Abstract
The association between dietary fat and fertility is not well studied. We evaluated intakes of total fat, saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids, trans fatty acids (TFA), ω-3 fatty acids, and ω-6 fatty acids in relation to fecundability in Danish and North American preconception cohort studies. Women who were attempting to become pregnant completed a validated food frequency questionnaire at baseline. Pregnancy status was updated bimonthly for 12 months or until pregnancy. Fecundability ratios (FR) and 95% confidence intervals were estimated using multivariable proportional probabilities regression. Intakes of total fat and saturated, monounsaturated, polyunsaturated, and ω-6 fatty acids were not appreciably associated with fecundability. TFA intake was associated with reduced fecundability in North American women (for the fourth quartile vs. the first, FR = 0.86, 95% confidence interval (CI): 0.71, 1.04) but not Danish women (for the fourth quartile vs. the first, FR = 1.04, 95% CI: 0.86, 1.25), though intake among Danish women was low. In North America, ω-3 fatty acid intake was associated with higher fecundability, but there was no dose-response relationship (among persons who did not use fish oil supplements: for the fourth quartile vs. the first, FR = 1.40, 95% CI: 1.13, 1.73); no association was found in Danish women, among whom low intake was rare. In the present study, high TFA intake and low ω-3 fatty acid intake were associated with reduced fecundity.
Keywords: fatty acids, fertility, internet, prospective studies, trans fatty acids
Approximately 10%–15% of couples experience infertility, which is clinically defined as inability to conceive after 12 months of unprotected intercourse (1). Fats comprise 30%–40% of daily energy intake in Western countries. They are essential components of cell membranes and can modulate the expression of enzymes involved in the metabolism of prostaglandins and steroid hormones, which are critical for reproduction (2). The association between dietary fat intake and fertility has not been studied extensively. Fat-rich diets have been associated with poor oocyte development, possibly related to the induction of oxidative stress in the follicular environment (3, 4). However, the type of fat likely matters. In a prospective cohort study of female nurses, a higher intake of trans fatty acids (TFAs) was associated with ovulatory infertility (5) and endometriosis (6). In animal studies, a higher intake of ω-3 fatty acids has been associated with improved markers of fertility (7–9), particularly in male rodents (8, 9), but evidence in humans is limited (10–14).
We assessed the association between dietary fat consumption and time to pregnancy (TTP) among women participating in preconception cohort studies in Denmark and North America. Specifically, we examined total dietary fat intake and intakes of major subtypes of fatty acids, including saturated fatty acid (SFA), polyunsaturated fatty acid (PUFA), monounsaturated fatty acid (MUFA), TFA, ω-3 fatty acids, and ω-6 fatty acids in relation to fecundability.
METHODS
Study population
Snart Foraeldre (“Soon Parents”) (SF) is a prospective cohort study of women who were attempting to become pregnant in Denmark. SF is an expansion of the Snart Gravid (“Soon Pregnant”) study, which has been described previously (15, 16). Recruitment for SF was Internet-based and began in 2011 with advertisements placed on Danish health-related websites and blogs. Enrollment and primary data collection were conducted via online self-administered questionnaires. Beginning in January 2013 (10 days after enrollment), participants were invited to complete a comprehensive food frequency questionnaire (FFQ) designed specifically for this population (17). Eligible women were 18–45 years of age, residents of Denmark, in a stable relationship with a male partner, planning a pregnancy, and not receiving fertility treatment.
From 3,128 eligible SF participants, we excluded 533 who did not complete at least 1 follow-up questionnaire, 52 whose last menstrual period (LMP) was more than 6 months before study entry, and 77 who had missing or implausible LMP information or who were pregnant at study entry. Furthermore, in an effort to avoid misclassification of diet due to subfertility, we limited our analyses to the 2,053 women who had been trying to conceive for 6 cycles or fewer at study entry. Among these, 1,166 women completed the FFQ once it was implemented (83% completion). We then excluded 24 women with implausible total energy intakes (<600 or >3,800 kcal/day) and 16 who had more than 12 missing food items on the FFQ, for a final analytic sample of 1,126 women. SF was approved by the Danish Data Protection Agency and the Institutional Review Board at Boston University Medical Center.
Pregnancy Study Online (PRESTO) is also an Internet-based preconception cohort study of North American women who were attempting to become pregnant; it was modeled after SF (18). Recruitment began in 2013. Eligible women were 21–45 years of age, residents of the United States or Canada, in a stable relationship with a male partner, planning a pregnancy, and not receiving fertility treatments. Ten days after completion of the baseline questionnaire, PRESTO participants were invited to complete the National Cancer Institute’s Dietary Health Questionnaire II (19), a Web-based FFQ. Of the 2,576 eligible participants who completed the baseline questionnaire, we excluded 487 women with no follow-up data, 30 whose baseline LMP was more than 6 months before study entry, and 41 with missing or implausible LMP data or who were pregnant at study entry. Of the 2,018 remaining women, we excluded 280 women who had been trying to conceive for more than 6 cycles at study entry. Among these, 1,310 completed the FFQ (75% completion). We additionally excluded 20 women with implausible total energy intakes (<600 or >3,800 kcal/day), for a final analytic sample of 1,290 women. PRESTO was approved by the Institutional Review Board at Boston University Medical Center. Participants in both studies provided online informed consent. Web Figure 1 (available at https://academic.oup.com/aje) is a flow chart of analytic exclusions for each cohort.
Baseline questionnaires for SF and PRESTO included information on demographic, lifestyle, and behavioral factors, as well as reproductive and medical histories. To determine pregnancy status, self-administered online follow-up questionnaires were completed every 8 weeks for 12 months or until a reported conception.
Assessment of fatty acid intake
Dietary fat intake was estimated using the nutrient composition of all food items in the FFQ and validated in each population (17, 19). Total dietary fat intake was calculated by summing all servings of fat from individual foods and mixed recipes. In SF, information about the fat content of specific foods was obtained from the Danish nutrient database (20). In PRESTO, we used the National Cancer Institute’s DIET*CALC software (version 1.5.0) (21) to estimate fat consumption.
In the SF dietary validation study, deattenuated correlation coefficients when comparing the FFQ data to 4-day food records were 0.63 for total fat, 0.61 for SFA, 0.59 for MUFA, and 0.49 for PUFA (17). In the Dietary Health Questionnaire II validation study, deattenuated correlation coefficients when comparing the FFQ data to repeated 24-hour dietary recalls were 0.66 for total fat, 0.66 for SFA, 0.62 for MUFA, and 0.64 for PUFA (19).
Assessment of TTP
We estimated TTP using data from the baseline and follow-up questionnaires. Women with regular menstrual cycles were asked to report their usual menstrual cycle length. Among women with irregular cycles, we estimated menstrual cycle length based on date of LMP at baseline and prospectively reported LMP dates during follow-up. We estimated TTP, in discrete menstrual cycles, using the following formula: [(reported cycles of pregnancy attempt time at baseline) + [(LMP date from most recent follow-up questionnaire − date of baseline questionnaire)/cycle length] + 1]. TTP was rounded to the nearest whole number.
Assessment of covariates
Information on potential confounders (including age, race/ethnicity (PRESTO only), educational level, household income, height, weight, physical activity level, smoking, alcohol consumption, marital status, last method of contraception, parity, and use of supplements (including fish oil supplements)) was reported on the baseline questionnaire. We calculated body mass index (BMI) as weight in kilograms divided by height in meters squared. In SF, total metabolic equivalent (MET)–hours per week were calculated using the International Physical Activity Questionnaire short-form by summing the MET-hours from walking, moderate physical activity, and vigorous physical activity (hours/week × 3.3 METs, 4 METs, and 8 METs, respectively) (22). In PRESTO, total MET-hours per week were calculated by multiplying the average number of hours per week spent engaging in various activities by METs estimated from the Compendium of Physical Activities (23, 24). Potential confounders examined in the 2 cohorts were identical except for race/ethnicity (ascertained in PRESTO only) and educational level, which was ascertained differently across the 2 studies.
Data analysis
We performed parallel analyses across the 2 cohorts. Dietary fat intakes were categorized into quartiles based on the data distribution of the percentage of energy from each type of dietary fat (25). We analyzed data on total dietary fat and subtypes of fat (SFA, PUFA, MUFA, and TFA), as well as ω-3 and ω-6 fatty acids. We also assessed the ratio of ω-6 to ω-3 fatty acids because the average intake of ω-6 has increased markedly in Western diets, whereas the average intake of ω-3 fatty acids has decreased over time (2, 26). ω-3 fatty acids may have anti-inflammatory effects, whereas ω-6 fatty acids (linoleic and arachidonic acid) tend to be proinflammatory (27). In addition to categorical analyses, we used restricted cubic splines to model the association between fat and fecundability without imposing linearity on the association (28).
Women contributed at-risk menstrual cycles to the analysis until they reported pregnancy or one of the following censoring events: initiation of fertility treatment, cessation of pregnancy attempts, withdrawal, loss to follow-up, or 12 cycles, whichever came first. To account for variation in pregnancy attempt time at study entry (range, 0–6 cycles) and to reduce bias from left truncation (29, 30), we based risk sets only on observed cycles at risk using the Anderson-Gill data structure (31). We used proportional probabilities regression models (32, 33) to estimate fecundability ratios (FR), defined as the ratio of the cycle-specific probability of conception comparing exposed women with unexposed women. This model controls for the decline in fecundability over time by adjusting for binary indicators of cycle number at risk.
