Abstract
Aims/hypothesis
Shift-work is associated with circadian rhythm disruption and an increased risk of obesity and type 2 diabetes. We sought to determine the effect of rotational shift-work on glucose metabolism in humans.
Methods
We studied 12 otherwise healthy nurses performing rotational shift-work using a randomised crossover study design. On each occasion, participants underwent an isotope-labelled mixed meal test during a simulated day shift and a simulated night shift, enabling simultaneous measurement of glucose flux and beta cell function using the oral minimal model. We sought to determine differences in fasting and postprandial glucose metabolism during the day shift vs the night shift.
Results
Postprandial glycaemic excursion was higher during the night shift (381±33 vs 580±48 mmol/l per 5 h, p<0.01). The time to peak insulin and C-peptide and nadir glucagon suppression in response to meal ingestion was also delayed during the night shift. While insulin action did not differ between study days, the beta cell responsivity to glucose (59±5 vs 44±4 × 10−9 min−1; p<0.001) and disposition index were decreased during the night shift.
Conclusions/interpretation
Impaired beta cell function during the night shift may result from normal circadian variation, the effect of rotational shift-work or a combination of both. As a consequence, higher postprandial glucose concentrations are observed during the night shift.
Keywords: Alpha cell function, Beta cell function, Circadian rhythm, Glucose tolerance, Insulin secretion, Shift-work
Introduction
Circadian disruption (i.e. a lack of synchrony between the endogenous circadian rhythm and the behavioural/environmental cycle) has been associated with an increased risk of type 2 diabetes and obesity [1, 2]. The pattern of food intake, exposure to light and (lack of) adequate sleep are all established inputs that affect circadian function [3, 4] and have been shown to negatively affect the regulation of glucose metabolism [5]. Large epidemiological studies suggest that shift-work is an independent risk factor for type 2 diabetes mellitus [1, 2]. In addition, induction of experimental circadian misalignment in otherwise healthy individuals leads to postprandial hyperglycaemia, accompanied by decreased insulin concentrations within a few weeks of exposure [6, 7]. Given the high prevalence of shift-work and night work in today’s society (for example, 68% of nurses in the Nurses’ Health Study II [8]), further studies are required to elucidate the mechanisms driving increased susceptibility to type 2 diabetes in humans experiencing work schedules associated with circadian disruption.
Circadian regulation of insulin secretion and action has long been appreciated as an important component of glucose homeostasis [5]. In humans, the circadian system is organised in a hierarchical manner [9]. Circadian pacemaker neurons localised to the suprachiasmatic nucleus of the hypothalamus synchronise (entrain) peripheral cell-autonomous circadian oscillators expressed in most cell types [10]. The molecular mechanism of the circadian clock is driven by a complex system of transcriptional, translational and post-translational feedback circuits operating with ~24 h periodicity [11]. The positive limb of this circuit includes the CLOCK and BMAL1 genes, which encode proteins that dimerise and initiate transcription through conserved promoter regions of ‘clock-controlled’ genes, thus providing temporal regulation of genes involved in diverse cellular processes [11]. Specifically, critical cellular pathways in beta cells are transcriptionally regulated by the circadian clock [12]. Both in vivo and in vitro data have shown that human pancreatic islets exhibit a robust 24 h circadian rhythm in insulin secretion independent of circulating glucose concentrations [13]. Moreover, beta cell responsivity to glucose is entrained by the light–dark cycle and circadian clock gene expression [14], whereas disruption of circadian rhythms and/or beta cell-specific deletion of Bmal1 impair glucose-stimulated insulin secretion and compromise beta cell survival and proliferative potential [15].
The circadian system also regulates insulin signalling and insulin action in tissues such as the liver, adipose and skeletal muscle that are critical for metabolic control in type 2 diabetes [16]. Consequently, disruption of circadian rhythms and/or tissue-specific clock gene deletion impair insulin action and induce glucose intolerance [17]. In the setting of impaired insulin action, the expression of key clock genes is altered in skeletal muscle and hepatic tissues [18]. In humans, shift-work increases the risk of obesity [1], but it is uncertain whether shift-work has direct effects on insulin action in humans [19]. Defects in both insulin secretion and insulin action contribute to the development of type 2 diabetes mellitus [20]; however, the underlying mechanism(s) by which shift-work increases this risk in humans is not established.
This study was designed to test the hypothesis that rotational shift-work raises postprandial glucose concentrations by decreasing beta cell function in non-diabetic humans. Beta cell function and glucose turnover were measured using the C-peptide minimal model together with a triple-tracer mixed meal [21] in volunteers during simulated day and night shifts. The timing of the experiment was consistent with the individuals’ work patterns so as to recapitulate physiological shift-work conditions. The study order was randomised but there was no other medical intervention. We report that beta cell responsivity to glucose was impaired during the night shift.
