||||||||||
| Cancer deathsi | ||||||||||||
| 50–79 yearse | ||||||||||||
| NHSf | 670 | 1,29,292 | 225 | 66,459 | 0.72 | 0.59, 0.87 | 1,168 | 5,17,309 | 47 | 37,808 | 0.89 | 0.65, 1.24 |
| WHI | 135 | 38,571 | 126 | 38,182 | 0.96 | 0.75, 1.22 | 111 | 44,400 | 133 | 47,500 | 1.10 | 0.86, 1.42 |
| P-heterogeneityg | 0.06 | 0.34 | ||||||||||
| 50–59 yearsh | ||||||||||||
| NHSf | 555 | 89,899 | 222 | 65,902 | 0.59 | 0.49, 0.72 | 1,055 | 4,08,373 | 46 | 37,470 | 0.79 | 0.57, 1.10 |
| WHI | 26 | 12,381 | 20 | 12,500 | 0.77 | 0.43, 1.39 | 72 | 51,429 | 70 | 70,000 | 0.71 | 0.38, 1.33 |
| P-heterogeneityg | 0.32 | 0.71 | ||||||||||
| Diabetes | ||||||||||||
| 50–79 yearse | ||||||||||||
| NHSf | 508 | 64,669 | 276 | 73,142 | 0.62 | 0.53, 0.73 | 1,687 | 3,35,667 | 206 | 73,670 | 0.63 | 0.53, 0.75 |
| WHIl | 527 | 34,000 | 449 | 33,507 | 0.86 | 0.76, 0.98 | 373 | 42,386 | 328 | 45,556 | 0.81 | 0.70, 0.94 |
| P-heterogeneityg | 0.001 | 0.02 | ||||||||||
| 50–59 yearsh | ||||||||||||
| NHSf | 428 | 50,152 | 245 | 65,531 | 0.64 | 0.54, 0.77 | 1,474 | 2,75,988 | 169 | 64,888 | 0.58 | 0.48, 0.71 |
| WHIl | 174 | 11,013 | 143 | 10,916 | 0.83 | 0.67, 1.04 | 125 | 14,706 | 118 | 15,946 | 0.85 | 0.66, 1.09 |
| P-heterogeneityg | 0.05 | 0.01 | ||||||||||
| Hip fracturem | ||||||||||||
| 50–79 yearse | ||||||||||||
| NHSf | 101 | 75,492 | 85 | 79,694 | 0.63 | 0.45, 0.86 | 373 | 3,70,656 | 48 | 78,106 | 0.52 | 0.37, 0.74 |
| WHI | 74 | 9,610 | 48 | 6,000 | 0.67 | 0.46, 0.96 | 75 | 44,118 | 53 | 48,182 | 0.67 | 0.47, 0.95 |
| P-heterogeneityg | 0.74 | 0.26 | ||||||||||
| All-cause mortalityi | ||||||||||||
| 50–79 yearse | ||||||||||||
| NHSf | 1,292 | 1,28,730 | 489 | 66,225 | 0.82 | 0.72, 0.93 | 2,381 | 5,16,372 | 85 | 37,772 | 0.96 | 0.76, 1.22 |
| WHI | 299 | 1,57,368 | 301 | 2,31,538 | 1.03 | 0.88, 1.21 | 238 | 44,906 | 250 | 48,077 | 0.97 | 0.81, 1.16 |
| P-heterogeneityg | 0.03 | 0.96 | ||||||||||
| 50–59 yearsh | ||||||||||||
| NHSf | 1,042 | 89,455 | 482 | 65,673 | 0.69 | 0.60, 0.79 | 2,140 | 4,07,525 | 84 | 37,433 | 0.86 | 0.68, 1.10 |
| WHI | 50 | 12,500 | 35 | 12,069 | 0.70 | 0.46, 1.09 | 48 | 15,484 | 35 | 16,667 | 0.67 | 0.43, 1.04 |
| P-heterogeneityg | 0.92 | 0.21 | ||||||||||
Abbreviations: CEE, conjugated equine estrogens; CI, confidence interval; HR, hazard ratio; HT, hormone therapy; MET, metabolic equivalents of task; MPA, medroxyprogesterone acetate; NHS, Nurses’ Health Study; WHI, Women's Health Initiative.
