Abstract
Reproductive tract infections have long been hypothesized to increase the risk of uterine fibroids. Few studies have been conducted, even for the common infection genital Chlamydia trachomatis (gCT), and only with self-reported gCT data. Our investigation used micro-immunofluorescence serology for gCT to characterize past exposure. We used cross-sectional enrollment data from a prospective fibroid study carried out in the Detroit, Michigan, area; ultrasound examinations systematically screened for fibroids. Participants were African-American women aged 23–34 years (recruited in 2010–2012). Age- and multivariable-adjusted logistic regression models were used to estimate odds ratios. A total of 1,587 women (94% of participants) had unequivocal gCT serology results; 22% had fibroids. Those who were seropositive for gCT were less likely to have fibroids (age-adjusted odds ratio = 0.68, 95% confidence interval: 0.54, 0.87; multivariable-adjusted odds ratio = 0.80, 95% confidence interval: 0.62, 1.03). Inverse associations were similar across categories of fibroid size, number, and total volume. Participant groups likely to have had multiple or severe infections (multiple serovar groups, more sex partners, clinically diagnosed chlamydia) all showed statistically significantly reduced odds of fibroids. A protective association of gCT with fibroids was unexpected but plausible. gCT infection might increase immune surveillance and eliminate early lesions. Further investigation on the relationship between fibroid development and reproductive tract infections is needed.
Keywords: Chlamydia trachomatis, serology, uterine fibroids
Uterine fibroids, hormonally dependent benign tumors of the uterine smooth muscle, are one of the most common gynecological conditions affecting reproductive-age women in the United States (1). However, their etiological causes are largely unknown. Infectious agents have been linked to several neoplasms, with approximately 18% of cancers estimated to be due to infection (2). Infection may also increase the risk of fibroid development. Such an association has been hypothesized since the 1930s regarding reproductive tract infections (RTIs). Both RTIs and fibroids disproportionately burden African-American women, and certain RTIs, such as genital Chlamydia trachomatis (gCT), are very common (approximately 3 million new gCT infections annually (3)) and can cause chronic pelvic infection.
The hypothesis that gCT could play a role in fibroid development is consistent with a theorized mechanism of infection-induced fibroid pathogenesis. gCT infection could stimulate an inflammatory immune response, which could facilitate the initiation of tissue damage resulting in tissue repair/regeneration (increased extracellular matrix, cell proliferation, decreased apoptosis), leading to the formation and growth of uterine fibroids (4, 5). gCT has been isolated from the upper genital tract, including the cervix (6) and endometrium (7, 8), and has also been found to infect the placenta (9). Furthermore, gCT has been shown to cause pelvic inflammatory disease and can lead to subepithelial inflammation, as well as epithelial ulceration and scarring (10).
However, the limited data on associations of RTIs, particularly gCT, with fibroid risk (11–13) have yielded inconsistent findings. One case-control study found suggestions of positive associations of fibroids with self-reported pelvic inflammatory disease (11) and gCT (11); a cross-sectional study found a suggestive positive association for gCT among white women (12). In a recent cross-sectional study, we found no positive associations between self-reported gCT or pelvic inflammatory disease and fibroids; in fact, women reporting a history of gCT tended to have fewer and smaller fibroids (13). These previous studies measured gCT history with self-reported questionnaire data, which can be plagued by recall error and misclassification due to the asymptomatic nature of gCT (14–17). A serological measure of exposure, identifying antibodies in the serum that remain after infection, would provide more accurate exposure assessment than self-reported gCT.
C. trachomatis antibodies persist years after infection (18); serological assays are sensitive and specific markers of past C. trachomatis infection (19). There are 19 serovars of C. trachomatis, divided into 3 serogroups based on genetic relatedness and serological cross-reactivity: serogroup B (serovars B, Ba, D, Da, E, L1, L2, and L2a), serogroup C (serovars A, C, H, I, Ia, J, K, and L3), and serogroup I (serovars F, G, and Ga). The majority of C. trachomatis genital infections in the United States are caused by serovars D–K, primarily D, E, and F (20). Serovars A–C are predominately associated with hyperendemic blinding trachoma, an eye infection typically found in the Middle East, Asia, and Africa; serovar B has also been found to cause genital infections (20). Serovars L1–L3 are exclusively associated with lymphogranuloma venereum, a systemic sexually transmitted infection that is endemic in parts of Africa, Asia, South America, and the Caribbean (21).
The aim of our study was to investigate the relationship between gCT and fibroids in a large cohort with ultrasound screening for fibroids and serological measurement of exposure. To our knowledge, this study was the first to investigate the association of fibroids with gCT exposure assessed serologically. We also explored the relationship between gCT seropositivity and number, size, and total volume of fibroids.
METHODS
Study participants and data collection
We used transvaginal ultrasound results, self-reported questionnaire data, and stored frozen serum specimens from participants in the Study of Environment, Lifestyle and Fibroids (SELF), an ongoing National Institute of Environmental Health Sciences investigation based in the Detroit, Michigan, area. SELF is a prospective cohort study of fibroid development; however, only baseline fibroid data were available at the time of this analysis. Enrollment data and specimen collection protocols have been described previously (22). In brief, from November 2010 to December 2012, the investigators recruited 1,696 African-American female volunteers aged 23–34 years. Women were ineligible for SELF if they had a previous diagnosis of uterine fibroids or a history of hysterectomy. Participants gave written informed consent. The study was approved by the institutional review boards of the National Institute of Environmental Health Sciences and the Henry Ford Health System (Detroit, Michigan).