Methods
Participants
After approval by the Mayo Institutional Review Board, healthy rotational shift-workers were recruited by internal advertisement from the Mayo Clinic, Rochester, USA. All volunteers worked the same hours with similar patterns (three consecutive 12 h shifts from either 07:00–19:00 hours or 19:00–07:00 hours), comprising at least three night shifts per month for at least 1 year. Informed written consent was obtained from all volunteers. Participants were studied on the third day of their scheduled shift so as to adhere to their shift-work pattern. Participants had no known active illness, no history of symptomatic microvascular or macrovascular disease and were not on medications that could affect glucose metabolism or absorption. Studies were performed at >2 and <6 weeks apart.
Experimental design
Participants were studied on two occasions in random order within 6 weeks (Fig. 1). On one occasion, volunteers were studied after two consecutive day shifts; on the other, after two consecutive night shifts. Each 3 day block of shift-work was preceded by 48 h of time off as per their usual work cycle. They were admitted to the clinical research unit at 20:00 hours (for the day shift study) or at 08:00 hours (for the night shift study) after the completion of their second shift. Participants consumed a standardised mixed meal (two scrambled eggs, 50 g ham and 70 g gelatin dessert) and then slept in darkened conditions. At 06:30 hours (for the day shift study) or 18:30 hours (for the night shift study; t=−180 min), a forearm vein was cannulated with an 18 g needle to enable infusions to be performed. An 18 g retrograde cannula was inserted into a vein of the dorsum of the contralateral hand. This was placed into a heated Plexiglas box maintained at 55°C to enable sampling of arterialised venous blood (see electronic supplementary material [ESM] Table 1). A primed continuous infusion of [6,6-2H2]glucose was initiated at 07:00 hours (for the day shift study) or 19:00 hours (for the night shift study). At t=0 (10:00 hours or 20:00 hours), participants consumed a mixed meal consisting of two scrambled eggs, 50 g ham and gelatin desert containing 70 g glucose enriched with [1-13C]glucose (4% enrichment). Simultaneously, an infusion of [6-3H]glucose was started and the infusion rate was varied to mimic the anticipated appearance of meal [1-13C]glucose. The rate of infusion of [6,6-2H2]glucose was altered to approximate the anticipated fall in endogenous glucose production (EGP), thereby minimising changes in specific activity (see ESM Table 2) [22].
Fig. 1.
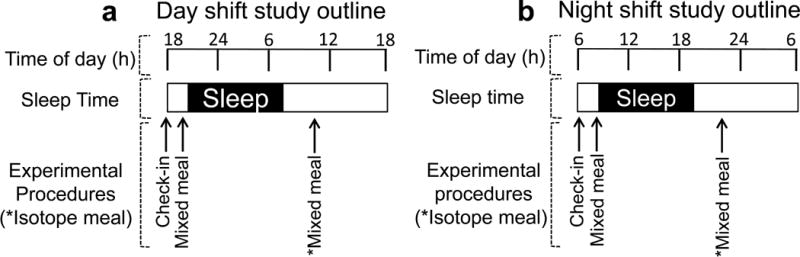
The experimental design used to mimic rotational shift-work. Participants were assessed after working two consecutive 12 h shifts during their third 12 h shift period for (a) the day shift study and (b) the night shift study
Consumption of the isotope meal was standardised. Participants repeatedly ate a spoonful of gelatin desert followed by a spoonful of ham and egg until the meal was finished. Participants were instructed to consume all of the meal within 15 min from the study start. At the end of the meal, participants drank 30 ml water. Blood samples were collected frequently throughout the study. At 15:00 hours or 03:00 hours, tracer infusions were stopped. Participants subsequently consumed a meal, all cannulas were removed and then participants left the clinical research unit.
Analytical techniques
Plasma samples were placed on ice, centrifuged at 4°C, separated and then stored at −20°C until assayed. Glucose concentrations were measured using the glucose oxidase method (Yellow Springs Instruments, Yellow Springs, OH, USA). Plasma insulin was measured using a chemiluminescence assay (Access Assay; Beckman, Chaska, MN, USA). Plasma glucagon and C-peptide concentrations were measured by Radio-Immunoassay (Linco Research, St Louis, MO, USA). Plasma [6,6-2H2]glucose and [1-13C]glucose enrichments were measured using gas chromatographic MS (Thermoquest, San Jose, CA, USA) to simultaneously monitor C-1 plus C-2 and C-3 – C-6 fragments, as described by Beylot et al [23]. In addition, [6-3H]glucose specific activity was measured by liquid scintillation counting after deproteinisation and anion exchange and cation exchange chromatography.
