Abstract
The right hemisphere of the human brain is known to be involved in processes underlying emotion and social cognition. Clinical neuropsychology investigations and brain lesion studies have linked a number of personality and social disorders to abnormal white matter (WM) integrity in the right hemisphere. Here, we tested the hypothesis that interpersonal competencies are associated with integrity of WM tracts in the right hemisphere of healthy young adults. Thirty-one participants underwent Diffusion Tensor Imaging (DTI) scanning. Fractional Anisotropy (FA) was used to quantify water diffusion. After the scanning session, participants completed the Adolescent Interpersonal Competence Questionnaire (AICQ). FA was subsequently correlated with AICQ scores using Tract Based Spatial Statistics (TBSS). Higher interpersonal competencies are related to higher WM integrity in several major tracts of the right hemisphere, in specific the uncinate fasciculus, the cingulum, the forceps minor, the infero-fronto occipital fasciculus, the inferior longitudinal fasciculus, and the superior longitudinal fasciculus. These results provide the first direct analysis of the neuroanatomical basis of interpersonal competencies and young adult self-reported skills in social contexts.
Introduction
Human beings are highly social animals, and in complex societies where social interaction is pervasive, nuanced, and extremely diverse, maintaining effective and sensitive social ties places a heavy burden on cognitive and emotional capacities of the individual. For example, developing and sustaining social relationships require competent and flexible social cognition including the ability to represent relationships between oneself and others and the capacity to apply those representations to effectively guide social behavior (Adolphs, 2001). Indeed, these social cognitions are central to what Buhrmester (1990) referred to as “interpersonal competence,” which encompasses the capacity to interact and communicate with others, to share personal views, to understand the emotions and opinions of others, and to cooperate with others or resolve conflict should it occur. Because these faculties constitute the building blocks of social relationships, inter-individual differences in interpersonal competence are linked to social rejection and isolation among both clinical and non-clinical samples of children (Kully-Martens, Denys, Treit, Tamana, & Rasmussen, 2012; Ladd, 1999) and adults (Anders & Tucker, 2000; Phelps & Hanley-Maxwell, 1997).
During the transition to adulthood, when young adults must navigate a vast and complex array of novel social contexts with sharply varying social protocols, deficiencies in interpersonal competence are likely particularly problematic. That is, transitioning adults are said to be caught “in between” childhood and adulthood (Maggs, Jager, Patrick, & Schulenberg, 2012; Shanahan, 2000), and as a result they face the difficult challenge of transitioning into adult settings (e.g., work) and into adult roles (e.g., spouse and parent), while maintaining, if not renegotiating, existing social ties from settings that are remnants of childhood (e.g., school/college, peer groups, the family of origin). Thus, deficits in interpersonal competence during young adulthood are associated with greater difficulty transitioning into college (Mahoney, Cairns, & Farmer, 2003; Parker, Summerfeldt, Hogan, & Majeski, 2004), work (Fitzgerald, Brown, Sonnega, & Ewart, 2005), and lasting romantic relationships (Collins & van Dulmen, 2006; Schneewind & Gerhard, 2002). Given the importance of interpersonal competence to young adult success and the fundamental roles that social and emotional cognition play in interpersonal competence, we were surprised to find that no social neuroscience studies have attempted to directly investigate brain mechanisms that underlie interpersonal competence. Because interpersonal competence entails the integration of cognitive and socioemotional resources, such as language processing, empathy, theory of mind, visual processing of socioemotional cues, and working memory, there is reason to believe that brain networks are involved in the development and maintenance of interpersonal competence.
Brain regions link to each other through bundles of myelinated nerve cell processes (or axons), which carry nerve impulses between neurons and constitute the so-called white matter (WM). Associations of behavioral and personality traits with WM have received increasing attention (Kanai & Rees, 2011; Loui, Li, Hohmann, & Schlaug, 2011). WM integrity (Charlton, McIntyre, Howe, Morris, & Markus, 2007; Deary et al., 2006; Schmithorst, Wilke, Dardzinski, & Holland, 2005) or hyper-connectivity (Loui, Li, Hohmann, et al., 2011) are well-known key indicators of higher information-processing efficiency in cognition at every stage of human development. Therefore, in the present study we looked at WM correlates throughout the whole brain, and examined whether individual differences in self-reported interpersonal competence relate to WM connectivity in a sample of healthy young adults. More specifically, given that several distinct lines of research have linked socioemotional cognitions to WM integrity in the right hemisphere of the brain, our main hypothesis was that interpersonal competence would be specifically associated with WM integrity in the right hemisphere.
Socioemotional Processing and the Right Hemisphere of the Brain
Individuals who are interpersonally competent are typically empathetic (Chow, Ruhl, & Buhrmester, 2013; de Wied, Branje, & Meeus, 2007) and display high levels of emotional intelligence, which includes the abilities to perceive, use, understand, and manage emotions (Brackett, Rivers, Shiffman, Lerner, & Salovey, 2006). Some components of empathy and emotional intelligence appear to reflect functioning of the human mirror neuron system (Iacoboni & Dapretto, 2006; Parkinson & Wheatley, 2012), and these aspects have been associated with the right hemisphere portion of the mirror neuron system (Cattaneo & Rizzolatti, 2009; Uddin, Iacoboni, Lange, & Keenan, 2007), which is believed to be central to understanding of self in relation to others. Additionally, social cognition has long been linked to right laterality (Decety & Lamm, 2007; Devinsky, 2000; Frith & Frith, 2012; Semrud-Clikeman, Fine, & Zhu, 2011; Semrud-Clikeman & Hynd, 1990; Weintraub & Mesulam, 1983; Winner, Brownell, Happe, Blum, & Pincus, 1998). Several comparative studies – some even involving phylogenetically distant species – indicate right hemispheric dominance in recognition of familiar social partners in processing information relative to other individuals as well as in the development of social competencies (Salva, Regolin, Mascalzoni, & Vallortigara, 2012; Vallortigara, 1992).
