Abstract
Small-scale production poultry operations are increasingly common worldwide. To investigate how these operations influence antimicrobial resistance and mobile genetic elements (MGEs), Escherichia coli isolates were sampled from small-scale production birds (raised in confined spaces with antibiotics in feed), household birds (no movement constraints; fed on scraps), and humans associated with these birds in rural Ecuador (2010–2012). Isolates were screened for genes associated with MGEs as well as phenotypic resistance to 12 antibiotics. Isolates from small-scale production birds had significantly elevated odds of resistance to 7 antibiotics and presence of MGE genes compared with household birds (adjusted odds ratio (OR) range = 2.2–87.9). Isolates from humans associated with small-scale production birds had elevated odds of carrying an integron (adjusted OR = 2.0; 95% confidence interval (CI): 1.06, 3.83) compared with humans associated with household birds, as well as resistance to sulfisoxazole (adjusted OR = 1.9; 95% CI: 1.01, 3.60) and trimethoprim/sulfamethoxazole (adjusted OR = 2.1; 95% CI: 1.13, 3.95). Stratifying by the presence of MGEs revealed antibiotic groups that are explained by biological links to MGEs; in particular, resistance to sulfisoxazole, trimethoprim/sulfamethoxazole, or tetracycline was highest among birds and humans when MGE exposures were present. Small-scale production poultry operations might select for isolates carrying MGEs, contributing to elevated levels of resistance in this setting.
Keywords: antimicrobial resistance, Escherichia coli, integrons, plasmids, poultry production
Antimicrobial resistance (AMR) threatens global health by increasing morbidity and mortality, complicating treatment, and increasing health-care costs (1). Therapeutic and nontherapeutic antibiotic use among humans and animals contribute to community and environmental reservoirs of AMR, such as those found in livestock operations (2). Antibiotic use among animals in the United States is 4 times higher by weight than among humans, and it remains largely unregulated and undocumented (3–6).
Poultry production is a quickly expanding form of livestock farming worldwide (7). In developed nations with industrial-scale operations, thousands of birds are often raised in confined spaces with feed or water laced with antibiotics (given for the purposes of disease prevention, treatment, and/or growth promotion). Higher frequencies of AMR have been found in poultry from these production operations compared with free-range birds not exposed to antibiotics (8). In addition, isolates from humans associated with different types of production poultry operations with varying degrees of antibiotic utilization have similar resistance frequencies and profiles to those found in corresponding poultry isolates (9) and higher resistance frequencies when compared with humans in the surrounding community not associated with production operations (10, 11). However, poultry farms worldwide are dominated by small-scale production operations (<100 birds), particularly in developing nations where poultry are raised for income and/or household consumption (12, 13). While a small number of studies have documented high levels of AMR in poultry in small-scale production operations (14–16), not all of these studies have appropriate nonproduction bird or human comparisons (nonclinical, from the immediate surrounding community), and so less is known about AMR development and transmission within these settings.
While many of these studies investigate resistant phenotypes, genetic factors also mark AMR spread. Mobile genetic elements (MGEs), such as integrons and plasmids, transfer genes conveying phenotypic resistance among diverse bacterial genera. Certain phenotypic resistance profiles might be associated with different MGEs; genes conveying resistance to tetracycline are often found on plasmids (17), and some integron types contain a gene conveying resistance to sulfonamides at the 3′ end of the integron structure (18). The high prevalence of MGEs documented in industrial-scale poultry operations from developed nations might contribute to elevated frequencies of AMR in these operations (19). A diverse array of AMR genes have been found in integrons located on plasmids, making them a common mechanism for horizontal spread of AMR and multiple-drug resistance (MDR) (20), and MGEs might be more important than bacterial cells in the transmission of AMR genes from livestock to humans (21).
The construction of a road in Esmeraldas province, Ecuador, has led to an influx of small-scale production poultry (chicken) operations using antibiotics in feed and water for growth promotion and prophylaxis. This increase provided an opportunity to observe how antibiotic use in small-scale production operations influences the epidemiology of AMR and MGEs in commensal gut bacteria of poultry and humans. A serial cross-sectional community-based study was conducted to examine how small-scale poultry production practices affect the frequency of MGEs and phenotypic AMR in poultry and human isolates across rural communities in northern Ecuador.
METHODS
Study area and design
The location and overall design of the parent case-control study have been described elsewhere (22, 23); briefly, the study included a total of 31 villages distributed along roads and river basins near Borbón, a major population hub in northern coastal Ecuador, that were visited between 2003 and 2012. This analysis was restricted to data from 20 of these villages, where fecal samples from both humans and chickens were collected (August 2010 to May 2012). Each village was visited for 15 days once a year during this period. Because the study period encompassed 20 months, some villages were visited once and others twice. Consent to participate was obtained from all households, and all study protocols were reviewed and approved by the University of Michigan Institutional Review Board and the Universidad San Francisco de Quito Bioethics Committee.
Poultry sample collection
Starting in August 2010, two types of birds were sampled from each village during study visits. Birds from small-scale production operations were production-breed broiler chickens and laying hens, raised in close contact with other birds within poultry coops, and were not allowed or able to leave the coop environment. Coop sizes ranged from tens to hundreds of birds (the majority housed 50–100). Diets of production birds were composed largely of formula feed containing antibiotics, and antibiotics were sometimes directly administered to these chickens in their water. Coop-level information on antibiotics included in feed was not available. Furthermore, the specific antibiotics used varied by brand and over time for a given brand. (Using mass spectrometry, a previous analysis of a commonly available feed for laying and broiler hens in the area revealed the presence of chloramphenicol, virginiamycin, lincomycin, and tetracycline (15).) In contrast, household chickens, kept for personal consumption (meat and eggs), did not have movement restrictions and had diets composed primarily of ground corn and kitchen scraps.
