Abstract
Blocked and event-related fMRI designs are both commonly used to localize language networks and determine hemispheric dominance in research and clinical settings. We compared activation profiles on a semantic judgement task using both designs in 43 healthy individuals to determine whether task design or subject-specific factors (i.e., age, sex, or language performance) influence activation patterns. We found high concordance between the two designs within core language regions, including the inferior frontal, posterior temporal, and basal temporal region. However, differences emerged within inferior parietal cortex. Subject-specific factors did not influence activation patterns, nor did they interact with task design. These results suggest that despite high concordance within perisylvian regions that are robust to subject-specific factors, methodological differences between blocked and event-related designs may contribute to parietal activations. These findings provide important information for researchers incorporating fMRI results into meta-analytic studies, as well as for clinicians using fMRI to guide pre-surgical planning.
Keywords: blocked design, event-related design, fMRI, language, semantics
1. Introduction
Two major types of experimental designs were employed to localize language networks and to identify the language-dominant hemisphere with functional magnetic resonance imaging (fMRI) are blocked and event-related designs. Both designs are frequently implemented in research and clinical settings and are thought to have different advantages. Blocked designs generally have large blood-oxygen-level-dependent (BOLD) signal changes relative to the baseline, resulting in high statistical power in a short time frame (e.g., Birn, Cox, & Bandettini, 2002; Friston, Zarahn, Josephs, Henson, & Dale, 1999). Thus, blocked designs may be more appropriate if the research goal is to localize a specific cognitive function or to detect subtle differences in BOLD response between different task conditions, especially in clinical settings that require efficiency (Chee, Venkatraman, Westphal, & Siong, 2003). Conversely, event-related designs are believed to have the advantage of reducing participant’s expectation of subsequent stimuli, providing greater specificity, and reducing motion artifacts when estimating the hemodynamic response (e.g., Birn et al., 2002; D’Esposito, Zarahn, & Aguirre, 1999; Friston et al., 1999; Liu, Frank, Wong, & Buxton, 2001). Thus, the choice of experimental design often depends upon the nature of the research or clinical question and the relative importance of each of these factors to answering the proposed question.
Despite the number of papers that have alluded to the relative merits of each design, only two papers have directly compared blocked versus event-related designs for identifying language networks in healthy controls or preoperative patients. In a small sample of young adults (n = 8–12 per group), Chee et al., (2003) used a semantic judgement task and found strong concordance in language activation patterns between blocked and event-related designs. In contrast, Tie et al., (2009) examined language processing in six healthy controls and eight patients with brain tumors using an antonym generation task and reported a relatively high degree of discordance between task designs. In fact, their event-related design produced more robust activations in putative language areas, including the inferior frontal gyrus and posterior superior temporal gyrus, relative to the blocked design. In addition, the blocked design was more likely to show activations outside of the core language network, including right frontal lobe and precuneus in a subset of healthy controls. These findings led them to conclude that their event-related design produced maps with both greater sensitivity and specificity to language networks relative to their blocked design.
Understanding the similarities and differences between blocked and event-related designs is critical for at least two reasons. First, this information can facilitate the comparison of fMRI findings across different studies aimed at identifying common brain regions involved in a specific cognitive function. Despite the growing number of systematic reviews and meta-analyses on fMRI activations associated with language and other cognitive functions, the variability in experimental designs is rarely considered (Costafreda, 2009). Second, fMRI is increasingly used in the clinical setting as a non-invasive tool for preoperative mapping of language networks and determination of language-dominance in pre-surgical planning for patients with epilepsy and brain tumors. Most published pre-surgical language fMRI studies utilize a blocked design (e.g., epilepsy: Desmond et al., 1995; Woermann et al., 2003; brain tumor: Stippich et al., 2007) because it is believed to be simpler to implement, time efficient, and has a higher detection power (e.g., Chee et al., 2003; Donaldson & Buckner, 2001; Tie et al., 2009). However, as noted above, there is some evidence that event-related designs may provide comparable or even higher detection power for determining language lateralization compared to blocked designs. Thus, understanding core differences between the two designs is critical for clinicians who must select the most robust task for clinical decision-making in the context of pre-surgical planning.
In present study, we build upon a surprisingly small literature that has compared blocked and event-related designs for identifying language networks and determining hemispheric language dominance in healthy controls. However, we augment the existing literature in two important ways. First, we include a large group of healthy controls (N = 43) who span a broad age range (19–72 years) and broad language performance (see Table 1) to parallel the variability seen in pre-surgical populations. Second, we stratify participants according to the demographic (i.e., age, sex) and language performance (i.e., high versus low performer) to explore whether there are main effects of these variables on language activation patterns or interactions between these subject-specific characteristics and fMRI experimental designs.
Table 1.
