Abstract
Influenza viruses are associated with a substantial global burden of morbidity and mortality every year. Estimates of influenza-associated mortality often vary between studies due to differences in study settings, methods, and measurement of outcomes. We reviewed 103 published articles assessing population-based influenza-associated mortality through searches of PubMed and Embase, and we identified considerable variation in the statistical methods used across studies. Studies using regression models with an influenza activity proxy applied 4 approaches to estimate influenza-associated mortality. The estimates increased with age and ranged widely, from −0.3–1.3 and 0.6–8.3 respiratory deaths per 100,000 population for children and adults, respectively, to 4–119 respiratory deaths per 100,000 population for older adults. Meta-regression analysis identified that study design features were associated with the observed variation in estimates. The estimates increased with broader cause-of-death classification and were higher for older adults than for children. The multiplier methods tended to produce lower estimates, while Serfling-type models were associated with higher estimates than other methods. No “average” estimate of excess mortality could reliably be made due to the substantial variability of the estimates, partially attributable to methodological differences in the studies. Standardization of methodology in estimation of influenza-associated mortality would permit improved comparisons in the future.
Keywords: epidemiologic methods, influenza, mortality, systematic reviews
It has been estimated that, globally, human seasonal influenzavirus infections result in 250,000−500,000 deaths annually, approximately 0.5%−1% of all deaths every year (1). The mortality impact of influenza is greatest at the extremes of age, that is, young children and old adults. Influenza-associated deaths can occur following primary viral pneumonia (2) or, more frequently, secondary bacterial pneumonia (3), or exacerbation of coexisting underlying medical conditions such as cardiovascular disease (4). However, influenza is not often listed as an underlying cause of death (5, 6). This is possibly due to the temporal delay between Influenzavirus infections and secondary consequences, such as bacterial pneumonia or myocardial infarction, or lack of access to laboratory testing (7). Therefore, studies of influenza-associated mortality often examine broader consequences of influenza epidemics in various causes of death, not limited to deaths recorded as “influenza.”
It is well understood that the impact of influenza on mortality varies across geographic locations and populations, because of differences in population structure, access to and quality of medical care, and the prevalence of underlying medical conditions (8). The mortality impact can also vary over time within geographic locations because of evolution in circulating viruses, changes in host immunity following prior epidemics, or changes in vaccination strategies (8). Thus, there can be no single “true” representative estimate of the mortality impact of influenza that is applied in all places and at all times. However, there may be a typical range within which the estimates of annual mortality impact in a particular location are likely to fall.
It is important to assess the mortality impact of influenza, as a key component of the overall burden of disease associated with influenza, to support planning and resource allocation. Variation in the mortality impact among different segments of the population can inform vaccination programs and other public health measures. In addition to understanding the impact of influenza on mortality, having a baseline measure of typical mortality can allow assessment of the impact of specific interventions on mortality (9), as part of economic evaluations of potential consequences of new control strategies (10).
Many studies, using various methods, have now been conducted to estimate the impact of influenza on population mortality. The objectives of this review were to identify published population-based studies of the impact of influenzavirus infections on mortality in defined populations, to describe and compare the methodological approaches used, to report the estimates of influenza-associated mortality, and to examine the influence of methodological factors on reported estimates.
METHODS
Search strategy and selection criteria
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (11) to conduct this review. Relevant studies were retrieved from MEDLINE (PubMed; National Library of Medicine, Bethesda, Maryland) and Embase (Excerpta Medica, Amsterdam, the Netherlands) on March 22, 2016. We searched for published journal articles using the following combination of search terms (1 and 2 and 3) in “all fields”:
1. “mortality” or “deaths” or “burden”
2. “influenza or “flu”
3. “excess” or “attributable” or “influenza-associated” or “influenza-related” or “influenza-attributed” or “influenza-attributable” or “associated with influenza” or “attributable to influenza” or “attributed to influenza” or “related to influenza” or “flu-associated” or “flu-related” or “associated with flu” or “attributable to flu” or “attributed to flu” or “related to flu.” No language restriction was applied to retrieved articles. Additional relevant studies were manually retrieved from the reference lists of papers identified from the 2 databases.
Two of the authors (L.L. and H.S.B.) independently screened all titles identified in the literature search. Abstracts and full texts of all potentially eligible articles were retrieved and reviewed for final inclusion. Disagreement in study selection between the 2 authors was resolved by a third author (J.Y.W.).
Eligible articles were those reporting population-based estimates of influenza-associated mortality from the following 4 causes of death: 1) pneumonia and influenza; 2) respiratory diseases; 3) respiratory and cardiovascular diseases; and 4) all causes. These causes are most commonly used in mortality studies of influenza. They represent a gradient of specificity, with pneumonia and influenza being the most specific, while the other 3 groups should incrementally capture a greater fraction of the full impact of influenza (12, 13). Studies were excluded if: 1) the full-text article was not available; 2) the estimates reported were not at the country or region level; 3) no annual influenza-associated excess mortality rates could be derived; 4) only laboratory-confirmed influenza deaths were reported; 5) influenza-associated excess mortality was only estimated for a specific risk population, such as pregnant women or those with underlying medical conditions; 6) the results could not be replicated by another published study; or 7) the study investigators reported on a subset of complete data published elsewhere. Reviews, book chapters, conference abstracts/summaries, commentaries, and letters were also excluded.
Data extraction
All data were extracted into a standardized form by 2 authors independently (L.L. and H.S.B.). The primary data extracted included technical details of the methods used for estimation of influenza-associated mortality and country/region-level estimates of the annual average influenza-associated mortality rate or death numbers in all ages, by age group and by selected cause of death, during a defined study period. If authors reported separate estimates for each year included in the study, we calculated an average estimate across the years studied (see Web Appendix 1, available at https://academic.oup.com/aje). The extracted data were not limited to positive estimates, since relatively wide 95% confidence intervals have been reported for certain age groups, such as children and young adults (14–16). Technical details included the analytical approaches used; how covariates, including temperature, humidity, respiratory syncytial virus activity, and other covariates, were controlled for; which proxy measure of influenza activity was used; and how the estimate of influenza-associated mortality was derived from the model. We extracted estimates for children (ages ≤14 years), adults (ages 15–64 years), and older adults (ages ≥65 years). Reported age-specific annual estimates of the influenza-associated mortality rate were classified as those for the group closest to one of these 3 predefined age groups if the reported age group did not match exactly. Methodological details were retrieved from the cited references if the selected study itself did not provide sufficient information. Statistical methods used in different studies to estimate influenza-associated mortality were classified as multiplier methods, regression models with an influenza activity proxy, Serfling-type models (in this review, we defined Serfling-type models as regression models with Fourier terms but without an influenza activity proxy), and other models without an influenza activity proxy. Details on how data were treated and how each of the methods was applied are provided in Web Appendix 1.
