Abstract
Authorized generics are identical in formulation to brand drugs, manufactured by the brand company but marketed as a generic. Generics, marketed by generic manufacturers, are required to demonstrate pharmaceutical and bioequivalence to the brand drug, but repetition of clinical trials is not required. This retrospective cohort study compared outcomes for generics and authorized generics, which serves as a generic vs. brand proxy that minimizes bias against generics. For the seven drugs studied between 1999-2014, 5,234 unique patients were on brand drug prior to generic entry and 4,900 (93.6%) switched to a generic. During the 12-months following the brand-to-generic switch, patients using generics vs. authorized generics were similar in terms of outpatient visits, urgent care visits, hospitalizations, and medication discontinuation. The likelihood of emergency department visits was slightly higher for authorized generics compared with generics. These data suggest that generics were clinically no worse than their proxy brand comparator.
Keywords: Generic, Authorized Generic, Switching, Medication Discontinuation, Hospitalization
INTRODUCTION
Generic drugs play an important role in controlling health care costs.(1, 2) Between 2003 and 2012, generic drug use realized savings of $1.2 trillion in health care expenditures.(3) In 2012 alone, health care savings of $217 billion was attributed to generic drug use.(3) Generic drugs also have been associated with better adherence than brand drugs.(4, 5) While the economic and adherence-related benefits of generic drug competition are clear, there is a perception among some health care providers and patients that there is a lack of clinical therapeutic equivalence between generic and brand drugs.(6, 7)
The U.S. Food and Drug Administration (FDA) has a rigorous approval process to ensure that a generic drug is therapeutically equivalent to a reference product (usually the brand drug) prior to releasing it into the market.(8) But, generic drugs, which are approved through an abbreviated new drug application (ANDA), are not required to provide preclinical and clinical data to establish safety and efficacy. Rather, generic drugs must show bioequivalence to the approved brand by demonstrating that the active ingredient has no significant difference in the rate and extent of absorption at the site of drug action typically in healthy individuals.(9) While ANDAs help expedite the generic drug approval process, bioequivalence studies are not designed to identify clinical outcome differences or detect adverse events that may occur at different rates with generic as opposed to brand drugs. Generic drugs are typically not required to contain the same inactive ingredients as the branded product approved through the new drug application (NDA) process, so it is possible that even though brand and generic products include the same active ingredients, differences in safety or efficacy related to variability in the formulation may exist. Research to conclusively support or refute differences in clinical outcomes for brand vs. generic products is limited.
A regulatory approach to address generic-brand equivalency concerns could be to modify the approval process to require comparative clinical trials. But, equivalence studies with clinical endpoints are less sensitive to formulation differences and would require considerably larger sample sizes than are currently used in bioequivalence studies. Requiring prospective head-to-head clinical studies for the approval of generic drugs would increase costs associated with bringing a generic drug to the market, and ultimately reduce competition and increase generic prices. Thus, changing the regulatory approval requirements in this way is not a viable solution. Post-marketing surveillance plays a valuable role in assessing lingering concerns regarding possible differences in the efficacy or incidence of adverse events between generic and branded drugs. However, patient and provider perceptions (i.e., public perception) that branded drugs are superior to generics (10-17) are believed to introduce bias in post-market assessment of efficacy and safety outcomes, making uncontrolled observational evaluation challenging.
One nuance in the generic drug market that provides an interesting research opportunity for overcoming the public perception bias is the advent of authorized generics. Authorized generics (AGs) are drugs that contain the same active and inactive ingredients as the branded product, authorized and manufactured under the same NDA, with the only difference being that they are labeled and marketed as generic drugs.(18) This compares to independent generics (i.e., “generics”), which are approved and marketed under an ANDA. Therefore, the safety and efficacy profiles between an AG and the branded drug should be identical, while the safety and efficacy profile of generics could plausibly (although unlikely) differ from an AG or branded drug. Since AGs and generics are similar in terms of their prescribing, dispensing, and cost to patients (i.e., they are both perceived to be “generics”),(19) the public perception bias and cost considerations should be equally applied to both products. Therefore, comparing their utilization patterns and outcomes provides a proxy for a brand vs. generic comparison that removes generic drug perception bias.
To our knowledge, no previous studies have systematically compared branded products, AGs, and generics to examine usage patterns and outcomes. The objectives of this study were to compare brand-to-generic switching patterns and, among those switching to a generic, compare outcomes for AG and generic drugs to determine if there is evidence that actual differences exist in terms of drug utilization patterns and markers of health services use that could be attributed to differences in therapeutic equivalence between drug products. We hypothesized that the rates of health services use and medication discontinuation would be similar for patients switching to an AG (i.e., brand drug proxy) as opposed to a generic.