Descriptive statistics for the demographic/performance characteristics for the participants in the blocked and event-related designs.
| Variables | Blocked | Event-Related |
|---|---|---|
| Sample Size | 21 | 22 |
| Demographic | ||
| Sex (Male/Female) | 9/12 | 11/11 |
| Handedness (Right/Left/Ambidextrous)+ | 19/1/1 | 21/1/0 |
| Language (Monolingual/Bilingual) | 14/7 | 17/5 |
| Age* | 37.14 (20 – 65) | 36.64 (19 – 72) |
| Education* | 16.05 (12 – 20) | 15.64 (13 – 20) |
| Language Performance | ||
| Language Composite* | 0.68 (−0.69 – 1.56) | 0.62 (−0.74 – 1.50) |
| Boston Naming Test (BNT)* | 0.29 (−1.00 – 2.00) | 0.20 (−1.00 – 2.00) |
| Auditory Naming Test (ANT)* | 0.51 (−0.77 – 0.94) | 0.07 (−0.77 – 0.94) |
| Category Fluency (CF)* | 0.98 (−1.33 – 3.00) | 1.11 (−1.33 – 2.67) |
| Letter Fluency (LF)* | 0.93 (−1.00 – 3.00) | 1.02 (−1.33 – 3.00) |
The handedness was measured by Edinburgh Handedness Inventory.
Mean (Minimum – Maximum)
2. Methods
2.1. Participants
This study was approved by the Institutional Review Board at the University of California, San Diego (UC San Diego) and informed consent was collected from all participants. A total of 50 healthy adults were recruited in this study. The final sample included 43 healthy adults; seven participants were excluded from the final analyses due to excessive head motion during fMRI scanning. Twenty-one participants completed the blocked design version of the task, whereas 22 completed the event-related design. All of the participants were screened for neurological or psychiatric conditions.
2.2. Materials and Procedures
2.2.1. Neuropsychological tasks
Participants were administered the Boston Naming Test, a visual confrontation naming measure (BNT; Kaplan, Goodglass, & Weintraub, 1983); Auditory Naming Test, an auditory naming test in which participants are provided with verbal cues (ANT; Hamberger & Seidel, 2003), and Category Fluency (CF) and Letter Fluency (LF) subtests from the Delis-Kaplan Executive Function System (Delis, Kaplan, & Kramer, 2001), as part of a larger neuropsychological test battery1. Age-corrected scaled scores were calculated for the CF, and LF and age and education-corrected scaled scores was calculated for the BNT based on normative data provided in the test manuals; education-corrected scaled scores were calculated for the ANT based on normative data published in Hamberger and Seidel (2003).
2.2.2. FMRI language task
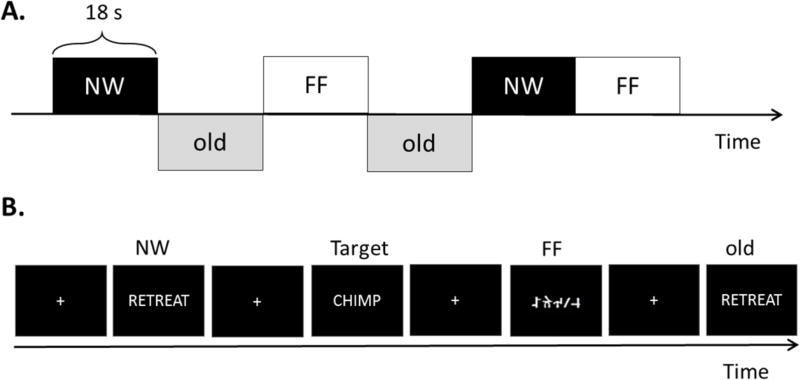
The blocked and event-related fMRI tasks were designed to be as comparable as possible, using the same stimuli, general timing, task instructions, and approximate length of each task run. During each task, four types of stimuli were presented visually on the screen as light gray letters on a black background in Arial font. These four types of stimuli included novel words (NW) that were presented only once, repeating words (old) that were presented more than once, false font (FF) stimuli, and target words (i.e., animals). The NW stimuli were nouns with 4 to 8 letters, with a written lexical frequency of 3–80 per 10 million (Francis & Kucera, 1982). The old words were repetitions of the novel words. The FF stimuli were comprised of alphabet-like characters that were matched in size and number of characters to each NW stimulus in order to control for visual features of the stimuli, but not lexical, syntactic, or semantic content (McDonald et al., 2009). The target words consisted of moderate to low frequency animal names. In the task, participants were asked to respond to the presence of target words by pressing a button. Presentation software (Neurobehavioral Systems, Inc, Albany, CA, U.S.A.) was used to present stimuli and collect participants’ responses. For the purpose of this study, the contrast between NW and FF stimuli was used as the primary contrast to model lexical-semantic processing in the blocked and event-related designs.