Fibroid assessment
As described previously (22), fibroids were assessed by study sonographers with transvaginal ultrasonography, the standard procedure for the detection and diagnosis of fibroids (23), at one of 3 Henry Ford Health System clinics. Focal fibroids 0.5 cm or more in diameter were measured in triplicate. For each measurement, the 3 perpendicular diameters (longitudinal, anterior-posterior, and transverse) were recorded.
Outcome definitions
The primary outcome of this study was the presence of fibroids (yes/no) at the enrollment transvaginal ultrasound. Participants with at least 1 fibroid greater than or equal to 0.5 cm in diameter at enrollment were considered to have fibroids, and all women without a fibroid greater than or equal to 0.5 cm in diameter were considered not to have fibroids. The secondary outcomes for this analysis were size of the largest fibroid (cm), number of fibroids, and total fibroid volume (cm3) (see Web Appendix 1, available at https://academic.oup.com/aje, for definitions).
Chlamydia assessment
The presence of serum immunoglobulin G antibodies to gCT was determined by means of a micro-immunofluorescence assay (24), which is the gold standard serological method. This assay can detect species-specific chlamydia antibodies and is the only test that also differentiates between the serovars of gCT.
An experienced laboratory technician at the University of Washington Chlamydia Laboratory (Seattle, Washington) performed the micro-immunofluorescence test. The laboratory’s antigen panel includes purified elementary bodies of C. trachomatis (serovars A, B, I, and H and groupings of serovars CJ, DE, KL3, FG, and L1L2), Chlamydia pneumoniae (serovar TW183), and Chlamydia psittaci (avian strain 6BC). Participants who tested positive (≥1:16 dilution) for any of the gCT serovars B, I, H, CJ, DE, KL3, and FG were considered seropositive for gCT infection. A negative antigen control was included with each set of antigens on the micro-immunofluorescence slides, and a positive serum control analysis was run with each set of sera on a given day.
C. psittaci was included in the antigen panel to monitor species cross-reactive antibody responses. We excluded participants with species cross-reactivity (where the maximum titer for serovars B–KL3 was ≥1:16 and identical to the C. psittaci titer) because they would not have had a conclusive gCT status. C. pneumoniae, a common respiratory infection, was used as a comparison for the gCT findings (see Web Appendix 1 for more details on the chlamydia assessment protocol).
C. trachomatis serological analysis was conducted for the 98% of participants with available enrollment blood samples (n = 1,661). Five batches of samples were sent to the laboratory. Each batch included blinded duplicate samples for quality control, and 3 batches included additional positive controls (Web Appendix 1). All of the blinded samples had the same gCT serostatus as their duplicate, and each positive control was positive for gCT based on our definition. After the 74 samples with the same serological titers for gCT and C. psittaci were excluded from gCT analyses, our primary analyses were performed on 1,587 participants. One hundred and sixty samples had the same titers for C. pneumoniae and C. psittaci and thus were excluded from C. pneumoniae analyses.
Statistical analyses
Standard descriptive statistics were calculated for all variables of interest. Missing data were minimal (<1%); complete-case analysis was performed. Ninety-five percent confidence intervals were estimated for all odds ratios. All analyses were conducted with SAS 9.3 (SAS Institute, Inc., Cary, North Carolina).
Primary analyses
Given the limited prior data, our primary aim was to identify associations rather than provide quantitative risk estimates. Thus, logistic regression was used to estimate the odds ratio for the relationship between gCT serostatus and fibroid presence. Although relative odds overestimate the relative risk for associations with an outcome as common as fibroids, the statistical test for association is valid. Covariates were determined on the basis of a review of the literature, and a directed acyclic graph (25) was used to provide a conceptual framework. A priori confounders included age (in years), age at menarche, parity, and use of depot medroxyprogesterone acetate. Alcohol consumption and education were tested as potential confounders. The alcohol variable reflected the drinking level each woman reported for the age(s) at which she was drinking the most (see details on covariates in Web Appendix 1). Body mass index and smoking have not been found to be consistently associated with fibroids in the literature (12) and were not considered important risk factors in this analysis.
After use of the 10% change-in-estimate approach, only the covariates included a priori remained in the final model. Logistic regression was used to estimate age- and multivariable-adjusted odds ratios for the relationship between C. pneumoniae serostatus and fibroid presence, for comparison with the C. trachomatis findings.
Sensitivity analyses
To evaluate the robustness of our findings, we examined multivariable-adjusted odds ratios for the association of gCT with fibroids in a series of sensitivity analyses. First, we repeated the primary analyses while using both stricter and more liberal definitions of gCT seropositivity (Web Table 1). Secondly, we excluded women who reported having undergone cervical treatment, such as cone biopsy, for removal of possible early cervical cancer lesions (226 of the 1,587 women were excluded, including 1 with missing data on cervical treatment) because it was previously found to be inversely associated with fibroids in SELF (26). Thirdly, to evaluate age as an effect modifier (due to a possible decline in antibody levels over time), we evaluated the association between gCT and fibroid presence within 2 age strata (23–29 years and 30–35 years).
We also conducted analyses to investigate the temporality of gCT exposure in relation to fibroid development. Smaller fibroids may have developed more recently as compared with larger fibroids; thus, we evaluated the association between gCT and fibroids among strata of the size of the largest fibroid (<2 cm and ≥2 cm). In addition, we looked at the association between gCT and fibroid presence within 3 strata of number of sexual partners before age 20 years (≤1, 2–5, or ≥6 sex partners). Participants with more sexual partners before age 20 years would have been more likely to be exposed to gCT prior to fibroid development.
