Abstract
The first step in established spermatogenesis is the production of progenitor cells by the stem cell population. The progenitor cells (undifferentiated A spermatogonia) expand in number via the formation of syncytial chains by mitosis. The mechanism by which these progenitor cells commit to meiosis and spermatogenesis is tightly controlled and results in complex morphological organization all of which is designed to efficiently achieve large numbers of spermatozoa. The major extrinsic factor that triggers the commitment to meiosis and establishes the structural complexity is retinoic acid (RA). Retinoic acid is produced from retinol via two oxidation steps in low abundance near its site of action. The action of RA on undifferentiated A spermatogonia results in the timed progression of these progenitor cells into the cycle of the seminiferous epithelium. We have utilized a drug WIN 18,446 that inhibits the second oxidation step in RA biosynthesis to block the progression of undifferentiated A spermatogonia in the mouse testis. As a result of this block the undifferentiated progenitor cells accumulate but do not differentiate into A1 spermatogonia. When the block is released and a bolus of RA is simultaneously administered the accumulated spermatogonia progress through the differentiation pathway in complete synchrony and maintain that synchrony with regard to stages of the cycle of the seminiferous epithelium for several months. This procedure allowed us to accumulate sufficient material to measure retinoic acid levels across the cycle and will allow us to isolate and analyze large number of progenitor cells proceeding synchronously down the pathway to meiosis. We have been able to show that the cycle of the seminiferous epithelium is established and maintained by pulses of RA that appear at stages VIII and IX of the cycle.
Keywords: Progenitor cells, Retinoic acid, Synchronous spermatogenesis
Sperm are produced in large numbers and at a fairly constant rate during the reproductive lifetime of mammals. The biological requirement for large numbers of sperm is driven by sperm competition and the relatively low numbers of sperm that reach the site of fertilization. Data on the actual numbers of sperm produced vary between species and within a species, but it is obvious that an active and effective stem cell population is required. Less obvious, yet just as critical, is the need for a complex organization that assures the efficient and continuous development of progenitor cells through meiosis and morphogenesis to become sperm. This complex organization was initially described in detail by LeBlond and Clermont in 1952 for the rat and has been termed the cycle of the seminiferous epithelium (Leblond and Clermont, 1952). Evolution has imposed certain structural and molecular constraints in the form of this cycle that satisfy the need for continuous production of large numbers of sperm. The molecular changes and cellular morphogenesis required to produce a functional vertebrate sperm once a progenitor cell is committed to enter meiosis is in the range of 30 to 60 days depending on the species, and limits the number of sperm an organism can produce (Clermont, 1972). Evolution has increased the efficiency of spermatogenesis by allowing progenitor cells to irreversibly commit to meiosis and subsequent sperm development several times (usually 4) during these 30 to 60 days. This process results in a series of repeated cellular associations (stages) in the seminiferous tubule and constitutes the cycle of the seminiferous epithelium, which increases sperm production by a factor of 4. Continuous sperm production is assured by staggering the timing of progenitor cell commitment along the length of the seminiferous tubule (spermatogenic wave). The result is a continuum of progenitor cells undergoing the commitment to meiosis balanced with sperm undergoing spermiation. This complex structure supporting spermatogenesis can be observed with some idiosyncrasies in the testes of essentially all mammals.