RESULTS
Across the seven drugs analyzed we identified 5,234 unique people and 5,544 unique person-drug combinations (Table 2) that were using an eligible brand drug and met other inclusion criteria at the time of generic entry. For the full cohort, the mean age was approximately 60 years and 71% were female (compared with 51.4% female overall in the SHP population). Characteristics of the patients switching to generic were similar to characteristics of the patients staying on brand, with the exception of defined daily dose, having an ED visit during the 6 months prior to generic entry, and the count of outpatient visits prior to generic entry. Compared with the patients not switching to generic (i.e., non-switchers), the brand-to-generic switchers tended to be on a higher mean daily dose (1.3 vs. 1.1 defined daily doses) and less likely to have had an ED visit (14% vs. 20%) or an outpatient visit (mean of 6 vs. 7) during the 6 months prior to generic entry (p<0.05). A total of 3,762 switches were from brand to generic (77%), while 1,138 switches were from brand to an AG (23%). The paroxetine and sertraline sub-cohorts accounted for the highest proportion of AG switches (48% and 40%, respectively).
Table 2.
Characteristics of brand to generic switchers and non-switchers (N=5234)
| Characteristic | Non-Switchers | All Switchers | Switch Type *
|
P-value: switchers vs. non-switchers | |
|---|---|---|---|---|---|
| Brand to AG | Brand to Generic | ||||
| Sample; n (%) | 334 (6%) | 4900 (94%) | 1138 (23%) | 3762 (77%) | - |
| Demographics | |||||
| Age; mean (SD) | 59 ± 17 | 60 ± 18 | 56 ± 18 | 61 ± 17 | 0.712 |
| Sex; n (%) male | 94 (28%) | 1421 (29%) | 355 (31%) | 1068 (28%) | 0.756 |
| Proportion of pre-index brand use; % | 59% | 60% | 62% | 59% | 0.731 |
| Pre-index defined daily dose (mean, SD) | 1.1 ± 0.7 | 1.3 ± 0.8 | 1.3 ± 0.8 | 1.2 ± 0.7 | <0.001 |
| Charlson comorbidity index (mean, SD) | 0.6 ± 1.1 | 0.6 ± 1.1 | 0.6 ± 1.1 | 0.6 ± 1.1 | 0.615 |
| Pre-index hospitalization; n (%) | 39 (12%) | 485 (10%) | 109 (10%) | 372 (10%) | 0.298 |
| Pre-index ED visit; n (%) | 68 (20%) | 696 (14%) | 190 (17%) | 508 (14%) | 0.003 |
| Pre-index outpatient visit count (median) | 7 | 6 | 6 | 6 | 0.007 |
| Switchers by Drug; n (%) ** | |||||
| Alendronate (n=930) | 57 (6%) | 873 (94%) | 41 (5%) | 832 (95%) | - |
| Amlodipine (n=1487) | 42 (3%) | 1445 (97%) | 289 (20%) | 1156 (80%) | - |
| Citalopram (n=813) | 69 (8%) | 744 (92%) | 74 (10%) | 670 (90%) | - |
| Gabapentin (n=279) | 55 (20%) | 224 (80%) | 25 (11%) | 199 (89%) | - |
| Paroxetine (n=669) | 39 (6%) | 630 (94%) | 302 (48%) | 328 (52%) | - |
| Sertraline (n=730) | 35 (5%) | 695 (95%) | 278 (40%) | 417 (60%) | - |
| Simvastatin (n=636) | 52 (8%) | 584 (92%) | 176 (30%) | 408 (70%) | - |
The percentages of switchers by switch type are reflected as a percentage of all switchers
Some individuals qualified for multiple drug cohorts. In the full cohort the first person-drug combination was selected and subsequent observations for other drugs were excluded. This results in 5234 unique patients in the full cohort, but 5544 unique person-drug combinations.
AG = authorized generic; “Generic” refers to all other independing generics filed under an Abbreviated New Drug Application (ANDA); ED = emergency department
Switching from brand to generic was common among this cohort, with 94% switching to generic during the first 12 months one was available. With the exception of gabapentin and simvastatin, the majority (i.e., 80-95%) of brand to generic switching occurred within the first 3 months following generic entry (Figure 1). For gabapentin and simvastatin, approximately 70% of switching occurred within 3 months following generic entry. For gabapentin the percentage of switchers continued to slowly increase through 12 months of follow-up, while for simvastatin there was a sharp increase in brand-to-generic switching after 6 months of generic availability. In the multivariable model of time to generic switching among the full cohort, the specific drug was the most consistent predictor (Table 3). For example, brand-to-generic switching was faster for alendronate (HR=1.25; 95% CI 1.15-1.36), amlodipine (HR=1.43; 95% CI 1.33-1.53), and sertraline (HR=1.17; 95% CI 1.07-1.27), but slower for citalopram (HR=0.78; 95% CI0.72-0.84), gabapentin (HR=0.67; 95% CI 0.58-0.77), paroxetine (HR=0.91; 95% CI0.83-0.99), and simvastatin (HR=0.71; 95% CI0.64-0.78). Patient-related factors associated with a more rapid brand-to-generic switch included having a higher defined daily dose (HR=1.09; 95% CI 1.05-1.13) and occurrence of a hospitalization during the 6 months prior to generic availability (HR=1.15; 95% CI 1.02-1.29).
Figure 1.