Secondary analyses
We also examined the association between gCT and size of the largest fibroid, number of fibroids, and total fibroid volume. Median values were used as category cutpoints. To estimate the odds ratios for the association of gCT with each outcome, we used multinomial logistic regression. We were also interested in severity of infection. Because most gCT infections are asymptomatic (27), self-reported infections are likely to be those that are symptomatic and potentially more severe. Thus, we evaluated the multivariable-adjusted odds ratios for the association between gCT and fibroid presence using a 3-level exposure variable: 0 = seronegative, 1 = not symptomatic (seropositive with no self-reported diagnosis), and 2 = symptomatic (seropositive with a self-reported diagnosis). In secondary analyses, we adjusted for the same covariates as in the primary analysis.
Exploratory analyses
We also conducted exploratory analyses to further understand the relationship between gCT and fibroids. We estimated the multivariable-adjusted odds ratios for the association between fibroid presence and the highest of the gCT titers (seronegative, 1:16–1:64, or 1:128–1:1,024) and the number of gCT serogroups (seronegative, 1 group, or multiple (2 or 3) groups).
RESULTS
The median age of women in our cohort was 29 years, and the median age at menarche was 12 years. The majority (61%) were parous, and over 40% had ever used depot medroxyprogesterone acetate. Fifty-seven percent (n = 907) of the participants were seropositive for gCT; seropositive women tended to be less educated and were more likely to have ever smoked, to be heavier alcohol drinkers, to be parous, to have ever used depot medroxyprogesterone acetate, to have had more sex partners before age 20 years, and to have been younger at first sex compared with those who were seronegative for gCT (Table 1).
Table 1.
Selected Enrollment Characteristics of 1,587a African-American Women Aged 23–34 Years According to Genital Chlamydia trachomatis Serostatus, Study of Environment, Lifestyle and Fibroids, Detroit, Michigan, 2010–2012
| Covariate | gCT Serostatus | |||
|---|---|---|---|---|
| Seropositive (n = 907) | Seronegative (n = 680) | |||
| No. of Women | % | No. of Women | % | |
| Age, years | ||||
| 23–26 | 274 | 30 | 211 | 31 |
| 27–30 | 309 | 34 | 235 | 35 |
| 31–35b | 324 | 36 | 234 | 34 |
| Education | ||||
| High school diploma or less | 254 | 28 | 95 | 14 |
| Some college or technical school | 470 | 52 | 324 | 48 |
| Bachelor’s degree or more | 182 | 20 | 261 | 38 |
| Missing data | 1 | 0 | ||
| Body mass indexc | ||||
| 15–24 | 154 | 17 | 164 | 24 |
| 25–29 | 199 | 22 | 130 | 19 |
| 30–34 | 169 | 19 | 137 | 20 |
| 35–80 | 385 | 42 | 249 | 37 |
| Smoking status | ||||
| Never smoker | 591 | 65 | 573 | 84 |
| Ever smoker | 316 | 35 | 107 | 16 |
| Peak alcohol consumptiond | ||||
| Light | 224 | 25 | 193 | 28 |
| Moderate | 265 | 29 | 254 | 37 |
| Heavy | 418 | 46 | 233 | 34 |
| Parity | ||||
| Nulliparous | 296 | 33 | 329 | 48 |
| Parous | 611 | 67 | 351 | 52 |
| DMPA use | ||||
| Never used | 455 | 50 | 455 | 67 |
| Ever used | 452 | 50 | 225 | 33 |
| Age at menarche, years | ||||
| 7–10e | 173 | 19 | 116 | 17 |
| 11–19 | 734 | 81 | 564 | 83 |
| No. of sex partners before age 20 years | ||||
| ≤1f | 148 | 16 | 259 | 38 |
| 2–5 | 471 | 52 | 309 | 45 |
| ≥6 | 287 | 32 | 110 | 16 |
| Missing data | 1 | 0 | 2 | 0 |
| Age at first intercourse, years | ||||
| ≤14 | 321 | 35 | 132 | 19 |
| 15–16 | 341 | 38 | 198 | 29 |
| ≥17f | 243 | 27 | 349 | 51 |
| Missing data | 2 | 0 | 1 | 0 |
Abbreviations: DMPA, depot medroxyprogesterone acetate; gCT, genital Chlamydia trachomatis.
a Of the 1,661 participants, 74 were excluded due to genus cross-reactivity (maximum C. trachomatis titer was greater than or equal to 1:16 and equal to Chlamydia psittaci titer).
b No one over age 34 years was recruited, but some 34-year-olds had turned 35 by the time they underwent ultrasonography.
c Weight (kg)/height (m)2.
d Alcohol consumption at the age at which the participant was drinking the most. Light drinkers were those who never consumed 10 or more alcoholic drinks in a year. Heavy drinkers were those who usually consumed 6 or more drinks on days when they drank or consumed 4 or more drinks per sitting at least 2–3 times per month. Moderate drinkers were all others.
e Categorized to identify early age at menarche, the category associated with fibroids.
f Includes participants who reported never having sex.
Twenty-two percent (n = 352) of women had fibroids discovered at ultrasound screening. The size of the largest fibroid was <2 cm for 59%; 61% had only 1 fibroid. The total fibroid volume was <2 cm3 for 49% of participants with fibroids.