Potential confounders were selected based on the literature and assessment of a causal graph (Web Figure 2). We included potential risk factors for subfertility that were associated with total fat intake. Final models were adjusted for age (<25, 25–29, 30–34, or ≥35 years), BMI (<20, 20–24, 25–29, or ≥30), smoking status (never, former, current occasional, or current regular smoker), parity (0 vs. ≥1 births), alcohol consumption (<1, 1–6, 7–13, or ≥14 drinks/week), physical activity level (<10, 10–19, 20–39, or ≥40 MET-hours/week), last contraceptive method (hormonal, barrier, or natural methods), intercourse frequency (<1, 1, 2–3, or ≥4 times per week), and marital status (married or living as married vs. not). PRESTO models were adjusted additionally for race/ethnicity (non-Hispanic white: yes vs. no), educational level (high school diploma or less, some college, college degree, or graduate school), and household income (<50,000, 50,000–99,999, 100,000–149,999, or ≥150,000 USD). SF models were adjusted additionally for vocational training (none, basic/semiskilled, and <3, 3–4, or ≥5 years of higher education) and household income (<12,500, 12,500–24,999, 25,000–39,999, 40,000–64,999, 65,000–80,000,r>80,000 Danish krones). Models for dietary fat were adjusted for total energy intake by including a continuous energy intake variable in the regression models (34). We additionally constructed models adjusted for total fat (to interpret the relative proportion of each fatty acid group) and for the remaining fatty acids (to interpret increases in each type of fatty acid, holding the rest constant). In the assessment of ω-3 fatty acids, additional analyses were restricted to persons who did not use fish oil supplements.
In secondary analyses, we stratified the models by pregnancy attempt time at study entry (<3 vs. 3–6 cycles) to assess the extent to which reverse causation could have explained our results (e.g., whether subfertility caused a change in fat intake). We also reasoned that findings might differ by age and obesity status, given their strong relationship to fecundability and evidence that they might modify fat-fertility associations (5, 14). Therefore, we stratified models by age (<30 vs. ≥30 years) and BMI (<25 vs. ≥25). Out of concern that parity could be a causal intermediate (35, 36), models were fit with and without adjustment for parity. Results from models in which we excluded intercourse frequency, a potential causal intermediate, were similar to those from the original models.
We used multiple imputation to impute missing covariate data (37). Covariate missingness in SF ranged from 0% (age and fat intake) to 6% (household income). In PRESTO, covariate missingness ranged from 0% (age, educational level, parity, marital status, and fat intake) to 3.3% (household income). Within each cohort, we used PROC MI to create 5 imputed data sets based on imputation models with at least 100 covariates. We combined coefficients and standard errors across the imputed data sets using PROC MIANALYZE. Analyses were conducted using SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina) (38).
RESULTS
During 2013–2016, a total of 1,290 PRESTO participants contributed 818 pregnancies and 5,579 menstrual cycles of pregnancy attempt time, and 1,126 SF participants contributed 774 pregnancies and 4,307 menstrual cycles of pregnancy attempt time. The distributions of total dietary fat intake were similar in the 2 cohorts except for TFA, the intake of which was markedly lower in SF, and for ω-3 fatty acids and saturated fat, the intakes of which were higher in SF. In PRESTO, mean percentages of energy intake were 37.58% (standard deviation (SD), 6.88) for total fat, 7.83% (SD, 2.11) for PUFA, 11.47% (SD, 2.37) for SFA, 14.90% (SD, 3.54) for MUFA, 6.89% (SD, 1.84) for ω-6 fatty acids, 0.87% (SD, 0.45) for ω-3 fatty acids, and 1.62% (SD, 0.49) for TFA. In SF, mean percentages of energy intake were 36.20% (SD, 5.50) from total fat, 5.66% (SD, 0.87) for PUFA, 14.25% (SD, 2.74) for SFA, 13.50% (SD, 2.51) for MUFA, 4.39% (SD, 0.69) for ω-6 fatty acids, 1.07% (SD,.26) for ω-3 fatty acids, and 0.60% (SD, 0.19) for TFA. The top 5 foods that contributed to each fat subtype are shown in Web Table 1.
Table 1 presents baseline characteristics of study participants according to quartiles of total dietary fat, TFA, and ω-3 fatty acid intakes. Intake of total dietary fat was positively associated with nulliparity and smoking in both cohorts. Although total dietary fat intake was associated with lower BMI and physical activity level in PRESTO, it was associated with higher BMI and physical activity level in SF. TFA intake was inversely associated with nulliparity, physical activity level, and income in both cohorts and with educational level and alcohol intake in PRESTO only. In PRESTO, intake of ω-3 fatty acids was positively associated with educational level and alcohol intake and inversely associated with BMI. Although intake of ω-3 fatty acids was associated with higher household income in PRESTO, it was associated with lower household income in SF. In PRESTO, TFA and ω-3 fatty acid intakes were lower among non-Hispanic whites than among other racial/ethnic groups.
Table 1.
Baseline Characteristics of Study Participants, Pregnancy Study Online (n = 1,290), United States and Canada, and Snart Foraeldre (n = 1,126), Denmark, 2013–2016
| Characteristic | % Energy From Total Dietary Fat | % Energy From Trans Fatty Acids | % Energy From ω-3 Fatty Acids | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRESTO | Snart Foraeldre | PRESTO | Snart Foraeldre | PRESTO | Snart Foraeldre | |||||||
| Q1 (n = 319) | Q4 (n = 323) | Q1 (n = 277) | Q4 (n = 278) | Q1 (n = 358) | Q4 (n = 283) | Q1 (n = 270) | Q4 (n = 277) | Q1 (n = 309) | Q4 (n = 344) | Q1 (n = 271) | Q4 (n = 282) | |
| Age, yearsa | 29.8 (4.0) | 30.6 (3.8) | 28.3 (4.3) | 28.0 (4.3) | 30.7 (4.0) | 29.4 (4.1) | 28.2 (4.1) | 28.1 (4.4) | 29.7 (4.0) | 30.7 (4.0) | 28.5 (4.5) | 28.8 (4.4) |
| Total energy, kcala | 1,533 (519) | 1,698 (514) | 1,905 (529) | 1,841 (573) | 1,550 (481) | 1,620 (550) | 1,800 (479) | 1,941 (588) | 1,568 (492) | 1,675 (543) | 1,835 (517) | 1,810 (548) |
| BMIa,b | 26.6 (6.8) | 25.7 (6.2) | 23.7 (4.7) | 24.4 (5.2) | 24.7 (5.3) | 28.4 (7.4) | 23.9 (4.7) | 24.5 (5.9) | 26.5 (6.8) | 25.2 (6.0) | 24.1 (5.1) | 24.2 (4.9) |
| Education, yearsc | ||||||||||||
| ≤12 | 2.5 | 1.2 | 15.2 | 12.6 | 0.8 | 3.2 | 15.9 | 15.5 | 1.6 | 1.7 | 18.1 | 11.0 |
| ≥17 | 44.8 | 49.9 | 38.6 | 39.6 | 55.0 | 39.9 | 38.9 | 34.7 | 44.0 | 54.1 | 42.4 | 37.6 |
| Never smoker | 78.4 | 75.9 | 76.2 | 70.9 | 79.9 | 78.5 | 79.3 | 67.2 | 80.9 | 77.9 | 70.9 | 71.3 |
| Alcohol intake of ≥7 drinks/week | 16.0 | 14.2 | 7.2 | 10.8 | 20.1 | 9.9 | 6.7 | 10.8 | 13.9 | 17.4 | 7.4 | 9.2 |
| User of fish oil supplements | 13.5 | 28.5 | 14.6 | 24.5 | 26.3 | 12.0 | 18.7 | 16.3 | 14.6 | 28.2 | 15.3 | 21.0 |
| Nulliparous | 72.1 | 76.8 | 62.5 | 69.4 | 78.2 | 66.8 | 67.4 | 62.5 | 69.3 | 72.7 | 63.8 | 69.2 |
| Intercourse frequency, times/week | ||||||||||||
| <1 | 18.2 | 23.2 | 15.9 | 15.5 | 20.7 | 19.4 | 16.7 | 19.1 | 20.1 | 17.7 | 22.1 | 11.4 |
| ≥4 | 13.8 | 11.2 | 13.4 | 19.4 | 15.4 | 14.1 | 16.7 | 16.3 | 16.2 | 13.4 | 16.6 | 20.6 |
| Physical activity, MET-hours/week | ||||||||||||
| <10 | 8.8 | 7.4 | 11.9 | 9.7 | 4.5 | 14.5 | 9.6 | 13.4 | 9.1 | 7.9 | 14.8 | 6.0 |
| ≥40 | 46.1 | 42.7 | 43.0 | 51.8 | 57.5 | 31.8 | 51.5 | 45.5 | 44.3 | 44.2 | 41.7 | 55.3 |
| Last method of contraception: OCs | 43.6 | 31.6 | 56.0 | 56.1 | 36.9 | 39.6 | 58.5 | 57.0 | 42.1 | 33.1 | 58.7 | 49.3 |
| Household income (PRESTO/SF)d | ||||||||||||
| <$50,000/<24,999 kr | 14.1 | 13.6 | 15.2 | 17.3 | 8.4 | 23.3 | 16.7 | 20.9 | 17.2 | 13.7 | 15.9 | 15.3 |
| ≥$150,000/≥65,000 kr | 17.9 | 22.6 | 23.5 | 20.9 | 26.3 | 13.1 | 24.1 | 17.3 | 14.9 | 24.1 | 26.9 | 20.2 |
| Married or living as married | 96.9 | 96.6 | 96.8 | 94.6 | 97.2 | 96.5 | 96.7 | 94.6 | 97.1 | 96.2 | 94.8 | 96.5 |
| Non-Hispanic whitee | 90.0 | 86.7 | 91.1 | 85.5 | 91.9 | 83.4 | ||||||
Abbreviations: BMI, body mass index; DDK, Danish Kroners; MET, metabolic equivalents; OCs, oral contraceptives; PRESTO, Pregnancy Study Online; Q, quartile; SF, Snart Foraeldre.
a Values are expressed as mean (standard deviation).
b Weight (kg)/height (m)2.
c Education in PRESTO and vocational training in SF.
d Household income for PRESTO is in US dollars and Snart Foraeldre is in Danish kroner.
e Information on race/ethnicity is not available for Snart Foraeldre.