To obtain poultry cloacal samples, teams visited all households with active small-scale production poultry coops as well as households with no small-scale production coops that were located as far as possible from active coops (one nonproduction household was sampled for each household with an active coop). At least 5 small-scale production chickens in each coop were sampled. For villages with no small-scale production operations present at the time of the visit, 3–10 households were chosen at random to sample household birds. The exact number was dependent on the proportion of households in the village that were raising household birds at the time, which ranged from 10%–30%.
Human sample collection
As part of the original case-control study, human stool samples were collected from individuals who had cases of diarrhea, identified through daily household visits during the 15-day village visits. We used the World Health Organization case definition of diarrhea, which requires 3 or more loose stools (self-reported or, for children <13 years of age, maternally reported) in a 24-hour period (24). A 10% random sample of the community was selected to identify controls, defined as individuals without diarrheal symptoms during the prior 6 days. Humans were linked to birds from small-scale production operations or household birds through a shared household identification number.
Markers for phenotypic AMR
Isolates of Escherichia coli from each human and chicken fecal sample were selected and grown on Chromocult agar (Merck, Darmstadt, Germany). Phenotypic resistance was measured for up to 5 isolates per chicken and (given the larger number of human samples collected over the entire study period) 1 isolate at random per human using disc-diffusion assays by standard antibiogram methods. Because the study comparisons were within bird and human groups (see below), any bias introduced by this approach should have been nondifferential. The following classes were tested: beta-lactams (ampicillin, amoxicillin/clavulanate acid, cefotaxime, cephalothin), quinolones (ciprofloxacin and enrofloxacin), aminoglycosides (gentamicin and streptomycin), sulfonamides (sulfisoxazole, sulfamethoxazole), trimethoprim (tested with sulfamethoxazole), chloramphenicol, and tetracycline. Clinical and Laboratory Standards Institute (CLSI) breakpoints were used to determine an isolate’s susceptibility or resistance to antibiotics from a clinical perspective (25). An isolate was defined as MDR if the isolate was resistant to 2 or more classes of antibiotics.
Markers for MGEs
To test for the presence of MGEs, genes associated with the following integrons and resistance genes were used as markers: class-1 integrons (int1), class-2 integrons (int2), and 2 tetracycline genes (class A: tetA, and class B: tetB). Presence or absence of each gene was determined using high-throughput dot-blot hybridization on the library-on-a-slide (LOS) array platform developed previously in our laboratory (26), with internal gene probes prepared with primers listed in Web Table 1, available at https://academic.oup.com/aje. Hybridization conditions and the analysis of the probing results have been described in detail elsewhere (26–29).
Statistical analysis
To examine the role of small-scale production operations on the prevalence of AMR in E. coli isolates, the AMR prevalence in isolates from small-scale production birds was compared with the prevalence in isolates from household birds. To investigate the role that small-scale production operations have on the prevalence of AMR isolates from household birds in the surrounding community, household birds were stratified by village type (household birds from villages with small-scale production operations and household birds from villages without small-scale production operations at the time of sample collection). To investigate how MGEs influence AMR levels, another stratified analysis was done stratifying isolates by both small-scale production and MGE status. AMR prevalence in isolates from humans associated with birds from small-scale production operations was compared with the prevalence of AMR in isolates from humans associated only with household birds. As with the poultry comparisons above, the latter group was also stratified by the presence or absence of a small-scale production operation in the village at the time of visit, as well as by both small-scale production and MGE status.
Multiple levels of clustering potentially occur for poultry (bird, household, and village-level) and human (household and village-level) isolates. To investigate potential clustering, odds ratios and 95% confidence intervals were generated by building nested mixed models with random intercepts for each level of clustering with PROC GLIMMIX in SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina). Evidence for clustering was assessed through significance of the household and village-level random effect variance. For isolates from both birds and humans, there was no evidence for clustering at the village level (i.e., the village-level random-effect variance was not significantly different from zero). Due to high correlation of isolates within birds that manifested in highly variable covariate effect estimates (data not shown), a random effect for birds was not used. Instead, isolates were collapsed across birds (i.e., if there was an isolate positive for a marker, then that bird was considered positive for that marker), and this bird-level observation was used for the models. Therefore, final mixed models for both poultry and humans included a random intercept only for the household.
To compare the odds of AMR in isolates from small-scale production birds (and associated humans) with those in household birds (and associated humans), the above models were used to calculate unadjusted and adjusted odds ratios and 95% confidence intervals. To calculate adjusted odds ratios, we controlled for variables based on hypothesized causal relationships between these variables and the exposure/outcome. Year of sample collection can influence the presence of a small-scale production operation and levels of AMR. Diarrheal case status of humans might be causally linked to AMR levels: If a person is sick and takes antibiotics, that might then select for AMR organisms (adjusting for a variable that is causally linked only to the outcome can increase statistical efficiency (30)). Model adjustments, therefore, included year of sample collection for isolates from birds and year of sample collection and original diarrheal case status for isolates from humans. In addition to the latter adjustment for humans (in model 1), because MGEs might be related to both small-scale production operations and AMR, we constructed a second model controlling for MGEs (model 2).
RESULTS
Poultry data
We collected 1,245 isolates from 376 chickens (186 small-scale production birds and 190 household birds) in the 20 villages. Higher prevalence of AMR for all markers was seen in isolates from small-scale production birds compared with those from household birds (Table 1). Unadjusted and adjusted odds ratios from mixed models showed significantly elevated odds for the presence of all MGE markers among small-scale production birds, and significantly elevated odds of resistance for 7 of 12 antibiotics tested (as well as MDR), compared with household birds (Table 2). Low prevalence of resistance was observed for amoxicillin/clavulanate acid, cephalothin, cefotaxime, enrofloxacin, and gentamicin, particularly among isolates from household birds; therefore, models for these antibiotics gave unstable odds ratios and are not reported.
Table 1.