a The comparison group included never users of HT who underwent a hysterectomy in the NHS and those randomized to the placebo group in the WHI CEE-alone trial (15).
b CEE (0.625 mg/day) use among women who underwent a hysterectomy.
c The comparison group included never users of HT with an intact uterus in the NHS and those randomized to the placebo group in the WHI CEE+MPA trial (15).
d CEE (0.625 mg/day) plus MPA (<10 mg/day) use among women with an intact uterus in the NHS. In the WHI CEE+MPA trial, the dosages were 0.625 mg/day of CEE and 2.5 mg/day of MPA for women with an intact uterus (15).
e Entry into the analysis was between 1980 and 1994. Women were enrolled as they became postmenopausal and were between the ages 50–79 years.
f The models adjusted for age, calendar time, smoking status (never, past, current 1–14 cigarettes/day, current 15–24 cigarettes/day, current >24 cigarettes/day), alcohol intake (g/day: 0, 0.1–4.9, 5–9.9, 10–14.9, ≥15), physical activity (MET-hours/week: <3, 3–8.9, 9–17.9, 18–26.9, ≥27), body mass index (weight (kg)/height (m)2; <21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–34.9, 35–39.9, ≥40), aspirin use for at least 1 day/month (yes/no), history of high blood pressure (yes/no), history of hypercholesterolemia (yes/no), history of type 2 diabetes mellitus (yes/no; all models except diabetes as an outcome), age at menopause (continuous), parental history of early myocardial infarction (yes/no), parental history of cancer (yes/no), duration of CEE use (in months), and duration of CEE+MPA use (in months).
gP for heterogeneity was derived from the Q test for heterogeneity proposed by DerSimonian and Laird (31).
h Entry into the analysis was between 1980 and 1994. Women were enrolled as they became postmenopausal and were between the ages 50–59 years.
i For all mortality endpoints, to avoid reverse-causality due to diagnosed disease, baseline HT use was the exposure variable.
j Excludes nonmelanoma skin cancer.
k The models adjusted for all of the variables in footnote (f) plus height (continuous), parity, age at first birth, body mass index at age 18 (continuous), history of benign breast disease, and mammogram in the previous cycle.
l Diabetes was self-reported in the WHI hormone therapy trials.
m Results are presented only for the age group 50–79 years because there were 5 or fewer cases in the WHI in the age group 50–59 years.
Figure 1.
Association between hormone therapy use (oral conjugated equine estrogens (CEE) (0.625 mg/day)) and health outcomes among postmenopausal women who underwent a hysterectomy, in the Nurses’ Health Study (1980–2002) (diamonds) and in the intervention phase of the Women's Health Initiative (WHI) (squares) CEE-alone trial (1) among participants aged 50–79 years (A) and aged 50–59 years (B) at entry into analysis. All NHS models adjusted for age, calendar time, smoking status, alcohol intake, physical activity, body mass index, aspirin use of at least 1 day/month, history of high blood pressure, history of hypercholesterolemia, history of type 2 diabetes mellitus (all models except diabetes as an outcome), age at menopause, parental history of early myocardial infarction (MI), parental history of cancer, duration of CEE use, and duration of CEE plus medroxyprogesterone acetate (MPA) use. Breast cancer models additionally adjusted for height, parity, age at first birth, body mass index at age 18, history of benign breast disease, and mammogram in the previous cycle. Results for total MI, colorectal cancer, cancer deaths, and all-cause mortality are not reported for the age group 50–79 years because a significant interaction by age was reported in the intervention phase of the WHI (1). Hip fracture results are shown only for the 50–79 years group because there were fewer than 5 cases in the WHI in the 50–59 years group (1). CI, confidence interval; HR, hazard ratio.