Statistical analysis
We constructed forest plots to examine the age-specific estimates of influenza-associated mortality rates across different causes of death. In addition, data were plotted within categories of the measured outcome and population. If 10 or more estimates were reported for a particular outcome-population category, they were plotted using violin plots. These plots are an extension of box plots and histograms that have the advantage of indicating the probability density of each reported or recalculated estimate, as well as their common numerical characteristics, including median values and interquartile ranges. If there were fewer than 10 estimates in an outcome-population category, then only the annual influenza-associated mortality rate from each included study was plotted. For studies that did not report the standard error of an estimate, we imputed the standard error using a linear regression model (Web Appendix 1). Statistical heterogeneity of the estimates among studies was assessed using Cochran’s Q test and the I2 statistic (17).
When heterogeneity was high, as recommended (18), we used meta-regression to identify factors associated with variations in the age-specific estimates of the influenza-associated mortality rate instead of computing a single summary estimate. In the model, the outcome was the age-specific estimate from each study, while the explanatory variables were study design features—including age group, whether the estimate was made for seasonal periods or the 2009 pandemic periods, cause of death, statistical modeling technique employed, income level, and climatic zone of the study country/region. The R2 statistic was used to indicate the proportion of heterogeneity explained by the covariates examined (19). We included estimates for the 2009 A(H1N1) pandemic in the meta-regression because there was a large number of studies with large sample sizes available for the 2009 pandemic. However, studies of the 1918, 1957, and 1968 pandemics were excluded from the meta-regression because of the relative paucity of studies for these pandemics and their smaller sample sizes. The potentially large variation in the estimates from these earlier pandemics may have obscured the relationships between influenza-associated mortality estimates and selected independent variables. All analyses were performed using R software, version 3.3.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
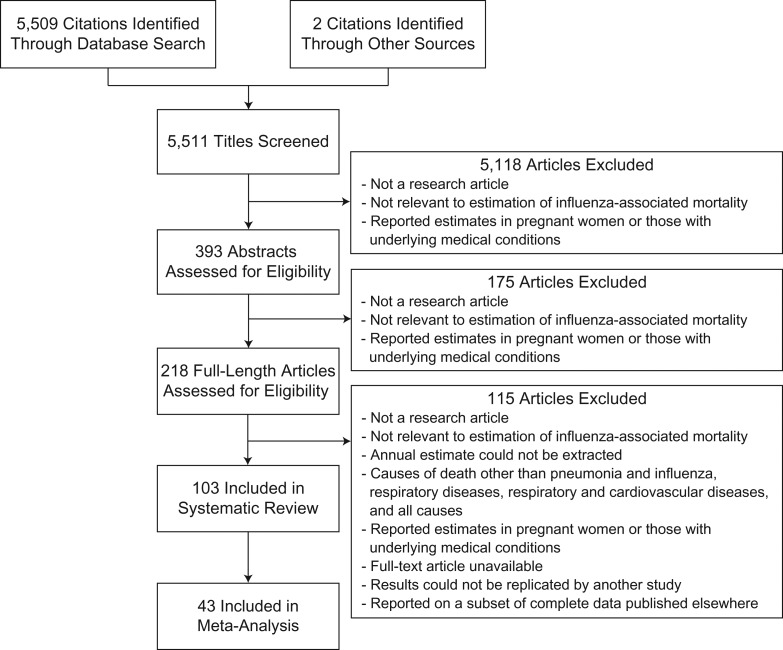
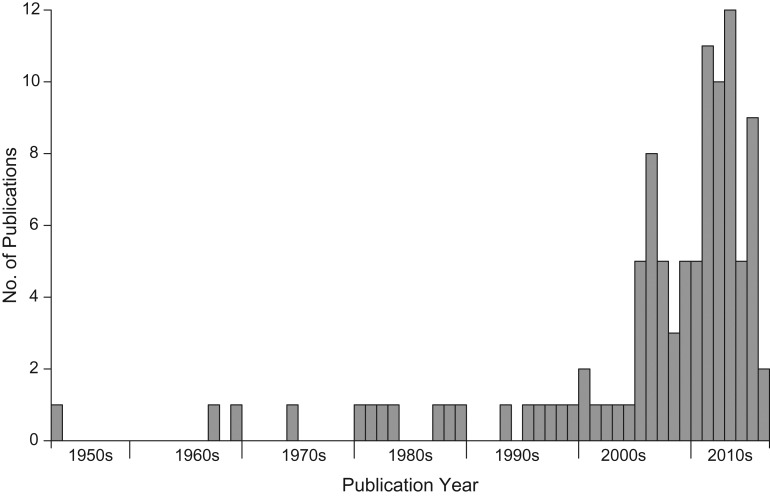
We identified 103 articles which met the inclusion criteria (Figure 1). Details of the studies included in this review are presented in Web Table 1. The majority of studies were published within the past 10 years (Figure 2). The first article identified was a study published in the 1950s by Collins and Lehmann (20) reporting influenza-associated excess deaths from a range of diseases, including chronic diseases. The included studies reported estimates from 39 countries/regions covering North and South America, Europe, Southern Africa, East and Southeast Asia, and Oceania. Two studies estimated the global mortality associated with the 2009 pandemic (8, 21), and 1 study assessed the global mortality impact of the 1957 pandemic (22).
Figure 1.
Selection of studies included in a systematic review of the impact of influenza on population mortality.
Figure 2.
Annual numbers of publications on studies of influenza-associated mortality as of March 22, 2016.
Large variation in estimates was observed among studies. The annual crude influenza-associated all-cause mortality rate for all ages, subdivided by period, ranged from 0 to 178 per 100,000 persons in seasonal periods; from 100 to 2,230 per 100,000 persons in the 1918 pandemic periods; from 23 to 123 per 100,000 persons in the 1957 pandemic periods; from 12 to 88 per 100,000 persons in the 1968 pandemic periods; and from −1 to 25 per 100,000 persons in the 2009 pandemic periods. By comparison, the age-standardized rate ranged from 3 to 81 per 100,000 persons in seasonal periods and from −1 to 31 per 100,000 persons in the 2009 pandemic periods. Three studies reported negative point estimates of influenza-associated mortality for children (14–16).
Various age group categorizations were used. We reclassified the reported age-specific estimates into 3 age groups. Reported estimates for persons aged ≤14, 1–14, 5–14, ≤17, ≤18, ≤19, or 0.5–19 years were grouped as those for “children”; estimates for persons aged 15–64, 15–59, 18–59, 20–59, 18–64, 19–64, or 20–64 years were grouped as those for “adults”; and estimates for persons aged ≥65 years or ≥60 years were grouped as those for “older adults.” Mean estimates of influenza-associated mortality increased with age. The estimated number of respiratory deaths per 100,000 population ranged from −0.3 to 1.3 for children, from 0.6 to 8.3 for adults, and from 4 to 119 for older adults (age ≥65 years). Influenza-associated mortality rates in older adults were generally higher than those in children and other adults, and the median estimate of seasonal influenza mortality burden for older adults increased when a broader cause-of-death outcome was applied (Web Figures 1–9). Age-standardized influenza-associated all-cause mortality rates were similar or higher in middle-income countries/regions than in high-income countries/regions (Web Figure 10).
Statistical modeling techniques
Four studies (8, 23–25) used multiplier methods to estimate influenza-associated mortality. These included multiplying the estimated number of symptomatic infections by the estimated ratio of hospitalizations to infections and the estimated ratio of deaths to hospitalizations. A brief technical description of how these approaches were implemented is given in Web Table 2.