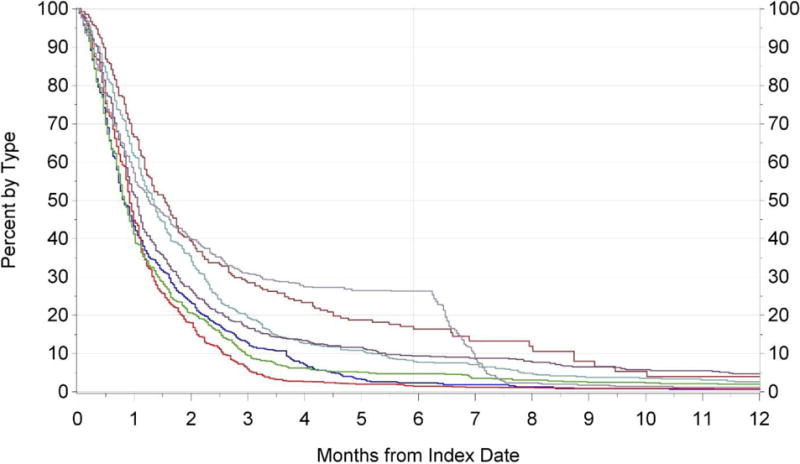
Time from generic entry (index date) to generic switch by drug type
Table 3.
Predictors of time to generic switching
| Characteristic (N=5234)* |
Hazard Ratio | 95% Confidence Interval | P-value | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Age (in years) | 1.00 | 1.00 | 1.00 | 0.9313 |
| Male | 0.97 | 0.91 | 1.03 | 0.3593 |
| Proportion of pre-index brand use; % | 0.91 | 0.81 | 1.04 | 0.158 |
| Pre-index defined daily dose | 1.09 | 1.05 | 1.13 | <0.0001 |
| Charlson comorbidity index | 0.98 | 0.95 | 1.01 | 0.1833 |
| Pre-index hospitalization | 1.15 | 1.02 | 1.29 | 0.0195 |
| Pre-index ED visit | 0.96 | 0.87 | 1.05 | 0.367 |
| Pre-index outpatient visit count | 1.00 | 1.00 | 1.00 | 0.8124 |
| Alendronate** | 1.25 | 1.15 | 1.36 | <0.0001 |
| Amlodipine | 1.43 | 1.33 | 1.53 | <0.0001 |
| Citalopram | 0.78 | 0.72 | 0.84 | <0.0001 |
| Gabapentin | 0.67 | 0.58 | 0.77 | <0.0001 |
| Paroxetine | 0.91 | 0.83 | 0.99 | 0.031 |
| Sertraline | 1.17 | 1.07 | 1.27 | 0.0006 |
| Simvastatin | 0.71 | 0.64 | 0.78 | <0.0001 |
ED = emergency department
Analyses include 5234 unique patients; for those (among the 5544) exposed to more than one of the seven drugs, the drug of first exposure is the drug analyzed.
In the absence of a specific comparison (control) drug, we present results for each drug contrasted with the combined cohort for the other six drugs. Results for each drug come from separate models, each using a unique indicator (e.g., Alendronate=1, all other drugs=0).
The patients who did not switch from brand to a generic were different from the switchers in terms of their mean observation time following generic entry. The mean observation time was 78 days for non-switchers, 220 days for switchers to AG, and 276 days for switchers to generic. On an annual basis the non-switchers had consistently higher rates of these outcomes than the switchers. However, since our analysis was designed to determine whether the type of generic drug (AG vs. generic) influenced outcomes we focused on the comparison of switchers to AG vs. switchers to generic rather than comparing non-switchers to switchers (Table 4). We did not observe differences between the AG and the generic switch groups in terms of the number of outpatient visits, the number of urgent care visits, the number of ED visits, the occurrence or number of hospitalizations, or the occurrence of medication discontinuation (P>0.05). However, we did observe a difference in the occurrence of all-cause ED visits for the AG and generic groups (P=0.006). In the switch to AG group, 27.6% (95% CI 24.5-30.8%) had an ED visit, while only 22.8% (95% CI 21.3-24.3%) had an ED visit in the switch to generic group.
Table 4.
Drug and health services utilization among non-switchers and switchers to authorized generic vs. generic
| Utilization | Non-Switchers | Switchers by Type
|
AG vs. Generic P-value | |
|---|---|---|---|---|
| Brand to AG | Brand to Generic | |||
| Annual number of all-cause outpatient visits (mean, 95% CI) | 20.8 (18.4-23.6) | 17.5 (16.6-18.5) | 17.4 (16.9-17.9) | 0.819 |
| Annual number of all-cause urgent care visits (mean, 95% CI) | 11.4 (8.2-15.8) | 0.6 (0.5-0.7) | 0.5 (0.5-0.6) | 0.140 |
| Annual all-cause emergency department visits | ||||
| Any visit (%, 95% CI) | 32.2 (23.8-41.9) | 27.6 (24.5-30.8) | 22.8 (21.3-24.3) | 0.006 |
| Number per year (mean, 95% CI) | 0.7 (0.4-1.0) | 0.5 (0.4-0.6) | 0.4 (0.4-0.5) | 0.074 |
| Annual all-cause hospitalizations | ||||
| Any visit (%, 95% CI) | 26.0 (18.1-35.8) | 17.7 (15.1-20.6) | 17.7 (16.4-19.1) | 0.997 |
| Number per year (mean, 95% CI) | 2.5 (1.4-4.6) | 1.4 (1.0-1.8) | 1.5 (1.3-1.7) | 0.641 |
| Medication discontinuation (%, 95% CI) | 99.4 (99.2-99.6) | 35.2 (32.0-38.5) | 34.8 (33.2-36.5) | 0.854 |
The mean observation time was 78 days for non-switchers, 220 days for switchers to AG, and 276 days for switchers to generic. Because of these differences in observation time the occurrence and number of outpatient, urgent care, emergency department, and hospital visits was estimated on an annual basis using univariate negative binomial regression for count variables and logistic regression for binary variables without adjusting for covariates. The difference in utilization between switchers to AG and switchers to generic was assessed via rate ratios for the negative binomial models and odds ratios for the logistic models, with statistical significance reflected by P<0.05.