Figure 2.
Association between hormone therapy use (oral conjugated equine estrogens (CEE) (0.625 mg/day) plus medroxyprogesterone acetate (MPA) (<10 mg/day)) and health outcomes among postmenopausal women with an intact uterus, in the Nurses’ Health Study (1980–2002) (squares) and in the intervention phase of the Women's Health Initiative (WHI) (diamonds) CEE+MPA trials (1) among participants aged 50–79 years (A) and 50–59 years (B) at entry into analysis. All NHS models adjusted for age, calendar time, smoking status, alcohol intake, body mass index, aspirin use of at least 1 day/month, history of high blood pressure, history of hypercholesterolemia, history of type 2 diabetes mellitus (all models except diabetes as an outcome), age at menopause, parental history of early myocardial infarction (MI), parental history of cancer, duration of CEE use, and duration of CEE+MPA use. Breast cancer models additionally adjusted for height, parity, age at first birth, body mass index at age 18, history of benign breast disease, and mammogram in the previous cycle. Hip fracture results are shown only for the 50–79 years group because there were fewer than 5 cases in the WHI in the 50–59 years group (1). CI, confidence interval; HR, hazard ratio.
Table 3.
Association Between Hormone Therapy Use and Total Myocardial Infarction Among Postmenopausal Women Aged 50–79 Years at Entry Into Analysisa and Those Who Were Less Than 10 Years Post Menopause Onset in the Nurses’ Health Study (1980–2002) and in the Intervention Phase of Women's Health Initiative Hormone Therapy Trials (15), United States
| Study | Never/Placebob | Current CEEc | HR | 95% CI | Never/Placebod | Current CEE+MPAe | HR | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cases | Person-Years | No. of Cases | Person-Years | No. of Cases | Person-Years | No. of Cases | Person-Years | |||||
| NHSf | 151 | 66,475 | 104 | 70,823 | 0.71 | 0.53, 0.94 | 574 | 3,47,680 | 86 | 76,204 | 0.89 | 0.68, 1.18 |
| WHI | 13 | 13,000 | 6 | 2,727 | 0.46 | 0.18, 1.22 | 30 | 16,667 | 24 | 24,000 | 0.91 | 0.54, 1.52 |
| P-heterogeneityg | 0.16 | 0.94 | ||||||||||
Abbreviations: CEE, conjugated equine estrogens; CI, confidence interval; HR, hazard ratio; HT, hormone therapy; MET, metabolic equivalents of task; MPA, medroxyprogesterone acetate; NHS, Nurses’ Health Study; WHI, Women's Health Initiative.
a Entry into analysis was between 1980 and 1994. Women were enrolled as they became postmenopausal and were between the ages 50–79 years.
b The comparison group included never users of HT in the NHS and those randomized to the placebo group in the WHI hormone therapy CEE-alone trial (15).
c CEE (0.625 mg/day) use among women who underwent a hysterectomy.
d The comparison group included never users of HT with an intact uterus in the NHS and those randomized to the placebo group in the WHI CEE+MPA trial (15).
e CEE (0.625 mg/day) plus MPA (<10 mg/day) use among women with an intact uterus in the NHS. In the WHI trial, the dosages were 0.625 mg/day of CEE and 2.5 mg/day of MPA for women with an intact uterus.
f The models adjusted for age, calendar time, smoking status (never, past, current 1–14 cigarettes/day, current 15–24 cigarettes/day, current >24 cigarettes/day), alcohol intake (g/day: 0, 0.1–4.9, 5–9.9, 10–14.9, ≥15), physical activity (MET-hours/week: <3, 3–8.9, 9–17.9, 18–26.9, ≥27), body mass index (weight (kg)/height (m)2; <21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–34.9, 35–39.9, ≥40), aspirin use for at least 1 day/month (yes/no), history of high blood pressure (yes/no), history of hypercholesterolemia (yes/no), history of type 2 diabetes mellitus (yes/no; all models except diabetes as an outcome), age at menopause (continuous), parental history of early myocardial infarction (yes/no), parental history of cancer (yes/no), duration of CEE use (in months), and duration of CEE+MPA use (in months).
gP for heterogeneity was derived from the Q test for heterogeneity proposed by DerSimonian and Laird (31).