There has been much speculation over the years about the mechanisms that generate and maintain the cycle of the seminiferous epithelium. It was proposed that biological clocks might be involved in regulating developmental decisions in each of the different cell types but no internal clocks or circadian rhythms have been found in the testis (Morse et al., 2003). In the rodent the cycle is generated by the transition of A undifferentiated spermatogonia (progenitor cells) to A1 differentiating spermatogonia. This transition appears to function like a trigger that irreversibly sends A1 spermatogonia through several mitoses and into meiosis. Once the trigger is pulled the development of the A1 spermatogonia into sperm is a carefully timed, species-specific event (Griswold, 2016). In the last few years we have shown that the extrinsic factor that functions as the trigger is retinoic acid (RA) (Zhou et al., 2008a; Zhou et al., 2008b). In the absence of RA, as a result of dietary restriction or chemical inhibition of synthesis, the progression of progenitor cells from A spermatogonia into A1 spermatogonia is blocked (Hogarth and Griswold, 2010; Hogarth and Griswold, 2013). Depleting RA in rodents using dietary restriction normally takes 2 to 3 months and results in severe global animal health issues. We have shown that the use of WIN 18,446, a bis-(dichloroacetyl)-diamine, can reduce testicular RA levels to ~10% of normal within hours (Hogarth et al., 2013; Hogarth et al., 2011; Kent et al., 2016). WIN 18,446 inhibits the aldehyde dehydrogenase enzymes that are required to convert retinal to RA. The half-life of RA in tissues appears to be about 30 min, so inhibition of synthesis quickly results in depletion of the final product. The net result in the rodent testis after weeks of dietary depletion (McLean et al., 2002; Morales and Griswold, 1987) or a few days of WIN 18,446 treatment is loss of any germ cells more advanced than A undifferentiated spermatogonia (Fig. 1). Following RA depletion via the diet, rodents can then be provided retinol or RA and trigger the A spermatogonia to proceed through the transition to A1 spermatogonia and spermatogenesis will recover. However, while there is a normal cycle of the seminiferous epithelium in recovered rodents, there is no spermatogenic wave and spermatogenesis is synchronous throughout the testis (Morales and Griswold, 1987). With a prolonged RA deficiency of several months there is the onset of tissue degradation and spermatogenesis cannot be restored. When WIN 18,446 is used to inhibit the synthesis of RA in mice that are 2 to 9 days of age the animals appear to have no health issues and the testis is of relatively normal size, but the A undifferentiated progenitor cells accumulate and there are no A1 differentiating spermatogonia present (Hogarth et al., 2013). We have been able to extend this treatment from 7 days as described above to 30 days and the A to A1 transition block is maintained. When this block is removed by administration of RA, spermatogenesis is restored and, as described above for the dietary restriction, the cycle of the seminiferous epithelium is normal but there is no spermatogenic wave because germ cell development is synchronous throughout the testis. We have carefully mapped the synchronous development of the A1spermatogonia to A2, A3, A4, Intermediate and Type B spermatogonia eventually forming preleptotene spermatocytes (Agrimson et al., 2016). The timing and development of these synchronous germ cells mimics those same cells in wild-type asynchronous testes. As a result, WIN 18,446/RA treatment of neonatal mice represents a powerful tool that will now enable the study of the early differentiation events that take place within spermatogonia between the commitment to and physical entry into meiosis.
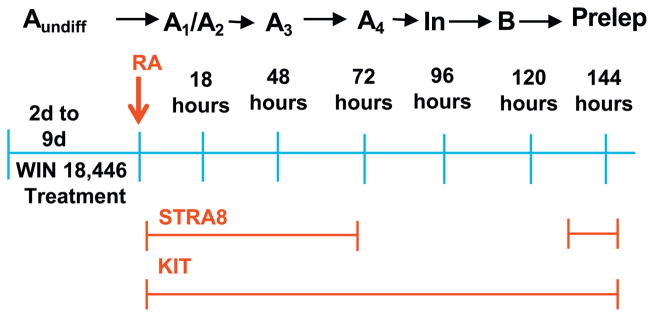
Fig. 1.
Summary of protocol for synchronizing the first round of spermatogenesis. Win 18,446 is administered to 2 day old mouse pups for 7 days. This treatment blocks the progression of Aundiff spermatogonia to A1 differentiating spermatogonia (Evans et al., 2014). At the end of the treatment period the mice are given an injection of retinoic acid to release the block and the accumulated spermatogonia proceed synchronously through spermatogonial differentiation with a timing characteristic of the first round (Agrimson et al., 2016). Stra8 is expressed in the initial differentiating spermatogonia and later in preleptotene spermatocytes. If allowed to develop into adults spermatogenesis remains synchronized for several months.