Calculations
The systemic rates of meal appearance (Meal Ra), EGP and glucose disappearance (Rd) were calculated using Steele’s model [24]. Meal Ra was calculated by multiplying the Ra of [1-13C]glucose (obtained from the infusion rate of [6-3H]glucose and the clamped plasma ratio of [6-3H]glucose and [1-13C]glucose) by the meal enrichment (ESM Fig. 1). The EGP rate was calculated from the infusion rate of [6,6-2H2]glucose and the ratio of [6,6-2H2]glucose to endogenous glucose concentration. Rd was calculated by subtracting the change in glucose mass from the overall rate of glucose appearance (i.e. Meal Ra + EGP). Values from −30 to 0 min were averaged and considered as basal. The area above basal (AAB) was calculated using the trapezoidal rule.
Net postprandial insulin action (insulin sensitivity index [Si]) was measured using the oral minimal model [21]. Beta cell responsivity indices were estimated using the oral C-peptide minimal model [21], incorporating age-associated changes in C-peptide kinetics [25]. The model assumes that insulin secretion comprises a static (ϕs) and a dynamic (ϕd) component with an index of total beta cell responsivity to glucose (Ф) derived from these two components. The disposition index (DI) was subsequently calculated by multiplying Ф by Si.
Statistical analysis
Data are presented as means ± SEM. The primary analyses compared changes in fasting, peak, time to peak and integrated hormone concentrations. Differences (day shift vs night shift) were assessed using a paired, two-tailed t test (for parametric values) or a Wilcoxon matched-pairs signed rank test (for non-parametric values). All rates are expressed per kilogram of lean body mass. The statistical analysis was performed using Primer 5 (GraphPad Software, San Diego, CA, USA). A p value of <0.05 was considered statistically significant. Data analysed came from participants with >90% complete datasets. If any data were missing, the missing variable was interpolated or the last observation was carried forward. Assuming similar variance in integrated glucose concentrations (mean ± SD, 421±62 mmol/l per 6 h) to those observed in prior studies [26–28], the inclusion of 12 participants would provide 80% power to detect a ~25% change in integrated glucose concentrations at α=0.05. In addition, assuming similar variation in DI to that observed previously [28] in a study of volunteers with type 2 diabetes (mean ± SD, 34±15 × 10−14 l kg−1 min−2 pmol−1), a paired study of 12 individuals would provide 80% power at an α=0.05 to detect an approximately 40% change (13 × 10−14 l kg−1 min−2 pmol−1). This is similar to the effect size observed with treatment with a dipeptidyl peptidase-4 inhibitor (a weak insulin secretagogue) [29].
Results
Volunteer characteristics
A total of 12 young (aged 25±1 years), overweight (BMI, 26.9±1.0 kg/m2; total weight, 73.8±4.9 kg; lean body mass 44.4±2.5 kg) but otherwise healthy nurses (10 women, two men) were studied. Participants had fasting glucose concentrations of 4.6±0.1 mmol/l and HbA1c concentrations of 5.0±0.1% (31±0.6 mmol/mol). Another two individuals gave written, informed consent but could not be included in the study because of scheduling conflicts.
Plasma glucose, insulin, C-peptide and glucagon concentrations during a mixed meal
Although fasting glucose concentrations did not differ between study days (Fig. 2a; day shift vs night shift, 4.9±0.1 vs 4.8±0.1 mmol/l, p=0.13), integrated postprandial glucose concentrations were higher during the night shift (day shift vs night shift, 381±33 vs 580±48 mmol/l per 5 h, p<0.01), with an accompanying delay in time to peak glucose concentrations (day shift vs night shift, 50±5 vs 69±6 mins, p<0.01).
Fig. 2.