Intakes of total dietary fat as well as SFAs, MUFAs, and PUFAs were not appreciably associated with fecundability in either cohort after adjustment for several potential confounders (Table 2). After further adjustment for total dietary fat in multivariable models, the highest quartile of SFA intake was associated with reduced fecundability in PRESTO (for the fourth quartile vs. the first, FR = 0.78, 95% confidence interval (CI): 0.62, 0.99) but not in SF (for the fourth quartile vs. the first, FR = 0.96, 95% CI: 0.73, 1.27).
Table 2.
Intake of Total Dietary Fat and Major Fat Subtypes in Relation to Fecundability, Pregnancy Study Online (n = 1,290), United States and Canada, and Snart Foraeldre (n = 1,126), Denmark, 2013–2016
| Study and Quartile | % Energy Median (Range) | No. of Women | No. of Pregnancies | No. of Cycles at Risk | FRa | 95% CI | FRb | 95% CI | FRc | 95% CI | FRd | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Fat | ||||||||||||
| PRESTO | ||||||||||||
| 1 | 30.2 (15.5–32.9) | 319 | 192 | 1,395 | 1.00 | Referent | 1.00 | Referent | ||||
| 2 | 34.9 (32.9–37.0) | 311 | 206 | 1,390 | 1.11 | 0.93, 1.33 | 1.10 | 0.92, 1.32 | ||||
| 3 | 39.4 (37.0–42.0) | 337 | 216 | 1,402 | 1.12 | 0.94, 1.34 | 1.12 | 0.93, 1.34 | ||||
| 4 | 45.4 (42.1–67.2) | 323 | 204 | 1,392 | 1.05 | 0.88, 1.26 | 1.08 | 0.90, 1.30 | ||||
| Snart Foraeldre | ||||||||||||
| 1 | 30.4 (15.1–32.7) | 277 | 196 | 1,078 | 1.00 | Referent | 1.00 | Referent | ||||
| 2 | 34.4 (32.7–35.7) | 268 | 183 | 1,071 | 0.92 | 0.77, 1.11 | 0.90 | 0.75, 1.08 | ||||
| 3 | 37.5 (35.7–39.4) | 303 | 214 | 1,081 | 1.10 | 0.92, 1.30 | 1.08 | 0.91, 1.28 | ||||
| 4 | 41.8 (39.4–66.4) | 278 | 181 | 1,077 | 0.94 | 0.78, 1.12 | 0.90 | 0.75, 1.08 | ||||
| Saturated Fat | ||||||||||||
| PRESTO | ||||||||||||
| 1 | 8.9 (4.3–10.0) | 332 | 205 | 1,390 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | ||
| 2 | 10.8 (10.0–11.5) | 333 | 224 | 1,396 | 1.07 | 0.90, 1.27 | 1.08 | 0.91, 1.28 | 1.02 | 0.85, 1.22 | ||
| 3 | 12.2 (11.5–13.0) | 323 | 211 | 1,396 | 1.03 | 0.87, 1.23 | 1.10 | 0.92, 1.31 | 1.01 | 0.83, 1.22 | ||
| 4 | 14.2 (13.1–22.0) | 302 | 178 | 1,397 | 0.87 | 0.72, 1.05 | 0.91 | 0.75, 1.10 | 0.78 | 0.62, 0.99 | ||
| Snart Foraeldre | ||||||||||||
| 1 | 11.4 (6.1–12.4) | 277 | 191 | 1,074 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | ||
| 2 | 13.2 (12.4–14.1) | 290 | 204 | 1,078 | 1.02 | 0.85, 1.21 | 0.94 | 0.79, 1.12 | 0.94 | 0.77, 1.15 | ||
| 3 | 14.8 (14.1–15.7) | 274 | 184 | 1,078 | 0.99 | 0.82, 1.18 | 0.96 | 0.80, 1.15 | 0.96 | 0.77, 1.20 | ||
| 4 | 17.0 (15.7–31.2) | 285 | 195 | 1,077 | 1.01 | 0.85, 1.21 | 0.96 | 0.80, 1.15 | 0.96 | 0.73, 1.27 | ||
| Monounsaturated Fat | ||||||||||||
| PRESTO | ||||||||||||
| 1 | 11.2 (4.5–12.4) | 317 | 198 | 1,390 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | ||
| 2 | 13.4 (12.4–14.4) | 310 | 193 | 1,396 | 0.98 | 0.82, 1.18 | 0.95 | 0.79, 1.14 | 0.91 | 0.75, 1.11 | ||
| 3 | 15.7 (14.4–17.0) | 334 | 215 | 1,400 | 1.05 | 0.88, 1.26 | 1.02 | 0.85, 1.22 | 0.94 | 0.75, 1.19 | ||
| 4 | 19.0 (17.0–32.4) | 329 | 212 | 1,393 | 1.04 | 0.87, 1.25 | 1.05 | 0.87, 1.26 | 0.93 | 0.68, 1.26 | ||
| Snart Foraeldre | ||||||||||||
| 1 | 11.0 (5.1–11.9) | 283 | 203 | 1,079 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | ||
| 2 | 12.6 (11.9–13.2) | 271 | 182 | 1,075 | 0.89 | 0.75, 1.07 | 0.86 | 0.71, 1.02 | 0.83 | 0.68, 1.02 | ||
| 3 | 13.9 (13.2–14.8) | 286 | 204 | 1,075 | 1.05 | 0.88, 1.24 | 1.01 | 0.85, 1.20 | 0.96 | 0.76, 1.22 | ||
| 4 | 16.1 (14.8–26.0) | 286 | 185 | 1,078 | 0.92 | 0.77, 1.10 | 0.89 | 0.74, 1.07 | 0.82 | 0.60, 1.13 | ||
| Polyunsaturated Fat | ||||||||||||
| PRESTO | ||||||||||||
| 1 | 5.7 (2.8–6.3) | 323 | 197 | 1,394 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | ||
| 2 | 6.9 (6.3–7.4) | 303 | 197 | 1,394 | 1.01 | 0.84, 1.21 | 0.95 | 0.79, 1.15 | 0.94 | 0.78, 1.13 | ||
| 3 | 8.1 (7.4–8.9) | 318 | 209 | 1,400 | 1.06 | 0.89, 1.27 | 1.01 | 0.84, 1.21 | 0.98 | 0.80, 1.20 | ||
| 4 | 10.1 (8.9–18.2) | 346 | 215 | 1,391 | 1.08 | 0.91, 1.30 | 1.07 | 0.89, 1.28 | 1.01 | 0.80, 1.28 | ||
| Snart Foraeldre | ||||||||||||
| 1 | 4.8 (2.5–5.1) | 277 | 189 | 1,070 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | ||
| 2 | 5.4 (5.1–5.6) | 271 | 194 | 1,069 | 1.00 | 0.84, 1.20 | 0.95 | 0.79, 1.14 | 0.95 | 0.79, 1.14 | ||
| 3 | 5.8 (5.6–6.1) | 292 | 204 | 1,088 | 1.05 | 0.88, 1.25 | 1.02 | 0.85, 1.22 | 1.02 | 0.84, 1.23 | ||
| 4 | 6.5 (6.1–10.0) | 286 | 187 | 1,080 | 0.95 | 0.79, 1.14 | 0.91 | 0.76, 1.10 | 0.91 | 0.74, 1.12 | ||
| Trans Fatty Acids | ||||||||||||
| PRESTO | ||||||||||||
| 1 | 1.1 (0.3–1.3) | 358 | 234 | 1,404 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | ||
| 2 | 1.5 (1.3–1.6) | 316 | 206 | 1,395 | 0.91 | 0.77, 1.08 | 0.92 | 0.78, 1.09 | 0.93 | 0.78, 1.10 | ||
| 3 | 1.8 (1.6–2.0) | 333 | 211 | 1,397 | 0.94 | 0.80, 1.12 | 0.97 | 0.82, 1.15 | 0.98 | 0.82, 1.16 | ||
| 4 | 2.2 (2.0–4.1) | 283 | 167 | 1,383 | 0.78 | 0.65, 0.93 | 0.86 | 0.71, 1.04 | 0.86 | 0.71, 1.04 | ||
| Snart Foraeldre | ||||||||||||
| 1 | 0.4 (0.2–0.5) | 270 | 187 | 1,062 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | ||
| 2 | 0.5 (0.5–0.6) | 304 | 215 | 1,122 | 1.09 | 0.91, 1.29 | 1.05 | 0.88, 1.26 | 1.06 | 0.88, 1.27 | ||
| 3 | 0.6 (0.6–0.7) | 275 | 180 | 1,054 | 1.01 | 0.84, 1.21 | 0.94 | 0.78, 1.14 | 0.96 | 0.78, 1.17 | ||
| 4 | 0.8 (0.7–1.9) | 277 | 192 | 1,069 | 1.07 | 0.89, 1.28 | 1.04 | 0.86, 1.25 | 1.06 | 0.85, 1.32 | ||
| ω-3 Fatty Acids | ||||||||||||
| PRESTO | ||||||||||||
| 1 | 0.5 (0.3–0.6) | 309 | 182 | 1,404 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 2 | 0.7 (0.6–0.7) | 327 | 213 | 1,412 | 1.19 | 0.99, 1.42 | 1.21 | 1.01, 1.46 | 1.21 | 1.00, 1.46 | 1.26 | 1.02, 1.54 |
| 3 | 0.8 (0.7–1.0) | 310 | 203 | 1,350 | 1.12 | 0.93, 1.34 | 1.14 | 0.95, 1.38 | 1.14 | 0.93, 1.38 | 1.19 | 0.96, 1.46 |
| 4 | 1.3 (1.0–4.4) | 344 | 220 | 1,413 | 1.19 | 0.99, 1.43 | 1.21 | 1.01, 1.46 | 1.20 | 0.96, 1.49 | 1.40 | 1.13, 1.73 |