The transcriptional response of A undifferentiated spermatogonia to RA is rapid and involves changes in the steady state levels of hundreds of different mRNAs (Evans et al., 2014). Within 4 h after RA administration to WIN 18,446-treated mice, major changes in transcript levels can be detected in germ cells as the transition to A1 spermatogonia occurs. RA triggers the timed progression of the A1 spermatogonia through a series of cell divisions culminating in the entry into meiosis. STRA8 mRNA and protein are expressed only in premeiotic germ cells and are excellent markers for the action of RA and the A to A1 transition. Stra8 mRNA increases as much as 15 fold within 4 h after RA treatment and peaks about 12 h after treatment. When the Stra8 gene is deleted there is a disruption at the A to A1 transition and at the entry into meiosis (Agrimson et al., 2016). So, for the normal commitment of progenitor cells to meiosis to occur, the induction of STRA8 by RA is required (Anderson et al., 2008; Mark et al., 2008). STRA8 can be found localized to either the nucleus or the cytoplasm and its function remains obscure (Tedesco et al., 2009). Stra8 has an apparent weak DNA binding activity and it is possible that it binds to chromatin and alters its structure but there is no evidence to support that hypothesis at this time. STRA8 is induced in A1 spermatogonia and in preleptotene spermatocytes at Stage VIII of the cycle of the seminiferous epithelium in the mouse. It was logical to assume that the levels of RA in the testis were highest at stages where both of these developmental steps occur but measurement of RA levels in tissues has been challenging (Griswold, 2016; Hogarth et al., 2015).
It has been shown that both germ cells and Sertoli cells respond to RA. In addition, Leydig cells contain enzymes for RA synthesis and degradation and signaling and respond to the presence or absence of RA (Griswold, unpublished). While the transcription of hundreds of genes is altered by RA in the testis, the most dramatic increases in mRNA levels occur in germ cells and include enzymes involved in RA synthesis and degradation such as retinol dehydrogenase 10 (RDH10) and Cyp26a1 (Evans et al., 2014).
RA is considered a morphogen and gradients of RA have been proposed to regulate many developmental processes but since levels in developing tissues are very low and transitory and RA is incredibly light sensitive, direct measurement of this gradient is technically not feasible given current technology. For example, direct measurement of RA gradients in seminiferous tubules would require mass isolation of each stage of the cycle. The amount of tissue and time required and the inability to perform these dissections in a light-protected environment make this approach impractical. However, using WIN 18,446 to synchronize spermatogenesis we were able to collect testes at various intervals between 42 and 50 days after RA injection and obtain testes comprised of only one or a few closely related stages of the cycle (Hogarth et al., 2015). In this way we were able to obtain enough tissue to allow the laboratory of our collaborator, Dr. Nina Isoherranen at the University of Washington, to use very sensitive liquid chromatography-mass spectrometry to measure levels of RA in the synchronized samples of testes. The data showed that there was a pulse of RA associated with Stages VIII–IX (approximately 30 pmoles/g tissue) of the cycle of the seminiferous epithelium that was 5 times the RA levels found in Stages I–VII (approximately 5 pmoles/g tissue). This result led to the concept that pulses of RA must move along the tubule correlated with the appearance of Stages VIII–IX of the cycle of the seminiferous epithelium (Griswold, 2016; Hogarth et al., 2015). We have proposed that the apparent movement of RA pulses along the tubule is likely the result of coordinated synthesis and degradation. This result was the first time a RA gradient proposed to regulate a number of developmental processes could be visualized and measured.
The presence of an RA gradient brings up questions about the cells and factors responsible for its synthesis and degradation. It is relatively clear based on mouse gene knockout models from a couple of laboratories that the RA pulse during the “first wave” or initiation of spermatogenesis is a result of the action of synthesis in the Sertoli cells. Deleting genes for retinol dehydrogenase 10 (Rdh10), or the retinaldehyde dehydrogenases (Aldh1a1, Aldh1a2, and Aldh1a3) specifically in Sertoli cells inhibits the development of the first wave. Deletion of either Rdh10 or Aldh1a1–3 initially blocks the A to A1 transition, resembling the block by WIN 14,446. Spermatogenesis can either resume spontaneously, occurring in the Rdh10 knockout mice, or following RA administration to the Aldh1a1–3 line (Raverdeau et al., 2012; Tong et al., 2013). A beta galactosidase expressing reporter mouse line has been instrumental in demonstrating that the asynchronous cycle of the seminiferous epithelium is established shortly after birth (Snyder et al., 2010). Transgenic mice that express beta galactosidase in cells that have active RA signaling (receptors and ligand) demonstrated that the A to A1 transition occurs in discrete patches of seminiferous tubules of 2–3 day old mice. These patches represent the first functional RA activity and the first appearance of the spermatogenic wave. However, if these mice are given exogenous RA the patches are eliminated and tubules transition into being continuously positive for beta galactosidase. If these animals are then left to recover to adulthood, synchronous spermatogenesis ensues. So, in these 2–3 day old mice the initial spermatogenic wave can be disrupted or eliminated by exogenous RA. However, by 8 days of age when the differentiating spermatogonia and first preleptotene spermatocytes appear, exogenous RA no longer can alter the spermatogenic wave (Davis et al., 2013; Snyder et al., 2011). This result and histological localization of RA synthetic enzymes in advanced germ cells suggested that the advanced germ cells could also be a source of RA (Sugimoto et al., 2012).