AG = authorized generic; “Generic” refers to all other independent generics filed under an Abbreviated New Drug Application (ANDA)
The difference in utilization outcomes between switchers to AG and switchers to generic was statistically assessed via rate ratios for the negative binomial models and odds ratios for the logistic models, adjusting for drug and other covariates (Figure 2). The adjusted comparison of health services utilization and medication discontinuation illustrates no statistically significant differences between the AG group and the generic group for outpatient visits, urgent care visits, all-cause hospitalization, and medication discontinuation. The differences in all-cause ED use comparing switchers to AG versus switchers to generic was marginally significant in the adjusted analyses, with an odds ratio of 1.33 (95% CI 1.11-1.61) for any ED visit and a rate ratio of 1.23 (95% CI 1.02-1.47) for the number of ED visits per year.
Figure 2. Adjusted comparison of health services utilization and medication discontinuation for authorized generic vs. generic.
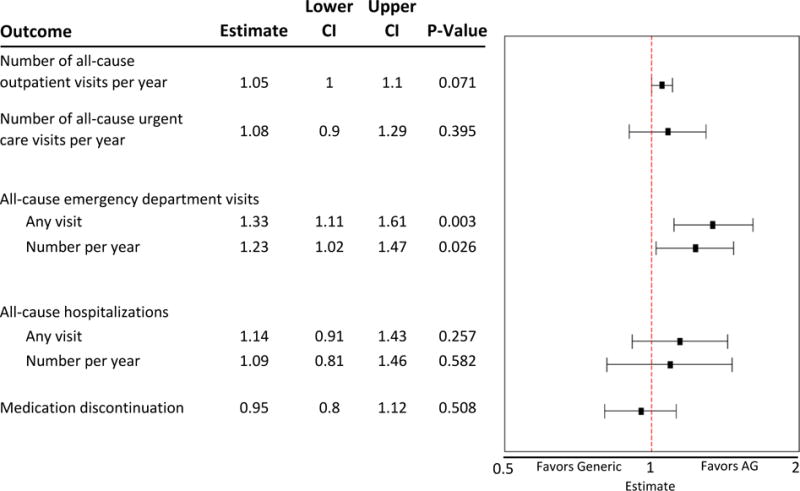
AG = authorized generic; “Generic” refers to all other independing generics filed under an Abbreviated New Drug Application (ANDA)
Estimates greater than 1 suggest that the outcome was more likely to occur in the AG group, while estimates less than 1 suggest that the outcome was more likely to occur in the generic group.
Considering the unexpected differences observed in ED visits between the AG and the generic groups, we explored whether individual drugs or other covariates might be particularly influential in this finding (see supplementary tables). Considering, for example, the adjusted odds ratios for any ED visits with individual drugs, we observed statistically significant differences for the AG group vs. the generic group only for two drugs; alendronate (OR=4.09; 95% CI2.18-13.16) and sertraline (OR=1.65; 95% CI 1.23-2.16) had a higher likelihood of ED visits for the AG group compared to the generic group.
DISCUSSION
In this study we compared brand-to-generic switching patterns and, among those switching to a generic, we compared AG and generic users with regard to subsequent measures of health services use and medication discontinuation. We conceptualized AG users to represent patients who were taking a drug identical in formulation to a brand drug, but who perceived they were using a generic drug. Therefore, comparison between AG and generic users indirectly represents a brand vs generic comparison after controlling for generic drug perception bias.(10-17) In our AG and generic comparison, we observed similar rates of outpatient visits, urgent care visits, hospitalizations, and medication discontinuation. While we observed a higher likelihood of using the ED and a higher number of ED visits among AG users compared to generic users, this finding still supports that generics did not have worse outcomes than the AGs.
The finding of higher likelihood of ED visits for patients switching to AG compared with generic is surprising and needs further scrutiny. For instance, it is possible that inactive ingredients used by the generic companies have a better safety profile than those used by the brand. But, it is also possible that even though we controlled for patient-related factors and pre-index health services use, there could be uncontrolled factors influencing who is getting an AG vs. a generic. For example, physicians and pharmacists may be more comfortable to prescribe and dispense generics produced by North American or European generic drug producers, yet we could not consistently measure and control for this factor. There also could be regional or health plan-related factors that influence which patients get an AG vs. a generic, and these differences might also be reflected in differential likelihood of using the ED. Health plan selection might also be correlated with pharmacy usage, and pharmacy supply may vary geographically or by type of pharmacy. When the AG and generic enter the market at the same time, pharmacies most likely will stock either the AG or the generic, and the decision of which product to stock could be influenced by wholesale distributors or buying groups. A plausible scenario to reflect AG vs. generic selection bias could be that hospital outpatient pharmacies might receive better pricing on AGs than community pharmacies, and therefore patients using the hospital outpatient pharmacy would be more likely to receive AGs compared with patients using a community pharmacy. Consequently, due to proximity of the hospital outpatient pharmacy with the ED, the likelihood to receive the AG may be related to the access to the ED or the likelihood to seek care at the ED. Future studies are needed to further explore potential unobserved confounding.