More specifically, abnormal WM integrity in the right hemisphere of the human brain has been linked to abnormalities in processing socioemotional information. For example, lesions in the right hemisphere are associated with deficits in social perception and understanding, such as recognition and expression of facial emotion (Montreys & Borod, 1998), affective prosody (Breitenstein, Daum, & Ackermann, 1998), and sarcasm (Shamay-Tsoory, Tomer, & Aharon-Peretz, 2005). Right hemisphere damage is also associated with impaired communication (Bartels-Tobin & Hinckley, 2005), lack of empathy (Rankin et al., 2006), and the inability to attribute mental states, such as desires, intentions, and beliefs, to oneself and to others (Happe, Brownell, & Winner, 1999; Lombardo, Chakrabarti, Bullmore, & Baron-Cohen, 2011; Weed, McGregor, Feldbaek Nielsen, Roepstorff, & Frith, 2010). Moreover, there is evidence to suggest that the interpersonal impairments associated with Asperger’s syndrome are the result of developmental abnormalities within the right cerebral hemisphere (Gunter, Ghaziuddin, & Ellis, 2002; McKelvey, Lambert, Mottron, & Shevell, 1995)
With respect to social cognition, several studies have identified the special role of the right prefrontal cortex. For example, showing participants pictures of eyes expressing friendly or hostile emotions activates the right orbitofrontal cortex (Wicker, Perrett, Baron-Cohen, & Decety, 2003). Additionally, Tranel, Bechara, and Denburg (2002) reported that patients with lesions in the right ventromedial prefrontal cortex displayed impairments in interpersonal behavior, but that patients with similar contralateral left lesions displayed no such impairments in social behavior.
Guided by the extant literature, we hypothesized that interpersonal competence is associated with the integrity of WM tracts in the right hemisphere of healthy young adults. The methodology we adopted accords with lines of research that link complex information about personality or attitudes measured off-line with brain structures measured with neuroimaging (Kanai & Rees, 2011). Of course, complex functions associated with social cognition cannot be understood solely in terms of localization of specialized brain areas working in isolation. Rather, a fundamental aspect in neural networks is connectivity between components, which determines the efficiency of the network as a whole. This basic concept is reflected in brain anatomy in terms of integrity of WM fibers connecting cerebral regions. To test our main hypothesis, we evaluated inter-individual differences in WM integrity using Fractional Anisotropy (FA), which provides information about the directionality of the diffusion of water molecules in the whole brain, and we then correlated this neuroanatomical information with an index of self-reported interpersonal competence as measured by the Adolescent Interpersonal Competence Questionnaire (Buhrmester, 1990).
Methods
Participants
Thirty-one healthy right-handed young adults (20 males) participated. They ranged in age from 19 to 29 years (M = 22.93, SD = 2.66). The study was approved by the University of Trento Ethical Committee, and all participants gave written informed consent.
Procedures and Measures
Image acquisition
Magnetic resonance images were acquired with a 4 T Bruker Medspec scanner (Bruker Medical, Ettlingen, Germany) using a birdcage-transmit, eight-channel receive head coil (USA Instruments, Inc., Ohio, USA). Each participant underwent a T1-weighted structural image (3D MPRAGE, 1×1×1mm3, TR = 2700 ms; TE = 4 ms; flip angle = 7°, generalized auto calibrating partially parallel acquisition (GRAPPA) factor 2, TI=1020 ms, bandwidth = 150 Hz/pixel, TA = 5 min) optimized for maximal contrast to noise ratio between gray and white matter at 4 T (Papinutto & Jovicich, 2008). In each session a diffusion weighted image (DWI) dataset was also acquired with a twice refocused 2D SE-EPI sequence (Reese, Heid, Weisskoff, & Wedeen, 2003) and the following acquisition parameters: TR=7000 ms, TE = 85 ms, GRAPPA factor 2, voxel size 2.5×2.5×2.5mm3, b-value 1000 s/mm2. Five images without any sensitizing diffusion gradient applied (b0) and 30 diffusion weighted images with diffusion gradients applied along unique directions that were defined by an electrostatic repulsion algorithm (Jones, 2004; Jones, Horsfield, & Simmons, 1999) were acquired, with an axial slice acquisition along the x-y plane of the static magnetic field reference frame. A field of view (FOV) of 240 mm2 and 50 contiguous slices enabled our covering the whole brain. A Full-Fourier acquisition was used to reduce cardiac pulsation artifacts (Robson & Porter, 2005). The total scan time lasted 270 seconds per acquisition.