Frequency of Antibiotic Resistance in Escherichia coli Isolates From Small-Scale Production Birds and Associated Humans in Rural Ecuador, 2010–2012
| Source and Marker | Type of Poultry | Type of Human Association | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Small-Scale Production (n = 619) | Household | Small-Scale Production (n = 45) | Household | |||||||||
| Small-Scale Production Villages (n = 246) | Nonproduction Villages (n = 380) | Small-Scale Production Villages (n = 385) | Nonproduction Villages (n = 369) | |||||||||
| No. of Samples | %a | No. of Samples | %a | No. of Samples | %a | No. of Samples | %a | No. of Samples | %a | No. of Samples | %a | |
| Microarray | ||||||||||||
| int1 | 269 | 43.5 | 27 | 11.0 | 22 | 5.8 | 13 | 28.9 | 75 | 19.5 | 63 | 17.1 |
| int2 | 65 | 10.5 | 6 | 2.4 | 2 | 0.5 | 4 | 8.9 | 14 | 3.6 | 12 | 3.3 |
| tetA | 307 | 49.6 | 54 | 22.0 | 53 | 13.9 | 15 | 33.3 | 88 | 22.9 | 64 | 17.3 |
| tetB | 88 | 14.2 | 19 | 7.7 | 23 | 6.1 | 7 | 15.6 | 54 | 14.0 | 46 | 12.5 |
| Antibiogram | ||||||||||||
| Sulfisoxazole | 401 | 64.8 | 43 | 17.5 | 37 | 9.7 | 22 | 48.9 | 134 | 34.8 | 119 | 32.2 |
| Trimethoprim/sulfamethoxazole | 368 | 59.5 | 39 | 15.9 | 32 | 8.4 | 21 | 46.7 | 116 | 30.1 | 104 | 28.2 |
| Tetracycline | 426 | 68.8 | 72 | 29.3 | 93 | 24.5 | 20 | 44.4 | 151 | 39.2 | 108 | 29.3 |
| Ampicillin | 279 | 45.1 | 29 | 11.8 | 29 | 7.6 | 16 | 35.6 | 131 | 34.0 | 115 | 31.2 |
| Chloramphenicol | 151 | 24.4 | 5 | 2.0 | 12 | 3.2 | 2 | 4.4 | 23 | 6.0 | 22 | 6.0 |
| Ciprofloxacin | 183 | 29.6 | 7 | 2.8 | 14 | 3.7 | 1 | 2.2 | 10 | 2.6 | 13 | 3.5 |
| Enrofloxacin | 196 | 31.7 | 7 | 2.8 | 13 | 3.4 | 2 | 4.4 | 10 | 2.6 | 13 | 3.5 |
| Streptomycin | 257 | 41.5 | 18 | 7.3 | 30 | 7.9 | 12 | 26.7 | 99 | 25.7 | 85 | 23.0 |
| Amoxicillin/clavulanate acid | 112 | 18.1 | 3 | 1.2 | 0 | 0.0 | 2 | 4.4 | 13 | 3.4 | 7 | 1.9 |
| Cephalothin | 201 | 32.5 | 8 | 3.3 | 16 | 4.2 | 8 | 17.8 | 66 | 17.1 | 49 | 13.3 |
| Cefotaxime | 13 | 2.1 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 0.5 |
| Gentamicin | 101 | 16.3 | 3 | 1.2 | 1 | 0.3 | 1 | 2.2 | 1 | 0.3 | 8 | 2.2 |
| MDR | 429 | 69.3 | 53 | 21.5 | 58 | 15.3 | 21 | 46.7 | 151 | 39.2 | 129 | 35.0 |
Abbreviations: int1, class-1 integron; int2, class-2 integron; MDR, multiple-drug resistance; tetA, tetracycline class A; tetB, tetracycline class B.
a Columns might not sum to the total, as an isolate can be positive for more than 1 genetic marker, and/or resistant to more than 1 antibiotic.
Table 2.
Odds Ratios From Mixed Modelsa Comparing Escherichia coli Isolates From Small-Scale Production Birds (n = 619) to Isolates From Household Birds (n = 626) in Rural Ecuador, 2010–2012
| Source and Marker | Small-Scale Production Bird vs. Household Bird | |||
|---|---|---|---|---|
| Unadjusted | Adjustedb | |||
| OR | 95% CI | OR | 95% CI | |
| Microarray | ||||
| int1 | 12.4 | 5.97, 25.7 | 14.1 | 6.44, 30.9 |
| int2 | 22.1 | 6.00, 81.4 | 25.8 | 6.71, 99.3 |
| tetA | 9.6 | 4.74, 19.5 | 9.6 | 4.63, 19.8 |
| tetB | 2.2 | 1.21, 3.86 | 2.2 | 1.18, 3.99 |
| Antibiogram | ||||
| Sulfisoxazole | 42.8 | 15.9, 115 | 44.1 | 15.8, 123 |
| Trimethoprim/sulfamethoxazole | 50.9 | 18.8, 137 | 52.7 | 18.9, 147 |
| Tetracycline | 5.3 | 2.69, 10.4 | 5.6 | 2.73, 11.3 |
| Ampicillin | 15.9 | 5.81, 43.3 | 15.6 | 5.59, 43.7 |
| Chloramphenicol | 17.3 | 6.40, 46.9 | 20.5 | 7.89, 53.0 |
| Ciprofloxacin | 19.2 | 6.59, 55.7 | 26.9 | 8.32, 87.1 |
| Streptomycin | 77.0 | 23.6, 251 | 87.9 | 25.7, 301 |
| MDR | 2.0 | 1.09, 3.69 | 2.2 | 1.13, 4.18 |
Abbreviations: CI, confidence interval; int1, class-1 integron; int2, class-2 integron; MDR, multiple-drug resistance; OR, odds ratio; tetA, tetracycline class A; tetB, tetracycline class B.
a Mixed models accounted for clustering at the household level; isolates were collapsed across birds due to high within-bird correlation. Models for 5 of 12 antibiotics not shown due to low frequencies.
b Adjustment includes year of sample collection.