In primary analyses, the odds of fibroids were lower among women who were seropositive for gCT than among those who were seronegative, although the association was weaker and not statistically significant in the multivariable model (age-adjusted odds ratio = 0.68, 95% confidence interval (CI): 0.54, 0.87; multivariable-adjusted odds ratio (aOR) = 0.80, 95% CI: 0.62, 1.03) (Table 2). Depot medroxyprogesterone acetate and parity, both of which had inverse associations with fibroid prevalence, had the strongest impact on the multivariable-adjusted association. In contrast to the inverse association between fibroids and gCT, we found no association between fibroids and C. pneumoniae in multivariable-adjusted analyses (Table 2), even when we varied the definition for C. pneumoniae (data not shown).
Table 2.
Relationships of Genital Chlamydia trachomatis and Chlamydia pneumoniae Serostatus With Uterine Fibroids Among African-American Women Aged 23–34 Years, Study of Environment, Lifestyle and Fibroids, Detroit, Michigan, 2010–2012
| Serostatus | No. of Women | Presence of Fibroids | Model 1a | Model 2b | |||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||||
| No. | % | ||||||
| Genital C. trachomatis-seropositivec | |||||||
| No | 680 | 174 | 26 | 1.00 | Referent | 1.00 | Referent |
| Yes | 907 | 178 | 20 | 0.68 | 0.54, 0.87 | 0.80 | 0.62, 1.03 |
| C. pneumoniae-seropositived | |||||||
| No | 447 | 107 | 24 | 1.00 | Referent | 1.00 | Referent |
| Yes | 1,054 | 232 | 22 | 0.89 | 0.68, 1.17 | 0.94 | 0.72, 1.24 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Results were adjusted for age.
b Results were adjusted for age, age at menarche, depot medroxyprogesterone acetate use, and parity.
c Of the 1,661 participants, 74 were excluded due to genus cross-reactivity (maximum C. trachomatis titer was greater than or equal to 1:16 and equal to Chlamydia psittaci titer).
d Of the 1,661 participants, 160 were excluded due to genus cross-reactivity (C. pneumoniae titer was greater than or equal to 1:8 and equal to C. psittaci titer).
The association between gCT and fibroids remained consistent across most of the sensitivity analyses that varied the definition of gCT (Web Table 1) and restricted/stratified the results (Table 3). When analyses were stratified by self-reported number of sex partners before age 20 years, we observed a potential dose-response relationship between gCT and fibroids. Women reporting the highest number of sex partners (≥6) before age 20 years had a more apparent inverse association between gCT and fibroids (aOR = 0.52, 95% CI: 0.29, 0.93) (Table 3).
Table 3.
Results of Sensitivity Analyses of the Association Between Uterine Fibroids and Genital Chlamydia trachomatis Serostatus Among African-American Women Aged 23–34 Years, Study of Environment, Lifestyle and Fibroids, Detroit, Michigan, 2010–2012
| No. of Women | Odds Ratioa | 95% Confidence Interval | |
|---|---|---|---|
| Full sample | 1,587b | 0.80 | 0.62, 1.03 |
| Exclusion of women with prior cervical treatment (n = 226) | 1,361 | 0.79 | 0.60, 1.03 |
| Stratification | |||
| By age group, yearsc | |||
| 23–29 | 892 | 0.74 | 0.51, 1.07 |
| ≥30 | 695 | 0.85 | 0.60, 1.21 |
| By size of the largest fibroid, cmd | |||
| None | 1,235 | 1.00 | Referent |
| <2 | 209 | 0.80 | 0.59, 1.09 |
| ≥2 | 143 | 0.79 | 0.55, 1.14 |
| By no. of sex partners before age 20 yearsd,e | |||
| 0–1 | 407 | 1.12 | 0.68, 1.82 |
| 2–5 | 780 | 0.81 | 0.56, 1.15 |
| ≥6 | 397 | 0.52 | 0.29, 0.93 |
a Adjusted for age, age at menarche, depot medroxyprogesterone acetate use, and parity.
b Of the 1,661 participants, 74 were excluded due to genus cross-reactivity (maximum C. trachomatis titer was greater than or equal to 1:16 and equal to Chlamydia psittaci titer).
c Test of possible decline in antibody levels over time.
d Test of temporality.
e Three participants had missing data for number of sex partners before age 20 years.
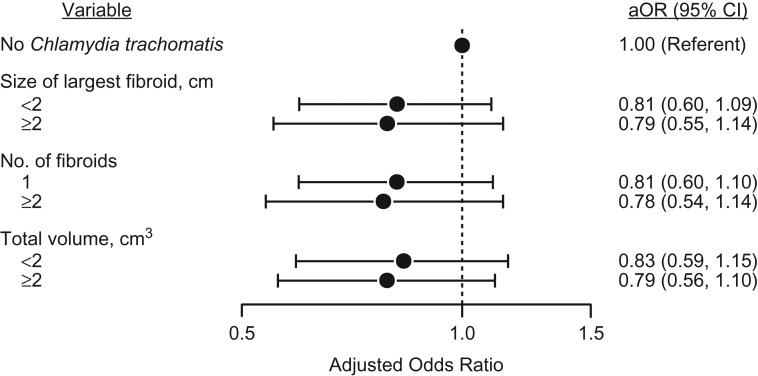
In secondary analyses, the inverse associations were similar across size, number, and volume of fibroids (Figure 1). The 49% of seropositive women who reported a gCT diagnosis (our measure of symptomatic gCT) had a stronger inverse association with fibroids (aOR = 0.72, 95% CI: 0.53, 0.99) than did seropositive women without a self-reported gCT diagnosis (Table 4).
Figure 1.