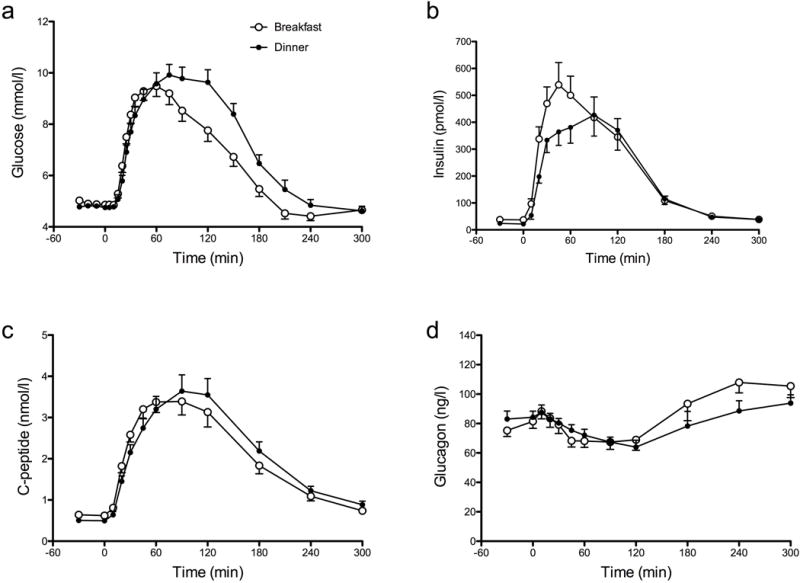
Glucose (a), insulin (b), C-peptide (c) and glucagon (d) concentrations during the day shift (white circles) and night shift (black circles). Values represent means ± SEMs
During the night shift, fasting insulin concentrations were lower (Fig. 2b; day shift vs night shift, 36±5 vs 23±3 pmol/l, p<0.01) and the time to peak insulin concentrations was delayed (day shift vs night shift, 61±9 vs 76±10 mins, p=0.02). Peak (day shift vs night shift, 623±68 vs 540±73 pmol/l, p=0.25) and integrated (day shift vs night shift, 57±8 vs 55±8 nmol/l per 5 h, p=0.68) postprandial insulin concentrations did not differ between study days. However, the increase in insulin concentration during the first 60 min after meal ingestion was lower during the night shift (day shift vs night shift, 20±8 vs 14±2 nmol/l per 5 h, p=0.02).
Similarly, fasting C-peptide concentrations were lower during the night shift (Fig. 2c; day shift vs night shift, 0.6±0.06 vs 0.5±0.04 nmol/l, p=0.01), with a significant delay in time to peak C-peptide concentrations (day shift vs night shift, 71±9 vs 96±7 mins, p=0.01). Consistent with insulin concentrations, peak and integrated postprandial C-peptide concentrations did not differ between study days.
Fasting glucagon concentrations (Fig. 2d; day shift vs night shift, 80±5 vs 84±5 ng/l, p=0.35) did not differ between study days. In addition, no differences in nadir (day shift vs night shift, 61±5 vs 62±4 ng/l, p=0.76) or integrated glucagon concentrations (day shift vs night shift, 2.6±1.8 vs 2.4±1.5 μg/l, p=0.07) were observed. However, the time to maximal glucagon suppression was increased during the night shift (day shift vs night shift, 85±8 vs 123±11 mins, p=0.01).
Net insulin action, beta cell responsivity and DI during the day and night shifts
Net insulin action (Si), as measured by the oral minimal model, did not differ between study days (day shift vs night shift, 7.2 ± 1.8 vs 6.0 ± 1.2 10−7 l kg−1 min per pmol−1, p = 0.50). The dynamic component of beta cell responsivity (ϕd; Fig. 3a) also did not differ between study days. However, the static component of beta cell responsivity to glucose was significantly impaired during the night shift (ϕs; day shift vs night shift, 51±5 vs 39±3 × 10−9 min−1, p<0.001; Fig. 3b).
Fig. 3.
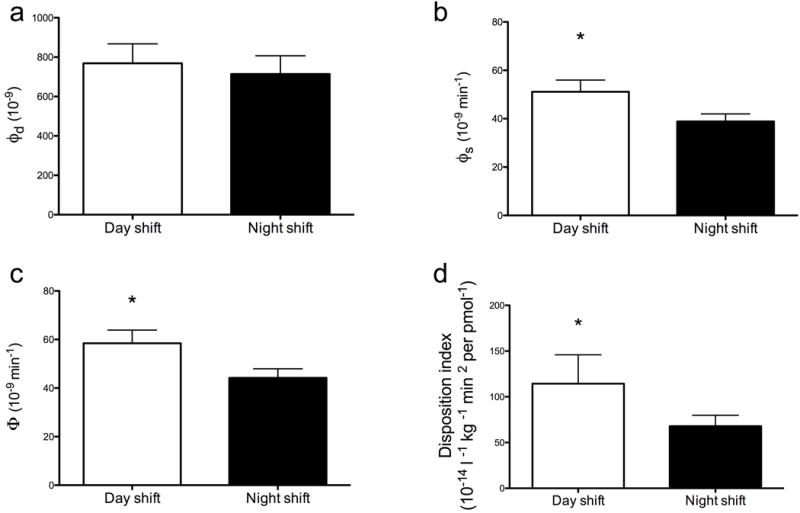
Indices of (a) dynamic (ϕd), (b) static (ϕs) and (c) total (Ф) beta cell responsivity, together with (d) DI during the day shift (white bars) and night shift (black bars). Values represent means ± SEMs. *p<0.05
Total beta cell responsivity (Φ; the sum of ϕd and ϕs) was decreased during the night shift study day (day shift vs night shift, 59±5 vs 44±4 × 10−9 min−1, p<0.001; Fig. 3c). When Φ was expressed as a function of the prevailing insulin action as DI, this variable was also significantly decreased during the night shift (Fig. 3d; day shift vs night shift, 114 ± 32 vs 68 ± 12 10−14 l kg−1 min−2 per pmol−1, p = 0.05) indicating impaired beta cell function.