| Snart Foraeldre | ||||||||||||
| 1 | 0.8 (0.4–0.9) | 271 | 188 | 1,051 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 2 | 1.0 (0.9–1.0) | 289 | 205 | 1,122 | 1.00 | 0.84, 1.19 | 0.96 | 0.80, 1.15 | 0.96 | 0.80, 1.15 | 0.98 | 0.81, 1.20 |
| 3 | 1.1 (1.0–1.2) | 284 | 198 | 1,080 | 1.02 | 0.85, 1.22 | 0.99 | 0.82, 1.19 | 0.99 | 0.82, 1.20 | 0.96 | 0.78, 1.18 |
| 4 | 1.3 (1.2–3.4) | 282 | 183 | 1,054 | 0.95 | 0.79, 1.14 | 0.92 | 0.76, 1.11 | 0.92 | 0.74, 1.13 | 0.96 | 0.78, 1.19 |
| ω-6 Fatty Acids | ||||||||||||
| PRESTO | ||||||||||||
| 1 | 5.0 (2.1–5.6) | 322 | 199 | 1,381 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | ||
| 2 | 6.1 (5.6–6.6) | 310 | 201 | 1,420 | 1.00 | 0.84, 1.20 | 0.97 | 0.81, 1.16 | 0.94 | 0.78, 1.14 | ||
| 3 | 7.2 (6.6–7.8) | 321 | 208 | 1,385 | 1.05 | 0.88, 1.26 | 1.04 | 0.86, 1.24 | 0.99 | 0.82, 1.21 | ||
| 4 | 8.9 (7.8–16.0) | 337 | 210 | 1,393 | 1.04 | 0.87, 1.25 | 1.03 | 0.85, 1.23 | 0.95 | 0.75, 1.19 | ||
| Snart Foraeldre | ||||||||||||
| 1 | 3.7 (2.0–3.9) | 271 | 181 | 1,066 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | ||
| 2 | 4.2 (3.9–4.3) | 280 | 203 | 1,071 | 1.09 | 0.91, 1.30 | 1.05 | 0.88, 1.26 | 1.06 | 0.88, 1.28 | ||
| 3 | 4.5 (4.3–4.7) | 281 | 184 | 1,093 | 0.96 | 0.79, 1.15 | 0.96 | 0.79, 1.16 | 0.97 | 0.80, 1.18 | ||
| 4 | 5.1 (4.7–8.4) | 294 | 206 | 1,077 | 1.08 | 0.90, 1.29 | 1.04 | 0.86, 1.25 | 1.06 | 0.86, 1.30 | ||
| Ratio of ω-3 to ω-6 Fatty Acids | ||||||||||||
| PRESTO | ||||||||||||
| 1 | 0.08 (0.04–0.09) | 274 | 161 | 1,236 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | ||
| 2 | 0.10 (0.10–0.11) | 389 | 254 | 1,676 | 1.09 | 0.91, 1.31 | 1.13 | 0.94, 1.36 | 1.13 | 0.94, 1.36 | ||
| 3 | 0.13 (0.12–0.14) | 332 | 221 | 1,422 | 1.13 | 0.94, 1.36 | 1.17 | 0.97, 1.41 | 1.17 | 0.97, 1.41 | ||
| 4 | 0.18 (0.15–0.48) | 295 | 182 | 1,245 | 1.10 | 0.90, 1.33 | 1.09 | 0.89, 1.33 | 1.07 | 0.88, 1.31 | ||
| Snart Foraeldre | ||||||||||||
| 1 | 0.20 (0.14–0.21) | 283 | 201 | 1,132 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | ||
| 2 | 0.23 (0.22–0.23) | 249 | 172 | 938 | 1.01 | 0.85, 1.22 | 0.99 | 0.82, 1.19 | 0.99 | 0.82, 1.19 | ||
| 3 | 0.25 (0.24–0.26) | 293 | 200 | 1,086 | 1.01 | 0.85, 1.21 | 0.99 | 0.83, 1.18 | 0.99 | 0.83, 1.19 | ||
| 4 | 0.29 (0.27–0.70) | 301 | 201 | 1,151 | 1.00 | 0.84, 1.20 | 1.00 | 0.84, 1.20 | 1.01 | 0.84, 1.21 | ||
Abbreviations: CI, confidence interval; FR, fecundability ratio; PRESTO, Pregnancy Study Online.
a Adjusted for total energy intake and age.
b Adjusted for energy intake, age, educational level, body mass index (weight (kg)/height (m)2), smoking status, total physical activity level, parity, alcohol intake, intercourse frequency, marital status, household income, last method of contraception, and race/ethnicity (PRESTO only).
c Adjusted for the covariates in footnote b and total fat intake.
d Adjusted for the covariates in footnote b and restricted to persons who did not use ω-3 fatty acid supplements (1,025 women (80%) in the PRESTO cohort and 922 women (82%) in the Snart Foraeldre cohort).
TFA intake was associated with reduced fecundability in PRESTO (for the fourth quartile vs. the first, FR = 0.86, 95% CI: 0.71, 1.04) but not in SF (for the fourth quartile vs. the first, FR = 1.04, 95% CI: 0.86, 1.25). Further adjustment for total fat had little effect on these estimates. Household income had the largest influence on the FR (increase in effect estimate = 10%).
In PRESTO, women in the lowest quartile of ω-3 fatty acid intake had lower fecundability than did women in the other quartiles, who had similar fecundability (for the fourth quartile vs. the first, FR = 1.21, 95% CI: 1.01, 1.46; for the top 3 quartiles vs. the first, FR = 1.19, 95% CI: 1.02, 1.39). There was little association between ω-3 fatty acid intake and fecundability in SF. Further adjustment for total fat intake produced similar results. Although use of fish oil supplements was not appreciably associated with fecundability in PRESTO (FR = 1.02, 95% CI: 0.87, 1.19) or SF (FR = 1.03, 95% CI: 0.88, 1.22), stronger associations between dietary ω-3 intake and fecundability were found among persons who did not take fish oil supplements in PRESTO (for the fourth quartile vs. the first, FR = 1.40, 95% CI: 1.13, 1.73; for the top 3 quartiles vs. the first,: FR = 1.27, 95% CI: 1.07, 1.51). Finally, there was little evidence that intake of ω-6 fatty acids or the ratio of ω-3 to ω-6 fatty acids appreciably influenced fecundability in either cohort. In a substitution model in PRESTO in which we replaced 1% of energy from ω-3 fatty acids with 1% of energy from TFA, the FR was 0.91 (95% CI: 0.78, 1.07).
Restricted cubic spline curves were generally consistent with results from categorical analyses (Figures 1 and 2). Multivariable models in which we adjusted for the remaining fatty acids yielded results similar to those from models adjusted for total fat and all other covariates (data not shown). Omitting parity from the models made little difference in the FRs (data not shown).
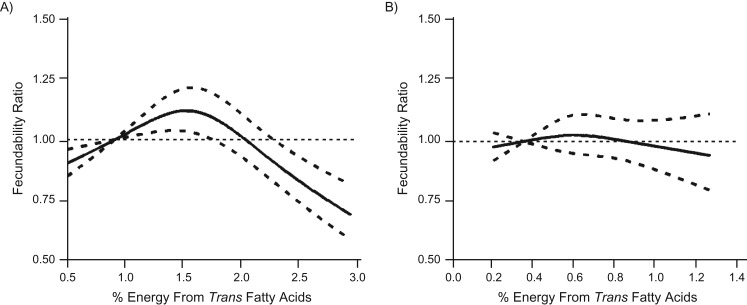
Figure 1.
Association between percentage of energy from trans fatty acids and fecundability, fitted by restricted cubic splines, Pregnancy Study Online (A), United States and Canada, and Snart Foraeldre (B), Denmark, 2013–2016. A) The reference level is 0.91 (5th percentile of the distribution). There are 3 knots located at 10th, 50th, and 90th percentiles (1.04, 1.57, and 2.25). The solid line indicates the fecundability ratio, and the dashed lines indicate the 95% confidence bounds. B) The reference level is 0.35 (5th percentile of the distribution). There are 3 knots located at 10th, 50th, and 90th percentiles (0.39, 0.57, and 0.84). The solid line indicates the fecundability ratio, and the dashed lines indicate the 95% confidence bounds.
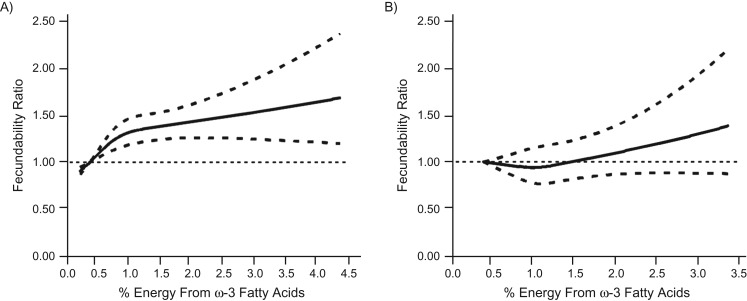
Figure 2.
