Abstract
Although alcohol exposure results in reduced mortality after traumatic brain injury (TBI) in animal models, clinical trials based on proposed mechanisms have been disappointing and have reported conflicting results. Methodological issues common to many of these clinical studies may have contributed to the spurious results. Our objective was to evaluate the association between blood alcohol concentration (BAC) and in-hospital mortality after TBI, and overcome methodological problems of prior studies. We conducted a retrospective cohort study on individuals treated for isolated TBI (n = 1,084) at the R Adams Cowley Shock Trauma Center (Baltimore, Maryland) from 1997 to 2012. We excluded individuals with injury to other body regions and examined multiple cutpoints of BAC. Our primary outcome was in-hospital mortality. In adjusted logistic regression models, the upper level of each blood alcohol categorization from 0.10 g/dL (odds ratio = 0.63, 95% confidence interval: 0.40, 0.97) through 0.30 g/dL (odds ratio = 0.25, 95% confidence interval: 0.08, 0.84) was associated with reduced risk of mortality after TBI compared with individuals with undetectable BAC. In sensitivity analyses among individuals without penetrating brain injuries (95% firearm-related) (n = 899), the protective association was eliminated. This study provides evidence that the observed protective association between BAC and in-hospital mortality after TBI resulted from bias introduced by inclusion of penetrating injuries.
Keywords: alcohol, mortality, traumatic brain injury
Alcohol consumption is a major risk factor for traumatic brain injury (TBI) (1). An estimated 38%–57% of individuals with TBI have a positive screen for blood alcohol at the time of injury, whereas 16%–66% have a history of alcohol abuse or heavy drinking (2–6). Although alcohol clearly increases the risk of injury, some studies suggest that alcohol may actually provide protective effects after injury. For example, animal studies demonstrated that low to moderate doses of alcohol had neuroprotective effects after TBI (7–9). However, evidence from clinical studies is controversial, as some studies report that alcohol reduces risk of in-hospital mortality after TBI, whereas others show no effect (3, 10–20).
The hypothesized neuroprotective effect of alcohol may be mediated through inhibition of the N-methyl-D-aspartic acid receptors (11). N-methyl-D-aspartic acid receptor activation after TBI results in increased levels of extracellular glutamate and aspartate, which initiates the biochemical chain reaction that ultimately results in neuronal death at the site of injury (11, 21, 22). Nonetheless, clinical trials of N-methyl-D-aspartic acid receptor antagonists conducted among individuals with TBI have not been efficacious in reducing either the progression of injury nor the occurrence of poor outcomes (23–25).
A serious methodological problem common to many published clinical studies is that blood alcohol concentration (BAC) was not assessed in all cases, and often, only 50 percent of the time (3, 12, 13, 15–20, 26, 27). Blood alcohol assessment is not random, and is related to individual or injury-related characteristics such as age (19, 27). Kraus et al. (27) reported that not only did blood alcohol assessment vary by sex and age, it also varied by mechanism of injury and severity of TBI, with more severely injured individuals being more likely to be tested but less likely to have a positive test. Furthermore, high BAC can depress the Glasgow Coma Scale score (28, 29). Intoxicated individuals may initially seem more severely injured than they actually are, which results in an admission bias that appears to be the protective effect of alcohol.
Another methodological complication common to previous clinical studies has been the difficulty of defining similar injuries for comparison by severity, especially when including individuals with injury to other body regions. Even studies that focused specifically on isolated TBI included individuals with relatively severe injury to other body regions (10, 12–16, 18–20). If injury to other body regions differed in severity or location by BAC, residual confounding of the effect of alcohol could have been present.
In contrast, the R Adams Cowley Shock Trauma Center (STC) in Baltimore, Maryland, routinely collects BAC on all admissions regardless of perceived level of severity or injury mechanism. Using data from the STC trauma registry, the objective of the present study was to overcome limitations of prior studies in evaluating the association between BAC and in-hospital mortality among individuals treated for TBI. To avoid possible confounding by other injury location or severity, we excluded individuals with injury to any other body region and focused the target population on isolated cases of TBI.
METHODS
Data source
The STC is the state-designated Primary Adult Resource Center for trauma care that treats more than 8,000 patients annually, which represents 33% of trauma cases in the state of Maryland. Although the STC is one of the busiest civilian trauma programs in the United States, 30% of cases treated there are only minor injuries, often with stays of less than 24 hours. We conducted a retrospective cohort study using data from the STC trauma registry which included demographic, clinical, injury, and procedure variables.
Study population
The present study included all STC admissions during 1997–2012 with a diagnosis of TBI (International Classification of Disease version 9, Clinical Modification codes 800, 801, 803, 804, 850–854.1, 950.1–950.3, 959.01), as per The Center for Disease Control and Prevention recommended definition (30, 31). These codes have been reported to be 89% sensitive to the detection of severe TBI and had a positive predictive value of 93% (32). In addition, we required an Abbreviated Injury Scale (AIS) head score of 3–6 to confirm TBI diagnosis, and excluded individuals with an AIS score greater than 0 for any other body region (33). The AIS scores are maintained in a trauma registry with internal validation by dedicated trauma coders. We also excluded individuals who were not transported directly to the STC from the scene of the injury because their BACs did not approximate those at the time of injury. The primary outcome was all-cause in-hospital mortality.