Figure 3.
Association between hormone therapy (HT) use and total myocardial infarction among postmenopausal women, aged 50–79 years at entry into analysis and less than 10 years since onset of menopause, in the Nurses’ Health Study (1980–2002) (diamonds) and in the intervention phase of the Women's Health Initiative (squares) HT trials (1). All NHS outcomes were adjusted for age, calendar time, smoking status, alcohol intake, body mass index, aspirin use of at least 1 day/month, history of high blood pressure, history of hypercholesterolemia, history of type 2 diabetes mellitus, age at menopause, parental history of early myocardial infarction, parental history of cancer, duration of oral conjugated equine estrogens (CEE) use, and duration of CEE plus medroxyprogesterone acetate (MPA) use. CI, confidence interval; HR, hazard ratio.
Risk for major cancer outcomes
In a primary analysis restricted to the age group 50–59 years, results in the NHS and WHI were similar for breast cancer, colorectal cancer, total cancer, and cancer deaths for users of both CEE alone and for CEE+MPA. However, in the broader age range of 50–79 years, the hazard ratios for HT use and cancer outcomes were largely discordant between the NHS and WHI, primarily reflecting differences in the underlying age structure for HT initiation.
Risk for other major health outcomes and all-cause mortality
In both the overall study population and the age group 50–59 years, current HT users of CEE alone or CEE+MPA had a lower risk of type 2 diabetes and hip fracture in both the NHS and WHI. For the association between CEE alone or CEE+MPA use and all-cause mortality, results tended to be more favorable for women aged 50–59 years than for the broader age group in both studies.
DISCUSSION
We present observational analyses from the NHS to simulate the inclusion and exclusion criteria and dose and formulation of HT used in the WHI trials. When analyses were conducted in the broader age range of 50–79 years, results were discordant for total MI and for several other outcomes. However, much of the apparent discordance in the WHI versus NHS findings disappeared when we restricted our analyses to the ages of 50–59 years at HT initiation, the age range when more than 60% of women initiated HT use in the NHS. Still, in this group, among women using CEE+MPA, the hazard ratio for total MI was in the direction of risk reduction in the NHS and risk elevation in the WHI. However, when we stratified our analyses by time since onset of menopause rather than age, results were concordant between the 2 studies, with hazard ratios for women close to menopause onset below 1.0 in both studies.