Association between percentage of energy from ω-3 fats and fecundability among those who did not consume fish oil supplements, fitted by restricted cubic splines, Pregnancy Study Online (A), United States and Canada, and Snart Foraeldre (B), Denmark, 2013–2016. A) The reference level is 0.43. There are 3 knots located at 10th, 50th, and 90th percentiles (0.49, 0.72, and 1.36). The solid line indicates the fecundability ratio, and the dashed lines indicate the 95% confidence bounds. After restriction to those who did not consume fish oil supplements, n = 1,025. B) The reference level is 0.43. There are 3 knots located at 10th, 50th, and 90th percentiles (0.81, 1.04, and 1.36). The solid line indicates the fecundability ratio, and the dashed lines indicate 95% confidence bounds. After restriction to those who did not consume fish oil supplements, n = 730.
When we stratified the data by attempt time at study entry, the associations of fecundability with TFA and ω-3 fatty acids were somewhat stronger among PRESTO participants with shorter attempt times, indicating little evidence of bias due to reverse causation (Table 3). Age stratification also produced relatively uniform findings (Table 4). In PRESTO, the inverse association between TFA intake and fecundability was stronger among lean women (Table 5). In contrast to the overall results, FRs increased monotonically with increasing intake of ω-3 fatty acids among lean women, but little association was observed among overweight women. In SF, findings were generally similar across overweight strata with the exception of ω-6 fatty acids, for which high intake was associated with increased fecundability among lean women but reduced fecundability among overweight/obese women.
Table 3.
Association Between Dietary Fat Intake and Fecundability, Stratified by Pregnancy Attempt Time at Study Entry, Pregnancy Study Online (n = 1,290), United States and Canada, and Snart Foraeldre (n = 1,126), Denmark, 2013–2016
| Study and Quartile | Median (Range) | Attempt Time at Study Entry | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <3 Cycles | 3–6 Cycles | ||||||||
| No. of Pregnancies | No. of Cycles at Risk | FRa | 95% CI | No. of Pregnancies | No. of Cycles at Risk | FRa | 95% CI | ||
| % Energy From Trans Fatty Acids | |||||||||
| PRESTO | |||||||||
| 1 | 1.1 (0.3–1.3) | 171 | 933 | 1.00 | Referent | 63 | 471 | 1.00 | Referent |
| 2 | 1.5 (1.3–1.6) | 155 | 950 | 0.97 | 0.80, 1.19 | 51 | 445 | 0.97 | 0.77, 1.23 |
| 3 | 1.8 (1.6–2.0) | 162 | 991 | 1.02 | 0.84, 1.25 | 49 | 406 | 0.94 | 0.75, 1.16 |
| 4 | 2.2 (2.0–4.1) | 114 | 973 | 0.80 | 0.63, 1.00 | 53 | 410 | 0.98 | 0.83, 1.16 |
| Snart Foraeldre | |||||||||
| 1 | 0.4 (0.2–0.5) | 145 | 786 | 1.00 | Referent | 42 | 276 | 1.00 | Referent |
| 2 | 0.5 (0.5–0.6) | 157 | 795 | 1.05 | 0.86, 1.29 | 58 | 327 | 1.05 | 0.89, 1.25 |
| 3 | 0.6 (0.6–0.7) | 128 | 748 | 0.91 | 0.73, 1.13 | 52 | 306 | 1.01 | 0.86, 1.20 |
| 4 | 0.8 (0.7–1.9) | 141 | 757 | 1.02 | 0.83, 1.26 | 51 | 312 | 1.04 | 0.87, 1.24 |
| % Energy From ω-3 Fatty Acids | |||||||||
| PRESTOb | |||||||||
| 1 | 0.5 (0.3–0.6) | 105 | 873 | 1.00 | Referent | 49 | 359 | 1.00 | Referent |
| 2 | 0.7 (0.6–0.7) | 127 | 777 | 1.43 | 1.12, 1.82 | 45 | 400 | 0.90 | 0.76, 1.08 |
| 3 | 0.8 (0.7–1.0) | 118 | 736 | 1.37 | 1.07, 1.75 | 35 | 310 | 0.90 | 0.68, 1.19 |
| 4 | 1.3 (1.0–4.4) | 117 | 647 | 1.52 | 1.18, 1.96 | 45 | 314 | 0.96 | 0.80, 1.15 |
| Snart Foraeldreb | |||||||||
| 1 | 0.8 (0.4–0.9) | 115 | 637 | 1.00 | Referent | 41 | 252 | 1.00 | Referent |
| 2 | 1.0 (0.9–1.0) | 121 | 664 | 0.94 | 0.75, 1.19 | 45 | 254 | 1.08 | 0.86, 1.35 |
| 3 | 1.1 (1.0–1.2) | 115 | 640 | 0.97 | 0.76, 1.23 | 45 | 284 | 1.02 | 0.83, 1.25 |
| 4 | 1.3 (1.2–3.4) | 106 | 541 | 0.97 | 0.76, 1.23 | 42 | 261 | 0.97 | 0.80, 1.19 |
| % Energy From Total Fat | |||||||||
| PRESTO | |||||||||
| 1 | 30.2 (15.5–32.9) | 138 | 943 | 1.00 | Referent | 54 | 452 | 1.00 | Referent |
| 2 | 34.9 (32.9–37.0) | 144 | 931 | 1.12 | 0.90, 1.39 | 62 | 459 | 0.99 | 0.75, 1.31 |
| 3 | 39.4 (37.0–42.0) | 161 | 940 | 1.18 | 0.95, 1.45 | 55 | 462 | 0.92 | 0.68, 1.23 |
| 4 | 45.4 (42.1–67.2) | 159 | 1,033 | 1.11 | 0.90, 1.38 | 45 | 359 | 0.98 | 0.72, 1.32 |
| Snart Foraeldre | |||||||||
| 1 | 30.4 (15.1–32.7) | 145 | 778 | 1.00 | Referent | 51 | 300 | 1.00 | Referent |
| 2 | 34.4 (32.7–35.7) | 138 | 773 | 0.95 | 0.77, 1.17 | 45 | 298 | 0.93 | 0.72, 1.19 |
| 3 | 37.5 (35.7–39.4) | 148 | 730 | 1.06 | 0.86, 1.30 | 66 | 351 | 1.03 | 0.88, 1.21 |
| 4 | 41.8 (39.4–66.4) | 140 | 805 | 0.92 | 0.74, 1.14 | 41 | 272 | 0.98 | 0.77, 1.25 |
Abbreviations: CI, confidence interval; FR, fecundability ratio; PRESTO, Pregnancy Study Online.
a Adjusted for energy intake, age, body mass index (weight (kg)/height (m)2), smoking status, total physical activity level, educational level, parity, alcohol intake, intercourse frequency, marital status, household income, last method of contraception, and race/ethnicity (PRESTO only).
b Restricted to persons who did not use fish oil supplements.
Table 4.
Association Between Dietary Fat Intake and Fecundability, Stratified by Age at Baseline, Pregnancy Study Online (n = 1,290), United States and Canada, and Snart Foraeldre (n = 1,126), Denmark, 2013–2016
| Study and Quartile | Median (Range) | Age at Baseline, years | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <30 | ≥30 | ||||||||
| No. of Pregnancies | No. of Cycles at Risk | FRa | 95% CI | No. of Pregnancies | No. of Cycles at Risk | FRa | 95% CI | ||
| % Energy From Trans Fatty Acids | |||||||||
| PRESTO | |||||||||
| 1 | 1.1 (0.3–1.3) | 101 | 550 | 1.00 | Referent | 133 | 854 | 1.00 | Referent |
| 2 | 1.5 (1.3–1.6) | 92 | 607 | 0.94 | 0.73, 1.22 | 114 | 788 | 0.99 | 0.79, 1.25 |
| 3 | 1.8 (1.6–2.0) | 109 | 693 | 1.00 | 0.78, 1.29 | 102 | 704 | 0.98 | 0.78, 1.25 |
| 4 | 2.2 (2.0–4.1) | 95 | 730 | 0.90 | 0.69, 1.18 | 72 | 653 | 0.82 | 0.62, 1.07 |
| Snart Foraeldre | |||||||||
| 1 | 0.4 (0.2–0.5) | 121 | 698 | 1.00 | Referent | 66 | 364 | 1.00 | Referent |
| 2 | 0.5 (0.5–0.6) | 136 | 662 | 1.09 | 0.87, 1.36 | 79 | 460 | 1.04 | 0.77, 1.41 |
| 3 | 0.6 (0.6–0.7) | 122 | 719 | 0.91 | 0.73, 1.15 | 58 | 335 | 1.13 | 0.81, 1.56 |
| 4 | 0.8 (0.7–1.9) | 124 | 722 | 0.92 | 0.73, 1.16 | 68 | 347 | 1.27 | 0.93, 1.73 |
| % Energy From ω-3 Fatty Acids | |||||||||
| PRESTOb | |||||||||
| 1 | 0.5 (0.3–0.6) | 91 | 657 | 1.00 | Referent | 63 | 575 | 1.00 | Referent |
| 2 | 0.7 (0.6–0.7) | 90 | 629 | 1.13 | 0.86, 1.50 | 82 | 548 | 1.37 | 1.01, 1.86 |
| 3 | 0.8 (0.7–1.0) | 75 | 499 | 1.06 | 0.80, 1.41 | 78 | 547 | 1.25 | 0.91, 1.71 |
| 4 | 1.3 (1.0–4.4) | 70 | 365 | 1.32 | 0.98, 1.77 | 92 | 596 | 1.34 | 0.98, 1.82 |
| Snart Foraeldreb | |||||||||
| 1 | 0.8 (0.4–0.9) | 103 | 562 | 1.00 | Referent | 53 | 327 | 1.00 | Referent |
| 2 | 1.0 (0.9–1.0) | 103 | 607 | 0.91 | 0.71, 1.16 | 63 | 311 | 1.21 | 0.86, 1.71 |
| 3 | 1.1 (1.0–1.2) | 112 | 620 | 0.96 | 0.75, 1.23 | 48 | 304 | 1.02 | 0.69, 1.50 |
| 4 | 1.3 (1.2–3.4) | 91 | 516 | 0.87 | 0.67, 1.12 | 57 | 286 | 1.15 | 0.79, 1.66 |
| % Energy From Total Fat | |||||||||
| PRESTO | |||||||||
| 1 | 30.2 (15.5–32.9) | 98 | 704 | 1.00 | Referent | 94 | 691 | 1.00 | Referent |
| 2 | 34.9 (32.9–37.0) | 103 | 710 | 1.07 | 0.83, 1.39 | 103 | 680 | 1.16 | 0.90, 1.50 |
| 3 | 39.4 (37.0–42.0) | 105 | 609 | 1.14 | 0.88, 1.47 | 111 | 793 | 1.06 | 0.83, 1.36 |
| 4 | 45.4 (42.1–67.2) | 91 | 557 | 1.07 | 0.82, 1.39 | 113 | 835 | 1.12 | 0.86, 1.45 |
| Snart Foraeldre | |||||||||
| 1 | 30.4 (15.1–32.7) | 127 | 695 | 1.00 | Referent | 69 | 383 | 1.00 | Referent |
| 2 | 34.4 (32.7–35.7) | 111 | 642 | 0.85 | 0.68, 1.07 | 72 | 429 | 1.00 | 0.73, 1.36 |
| 3 | 37.5 (35.7–39.4) | 132 | 704 | 0.96 | 0.77, 1.20 | 82 | 377 | 1.21 | 0.91, 1.61 |
| 4 | 41.8 (39.4–66.4) | 133 | 760 | 0.87 | 0.70, 1.08 | 48 | 317 | 0.90 | 0.64, 1.27 |
Abbreviations: CI, confidence interval; FR, fecundability ratio; PRESTO, Pregnancy Study Online.