Rates of EGP, meal Ra and Rd during a mixed meal
Fasting EGP was lower (p=0.02) during the night shift (day shift vs night shift, 16.5±0.7 vs 14.4±0.6 μmol kg−1 min−1; Fig. 4a). However, there was no difference in suppression to nadir values following meal ingestion (day shift vs night shift, 4.6±0.4 vs 4.2±0.4 μmol kg−1 min−1, p=0.48). Post hoc analysis suggested delayed recovery of EGP to fasting values during the night shift (e.g. at 300 min: day shift vs night shift, 16.5±2.8 vs 8.8±1.1 μmol kg−1 min−1, p=0.03), probably due to the higher glucose concentrations present.
Fig. 4.
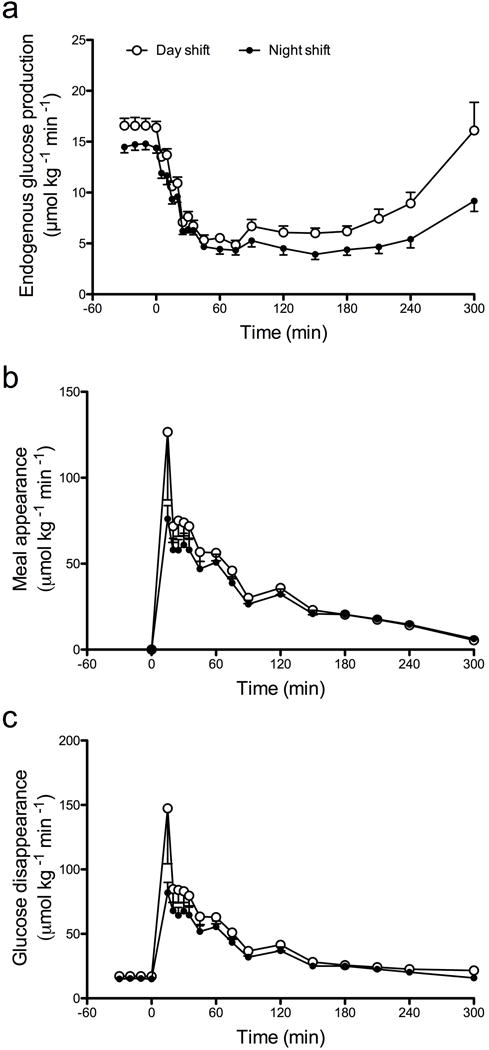
EGP (a), rate of meal appearance (b) and rate of glucose disappearance (c) during the day shift (white circles) and night shift (black circles). Values represent means ± SEMs.
Neither peak (day shift vs night shift, 123±31 vs 69±8 μmol kg−1 min−1, p=0.10) nor integrated (day shift vs night shift, 9.1±0.9 vs 7.7±0.6 mmol per 5 h, p=0.24) meal appearance differed between study days (Fig. 4b). Post hoc analysis of integrated rates of meal appearance during the first 60 min after meal ingestion did not demonstrate any difference between study days (day shift vs night shift, 3.7±0.6 vs 2.7±0.3 mmol per 1 h, p=0.12).
There was also no difference in the peak (day shift vs night shift, 132±31 vs 77±8 μmol kg−1 min−1, p=0.10) or integrated AAB (day shift vs night shift, 4.9±1.0 vs 4.8±0.5 mmol per 5 h, p=0.88) for glucose disappearance during the night shift study day compared with the day shift (Fig. 4c). Post hoc analysis of the integrated incremental (AAB) rates of glucose disappearance during the first 60 min after meal ingestion did not demonstrate any difference between study days (day shift vs night shift, 3.1±0.5 vs 2.2±0.3 mmol per 1 h, p=0.16).
Discussion
In this study of otherwise healthy rotational shift nurses, we demonstrate that an identical energy challenge during the night shift study day resulted in higher postprandial glucose concentrations compared with the day shift study day. This was due to impaired beta cell responsivity to glucose, specifically the component of beta cell responsivity (ϕs) that is thought to represent insulin synthesis and secretion in response to hyperglycaemia [21]. This resulted in blunted increases in insulin and C-peptide concentrations during the first hour after meal ingestion. The decreased and delayed secretion of insulin observed during the night shift closely resembles the pattern observed in people with impaired glucose tolerance or diabetes [30]. Taken together, these data suggest that during the night shift study, postprandial glucose concentrations were increased relative to the day shift study. This was explained by decreased beta cell function during the night shift study. The results are in keeping with a prior study in which shift-work (defined as at least three night shifts per month) significantly increased the risk of type 2 diabetes [1].