We identified 54 studies using regression models with an influenza activity proxy (Table 1). Among them, 21 used polynomial and trigonometric functions of time to control for temporal trends and seasonality in mortality, respectively. The majority of the generalized linear models assumed that mortality followed a normal distribution with an identity link, or a Poisson distribution with a log link. Temperature, humidity (absolute humidity (16, 26–29) or relative humidity (15, 30–34)), and respiratory syncytial virus activity (6, 13, 16, 27, 28, 31, 32, 35–45) were the covariates most commonly included in the regression models. More than 10 different influenza activity proxies were used in the reviewed studies. The most commonly used influenza activity proxy (33.3%) was the proportion of sentinel samples positive for influenza (Web Figure 11). Thirty-four studies using generalized linear models assumed no time lag between influenza activity and population mortality (Table 1). Details of the studies using regression models with an influenza activity proxy are shown in Web Appendix 2.
Table 1.
Characteristics of the Studies Selected to Estimate Influenza-Associated Excess Mortality
| Characteristic | No. of Studies (n = 103)a | Reference(s) |
|---|---|---|
| Statistical modeling technique | ||
| Multiplier method | 4 | 8, 23–25 |
| Regression model with a proxy for influenza activity | 54 | 6, 13–16, 21, 26–45, 62, 64, 68, 69, 71–94 |
| Serfling-type model | 28 | 35, 38, 40, 48, 54, 61, 62, 88, 95–114 |
| Other | 28 | 20, 22, 31, 46–63, 88, 115–120 |
| Consideration of effects of time in regression models with an influenza activity proxy | ||
| Yes | 51 | 6, 13–16, 21, 26–45, 62, 64, 68, 69, 71–81, 83–88, 90–93 |
| No | 3 | 82, 89, 94 |
| Time lag between influenza activity and outcome in regression models | ||
| 0 week/month/year | 35 | 6, 13, 14, 21, 30, 32–35, 37–44, 62, 64, 71–73, 75, 77–80, 82–84, 86–90 |
| 1 week | 6 | 16, 27–29, 31, 81 |
| Otherb | 14 | 15, 26, 32, 36, 45, 68, 69, 74, 76, 85, 91–94 |
| Whether RSV activity was considered in the regression models with an influenza activity proxy | ||
| Yes | 18 | 6, 13, 16, 27, 28, 31, 32, 35–45 |
| No | 38 | 6, 14, 15, 21, 26, 29, 30, 32–34, 62, 64, 68, 69, 71–94 |
| Income level of country/region | ||
| Low | 1 | 60 |
| Middle | 16 | 15, 21, 26, 35, 43, 44, 60, 90, 94, 96, 99, 103, 105, 116–118 |
| High | 89 | 6, 13, 14, 16, 20, 21, 23–25, 27–42, 45–64, 68, 69, 71–89, 91–93, 97–102, 104–115, 119, 120 |
| Otherc | 4 | 8, 21, 22, 95 |
| Climatic zone | ||
| Temperate | 81 | 6, 13, 14, 20, 21, 23–25, 29, 35–42, 45, 48–64, 68, 69, 71–81, 83–94, 96–100, 104–119 |
| Tropical/subtropical | 25 | 15, 16, 21, 26–28, 30–35, 43, 44, 46, 47, 55, 60, 82, 99, 101–103, 105, 120 |
| Otherc | 4 | 8, 21, 22, 95 |
Abbreviation: RSV, respiratory syncytial virus.
a Only statistical methods used in the main analysis of each study were included in our analysis. Multiple statistical methods or models could have been used in a single study to estimate influenza-associated mortality; therefore, different methods/models were counted separately in our data analysis. Similarly, some studies could also have reported estimates for more than 1 country or region, so those studies would have been counted more than once in the table. Therefore, the subtotal in each category may differ from the total number of studies (n = 103).
b Refers to studies applying a lag of 2 weeks; studies applying multiple lags; studies applying a moving average proxy for influenza activity in week 0 through week −2 with equal weights or in week −1 through week −2 with unequal weights; and studies without an indication of a lag.
c Refers to studies reporting a combined estimate for multiple countries/regions.
Thirty-five studies provided estimates of the influenza-associated excess mortality from models without an influenza activity proxy (Table 1). Of these, 28 used Serfling-type models. Most studies (15/28) using Serfling-type models assumed that the mortality followed a normal distribution with an identity link, and they used a linear or quadratic function of time and a trigonometric function of time to control for the trend in mortality and the seasonality of mortality, respectively. Details of studies using Serfling-type models are given in Web Appendix 2.
Four studies applied moving average methods (20, 46–48), 6 used relative mortality distribution models (49–54), and 10 applied incidence rate-difference models (31, 55–63).
Most of the included studies reported estimates for high-income countries/regions, while only 1 study (60) provided the 1918–1920 pandemic influenza mortality in a low-income country/region (Sri Lanka). Eighty-one studies gave estimates for temperate countries/regions, while 25 studies reported estimates for tropical or subtropical countries/regions.
Derivation of influenza-associated mortality
The majority of studies using Serfling-type models estimated the excess mortality by subtracting the baseline mortality from the observed mortality when the observed mortality was greater than the upper limit of the baseline mortality 95% confidence interval.
Four approaches were applied in studies that used regression models with an influenza activity proxy to estimate the excess mortality: 1) the observed mortality minus the predicted baseline mortality estimated from the fitted regression model in which influenza activity was assumed to be zero, μ0,t; 2) subtraction of μ0,t from the predicted mortality estimated from a model that fitted the observed influenza activity μt, that is, μt − μ0,t; 3) the same method as method 2, but the estimate of excess mortality for a particular year would be set to zero if the annual number of excess deaths for that year was negative; and 4) the same method as method 2, but the estimate of excess mortality at a particular time t would be set to zero if a negative excess mortality was obtained at time t.
Meta-regression analysis
The results of the Cochran Q test indicated the presence of heterogeneity in the age-specific estimates (children: Q = 425, P < 0.001; adults: Q = 6,502, P < 0.001; older adults: Q = 16,449, P < 0.001), and the I2 statistic indicated that 100% of variance in the estimates was attributable to study heterogeneity rather than chance. The potential factors explored in the meta-regression analysis—age group, seasonal periods versus the 2009 pandemic periods, cause of death used, income level and climatic zone of the study country/region, and the statistical modeling technique employed—explained 57.3% of the heterogeneity in the estimates. This suggested that estimates of influenza-associated mortality derived from the multiplier methods were generally smaller than those estimated from other models while estimates from the Serfling-type models were generally higher than those estimated from other models (Table 2). The heterogeneity was also observed within the estimates from each category of statistical method (regression models with an influenza activity proxy: Q = 34,163, P < 0.001; Serfling-type models: Q = 7,602, P < 0.001; other models: Q = 4,142, P < 0.001). The estimates were higher during seasonal influenza periods than in the 2009 pandemic periods and higher for older adults. Estimates of influenza-associated mortality were also increased when the model adopted a broader cause of death. The estimates for middle-income and tropical/subtropical countries/regions were higher than those in high-income and temperate countries/regions, respectively, but the differences were not statistically significant.