To investigate the role of small-scale production operations on household chickens, household birds were stratified by the presence of a small-scale production operation in the village (n = 68) or the absence of a small-scale production operation in the village (n = 122) at the time of the visit (Table 3). Household chickens from villages with small-scale production operations had significantly higher odds of resistance to 2 of the 4 MGE markers (for int1, adjusted odds ratio (OR) = 3.1 (95% confidence interval (CI): 1.27, 7.69), and for tetA, adjusted OR = 2.6 (95% CI: 1.20, 5.66)) compared with household chickens from nonproduction villages. In addition, higher odds of phenotypic resistance were observed for MDR (adjusted OR = 2.4, 95% CI: 1.07, 5.40) and for 2 antibiotics (for sulfisoxazole, adjusted OR = 3.3 (95% CI: 1.35, 8.02), and for trimethoprim/sulfamethoxazole, adjusted OR = 3.3 (95% CI: 1.35, 7.99)).
Table 3.
Odds Ratios From Mixed Modelsa Comparing Escherichia coli Isolates in Rural Ecuador, 2010–2012
| Source and Marker | Small-Scale Production Bird | Household Bird | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjustedb | Unadjusted | Adjustedb | |||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Microarray | ||||||||
| int1 | 18.4 | 7.74, 43.9 | 25.4 | 9.32, 69.1 | 2.5 | 1.06, 5.90 | 3.1 | 1.27, 7.69 |
| int2 | 44.0 | 5.44, 355 | 56.1 | 6.61, 475 | 3.8 | 0.32, 45.9 | 4.6 | 0.38, 55.3 |
| tetA | 13.8 | 6.09, 31.2 | 15.1 | 6.38, 35.8 | 2.4 | 1.14, 5.10 | 2.6 | 1.20, 5.66 |
| tetB | 2.8 | 1.38, 5.80 | 3.0 | 1.39, 6.52 | 1.9 | 0.81, 4.55 | 2.0 | 0.82, 4.91 |
| Antibiogram | ||||||||
| Sulfisoxazole | 65.7 | 21.5, 201 | 82.8 | 24.0, 285 | 2.8 | 1.20, 6.46 | 3.3 | 1.35, 8.02 |
| Trimethoprim/sulfamethoxazole | 78.0 | 25.5, 239 | 98.5 | 28.7, 339 | 2.8 | 1.20, 6.41 | 3.3 | 1.35, 7.99 |
| Tetracycline | 6.1 | 2.91, 12.9 | 6.9 | 3.08, 15.7 | 1.5 | 0.70, 3.07 | 1.6 | 0.75, 3.49 |
| Ampicillin | 21.0 | 6.70, 65.6 | 22.2 | 6.61, 74.7 | 1.9 | 0.74, 4.90 | 2.0 | 0.74, 5.34 |
| Chloramphenicol | 16.2 | 5.43, 48.4 | 21.9 | 7.43, 64.4 | 0.8 | 0.23, 3.03 | 1.2 | 0.34, 4.19 |
| Ciprofloxacin | 16.1 | 5.11, 51.1 | 25.2 | 6.92, 92.1 | 0.6 | 0.16, 2.51 | 0.9 | 0.21, 3.44 |
| Streptomycin | 79.9 | 23.0, 278 | 102.0 | 26.5, 392 | 1.1 | 0.42, 2.87 | 1.4 | 0.52, 3.67 |
| MDR | 2.7 | 1.34, 5.38 | 3.4 | 1.53, 7.33 | 2.0 | 0.95, 4.41 | 2.4 | 1.07, 5.40 |
Abbreviations: CI, confidence interval; int1, class-1 integron; int2, class-2 integron; MDR, multiple-drug resistance; OR, odds ratio; tetA, tetracycline class A; tetB, tetracycline class B.
a Models compared E. coli isolates from small-scale production birds (n = 619) to isolates from household birds in villages without small-scale production operations (n = 380) and household birds in villages with small-scale production operations (n = 246) to isolates from household birds in villages without small-scale production operations (n = 380). Mixed models accounted for clustering at the household level; isolates were collapsed across birds due to high within-bird correlation. Reference group was household birds in villages without small-scale production operations. Models for 5 of 12 antibiotics not shown due to low frequencies.
b Adjustment includes year of sample collection.
Human data
Human isolates (n = 799) were linked in the database to small-scale production operation or household birds through a shared household identification number. There were 45 humans associated with production birds and 754 associated with household birds. The prevalences of AMR to 8 of 12 antibiotics and 4 of 4 MGE markers were higher for isolates from humans associated with production birds compared with those associated with household birds (Table 1). Odds ratios from nested mixed models are shown in Table 4. As with the poultry data, there was low AMR prevalence for a subset of antibiotics, and these models are not reported. There was also low prevalence of int2 and tetB; these were combined with int1 and tetA for a single category for integrons and one for tetracycline genes (Table 4). Models with adjustment showed increased odds of the presence of an integron (adjusted OR = 2.0, 95% CI: 1.06, 3.83), sulfisoxazole resistance (adjusted OR = 1.9, 95% CI: 1.01, 3.60), and trimethoprim/sulfamethoxazole resistance (adjusted OR = 2.1, 95% CI: 1.13, 3.95) among humans associated with small-scale production birds compared with humans associated with household birds. Similar patterns were observed upon stratifying humans associated with household birds by village type (Table 5), with higher odds of tetA/B becoming significant when comparing humans associated with small-scale production birds with humans associated with household birds in nonproduction villages (model 1: adjusted OR = 2.0, 95% CI: 1.02, 3.78). Only tetracycline resistance (model 1: adjusted OR = 1.5, 95% CI: 1.03, 2.08) had significantly elevated odds when comparing humans associated with household birds in small-scale production villages with those in nonproduction villages.
Table 4.