a Adjusted for energy intake, age, body mass index (weight (kg)/height (m)2), smoking status, total physical activity level, educational level, parity, alcohol intake, intercourse frequency, marital status, household income, last method of contraception, and race/ethnicity (PRESTO only).
b Restricted to persons who did not use fish oil supplements.
Table 5.
Association Between Dietary Fat Intake and Fecundability, Stratified by Body Mass Index, Pregnancy Study Online (n = 1,290), United States and Canada, and Snart Foraeldre (n = 1,126), Denmark, 2013–2016
| Study and Quartile | Median (Range) | Body Mass Indexa | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <25 | ≥25 | ||||||||
| No. of Pregnancies | No. of Cycles at Risk | FRb | 95% CI | No. of Pregnancies | No. of Cycles at Risk | FRb | 95% CI | ||
| % Energy From Trans Fatty Acids | |||||||||
| PRESTO | |||||||||
| 1 | 1.1 (0.3–1.3) | 159 | 828 | 1.00 | Referent | 75 | 576 | 1.00 | Referent |
| 2 | 1.5 (1.3–1.6) | 115 | 766 | 0.79 | 0.64, 0.99 | 91 | 629 | 1.05 | 0.92, 1.19 |
| 3 | 1.8 (1.6–2.0) | 127 | 790 | 0.86 | 0.69, 1.06 | 84 | 607 | 1.05 | 0.90, 1.21 |
| 4 | 2.2 (2.0–4.1) | 84 | 547 | 0.80 | 0.69, 1.03 | 83 | 836 | 0.97 | 0.82, 1.15 |
| Snart Foraeldre | |||||||||
| 1 | 0.4 (0.2–0.5) | 131 | 773 | 1.00 | Referent | 56 | 289 | 1.00 | Referent |
| 2 | 0.5 (0.5–0.6) | 153 | 801 | 1.11 | 0.90, 1.37 | 62 | 321 | 0.95 | 0.69, 1.31 |
| 3 | 0.6 (0.6–0.7) | 132 | 728 | 1.02 | 0.82, 1.27 | 48 | 326 | 0.75 | 0.53, 1.07 |
| 4 | 0.8 (0.7–1.9) | 123 | 725 | 1.07 | 0.86, 1.34 | 69 | 344 | 1.21 | 0.64, 2.31 |
| % Energy From ω-3 Fatty Acids | |||||||||
| PRESTOc | |||||||||
| 1 | 0.5 (0.3–0.6) | 87 | 593 | 1.00 | Referent | 67 | 639 | 1.00 | Referent |
| 2 | 0.7 (0.6–0.7) | 86 | 543 | 1.13 | 0.85, 1.49 | 86 | 634 | 1.11 | 0.86, 1.43 |
| 3 | 0.8 (0.7–1.0) | 88 | 535 | 1.18 | 0.90, 1.56 | 65 | 511 | 1.06 | 0.86, 1.30 |
| 4 | 1.3 (1.0–4.4) | 112 | 576 | 1.41 | 1.09, 1.83 | 50 | 385 | 1.03 | 0.83, 1.28 |
| Snart Foraeldrec | |||||||||
| 1 | 0.8 (0.4–0.9) | 109 | 633 | 1.00 | Referent | 47 | 256 | 1.00 | Referent |
| 2 | 1.0 (0.9–1.0) | 117 | 654 | 0.98 | 0.77, 1.25 | 49 | 264 | 1.02 | 0.71, 1.46 |
| 3 | 1.1 (1.0–1.2) | 113 | 653 | 1.00 | 0.79, 1.28 | 47 | 271 | 0.84 | 0.56, 1.25 |
| 4 | 1.3 (1.2–3.4) | 100 | 539 | 1.00 | 0.78, 1.28 | 48 | 263 | 0.93 | 0.62, 1.39 |
| % Energy From ω-6 Fatty Acids | |||||||||
| PRESTO | |||||||||
| 1 | 5.0 (2.1–5.6) | 111 | 704 | 1.00 | Referent | 88 | 677 | 1.00 | Referent |
| 2 | 6.1 (5.6–6.6) | 116 | 738 | 0.95 | 0.75, 1.20 | 85 | 682 | 0.98 | 0.86, 1.11 |
| 3 | 7.2 (6.6–7.8) | 123 | 682 | 1.10 | 0.87, 1.39 | 85 | 703 | 0.98 | 0.86, 1.11 |
| 4 | 8.9 (7.8–16.0) | 135 | 807 | 1.03 | 0.81, 1.30 | 75 | 586 | 0.99 | 0.86, 1.13 |
| Snart Foraeldre | |||||||||
| 1 | 3.7 (2.0–3.9) | 124 | 776 | 1.00 | Referent | 57 | 290 | 1.00 | Referent |
| 2 | 4.2 (3.9–4.3) | 141 | 772 | 1.10 | 0.89, 1.37 | 62 | 299 | 0.94 | 0.68, 1.31 |
| 3 | 4.5 (4.3–4.7) | 137 | 813 | 1.04 | 0.84, 1.30 | 47 | 280 | 0.81 | 0.57, 1.15 |
| 4 | 5.1 (4.7–8.4) | 137 | 666 | 1.26 | 1.01, 1.57 | 69 | 411 | 0.73 | 0.53, 1.02 |
| Ratio of ω-3 to ω-6 Fatty Acids | |||||||||
| PRESTO | |||||||||
| 1 | 0.1 (0.0–0.1) | 94 | 660 | 1.00 | Referent | 67 | 576 | 1.00 | Referent |
| 2 | 0.1 (0.1–0.1) | 150 | 827 | 1.26 | 1.00, 1.60 | 104 | 849 | 0.99 | 0.86, 1.14 |
| 3 | 0.1 (0.1–0.1) | 125 | 715 | 1.25 | 0.98, 1.60 | 96 | 707 | 1.00 | 0.88, 1.14 |
| 4 | 0.2 (0.2–0.5) | 116 | 729 | 1.19 | 0.93, 1.53 | 66 | 516 | 0.97 | 0.78, 1.20 |
| Snart Foraeldre | |||||||||
| 1 | 0.2 (0.1–0.2) | 142 | 765 | 1.00 | Referent | 59 | 367 | 1.00 | Referent |
| 2 | 0.2 (0.2–0.2) | 116 | 646 | 0.95 | 0.76, 1.18 | 56 | 292 | 1.12 | 0.79, 1.58 |
| 3 | 0.3 (0.2–0.3) | 134 | 785 | 0.93 | 0.75, 1.15 | 66 | 301 | 1.33 | 0.94, 1.87 |
| 4 | 0.3 (0.3–0.7) | 147 | 831 | 0.97 | 0.79, 1.19 | 54 | 320 | 1.08 | 0.76, 1.55 |
| % Energy From Total Fat | |||||||||
| PRESTO | |||||||||
| 1 | 30.2 (15.5–32.9) | 113 | 749 | 1.00 | Referent | 79 | 646 | 1.00 | Referent |
| 2 | 34.9 (32.9–37.0) | 116 | 694 | 1.15 | 0.90, 1.47 | 90 | 696 | 1.07 | 0.89, 1.28 |
| 3 | 39.4 (37.0–42.0) | 127 | 764 | 1.06 | 0.84, 1.33 | 89 | 638 | 1.05 | 0.86, 1.28 |
| 4 | 45.4 (42.1–67.2) | 129 | 724 | 1.20 | 0.95, 1.52 | 75 | 668 | 0.99 | 0.85, 1.14 |
| Snart Foraeldre | |||||||||
| 1 | 30.4 (15.1–32.7) | 136 | 806 | 1.00 | Referent | 60 | 272 | 1.00 | Referent |
| 2 | 34.4 (32.7–35.7) | 141 | 807 | 0.96 | 0.78, 1.19 | 42 | 264 | 0.79 | 0.55, 1.15 |
| 3 | 37.5 (35.7–39.4) | 145 | 727 | 1.18 | 0.96, 1.45 | 69 | 354 | 0.88 | 0.63, 1.22 |
| 4 | 41.8 (39.4–66.4) | 117 | 687 | 1.01 | 0.81, 1.27 | 64 | 390 | 0.72 | 0.51, 1.00 |
Abbreviations: CI, confidence interval; FR, fecundability ratio; PRESTO, Pregnancy Study Online.
a Weight (kg)/height (m)2.
b Adjusted for energy intake, age, body mass index, smoking status, total physical activity level, educational level, parity, alcohol intake, intercourse frequency, marital status, household income, last method of contraception, and race/ethnicity (PRESTO only).
c Restricted to persons who did not use fish oil supplements.