Our analyses pooled data for seven drugs, which gave us the opportunity to study a large population of users of various commonly used drugs with AGs in the market. However, the group of drugs we studied influenced the cohort characteristics. For example, our cohort was comprised of 71% females, compared with 51.4% females in the overall SHP population. This appeared to be consistent with previously documented higher rates of female prescribing of alendronate for osteoporosis (20) and antidepressants (e.g., citalopram, paroxetine, and sertraline) for conditions such as depression or anxiety.(21) Possible differences between a brand and generic also could be isolated to specific products rather than generalized across all drugs. To address this concern we replicated our analyses for each individual drug. Although the results are too lengthy for presentation here (see supplementary data), these analyses were generally consistent with the pooled analyses with the exception of the ED visit outcome. The individual drug analyses illustrated that the higher likelihood of an ED visit and the higher number of ED visits for AG vs. generic was driven by alendronate and amlodipine, while simvastatin illustrated an opposite relationship whereby ED visits were less common for those on the AG vs. the generic. This emphasizes the importance of cautious interpretation of the pooled data, and suggests that future evaluations should consider products individually. Moreover, future research should consider that the group of generic drugs also may be heterogeneous and assess individual generic drugs and formulations. This has been illustrated with historical examples such as bupropion and methylphenidate, whereby post-marketing data led to a delayed determination that specific generic formulations were not equivalent to the branded reference drug.(22-24)
Another complexity in making comparisons across different drugs relates to the timing of generic drug availability. In many cases, the AG enters the market at the same time as the first generic. In our analyses, amlodipine, citalopram, gabapentin, sertraline, and simvastatin had the AG enter the market at the same time as the first generic. For alendronate and paroxetine, however, the AG entered the market 6 months prior to the first generic (Table 1). In both of these drugs, the 6-month delay in entry of the generic could have influenced switching patterns and could have introduced additional confounding in our analyses.
Table 1.
Products with authorized generics assessed in this study
| Drug | Form/Strentghs | Therapeutic Class | Date of First Reference Listed Drug (Brand) | Date of First Authorized Generic (AG) | Date of First ANDA-Approved Generic (Generic) | Number of FDA ANDA Approvals* |
|---|---|---|---|---|---|---|
| Alendronate | Tablets, 35 & 70MG | Osteoporosis | 9/29/95 | 2/6/08 | 8/4/08 | 13 |
| Amlodipine | Tablets, 2.5, 5 & 10MG | Cardiovascular | 7/31/92 | 3/23/07 | 3/23/07 | 34 |
| Citalopram | Tablets, 10, 20 & 40MG | Antidepressant | 7/17/98 | 10/28/04 | 10/28/04 | 25 |
| Gabapentin | Capsules, 100, 300 & 400MG | Antiepileptic | 12/30/93 | 10/8/04 | 10/4/04 | 18 |
| Paroxetine | Tablets, 10, 20, 30 & 40MG | Antidepressant | 12/29/92 | 3/5/03 | 9/8/03 | 12 |
| Sertraline | Tablets, 25, 50 & 100MG | Antidepressant | 12/30/91 | 8/14/06 | 8/14/06 | 26 |
| Simvastatin | Tablets, 5, 10, 20, 40 & 80MG | Cardiovascular | 12/23/91 | 6/23/06 | 6/23/06 | 14 |
Number of Abbreviated New Drug Applications (ANDAs) approved by the FDA for each drug as of December 31, 2014. A sponsor company may have multiple ANDAs fror the same drug.
AG = authorized generic; “Generic” refers to all other independing generics filed under an ANDA
We observed a rapid rate of switching from brand to generic among the insured population we studied. This is likely a sign of effective formulary management by the health plan. We also observed that the patients who did not switch from brand to generic were quite different from the generic switchers in that they had shorter eligible follow-up time, lower defined daily dose, higher percentage of pre-index ED visits, and a higher count of pre-index outpatient visits. While we could have controlled for these factors when trying to compare outcomes of the generic switchers and non-switchers, the small sample size and high health services utilization in the non-switcher group did not allow for a meaningful comparison with the switcher groups. Future research might consider using patients with stable brand drug use prior to generic availability as their own controls for assessing outcomes after switching to a generic drug.