The Rdh10 or Aldh1a1–3 knockout mice accumulate A undifferentiated spermatogonia but if given a single injection of RA, the A to A1 transition is triggered and sperm ultimately form. In addition to the first wave, spermatogenesis continues unimpeded for months after the single RA injection but in the Aldh1a1–3 knockout spermiogenesis remains abnormal (Raverdeau et al., 2012; Tong et al., 2013). One explanation for a single dose of retinoic acid triggering recurring spermatogenesis is that the advanced germ cells from the first wave could become the source of RA for subsequent waves. Based on all of the data the most likely germ cells to be the source of RA after the first wave are the preleptotene spermatocytes that appear in Stage VIII of the cycle, coincident with the pulse of RA (Davis et al., 2013). Thus the design of this cyclic system may involve initiation by the Sertoli cells that control the patchy appearance of the retinoid signaling system in 2–3 day old mice followed by the cyclic synthesis of RA by preleptotene spermatocytes at every appearance of Stage VIII of the cycle. Several aspects of this model are still unsupported by direct experimental data. First, the factors involved in the initial patchy appearance of the retinoid signaling are unknown. Second, there is no direct proof that preleptotene spermatocytes synthesize RA. However, some aspects of this model are useful to clarify our current understanding. First, in the absence of advanced germ cells the initial priming of the cycle by Sertoli cells followed by the maintenance of the cycle by the later germ cells fits the known data. Second, the patchy nature of the development of RA signaling can be over-ridden by a bolus of exogenous RA, suggesting that it is the RA itself that is limiting. Third, anytime there are no germ cells developed beyond A undifferentiated spermatogonia a bolus of exogenous RA will result in synchronous spermatogenesis in the adult (Hogarth et al., 2015). So RA treatment of adult vitamin A deficient mice, perinatal mice 2 to 6 days of age, 2 day old mice treated with WIN 18,446 or RDH10 Sertoli cell specific knockout mice leads to synchronous spermatogenesis in the adult. In all cases of induced synchrony the spermatogenesis reverts toward normal cycling with time (several months).
In some ways the functional units of control of the transition of A spermatogonia to A1 spermatogonia are reminiscent of what was required to start and run an old model T automobile. The engine had to be started by a hand crank that got the internal cylinders firing and maintaining the running of the engine. Once started by this external crank the cylinders fired sequentially and maintained a smooth rotation of the crankshaft. If all cylinders fired at once as is seen in the synchronized testis the automobile could move but did so in a very herky-jerky manner. Whenever the engine quit running it had to be started again by the crank (RA from Sertoli cells).
Acknowledgments
Our work in this review was supported by grants from NIH: HD10808 and U5442454.