Blocked Design
Two runs of the blocked design task were presented (see Figure 1A). In each run, ten blocks of each of the three stimulus types were presented (i.e., NW, FF, old words). Each block consisted of 15 NW, FF, or old word stimuli plus 3 target words. A total of 150 NW, 150 FF, 150 old words, and 90 target words were presented. Each stimulus was presented for 1000-ms each. The blocks of these three stimulus types were presented in pseudo-random order (see Figure 1A for example). The entire blocked design took 8 minutes to complete.
Fig. 1.
Two fMRI task designs. NW = New Word; FF = False Font; old = Old Words. A: blocked design. Each block consisted of 15 stimuli with 3 target words. B: event-related design.
Event-Related Design
Two runs of the event-related design task were also presented (see Figure 1B). Each run consisted of 80 NW, 80 FF, 80 old words, and 24 target words. The stimuli were presented in pseudo-random order with a 900-ms stimulus onset asynchrony followed by a 600-ms crosshair. Temporal jittering with 198 500-ms null baseline trials (presenting only a visual crosshair) was optimally inserted throughout the runs, using the program Optseq2 (Dale, Fischl, & Sereno, 1999). The entire event-related design took 9 minutes to complete.
2.3. Data Acquisition
All imaging was performed on a General Electric Discovery MR750 3T scanner with an 8-channel phased-array head coil at the Keck Center for Functional MRI at UC San Diego. The sequence of image acquisition was a conventional three-plane localizer, GE calibration scan, a T1-weighted 3D customized FSPGR structural sequence (TR = 8.08 ms, TE = 3.16 ms, TI = 600 ms, flip angle = 8°, FOV = 256 mm, matrix = 256 × 192, slice thickness = 1.2 mm), and two functional T2*-sensitive echo-planar imaging (EPI) scans (TR = 3000 ms, TE = 30 ms, flip angle = 90°, FOV = 220 mm, matrix = 64 × 64, slice thickness = 2.5 mm). The fMRI scans were acquired for each individual using two different phase encoding directions (forward and reverse) to correct for geometric distortions in the EPI images (Holland, Kuperman, & Dale, 2010). The order and combination of the two phase encoding directions and two runs of tasks were counterbalanced across the participants to control for order effects.
2.4. Imaging Data Processing
2.4.1. Volumetric MRI
Individual T1-weighted images were used to construct models of each participant’s cortical surfaces using FreeSurfer software 5.1.0 (http://surfer.nmr.mgh.harvard.edu) (Dale et al., 1999; Dale & Sereno, 1993). The reconstructed surfaces were visually inspected for any defects and manually edited according to established software guidelines. The cortical surface was then parcellated into regions of interest (ROIs) according to the Destrieux atlas (Destrieux, Fischl, Dale, & Halgren, 2010).
2.4.2. Functional MRI
The fMRI data analysis was carried out using Analysis of Functional NeuroImages (AFNI; Cox, 1996), SUMA (Saad & Reynolds, 2012), and Matlab programs (MathWorks, Natick, MA).
Processing
Each participant’s data were preprocessed with the following steps. In order to accurately co-register functional and structural MRI datasets, any distortions caused by gradient nonlinearities and B0 magnetic field inhomogeneities in both functional and structural MRI were minimized according to Holland et al. (2010). Head motion between scans was removed by rigid-body registration to the first functional run and head motion within scan was removed using AFNI’s 3dvolreg. Each time series was shifted so that each slice was aligned to the first acquired slice using AFNI’s 3dTshift. EPI datasets were aligned to one another and to the T1-weighted images, and then were resampled to 2.5 mm3 isotropic voxels. Both cortical parcellations and subcortical volume segmentations were imported, aligned, and applied to the EPI using SUMA’s @SUMA_Make_Spec_FS. Each participant’s anatomical and functional data were transformed into N27 atlas space (Mazziotta et al., 2001) for group analyses. The functional data for each participant were then smoothed using a 4-mm full-width half maximum (FWHM) Gaussian kernel in AFNI’s 3dBlurToFWHM program. This program first estimates the smoothness of the functional data, then smooths it with a 4-mm FWHM in the x, y, z direction, such that the degree of smoothness is consistent across participants. The data were then scaled by computing the mean of each voxel time series in order to calculate the percent signal change using AFNI’s 3dTstat and 3dcalc functions. Then, the preprocessed time series data for each participant were analyzed based on the General Linear Model using the BLOCK response function in AFNI’s 3dDeconvolve. The function of BLOCK (18,1) was used for the blocked design, and the BLOCK (1, 1) function was used for the event-related design. In addition, two strategies were used to correct head motion within scan. First, large motion events, defined as the time points within each EPI time-series in which the euclidean-normalized derivative of the participant’s motion parameters was greater than 0.3 (roughly 0.3 mm motion), were excluded from the deconvolution analysis. The TR immediately before the motion-contaminated TR was also excluded. In the blocked design, one more step was introduced to correct for head motion: if more than 3 large motion events (9 seconds) occurred within one block, the whole block was excluded from the deconvolution analysis. The second strategy consisted of using six additional regressors to model motion residuals. Statistical activation maps were generated by calculating linear contrasts, which provided the differences between the mean regression coefficients for the effects of interest. The NW vs. FF (NW-FF) contrast was generated to identify the regions associated with lexical-semantic processing.