We believe our analysis had several key strengths that should be considered in light of the challenges and limitations previously described. First, we believe that use of the AG as a brand drug proxy is a novel way to study generic drug equivalence in the post-marketing environment. While this research method may not be confirmatory in assessing problems with brand and generic clinical equivalence, we believe it could be a valuable surveillance tool to trigger further investigation when differences between AG and generic drugs are identified. Second, it should not be overlooked that the majority of outcome measures we evaluated suggested that patients using AG drugs had similar outcomes as patients using generic drugs. This is a reassuring indicator that generic drugs are generally tolerable (i.e., indirectly measured by medication discontinuation rates) and have similar risk of need for higher levels of care (i.e., all-cause hospitalization) as their corresponding AG drugs that are chemically identical to the branded reference drug. Finally, by linking insurance claims data with electronic medical record data we were able to robustly capture medication and health services use. We believe this allowed for a more comprehensive measurement than using either of these data sources on their own.(25)
In conclusion, this study found similar likelihood of hospitalization and medication discontinuation between AG and generic drugs, which indirectly supports similar outcomes for generic compared with brand drugs. The finding of higher ED visits with AG compared with generic drugs needs further investigation.
METHODS
Study Design
We conducted a series of retrospective cohort studies among patients receiving select branded drugs prior to generic drug entry. Included drugs were selected based on evidence that both an AG and generic were marketed at an overlapping point between the years 1999 and 2014 (Table 1). These drugs included alendronate, amlodipine, citalopram, gabapentin, paroxetine, sertraline, and simvastatin. The sample of drugs was not based on similarities in pharmacological action or therapeutic uses, but rather we considered drugs with a sufficient sample size of users in our data set at the time of generic entry (i.e., the date of first U.S. marketing of an AG or generic). The date of the first generic prescription claim in our data was considered the index date for each drug, and this date marked the beginning of follow-up to evaluate brand-to-generic switching patterns over the subsequent 30 months. For patients who switched to a generic, the date of each individual’s first generic prescription claim marked the index date for evaluating subsequent outcomes with that product. For comparison with patients that did not switch to generic, we marked the beginning of the follow-up period by using a randomly selected imputed counterfactual switch date. Health services utilization and medication discontinuation were measured for up to 12 months following the brand-to-generic switch or the counterfactual date (for non-switching patients). The study was approved by the Institutional Review Boards of the Marshfield Clinic Research Foundation and Auburn University, and by the U.S. FDA Research Involving Human Subjects Committee (RIHSC).
Data
Administrative claims data from a regional insurance provider (Security Health Plan (SHP)) were combined with electronic health record (EHR) data from the Marshfield Clinic (MC), which is an integrated health care delivery system that provides the majority of healthcare services to 1.5 million patients residing in more than 50 locations in northern, central, and western Wisconsin. The MC has coded diagnoses dating back to the early 1960s, and a fully modern integrated EHR and data warehouse beginning in the 1990s. On average 102,700 SHP insured beneficiaries have full year insurance coverage, with claims data including inpatient, outpatient, and pharmacy claims. Approximately 65% of the SHP population having full year insurance coverage have evidence of use of MC clinics and their providers as reflected by at least one recorded diagnosis in their EHR record. Drug use was measured and classified based on the SHP claims data that include the National Drug Code (NDC) for the dispensed drug, and health services utilization and related covariates were measured using both the SHP claims data and the MC EHR data.
Study Sample
Data from 1999 through 2014 were used to apply the following inclusion criteria: 1) continuous enrollment in SHP in the 6 months prior to generic introduction, with continuous enrollment defined as no gaps in enrollment greater than 31 days; 2) continuous enrollment through at least the first eligible prescription fill following generic availability; 3) at least 1 encounter per year in the MC system; 4) at least 1 brand prescription of interest during the 6 months pre-generic availability; and 5) at least 1 prescription fill of a medication in the therapeutic area within 12 months following generic availability.
Main Outcome Measures
Generic switch was defined as a patient switching from the branded drug to an AG or generic during the 30-month period after each drug’s index date. We assumed that the dispensing date of the AG or generic reflected the date the patient began taking the respective generic drug. Patients who stayed on the branded drugs until the end of observation were defined as non-switchers. Treatment discontinuation was defined as a gap in medication supply exceeding 90 days during the 12-month period after the initial generic switch (or following the counterfactual switch date for the non-switchers).(26, 27) For both brand-to-generic switchers and non-switchers, patients discontinuing treatment were censored at the time of discontinuation in analyses of health services use. This measure of treatment discontinuation was used as a proxy to measure possible differences in efficacy or tolerability between switchers to an AG and generic drug.
Health services utilization was quantified during the 12-month period after the initial generic switch or counterfactual switch date using both the EHR data and SHP claims. Utilization measures included all-cause hospitalization and emergency department (ED) visits, number of urgent care visits, and number of outpatient visits. All-cause hospitalizations and ED visits were measured as both binary and count variables.
Covariates
Covariates were measured during the 6-month pre-index period. These included demographics (age and sex), the proportion of other prescriptions the patient received that were filled with a brand drug, defined daily dosage of the last prescription prior to generic switch,(28) Charlson comorbidity index,(29) any pre-index all-cause hospitalizations, any pre-index all-cause ED visits, and the number of pre-index outpatient visits.