Odds Ratios From Mixed Modelsa Comparing Escherichia coli Isolates From Humans Associated With Small-Scale Production Birds (n = 45) to Isolates From Humans Associated With Household Birds (n = 754) in Rural Ecuador, 2010–2012
| Source and Marker | Human Associated with Small-Scale Production Birds vs. Human Associated with Household Birds | |||
|---|---|---|---|---|
| Unadjusted | Adjustedb | |||
| OR | 95% CI | OR | 95% CI | |
| Microarray | ||||
| int1/2 | 2.0 | 1.08, 3.88 | 2.0 | 1.06, 3.83 |
| tetA/B | 1.7 | 0.91, 3.10 | 1.6 | 0.89, 3.04 |
| Antibiogram | ||||
| Sulfisoxazole | 1.9 | 1.03, 3.47 | 1.9 | 1.01, 3.60 |
| Trimethoprim/sulfamethoxazole | 2.1 | 1.16, 3.90 | 2.1 | 1.13, 3.95 |
| Tetracycline | 1.5 | 0.83, 2.81 | 1.4 | 0.77, 2.66 |
| Ampicillin | 1.1 | 0.61, 2.14 | 1.1 | 0.60, 2.18 |
| Streptomycin | 1.1 | 0.57, 2.23 | 1.1 | 0.56, 2.24 |
| MDR | 1.5 | 0.81, 2.72 | 1.5 | 0.78, 2.74 |
Abbreviations: CI, confidence interval; int1/2, class-1 or class-2 integron; MDR, multiple-drug resistance; OR, odds ratio; tetA/B, tetracycline class A or B.
a Mixed models accounted for clustering at the household level. Models for 7 of 12 antibiotics not shown due to low frequencies.
b Adjustment includes year of sample collection and diarrheal case status.
Table 5.
Odds Ratios From Mixed Modelsa Comparing Escherichia coli Isolates in Rural Ecuador, 2010–2012
| Source and Marker | Human Associated With Small-Scale Production Birds | Human Associated With Household Birds | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Model 1b | Model 2c | Unadjusted | Model 1b | Model 2c | |||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Microarray | ||||||||||||
| int1/2 | 2.2 | 1.13, 4.27 | 2.1 | 1.07, 4.30 | 1.1 | 0.81, 1.63 | 1.1 | 0.74, 1.66 | ||||
| tetA/B | 1.9 | 1.03, 3.64 | 2.0 | 1.02, 3.78 | 1.3 | 0.96, 1.78 | 1.3 | 0.92, 1.88 | ||||
| Antibiogram | ||||||||||||
| Sulfisoxazole | 2.1 | 1.06, 4.06 | 1.9 | 0.95, 3.67 | 1.5 | 0.65, 3.31 | 1.1 | 0.82, 1.54 | 1.0 | 0.67, 1.39 | 0.9 | 0.59, 1.39 |
| Trimethoprim/sulfamethoxazole | 2.3 | 1.17, 4.52 | 2.0 | 1.03, 3.92 | 1.6 | 0.71, 3.66 | 1.1 | 0.80, 1.53 | 0.9 | 0.63, 1.34 | 0.8 | 0.53, 1.29 |
| Tetracycline | 1.9 | 1.03, 3.64 | 1.8 | 0.95, 3.55 | 1.2 | 0.03, 48.8 | 1.6 | 1.15, 2.12 | 1.5 | 1.03, 2.08 | 1.4 | 0.29, 7.22 |
| Ampicillin | 1.3 | 0.63, 2.60 | 1.2 | 0.59, 2.47 | 0.9 | 0.39, 1.86 | 1.1 | 0.83, 1.58 | 1.1 | 0.73, 1.53 | 1.0 | 0.69, 1.53 |
| Streptomycin | 1.2 | 0.60, 2.46 | 1.2 | 0.57, 2.51 | 0.9 | 0.40, 1.96 | 1.2 | 0.83, 1.62 | 1.1 | 0.75, 1.64 | 1.1 | 0.71, 1.64 |
| MDR | 1.6 | 0.87, 3.05 | 1.5 | 0.78, 2.96 | 1.1 | 0.47, 2.47 | 1.2 | 0.89, 1.62 | 1.1 | 0.75, 1.51 | 1.0 | 0.68, 1.57 |
Abbreviations: CI, confidence interval; int1/2, class-1 or class-2 integron; MDR, multiple-drug resistance; OR, odds ratio; tetA/B, tetracycline class A or B.
a Models compared isolates from humans associated with small-scale production birds (n = 45) to isolates from humans associated with household birds in villages without small-scale production operations (n = 369) and humans associated with household birds in villages with small-scale production operations (n = 385) to isolates from humans associated with household birds in villages without small-scale production operations (n = 369). Mixed models accounted for clustering at the household level. Reference group is humans associated with household birds in villages without small-scale production operations. Models for 7/12 antibiotics not shown due to low frequencies.
b Model 1 includes year of sample collection and diarrheal case status.
c Model 2 includes year of sample collection, diarrheal case status, and presence of an integron (the model for tetracycline includes presence of tetA/B).