Exposure
BAC assays are routinely performed on all admissions to the STC. As a continuous variable, BAC, measured in grams per deciliter, is highly skewed with many zero (negative test) values. This prompts examination of different dichotomous and categorical cutpoints.
TBI Severity
The AIS head score includes injuries to the scalp, skull, brain, intracranial vessels, and cranial nerves. It is coded at hospital discharge using evidence from physical examinations, as well as computed tomography and magnetic resonance imaging scans obtained by a trained AIS coder, which provide an accurate description of the TBI. Anatomic TBI severity was determined using the highest AIS head score. To focus specifically on TBI, we excluded individuals with AIS head scores of 1 or 2, which primarily code injuries to the scalp, cranial nerves, and sometimes the skull. The Glasgow Coma Scale measures neurologic deficit in eye opening, as well as verbal and motor response (34). It is often used as an initial measure of TBI severity and was measured on admission to the STC.
Covariates
International Classification of Disease, Ninth Revision, Clinical Modification E codes were used to define the mechanism of injury. We created variables that indicated motor vehicle transport collisions, falls, assaults, and “other injuries or accidents.” We also included an indicator for injury type: blunt, penetrating, or other.
Comorbid conditions were gathered from STC admission assessment data (self or proxy report) and coded using codes from the International Classification of Disease, Ninth Revision, Clinical Modification. From these, we created the following indicator variables: alcohol dependence, Alzheimer’s disease and related dementias, cardiac arrhythmia, chronic obstructive pulmonary disease, diabetes, depression, heart failure, hypertension, ischemic heart disease, neurologic disorders (including Parkinson’s disease and epilepsy), and stroke.
Data analysis
We assessed the distributions of all variables and made comparisons between individuals with undetectable BAC (considered to be BAC-negative, or BAC−) and those with a positive test (considered to be BAC-positive, or BAC+) using χ2 or Student’s t tests as appropriate. Similarly, we tested associations between covariates and in-house mortality, and assessed the unadjusted associations between different BAC categorizations and mortality. We also assessed this association while leaving BAC as a continuous variable. Variables not in the causal pathway that were associated with both BAC and mortality were considered for inclusion in our final logistic regression model. Although used in many prior studies as covariates, variables that come after the alcohol exposure such as injury type, severity measures, and clinical variables at STC admission may be in the causal pathway and are therefore not potential confounders (35, 36). We modelled the log-odds of in-hospital mortality as a function of BAC categories and selected predictors. In all cases, the reference group had undetectale BAC. We assessed effect modification by age and sex by creating interaction terms in the logistic model. A P value of less than 0.05 for an interaction term was considered evidence of effect modification. Our final adjusted model contained an indicator variable for the BAC categorization, age, sex, race, and history of alcohol dependence and abuse.
In addition to testing multiple cutpoints for BAC, we conducted sensitivity analyses. A small number of individuals (n = 18), consisting of 2% of the population, were missing the BAC assessment. To determine whether exclusion of these individuals biased our results, we assigned them to each BAC category in turn. During data exploration, we observed that individuals with penetrating injuries (95% of which were due to firearm injuries) were more likely to be BAC−, yet were also more likely to have high AIS scores and in-hospital mortality. To determine whether this may have introduced selection bias, we restratified the analyses according to penetrating injury type. Finally, we reconducted an analyses which included individuals with injury to other body parts. These individuals were excluded from our primary analyses because of concern that severity of injury to other body parts might bias the association between BAC and in-hospital mortality. We stratified analyses by penetrating injury as well as by severity of injury to other body parts.
The present study was approved by the Institutional Review Board of the University of Maryland, Baltimore. Data analysis was performed using SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina) and a P value of <0.05 was considered statistically significant.
RESULTS
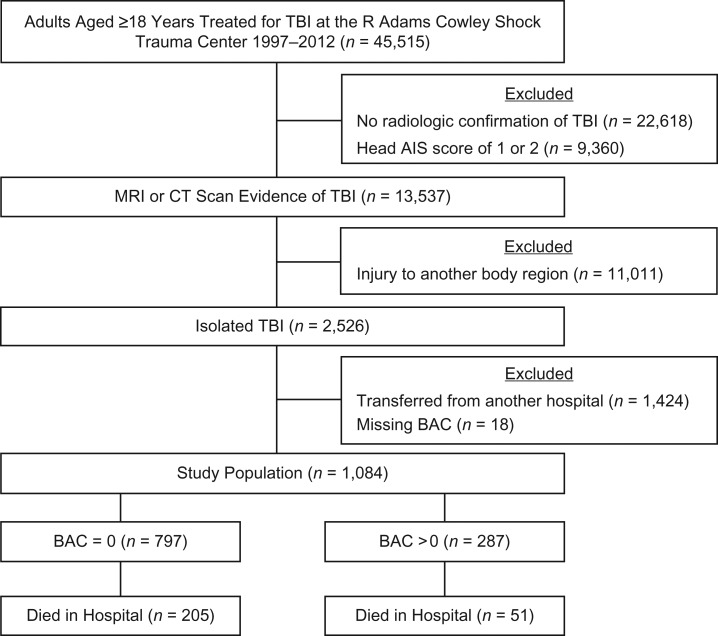
There were 45,515 individuals aged 18 years and older who were treated for TBI at the STC from 1997 to 2012 (Figure 1). Of these, 22,618 (50%) lacked evidence of head injury and 9,360 (21%) had an AIS head score of 1 or 2, which left 13,537 (30%) with confirmed TBI. Out of these, 11,011 (81%) had sustained injury to other body regions and 1,424 (11%) were transferred from another hospital. After excluding the 18 (2%) individuals with missing BAC evaluations, 1,084 (8% of 13,537) adults with isolated TBI remained and formed our study sample. The majority (74%) of the study sample were BAC− (n = 797).