DTI preprocessing
All diffusion-weighted images were processed using tools from the FMRIB software library (FSL, version 4.1.5; http://www.fmrib.ox.ac.uk/fsl) running on a Linux operating system. First, the DICOM files were converted to the nifti format using an open source DICOM-to-nifti converter (http://www.mccauslandcenter.sc.edu/mricro/mricron/index.html). Then, each dataset was corrected for head movement and eddy current distortions using an affine transformation of each diffusion weighted image and b0 image to the first b0 image, used as reference. Second, a binary brain mask was generated from the non-diffusion weighted image by using the BET brain extraction tool (Smith, 2002). Following these steps, a diffusion tensor model was fitted independently for each voxel within the brain mask, and images of Fractional Anisotropy (FA) were generated for each participant. FA describes the degree of anisotropy of the water diffusion within a voxel and is considered a reliable index of micro-structural integrity of WM and a measure of directional strength of the local tract structure. FA values range from 0 (minimum coherence in the WM structures) to 1 (maximum coherence in WM structures). As an additional test for the relative contribution of parallel and radial diffusivity to FA, for each participant we separately computed Mean Diffusivity (MD), Radial Diffusivity (RD), and Axial Diffusivity (AD) globally in the whole brain. Additionally, we computed RD and AD locally in small ROIs selected around the central coordinates of the first 5 clusters reported in Table 1, and we summed the results. Voxelwise statistical analysis of the diffusivity data was carried out using Tract-based spatial statistics (TBSS; Smith et al., 2006). TBSS is a technique that aims to improve the sensitivity, objectivity, and interpretability of analysis in multi-participant diffusion imaging studies. TBSS has been proposed to reduce problems related to possible misalignment of different participants’ co-registered data through an optimized non-linear registration followed by projection onto an alignment-invariant tract representation. In this way, the TBSS method allows for valid conclusions to be drawn from the subsequent voxel-wise analysis.
Table 1. Clusters where FA and interpersonal competence correlated significantly.
Clusters where FA and interpersonal competence were significantly correlated (p<0.05). Larger clusters have smaller numbers (1-12). The column “size (voxels)” indicates how many voxels are contained in each cluster. The column “corrected p-value” refers to the p-value associated with the maximum “intensity” voxel within the cluster after correction for multiple comparisons using Threshold-Free Cluster Enhancement (TFCE). The columns “X” “Y” and “Z” indicate the MNI coordinates of the maximum intensity voxel in each cluster; coordinates are expressed in standard space (mm). The last column reports the WM (white matter) labels taken from the JHU white-matter tractography atlas. According to the atlas, the clusters contain voxels belonging to six WM tracts: Uncinate Fasciculus (UF), Cingulum (CM), Forceps Major (FMa), Inferior Fronto Occipital Fasciculus (IFOF), Inferior Longitudinal Fasciculus (ILF), and Superior Longitudinal Fasciculus (SLF).
| Cluster number | Size (Voxels) | Corrected p-value | PEAK X | PEAK Y | PEAK Z | HEMISPHERE & LOBE | TRACT LOCATION |
|---|---|---|---|---|---|---|---|
| 1 | 10786 | 0.016 | 8 | 36 | 47 | R Frontal | CM |
| 2 | 1123 | 0.021 | 28 | −89 | −7 | R Occipital | ILF |
| 3 | 864 | 0.018 | 47 | −50 | 0 | R Temporal | SLF |
| 4 | 351 | 0.028 | 35 | −28 | −23 | R Temporal | ILF |
| 5 | 143 | 0.028 | 54 | −20 | 1 | R Temporal | ILF |
| 6 | 115 | 0.028 | 44 | −17 | −17 | R Temporal | ILF |
| 7 | 80 | 0.028 | 44 | −32 | 10 | R Temporal | ILF |
| 8 | 64 | 0.029 | 58 | −36 | 17 | R Temporal | SLF |
| 9 | 50 | 0.021 | 15 | 6 | −19 | R Frontal | UF |
| 10 | 14 | 0.03 | 18 | −102 | 8 | R Occipital | FMa |
| 11 | 11 | 0.03 | 45 | 5 | −34 | R Temporal | ILF |
| 12 | 9 | 0.03 | 10 | −83 | 30 | R Occipital | FMa |
Briefly, TBSS for FA consists of the following steps: (1) identification of the most typical participant in the group as target for all the non-linear registration. This participant is selected minimizing the amount of warping required for all other participants to be co-registered with the target. (2) Alignment of all participants’ FA images to the target using both linear and non-linear transformations (Andersson, Jenkinson, & Smith, 2007a, 2007b) and subsequent affine-transformation to the standard Montreal Neurological Institute (MNI) space. (3) Averaging of the aligned individual FA image and generation of a skeleton representing white matter tracts common to all participants. In our case, the mean skeleton image was created using an inferior FA threshold of 0.2. (4) Projection of each participant’s aligned FA data onto the skeleton. (5) Group comparison using voxel-wise cross-participant statistic.
Statistical analysis
We performed cross-subject analyses to relate voxelwise measures of diffusivity values (FA, MD, AD, RD) to interpersonal competence using the General Linear Model (GLM) tool in FSL in conjunction with permutation-based tests using Randomise (5000 permutations). The cluster-size analysis results were corrected for multiple comparisons across space (p<0.05) using Threshold-Free Cluster Enhancement (TFCE). Clusters where local diffusivity measures differed as a function of scores in interpersonal competence were labeled using a stereotaxic white matter atlas (Mori et al. 2008).
Interpersonal competence
Interpersonal competence was assessed with the Adolescent Interpersonal Competence Questionnaire (AICQ; Buhrmester, 1990), which has been widely used in young adult samples (Daley & Hammen, 2002; Lopes et al., 2004). The AICQ is a 40-item measure with 5 subscales: self-disclosure, providing emotional support to friends, management of conflicts, negative assertion, and initiation of friendships. Items were rated on a 5-point scale (1 = Poor at this, would be so uncomfortable and unable to handle this situation that it would be avoided if possible; 5 = Extremely good at this, would feel very comfortable and could handle this situation very well). The total scale as well as the subscales all displayed excellent reliability (e.g., in each case the Cronbach alpha was .85 or higher).