Table 2.
Results of a Meta-Regression Analysis Conducted to Identify Variables That Influence Estimates of Influenza-Associated Mortality
| Variable | Change in Annual No. of Excess Deaths (per 100,000 Persons) | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age group, yearsa | |||
| Children (ages ≤14 years) | 0 | Referent | |
| Adults (ages 15–64 years) | 2 | −10, 15 | 0.694 |
| Older adults (ages ≥65 years) | 57 | 46, 67 | <0.001 |
| Whether the estimate was for seasonal periods or pandemic periods | |||
| Seasonal | 0 | Referent | |
| 2009 pandemic | −23 | −33, −13 | <0.001 |
| Cause of death | |||
| Pneumonia and influenza | 0 | Referent | |
| Respiratory diseases | 11 | −2, 25 | 0.105 |
| Respiratory and cardiovascular diseases | 29 | 16, 42 | <0.001 |
| All causes | 43 | 32, 54 | <0.001 |
| Statistical modeling technique | |||
| Multiplier method | 0 | Referent | |
| Regression model with a proxy for influenza activity | 30 | −5, 65 | 0.096 |
| Serfling-type model | 40 | 3, 77 | 0.033 |
| Other | 30 | −6, 67 | 0.101 |
| Income level of country/regionb | |||
| High | 0 | Referent | |
| Middle | 3 | −14, 19 | 0.766 |
| Climatic zone | |||
| Temperate | 0 | Referent | |
| Tropical/subtropical | 5 | −7, 18 | 0.408 |
a Only studies reporting age-specific estimates of the influenza-associated mortality rate were included in the analysis.
b Income level in the middle year of the study period as reported by the World Bank (121) was used to classify the income level of a country or region. If no national income data were available for a specific year, the year closest to the study period with national income reported by the World Bank was used for the analysis.
DISCUSSION
We reviewed 103 published studies of influenza-associated mortality and observed substantial variation among the methods used. Estimates of influenza-associated mortality varied considerably across studies; the annual age-standardized all-cause excess mortality rates ranged from 3 per 100,000 persons to 81 per 100,000 persons in seasonal periods and from −1 per 100,000 persons to 31 per 100,000 persons in the 2009 pandemic periods (Web Figure 2). No “average” estimate of excess mortality could reliably be made due to the substantial heterogeneity of the estimates, and a more comprehensive modeling approach would be needed to extrapolate annual estimates of global mortality taking into account the variability between locations and years. The meta-regression indicated that modeling techniques were an important predictor of mortality estimates.
Consistent with previous studies (16, 29, 64), our review identified that the influenza-associated mortality burden was highest in older adults. This finding supports policies that recommend vaccination for the elderly, who are generally at higher risk of serious complications from influenza (65). Influenza was reported to be associated with 0.1 laboratory-confirmed pediatric deaths per 100,000 persons annually in the United States in 2004–2012 (66), a figure which was generally lower than the annual average influenza-associated all-cause mortality rate (0.7 per 100,000 persons) estimated for children in high-income temperate countries/regions in our study. The observed difference between the laboratory-confirmed influenza deaths and the estimates of all-cause mortality from statistical models could be due to a substantial number of clinically undocumented deaths which were actually associated with influenzavirus infection. However, death rates for laboratory-confirmed influenza in children were very close to the influenza-associated pediatric mortality estimated from multiple models (Web Figure 2). This might be explained by the observation that fatal influenzavirus infections in children were likely to have respiratory presentations and most of these deaths were more likely to be confirmed and reported (67). Negative estimates of influenza-associated mortality with relatively wide 95% confidence intervals were observed for children in some studies (14–16). This may be attributable to the difficulty in differentiating seasonal excesses in childhood mortality, which is generally low. Therefore, greater uncertainty in influenza-associated mortality estimates for children would be expected in milder influenza seasons.
On average, the estimated numbers of influenza-associated all-cause excess deaths were higher than the cause-specific estimates. However, compared with cause-specific estimates, greater variation was observed in all-cause excess mortality estimates. Influenzavirus infection may only marginally contribute to all-cause mortality but is more specifically associated with deaths caused by respiratory diseases or pneumonia (12, 68). The use of specific conditions to measure influenza-associated mortality may substantially underestimate the population-level impact of influenza (68). The impact of influenza on causes of death not included in this review deserves further investigation to establish potential associations between influenza and other diseases (16, 31, 69). Conversely, conditions clearly not associated with influenza would be eligible for use as negative controls in cohort studies (70). We therefore suggest that in future studies, researchers report estimates of influenza-associated mortality for a broader range of outcomes to provide a more comprehensive picture of the burden of influenza on mortality.
The meta-regression analysis suggested that the estimates of mortality burden vary by study design. Most of the included studies used time-series data in regression models to estimate the excess mortality associated with influenza—that is, the excess mortality estimated from the observed mortality or the predicted mortality with and without incorporation of influenzavirus activities. In contrast, fewer studies applied multiplier methods using cross-sectional data to quantify the mortality burden. The multiplier method seemed to be associated with smaller estimates of influenza mortality burden compared with those from other methods. Further studies may be needed to investigate the possible impact of the methods on estimation of the disease burden.
In our study, estimates of influenza-associated excess mortality were generally higher from the models that did not include a proxy measure of influenzavirus activity (Table 2). Such studies often estimated influenza-associated mortality as the difference between the observed population mortality and a baseline mortality that was mainly derived from time periods without apparent influenzavirus activity. However, the excess deaths estimated during influenza seasons might have also resulted from other underlying causes, such as low temperature or cocirculating pathogens (16, 62). In addition, estimation of the baseline mortality solely dependent on data collected in interseasonal periods may not fully capture patterns in the baseline mortality, leading to a potential bias in estimates of excess mortality.
In models with an influenza activity proxy, the influenza-associated excess mortality was estimated as either (method 1) subtraction of the predicted mortality Y with the influenza activity proxy X set to zero from the predicted mortality under the fitted model—that is, E(Y|X) − E(Y|X = 0) (6, 13, 15, 16, 26–29, 35, 37, 38, 40–44, 62, 64, 71–78)—or (method 2) subtraction of the predicted baseline mortality from the observed mortality—that is, Y − E(Y|X = 0) (30–34, 69, 79–88). Estimates derived from method 1 should represent the specific contribution from influenza, without residual errors in Y. In contrast, estimates from method 2 would have the same point estimate but potentially greater uncertainty or variability owing to the persistence of residual errors in Y.
Some studies used zeros to replace negative estimates of the weekly excess mortality. Replacing negative estimates (i.e., reduced mortality) with zero has been justified by the biological argument that influenza epidemics should increase but not reduce mortality, although this depends to some extent on the cause of death examined. Some studies identified particular causes of death, such as unintentional injuries or bone fracture, as negative controls (16, 31, 69). Influenza-associated mortality under these causes would be expected to have point estimates close to zero, with uncertainty intervals generally overlapping zero. This is a useful approach. However, if negative estimates are replaced with zeroes it is not possible to confirm a null association of influenza with a control outcome, or potentially a positive association of influenza with other outcomes, because a positive association was already assumed when replacing negative values. In some studies, similar manipulations were made on annual estimates of the negative influenza-associated excess mortality (36, 45, 68), and this might lead to some overestimation of the average excess mortality.