Figure 1.
Flow chart showing inclusion criteria for adult admissions with isolated traumatic brain injury, R Adams Cowley Shock Trauma Center, Baltimore, Maryland, 1997–2012. AIS, Abbreviated Injury Scale; BAC, blood alcohol concentration; CT, computed tomography; MRI, magnetic resonance imaging; TBI, traumatic brain injury.
Individuals who were BAC+ differed from BAC− individuals in demographic, clinical, and injury characteristics. They were younger, with a mean of 43.5 (standard deviation, 17.5) years versus 51.5 (standard deviation, 22.6) years (P < 0.001), and a greater proportion were male (83% vs. 68%, P < 0.001) (Table 1). A greater proportion of BAC+ individuals had alcohol abuse or dependence disorder (29% vs. 3%, P < 0.001) but otherwise had fewer comorbidities. In terms of injury characteristics, there were no differences between BAC+ and BAC− individuals except the mechanism of injury (Table 2). BAC+ individuals were more likely to have been injured in an assault (28% vs. 19%, P = 0.02). Distribution of AIS scores did not differ between those who died in hospital (discharged dead) and those who lived when stratified by BAC (Table 3).
Table 1.
Demographic and Clinical Characteristics of Adults Admitted With Isolated Traumatic Brain Injury by Alcohol Intoxication (n = 1,084), R Adams Cowley Shock Trauma Center, Baltimore, Maryland, 1997–2012
| Characteristic | BAC = 0 (n = 797) | BAC >0 (n = 287) | P Valuea | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Ageb, years | 51.5 (22.6) | 43.5 (17.5) | <0.001 | ||
| Age group, years | <0.001 | ||||
| <30 | 182 | 23 | 85 | 30 | |
| 30–64 | 346 | 43 | 165 | 57 | |
| ≥65 | 269 | 34 | 37 | 13 | |
| Sex | <0.001 | ||||
| Female | 252 | 32 | 49 | 17 | |
| Male | 545 | 68 | 238 | 83 | |
| Race | 0.39 | ||||
| White | 506 | 63 | 169 | 59 | |
| Black | 239 | 30 | 97 | 34 | |
| Other | 52 | 7 | 21 | 7 | |
| Admission years | 0.38 | ||||
| 1997–2002 | 252 | 32 | 84 | 29 | |
| 2003–2007 | 248 | 31 | 83 | 29 | |
| 2008–2012 | 296 | 37 | 120 | 42 | |
| Comorbid conditions | |||||
| Alcohol dependence | 24 | 3 | 84 | 29 | <0.001 |
| Diabetes | 75 | 9 | 8 | 3 | <0.001 |
| Depression | 35 | 4 | 15 | 5 | 0.56 |
| Hypertension | 201 | 25 | 46 | 16 | 0.002 |
| Ischemic heart disease | 20 | 3 | 3 | 1 | 0.14 |
| Neurologic disorders | 34 | 4 | 11 | 4 | 0.75 |
| Stroke | 23 | 3 | 2 | 1 | 0.03 |
| Systolic blood pressure at admission, mm Hgb | 149.2 (49.3) | 146.9 (33.0) | 0.38 | ||
| Length of stay, days | 0.03 | ||||
| <1 (early death) | 117 | 15 | 23 | 8 | |
| <2 | 263 | 33 | 108 | 38 | |
| 2–13 | 327 | 41 | 119 | 41 | |
| ≥14 | 90 | 11 | 37 | 13 | |
| Expired at discharge | 205 | 26 | 51 | 18 | 0.007 |
| Time to death, daysc,d | 0.6 (2.4) | 1.5 (4.2) | 0.09 | ||
Abbreviation: BAC, blood alcohol concentration.
aP value from χ2 goodness of fit, Student’s t test, or Wilcoxon rank sum.
b Values are expressed as mean (standard deviation).
c Values are expressed as median (interquartile range).
d In-hospital deaths.
Table 2.