Small-scale production operations, AMR, and MGEs
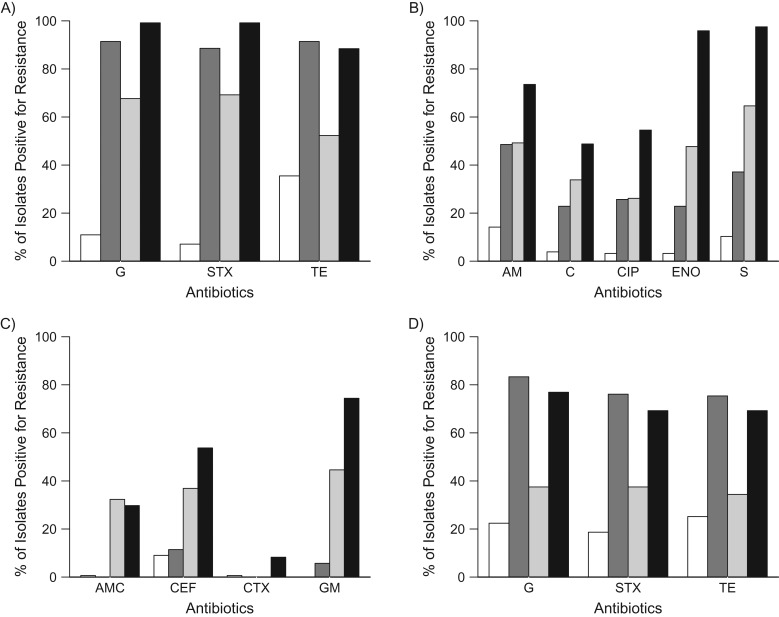
Elevated levels of markers for MGEs, particularly isolates positive for int1 and tetA, were consistently observed in small-scale production birds and associated humans compared with household birds and associated humans. To explore how MGEs might facilitate AMR in isolates from poultry and humans, the prevalence of phenotypic AMR by small-scale production exposure was further stratified by the presence or absence of MGEs (results for int1 are shown in Figure 1). Three distinct groups of phenotypic AMR emerged when using all isolates from poultry, which we refer to here as group 1, group 2, and group 3. Resistance to group 1 antibiotics (sulfisoxazole, trimethoprim/sulfamethoxazole, and tetracycline) had the highest levels of resistance whenever an integron was present, regardless of poultry type (Figure 1A). In group 2 (Figure 1B), both the presence of small-scale production operations and int1 increased the prevalence of AMR. Resistance to group 3 antibiotics was high with production exposure, but sometimes both production and integron exposures were required (Figure 1C). Similar grouping patterns were also seen when stratifying by int2, tetA, and tetB (data not shown). In humans, there were low frequencies of resistance to group 2 and 3 antibiotics, and so similar patterns failed to emerge for these groups (data not shown). However, the prevalence of AMR for sulfisoxazole, trimethoprim/sulfamethoxazole, and tetracycline (group 1) in human isolates showed similar patterns to those seen in poultry isolates, with the highest AMR prevalence (approximately 70%–80%) occurring when MGEs were present, intermediate prevalence (35%–40%) occurring when only small-scale production operations were present, and the lowest prevalence (approximately 20%) present when neither small-scale production operations nor MGEs were present (Figure 1D).
Figure 1.
Prevalence of antimicrobial resistance in E. coli isolates from small-scale production and household birds, stratified by the presence or absence of class-1 integrons (int1), in rural Ecuador, 2010–2012. A) For poultry isolates in group 1, the association of small-scale production operations appeared to be driven largely by the presence or absence of int1. B) For poultry isolates in group 2, the association of small-scale production operations and int1 was roughly additive. C) For poultry isolates in group 3, while small-scale production operations resulted in high resistance levels, both int1 and small-scale production operation exposures were often needed to reach high levels of resistance. D) Resistance patterns in isolates from humans associated with small-scale production or household birds for group 1 antibiotics mirrored those seen in isolates from poultry (see (A)). White: household birds, int1-negative; dark gray: household birds, int1-positive; light gray: small-scale production birds, int1-negative; black: small-scale production birds, int1-positive. AM, ampicillin; AMC, amoxicillin/clavulanate acid; C, chloramphenicol; CEF, cephalothin; CIP, ciprofloxacin; CTX, cefotaxime; ENO, enrofloxacin; G, sulfisoxazole; GM, gentamicin; S, streptomycin; STX, trimethoprim/sulfamethoxazole; TE, tetracycline.
Given our observations that small-scale production operations are associated with elevated odds of MGEs (Tables 2–5), and that MGEs are associated with elevated levels of AMR (Figure 1), a second adjusted model for humans was created in which presence/absence of MGEs was added (Table 5, model 2). Attenuated odds ratios and a loss of (moderate to weak) significance for sulfisoxazole, trimethoprim/sulfamethoxazole, and tetracycline for humans associated with production birds (and tetracycline for humans associated with household birds in production villages) were observed.
DISCUSSION
This study suggests that production operations on a smaller scale, and not just industrial-scale poultry operations, are associated with increases in resistant isolates in poultry and humans associated with these operations. In addition, the MGE and phenotypic data presented here highlighted a potentially important role of small-scale production operations within the study site, namely an elevated occurrence of MGEs in E. coli carried by production birds and associated humans. Integrons house a wide array of resistance genes (20), which can result in the expression of resistant phenotypes not directly related to antibiotics used in production farming. In a study of livestock farms with heavy use of antibiotics, all types of resistance genes were enriched, and such enrichment was highly correlated with the abundance of MGEs such as transposases (31), often linked with integrons (32).
The ability of class-1 integrons to carry AMR genes is the most likely explanation for our observed relationship between int1, small-scale production operations, and AMR (Figure 1). For group 1 antibiotics (sulfisoxazole, trimethoprim/sulfamethoxazole, and tetracycline), the presence of int1 was associated with an AMR prevalence of approximately 90% from bacterial isolates taken from birds, regardless of poultry type. This finding can be explained by physical links to MGEs. All class-1 integrons incorporate a sulfonamide (sul) resistance gene as part of their structure, and often carry dihydrofolate reductase (dfr) genes (conveying resistance to trimethoprim) (33). In addition, tetracycline resistance genes are often found on the same conjugative plasmids as integrons (34, 35).
In contrast to group 1, for group 2 antibiotics, the presence of int1 contributed to some increases of resistance in isolates from poultry, while additional exposure to small-scale production operations resulted in even higher resistance levels. This finding is plausible because, while genes conferring resistance to the group 2 antibiotics have been found on class-1 integrons, some are less common than the sul and dfr genes (33). For group 3 antibiotics, exposures to small-scale production operations or int1 genes alone tended to result in minor increases in resistance. Only when both small-scale production operations and integrons were present did we observe high levels of resistance, suggesting that even antibiotics with overall low AMR levels in a community can achieve higher levels given the presence of both these exposures.