References
- Agrimson KS, et al. Characterizing the Spermatogonial response to retinoic acid during the onset of spermatogenesis and following synchronization in the neonatal mouse testis. Biol Reprod. 2016;95(4):229–236. doi: 10.1095/biolreprod.116.141770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EL, et al. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A. 2008;105(39):14976–14980. doi: 10.1073/pnas.0807297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont Y. Kinetics of spermatogenesis in Mammmals. Physiol Rev. 1972;52:198–204. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- Davis JC, et al. Induction of spermatogenic synchrony by retinoic acid in neonatal mice. Spermatogenesis. 2013;3(1):e23180. doi: 10.4161/spmg.23180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, et al. Riding the spermatogenic wave: profiling gene expression within neonatal germ and Sertoli cells during a synchronized initial wave of spermatogenesis in mice. Biol Reprod. 2014;90(5) doi: 10.1095/biolreprod.114.118034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MD. Spermatogenesis: the commitment to meiosis. Physiol Rev. 2016;96(1):1–17. doi: 10.1152/physrev.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth CA, Griswold MD. The key role of vitamin A in spermatogenesis. J Clin Invest. 2010;120(4):956–962. doi: 10.1172/JCI41303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth CA, Griswold MD. Retinoic acid regulation of male meiosis. Curr Opin Endocrinol Diabetes Obes. 2013;20(3):217–223. doi: 10.1097/MED.0b013e32836067cf. [DOI] [PubMed] [Google Scholar]
- Hogarth CA, et al. Suppression of Stra8 expression in the mouse gonad by WIN 18,446. Biol Reprod. 2011;84(5):957–965. doi: 10.1095/biolreprod.110.088575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth CA, et al. Turning a spermatogenic wave into a tsunami: synchronizing murine spermatogenesis using WIN 18,446. Biol Reprod. 2013;88(2):40. doi: 10.1095/biolreprod.112.105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth CA, et al. Processive pulses of retinoic acid propel asynchronous and continuous murine sperm production. Biol Reprod. 2015;92(2):37. doi: 10.1095/biolreprod.114.126326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent T, et al. ALDH enzyme expression is independent of the spermatogenic cycle and their inhibition causes misregulation of murine spermatogenic processes. Biol Reprod. 2016;94(1):1–13. doi: 10.1095/biolreprod.115.131458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci. 1952;55:548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- Mark M, et al. STRA8-deficient spermatocytes initiate, but fail to complete, meiosis and undergo premature chromosome condensation. J Cell Sci. 2008;121(Pt 19):3233–3242. doi: 10.1242/jcs.035071. [DOI] [PubMed] [Google Scholar]
- McLean DJ, Russell LD, Griswold MD. Biological activity and enrichment of spermatogonial stem cells in vitamin A-deficient and hyperthermia-exposed testes from mice based on colonization following germ cell transplantation. Biol Reprod. 2002;66(5):1374–1379. doi: 10.1095/biolreprod66.5.1374. [DOI] [PubMed] [Google Scholar]
- Morales CR, Griswold MD. Retinol induces stage synchronization in seminiferous tubules of vitamin a deficient rats. Ann N Y Acad Sci. 1987;513:292–293. [Google Scholar]
- Morse D, et al. No circadian rhythms in testis: period1 expression is clock independent and developmentally regulated in the mouse. Mol Endocrinol. 2003;17(1):141–151. doi: 10.1210/me.2002-0184. [DOI] [PubMed] [Google Scholar]
- Raverdeau M, et al. Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc Natl Acad Sci U S A. 2012;109(41):16582–16587. doi: 10.1073/pnas.1214936109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Small C, Griswold MD. Retinoic acid availability drives the asynchronous initiation of spermatogonial differentiation in the mouse. Biol Reprod. 2010;83(5):783–790. doi: 10.1095/biolreprod.110.085811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, et al. Exposure to retinoic acid in the neonatal but not adult mouse results in synchronous spermatogenesis. Biol Reprod. 2011;84(5):886–893. doi: 10.1095/biolreprod.110.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto R, Nabeshima Y, Yoshida S. Retinoic acid metabolism links the periodical differentiation of germ cells with the cycle of Sertoli cells in mouse seminiferous epithelium. Mech Dev. 2012;128(11–12):610–624. doi: 10.1016/j.mod.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Tedesco M, et al. STRA8 shuttles between nucleus and cytoplasm and displays transcriptional activity. J Biol Chem. 2009;284(51):35781–35793. doi: 10.1074/jbc.M109.056481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong MH, et al. Retinol dehydrogenase 10 is indispensible for spermatogenesis in juvenile males. Proc Natl Acad Sci U S A. 2013;110(2):543–548. doi: 10.1073/pnas.1214883110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, et al. Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol Reprod. 2008a;78(3):537–545. doi: 10.1095/biolreprod.107.064337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, et al. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol Reprod. 2008b;79(1):35–42. doi: 10.1095/biolreprod.107.066795. [DOI] [PMC free article] [PubMed] [Google Scholar]