Injury Characteristics of Adults Admitted With Isolated Traumatic Brain Injury by Alcohol Intoxication (n = 1,084), R Adams Cowley Shock Trauma Center, Baltimore, Maryland, 1997–2012
| Characteristic | BAC = 0 (n = 797) | BAC >0 (n = 287) | P Valuea | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Admission Glasgow Coma Scale score | 0.26 | ||||
| 14–15 | 416 | 52 | 135 | 47 | |
| 9–13 | 129 | 16 | 47 | 16 | |
| 3–8 | 252 | 32 | 105 | 37 | |
| AIS head score | 0.44 | ||||
| 3 | 186 | 23 | 76 | 26 | |
| 4 | 342 | 43 | 124 | 43 | |
| ≥5 | 269 | 34 | 87 | 30 | |
| Injury type | 0.54 | ||||
| Blunt | 643 | 81 | 240 | 84 | |
| Penetrating | 142 | 18 | 43 | 15 | |
| Other | 12 | 2 | 4 | 1 | |
| Cause of injury | 0.02 | ||||
| Motor vehicle collision | 134 | 17 | 42 | 15 | |
| Falls | 373 | 47 | 126 | 44 | |
| Assault | 155 | 19 | 80 | 28 | |
| Other causes | 134 | 17 | 42 | 15 | |
Abbreviations: AIS, Abbreviated Injury Scale; BAC, blood alcohol concentration.
aP value from χ2 goodness of fit or Student’s t test.
Table 3.
Stratified Association Between Positive Blood Alcohol Concentration and Mortality After Isolated Traumatic Brain Injury, Controlling for Abbreviated Injury Scale Score, Among Admitted Adults (n = 1,084), R Adams Cowley Shock Trauma Center, Baltimore, Maryland, 1997–2012
| AIS Score | Survived (n = 828) | Died (n = 256) | ||||||
|---|---|---|---|---|---|---|---|---|
| BAC− | BAC+ | BAC− | BAC+ | |||||
| No. | % | No. | % | No. | % | No. | % | |
| 3 | 178 | 30 | 75 | 32 | 8 | 4 | 1 | 2 |
| 4 | 307 | 52 | 116 | 49 | 35 | 17 | 8 | 16 |
| 5 or 6 | 107 | 18 | 45 | 19 | 162 | 79 | 42 | 82 |
Abbreviations: AIS, Abbreviated Injury Scale; BAC–, undetectable blood alcohol concentration; BAC+, positive blood alcohol concentration.
The distribution of BAC was highly skewed because of the large number of undetectable assessments. When individuals who were BAC− were removed, the distribution approached normality with a mean BAC of 0.19 (standard deviation, 0.10) g/dL. Distributions of BAC categories are reported in Table 4.
Table 4.
Adjusted and Unadjusted Odds Ratios for Mortality After Isolated Traumtic Brain Injury Associated With Blood Alcohol Concentration Among Admitted Adults (n = 1,084), R Adams Cowley Shock Trauma Center, Baltimore, Maryland, 1997–2012
| BAC Category, g/dL | No. | % | Unadjusted | Adjusteda | ||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| Negative | 797 | 74 | 1.00 | Referent | 1.00 | Referent |
| >0 | 287 | 26 | 0.62 | 0.44, 0.88 | 0.80 | 0.55, 1.16 |
| <0.05 | 29 | 3 | 1.77 | 0.82, 3.80 | 1.73 | 0.79, 3.81 |
| ≥0.05 | 252 | 4 | 0.53 | 0.37, 0.77 | 0.69 | 0.46, 1.03 |
| <0.10 | 64 | 6 | 1.22 | 0.70, 2.13 | 1.40 | 0.78, 2.50 |
| ≥0.10 | 223 | 21 | 0.48 | 0.24, 0.83 | 0.63 | 0.40, 0.97 |
| <0.15 | 109 | 10 | 1.00 | 0.63, 1.58 | 1.27 | 0.78, 2.06 |
| ≥0.15 | 178 | 16 | 0.43 | 0.27, 0.69 | 0.54 | 0.33, 0.89 |
| <0.20 | 152 | 14 | 1.03 | 0.70, 1.53 | 1.32 | 0.86, 2.02 |
| ≥0.20 | 135 | 12 | 0.26 | 0.14, 0.48 | 0.32 | 0.17, 0.62 |
| <0.25 | 205 | 19 | 0.81 | 0.56, 1.17 | 1.06 | 0.71, 1.57 |
| ≥0.25 | 82 | 8 | 0.23 | 0.10, 0.53 | 0.27 | 0.11, 0.65 |
| <0.30 | 244 | 23 | 0.71 | 0.50, 1.01 | 0.92 | 0.63, 1.35 |
| ≥0.30 | 43 | 4 | 0.22 | 0.07, 0.71 | 0.25 | 0.08, 0.84 |
Abbreviations: BAC, blood alcohol concentration; CI, confidence interval; OR, odds ratio.
a All models were adjusted for age, sex, race, and alcohol dependence disorder.
In our sample, 95% of penetrating injuries were caused by firearms. The remaining 5% were caused by cutting or piercing instruments. Individuals with penetrating injuries (n = 185) differed from those with nonpenetrating injuries (n = 899). They were more likely to have AIS head scores of 5 or 6, which indicated the highest TBI severity level (79% vs 23%, P < 0.001) and were more likely to die at discharge (66% vs. 15%, P < 0.001). They were more likely to be BAC− (77% vs. 73%) and less likely to have BAC >0.1 g/dL (16% vs. 22%, P = 0.007 across all alcohol groups). Suicides, of which 78% were successful, accounted for 35% of penetrating injuries and were more likely to be associated with BAC+ (28% vs 21%, P = 0.3) compared with other penetrating injuries; however, this association was not statistically significant.