Some studies used identity link or log link functions in the generalized linear regression models. The identity link function might be preferred for theoretical reasons if one assumes an additive effect of influenzavirus activity on mortality; that is, the number of excess deaths is assumed to be directly proportional to the number of infections rather than the logarithm of the number of infections (69). This approach presupposes that the influenza activity proxy should preferably be a direct correlate of the incidence of infection, an assumption that is rarely stated. For models that estimate excess mortality by subtracting baseline mortality (assuming influenza activity equals zero) from predicted mortality, the log link function may be less appropriate because it assumes a multiplicative effect of influenza and other covariates on mortality (16). A theoretical examination of the methodology used in excess mortality studies would be valuable. Although we observed no major differences between mortality estimates made using the 2 link functions, simulations may identify the conditions under which large discrepancies could arise (27).
This study had several limitations. First, we used 3 predefined age groups for easier comparison of age-specific excess estimates from different studies. Sometimes the incomplete matching of the age classifications reported in the included studies might have obscured differences in the estimates between age groups. Second, standard errors were not provided for all estimates, and we estimated these for some studies using a regression model (Web Appendix 1). Differences between true and estimated standard errors may have influenced the results of the meta-regression. Third, although we classified studies according to 4 broad causes of death, the exact diagnostic codes used varied across studies. Fourth, we did not examine the influence of predominant virus types/subtypes in the study period because they were not usually reported in the included studies. This is likely to be an important source of variation in mortality estimates, as mortality is typically higher in years when influenza A(H3N2) virus predominates (6, 16, 69). Further studies may be needed to assess the temporal variation in influenza-associated mortality and the potential influence of different predominant strains on disease burden. Fifth, the age-standardized annual estimate of influenza-associated mortality was based on the individual estimates extracted from the selected studies and may have been calculated from influenza seasons of varying lengths. Finally, in the meta-regression, we included multiple estimates from the same study without accounting for potential clustering.
In conclusion, methodological differences may account for much of the variation seen among reported estimates of influenza-associated mortality. Standardization of the methodology used for estimation would allow a more valid comparison of the estimates.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: WHO Collaborating Center for Infectious Disease Epidemiology and Control, School of Public Health, Li Ka Shing Faculty of Medicine, University of Hong Kong, Hong Kong Special Administrative Region, China (Li Li, Jessica Y. Wong, Peng Wu, Helen S. Bond, Eric H. Y. Lau, Benjamin J. Cowling); WHO Collaborating Center for Reference and Research on Influenza, Peter Doherty Institute for Infection and Immunity, Melbourne, Victoria, Australia (Sheena G. Sullivan); and Department of Epidemiology, Fielding School of Public Health, University of California, Los Angeles, Los Angeles, California (Sheena G. Sullivan).
S.G.S. and B.J.C. are joint senior authors.
This work was supported by the Health and Medical Research Fund, Food and Health Bureau, Government of the Hong Kong Special Administrative Region (grant HKS-16-E08); the Research Grants Council of the Hong Kong Special Administrative Region (project T11-705/14N); and the Harvard Center for Communicable Disease Dynamics through the National Institute of General Medical Sciences, US National Institutes of Health (grant U54 GM088558).
B.J.C. has received research funding from Sanofi Pasteur (Lyon, France) and consults for Crucell NV (Leiden, the Netherlands).
REFERENCES
- 1. World Health Organization Influenza (seasonal). http://www.who.int/mediacentre/factsheets/fs211/en/. Accessed April 28, 2016.
- 2. Metersky ML, Masterton RG, Lode H, et al. . Epidemiology, microbiology, and treatment considerations for bacterial pneumonia complicating influenza. Int J Infect Dis. 2012;16(5):e321–e331. [DOI] [PubMed] [Google Scholar]
- 3. Lee KH, Gordon A, Foxman B. The role of respiratory viruses in the etiology of bacterial pneumonia: an ecological perspective. Evol Med Public Health. 2016;2016(1):95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Warren-Gash C, Smeeth L, Hayward AC. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis. 2009;9(10):601–610. [DOI] [PubMed] [Google Scholar]
- 5. Thompson WW, Comanor L, Shay DK. Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. J Infect Dis. 2006;194(suppl 2):S82–S91. [DOI] [PubMed] [Google Scholar]
- 6. Thompson WW, Shay DK, Weintraub E, et al. . Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003;289(2):179–186. [DOI] [PubMed] [Google Scholar]
- 7. Jhung MA, Swerdlow D, Olsen SJ, et al. . Epidemiology of 2009 pandemic influenza A (H1N1) in the United States. Clin Infect Dis. 2011;52(suppl 1):S13–S26. [DOI] [PubMed] [Google Scholar]
- 8. Dawood FS, Iuliano AD, Reed C, et al. . Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012;12(9):687–695. [DOI] [PubMed] [Google Scholar]
- 9. Reichert TA, Sugaya N, Fedson DS, et al. . The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med. 2001;344(12):889–896. [DOI] [PubMed] [Google Scholar]
- 10. Baguelin M, Flasche S, Camacho A, et al. . Assessing optimal target populations for influenza vaccination programmes: an evidence synthesis and modelling study. PLoS Med. 2013;10(10):e1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, et al. . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- 12. Simonsen L, Viboud C. The art of modeling the mortality impact of winter-seasonal pathogens. J Infect Dis. 2012;206(5):625–627. [DOI] [PubMed] [Google Scholar]
- 13. Charu V, Simonsen L, Lustig R, et al. . Mortality burden of the 2009–10 influenza pandemic in the United States: improving the timeliness of influenza severity estimates using inpatient mortality records. Influenza Other Respir Viruses. 2013;7(5):863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mølbak K, Widgren K, Jensen KS, et al. . Burden of illness of the 2009 pandemic of influenza A (H1N1) in Denmark. Vaccine. 2011;29(suppl 2):B63–B69. [DOI] [PubMed] [Google Scholar]
- 15. Cooper BS, Kotirum S, Kulpeng W, et al. . Mortality attributable to seasonal influenza A and B infections in Thailand, 2005–2009: a longitudinal study. Am J Epidemiol. 2015;181(11):898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu P, Goldstein E, Ho LM, et al. . Excess mortality associated with influenza A and B virus in Hong Kong, 1998–2009. J Infect Dis. 2012;206(12):1862–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 18. Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed Philadelphia, PA: Lippincott, Williams & Wilkins; 2008. [Google Scholar]