The selection of MGEs due to small-scale production operations might be an evolutionary response to exposure to a large variety of antibiotics. In this study site, various types of antibiotics were detected in feed used in production coops (15). Such broad antibiotic use in this setting might select bacterial isolates in birds carrying MGEs, which in turn might influence AMR in bacterial isolates from humans. This agrees with recent evidence showing that transmission of AMR from animals to humans might be driven more by MGEs than cross-colonization by resistant strains (21). The higher prevalence of tetA and tetB genes in isolates from small-scale production birds could also be explained by antibiotic use, because tetracycline has been previously detected in feed used in small-scale production operations in this area (15).
E.coli isolates from humans associated with small-scale production birds had higher levels of MGEs and phenotypic AMR than those from humans associated with household birds, particularly those linked to MGEs from Figure 1 (sulfisoxazole and trimethoprim/sulfamethoxazole) (Tables 4 and 5). The lack of significant differences for other markers might be due to the small number of samples from humans associated with production birds (n = 45). After adjusting for the presence of MGEs, the relationships between small-scale production operations and phenotypic resistance were attenuated, indicating overlap in the variance in the outcome (AMR) explained by presence of MGEs and these operations. One plausible explanation for this overlap is that small-scale production operations increase the prevalence of isolates containing MGEs, which in turn increases prevalence of AMR isolates.
Isolates from household birds in small-scale production villages had significantly higher odds of resistance than samples from those in nonproduction villages for int1, tetA, sulfisoxazole, trimethoprim/sulfamethoxazole, and MDR (Table 3). In addition, humans associated with household birds in small-scale production villages had elevated odds of tetracycline resistance (Table 5). Together, these results are indirect evidence of environmental spread of AMR at the community level from small-scale production operations, possibly through soil, air, water, or meat.
A small number of studies have studied AMR patterns in chickens and/or humans associated with small-scale production operations in developing countries (14–16). We add a new perspective by sampling E. coli isolates from three groups of birds (small-scale production birds and household birds residing in small-scale production and nonproduction villages) and from corresponding humans from the community rather than clinics. In this region of Ecuador, high levels of AMR in isolates from these groups were observed despite the small-scale nature of the operations, suggesting that high levels of AMR might be found in small-scale production operations elsewhere in the developing world. Given the growing interest in utilizing poultry as a tool for economic development (36–38), the results presented here are important when considering policy implications, especially in nations that have not yet enacted or enforced regulations on nontherapeutic antibiotic use.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, Michigan (Kara A. Moser, Ian Spicknall, Carl F. Marrs, Betsy Foxman, Joseph N. S. Eisenberg); Department of Epidemiology and Biostatistics, Michigan State University, East Lansing, Michigan (Lixin Zhang); Department of Microbiology and Molecular Genetics, Michigan State University, East Lansing, Michigan (Lixin Zhang); Department of Environmental Health, Rollins School of Public Health, Emory University, Atlanta, Georgia (Nikolay P. Braykov, Karen Levy); Instituto de Microbiología, Universidad San Francisco de Quito, Quito, Ecuador (Gabriel Trueba); Centro de Biomedicina, Universidad Central del Ecuador, Quito, Ecuador (William Cevallos); Department of Emergency Medicine, University of Michigan, Ann Arbor, Michigan (Jason Goldstick); Injury Research Center, University of Michigan, Ann Arbor, Michigan (Jason Goldstick); and Department of Anthropology, Trinity College, Hartford, Connecticut (James Trostle).
This work was supported by the National Institute of Allergy and Infectious Diseases (grants R01AI050038 and 1K01AI103544), and the National Science Foundation, Ecology and Evolution of Infectious Diseases program (grant 08119234).
We thank the Ecología, Desarrollo, Salud y Sociedad (EcoDESS) project research team for their invaluable contributions to data collection and lab personnel at the Instituto de Microbiología, Universidad San Francisco de Quito (Quito, Ecuador), and the Department of Epidemiology, University of Michigan School of Public Health (Ann Arbor, Michigan), for their efforts in sample processing.
Conflict of interest: none declared.
Abbreviations
- AMR
antimicrobial resistance
- CI
confidence interval
- int1
class-1 integron
- int2
class-2 integron
- MDR
multiple-drug resistance
- MGE
mobile genetic elements
- OR
odds ratio
- tetA
tetracycline class A
- tetB
tetracycline class B
REFERENCES
- 1. de Kraker ME, Davey PG, Grundmann H, et al. . Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Med. 2011;8(10):e1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wellington EM, Boxall AB, Cross P, et al. . The role of the natural environment in the emergence of antibiotic resistance in gram-negative bacteria. Lancet Infect Dis. 2013;13(2):155–165. [DOI] [PubMed] [Google Scholar]
- 3. Gilchrist MJ, Greko C, Wallinga DB, et al. . The potential role of concentrated animal feeding operations in infectious disease epidemics and antibiotic resistance. Environ Health Perspect. 2007;115(2):313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alcorn T. Antibiotic use in livestock production in the USA. Lancet Infect Dis. 2012;12(4):273–274. [DOI] [PubMed] [Google Scholar]
- 5. Webster P. Poultry, politics, and antibiotic resistance. Lancet. 2009;374(9692):773–774. [DOI] [PubMed] [Google Scholar]
- 6. Leung E, Weil DE, Raviglione M, et al. . The WHO policy package to combat antimicrobial resistance. Bull World Health Organ. 2011;89(5):390–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steinfeld H, Wassenaar T, Jutzi S. Livestock production systems in developing countries: status, drivers, trends. Rev Sci Tech. 2006;25(2):505–516. [DOI] [PubMed] [Google Scholar]
- 8. Luangtongkum T, Morishita TY, Ison AJ, et al. . Effect of conventional and organic production practices on the prevalence and antimicrobial resistance of Campylobacter spp. in poultry. Appl Environ Microbiol. 2006;72(5):3600–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van den Bogaard AE, London N, Driessen C, et al. . Antibiotic resistance of faecal Escherichia coli in poultry, poultry farmers and poultry slaughterers. J Antimicrob Chemother. 2001;47(6):763–771. [DOI] [PubMed] [Google Scholar]
- 10. Levy SB, FitzGerald GB, Macone AB. Changes in intestinal flora of farm personnel after introduction of a tetracycline-supplemented feed on a farm. N Engl J Med. 1976;295(11):583–588. [DOI] [PubMed] [Google Scholar]
- 11. Price LB, Graham JP, Lackey LG, et al. . Elevated risk of carrying gentamicin-resistant Escherichia coli among US poultry workers. Environ Health Perspect. 2007;115(12):1738–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sonaiya EB, Swan SEJ. Small-Scale Poultry Production: Technical Guide Rome, Italy: Food and Agriculture Organization of the United Nations (FEO); 2004.