Despite changes in beta cell function, we did not observe significant changes in meal appearance or EGP. Meal appearance is altered by the rate of gastric emptying and by splanchnic extraction of ingested glucose. It is possible that equal and opposite changes in either variable between study days resulted in no net change in meal appearance. However, in the absence of changes in peak rates of and time to peak meal appearance, a change in gastric emptying induced by shift change seems unlikely [28].
More significantly, fasting EGP was lower during the night shift. As EGP represents the sum of gluconeogenesis and glycogenolysis, future studies will be required to ascertain the relative contribution of either process to EGP during a diurnal cycle or in situations of circadian misalignment. The changes in EGP were not accompanied by changes in postprandial rates of EGP or postprandial changes in insulin action (quantified by the minimal model as Si). Consequently, DI (which expresses beta cell responsivity as a function of insulin action) was impaired during the night shift compared with the day shift.
It is possible that our experiment was underpowered to detect small changes in glucose metabolism (despite changes in islet function) because tracer-based measurement are limited by requiring larger numbers of participants to reliably detect an effect on glucose metabolism [31]. An alternative explanation is that in otherwise healthy, young non-diabetic individuals, glucose effectiveness (i.e. the ability of glucose per se to stimulate its own uptake and suppress its own release) is sufficiently unimpaired so that stimulation of glucose disappearance and suppression of EGP is relatively unaffected despite decreased insulin secretion [32]. This phenomenon has been observed in other situations in which significant decreases in insulin secretion are accompanied by small changes in postprandial glucose metabolism [27].
Are these observations a direct effect of shift-work or a maladaptive response to stressors associated with shift-work, such as sleep deprivation or increased energy intake? The experimental design ensured that participants experienced an equivalent amount of sleep prior to each experiment. Moreover, energy intake and meal composition were identical for both study days, so these potential confounders cannot explain the experimental results. An alternative explanation is based on the observation that in healthy volunteers higher postprandial glucose excursion occurs in the evening [33–35]. However, the cause is variously reported as altered insulin secretion, insulin action or both across studies.
The study by Saad et al used techniques similar to ours to measure beta cell function and fasting and postprandial glucose metabolism, while also controlling for meal size, composition and physical activity [33]. These authors reported that beta cell responsivity and DI were higher at breakfast than at other times of the day, suggesting a diurnal pattern of beta cell function in non-diabetic individuals. This was accompanied by higher postprandial glucagon excursions after breakfast compared with other meals. However, the significance of this particular finding remains uncertain. In the current study, despite higher postprandial glucose concentrations during the night shift study, glucagon suppression was delayed compared with the day shift study but the actual nadir values did not differ. Whether delayed glucagon suppression is a consequence of impaired beta cell function is uncertain. Recently, it has been associated with impaired insulin action [36]; however, no effect of shift change on insulin action was observed in the current study.
It is possible that our experimental results reflect the normal diurnal decline in beta cell function (in otherwise healthy humans), as reported previously [33], and that 2 days of night shift-work is insufficient to alter or invert this circadian variation. Whether our findings represent alterations in insulin exocytosis and/or islet protein processing in the endoplasmic reticulum remains uncertain [37, 38]. Previously, Morris et al simulated night shift-work with an inverted behavioural cycle and reported higher postprandial glucose concentrations at 20:00 hours than at 08:00 hours, accompanied by lower insulin concentrations, independent of activity [39]. This agrees with our current findings, although insulin concentrations are a poor surrogate of insulin secretion because they represent the end result of insulin secretion and hepatic insulin extraction [21].
Previous epidemiological data showed that rotational shift-workers have a higher risk of type 2 diabetes mellitus [1], although, a more recent Danish study reported a higher risk of developing type 2 diabetes mellitus (independent of BMI) in night shift-workers compared with both day and rotational shift nurses over a 15 year period [40]. To date, there is a relative dearth of studies examining glucose metabolism and beta cell function in chronic shift-workers, and the conclusions that can be drawn are limited by the design of these studies. For example, Simon et al examined 24 h rhythms of glucose and insulin secretion in chronic night workers receiving continuous enteral nutrition [41]. The authors concluded that these volunteers had failed to completely adapt their 24 h rhythm of insulin secretion to their behavioural cycle. These differences were not explained by any difference in sleep variables, suggesting that sleep and circadian effects alter beta cell function through separate mechanisms.
Other investigators measured glucose and insulin concentration over periods of >48 h to demonstrate the circadian rhythmicity of insulin secretion [13, 42]. However, these studies used i.v. glucose infusions and did not directly measure beta cell function in response to a meal challenge. Nevertheless, they independently suggested diurnal variation in insulin secretion, compatible with the results obtained in the current study.