We used a composite measure of interpersonal competence that incorporated all 5 AICQ subscales. After calculating mean scores for each subscale, we conducted a confirmatory factor analysis within Mplus (Muthén & Muthén, 1998-2009) that loaded each of the 5 subscale means onto a single latent factor. Using the FSCORE command within Mplus, we then outputted the latent factor scores so that they could be used in subsequent analyses. This latent factor approach for calculating a composite AICQ measure is superior to merely calculating a global mean score (i.e., the mean of AICQ’s 40 items), because only the latent factor approach adjusts for measurement error and thereby increases both power and measurement reliability (Kline, 2010).
Identification of white matter tracts
Identification of white matter tracts in which there was a correlation between AICQ and diffusivity measures (FA, MD, AD, RD) was based on the John Hopkins University (JHU) white matter tractography probabilistic atlases, available within the FSL toolboxes (Hua et al., 2008; Wakana et al., 2007). These atlases allow voxel by voxel categorization to different major WM tracts within certain probabilities.
Results
Voxel-wise analysis in TBSS revealed significant differences within participants in mean FA indicating that higher interpersonal competence - as measured by the AICQ (Buhrmester, 1990) - is correlated with higher WM integrity (p < 0.05, corrected). We hypothesized that the difference in WM integrity should be localized exclusively in the right hemisphere (Figure 1). Specifically, higher FA values were found in voxels belonging to six major WM tracts of the right hemisphere: the Uncinate Fasciculus (UF), the Cingulum (CM), the Forceps Minor (FM), the Infero-Fronto Occipital Fasciculus (IFOF), the Inferior Longitudinal Fasciculus (ILF), and the Superior Longitudinal Fasciculus (SLF). The significant voxels obtained from the voxelwise TBSS analysis were grouped in clusters and are reported in Table 1.
Figure 1.
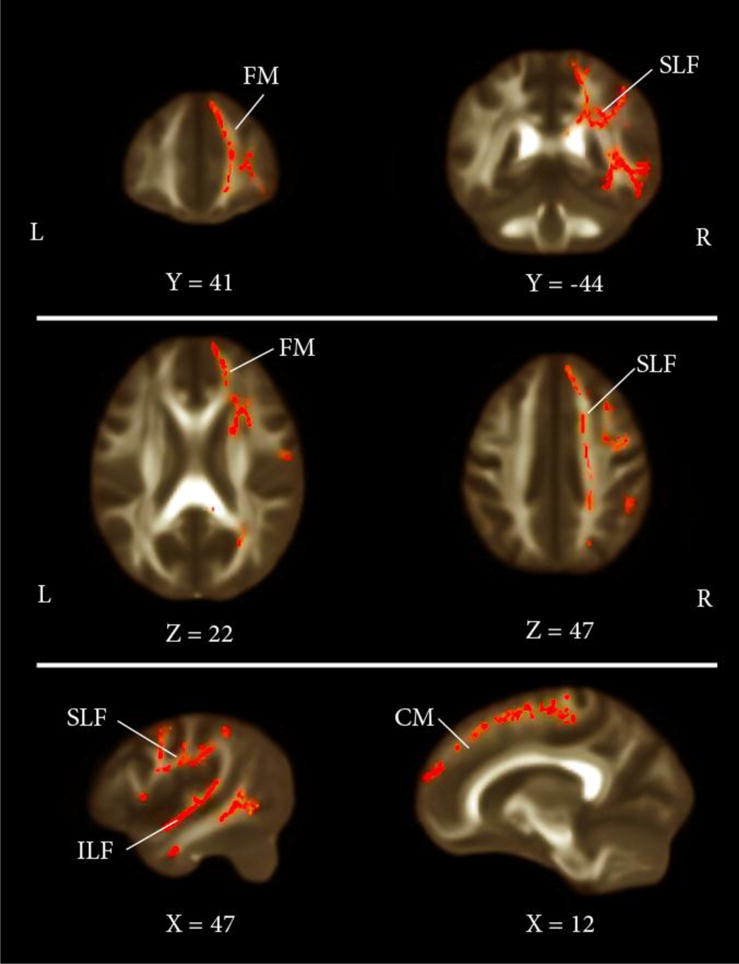
Coronal, axial, and sagittal views (from top to bottom) of the t statistics map of FA comparison between participants (p<0.03 corrected). The background image is the MNI template. Red voxels represent regions in which higher FA values are associated with higher scores in AICQ. The images are reported in the neurological orientation (left side of the brain is on the left of the coronal and axial views). Some of traits include Forceps Minor (FM), Superior Longitudinal Fasciculus (SLF), Inferior Longitudinal Fasciculus (ILF), and Cingulum (CM), all in the right hemisphere. X, Y and Z coordinates are based on the atlas of the Montreal Neurological Institute (MNI).
The global TBSS analysis of MD, AD, and RD showed no significant correlation with AICQ (p < 0.05, corrected). Without the correction for multiple comparisons, too conservative to evidence effects on the three metrics, RD showed a significant correlation with the AICQ in many voxels (p < 0.05, uncorrected). An additional local analysis of relative contributions of parallel and radial diffusivity to FA within the first five clusters reported in Table 1 showed that AD and RD values were both anticorrelated to the AICQ measure (see supplementary Figure 1). This result was more evident for RD (r =−0.62, p=0.00019) than for AD (r = −0.31, p=0.093).