Relationships of size of the largest fibroid, number of fibroids, and total fibroid volume with genital Chlamydia trachomatis serostatus among 23- to 34-year-old African-American women with uterine fibroids (n = 352), Study of Environment, Lifestyle and Fibroids, Detroit, Michigan, 2010–2012. Multivariable-adjusted odds ratios (aORs) were adjusted for age, age at menarche, depot medroxyprogesterone acetate use, and parity. Bars, 95% confidence intervals (CIs).
Table 4.
Relationships of Genital Chlamydia trachomatis Symptoms and Number of Serogroups With Uterine Fibroids Among African-American Women Aged 23–34 Years, Study of Environment, Lifestyle and Fibroids, Detroit, Michigan, 2010–2012
| Category and gCT Serostatus | No. of Women | Presence of Fibroids | Odds Ratioa | 95% Confidence Interval | |
|---|---|---|---|---|---|
| No. | % | ||||
| gCT symptomsb,c | |||||
| Seronegative | 680 | 174 | 26 | 1.00 | Referent |
| Seropositive | |||||
| Asymptomatic | 459 | 100 | 22 | 0.88 | 0.65, 1.18 |
| Symptomaticd | 446 | 78 | 17 | 0.72 | 0.53, 0.99 |
| No. of serogroupse | |||||
| Seronegative | 680 | 174 | 26 | 1.00 | Referent |
| Seropositive | |||||
| 1 serogroup | 310 | 62 | 20 | 0.76 | 0.54, 1.07 |
| 2 or 3 serogroups | 310 | 54 | 17 | 0.69 | 0.49, 0.99 |
Abbreviations: gCT, genital Chlamydia trachomatis.
a Adjusted for age, age at menarche, depot medroxyprogesterone acetate use, and parity.
b Of the 1,661 participants, 74 were excluded due to genus cross-reactivity (maximum C. trachomatis titer was greater than or equal to 1:16 and equal to Chlamydia psittaci titer); 2 were excluded because of missing self-reported data on gCT.
c Limited to participants with data on gCT serostatus and self-report.
d Participants with a self-reported diagnosis of gCT (n = 2 where self-report was missing).
e Of the 1,661 participants, 74 were excluded due to genus cross-reactivity (maximum C. trachomatis titer was greater than or equal to 1:16 and equal to C. psittaci titer); 286 were excluded because no serovar pattern was available; and 1 was excluded because the serovar pattern was L1L2, which is an indication of systemic C. trachomatis, not gCT.
The exploratory analysis revealed that participants with 2 or 3 serogroups had a more apparent inverse association with fibroids (aOR = 0.69, 95% CI: 0.49, 0.99) than those with 1 serogroup (Table 4). However, those with the highest titers did not have the strongest inverse association (Web Table 2).
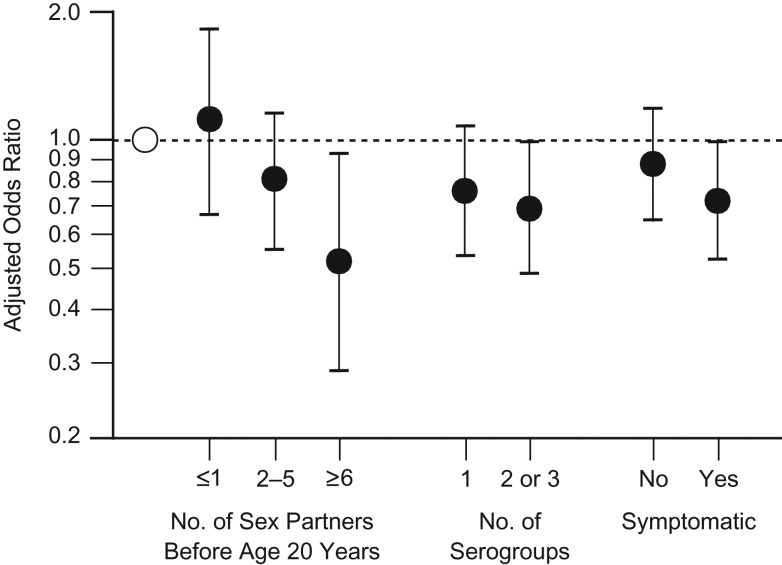
Overall, women with more infections and more severe infections evidenced the strongest protective associations (Figure 2).
Figure 2.
Odds of uterine fibroid development according to number and severity of genital Chlamydia trachomatis (gCT) infections among 23- to 34-year-old African-American women, Study of Environment, Lifestyle and Fibroids, Detroit, Michigan, 2010–2012. Participants who were more likely to have had multiple gCT infections (more sex partners, more serogroups) or more severe gCT infections (symptomatic) showed statistically significantly reduced odds of fibroids. Multivariable-adjusted odds ratios were adjusted for age, age at menarche, depot medroxyprogesterone acetate use, and parity. White circle, gCT-seronegative women (referent); black circles, gCT-seropositive women. Bars, 95% confidence intervals.
DISCUSSION
Our results suggest a robust association of gCT seropositivity with decreased odds of uterine fibroids. Participants who were more likely to have been infected more often (those reporting more sex partners before the age of 20 years and those with multiple serogroups) and/or who probably had more severe infections (infections that came to clinical attention) had the strongest reduced odds of fibroids.
Our findings are consistent with those of a previous study of self-reported RTIs in this same population which suggested that women self-reporting gCT tended to have fewer and smaller fibroids (13). In the 2 other studies that have investigated the association between self-reported gCT and fibroid presence, the results suggested positive associations between gCT and fibroids (11, 12). A clinic-based case-control study of premenopausal women aged 18–55 years showed a positive though nonsignificant association between gCT and clinically detected fibroids (11). The clinic-based study included a much broader age range than our population and did not screen for fibroids. The Uterine Fibroid Study (12), a cross-sectional study that used ultrasound to screen randomly selected members of an urban health plan aged 35–49 years for fibroids, found a nonsignificant positive association of self-reported gCT with fibroids in white women (12). The Uterine Fibroid Study included an older age group than the women in our study and did not find an association in African-American women. A small pilot study found no evidence of latent gCT infection in immunostained fibroid tissue specimens from 20 Uterine Fibroid Study participants who had reported a history of sexually transmitted disease or multiple sex partners (12).