In unadjusted models, BAC greater than 0 was protective against mortality (odds ratio (OR) = 0.62, 95% confidence interval (CI): 0.44, 0.88) (Table 4). In the adjusted regression models, the higher level of each BAC categorization from 0.10 g/dL (OR = 0.63, 95% CI: 0.40, 0.97) through 0.30 g/dL (OR = 0.25, 95% CI: 0.08, 0.84) was associated with reduced risk of mortality after TBI compared with BAC− individuals (Table 4). BAC of 0.05 g/dL or greater was associated with decreased risk of mortality (OR = 0.69, 95% CI: 0.46, 1.03), but this association was not statistically significant. In contrast, the lower level of each BAC categorization was not significantly associated with risk of mortality after isolated TBI compared with BAC− individuals regardless of cutpoint. When we examined BAC as a dichotomous variable in our adjusted regression models, positive blood alcohol was associated with reduced risk of in-hospital mortality after isolated TBI (OR = 0.80, 95% CI: 0.55, 1.16) compared with negative blood alcohol, but this association was not statistically significant.
In sensitivity analyses, although assigning individuals with missing BAC to different BAC categories did not change results, stratifying by penetrating injury significantly impacted results (Table 5). Among individuals with nonpenetrating injury, the higher level of each BAC categorization was no longer associated with reduced mortality, whereas the lower level of each BAC categorization from 0.10 g/dL (OR = 2.67, 95% CI: 1.25, 5.73) to 0.25 g/dL (OR = 1.83, 95% CI: 1.06, 3.16) was associated with increased risk of in-hospital mortality. Among individuals with penetrating injuries, positive blood alcohol was associated with a decreased risk of mortality. We could not estimate the associations between BAC levels above 0.2 g/dL and mortality among individuals with penetrating injuries because of questionable model fit.
Table 5.
Results of Sensitivity Analyses on Mortality After Isolated Traumatic Brain Injury Associated With Blood Alcohol Concentration, Stratified by Penetrating Injury, Among Adult Admissions (n = 1,084), R Adams Cowley Shock Trauma Center, Baltimore, Maryland, 1997–2012
| BAC Category, g/dL | NonPenetrating Injuries (n = 899) | Penetrating Injuries (n = 185) | ||
|---|---|---|---|---|
| Adjusteda OR | 95% CI | Adjusteda OR | 95% CI | |
| Negative | 1.00 | Referent | 1.00 | Referent |
| >0 | 1.46 | 0.88, 2.41 | 0.30 | 0.14, 0.65 |
| <0.05 | 2.55 | 0.93, 7.04 | 1.14 | 0.21, 6.35 |
| ≥0.05 | 1.30 | 0.76, 2.24 | 0.22 | 0.10, 0.52 |
| <0.1 | 2.67 | 1.25, 5.73 | 0.47 | 0.15, 1.53 |
| ≥0.1 | 1.13 | 0.63, 2.03 | 0.24 | 0.10, 0.59 |
| <0.15 | 2.42 | 1.26, 4.64 | 0.32 | 0.13, 0.80 |
| ≥0.15 | 0.99 | 0.52, 1.89 | 0.28 | 0.10, 0.83 |
| <0.2 | 2.25 | 1.25, 4.02 | 0.39 | 0.17, 0.90 |
| ≥0.2 | 0.78 | 0.36, 1.67 | 0.10 | 0.02, 0.52 |
| <0.25 | 1.83 | 1.06, 3.16 | N/Ab | N/Ab |
| ≥0.25 | 0.79 | 0.32, 1.96 | N/Ab | N/Ab |
| <0.3 | 1.60 | 0.95, 2.69 | N/Ab | N/Ab |
| ≥0.3 | 0.80 | 0.23, 2.78 | N/Ab | N/Ab |
Abbreviations: BAC, blood alcohol concentration; CI, confidence interval; N/A, not applicable; OR, odds ratio.
a All models were adjusted for age, sex, race, and alcohol dependence disorder.
b Questionable model fit.
Our final sensitivity analysis examined the impact of excluding individuals with injury to other body parts. Similar to our primary analysis, we observed a protective association between positive blood alcohol and in-hospital mortality at almost all BAC categorization levels (Web Table 1, available at https://academic.oup.com/aje). Stratifying by penetrating injury, the observed protective association was attenuated, but still statistically significant in the upper, but not lower, level of each BAC categorization until BAC was 0.15 g/dL, at which point it switched and was present in the lower but not upper level of those with nonpenetrating injury (Web Table 2). A similar pattern was observed among those with penetrating injury (Web Table 2). Stratifying by severity of injury to other body parts and penetrating injury eliminated the observed protective association among individuals with nonpenetrating injury in all except those with the highest level of severity to other body regions (AIS score 4, 5, and 6) (Web Tables 3–5). Conversely, the stratification eliminated the observed protective association between positive blood alcohol and in-hospital mortality among individuals with penetrating injury except among those with the lowest (AIS score 0, 1, and 2) levels of injury to other body parts (Web Tables 3–5).