The discordance between observational studies and randomized clinical trials of HT on chronic disease risk has raised questions about the validity and credibility of evidence from observational studies (32–35). As was highlighted in the recent reanalysis and extended follow-up of the WHI (15) and in our current analysis, 2 key factors that can account for these discrepancies are age and time since onset of menopause at HT initiation. Women who initiated HT in the NHS were generally younger than age 55 years and within 2–3 years of menopause onset. Conversely, women in the WHI had an average age of 63 at screening, and most (64%–82%) were more than 10 years past menopause onset. The role of age and time since menopause onset in modifying the effect of HT on chronic disease, particularly cardiovascular disease, may have biological underpinnings. This phenomenon, referred to as the “timing hypothesis,” postulates that estrogen has a differential effect on vascular and inflammatory cell biology during the early and late stages of atherosclerosis progression. During the nascent stages of vascular disease (as seen in younger women or in early stages of menopause), estrogen therapy can improve or reverse endothelial dysfunction and slow progression of atherosclerosis (36–39). However, in advanced atherosclerotic lesions, often seen in older women, delayed initiation of HT may exacerbate inflammation, precipitate rupture of vulnerable plaques, and promote thrombo-occlusive events (36, 37). Supporting evidence for the timing hypothesis derives from studies in monkeys (40), mice (41), and ovariectomized rabbits (42–44), where early compared with delayed administration of 17-β estradiol had differential effects on atherosclerosis progression. Recent data from the Early Versus Late Intervention Trial with Estradiol (ELITE) trial demonstrated that oral estradiol therapy was associated with less progression of subclinical atherosclerosis than was placebo when such therapy was initiated within 6 years of menopause but not later (≥10 years). Still, there were no differences in coronary artery disease, assessed by coronary artery stenosis, after 5 years of therapy (45). Although we did not have sufficient power to examine the effects of HT on risk of MI in women who initiated HT many years after menopause (≥20 years), the most recent WHI report indicates a higher risk of total MI (HR = 1.99, 95% CI: 1.32, 3.02) among users of CEE+MPA in this group (15).
Although differences in age and time since menopause at HT initiation seem to account for most of the apparent discrepancies in the findings of the WHI HT trials and NHS observational studies, other key design issues remain. For example, in a previous NHS report that included women with prevalent coronary disease, HT was still associated with a lower risk of CHD among women who initiated HT within 4 years of menopause (for CEE, HR = 0.62, 95% CI: 0.52, 0.76; for CEE+MPA, HR = 0.71, 95% CI: 0.56, 0.89) (46). However, the younger age at initiation of HT, and the inclusion of a variety of HT regimens and doses in these analyses, may explain discrepancies with WHI. Contrary to our current findings of a null association between HT and stroke among women aged 50–59 years, a previous NHS report in the same age group documented a significant elevation in risk of stroke among CEE users (HR = 1.58, 95% CI: 1.06, 2.37) (47). However, this report did not restrict analyses to women aged 50–79 years, and the reference group included never users, irrespective of their hysterectomy status. In both NHS and WHI, CEE+MPA use was associated with a higher risk of invasive breast cancer, but the hazard ratio was slightly higher in NHS than in WHI. Although we attempted to adjust for mammographic screening differences residual confounding by differential screening rates in users and nonusers of HT remains possible.
Our findings need to be interpreted in the context of a few limitations. First, given the observational nature of our analysis, residual and unknown confounding remain a possibility. Still, we carefully selected our confounders based on prior knowledge of established risk factors. We also noted that age-adjusted estimates for MI and invasive breast cancer were attenuated by 13%–31% upon adjustment for confounders, raising the possibility of further attenuation with more precise measures of the confounders rather than self-report. However, all questionnaires used to capture these data were previously validated in our cohort (29, 48, 49). Further, we believe that the high educational status of our cohort participants and their knowledge of health allow them to provide reliable information on the questionnaires and minimize confounding by socioeconomic status. Second, due to the typical use of HT, we did not have statistical power to examine the potential influence of beginning HT on chronic disease at older ages or at increasingly longer intervals since menopause. Nonetheless, results from a previous NHS report indicate that, as observed in WHI, continuous estrogen/progestin therapy may not have cardiovascular benefits in women aged ≥60 years or who are ≥10 years beyond menopause onset (50). In another reanalysis of the NHS data, a significant trend of higher risk of CHD with increasing time since menopause (P-trend = 0.04) was documented (46). Finally, because HT use was measured only every 2 years, we could not assess the acute effects of HT use on chronic disease, particularly MI risk (9). However, our prior sensitivity analysis showed that cases occurring among women initiating HT use within a 2-year interval would not have appreciably influenced our findings (46).