Statistical Analysis
Descriptive statistics were used to summarize characteristics of the switchers and non-switchers, stratifying the switchers by whether they switched to an AG or generic. Independent sample t-tests and Chi-square tests were used to compare continuous and categorical characteristics, respectively, between the switcher and non-switcher group. Time to generic switching was evaluated using the Kaplan–Meier method, a conditional probability approach that we used to measure the fraction of patients that did not switch from brand-to-generic over the 12-month time period following generic entry. Multivariable Cox proportional hazards models were used to evaluate factors associated with the time to generic switch, reporting the median estimated hazard ratio (HR) and 95% confidence interval (CI) across 1000 bootstrapped samples. Drugs were first considered as individual cohorts, and then combined as an aggregated cohort with time anchored to the index date of generic entry for each drug. Combined analyses controlled for individual drug effects. Unless otherwise specified, results are shown for the combined population.
Among the sample of brand-to-generic switchers, the AG and generic groups were compared using multivariable models to assess health services utilization and medication discontinuation outcomes. Because of differences in observation time between groups, we estimated the occurrence and number of outpatient, urgent care, emergency department, and hospital visits on an annual basis using univariate negative binomial regression for count variables and logistic regression for binary variables. For binary outcome variables (hospitalization, ED events, and medication discontinuation), generalized logistic regression was used to fit a cumulative logit model reporting the median odds ratio (OR) and 95% CI across 20 bootstrapped samples. Negative binomial regression was used to model count variables (number of outpatient or urgent care visits), reporting the median rate ratio (RR) and 95% CI across 20 bootstrapped samples. Because of small sample sizes in some drug-specific switch groups, the number of covariates in these models was reduced to include only age, defined daily dose, and Charlson score since these were the most influential variables in bivariable analyses. All analyses were performed using SAS 9.3 (SAS Institute, Inc., Cary, NC) and the statistical significance was set at P<0.05.
Supplementary Material
STUDY HIGHLIGHTS.
What is the current knowledge on the topic?
Generic drugs save healthcare dollars, but some patients and providers question whether outcomes are the same as brands. Post-marketing comparisons of brand and generic outcomes are limited by generic drug perception biases. These biases can be addressed by studying AGs, which are chemically identical to brand drugs but still perceived as generics.
What question did this study address?
Are there differences in outcome measures between AGs (i.e., brand proxy) and generic drugs?
What this study adds to our knowledge
Patients using AGs were similar to patients on generics in terms of outpatient visits, urgent care visits, hospitalizations, and medication discontinuation, but the likelihood of emergency department visits was slightly higher for AGs compared with generics.
How this might change clinical pharmacology or translational science
Post-marketing surveillance can compare AGs vs. generics as a way to minimize bias in observational designs.
Acknowledgments
We would like to thank Motiur Rahman at Auburn University for his assistance with figure production.
Footnotes
CONFLICT OF INTEREST
In the past 3 years, Richard Hansen has provided expert testimony for Boehringer Ingelheim. No other authors declare a potential conflict of interest. Funding was made possible by the U.S. Food and Drug Administration through grant U01FD005272. Views expressed in written materials or publications and by speakers do not necessarily reflect the official policies of the Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
AUTHOR CONTRIBUTION
Wrote/edited Manuscript: RH, JQ, RB, JL, ESV, SD, SR, DP, PP
Designed Research: RH, JQ, RB, ESV, SD, SR, DP, PP
Performed Research: RB, JL, PP
Analyzed Data: RB
Interpreted Results: RH, JQ, RB, SD, SR, DP, PP
References
- 1.Fischer MA, Avorn J. Potential savings from increased use of generic drugs in the elderly: what the experience of Medicaid and other insurance programs means for a Medicare drug benefit. Pharmacoepidemiology and drug safety. 2004;13(4):207–14. doi: 10.1002/pds.872. [DOI] [PubMed] [Google Scholar]
- 2.Haas JS, Phillips KA, Gerstenberger EP, Seger AC. Potential savings from substituting generic drugs for brand-name drugs: medical expenditure panel survey, 1997-2000. Annals of internal medicine. 2005;142(11):891–7. doi: 10.7326/0003-4819-142-11-200506070-00006. [DOI] [PubMed] [Google Scholar]
- 3.Generic Pharmaceutical Association. Generic drug savings in the US - Fifth Annual Edition. 2013 Available from: http://www.gphaonline.org/media/cms/2013_Savings_Study_12.19.2013_FINAL.pdf.
- 4.Gagne JJ, Choudhry NK, Kesselheim AS, Polinski JM, Hutchins D, Matlin OS, et al. Comparative effectiveness of generic and brand-name statins on patient outcomes: a cohort study. Annals of internal medicine. 2014;161(6):400–7. doi: 10.7326/M13-2942. [DOI] [PubMed] [Google Scholar]
- 5.Shrank WH, Hoang T, Ettner SL, Glassman PA, Nair K, DeLapp D, et al. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med. 2006;166(3):332–7. doi: 10.1001/archinte.166.3.332. [DOI] [PubMed] [Google Scholar]
- 6.Crawford P, Feely M, Guberman A, Kramer G. Are there potential problems with generic substitution of antiepileptic drugs? A review of issues. Seizure : the journal of the British Epilepsy Association. 2006;15(3):165–76. doi: 10.1016/j.seizure.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Del Tacca M, Pasqualetti G, Di Paolo A, Virdis A, Massimetti G, Gori G, et al. Lack of pharmacokinetic bioequivalence between generic and branded amoxicillin formulations. A post-marketing clinical study on healthy volunteers. British journal of clinical pharmacology. 2009;68(1):34–42. doi: 10.1111/j.1365-2125.2009.03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. Food and Drug Administration. How drugs are developed and approved. 2014 Available from: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/default.htm.