- 19. Cooper H, Hedges LV, Valentine JC. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed New York, NY: Russell Sage Foundation; 2009. [Google Scholar]
- 20. Collins SD, Lehmann J. Excess deaths from influenza and pneumonia and from important chronic diseases during epidemic periods, 1918–51. Public Health Monogr. 1953;10:1–21. [PubMed] [Google Scholar]
- 21. Simonsen L, Spreeuwenberg P, Lustig R, et al. . Global mortality estimates for the 2009 influenza pandemic from the GLaMOR project: a modeling study. PLoS Med. 2013;10(11):e1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Viboud C, Simonsen L, Fuentes R, et al. . Global mortality impact of the 1957–1959 influenza pandemic. J Infect Dis. 2016;213(5):738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Presanis AM, Pebody RG, Paterson BJ, et al. . Changes in severity of 2009 pandemic A/H1N1 influenza in England: a Bayesian evidence synthesis. BMJ. 2011;343:d5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reed C, Chaves SS, Daily Kirley P, et al. . Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One. 2015;10(3):e0118369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shrestha SS, Swerdlow DL, Borse RH, et al. . Estimating the burden of 2009 pandemic influenza A (H1N1) in the United States (April 2009–April 2010). Clin Infect Dis. 2011;52(suppl 1):S75–S82. [DOI] [PubMed] [Google Scholar]
- 26. Aungkulanon S, Cheng PY, Kusreesakul K, et al. . Influenza-associated mortality in Thailand, 2006–2011. Influenza Other Respir Viruses. 2015;9(6):298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wong JY, Wu P, Nishiura H, et al. . Infection fatality risk of the pandemic A(H1N1)2009 virus in Hong Kong. Am J Epidemiol. 2013;177(8):834–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu P, Goldstein E, Ho LM, et al. . Excess mortality impact of two epidemics of pandemic influenza A(H1N1pdm09) virus in Hong Kong. Influenza Other Respir Viruses. 2014;8(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park M, Wu P, Goldstein E, et al. . Influenza-associated excess mortality in South Korea. Am J Prev Med. 2016;50(4):e111–e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chow A, Ma S, Ling AE, et al. . Influenza-associated deaths in tropical Singapore. Emerg Infect Dis. 2006;12(1):114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wong CM, Chan KP, Hedley AJ, et al. . Influenza-associated mortality in Hong Kong. Clin Infect Dis. 2004;39(11):1611–1617. [DOI] [PubMed] [Google Scholar]
- 32. Wong CM, Peiris JS, Yang L, et al. . Effect of influenza on cardiorespiratory and all-cause mortality in Hong Kong, Singapore and Guangzhou. Hong Kong Med J. 2012;18(suppl 2):8–11. [PubMed] [Google Scholar]
- 33. Yang L, Chan KP, Cowling BJ, et al. . Excess mortality associated with the 2009 pandemic of influenza A(H1N1) in Hong Kong. Epidemiol Infect. 2012;140(9):1542–1550. [DOI] [PubMed] [Google Scholar]
- 34. Yang L, Ma S, Chen PY, et al. . Influenza associated mortality in the subtropics and tropics: results from three Asian cities. Vaccine. 2011;29(48):8909–8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng PY, Palekar R, Azziz-Baumgartner E, et al. . Burden of influenza-associated deaths in the Americas, 2002–2008. Influenza Other Respir Viruses. 2015;9(suppl 1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Green HK, Andrews N, Fleming D, et al. . Mortality attributable to influenza in England and Wales prior to, during and after the 2009 pandemic. PLoS One. 2013;8(12):e79360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kessaram T, Stanley J, Baker MG. Estimating influenza-associated mortality in New Zealand from 1990 to 2008. Influenza Other Respir Viruses. 2015;9(1):14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lopez-Cuadrado T, de Mateo S, Jimenez-Jorge S, et al. . Influenza-related mortality in Spain, 1999–2005. Gac Sanit. 2012;26(4):325–329. [DOI] [PubMed] [Google Scholar]
- 39. Matias G, Taylor R, Haguinet F, et al. . Estimates of mortality attributable to influenza and RSV in the United States during 1997–2009 by influenza type or subtype, age, cause of death, and risk status. Influenza Other Respir Viruses. 2014;8(5):507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Newall AT, Viboud C, Wood JG. Influenza-attributable mortality in Australians aged more than 50 years: a comparison of different modelling approaches. Epidemiol Infect. 2010;138(6):836–842. [DOI] [PubMed] [Google Scholar]
- 41. Newall AT, Wood JG, Macintyre CR. Influenza-related hospitalisation and death in Australians aged 50 years and older. Vaccine. 2008;26(17):2135–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schanzer DL, Sevenhuysen C, Winchester B, et al. . Estimating influenza deaths in Canada, 1992–2009. PLoS One. 2013;8(11):e80481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tempia S, Walaza S, Viboud C, et al. . Mortality associated with seasonal and pandemic influenza and respiratory syncytial virus among children <5 years of age in a high HIV prevalence setting—South Africa, 1998–2009. Clin Infect Dis. 2014;58(9):1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tempia S, Walaza S, Viboud C, et al. . Deaths associated with respiratory syncytial and influenza viruses among persons ≥5 years of age in HIV-prevalent area, South Africa, 1998–2009. Emerg Infect Dis. 2015;21(4):600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wijngaard CC, Asten L, Koopmans MP, et al. . Comparing pandemic to seasonal influenza mortality: moderate impact overall but high mortality in young children. PLoS One. 2012;7(2):e31197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee VJ, Yap J, Ong JB, et al. . Influenza excess mortality from 1950–2000 in tropical Singapore. PLoS One. 2009;4(12):e8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Linhart Y, Shohat T, Bromberg M, et al. . Excess mortality from seasonal influenza is negligible below the age of 50 in Israel: implications for vaccine policy. Infection. 2011;39(5):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Simonsen L, Clarke MJ, Schonberger LB, et al. . Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis. 1998;178(1):53–60. [DOI] [PubMed] [Google Scholar]
- 49. Ohmi K, Marui E. Estimation of the excess death associated with influenza pandemics and epidemics in Japan after World War II: relation with pandemics and the vaccination system [in Japanese]. Nihon Koshu Eisei Zasshi. 2011;58(10):867–878. [PubMed] [Google Scholar]
- 50. Tachibana T, Kawaminami K, Minowa M. Excess mortality from influenza epidemics in Japan, 1980–1994 [in Japanese]. Nihon Koshu Eisei Zasshi. 1999;46(4):263–274. [PubMed] [Google Scholar]
- 51. Takahashi M, Nagai M. Estimation of excess mortality associated with influenza epidemics specific for sex, age and cause of death in Japan during 1987–2005 [in Japanese]. Nihon Eiseigaku Zasshi. 2008;63(1):5–19. [DOI] [PubMed] [Google Scholar]
- 52. Takahashi M, Tango T. Comparative study of new method and the Kawai and Fukutomi methods for estimating excess mortality associated with influenza-epidemics, based upon national vital statistics from 1975 to 1997 [in Japanese]. Nippon Koshu Eisei Zasshi. 2001;48(10):816–826. [PubMed] [Google Scholar]