Our study had several strengths. To our knowledge, it was the first study to investigate the relationship between gCT and fibroids using an immunological measure of exposure. Antibody titers persist for years postinfection (28) and therefore can identify gCT infections that occurred years prior to enrollment. Our blinded quality control samples demonstrated low measurement error in gCT serostatus. We also had information on gCT serogroups and titers. Previous studies have used only self-reported diagnosis of gCT as the exposure measurement, which is problematic due to the high prevalence of asymptomatic infection (27) and moderate specificity (e.g., 21% of seronegative women in our study reported having been diagnosed with gCT). Furthermore, we used a standard and valid measure of fibroid status based on systematic ultrasound screening rather than fibroids clinically detected because of symptoms or incidental detection. The number, diameter, and volume of the fibroids were systematically measured, so we could examine associations with these characteristics. Our sample size was sufficient to provide good precision for the main hypotheses. Our study also had extensive data for assessment of potential confounding, minimal missing data, and sensitivity analyses for evaluating potential bias.
Our study also had limitations. First, it was a cross-sectional analysis. Thus, the temporal relationship between gCT infection and fibroid development is unknown. However, reverse causation (fibroids developing first and inducing protection against gCT) has no plausible mechanism, and the timing is unlikely. gCT infection was likely to have begun early. Approximately 63% of new gCT infections in the United States occur among 15- to 24-year-olds (3), and in our study, the median time between first self-reported gCT diagnosis and study enrollment was 9 years. In contrast, development of fibroids was likely to occur relatively later. Fibroids are rarely seen in African Americans before the mid-20s (22, 29), and most of the fibroids detected in our study were small, suggesting new development.
Secondly, selection bias from the exclusion of women with previously diagnosed fibroids could be a potential problem. If those excluded were substantially more likely to have had a prior gCT infection than those enrolled, such selection could have created an artifactual inverse association. However, when we estimated gCT infection rates that would have been necessary in the excluded women to have substantially changed our results, we found that nearly 80% of those excluded would have had to have been gCT-seropositive (see details in Web Appendix 2 and Web Tables 3 and 4). Such a high infection rate is very unlikely. Additionally, because the risk of fibroids increases with age, there would have been many more older women excluded because of a prior fibroid diagnosis than younger women. Therefore, if the inverse association was due to selection bias, we would expect that inverse association to have been stronger in the older women, which we did not find in our sensitivity analyses. In addition, a large proportion of women who are seropositive for gCT are unaware of their past infection (15), making it very unlikely that women without fibroids who had a history of gCT exposure would be more likely to enroll in the study than those without gCT; thus, there was limited potential for this type of selection bias.
Third, uncontrolled confounding is a potential limitation, but the study’s extensive data collection allowed us to control for all well-documented risk factors for fibroids and to investigate other variables as well. In our previous study, there were no strong associations for any self-reported RTIs (13) or with herpes measured serologically (30); thus, we did not include other RTIs in our models. Finally, our sample comprised volunteers, thus potentially limiting generalizability. However, in our sample, the self-reported frequency of a history of gCT infection was 38% and the seroprevalence of gCT was 57%, both consistent with other reports (15, 31). In addition, the 22% prevalence of fibroids in our cohort at ultrasound screening falls within the range of prior US studies that conducted ultrasound screening (12, 29, 32, 33).
The inverse association we found was unexpected and contrary to our hypothesis but is plausible. There has long been substantial evidence that bacterial infections can lead to the spontaneous regression of some tumors (34). The infecting organism could directly induce antitumor effects, or the infection could lead to enhancement of immune surveillance (34). Altough little is known about gCT infection of the uterus, especially the myometrium, finding gCT in the upper genital tract is not uncommon (7, 8); the association between gCT and pelvic inflammatory disease is very well known (35). Smooth muscle cells are a target for C. pneumoniae (36–38); thus, it is plausible that C. trachomatis could infect uterine smooth muscle cells. In mouse models of chlamydia infection, endometrial infection appears to lead to subendometrial macrophage influx (39). gCT appears to persist for at least a year in most women and even up to 5 years (40) and thus could generate a chronic inflammatory immune response that might eliminate initial mutated cells. Preneoplastic lesions have been found to be more susceptible to apoptosis than normal cells (41, 42). However, this mechanism would rely on timing of infection during a period in fibroid development when lesions are small. Women with more infections or more severe infections would have a greater probability of being infected with gCT during such a developmental window of the tumor. Women with more severe infections evidenced the strongest protective associations. Information on the timing of infection is needed to better evaluate this potential mechanism.
In conclusion, in a large cohort of African-American women aged 23–34 years, gCT seropositivity was inversely associated with the presence of fibroids. To investigate this finding further, researchers could use fibroid animal models to determine the impact of an ascending genital chlamydial infection, especially in the very early stages of fibroid development. The rat and guinea pig models have been used for both fibroids (43, 44) and chlamydia infection (45, 46), so there is potential for these 2 conditions to be modeled together. In addition, prospective evaluation of the relationship between RTIs and fibroid development in women is needed.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Kristen R. Moore, Jennifer S. Smith, Stephen R. Cole, Victor J. Schoenbach); Department of Microbiology and Immunology, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Dirk P. Dittmer); and Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (Kristen R. Moore, Donna D. Baird).