DISCUSSION
For this large study of individuals treated for isolated TBI at a major urban trauma center, in which 98% of cases had a BAC assessment, inclusion of individuals with penetrating brain injuries produced a spurious protective association between BAC and risk of in-hospital mortality. This means that all but 2% of individuals underwent BAC assessment, unlike many prior studies. We also excluded transfers from other hospitals, and focused specifically on isolated cases of TBI by excluding individuals with injury to other body regions (3, 10, 15, 16, 18–20). Even with these improvements, the effect size we observed in our main analysis for the protective association between blood alcohol and mortality after TBI was remarkably consistent with other studies. This suggests that the bias we observed in the present study may have been present in other studies as well (3, 12, 13, 15, 16, 19, 26). In sensitivity analysis including individuals with injury to other body regions, we observed significant variation in the observed protective association between positive blood alcohol and in-hospital mortality, which supported our contention that including these individuals could introduce bias. The presence of bias and inclusion of variables that may be in the causal pathway in regression models are major problems common in prior studies. Because alcohol consumption may both predispose individuals to injury, and influence the severity of injury, traditional adjustment for this factor likely introduced an over-adjustment bias in multivariable logistic regression (35).
In contrast with results from our sensitivity analyses, Tien et al. (10) reported that the highest BAC categorization (≥0.23 g/dL) was associated with increased risk of death (OR = 1.73, 95% CI: 1.05, 2.84), whereas the lower BAC categorization (<0.23 g/dL) was associated with a protective effect (OR = 0.76, 95% CI: 0.52, 0.98) among individuals with severe TBI. Unlike the present study, participants in the Tien et al. study (10) also had severe injury to other body regions as evidenced by an average Injury Severity Scale score of 37 in the alcohol negative group. This suggests that injury to other body regions may modify the effect of alcohol on mortality. We excluded individuals with injuries to any other part of the body and did not examine this potential interaction in order to avoid confounding, with the goal of providing a more clear relationship between alcohol, TBI, and death.
Some prior studies did not find a protective association between alcohol and mortality. Jurkovich et al. (17) compared individuals with BAC 0.2 g/dL or less to those with BAC greater than 0.2 g/dL among all trauma cases at a Level 1 trauma center. However, grouping BAC− individuals with those with BAC 0.2 g/dL and lower may have obscured any observed association from blood alcohol. Furthermore, our study focused specifically on isolated cases of TBI (a group for whom there is significant pre-clinical evidence of the protective effect of alcohol) rather than a heterogeneous group of trauma cases (7–9). Chen et al. (18) matched individuals with positive BAC and nondetectable BAC on age, sex, and race, among a cohort of individuals with TBI. In that study, BAC was only assessed in 26% of the individuals with TBI. Because BAC testing is associated with injury severity and mechanism, this may have biased results (18, 27). Shandro et al. (20) resolved the problem of missing BAC values in a large multicenter study using multiple imputation based on demographic, clinical, and injury-related characteristics and reported no significant association between BAC and in-hospital mortality.
We observed a protective association between positive blood alcohol and mortality among individuals with penetrating injuries, but possible explanations are lacking. A recently conducted systematic review of the literature on alcohol and firearm violence (37) suggested that blood alcohol is associated with suicide and self-inflicted injury but not with assault or homicide by firearm. We observed no significant difference in BAC levels between suicides and other penetrating injuries, although this may have been due to small sample size. A study (38) that focused specifically on intentional TBI reported that a history of substance or alcohol abuse was more likely among individuals with self-inflicted injury (12%) and other-inflicted injury (10%), compared with nonintentional injury (7%) (P < 0.001), although it did not assess BAC at injury.
Injury type is technically not a confounder because it occurs after alcohol consumption; however, individuals with penetrating injury (95% gunshot wounds) were more likely to both have undetectable BAC and to die, which introduced bias (39). Upon excluding individuals with penetrating injury, we observed that the lower level of each BAC categorization was associated with increased risk of in-hospital mortality. The lack of an effect at the higher BAC categorization may be due to selection bias operating at the level of transport to the trauma center. Individuals who are intoxicated may be more likely to be transported to a trauma center due to lower Glasgow Coma Scale scores, which could have been due to the alcohol instead of a TBI (17, 28, 29). However, these individuals may be less severely injured and less likely to die, which masked the risk of mortality associated with higher BAC.
Limitations of the present study include the possibility of additional bias or residual confounding. Data in the STC trauma registry are highly accurate for injury-related variables; however, they may be less accurate in documentation of comorbidities that is sometimes obtained from proxy reports. Our study was conducted at a single trauma center with a unique patient population. Although the STC treats 33% of trauma cases in the state of Maryland and is one of the busiest civilian trauma programs in the United States, it is possible that results from the present study may not generalize to other locations. However, the characteristics of STC patients are comparable to patients treated in other trauma centers throughout the United States (40).
Nonetheless, strengths of the present study include a high BAC testing rate that minimizes assessment bias present in other studies. We focused exclusively on isolated cases of TBI, which eliminated the confounding effect of injury to other body regions. We excluded individuals who were transferred from other hospitals to ensure that the assessment approximated the BAC at the time of injury. The study was conducted at a single trauma center, which reduced variability in treatment of TBI. In addition to examining several BAC cutpoints, we conducted sensitivity analyses to investigate different assumptions of our models, which did not demonstrate significant changes to our primary findings.