In conclusion, the increase in postprandial glucose concentrations observed during the night shift study day is explained by decreased beta cell function. These changes may represent circadian variations in insulin secretory capacity driven by changes in beta cell clock gene expression, as recently reported [38]. Further studies will be necessary to determine the mechanism(s) of diurnal decline in beta cell function and whether exposure to more prolonged patterns of chronic shift-work promotes sustained or greater decreases in beta cell function.
Supplementary Material
Acknowledgments
We gratefully acknowledge the contributions of our study coordinator P. D. Giesler and our research technicians J. Laugen and G. de Foster, and thank M. M. Davis for secretarial assistance (all from the Endocrine Research Unit, Mayo Clinic).
Funding
This study was funded by awards from the Mayo Clinic General Clinical Research Center (UL1 TR000135). AV is supported by the National Institutes of Health (grant no. DK78646) and RTV is supported by training grant (no. 5T32DK007352-37).
Abbreviations
- Ф
Beta cell responsivity to glucose
- ϕd
Dynamic component of insulin secretion
- ϕs
Static component of insulin secretion
- AAB
Area above basal
- DI
Disposition index
- EGP
Endogenous glucose production
- Meal Ra
Rate of meal appearance
- Rd
Rate of glucose disappearance
- Si
Insulin sensitivity index
Footnotes
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Duality of interest statement
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
AV designed (assisted by AM and RAR) and oversaw the study; AS ran the study; CC, CDM, MCL and RTV assisted with data acquisition, AS and AV researched the data; MCL, CDM, RTV and CC analysed and interpreted the data; AS drafted the manuscript; AM, CC, CDM, MCL, RAR and RTV reviewed and/or edited the manuscript; and AV wrote the final version of the manuscript. All authors gave final approval of the version to be published. AV is the guarantor of this work and, as such, had full access to all of the study data and takes responsibility for data integrity and the accuracy of the data analysis.
References
- 1.Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8:e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suwazono Y, Dochi M, Oishi M, Tanaka K, Kobayashi E, Sakata K. Shiftwork and impaired glucose metabolism: a 14-year cohort study on 7104 male workers. Chronobiol Int. 2009;26:926–941. doi: 10.1080/07420520903044422. [DOI] [PubMed] [Google Scholar]
- 3.Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23:1048–1059. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonken LK, Nelson RJ. The effects of light at night on circadian clocks and metabolism. Endocr Rev. 2014;35:648–670. doi: 10.1210/er.2013-1051. [DOI] [PubMed] [Google Scholar]
- 5.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buxton OM, Cain SW, O’Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra143. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujishiro K, Lividoti Hibert E, Schernhammer E, Rich-Edwards JW. Shift work, job strain and changes in the body mass index among women: a prospective study. Occup Environ Med. 2016 doi: 10.1136/oemed-2016-103747. [DOI] [PubMed] [Google Scholar]
- 9.Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 10.Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001;2:521–526. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perelis M, Ramsey KM, Marcheva B, Bass J. Circadian transcription from beta cell function to diabetes pathophysiology. J Biol Rhythms. 2016;31:323–336. doi: 10.1177/0748730416656949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boden G, Ruiz J, Urbain JL, Chen X. Evidence for a circadian rhythm of insulin secretion. Am J Physiol. 1996;271:E246–E252. doi: 10.1152/ajpendo.1996.271.2.E246. [DOI] [PubMed] [Google Scholar]
- 14.Qian J, Block GD, Colwell CS, Matveyenko AV. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes. 2013;62:3469–3478. doi: 10.2337/db12-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rakshit K, Hsu TW, Matveyenko AV. Bmal1 is required for beta cell compensatory expansion, survival and metabolic adaptation to diet-induced obesity in mice. Diabetologia. 2016;59:734–743. doi: 10.1007/s00125-015-3859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian J, Scheer FA. Circadian system and glucose metabolism: implications for physiology and disease. Trends Endocrinol Metab. 2016;27:282–293. doi: 10.1016/j.tem.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Zhou B, Yan M, et al. CLOCK and BMAL1 regulate muscle insulin sensitivity via SIRT1 in male mice. Endocrinology. 2016;157:2259–2269. doi: 10.1210/en.2015-2027. [DOI] [PubMed] [Google Scholar]