Discussion
The main goal of the present study was to investigate associations between interpersonal competence of healthy young adults and their WM structural connectivity. Given that interpersonal competence depends on the integration of cognitive, affective, and social competencies, it is presumably served by distant brain regions working in concert. We hypothesized that interpersonal competence would be associated with higher integrity of WM pathways connecting distant brain regions in the whole brain. Specifically, in line with other behavioral, neuroimaging, and lesion studies that identify right laterality in social competence, we expected to find more pronounced associations of social competence with WM integrity in the right hemisphere. Here we found that increased FA was associated with greater social competence in specific clusters identified using probabilistic atlases. Additional local RD and AD diffusivity analyses in the first five clusters reported in Table 1 suggest that this result is primarily driven by a negative correlation between RD and the AICQ. RD, like FA, is a parameter that is generally linked to myelination and axonal packing, whereas AD can vary with fiber diameter and axon coherence (Beaulieu, 2002; Song et al., 2005; Takahashi, Ono, Harada, Maeda, & Hackney, 2000). Thus, in several studies (Eluvathingal, Hasan, Kramer, Fletcher, & Ewing-Cobbs, 2007; Lebel & Beaulieu, 2011; Lebel, Walker, Leemans, Phillips, & Beaulieu, 2008; Mukherjee et al., 2001) longitudinal increases of FA, paired with reductions of RD and AD remaining constant, have been interpreted in terms of an increase in myelination from childhood into adolescence and young adulthood. Therefore, our results suggest that interpersonal competence might be associated to greater integrity of WM pathways connecting distant brain regions in the whole brain, possibly because of more complete maturation of white matter in terms of increased myelination into young adulthood. In particular, we found that AICQ correlated with several major tracts of the right hemisphere, including the uncinate fasciculus, the cingulum, the forceps minor, the infero-fronto occipital fasciculus, the inferior longitudinal fasciculus, the superior longitudinal fasciculus.
The anatomical structure of these tracts connects several regions which form the neural basis of several cognitive, emotional, and social functions, such as perception, language, modulation of social stimuli, auditory and visual association cortices, executive functions, and emotion regulation, all of which are of great relevance to interpersonal competence. We discuss these WM tracts and their functions in greater detail, and how efficiency in these pathways indicates prioritized information processing of interpersonal and social competence.
The UF connects the orbitofrontal cortex, the hippocampus, and the amygdala (Mori et al., 2008). Given that the UF is a part of the limbic system (Catani, Howard, Pajevic, & Jones, 2002; Hasan et al., 2009), its major purpose is believed to lie in emotional functioning, in particular sharing affective states which typically characterizes empathy (Decety & Svetlova, 2012). Disruption of the UF tract in people affected by autism spectrum disorder (ASD; Ameis et al., 2011) has further confirmed its association with socioemotional behavior.
The CM connects the cingulate gyrus to the entorhinal cortex, facilitating communication between sections of the limbic system (Mori et al., 2008). It has been identified with processing emotional information as well as performing error monitoring in the service of cognitive control (Metzler-Baddeley et al., 2012). Specific right CM lesions have been found to relate to impaired social functioning in children (Angelini, Mazzucchi, Picciotto, Nardocci, & Broggi, 1981).
The FM is a part of the anterior region of the corpus callosum. In particular, it connects - via the most anterior part of the corpus callosum (the genu) - orbitofrontal areas involved in emotional and executive control (Park et al., 2008), which is a fundamental function in socioemotional competence.
IFOF and ILF jointly connect occipital and temporal cortices, and IFOF also connects with the frontal lobe and the posterior part of the parietal lobe (Mori et al., 2008). Thus, they are two of the largest and longest association fiber bundles in the human brain. Damage to these long association fibers has been linked to impairment in processing visual emotional cues (Bauer, 1982) and facial expressions of emotions (Philippi, Mehta, Grabowski, Adolphs, & Rudrauf, 2009; Thomas et al., 2009). Damaged ILF has also been associated with ASD (Cheung et al., 2009).
The SLF is one of the major intra-hemispheric pathways that connects parietal, temporal, and frontal lobes and is effectively a bundle of fibers carrying most high-level processing of information taking place in the human brain (Mori et al., 2008). The right SLF has been determined to be involved in processing tones and melodic information, particularly in pitch-based grammar learning (Loui, Li, & Schlaug, 2011), thus suggesting a major role of these connections in processing emotion and communication during language learning, development, and understanding others.
In overview, the most important finding to emerge in the present study was that right lateralization in WM integrity is associated with interpersonal competence. This finding supports evidence in the literature that points to the fundamental role of the right hemisphere in social cognition (e.g., Frith & Frith, 2012). The finding may have implications for theories claiming that the right hemisphere plays a major role in modulating emotion and nonverbal communication during the first interpersonal relationship that every human being experiences, namely the infant-mother relationship (Schore, 1997, 2000, 2009). According to this line of research, the development of emotional and social intelligence in the individual - from childhood to adulthood - depends on the quality of their relationship with a principal caregiver, and those socioemotional competencies heavily rely on right brain function. Our results support this hypothesis, highlighting the association between WM in the right hemisphere and interpersonal competence. Shore (2001) also suggested that dysfunction in the development of the right hemisphere might affect infant mental health and lead to psychosis and social difficulties in later stages of development. This suggestion might explain the large literature which associates right hemisphere under-connectivity with personality disorder, and it is supported by evidence of reduced WM connectivity of the right hemisphere in animal and human studies with early deprivation of maternal care (Helmeke, Poeggel, & Braun, 2001) and orphanage care (Govindan, Behen, Helder, Makki, & Chugani, 2010).