We observed a protective association between blood alcohol and in-hospital mortality in our main analysis of 1,084 adults who were treated at an urban trauma center for isolated TBI; however, further investigation suggested that this association was spurious, produced by the inclusion of individuals with penetrating brain injury. This spurious association may explain the failure of N-methyl-D-aspartic acid receptor antagonists to reduce progression of TBI and suggests that overadjustment bias may have been present in other clinical studies of TBI (3, 10, 15, 16, 19, 23–25). Furthermore, our study highlights the importance of detailed bivariate exploratory analysis in addition to a comprehensive approach to building regression models.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology and Public Health, School of Medicine, University of Maryland, Baltimore, Maryland (Jennifer S. Albrecht, Gordon S. Smith); Department of Public Health Sciences, Loyola University, Chicago, Illinois (Majid Afshar); Department of Surgery, Division of Surgical Critical Care, R Adams Cowley Shock Trauma Center, University of Maryland Medical Center, Baltimore, Maryland (Deborah M. Stein); and Shock, Trauma and Anesthesiology Research (STAR) Organized Research Center, National Study Center for Trauma and Emergency Medical Services, University of Maryland, Baltimore, Maryland (Gordon S. Smith).
This work was supported by the National Institutes of Health (grants K12HD43489 (to J.S.A.), K23AA024503 (to M.A.), and R01AA18707 (to G.S.S.)) and the Agency for Healthcare Research and Quality (grant 1K01HS024560 (to J.S.A.)).
Conflict of interest: none declared.
Abbreviations
- AIS
Abbreviated Injury Scale
- BAC
blood alcohol concentration
- BAC–
undetectable blood alcohol concentration
- BAC+
positive blood alcohol concentration
- CI
confidence interval
- OR
odds ratio
- STC
R Adams Cowley Shock Trauma Center
- TBI
traumatic brain injury
REFERENCES
- 1. Rapoport MJ. Depression following traumatic brain injury: epidemiology, risk factors and management. CNS Drugs. 2012;26(2):111–121. [DOI] [PubMed] [Google Scholar]
- 2. Corrigan JD. Substance abuse as a mediating factor in outcome from traumatic brain injury. Arch Phys Med Rehabil. 1995;76(4):302–309. [DOI] [PubMed] [Google Scholar]
- 3. Salim A, Ley EJ, Cryer HG, et al. Positive serum ethanol level and mortality in moderate to severe traumatic brain injury. Arch Surg. 2009;144(9):865–871. [DOI] [PubMed] [Google Scholar]
- 4. Kolakowsky-Hayner SA, Gourley EV 3rd, Kreutzer JS, et al. Pre-injury substance abuse among persons with brain injury and persons with spinal cord injury. Brain Inj. 1999;13(8):571–581. [DOI] [PubMed] [Google Scholar]
- 5. Lange RT, Iverson GL, Franzen MD. Short-term neuropsychological outcome following uncomplicated mild TBI: effects of day-of-injury intoxication and pre-injury alcohol abuse. Neuropsychology. 2007;21(5):590–598. [DOI] [PubMed] [Google Scholar]
- 6. Bombardier CH, Rimmele CT, Zintel H. The magnitude and correlates of alcohol and drug use before traumatic brain injury. Arch Phys Med Rehabil. 2002;83(12):1765–1773. [DOI] [PubMed] [Google Scholar]
- 7. Kelly DF, Kozlowski DA, Haddad E, et al. Ethanol reduces metabolic uncoupling following experimental head injury. J Neurotrauma. 2000;17(4):261–272. [DOI] [PubMed] [Google Scholar]
- 8. Gottesfeld Z, Moore AN, Dash PK. Acute ethanol intake attenuates inflammatory cytokines after brain injury in rats: a possible role for corticosterone. J Neurotrauma. 2002;19(3):317–326. [DOI] [PubMed] [Google Scholar]
- 9. Zink BJ,Walsh RF, Feustel PJ. Effects of ethanol in traumatic brain injury. J Neurotrauma. 1993;10(3):275–286. [DOI] [PubMed] [Google Scholar]
- 10. Tien HCN, Tremblay LN, Rizoli SB, et al. Association between alcohol and mortality in patients with severe traumatic head injury. Arch Surg. 2006;141(12):1185–1191. [DOI] [PubMed] [Google Scholar]
- 11. Opreanu RC, Kuhn D, Basson MD. Influence of alcohol on mortality in traumatic brain injury. J Am Coll Surg. 2010;210(6):997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Phelan K, McArthur DL, Chang CW, et al. The impact of substance abuse on mortality in patients with severe traumatic brain injury. J Trauma. 2008;65(3):674–677. [DOI] [PubMed] [Google Scholar]
- 13. Brennan JH, Bernard S, Cameron PA, et al. Ethanol and isolated traumatic brain injury. J Clin Neurosci. 2015;22(9):1375–1381. [DOI] [PubMed] [Google Scholar]
- 14. Talving P, Plurad D, Barmparas G, et al. Isolated severe traumatic brain injuries: association of blood alcohol levels with the severity of injuries and outcomes. J Trauma. 2010;68(2):357–362. [DOI] [PubMed] [Google Scholar]