- 19.Dowling HJ, Fried SK, Pi-Sunyer FX. Insulin resistance in adipocytes of obese women: effects of body fat distribution and race. Metabolism. 1995;44:987–995. doi: 10.1016/0026-0495(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 20.Sathananthan A, Man CD, Zinsmeister AR, et al. A concerted decline in insulin secretion and action occurs across the spectrum of fasting and postchallenge glucose concentrations. Clin Endocrinol (Oxf) 2012;76:212–219. doi: 10.1111/j.1365-2265.2011.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R. The oral minimal model method. Diabetes. 2014;63:1203–1213. doi: 10.2337/db13-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vella A, Rizza RA. Application of isotopic techniques using constant specific activity or enrichment to the study of carbohydrate metabolism. Diabetes. 2009;58:2168–2174. doi: 10.2337/db09-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beylot M, Previs SF, David F, Brunengraber H. Determination of the 13C-labeling pattern of glucose by gas chromatography-mass spectrometry. Anal Biochem. 1993;212:526–531. doi: 10.1006/abio.1993.1363. [DOI] [PubMed] [Google Scholar]
- 24.Steele R, Bjerknes C, Rathgeb I, Altszuler N. Glucose uptake and production during the oral glucose tolerance test. Diabetes. 1968;17:415–421. doi: 10.2337/diab.17.7.415. [DOI] [PubMed] [Google Scholar]
- 25.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41:368–377. doi: 10.2337/diab.41.3.368. [DOI] [PubMed] [Google Scholar]
- 26.Bock G, Dalla Man C, Micheletto F, et al. The effect of DPP-4 inhibition with sitagliptin on incretin secretion and on fasting and postprandial glucose turnover in subjects with impaired fasting glucose. Clin Endocrinol (Oxf) 2010;73:189–196. doi: 10.1111/j.1365-2265.2009.03764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah M, Law JH, Micheletto F, et al. Contribution of endogenous glucagon-like peptide 1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes. 2014;63:483–493. doi: 10.2337/db13-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smushkin G, Sathananthan M, Piccinini F, et al. The effect of a bile acid sequestrant on glucose metabolism in subjects with type 2 diabetes. Diabetes. 2013;62:1094–1101. doi: 10.2337/db12-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalla Man C, Bock G, Giesler PD, et al. Dipeptidyl peptidase-4 inhibition by vildagliptin and the effect on insulin secretion and action in response to meal ingestion in type 2 diabetes. Diabetes Care. 2009;32:14–18. doi: 10.2337/dc08-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bock G, Dalla Man C, Campioni M, et al. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 2006;55:3536–3549. doi: 10.2337/db06-0319. [DOI] [PubMed] [Google Scholar]
- 31.Vella A. Mechanism of action of DPP-4 inhibitors – new insights. J Clin Endocrinol Metab. 2012;97:2626–2628. doi: 10.1210/jc.2012-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basu A, Caumo A, Bettini F, et al. Impaired basal glucose effectiveness in NIDDM: contribution of defects in glucose disappearance and production, measured using an optimized minimal model independent protocol. Diabetes. 1997;46:421–432. doi: 10.2337/diab.46.3.421. [DOI] [PubMed] [Google Scholar]
- 33.Saad A, Dalla Man C, Nandy DK, et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes. 2012;61:2691–2700. doi: 10.2337/db11-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Service FJ, Hall LD, Westland RE, et al. Effects of size, time of day and sequence of meal ingestion on carbohydrate tolerance in normal subjects. Diabetologia. 1983;25:316–321. doi: 10.1007/BF00253193. [DOI] [PubMed] [Google Scholar]
- 35.Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol. 1992;262:E467–E475. doi: 10.1152/ajpendo.1992.262.4.E467. [DOI] [PubMed] [Google Scholar]
- 36.Faerch K, Vistisen D, Pacini G, et al. Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation. Diabetes. 2016;65:3473–3481. doi: 10.2337/db16-0240. [DOI] [PubMed] [Google Scholar]
- 37.Rakshit K, Qian J, Ernst J, Matveyenko AV. Circadian variation of the pancreatic islet transcriptome. Physiol Genomics. 2016;48:677–687. doi: 10.1152/physiolgenomics.00019.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perelis M, Marcheva B, Ramsey KM, et al. Pancreatic beta cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015;350:aac4250. doi: 10.1126/science.aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris CJ, Purvis TE, Mistretta J, Scheer FA. Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. J Clin Endocrinol Metab. 2016;101:1066–1074. doi: 10.1210/jc.2015-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen AB, Stayner L, Hansen J, Andersen ZJ. Night shift work and incidence of diabetes in the Danish Nurse Cohort. Occup Environ Med. 2016;73:262–268. doi: 10.1136/oemed-2015-103342. [DOI] [PubMed] [Google Scholar]
- 41.Simon C, Weibel L, Brandenberger G. Twenty-four-hour rhythms of plasma glucose and insulin secretion rate in regular night workers. Am J Physiol Endocrinol Metab. 2000;278:E413–420. doi: 10.1152/ajpendo.2000.278.3.E413. [DOI] [PubMed] [Google Scholar]
- 42.Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88:934–942. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