This work was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health (grant 10-E-N044). Funding also came from American Recovery and Reinvestment Act funds designated for National Institutes of Health research.
We thank Drs. Lauren Wilson and Christine Parks for reviewing a draft of the manuscript. We thank Linda Cles at the University of Washington Chlamydia Laboratory for conducting the serological analyses. We thank Drs. Toni Darville and Uma Nagarajan at the University of North Carolina at Chapel Hill for their expertise regarding C. trachomatis immunology. We also thank our collaborators and study staff at the Henry Ford Health System (Detroit, Michigan) and Social and Scientific Systems (Research Triangle Park, North Carolina), specifically Deborah Cousins at Social and Scientific Systems, for help with data quality control.
Conflict of interest: none declared.
Abbreviations
- aOR
multivariable-adjusted odds ratio
- CI
confidence interval
- gCT
genital Chlamydia trachomatis
- RTI
reproductive tract infection
- SELF
Study of Environment, Lifestyle and Fibroids
REFERENCES
- 1. Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293–298. [DOI] [PubMed] [Google Scholar]
- 2. International Agency for Research on Cancer Infections—Infections and Cancer Epidemiology Group. 2013. http://www.iarc.fr/en/research-groups/ICE/index.php. Accessed November 11, 2014.
- 3. Centers for Disease Control and Prevention Incidence, Prevalence, and Cost of Sexually Transmitted Infections in the United States Atlanta, GA: Centers for Disease Control and Prevention; 2013. http://www.cdc.gov/std/stats/STI-Estimates-Fact-Sheet-Feb-2013.pdf. Accessed June 20, 2013.
- 4. Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195(2):415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wegienka G. Are uterine leiomyoma a consequence of a chronically inflammatory immune system? Med Hypotheses. 2012;79(2):226–231. [DOI] [PubMed] [Google Scholar]
- 6. Marrazzo JM, Martin DH. Management of women with cervicitis. Clin Infect Dis. 2007;44(suppl 3):S102–S110. [DOI] [PubMed] [Google Scholar]
- 7. Jones RB, Mammel JB, Shepard MK, et al. . Recovery of Chlamydia trachomatis from the endometrium of women at risk for chlamydial infection. Am J Obstet Gynecol. 1986;155(1):35–39. [DOI] [PubMed] [Google Scholar]
- 8. Kiviat NB, Wølner-Hanssen P, Eschenbach DA, et al. . Endometrial histopathology in patients with culture-proved upper genital tract infection and laparoscopically diagnosed acute salpingitis. Am J Surg Pathol. 1990;14(2):167–175. [DOI] [PubMed] [Google Scholar]
- 9. Rours GI, de Krijger RR, Ott A, et al. . Chlamydia trachomatis and placental inflammation in early preterm delivery. Eur J Epidemiol. 2011;26(5):421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stamm WE. Chlamydia trachomatis infections of the adult In: Holmes KK, Sparling PF, Stamm WE, et al., eds. Sexually Transmitted Diseases. 4th ed.New York, NY: McGraw-Hill Medical; 2008:575–593. [Google Scholar]
- 11. Faerstein E, Szklo M, Rosenshein NB. Risk factors for uterine leiomyoma: a practice-based case-control study. II. Atherogenic risk factors and potential sources of uterine irritation. Am J Epidemiol. 2001;153(1):11–19. [DOI] [PubMed] [Google Scholar]
- 12. Laughlin SK, Schroeder JC, Baird DD. New directions in the epidemiology of uterine fibroids. Semin Reprod Med. 2010;28(3):204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moore KR, Cole SR, Dittmer DP, et al. . Self-reported reproductive tract infections and ultrasound diagnosed uterine fibroids in African-American women. J Womens Health (Larchmt). 2015;24(6):489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown JL, Sales JM, DiClemente RJ, et al. . Predicting discordance between self-reports of sexual behavior and incident sexually transmitted infections with African American female adolescents: results from a 4-city study. AIDS Behav. 2012;16(6):1491–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frisse AC, Marrazzo JM, Tutlam NT, et al. . Validity of self-reported history of Chlamydia trachomatis infection. Am J Obstet Gynecol. 2017;216(4):393.e1–393.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harrington KF, DiClemente RJ, Wingood GM, et al. . Validity of self-reported sexually transmitted diseases among African American female adolescents participating in an HIV/STD prevention intervention trial. Sex Transm Dis. 2001;28(8):468–471. [DOI] [PubMed] [Google Scholar]
- 17. Hong Y, Fang X, Zhou Y, et al. . Factors associated with sexually transmitted infection underreporting among female sex workers in China. J Women’s Health (Larchmt). 2011;20(1):129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clad A, Freidank HM, Kunze M, et al. . Detection of seroconversion and persistence of Chlamydia trachomatis antibodies in five different serological tests. Eur J Clin Microbiol Infect Dis. 2000;19(12):932–937. [DOI] [PubMed] [Google Scholar]
- 19. Black CM. Current methods of laboratory diagnosis of Chlamydia trachomatis infections. Clin Microbiol Rev. 1997;10(1):160–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schachter J, Stephens RS. Biology of Chlamydia trachomatis In: Holmes KK, Sparling PF, Stamm WE, et al., eds. Sexually Transmitted Diseases. 4th ed.New York, NY: McGraw-Hill Medical; 2008:555–574. [Google Scholar]
- 21. Gaydos C, Essig A. Chlamydiaceae In: Versalovic J, Carroll KC, Funke G, et al., eds. Manual of Clinical Microbiology. 10th ed.Washington, DC: ASM Press; 2011:986–1000. [Google Scholar]