In summary, our findings suggest that the discrepancies between the WHI and NHS could largely be ascribed to differences in timing of HT initiation in relation to age or onset of menopause. This is important because HT continues to be endorsed by numerous professional organizations for the management of moderate to severe vasomotor symptoms, which are most prevalent in early menopause (51–54). Among women aged 50–59 years, the lower risk of mortality with CEE use warrants further examination. Still, given the complex pattern of risks and benefits associated with HT, current findings do not support its use for chronic disease prevention.
ACKNOWLEDGMENTS
Author affiliations: Department of Nutrition, Harvard T.H. Chan School of Public Health, Harvard University, Boston, Massachusetts (Shilpa N. Bhupathiraju, Meir J. Stampfer, Frank B. Hu, Walter C. Willett); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Harvard University, Boston, Massachusetts (Francine Grodstein, Meir J. Stampfer, Frank B. Hu, Walter C. Willett, JoAnn E. Manson); Department of Biostatistics, Harvard T.H. Chan School of Public Health, Harvard University, Boston, Massachusetts (Bernard A. Rosner); Channing Division of Network Medicine, Harvard Medical School, Boston, Massachusetts (Shilpa N. Bhupathiraju, Francine Grodstein, Bernard A. Rosner, Meir J. Stampfer, Frank B. Hu, Walter C. Willett, JoAnn E. Manson); and Division of Preventive Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts (JoAnn E. Manson).
This study was supported by the National Institutes of Health (grants UM1 CA186107, P01 CA87969, R01 HL034594, and R01 HL088521).
We thank the participants and staff of the Nurses’ Health Study for their valuable contributions as well as the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming.
The authors assume full responsibility for analyses and interpretation of these data.
Conflict of interest: none declared.
Abbreviations
- CEE
conjugated equine estrogens
- CHD
coronary heart disease
- CI
confidence interval
- HR
hazard ratio
- HT
hormone therapy
- MI
myocardial infarction
- MPA
medroxyprogesterone acetate
- NHS
Nurses’ Health Study
- WHI
Women's Health Initiative
REFERENCES
- 1. Hunt K, Vessey M, McPherson K. Mortality in a cohort of long-term users of hormone replacement therapy: an updated analysis. Br J Obstet Gynaecol. 1990;97(12):1080–1086. [DOI] [PubMed] [Google Scholar]
- 2. Colditz GA, Hankinson SE, Hunter DJ, et al. . The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332(24):1589–1593. [DOI] [PubMed] [Google Scholar]
- 3. Wu O. Postmenopausal hormone replacement therapy and venous thromboembolism. Gend Med. 2005;2(suppl A):S18–S27. [DOI] [PubMed] [Google Scholar]
- 4. Grodstein F, Stampfer MJ, Falkeborn M, et al. . Postmenopausal hormone therapy and risk of cardiovascular disease and hip fracture in a cohort of Swedish women. Epidemiology. 1999;10(5):476–480. [PubMed] [Google Scholar]
- 5. Grodstein F, Manson JE, Colditz GA, et al. . A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133(12):933–941. [DOI] [PubMed] [Google Scholar]
- 6. Grodstein F, Stampfer MJ, Manson JE, et al. . Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335(7):453–461. [DOI] [PubMed] [Google Scholar]
- 7. Anderson GL, Limacher M, Assaf AR, et al. . Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. [DOI] [PubMed] [Google Scholar]
- 8. Rossouw JE, Anderson GL, Prentice RL, et al. . Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. [DOI] [PubMed] [Google Scholar]
- 9. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med. 2003;348(7):645–650. [DOI] [PubMed] [Google Scholar]