Our study shows that WM integrity in several key tracts of the right hemisphere correlate with self-assessed interpersonal competence. Such individual differences might arise for a variety of reasons. They might be the effect of repeated behavioral patterns that favor interpersonal competence, as in continuous practice of those skills. This view is supported by several cognitive and affective neuroscience studies showing that the brain is highly plastic and that its interactions with the environment preserve gray matter from decaying (Pascual-Leone, Amedi, Fregni, & Merabet, 2005), and promote the formation of new and more efficient connections in white matter (Scholz, Klein, Behrens, & Johansen-Berg, 2009). Alternatively, such individual differences in WM integrity might reflect genetic causes that predispose people to more effective interpersonal competencies. Finally, they might result from both experiences that simultaneously promote more effective interpersonal competencies and right hemisphere development (e.g., parenting or favorable economic circumstances).
Interpersonal competence is not an isolated function, but it is linked to a number of other cognitive and socio-emotional skills, such as language processing, empathy, theory of mind, visual processing of relational cues, and working memory. Thus, our finding that several major brain white matter tracts are correlated with high levels of interpersonal competence should not be taken as indicative that these connections are specific or exclusive to this function; on the contrary, these are key fiber bundles, which play fundamental roles in other domains. Additionally, given the complexity of interpersonal interactions, and the number of different factors that play important parts in them (biological, cognitive, emotional, and social), further assessments of associations between interpersonal competence and brain structure call for longitudinal, multicultural, and additional functional neuroimaging investigations.
Supplementary Material
Acknowledgments
For Charles G. Gross with admiration and affection in equal measure. This research was supported by the Intramural Research Program of the NIH, NICHD.
Source of funding
This research was supported by the Intramural Research Program of the NIH, NICHD, by the Center for Mind/Brain Sciences of the University of Trento (Italy), and by the Department of Psychology and Cognitive Science of the University of Trento (Italy).
Footnotes
Statement of Conflicts of Interest
We declare no conflict of interest relatively to this study.
References
- Adolphs R. The neurobiology of social cognition. Current opinion in neurobiology. 2001;11(2):231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Ameis SH, Fan J, Rockel C, Voineskos AN, Lobaugh NJ, Soorya L, et al. Impaired structural connectivity of socio-emotional circuits in autism spectrum disorders: a diffusion tensor imaging study. PLoS One. 2011;6(11):e28044. doi: 10.1371/journal.pone.0028044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders SL, Tucker JS. Adult attachment style, interpersonal communication competence, and social support. Personal Relationships. 2000;7(4):379–389. [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. FMRIB technical report TR07JA1. 2007a from www.fmrib.ox.ac.uk/analysis/techrep.
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. 2007b from www.fmrib.ox.ac.uk/analysis/techrep.
- Angelini L, Mazzucchi A, Picciotto F, Nardocci N, Broggi G. Focal lesion of the right cingulum: a case report in a child. Journal of neurology, neurosurgery, and psychiatry. 1981;44(4):355–357. doi: 10.1136/jnnp.44.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels-Tobin LR, Hinckley JJ. Cognition and discourse production in right hemisphere disorder. Journal of Neurolinguistics. 2005;18(6):461–477. [Google Scholar]
- Bauer RM. Visual hypoemotionality as a symptom of visual-limbic disconnection in man. Archives of neurology. 1982;39(11):702–708. doi: 10.1001/archneur.1982.00510230028009. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in biomedicine. 2002;15(7-8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Brackett MA, Rivers SE, Shiffman S, Lerner N, Salovey P. Relating emotional abilities to social functioning: a comparison of self-report and performance measures of emotional intelligence. Journal of Personality and Social Psychology. 2006;91(4):780–795. doi: 10.1037/0022-3514.91.4.780. [DOI] [PubMed] [Google Scholar]
- Breitenstein C, Daum I, Ackermann H. Emotional processing following cortical and subcortical brain damage: contribution of the fronto-striatal circuitry. Behavioural neurology. 1998;11(1):29–42. doi: 10.1155/1998/579029. [DOI] [PubMed] [Google Scholar]
- Buhrmester D. Intimacy of friendship, interpersonal competence, and adjustment during preadolescence and adolescence. Child development. 1990;61(4):1101–1111. [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17(1):77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Rizzolatti G. The mirror neuron system. Archives of neurology. 2009;66(5):557–560. doi: 10.1001/archneurol.2009.41. [DOI] [PubMed] [Google Scholar]
- Charlton RA, McIntyre DJ, Howe FA, Morris RG, Markus HS. The relationship between white matter brain metabolites and cognition in normal aging: the GENIE study. Brain Research. 2007;1164:108–116. doi: 10.1016/j.brainres.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Cheung C, Chua SE, Cheung V, Khong PL, Tai KS, Wong TK, et al. White matter fractional anisotropy differences and correlates of diagnostic symptoms in autism. Journal of child psychology and psychiatry, and allied disciplines. 2009;50(9):1102–1112. doi: 10.1111/j.1469-7610.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- Chow CM, Ruhl H, Buhrmester D. The mediating role of interpersonal competence between adolescents’ empathy and friendship quality: a dyadic approach. Journal of adolescence. 2013;36(1):191–200. doi: 10.1016/j.adolescence.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WA, van Dulmen M. “The Course of True Love(s)…”: Origins and Pathways in the Development of Romantic Relationships. In: Booth ACCA, editor. Romance and sex in adolescence and emerging adulthood: Risks and opportunities. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 2006. pp. 63–86. [Google Scholar]