- 22. Baird DD, Harmon QE, Upson K, et al. . A prospective, ultrasound-based study to evaluate risk factors for uterine fibroid incidence and growth: methods and results of recruitment. J Womens Health (Larchmt). 2015;24(11):907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dueholm M, Lundorf E, Hansen ES, et al. . Accuracy of magnetic resonance imaging and transvaginal ultrasonography in the diagnosis, mapping, and measurement of uterine myomas. Am J Obstet Gynecol. 2002;186(3):409–415. [DOI] [PubMed] [Google Scholar]
- 24. Wang SP, Grayston JT. Human serology in Chlamydia trachomatis infection with microimmunofluorescence. J Infect Dis. 1974;130(4):388–397. [DOI] [PubMed] [Google Scholar]
- 25. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 26. Moore KR, Smith JS, Laughlin-Tommaso SK, et al. . Cervical neoplasia-related factors and decreased prevalence of uterine fibroids among a cohort of African American women. Fertil Steril. 2014;101(1):208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med. 2003;36(4):502–509. [DOI] [PubMed] [Google Scholar]
- 28. Clad A, Freidank HM, Kunze M, et al. . Detection of seroconversion and persistence of Chlamydia trachomatis antibodies in five different serological tests. Eur J Clin Microbiol Infect Dis. 2000;19(12):932–937. [DOI] [PubMed] [Google Scholar]
- 29. Laughlin SK, Baird DD, Savitz DA, et al. . Prevalence of uterine leiomyomas in the first trimester of pregnancy: an ultrasound-screening study. Obstet Gynecol. 2009;113(3):630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moore KR, Smith JS, Cole SR, et al. . Herpes simplex virus type 2 seroprevalence and ultrasound-diagnosed uterine fibroids in a large population of young African-American women. Am J Epidemiol. 2016;183(11):961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peterman TA, Newman DR, Torrone E, et al. . Cumulative risk of chlamydial infection among young women in Florida, 2000–2011. J Adolesc Health. 2014;55(2):241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baird DD, Dunson DB, Hill MC, et al. . High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107. [DOI] [PubMed] [Google Scholar]
- 33. Marsh EE, Ekpo GE, Cardozo ER, et al. . Racial differences in fibroid prevalence and ultrasound findings in asymptomatic young women (18–30 years old): a pilot study. Fertil Steril. 2013;99(7):1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kucerova P, Cervinkova M. Spontaneous regression of tumour and the role of microbial infection—possibilities for cancer treatment. Anticancer Drugs. 2016;27(4):269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haggerty CL, Gottlieb SL, Taylor BD, et al. . Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis. 2010;201(suppl 2):S134–S155. [DOI] [PubMed] [Google Scholar]
- 36. Assar O, Nejatizadeh A, Dehghan F, et al. . Association of Chlamydia pneumoniae infection with atherosclerotic plaque formation. Glob J Health Sci. 2015;8(4):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Borel N, Summersgill JT, Mukhopadhyay S, et al. . Evidence for persistent Chlamydia pneumoniae infection of human coronary atheromas. Atherosclerosis. 2008;199(1):154–161. [DOI] [PubMed] [Google Scholar]
- 38. Yamashita K, Ouchi K, Shirai M, et al. . Distribution of Chlamydia pneumoniae infection in the athersclerotic carotid artery. Stroke. 1998;29(4):773–778. [DOI] [PubMed] [Google Scholar]
- 39. Morrison SG, Morrison RP. In situ analysis of the evolution of the primary immune response in murine Chlamydia trachomatis genital tract infection. Infect Immun. 2000;68(5):2870–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Molano M, Meijer CJ, Weiderpass E, et al. . The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a 5-year follow-up study. J Infect Dis. 2005;191(6):907–916. [DOI] [PubMed] [Google Scholar]
- 41. Grasl-Kraupp B, Bursch W, Ruttkay-Nedecky B, et al. . Food restriction eliminates preneoplastic cells through apoptosis and antagonizes carcinogenesis in rat liver. Proc Natl Acad Sci USA. 1994;91(21):9995–9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Preston GA, Lang JE, Maronpot RR, et al. . Regulation of apoptosis by low serum in cells of different stages of neoplastic progression: enhanced susceptibility after loss of a senescence gene and decreased susceptibility after loss of a tumor suppressor gene. Cancer Res. 1994;54(15):4214–4223. [PubMed] [Google Scholar]
- 43. Howe SR, Gottardis MM, Everitt JI, et al. . Rodent model of reproductive tract leiomyomata. Establishment and characterization of tumor-derived cell lines. Am J Pathol. 1995;146(6):1568–1579. [PMC free article] [PubMed] [Google Scholar]
- 44. Porter KB, Tsibris JC, Nicosia SV, et al. . Estrogen-induced guinea pig model for uterine leiomyomas: do the ovaries protect? Biol Reprod. 1995;52(4):824–832. [DOI] [PubMed] [Google Scholar]
- 45. Kaushic C, Murdin AD, Underdown BJ, et al. . Chlamydia trachomatis infection in the female reproductive tract of the rat: influence of progesterone on infectivity and immune response. Infect Immun. 1998;66(3):893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mount DT, Bigazzi PE, Barron AL. Infection of genital tract and transmission of ocular infection to newborns by the agent of guinea pig inclusion conjunctivitis. Infect Immun. 1972;5(6):921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.