- 10. Fletcher RH, Fletcher SW, Fletcher GS. Clinical Epidemiology: The Essentials. 5th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 11. Willett WC, Manson JE, Grodstein F, et al. . Re: “combined postmenopausal hormone therapy and cardiovascular disease: toward resolving the discrepancy between observational studies and the women's health initiative clinical trial”. Am J Epidemiol. 2006;163(11):1067–1068. [DOI] [PubMed] [Google Scholar]
- 12. Grodstein F, Manson JE, Stampfer MJ, et al. . The discrepancy between observational studies and randomized trials of menopausal hormone therapy. Ann Intern Med. 2004;140(9):764–765. [DOI] [PubMed] [Google Scholar]
- 13. Vandenbroucke JP. The HRT controversy: observational studies and RCTs fall in line. Lancet. 2009;373(9671):1233–1235. [DOI] [PubMed] [Google Scholar]
- 14. Tuomikoski P, Mikkola TS. Postmenopausal hormone therapy and coronary heart disease in early postmenopausal women. Ann Med. 2014;46(1):1–7. [DOI] [PubMed] [Google Scholar]
- 15. Manson JE, Chlebowski RT, Stefanick ML, et al. . Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belanger CF, Hennekens CH, Rosner B, et al. . The Nurses’ Health Study. Am J Nurs. 1978;78(6):1039–1040. [PubMed] [Google Scholar]
- 17. Belanger C, Speizer FE, Hennekens CH, et al. . The Nurses’ Health Study: current findings. Am J Nurs. 1980;80(7):1333. [DOI] [PubMed] [Google Scholar]
- 18. Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 19. Working Group on Ischaemic Heart Disease Registers, World Health Organization Regional Office for Europe Ischemic Heart Disease Registers: Report of the Fifth Working Group (including a Second Revision of the Operating Protocol). Copenhagen, Denmark; April 26–29, 1971. (Fifth Working Group on Ischaemic Heart Disease Registers). [Google Scholar]
- 20. Alpert JS, Thygesen K, Antman E, et al. . Myocardial infarction redefined–a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959–969. [DOI] [PubMed] [Google Scholar]
- 21. Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke. 1981;12(2 Pt 2 suppl 1):I13–I44. [PubMed] [Google Scholar]
- 22. Puckett CD. The Educational Annotation of ICD-9-CM; Diseases and Procedures Tabular Lists. Reno, NV: Channel Publishing, Ltd, 1986. [Google Scholar]
- 23. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28(12):1039–1057. [DOI] [PubMed] [Google Scholar]
- 24. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–1197. [DOI] [PubMed] [Google Scholar]
- 25. Manson JE, Rimm EB, Stampfer MJ, et al. . Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338(8770):774–778. [DOI] [PubMed] [Google Scholar]
- 26. Stampfer MJ, Willett WC, Speizer FE, et al. . Test of the National Death Index. Am J Epidemiol. 1984;119(5):837–839. [DOI] [PubMed] [Google Scholar]
- 27. Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax nationwide death search. Am J Epidemiol. 1994;140(11):1016–1019. [DOI] [PubMed] [Google Scholar]
- 28. Willett W, Stampfer MJ, Bain C, et al. . Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117(6):651–658. [DOI] [PubMed] [Google Scholar]
- 29. Colditz GA, Stampfer MJ, Willett WC, et al. . Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol. 1987;126(2):319–325. [DOI] [PubMed] [Google Scholar]
- 30. Zahl PH, Jørgensen KJ, Maehlen J, et al. . Biases in estimates of overdetection due to mammography screening. Lancet Oncol. 2008;9(3):199–201. [DOI] [PubMed] [Google Scholar]
- 31. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 32. Col NF, Pauker SG. The discrepancy between observational studies and randomized trials of menopausal hormone therapy: did expectations shape experience. Ann Intern Med. 2003;139(11):923–929. [DOI] [PubMed] [Google Scholar]
- 33. Banks E, Canfell K. Invited commentary: hormone therapy risks and benefits—the Women's Health Initiative findings and the postmenopausal estrogen timing hypothesis. Am J Epidemiol. 2009;170(1):24–28. [DOI] [PubMed] [Google Scholar]