- de Wied M, Branje SJ, Meeus WH. Empathy and conflict resolution in friendship relations among adolescents. Aggressive behavior. 2007;33(1):48–55. doi: 10.1002/ab.20166. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Bastin ME, Pattie A, Clayden JD, Whalley LJ, Starr JM, et al. White matter integrity and cognition in childhood and old age. Neurology. 2006;66(4):505–512. doi: 10.1212/01.wnl.0000199954.81900.e2. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2007;13(6):580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Decety J, Svetlova M. Putting together phylogenetic and ontogenetic perspectives on empathy. Developmental cognitive neuroscience. 2012;2(1):1–24. doi: 10.1016/j.dcn.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O. Right Cerebral Hemisphere Dominance for a Sense of Corporeal and Emotional Self. Epilepsy & Behavior. 2000;1(1):60–73. [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cerebral Cortex. 2007;17(12):2760–2768. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald ST, Brown KM, Sonnega JR, Ewart CK. Early antecedents of adult work stress: social-emotional competence and anger in adolescence. Journal of behavioral medicine. 2005;28(3):223–230. doi: 10.1007/s10865-005-4658-x. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Mechanisms of social cognition. Annual review of psychology. 2012;63:287–313. doi: 10.1146/annurev-psych-120710-100449. [DOI] [PubMed] [Google Scholar]
- Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT. Altered water diffusivity in cortical association tracts in children with early deprivation identified with Tract-Based Spatial Statistics (TBSS) Cerebral Cortex. 2010;20(3):561–569. doi: 10.1093/cercor/bhp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter HL, Ghaziuddin M, Ellis HD. Asperger syndrome: tests of right hemisphere functioning and interhemispheric communication. Journal of autism and developmental disorders. 2002;32(4):263–281. doi: 10.1023/a:1016326701439. [DOI] [PubMed] [Google Scholar]
- Happe F, Brownell H, Winner E. Acquired ‘theory of mind’ impairments following stroke. Cognition. 1999;70(3):211–240. doi: 10.1016/s0010-0277(99)00005-0. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Iftikhar A, Kamali A, Kramer LA, Ashtari M, Cirino PT, et al. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Research. 2009;1276:67–76. doi: 10.1016/j.brainres.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmeke C, Poeggel G, Braun K. Differential emotional experience induces elevated spine densities on basal dendrites of pyramidal neurons in the anterior cingulate cortex of Octodon degus. Neuroscience. 2001;104(4):927–931. doi: 10.1016/s0306-4522(01)00201-9. [DOI] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nature reviews. Neuroscience. 2006;7(12):942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2004;51(4):807–815. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1999;42(3):515–525. [PubMed] [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nature reviews. Neuroscience. 2011;12(4):231–242. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Kully-Martens K, Denys K, Treit S, Tamana S, Rasmussen C. A review of social skills deficits in individuals with fetal alcohol spectrum disorders and prenatal alcohol exposure: profiles, mechanisms, and interventions. Alcoholism, clinical and experimental research. 2012;36(4):568–576. doi: 10.1111/j.1530-0277.2011.01661.x. [DOI] [PubMed] [Google Scholar]
- Ladd GW. Peer relationships and social competence during early and middle childhood. Annual review of psychology. 1999;50:333–359. doi: 10.1146/annurev.psych.50.1.333. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Baron-Cohen S. Specialization of right temporo-parietal junction for mentalizing and its relation to social impairments in autism. Neuroimage. 2011;56(3):1832–1838. doi: 10.1016/j.neuroimage.2011.02.067. [DOI] [PubMed] [Google Scholar]
- Loui P, Li HC, Hohmann A, Schlaug G. Enhanced cortical connectivity in absolute pitch musicians: a model for local hyperconnectivity. Journal of Cognitive Neuroscience. 2011;23(4):1015–1026. doi: 10.1162/jocn.2010.21500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loui P, Li HC, Schlaug G. White matter integrity in right hemisphere predicts pitch-related grammar learning. Neuroimage. 2011;55(2):500–507. doi: 10.1016/j.neuroimage.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggs JL, Jager J, Patrick ME, Schulenberg J. Social role patterning in early adulthood in the USA: adolescent predictors and concurrent wellbeing across four distinct configurations. Longitudinal and life course studies. 2012;3(2):190–210. doi: 10.14301/llcs.v3i2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JL, Cairns BD, Farmer TW. Promoting interpersonal competence and educational success through extracurricular activity participation. Journal of Educational Psychology. 2003;95(2):409–418. [Google Scholar]
- McKelvey JR, Lambert R, Mottron L, Shevell MI. Right-hemisphere dysfunction in Asperger’s syndrome. Journal of Child Neurology. 1995;10(4):310–314. doi: 10.1177/088307389501000413. [DOI] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Jones DK, Steventon J, Westacott L, Aggleton JP, O’Sullivan MJ. Cingulum microstructure predicts cognitive control in older age and mild cognitive impairment. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(49):17612–17619. doi: 10.1523/JNEUROSCI.3299-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montreys CR, Borod JC. A preliminary evaluation of emotional experience and expression following unilateral brain damage. The International journal of neuroscience. 1998;96(3-4):269–283. doi: 10.3109/00207459808986474. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, Almli CR, et al. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221(2):349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- Papinutto ND, Jovicich J. Paper presented at the European society for magnetic resonance in medicine and biology, Valencia. 2008. Optimization of brain tissue contrast in structural images at 4T: a computer simulation and validation study. [Google Scholar]