2.5. Group Comparisons in fMRI
Two strategies were used to compare the activation differences between the blocked and event-related designs. The first generated statistical activation maps for each group to visualize the differences between the two experimental designs across the cortical surface. The second strategy employed an anatomical region of interest (ROI) analysis to quantify the differences between two designs in terms of overall signal strength and lateralization of the responses.
Whole Brain Activation Maps
The group activation patterns for two experimental designs were generated using the mixed effects model in AFNI’s 3dANOVA2. The statistical maps were corrected for multiple comparisons by using a combined significance level of p < .01 and a cluster size > 31, for a corrected α of .05 as determined by AFNI’s 3dClustSim.
ROI Analyses
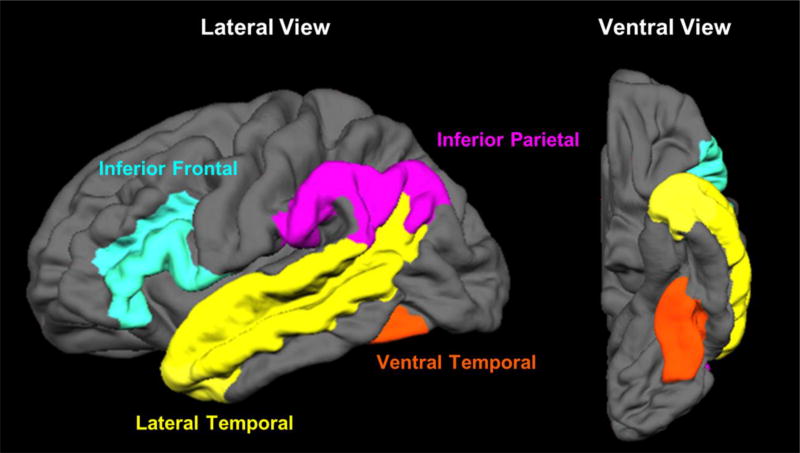
Multiple-parcelled Destrieux regions were combined to create four ROIs: ventral temporal (VT), lateral temporal (LT), inferior parietal (IP), and inferior frontal (IF) regions (see Figure 2). Selection of these ROIs was guided by previous fMRI and magnetoencephalography (MEG) studies that have identified these four regions as critical to different aspects of language processing (Chang et al., 2017; McDonald et al., 2010; Thesen et al., 2012). These regions involve visual word form processing, lexical access, and phonological processing (e.g., Dehaene & Cohen, 2011; Poldrack et al., 1999; Thesen et al., 2012). Furthermore, a meta-analysis of 120 functional neuroimaging studies showed that these four ROIs are core to lexical-semantic processing (Binder, Desai, Graves, & Conant, 2009). To assess the activation within each ROI, each participant’s statistical maps were first corrected for multiple comparisons by using a combined significance level of p < .01 and a cluster size > 19, for a corrected α of .05 as determined by 3dClustSim. The number of activated voxels were then counted within each of the ROIs for each hemisphere. Laterality indices (LI: [L-R]/[L+R]) were calculated between the two hemispheres and for all four ROIs to provide a measure of hemispheric dominance for language. Positive LIs indicate a leftward asymmetry in activations, whereas a negative LI indicates a rightward asymmetry.
Fig. 2.
Four ROIs. The inferior frontal region consists of the opercular, orbital, and triangular part of the inferior frontal gyrus (index 12, 13, and 14), inferior frontal sulcus (index 52), horizontal and vertical ramus of the anterior segments of the lateral sulcus (index 39 and 40) in the Destrieux atlas. The temporo-parietal region consists of the angular gyrus (index 25), supramarginal gyrus (index 26), and sulcus intermedius primus of Jensen (index 55). The lateral temporal region consists of the lateral aspect of the superior temporal gyrus (index 34), middle temporal gyrus (index 38) and superior temporal sulcus (index 73). The ventral temporal region consists of the lateral occipito-temporal gyrus (index 21) and lateral occipito-temporal sulcus (index 60).
2.6. Statistical Analyses
Between Group Difference
Group differences between the blocked and event-related designs were tested for neuropsychological measures of language function and for each of the LIs across the ROIs in fMRI with either a Welch U test or Wilcoxon-Mann-Whitney (WMW) test, according to recommendations in Skovlund and Fenstad (2001). The equality of variances between the two groups was assessed by Levene’s test. A WMW test was performed and the Z value was reported if the variances were equal, whereas a Welch U test was performed and the F value was reported if the variances were unequal.