DISCUSSION
In the present study, there was little evidence of an association between fecundability and intakes of total fat, SFAs, MUFAs, PUFAs, or ω-6 fatty acids. However, there was some suggestion that high TFA intake and low ω-3 fatty acid intake were associated with reduced fecundability among North American women. These associations were slightly stronger among lean women and women with shorter attempt times at study entry, but they did not vary materially by age or with further adjustment for total fat intake. Controlling for socioeconomic status, particularly household income, attenuated these associations. There was little association between use of fish oil supplements and fecundability.
Our findings for TFA intake are consistent with those from the Nurses’ Health Study II (5), a prospective cohort study in which TFA intake was associated with an increased risk of ovulatory infertility during a 9-year period (1991–1999) (5). Because we examined all types of subfertility, not just ovulatory infertility, associations may have been attenuated or obscured if intakes of specific subtypes of fat have an effect on ovarian function but not on other types of subfertility (e.g., tubal blockage). TFAs were eliminated from the Danish food supply in 2003 (39), and their low prevalence could explain the lack of association between TFA intake and fecundability in SF. Although food labeling for TFA is required in North America and restaurant use has been reduced, TFAs have not been banned.
Our findings for ω-3 fatty acids agree with those from a cohort of 259 regularly menstruating women in the BioCycle Study, in which dietary intake of docosapentaenoic acid—a fatty acid commonly found in fish—was associated with a lower risk of anovulation. In addition, higher dietary intake of total marine ω-3 polyunsaturated fats—specifically eicosapentaenoic acid, docosahexaenoic acid, and docosapentaenoic acid—was associated with increased luteal-phase progesterone concentrations (13). To our knowledge, there are no other studies of ω-3 fatty acids and reproductive-related endpoints in women. Our results for ω-6 fatty acids conflict with those from a study of infertile women, in which higher preconception intakes of ω-6 fatty acid and linoleic acid (PUFA) were associated with a higher probability of pregnancy among 46 overweight/obese women who were undergoing in vitro fertilization (14). Finally, the lack of association between fish oil supplements and fecundability is difficult to explain. Supplements can vary widely in their quality and content (40), which would lead to misclassification, and individuals who take supplements might have vastly different diets or family histories of illnesses (e.g., heart disease), which could have produced unmeasured confounding. Finally, if supplements contain more contaminants (e.g., mercury in fish) than fish themselves, any beneficial effects on fertility could be negated.
Strengths of the present study include enrollment during the preconception period, with more than two-thirds of women enrolled during their first 3 cycles of attempted pregnancy. Dietary assessment before pregnancy avoids recall bias. Cohort retention exceeded 80% in both cohorts during the follow-up period. The median length of follow-up was identical (9 cycles) for those in the highest and lowest quartiles of total fat intake. Data were collected on a wide range of potential confounders. FFQs were validated in each population, showing high relative validity of nutrients of interest.
Although the FFQ is the most feasible method for assessing long-term diet in large epidemiologic studies (34, 41, 42), some misclassification of diet is expected. It should also be noted that country-specific dietary databases were used in the 2 studies. Estimation of macronutrients, including fatty acids, are based on laboratory analysis with extrapolation to similar foods; thus, we do not expect such differences to have materially affected the results. Because diet was assessed prospectively relative to the outcome, any misclassification in our studies was likely to be nondifferential and would attenuate extreme categories of fat intake towards the null. Residual confounding is another possible explanation of our findings. Consumption of TFA—which is typically found in highly processed foods—is associated with unhealthful lifestyle practices. Thus, confounding by unmeasured covariates associated with unhealthful lifestyle factors that were not captured by measured variables such as sedentariness, adiposity, and energy intake may have biased our results. Another limitation is that we did not control for the male partners’ dietary intakes, which may be correlated with female partners’ intakes. Epidemiologic studies have shown associations between poor semen quality and both high intake of TFA (43, 44) and docosahexaenoic acid deficiency (10–12). Finally, there could be nondietary factors that explain the inconsistent results across cohorts. For example, factors by which Danes differ from North Americans (e.g., lower stress and pollution levels, reduced socioeconomic inequality) could interact with dietary fat intake and produce different associations with fecundity.
FFQ estimates for fat intake in our cohorts were consistent with national data on reproductive-aged women (45–47). These observations, coupled with the finding that our results did not vary materially by other covariates, suggest that our results are generalizable to other reproductive-aged women. Web-based recruitment has been criticized because of differences in the characteristics of persons with and without Internet access (48). However, this recruitment method would not affect the validity of the study results unless the relationship between diet and fertility differed substantially between those who did and did not use the Internet, which is unlikely (49). The same argument can be made regarding differences between women who did and did not plan pregnancy. Our study (50) and others (51, 52) have shown that even when participation at cohort entry is related to characteristics such as age, parity, or smoking, measures of association are not biased because of self-selection. Concerns about selection bias stemming from length-biased recruitment of couples with longer TTP or misclassification arising from changes in diet over time because of subfertility can be assessed by stratifying by attempt time at study entry. Selected results were slightly stronger among women with shorter attempt times at study entry, providing little support for selection bias.
Our findings are biologically plausible. TFA intake has been associated with inflammation (53) and insulin resistance (54), both of which are known to impair ovulatory function. In addition to their own anti-inflammatory effects (55), ω-3 fatty acids may improve fecundity by reducing the likelihood of anovulation (13), supporting optimal endogenous progesterone levels (13), and increasing the prostacyclin/thromboxane ratio (56), thereby increasing uterine blood flow (57).
In summary, 2 prospective cohort studies of women from North America and Denmark who were attempting to become pregnant showed little evidence of an association between fecundability and intakes of total dietary fat, SFA, MUFA, PUFA, and ω-6 fatty acids. High intake of TFAs and low intake of ω-3 fatty acids were associated with reduced fecundability among North Americans only. The low prevalence of TFA intake in Denmark limited our ability to assess its association with fecundability in this population. The higher median intake of ω-3 fatty acids in Denmark and its lack of association with fecundability is consistent with our finding of a threshold effect affecting the lowest quartile of intake in the North American cohort. Our findings agree with those from the small number of previous studies of this topic, but further research is warranted to confirm our results.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, School of Public Health, Boston University, Boston, Massachusetts (Lauren A. Wise, Amelia K. Wesselink, Craig J. McKinnon, Kristen A. Hahn, Kenneth J. Rothman, Elizabeth E. Hatch); Department of Clinical Laboratory and Nutritional Sciences, College of Health Sciences, University of Massachusetts Lowell, Lowell, Massachusetts (Katherine L. Tucker, Shilpa Saklani); Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark (Ellen M. Mikkelsen, Heidi Cueto, Anders H. Riis, Henrik Toft Sørensen); Division of Risk Assessment and Nutrition, National Food Institute, Technical University of Denmark, Søborg, Denmark (Ellen Trolle); and RTI Health Solutions, Research Triangle Park, North Carolina (Kenneth J. Rothman).
This work was supported by the National Institute of Child Health and Human Development (NICHD) (grants R21-050264, R01-HD060680, R21-HD072326, and R01-HD086742) and the Danish Medical Research Council (grant 271-07-0338). A.K.W. and K.A.H. were supported in part by NICHD grant T32-HD052458 through the Boston University Reproductive, Perinatal and Pediatric Epidemiology Training Program. H.T.S. was supported by a grant from the Program for Clinical Research Infrastructure established by the Lundbeck and the Novo Nordisk Foundation.
We thank the Snart Foraeldre and Pregnancy Study Online (PRESTO) staff. We thank Thomas Jensen and Michael Bairos for their technical support in developing the Web-based infrastructure of the studies, Vibeke Knudsen and Tue Christensen for assistance with the development, testing, and processing of the Danish food frequency questionnaire, and Dr. Amy Subar and Ken Bishop for sharing the Dietary Health Questionnaire II and its software programs.