- Park H-J, Kim JJ, Lee S-K, Seok JH, Chun J, Kim DI, et al. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Human Brain Mapping 2008. 2008;29:503e16. doi: 10.1002/hbm.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J, Summerfeldt L, Hogan M, Majeski S. Emotional intelligence and academic success: examining the transition from high school to university. Personality and Individual Differences. 2004;36(1) [Google Scholar]
- Parkinson C, Wheatley T. Relating Anatomical and Social Connectivity: White Matter Microstructure Predicts Emotional Empathy. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs347. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annual Review of Neuroscience. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Phelps LA, Hanley-Maxwell C. School-to-Work Transitions for Youth with Disabilities: A Review of Outcomes and Practices. Review of Educational Research. 1997;67(2):197–226. [Google Scholar]
- Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(48):15089–15099. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, et al. Structural anatomy of empathy in neurodegenerative disease. Brain : a journal of neurology. 2006;129(Pt 11):2945–2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2003;49(1):177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Robson MD, Porter DA. Reconstruction as a source of artifact in non-gated single-shot diffusion-weighted EPI. Magnetic resonance imaging. 2005;23(9):899–905. doi: 10.1016/j.mri.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Salva OR, Regolin L, Mascalzoni E, Vallortigara G. Cerebral and behavioural asymmetries in animal social recognition. Comparative Cognition & Behavior Reviews. 2012;7:110–138. [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Human Brain Mapping. 2005;26(2):139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneewind KA, Gerhard AK. Relationship Personality, Conflict Resolution, and Marital Satisfaction in the First 5 Years of Marriage*. Family Relations. 2002;51(1):63–71. [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nature neuroscience. 2009;12(11):1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schore AN. Early organization of the nonlinear right brain and development of a predisposition to psychiatric disorders. Development and psychopathology. 1997;9(4):595–631. doi: 10.1017/s0954579497001363. [DOI] [PubMed] [Google Scholar]
- Schore AN. Attachment and the regulation of the right brain. Attachment & human development. 2000;2(1):23–47. doi: 10.1080/146167300361309. [DOI] [PubMed] [Google Scholar]
- Schore AN. Relational trauma and the developing right brain: an interface of psychoanalytic self psychology and neuroscience. Annals of the New York Academy of Sciences. 2009;1159:189–203. doi: 10.1111/j.1749-6632.2009.04474.x. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Fine JG, Zhu DC. The role of the right hemisphere for processing of social interactions in normal adults using functional magnetic resonance imaging. Neuropsychobiology. 2011;64(1):47–51. doi: 10.1159/000325075. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Hynd GW. Right hemispheric dysfunction in nonverbal learning disabilities: social, academic, and adaptive functioning in adults and children. Psychological bulletin. 1990;107(2):196–209. doi: 10.1037/0033-2909.107.2.196. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Aharon-Peretz J. The neuroanatomical basis of understanding sarcasm and its relationship to social cognition. Neuropsychology. 2005;19(3):288–300. doi: 10.1037/0894-4105.19.3.288. [DOI] [PubMed] [Google Scholar]
- Shanahan MJ. Pathways to adulthood in changing societies: Variability and mechanisms in life course perspective. Annual Review of Sociology. 2000;26:667–692. [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26(1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Ono J, Harada K, Maeda M, Hackney DB. Diffusional anisotropy in cranial nerves with maturation: quantitative evaluation with diffusion MR imaging in rats. Radiology. 2000;216(3):881–885. doi: 10.1148/radiology.216.3.r00se41881. [DOI] [PubMed] [Google Scholar]
- Thomas C, Avidan G, Humphreys K, Jung KJ, Gao F, Behrmann M. Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nature neuroscience. 2009;12(1):29–31. doi: 10.1038/nn.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex; a journal devoted to the study of the nervous system and behavior. 2002;38(4):589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends in cognitive sciences. 2007;11(4):153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Vallortigara G. Right hemisphere advantage for social recognition in the chick. Neuropsychologia. 1992;30(9):761–768. doi: 10.1016/0028-3932(92)90080-6. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed E, McGregor W, Feldbaek Nielsen J, Roepstorff A, Frith U. Theory of Mind in adults with right hemisphere damage: What’s the story? Brain and language. 2010;113(2):65–72. doi: 10.1016/j.bandl.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Mesulam MM. Developmental learning disabilities of the right hemisphere. Emotional, interpersonal, and cognitive components. Archives of neurology. 1983;40(8):463–468. doi: 10.1001/archneur.1983.04210070003003. [DOI] [PubMed] [Google Scholar]
- Wicker B, Perrett DI, Baron-Cohen S, Decety J. Being the target of another’s emotion: a PET study. Neuropsychologia. 2003;41(2):139–146. doi: 10.1016/s0028-3932(02)00144-6. [DOI] [PubMed] [Google Scholar]
- Winner E, Brownell H, Happe F, Blum A, Pincus D. Distinguishing lies from jokes: theory of mind deficits and discourse interpretation in right hemisphere brain-damaged patients. Brain and language. 1998;62(1):89–106. doi: 10.1006/brln.1997.1889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