- 53. Takahashi M, Tango T. Estimation of excess mortality associated with influenza-epidemics by age and cause specific death in Japan, 1975–1999 [in Japanese]. Nihon Eiseigaku Zasshi. 2002;57(3):571–584. [DOI] [PubMed] [Google Scholar]
- 54. Zucs P, Buchholz U, Haas W, et al. . Influenza associated excess mortality in Germany, 1985–2001. Emerg Themes Epidemiol. 2005;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Andreasen V, Simonsen L. The perils of using annual all-cause mortality data to estimate pandemic influenza burden. Vaccine. 2011;29(suppl 2):B49–B55. [DOI] [PubMed] [Google Scholar]
- 56. Bonmarin I, Belchior E, Levy-Bruhl D. Impact of influenza vaccination on mortality in the French elderly population during the 2000–2009 period. Vaccine. 2015;33(9):1099–1101. [DOI] [PubMed] [Google Scholar]
- 57. Fleming DM. The contribution of influenza to combined acute respiratory infections, hospital admissions, and deaths in winter. Commun Dis Public Health. 2000;3(1):32–38. [PubMed] [Google Scholar]
- 58. Fleming DM, Pannell RS, Cross KW. Mortality in children from influenza and respiratory syncytial virus. J Epidemiol Community Health. 2005;59(7):586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jansen AG, Sanders EA, Hoes AW, et al. . Influenza- and respiratory syncytial virus-associated mortality and hospitalisations. Eur Respir J. 2007;30(6):1158–1166. [DOI] [PubMed] [Google Scholar]
- 60. Murray CJ, Lopez AD, Chin B, et al. . Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918–20 pandemic: a quantitative analysis. Lancet. 2006;368(9554):2211–2218. [DOI] [PubMed] [Google Scholar]
- 61. Richard SA, Sugaya N, Simonsen L, et al. . A comparative study of the 1918–1920 influenza pandemic in Japan, USA and UK: mortality impact and implications for pandemic planning. Epidemiol Infect. 2009;137(8):1062–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thompson WW, Weintraub E, Dhankhar P, et al. . Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respir Viruses. 2009;3(1):37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mamelund SE, Iversen BG. Morbidity and mortality in pandemic influenza in Norway [in Norwegian]. Tidsskr Nor Laegeforen. 2000;120(3):360–363. [PubMed] [Google Scholar]
- 64. Centers for Disease Control and Prevention Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR Morb Mortal Wkly Rep. 2010;59(33):1057–1062. [PubMed] [Google Scholar]
- 65. Centers for Disease Control and Prevention What you should know and do this flu season if you are 65 years and older. http://www.cdc.gov/flu/about/disease/65over.htm. Accessed April 28, 2016.
- 66. Wong KK, Jain S, Blanton L, et al. . Influenza-associated pediatric deaths in the United States, 2004–2012. Pediatrics. 2013;132(5):796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bhat N, Wright JG, Broder KR, et al. . Influenza-associated deaths among children in the United States, 2003–2004. N Engl J Med. 2005;353(24):2559–2567. [DOI] [PubMed] [Google Scholar]
- 68. Hardelid P, Pebody R, Andrews N. Mortality caused by influenza and respiratory syncytial virus by age group in England and Wales 1999–2010. Influenza Other Respir Viruses. 2013;7(1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Goldstein E, Viboud C, Charu V, et al. . Improving the estimation of influenza-related mortality over a seasonal baseline. Epidemiology. 2012;23(6):829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lipsitch M, Jha A, Simonsen L. Observational studies and the difficult quest for causality: lessons from vaccine effectiveness and impact studies. Int J Epidemiol. 2016;45(6):2060–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Brinkhof MW, Spoerri A, Birrer A, et al. . Influenza-attributable mortality among the elderly in Switzerland. Swiss Med Wkly. 2006;136(19-20):302–309. [DOI] [PubMed] [Google Scholar]
- 72. Carrat F, Valleron AJ. Influenza mortality among the elderly in France, 1980–90: how many deaths may have been avoided through vaccination? J Epidemiol Community Health. 1995;49(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dushoff J, Plotkin JB, Viboud C, et al. . Mortality due to influenza in the United States—an annualized regression approach using multiple-cause mortality data. Am J Epidemiol. 2006;163(2):181–187. [DOI] [PubMed] [Google Scholar]
- 74. Foppa IM, Cheng PY, Reynolds SB, et al. . Deaths averted by influenza vaccination in the US during the seasons 2005/06 through 2013/14. Vaccine. 2015;33(26):3003–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Foppa IM, Hossain MM. Revised estimates of influenza-associated excess mortality, United States, 1995 through 2005. Emerg Themes Epidemiol. 2008;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lemaitre M, Carrat F, Rey G, et al. . Mortality burden of the 2009 A/H1N1 influenza pandemic in France: comparison to seasonal influenza and the A/H3N2 pandemic. PLoS One. 2012;7(9):e45051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schanzer DL, Tam TW, Langley JM, et al. . Influenza-attributable deaths, Canada 1990–1999. Epidemiol Infect. 2007;135(7):1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sprenger MJ, Mulder PG, Beyer WE, et al. . Impact of influenza on mortality in relation to age and underlying disease, 1967–1989. Int J Epidemiol. 1993;22(2):334–340. [DOI] [PubMed] [Google Scholar]
- 79. Alling DW, Blackwelder WC, Stuart-Harris CH. A study of excess mortality during influenza epidemics in the United States, 1968–1976. Am J Epidemiol. 1981;113(1):30–43. [DOI] [PubMed] [Google Scholar]
- 80. Gran JM, Iversen B, Hungnes O, et al. . Estimating influenza-related excess mortality and reproduction numbers for seasonal influenza in Norway, 1975–2004. Epidemiol Infect. 2010;138(11):1559–1568. [DOI] [PubMed] [Google Scholar]
- 81. Gran JM, Kacelnik O, Grjibovski AM, et al. . Counting pandemic deaths: comparing reported numbers of deaths from influenza A(H1N1)pdm09 with estimated excess mortality. Influenza Other Respir Viruses. 2013;7(6):1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Li CK, Choi BC, Wong TW. Influenza-related deaths and hospitalizations in Hong Kong: a subtropical area. Public Health. 2006;120(6):517–524. [DOI] [PubMed] [Google Scholar]
- 83. Mann AG, Mangtani P, Russell CA, et al. . The impact of targeting all elderly persons in England and Wales for yearly influenza vaccination: excess mortality due to pneumonia or influenza and time trend study. BMJ Open. 2013;3(8):e002743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nicholson KG. Impact of influenza and respiratory syncytial virus on mortality in England and Wales from January 1975 to December 1990. Epidemiol Infect. 1996;116(1):51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Quandelacy TM, Viboud C, Charu V, et al. . Age- and sex-related risk factors for influenza-associated mortality in the United States between 1997–2007. Am J Epidemiol. 2014;179(2):156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tillett HE, Smith JW, Clifford RE. Excess morbidity and mortality associated with influenza in England and Wales. Lancet. 1980;1(8172):793–795. [DOI] [PubMed] [Google Scholar]