Interaction between Experimental Design and Demographic/Performance Characteristics
To explore whether there was an interaction between fMRI design and either demographic (age, sex) or language performance characteristics, the mixed effect model was used. Principal component analysis was used to generate a composite score to represent language ability by reducing the number of neuropsychological variables (BNT, ANT, CF, and LF) to a single score with the highest eigenvalue. This newly generated principal component (PC) variable was named “Language PC”. The high language ability group included those who obtained a Language PC at or above the 50th percentile, whereas the low ability group included those who obtained a Language PC below the 50th percentile. The mixed effect model consisted of three fixed factors (i.e., design, one of the demographic/performance characteristics, and the interaction between design and demographic/performance characteristics) and two random factors (i.e., subject, ROI). The ROI was treated as a random effect because the mixed effects model accounts for the differential activation among the four ROIs. In other words, it takes into account the variability of activation in the four ROIs.
3. Results
Participants in the two experimental designs were comparable in their demographic characteristics (see Table 1). The mean age and mean years of education of the blocked design group was not statistically different from the event-related design group, t(41) = −0.12, p = 0.91, t(30) = −0.52, p = 0.61, respectively. The distribution of sex and handedness were not statistically different between the groups (sex: χ2 = 0.22, p = 0.64; handedness: χ2 = 1.46, p = 0.48) and they did not differ in language ability (Language PC: Z = 0.73, p = 0.47), as measured by neuropsychological tests of visual and auditory naming and verbal fluency (BNT: Z = 0.32, p = 0.75; ANT: Z = 1.61, p = 0.11; CF: Z = −0.43, p = 0.67; LF: Z = −0.54, p = 0.59). Scores on all four language measures ranged from low average (SS = 6 to 7) to high average/superior (SS > 15) in both groups. The eigenvalues of principal component analysis were 1.91, 1.06, 0.69, and 0.35, and the first principal component accounted for 48% of the total variance using the four language measures. The loading values of the four language measures on the first principal component were: 0.52 in BNT, 0.27 in ANT, 0.50 in LF, and 0.64 in CF.
3.1. Behavioral Data in Scanner
The accuracy of the behavioral data in the scanner was computed using d prime (Stanislaw & Todorov, 1999). There was no group difference between the blocked (M = 3.84, SD = 1.12) and event-related (M = 4.35, SD = 1.04) designs in the accuracy of the behavioral data in the scanner, Z = −1.61, p = 0.108.
3.2. Group Differences in Block and Event-Related Design
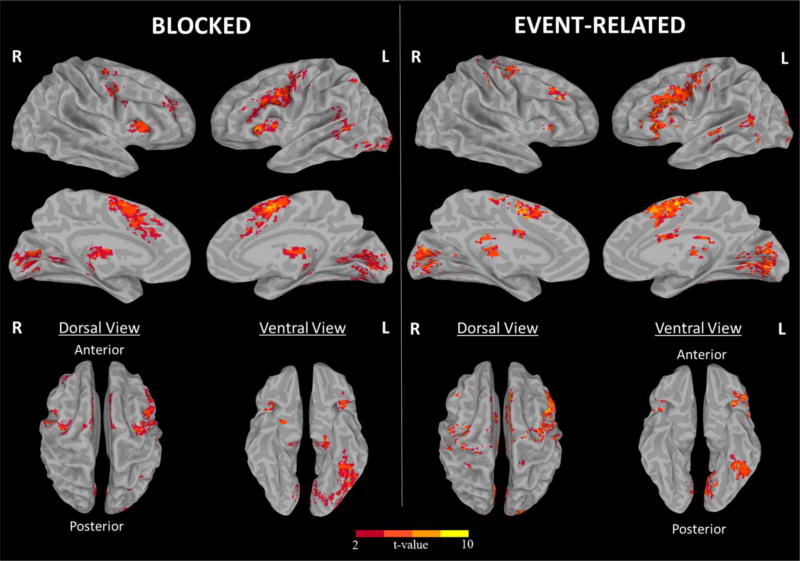
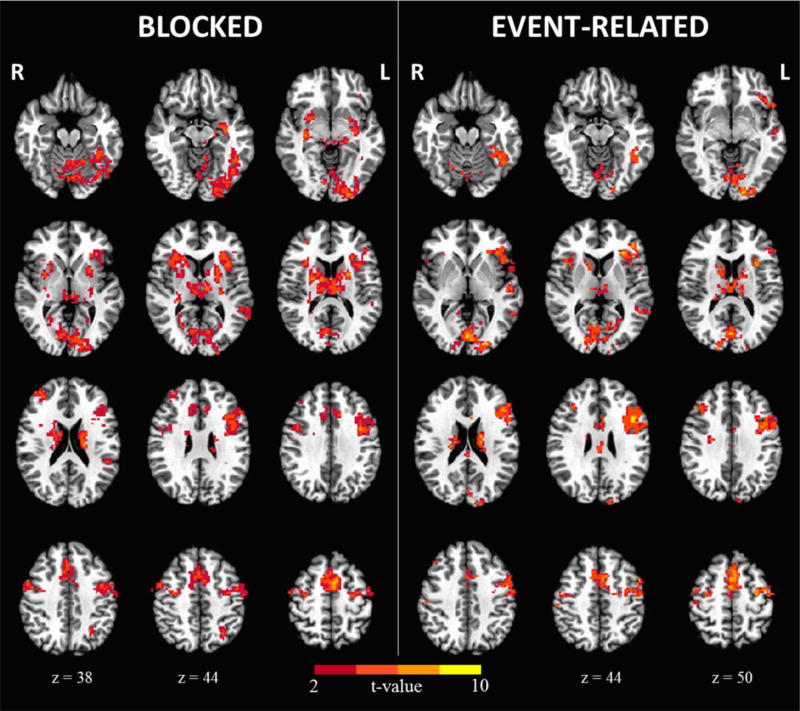