- 34. Prentice RL, Langer R, Stefanick ML, et al. . Combined postmenopausal hormone therapy and cardiovascular disease: toward resolving the discrepancy between observational studies and the Women's Health Initiative clinical trial. Am J Epidemiol. 2005;162(5):404–414. [DOI] [PubMed] [Google Scholar]
- 35. Petitti DB, Freedman DA. Invited commentary: how far can epidemiologists get with statistical adjustment. Am J Epidemiol. 2005;162(5):415–418. [DOI] [PubMed] [Google Scholar]
- 36. Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340(23):1801–1811. [DOI] [PubMed] [Google Scholar]
- 37. Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308(5728):1583–1587. [DOI] [PubMed] [Google Scholar]
- 38. Mikkola TS, Clarkson TB. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovasc Res. 2002;53(3):605–619. [DOI] [PubMed] [Google Scholar]
- 39. Hodis HN, Mack WJ, Shoupe D, et al. . Abstract 13283: testing the menopausal hormone therapy timing hypothesis: the early versus late intervention trial with estradiol. Circulation. 2014;130(suppl 2):A13283. [Google Scholar]
- 40. Clarkson TB, Anthony MS, Klein KP. Hormone replacement therapy and coronary artery atherosclerosis: the monkey model. Br J Obstet Gynaecol. 1996;103(suppl 13):53–57. [PubMed] [Google Scholar]
- 41. Rosenfeld ME, Kauser K, Martin-McNulty B, et al. . Estrogen inhibits the initiation of fatty streaks throughout the vasculature but does not inhibit intra-plaque hemorrhage and the progression of established lesions in apolipoprotein E deficient mice. Atherosclerosis. 2002;164(2):251–259. [DOI] [PubMed] [Google Scholar]
- 42. Haarbo J, Christiansen C. The impact of female sex hormones on secondary prevention of atherosclerosis in ovariectomized cholesterol-fed rabbits. Atherosclerosis. 1996;123(1-2):139–144. [DOI] [PubMed] [Google Scholar]
- 43. Haarbo J, Leth-Espensen P, Stender S, et al. . Estrogen monotherapy and combined estrogen-progestogen replacement therapy attenuate aortic accumulation of cholesterol in ovariectomized cholesterol-fed rabbits. J Clin Invest. 1991;87(4):1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hanke H, Kamenz J, Hanke S, et al. . Effect of 17-beta estradiol on pre-existing atherosclerotic lesions: role of the endothelium. Atherosclerosis. 1999;147(1):123–132. [DOI] [PubMed] [Google Scholar]
- 45. Hodis HN, Mack WJ, Henderson VW, et al. . Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt). 2006;15(1):35–44. [DOI] [PubMed] [Google Scholar]
- 47. Grodstein F, Manson JE, Stampfer MJ, et al. . Postmenopausal hormone therapy and stroke: role of time since menopause and age at initiation of hormone therapy. Arch Intern Med. 2008;168(8):861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rimm EB, Stampfer MJ, Colditz GA, et al. . Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–473. [DOI] [PubMed] [Google Scholar]
- 49. Wolf AM, Hunter DJ, Colditz GA, et al. . Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–999. [DOI] [PubMed] [Google Scholar]
- 50. Hernan MA, Alonso A, Logan R, et al. . Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19(6):766–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. North American Menopause Society The 2012 hormone therapy position statement of: the North American Menopause Society. Menopause. 2012;19(3):257–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goodman NF, Cobin RH, Ginzburg SB, et al. . American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of menopause. Endocr Pract. 2011;17(suppl 6):1–25. [DOI] [PubMed] [Google Scholar]
- 53. de Villiers TJ, Gass ML, Haines CJ, et al. . Global consensus statement on menopausal hormone therapy. Climacteric. 2013;16(2):203–204. [DOI] [PubMed] [Google Scholar]
- 54. Santen RJ, Allred DC, Ardoin SP, et al. . Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95(7 suppl 1):s1–s66. [DOI] [PMC free article] [PubMed] [Google Scholar]