Conflict of interest: none declared.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- FFQ
food frequency questionnaire
- FR
fecundability ratio
- LMP
last menstrual period
- MET
metabolic equivalent
- MUFA
monounsaturated fatty acid
- PRESTO
Pregnancy Study Online
- PUFA
polyunsaturated fatty acid
- SD
standard deviation
- SF
Snart Foraeldre
- SFA
saturated fatty acid
- TFA
trans fatty acid
- TTP
time to pregnancy
REFERENCES
- 1. Thoma ME, McLain AC, Louis JF, et al. . Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324.e1–1331.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod. 2007;77(2):190–201. [DOI] [PubMed] [Google Scholar]
- 3. Kazemi A, Ramezanzadeh F, Nasr-Esfahani MH, et al. . Does dietary fat intake influence oocyte competence and embryo quality by inducing oxidative stress in follicular fluid? Iran J Reprod Med. 2013;11(12):1005–1012. [PMC free article] [PubMed] [Google Scholar]
- 4. Jungheim ES, Macones GA, Odem RR, et al. . Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertil Steril. 2011;95(6):1970–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chavarro JE, Rich-Edwards JW, Rosner BA, et al. . Dietary fatty acid intakes and the risk of ovulatory infertility. Am J Clin Nutr. 2007;85(1):231–237. [DOI] [PubMed] [Google Scholar]
- 6. Missmer SA, Chavarro JE, Malspeis S, et al. . A prospective study of dietary fat consumption and endometriosis risk. Hum Reprod. 2010;25(6):1528–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Skaznik-Wikiel ME, Rudolph MC, Swindle DC, et al. . Elevated serum levels of biologically active omega-3 fatty acids are associated with better ovarian reserve. Fertil Steril. 2016;106(3):e66. [Google Scholar]
- 8. Roqueta-Rivera M, Abbott TL, Sivaguru M, et al. . Deficiency in the omega-3 fatty acid pathway results in failure of acrosome biogenesis in mice. Biol Reprod. 2011;85(4):721–732. [DOI] [PubMed] [Google Scholar]
- 9. Roqueta-Rivera M, Stroud CK, Haschek WM, et al. . Docosahexaenoic acid supplementation fully restores fertility and spermatogenesis in male delta-6 desaturase-null mice. J Lipid Res. 2010;51(2):360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zalata AA, Christophe AB, Depuydt CE, et al. . The fatty acid composition of phospholipids of spermatozoa from infertile patients. Mol Hum Reprod. 1998;4(2):111–118. [DOI] [PubMed] [Google Scholar]
- 11. Conquer JA, Martin JB, Tummon I, et al. . Fatty acid analysis of blood serum, seminal plasma, and spermatozoa of normozoospermic vs. asthenozoospermic males. Lipids. 1999;34(8):793–799. [DOI] [PubMed] [Google Scholar]
- 12. Eslamian G, Amirjannati N, Rashidkhani B, et al. . Dietary fatty acid intakes and asthenozoospermia: a case-control study. Fertil Steril. 2015;103(1):190–198. [DOI] [PubMed] [Google Scholar]
- 13. Mumford SL, Chavarro JE, Zhang C, et al. . Dietary fat intake and reproductive hormone concentrations and ovulation in regularly menstruating women. Am J Clin Nutr. 2016;103(3):868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moran LJ, Tsagareli V, Noakes M, et al. . Altered preconception fatty acid intake is associated with improved pregnancy rates in overweight and obese women undertaking in vitro fertilisation. Nutrients. 2016;8(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huybrechts KF, Mikkelsen EM, Christensen T, et al. . A successful implementation of e-epidemiology: the Danish pregnancy planning study “Snart-Gravid”. Eur J Epidemiol. 2010;25(5):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mikkelsen EM, Hatch EE, Wise LA, et al. . Cohort profile: the Danish web-based pregnancy planning study–“Snart-Gravid”. Int J Epidemiol. 2009;38(4):938–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Knudsen VK, Hatch EE, Cueto H, et al. . Relative validity of a semi-quantitative, web-based FFQ used in the “Snart Forældre” cohort – a Danish study of diet and fertility. Public Health Nutr. 2016;19(6):1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wise LA, Rothman KJ, Mikkelsen EM, et al. . Design and conduct of an internet-based preconception cohort study in North America: Pregnancy Study Online. Paediatr Perinat Epidemiol. 2015;29(4):360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Subar AF, Thompson FE, Kipnis V, et al. . Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–1099. [DOI] [PubMed] [Google Scholar]
- 20.Danish National Food Database. Lyngby, Denmark: Technical University of Denmark; http://frida.fooddata.dk/. Accessed March 7, 2015. [Google Scholar]
- 21. National Cancer Institute Diet History Questionnaire: Diet*Calc Software version 1.5.0. Rockville, MD: National Cancer Institute; 2012. http://riskfactor.cancer.gov/DHQ/dietcalc/. Accessed October 19, 2012. [Google Scholar]
- 22. Craig CL, Marshall AL, Sjöström M, et al. . International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. [DOI] [PubMed] [Google Scholar]
- 23. Ainsworth BE, Haskell WL, Whitt MC, et al. . Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 suppl):S498–S504. [DOI] [PubMed] [Google Scholar]
- 24. McKinnon CJ, Hatch EE, Rothman KJ, et al. . Body mass index, physical activity and fecundability in a North American preconception cohort study. Fertil Steril. 2016;106(2):451–459. [DOI] [PubMed] [Google Scholar]
- 25. Willett WC, Stampfer MJ. Implications of total energy intake for epidemiologic analysis In: Willett WC, ed. Nutritional Epidemiology. 2nd ed New York, NY: Oxford University Press; 1998:273–301. [Google Scholar]
- 26. Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). 2008;233(6):674–688. [DOI] [PubMed] [Google Scholar]
- 27. Simopoulos AP. The omega-6/omega-3 fatty acid ratio, genetic variation, and cardiovascular disease. Asia Pac J Clin Nutr. 2008;17(suppl 1):131–134. [PubMed] [Google Scholar]
- 28. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. [DOI] [PubMed] [Google Scholar]
- 29. Schisterman EF, Cole SR, Ye A, et al. . Accuracy loss due to selection bias in cohort studies with left truncation. Paediatr Perinat Epidemiol. 2013;27(5):491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Howards PP, Hertz-Picciotto I, Poole C. Conditions for bias from differential left truncation. Am J Epidemiol. 2007;165(4):444–452. [DOI] [PubMed] [Google Scholar]
- 31. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 32. Weinberg CR, Baird DD, Wilcox AJ. Re: “Effects of caffeine consumption on delayed conception”. Am J Epidemiol. 1996;144(8):799. [DOI] [PubMed] [Google Scholar]
- 33. Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed New York, NY: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 34. Willett W. Nutritional Epidemiology. 2nd ed New York, NY: Oxford University Press; 1998. [Google Scholar]
- 35. Weinberg CR. Toward a clearer definition of confounding. Am J Epidemiol. 1993;137(1):1–8. [DOI] [PubMed] [Google Scholar]
- 36. Howards PP, Schisterman EF, Poole C, et al. . “Toward a clearer definition of confounding” revisited with directed acyclic graphs. Am J Epidemiol. 2012;176(6):506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou XH, Eckert GJ, Tierney WM. Multiple imputation in public health research. Stat Med. 2001;20(9–10):1541–1549. [DOI] [PubMed] [Google Scholar]
- 38. SAS SAS/STAT® 9.4 User’s Guide Cary, NC: SAS Institute; 2014. [Google Scholar]
- 39. L’Abbé MR, Stender S, Skeaff CM, et al. . Approaches to removing trans fats from the food supply in industrialized and developing countries. Eur J Clin Nutr. 2009;63:S50–S67.19190645 [Google Scholar]
- 40. Kleiner AC, Cladis DP, Santerre CR. A comparison of actual versus stated label amounts of EPA and DHA in commercial omega-3 dietary supplements in the United States. J Sci Food Agric. 2015;95(6):1260–1267. [DOI] [PubMed] [Google Scholar]
- 41. Willett W. Invited commentary: OPEN questions. Am J Epidemiol. 2003;158(1):22–24. [Google Scholar]
- 42. Willett WC, Sampson L, Stampfer MJ, et al. . Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 43. Chavarro JE, Furtado J, Toth TL, et al. . Trans-fatty acid levels in sperm are associated with sperm concentration among men from an infertility clinic. Fertil Steril. 2011;95(5):1794–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chavarro JE, Mínguez-Alarcón L, Mendiola J, et al. . Trans fatty acid intake is inversely related to total sperm count in young healthy men. Hum Reprod. 2014;29(3):429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moskal A, Pisa PT, Ferrari P, et al. . Nutrient patterns and their food sources in an International study setting: report from the EPIC study. PLoS One. 2014;9(6):e98647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang DD, Leung CW, Li Y, et al. . Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174(10):1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rehm CD, Penalvo JL, Afshin A, et al. . Dietary intake among US adults, 1999–2012. JAMA. 2016;315(23):2542–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Keiding N, Slama R. Commentary: time-to-pregnancy in the real world. Epidemiology. 2015;26(1):119–121. [DOI] [PubMed] [Google Scholar]
- 49. Rothman KJ, Gallacher JE, Hatch EE. Why representativeness should be avoided. Int J Epidemiol. 2013;42(4):1012–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hatch EE, Hahn KA, Wise LA, et al. . Evaluation of selection bias in an internet-based study of pregnancy planners. Epidemiology. 2016;27(1):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nohr EA, Frydenberg M, Henriksen TB, et al. . Does low participation in cohort studies induce bias? Epidemiology. 2006;17(4):413–418. [DOI] [PubMed] [Google Scholar]
- 52. Nilsen RM, Vollset SE, Gjessing HK, et al. . Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23(6):597–608. [DOI] [PubMed] [Google Scholar]
- 53. Mozaffarian D, Pischon T, Hankinson SE, et al. . Dietary intake of trans fatty acids and systemic inflammation in women. Am J Clin Nutr. 2004;79(4):606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lefevre M, Lovejoy JC, Smith SR, et al. . Comparison of the acute response to meals enriched with cis- or trans-fatty acids on glucose and lipids in overweight individuals with differing FABP2 genotypes. Metabolism. 2005;54(12):1652–1658. [DOI] [PubMed] [Google Scholar]
- 55. Li K, Huang T, Zheng J, et al. . Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor alpha: a meta-analysis. PLoS One. 2014;9(2):e88103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jiang J, Li K, Wang F, et al. . Effect of marine-derived n-3 polyunsaturated fatty acids on major eicosanoids: a systematic review and meta-analysis from 18 randomized controlled trials. PLoS One. 2016;11(1):e0147351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu HS, Chu TY, Yu MH, et al. . Thromboxane and prostacyclin in maternal and fetal circulation in pre-eclampsia. Int J Gynaecol Obstet. 1998;63(1):1–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.