- 15. Berry C, Salim A, Alban R, et al. Serum ethanol levels in patients with moderate to severe traumatic brain injury influence outcomes: a surprising finding. Am Surg. 2010;76(10):1067–1070. [PubMed] [Google Scholar]
- 16. Berry C, Ley EJ, Margulies DR, et al. Correlating the blood alcohol concentration with outcome after traumatic brain injury: too much is not a bad thing. Am Surg. 2011;77(10):1416–1419. [PubMed] [Google Scholar]
- 17. Jurkovich GJ, Rivara FP, Gurney JG, et al. The effect of acute alcohol intoxication and chronic alcohol abuse on outcome from trauma. JAMA. 1993;270(1):51–56. [PubMed] [Google Scholar]
- 18. Chen CM, Yi HY, Yoon YH, et al. Alcohol use at time of injury and survival following traumatic brain injury: results from the National Trauma Data Bank. J Stud Alcohol Drugs. 2012;73(4):531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lustenberger T, Inaba K, Barmparas G, et al. Ethanol intoxication is associated with a lower incidence of admission coagulopathy in severe traumatic brain injury patients. J Neurotrauma. 2011;28(9):1699–1706. [DOI] [PubMed] [Google Scholar]
- 20. Shandro JR, Rivara FP, Wang J, et al. Alcohol and risk of mortality in patients with traumatic brain injury. J Trauma. 2009;66(6):1584–1590. [DOI] [PubMed] [Google Scholar]
- 21. Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil. 2005;20(1):76–94. [DOI] [PubMed] [Google Scholar]
- 22. Werner C, Englehard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4–9. [DOI] [PubMed] [Google Scholar]
- 23. Yurkewicz L, Weaver J, Bullock MR, et al. The effect of the selective NMDA receptor antagonist traxoprodil in the treatment of traumatic brain injury. J Neurotrauma. 2005;22(12):1428–1443. [DOI] [PubMed] [Google Scholar]
- 24. Willis C, Lybrand S, Bellamy N. Excitatory amino acid inhibitors for traumatic brain injury. Cochrane Database Syst Rev. 2004;(1):CD003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morris GF, Bullock R, Marshall SB, et al. Failure of the competitive N-methyl-D-aspartate antagonist Selfotel (CGS 19755) in the treatment of severe head injury: results of two phase III clinical trials. The Selfotel Investigators. J Neurosurg. 1999;91(5):737–743. [DOI] [PubMed] [Google Scholar]
- 26. Raj R, Skrifvars MB, Kivisaari R, et al. Acute alcohol intoxication and long-term outcome in patients with traumatic brain injury. J Neurotrauma. 2015;32(2):95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kraus JF, Morgenstern H, Fife D, et al. Blood alcohol tests, prevalence of involvement, and outcomes following brain injury. Am J Public Health. 1989;79(3):294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alexander S, Kerr ME, Yonas H, et al. The effects of admission alcohol level on cerebral blood flow and outcomes after severe traumatic brain injury. J Neurotrauma. 2004;21(5):575–583. [DOI] [PubMed] [Google Scholar]
- 29. Rundhaug NP, Moen KG, Skandsen T, et al. Moderate and severe traumatic brain injury: effect of blood alcohol concentration on Glasgow Coma Scale score and relation to computed tomography findings. J Neurosurg. 2015;122(1):211–218. [DOI] [PubMed] [Google Scholar]
- 30. Thurman DJ, Sniezek JE, Johnson D, et al. Guidelines for Surveillance of Central Nervous System Injury. Atlanta, GA: Centers for Disease Control and Prevention; 1995. [Google Scholar]
- 31. Marr A, Coronado V, eds. Central Nervous System Injury Surveillance Data Submission Standards—2002. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2004. [Google Scholar]
- 32. Carroll CP, Cochran JA, Guse CE, et al. Are we underestimating the burden of traumatic brain injury? Surveillance of severe traumatic brain injury using centers for disease control international classification of disease, ninth revision, clinical modification, traumatic brain injury codes. Neurosurgery. 2012;71(6):1064–1070. [DOI] [PubMed] [Google Scholar]
- 33. Rating the severity of tissue damage. I. The abbreviated scale. JAMA. 1971;215(2):277–280. [DOI] [PubMed] [Google Scholar]
- 34. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. [DOI] [PubMed] [Google Scholar]
- 35. Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed Philadelphia, PA: Lippincott, Williams & Wilkins; 2008. [Google Scholar]
- 37. Brannas CC, Han S, Wiebe DJ. Alcohol use and firearm violence. Epidemiol Rev. 2016;38(1):32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim H, Colantonio A. Intentional traumatic brain injury in Ontario, Canada. J Trauma. 2008;65(6):1287–1292. [DOI] [PubMed] [Google Scholar]
- 39. Part 2: Prognosis in penetrating brain injury. J Trauma. 2001;51(2 suppl):S44–S86. [PubMed] [Google Scholar]
- 40. Champion HR, Copes WS, Sacco WJ, et al. The major trauma outcome study: establishing national norms for trauma care. J Trauma. 1990;30(11):1356–1365. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.