- 13. Sonaiya F. Smallholder family poultry as a tool to initiate rural development. Presented at the International Conference Poultry in the Twenty-first Century: avian influenza and beyond, Bangkok, Thailand, November 5–7, 2007.
- 14. Kariuki S, Gilks CF, Kimari J, et al. . Plasmid diversity of multi-drug-resistant Escherichia coli isolated from children with diarrhoea in a poultry-farming area in Kenya. Ann Trop Med Parasitol. 1997;91(1):87–94. [DOI] [PubMed] [Google Scholar]
- 15. Braykov NP, Eisenberg JN, Grossman M, et al. . Antibiotic resistance in animal and environmental samples associated with small-scale poultry farming in northwestern Ecuador. mSphere. 2016;1(1):e00021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bernadether TR, Douglas RC, Gaspary OM, et al. . Comparison of the prevalence of antibiotic-resistant Escherichia coli isolates from commercial-layer and free-range chickens in Arusha district, Tanzania. Afr J Microbiol Res. 2016;10(34):1422–1429. [Google Scholar]
- 17. Mendez B, Tachibana C, Levy SB. Heterogeneity of tetracycline resistance determinants. Plasmid. 1980;3(2):99–108. [DOI] [PubMed] [Google Scholar]
- 18. Mazel D. Integrons: agents of bacterial evolution. Nat Rev Microbiol. 2006;4(8):608–620. [DOI] [PubMed] [Google Scholar]
- 19. Nandi S, Maurer JJ, Hofacre C, et al. . Gram-positive bacteria are a major reservoir of Class 1 antibiotic resistance integrons in poultry litter. Proc Natl Acad Sci USA. 2004;101(18):7118–7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Randall LP, Cooles SW, Osborn MK, et al. . Antibiotic resistance genes, integrons and multiple antibiotic resistance in thirty-five serotypes of Salmonella enterica isolated from humans and animals in the UK. J Antimicrob Chemother. 2004;53(2):208–216. [DOI] [PubMed] [Google Scholar]
- 21. de Been M, Lanza VF, de Toro M, et al. . Dissemination of cephalosporin resistance genes between Escherichia coli strains from farm animals and humans by specific plasmid lineages. PLoS Genet. 2014;10(12):e1004776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eisenberg JN, Cevallos W, Ponce K, et al. . Environmental change and infectious disease: how new roads affect the transmission of diarrheal pathogens in rural Ecuador. Proc Natl Acad Sci USA. 2006;103(51):19460–19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eisenberg JN, Goldstick J, Cevallos W, et al. . In-roads to the spread of antibiotic resistance: regional patterns of microbial transmission in northern coastal Ecuador. J R Soc Interface. 2012;9(70):1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers Geneva, Switzerland: World Health Organization (WHO); 2005.
- 25. Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. Vol. 31. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 26. Zhang L, Srinivasan U, Marrs CF, et al. . Library on a slide for bacterial comparative genomics. BMC Microbiol. 2004;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kong Y, Cave MD, Zhang L, et al. . Population-based study of deletions in five different genomic regions of Mycobacterium tuberculosis and possible clinical relevance of the deletions. J Clin Microbiol. 2006;44(11):3940–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sandstedt SA, Zhang L, Patel M, et al. . Comparison of laboratory-based and phylogenetic methods to distinguish between Haemophilus influenzae and H. haemolyticus. J Microbiol Methods. 2008;75(2):369–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang L, Xie J, Patel M, et al. . Nontypeable Haemophilus influenzae genetic islands associated with chronic pulmonary infection. PLoS One. 2012;7(9):e44730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu YG, Johnson TA, Su JQ, et al. . Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc Natl Acad Sci USA. 2013;110(9):3435–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dawes FE, Kuzevski A, Bettelheim KA, et al. . Distribution of class 1 integrons with IS26-mediated deletions in their 3′-conserved segments in Escherichia coli of human and animal origin. PLoS One. 2010;5(9):e12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Partridge SR, Tsafnat G, Coiera E, et al. . Gene cassettes and cassette arrays in mobile resistance integrons. FEMS Microbiol Rev. 2009;33(4):757–784. [DOI] [PubMed] [Google Scholar]
- 34. Schmidt AS, Bruun MS, Dalsgaard I, et al. . Incidence, distribution, and spread of tetracycline resistance determinants and integron-associated antibiotic resistance genes among motile aeromonads from a fish farming environment. Appl Environ Microbiol. 2001;67(12):5675–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sunde M, Norström M. The prevalence of, associations between and conjugal transfer of antibiotic resistance genes in Escherichia coli isolated from Norwegian meat and meat products. J Antimicrob Chemother. 2006;58(4):741–747. [DOI] [PubMed] [Google Scholar]
- 36. Scanes CG. Contribution of poultry to quality of life and economic development in the developing world. Poult Sci. 2007;86(11):2289–2290. [DOI] [PubMed] [Google Scholar]
- 37. Mcleod A, Thieme O, Mack SD. Structural changes in the poultry sector: will there be smallholder poultry development in 2030? Worlds Poult Sci J. 2009;65(2):191–200. [Google Scholar]
- 38. Gates B. Why I Would Raise Chickens. https://www.gatesnotes.com/Development/Why-I-Would-Raise-Chickens. Published June 7, 2016. Accessed October 4, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.