Surface and voxel-wise maps of the NW-FF contrast are shown in Figures 3 and 4 for both the blocked and event-related designs. As can be seen, our semantic judgement task activated a highly similar network of cortical regions across designs that included the left inferior frontal, left inferior parietal, left lateral temporal, and left ventral temporal (fusiform) regions. In addition, weaker activations are observed in the right inferior frontal, middle frontal, and precentral regions in both designs. Overall, the blocked design resulted in a stronger activation pattern relative to the event-related design in terms of the total of number of voxels and t values. In addition, areas of supra-threshold activation were identified within the basal ganglia and cerebellum in the blocked design that are less visible in the event-related design. Peak coordinates and regions for each design are provided in the Table 1 in the Supplementary.
Fig. 3.
The surface fMRI activation maps for the blocked and event-related designs.
Fig. 4.
The voxel-wise fMRI activation maps for the blocked and event-related designs.
ROI analysis
Descriptive and inferential statistics for the activations in both left and right hemispheres and laterality indices in the four ROIs are presented for each group in Table 2. The mean activation in the blocked design was greater than the mean activation in the event-related design in the IF, LT, and VT ROIs within the left hemisphere, and all four of the ROIs in the right hemisphere. Laterality indices were positive for all four ROIs in both designs. Paired t-tests revealed that in the blocked design, the activations were significantly left-lateralized in the IF (t(20) = −4.41, p < 0.001), LT (t(20) = −5.82, p < 0.001), and VT (t(20) = −6.77, p < 0.001) regions, whereas they were bilateral in the IP region (t(20) = −1.05, p = 0.3). In the event-related design, the activations were strongly left-lateralized in all four ROIs (IF: t(21) = −4.79, p < 0.001; IP: t(21) = −2.55, p = 0.019; IF: t(21) = −3.82, p = 0.001; IF: t(21) = −4.05, p < 0.001). There were no group differences in laterality of the IF, LT, and VT activations between the groups. However, there was a significantly greater (leftward) asymmetry within the IP region in the event-related design compared to the block design.
Table 2.
Descriptive and inferential statistics for the numbers of activated voxels and laterality index in each ROI: inferior frontal (IF), inferior parietal (IP), lateral temporal (LT), and ventral temporal (VT).
| Variables | Blocked Mean (SD) |
Event-Related Mean (SD) |
Statistical Result | p |
|---|---|---|---|---|
| Left Hemisphere | ||||
| IF | 142.0 (86.7) | 78.6 (67.1) | Z = 2.44 | .015* |
| IP | 33.4 (46.5) | 24.3 (31.2) | Z = 0.58 | .559 |
| LT | 153.8 (126.2) | 65.0 (67.5) | F(1, 31.3) = 8.16 | .008** |
| VT | 127.0 (84.5) | 34.2 (39.6) | F(1, 28.1) = 21 | < .001*** |
|
| ||||
| Right Hemisphere | ||||
| IF | 68.3 (80.8) | 20.6 (27.1) | F(1, 24.3) = 6.62 | .017* |
| IP | 23.8 (34.8) | 8.7 (16.9) | Z = 2.4 | .017* |
| LT | 58.3 (57.1) | 25.5 (43.7) | Z = 2.4 | .016* |
| VT | 25.1 (28.1) | 1.1 (2.5) | F(1, 20.3) = 15.21 | < .001*** |
|
| ||||
| Laterality Index | ||||
| IF | 0.45 (0.37) | 0.53 (0.47) | Z = −0.89 | .375 |
| IP | 0.15 (0.48) | 0.45 (0.40) | Z = −2.01 | .045* |
| LT | 0.32 (0.24) | 0.50 (0.41) | F(1, 35.7) = 0.27 | .608 |
| VT | 0.68 (0.29) | 0.66 (0.38) | Z = −0.05 | .961 |
Note: In the column of statistical result, Z value is the result of Wilcoxon-Mann-Whitney test, and F value is the result of Welch U test.