- 87. Tillett HE, Smith JW, Gooch CD. Excess deaths attributable to influenza in England and Wales: age at death and certified cause. Int J Epidemiol. 1983;12(3):344–352. [DOI] [PubMed] [Google Scholar]
- 88. Choi K, Thacker SB. Mortality during influenza epidemics in the United States, 1967–1978. Am J Public Health. 1982;72(11):1280–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pitman RJ, Melegaro A, Gelb D, et al. . Assessing the burden of influenza and other respiratory infections in England and Wales. J Infect. 2007;54(6):530–538. [DOI] [PubMed] [Google Scholar]
- 90. Yu H, Feng L, Viboud CG, et al. . Regional variation in mortality impact of the 2009 A(H1N1) influenza pandemic in China. Influenza Other Respir Viruses. 2013;7(6):1350–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. van Asten L, van den Wijngaard C, van Pelt W, et al. . Mortality attributable to 9 common infections: significant effect of influenza A, respiratory syncytial virus, influenza B, norovirus, and parainfluenza in elderly persons. J Infect Dis. 2012;206(5):628–639. [DOI] [PubMed] [Google Scholar]
- 92. Muscatello DJ, Newall AT, Dwyer DE, et al. . Mortality attributable to seasonal and pandemic influenza, Australia, 2003 to 2009, using a novel time series smoothing approach. PLoS One. 2014;8(6):e64734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nielsen J, Mazick A, Glismann S, et al. . Excess mortality related to seasonal influenza and extreme temperatures in Denmark, 1994–2010. BMC Infect Dis. 2011;11:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ivan A, Freund S, Ionescu T, et al. . Problem of excess mortality in influenza [in Romanian]. Viata Med. 1969;16(21):1483–1491. [Google Scholar]
- 95. Mazick A, Gergonne B, Wuillaume F, et al. . Higher all-cause mortality in children during autumn 2009 compared with the three previous years: pooled results from eight European countries. Euro Surveill. 2010;15(5):19480. [PubMed] [Google Scholar]
- 96. Azziz-Baumgartner E, Cabrera AM, Cheng PY, et al. . Incidence of influenza-associated mortality and hospitalizations in Argentina during 2002–2009. Influenza Other Respir Viruses. 2013;7(5):710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Viboud C, Grais RF, Lafont BA, et al. . Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis. 2005;192(2):233–248. [DOI] [PubMed] [Google Scholar]
- 98. Kuo HW, Schmid D, Liu YL, et al. . Influenza-related excess mortality, Austria 2001 till 2009. Wien Klin Wochenschr. 2011;123(19-20):593–598. [DOI] [PubMed] [Google Scholar]
- 99. Ansart S, Pelat C, Boelle PY, et al. . Mortality burden of the 1918–1919 influenza pandemic in Europe. Influenza Other Respir Viruses. 2009;3(3):99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Viboud C, Tam T, Fleming D, et al. . 1951 influenza epidemic, England and Wales, Canada, and the United States. Emerg Infect Dis. 2006;12(4):661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rizzo C, Bella A, Viboud C, et al. . Trends for influenza-related deaths during pandemic and epidemic seasons, Italy, 1969–2001. Emerg Infect Dis. 2007;13(5):694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rizzo C, Viboud C, Montomoli E, et al. . Influenza-related mortality in the Italian elderly: no decline associated with increasing vaccination coverage. Vaccine. 2006;24(42-43):6468–6475. [DOI] [PubMed] [Google Scholar]
- 103. Charu V, Chowell G, Palacio Mejia LS, et al. . Mortality burden of the A/H1N1 pandemic in Mexico: a comparison of deaths and years of life lost to seasonal influenza. Clin Infect Dis. 2011;53(10):985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nogueira PJ, Nunes B, Machado A, et al. . Early estimates of the excess mortality associated with the 2008–9 influenza season in Portugal. Euro Surveill. 2009;14(18):19194. [DOI] [PubMed] [Google Scholar]
- 105. Cohen C, Simonsen L, Kang JW, et al. . Elevated influenza-related excess mortality in South African elderly individuals, 1998–2005. Clin Infect Dis. 2010;51(12):1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Simón Méndez L, López-Cuadrado T, López Perea N, et al. . Premature mortality excess related to influenza in Spain during an interpandemic period [in Spanish]. Rev Esp Salud Publica. 2012;86(2):153–163. [DOI] [PubMed] [Google Scholar]
- 107. Chowell G, Erkoreka A, Viboud C, et al. . Spatial-temporal excess mortality patterns of the 1918–1919 influenza pandemic in Spain. BMC Infect Dis. 2014;14:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. León-Gómez I, Delgado-Sanz C, Jiménez-Jorge S, et al. . Excess mortality associated with influenza in Spain in winter 2012 [in Spanish]. Gac Sanit. 2015;29(4):258–265. [DOI] [PubMed] [Google Scholar]
- 109. Nguyen AM, Noymer A. Influenza mortality in the United States, 2009 pandemic: burden, timing and age distribution. PLoS One. 2013;8(5):e64198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Housworth J, Langmuir AD. Excess mortality from epidemic influenza, 1957–1966. Am J Epidemiol. 1974;100(1):40–48. [DOI] [PubMed] [Google Scholar]
- 111. Lui KJ, Kendal AP. Impact of influenza epidemics on mortality in the United States from October 1972 to May 1985. Am J Public Health. 1987;77(6):712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Simonsen L, Reichert TA, Viboud C, et al. . Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med. 2005;165(3):265–272. [DOI] [PubMed] [Google Scholar]
- 113. Serfling RE, Sherman IL, Houseworth WJ. Excess pneumonia-influenza mortality by age and sex in three major influenza A2 epidemics, United States, 1957–58, 1960 and 1963. Am J Epidemiol. 1967;86(2):433–441. [DOI] [PubMed] [Google Scholar]
- 114. Simonsen L, Clarke MJ, Williamson GD, et al. . The impact of influenza epidemics on mortality: introducing a severity index. Am J Public Health. 1997;87(12):1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Egger M, Jennings S, Spuhler T, et al. . Mortality in influenza epidemics in Switzerland 1969–1985 [in German]. Schweiz Med Wochenschr. 1989;119(13-14):434–439. [PubMed] [Google Scholar]
- 116. Imaz MS, Eimann M, Poyard E, et al. . Influenza associated excess mortality in Argentina: 1992–2002 [in Spanish]. Rev Chilena Infectol. 2006;23(4):297–306. [DOI] [PubMed] [Google Scholar]
- 117. Kyncl J, Prochazka B, Goddard NL, et al. . A study of excess mortality during influenza epidemics in the Czech Republic, 1982–2000. Eur J Epidemiol. 2005;20(4):365–371. [DOI] [PubMed] [Google Scholar]
- 118. Nunes B, Viboud C, Machado A, et al. . Excess mortality associated with influenza epidemics in Portugal, 1980 to 2004. PLoS One. 2011;6(6):e20661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Stroup DF, Thacker SB, Herndon JL. Application of multiple time series analysis to the estimation of pneumonia and influenza mortality by age 1962–1983. Stat Med. 1988;7(10):1045–1059. [DOI] [PubMed] [Google Scholar]
- 120. Lee VJ, Chen MI, Chan SP, et al. . Influenza pandemics in Singapore, a tropical, globally connected city. Emerg Infect Dis. 2007;13(7):1052–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. World Bank World Bank country and lending groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed November 21, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.