- 9.U.S. Food Drug Administration. Abbreviated new drug application (ANDA): generics. 2014 Available from: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/default.htm.
- 10.Berg MJ, Gross RA, Haskins LS, Zingaro WM, Tomaszewski KJ. Generic substitution in the treatment of epilepsy: patient and physician perceptions. Epilepsy & behavior : E&B. 2008;13(4):693–9. doi: 10.1016/j.yebeh.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Sewell K, Andreae S, Luke E, Safford MM. Perceptions of and barriers to use of generic medications in a rural African American population, Alabama, 2011. Preventing chronic disease. 2012;9:E142. doi: 10.5888/pcd9.120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keenum AJ, Devoe JE, Chisolm DJ, Wallace LS. Generic medications for you, but brand-name medications for me. Research in social & administrative pharmacy : RSAP. 2012;8(6):574–8. doi: 10.1016/j.sapharm.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Shrank WH, Cox ER, Fischer MA, Mehta J, Choudhry NK. Patients’ perceptions of generic medications. Health Aff (Millwood) 2009;28(2):546–56. doi: 10.1377/hlthaff.28.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brennan TA, Lee TH. Allergic to generics. Annals of internal medicine. 2004;141(2):126–30. doi: 10.7326/0003-4819-141-2-200407200-00011. [DOI] [PubMed] [Google Scholar]
- 15.O’Malley AJ, Frank RG, Kaddis A, Rothenberg BM, McNeil BJ. Impact of alternative interventions on changes in generic dispensing rates. Health services research. 2006;41(5):1876–94. doi: 10.1111/j.1475-6773.2006.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunne S, Shannon B, Dunne C, Cullen W. Patient perceptions of generic medicines: a mixed-methods study. Patient. 2014;7(2):177–85. doi: 10.1007/s40271-013-0042-z. [DOI] [PubMed] [Google Scholar]
- 17.Faasse K, Martin LR, Grey A, Gamble G, Petrie KJ. Impact of brand or generic labeling on medication effectiveness and side effects. Health Psychol. 2016;35(2):187–90. doi: 10.1037/hea0000282. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Food Drug Administration. List of authorized generic drugs. 2014 Available from: http://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/abbreviatednewdrugapplicationandagenerics/ucm126389.htm.
- 19.Federal Trade Commission. Authorized generic drugs: short-term effects and long-term impact. 2011 Available from: https://www.ftc.gov/sites/default/files/documents/reports/authorized-generic-drugs-short-term-effects-and-long-term-impact-report-federal-trade-commission/authorized-generic-drugs-short-term-effects-and-long-term-impact-report-federal-trade-commission.pdf.
- 20.Guggenbuhl P. Osteoporosis in males and females: Is there really a difference? Joint Bone Spine. 2009;76(6):595–601. doi: 10.1016/j.jbspin.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Pratt LA, Brody DJ, Gu Q. Antidepressant use in persons aged 12 and over: United States, 2005-2008. (NCHS Data Brief No. 76).National Center for Health Statistics, Centers for Disease Control and Prevention. 2011 [PubMed] [Google Scholar]
- 22.Seoane-Vazquez E, Rodriguez-Monguio R, Hansen R. Interchangeability, Safety and Efficacy of Modified-Release Drug Formulations in the USA: The Case of Opioid and Other Nervous System Drugs. Clin Drug Investig. 2016;36(4):281–92. doi: 10.1007/s40261-015-0374-7. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Food Drug Administration. Update: bupropion hydrochloride extended-release 300mg bioequivalence studies. 2013 Available from: http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm322161.htm.
- 24.U.S. food and Drug Administration. Methylphenidate hydrochloride extended release tablets (generic Concerta) made by Mallinckrodt and Kudco. 2014 Available from: http://www.fda.gov/drugs/drugsafety/ucm422568.htm.
- 25.Lin KJ, Schneeweiss S. Considerations on the analysis of longitudinal electronic heath records linked to claims data to study the effectiveness and safety of drugs. Clin Pharmacol Ther. 2016 doi: 10.1002/cpt.359. [DOI] [PubMed] [Google Scholar]
- 26.Trivedi D, Landsman-Blumberg P, Darkow T, Smith D, McMorrow D, Mullins CD. Adherence and persistence among chronic myeloid leukemia patients during second-line tyrosine kinase inhibitor treatment. J Manag Care Spec Pharm. 2014;20(10):1006–15. doi: 10.18553/jmcp.2014.20.10.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson WW, Song X, Coleman CI, Thomson E, Smith DM, Damaraju CV, et al. Medication persistence and discontinuation of rivaroxaban versus warfarin among patients with non-valvular atrial fibrillation. Curr Med Res Opin. 2014;30(12):2461–9. doi: 10.1185/03007995.2014.933577. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Defined daily dose definition and general considerations. Available from: http://www.whocc.no/ddd/definition_and_general_considera/.
- 29.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. American journal of epidemiology. 2011;173(6):676–82. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