< .001;
< .01;
< .05
3.3. Exploring Interactions between Demographic/Performance Characteristics and Experimental Design
Mixed effect models demonstrated that there were no interactions between experimental design and sex (F(1, 39) = 0.88; p = 0.36), age (F(1, 39) = 2.6; p = 0.11), or language ability (F(1, 30) = 0.06; p = 0.81). Since there was no interaction between these variables and fMRI design, main effects were reported. There were no main effect of sex (F(1, 39) = 0.26; p = 0.61), age (F(1, 39) = 0.36, p = 0.55), or language ability (F(1, 30) = 1.34; p = 0.26).
4. Discussion
In this study, we used a semantic decision task to investigate whether the topology of the language network and/or hemispheric dominance varies as a function of the two most common fMRI designs (i.e., blocked versus event-related). We also investigated whether key demographic variables (age, gender) or language performance influenced language activations or varied by task design. At both the whole brain and ROI levels, our data revealed that blocked and event-related designs show a remarkably similar spatial pattern of responses across the cortical surface. As shown in Figure 3, the activations were strongly left lateralized and maximal within IF, LT, and VT regions for both designs, with weaker activations apparent within inferior frontal, middle frontal and precentral regions of the right hemisphere. These left-lateralized, region-specific activations are consistent with the results found in a meta-analysis of 120 fMRI studies of the lexical-semantic system (Binder et al., 2009) that have implicated the IF, LT, and VT regions in a complex language network responsible for phonological/syntactic, receptive, and visual word form processing, respectively.
Similar to previous studies, we found that the blocked design resulted in greater detection power compared to the event-related design, as evidenced by the greater number of suprathreshold activations observed across ROIs (Birn et al., 2002; Friston et al., 1999). In fact, detection power in terms of activated voxels within the blocked design was about 2 times higher than that observed in the event-related design in most regions. Conversely, our data do not necessarily support the notion of greater specificity in the event-related design. As observed in Figure 4, both our blocked and event-related designs show activations outside our a priori language regions, including the basal ganglia and cerebellum. Although the contribution of these extra-sylvian regions to language processing is not as well established, there is mounting evidence that both regions are involved in reading and language processing (Abdullaev & Melnichuk, 1997; Booth, Wood, Lu, Houk, & Bitan, 2007; Chen & Desmond, 2005a, 2005b; Moro et al., 2001; Poldrack et al., 1999; Tettamanti et al., 2005). Specifically, the putamen is thought to play a key role in the initiation of phonological representations in the left inferior frontal gyrus and left lateral temporal cortex while the cerebellum has been implicated in the amplification and refinement of this signal to facilitate correct lexical decision-making (Booth et al., 2007). Thus, these regions may be critical to phonological processing for articulatory planning and control. It is of note that the blocked design resulted in greater activations in both of these regions compared to the event-related design. This difference may reflect the greater demands placed on articulatory processing while reading blocks of NW relative to when these words are scattered in the event-related design. However, a more parsimonious explanation is that the higher detection power of the blocked design led to this perceived difference in activations (which were present, but weaker in the event-related design) (Friston et al., 1999).
Despite the stronger activations in the blocked design relative to event-related design across ROIs, both tasks showed comparable estimates of hemispheric language dominance. That is, our results did not reveal group differences in the laterality indices calculated in three out of four ROIs (i.e., IF, VT, and LT), suggesting that the two designs may provide comparable results in the context of preoperative planning. The one exception to this is that we found a greater leftward asymmetry in the IP region in the event-related compared to the blocked design—a region that showed much weaker activations overall compared with the other ROIs. The IP region has been identified as a higher-level, supramodal integration hub implicated in aspects of both language and non-language processing, including complex information integration, sentence comprehension, discourse, problem solving, planning and attention (e.g., Binder et al., 2009; Rushworth, Krams, & Passingham, 2001; Rushworth, Nixon, Renowden, Wade, & Passingham, 1997). However, why laterality differences within this region would emerge as a function of task design is not clear. One possibility is that this weak group difference at the ROI level (which is not captured in the surface-based maps) may reflect multiple testing, as it would not survive Bonferroni correction. A second possibility is that differences in attentional demands between the two tasks may have resulted in differential IP activations, especially for motor attention. Motor attention has been defined as the preparation and redirection of movement, which involves the left inferior parietal lobe, especially in the supramarginal region. In particular, the event-related design may place greater demands on attentional processing and motor monitoring because any stimulus could follow any other stimulus. This may be especially the case for NWs which are more difficult to distinguish from targets than are FF. On the other hand, stimuli in the blocked design are much more predictable with a very low target to non-target ratio (i.e., 3 targets per block). Thus, lower attentional demands (and less anticipation of a motor response) in both conditions may be driving this task-related difference. In addition, the box-car method of analysis in blocked design models averages activation over a time course. If there are only a few instances of target words, the higher levels of activation caused by these stimuli may be washed out by the majority of lower activating stimuli in the block. However, it is also possible that the event-related design detected some other aspect of semantic or cognitive processing that was not captured by our blocked design. The overall weak activations within this ROI indicate that the IP activations are likely not core to language processing, but rather reflect some secondary cognitive process.
Interestingly, language activation profiles did not vary by sex, age, or level of language ability, nor were there any interactions between these variables and experimental design. A handful of studies have reported that females have a more bilateral pattern of language representation than males (e.g., Kulynych, Vladar, Jones, & Weinberger, 1994; Levy, 1969), which would result in lower laterality indices for females. However, a meta-analysis of fourteen fMRI studies that included 377 healthy males and 422 females was congruent with our findings and did not support a sex difference in language lateralization (Sommer, Aleman, Bouma, & Kahn, 2004). It has also been hypothesized that language laterality changes as a function of age. A recent study found that language lateralization to the dominant hemisphere increased between the ages 5 and 20 years, plateaus between 20 and 25 years, and then slowly declines from 25 and 70 years (Szaflarski, Holland, Schmithorst, & Byars, 2006). Our results do not support this pattern either in that we did not find language dominance to vary by age in a sample of individuals 19 to 72 years. However, it is of note that this inconsistency between our study and previous literature may be due to the relatively small sample size in our study and the matched language performance in the different age range. Finally, our results suggest that the degree of language lateralization does not differ between individuals with higher versus lower language performance, nor does it vary as a function of experimental design. In our study, individuals ranged from low average to superior on common neuropsychological measures of language (i.e., naming and verbal fluency). Thus, we captured a broad range of language abilities in our sample, reflecting much of the heterogeneity observed in pre-surgical populations. However, it is of note that these results may not translate well to patients with severe language deficits. In summary, language laterality within core perisylvian ROIs appears robust to not only task design, but a range of other important individual characteristics that could be important for preoperative planning.
Major advantages of our study include a much larger sample size than has been included in previous blocked versus event-related studies, with groups well-matched in key demographic variables (sex, age, education) and language performance. Contrary to Tie et al.’s (2009) study that used fixation as the control condition, we used a contrast condition that controlled for visual, but not lexical, syntactic, or sematic features (i.e., false fonts) and may have helped to increase the specificity of our blocked design. However, there are several limitations of this study that should be noted. First, different participants were used in the blocked versus event-related designs, resulting in a between-subject analysis. Thus, it is possible that there were important differences between the groups that were not accounted for in our participant matching. However, a within-subject design has its own limitations in that participants are exposed to both designs, resulting in practice effects when the same stimuli are used in each design. Second, there were slight differences between the blocked and event-related designs in both scan length and number of stimuli. However, we matched the tasks as closely as possible in order to conform to the optimal design for each task type. Third, although we selected a well-validated semantic judgement task and ROIs that have been previously used to map language functions in previous studies, it is possible that greater differences between our blocked and event-related designs would have emerged had we selected a different task (e.g., verb generation) or different ROIs.
5. Conclusions
Taken together, our results suggest that blocked and event-related designs show highly similar activation patterns across the cortical surface and yield comparable laterality indices in language regions critical to preoperative planning. We also demonstrate that these patterns and differences in task design are robust to sex, age and language ability. However, we also highlighted the importance of carefully considering other methodological factors between the two designs that could result in non-language related activations (e.g., attentional processing) that may confound some language-related ROIs.
Supplementary Material
Statement of Significance.
Despite high concordance between blocked and event-related designs within perisylvian regions that are robust to subject-specific factors, methodological differences between these two designs may contribute to the activations outside the core regions. These findings may aid researchers in future fMRI meta-analytic studies, as well as guide clinicians in pre-surgical planning.
Highlights.
Blocked and event-related designs generated comparable language network activation
Other cognitive process may emerge due to the differences between two fMRI designs
Sex, age, and language performance did not interact with the two fMRI designs
Acknowledgments
The authors thank Nobuko Kemmotsu and N. Erkut Kucukboyaci for assistance with fMRI task programming and data collection.
Funding: This work was supported by the National Institutes of Health [R01 NS065838 to C.R.M.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Language performance were measured by the four neuropsychological tasks of language, which was performed on 39 participants. Three participants did not perform all the neuropsychological tasks; two participants did not perform Boston Naming Test, and one participant did not perform Auditory Naming Test.
References
- Abdullaev YG, Melnichuk KV. Cognitive operations in the human caudate nucleus. Neuroscience Letters. 1997;234(2–3):151–155. doi: 10.1016/s0304-3940(97)00680-0. https://doi.org/10.1016/S0304-3940(97)00680-0. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. https://doi.org/10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Cox RW, Bandettini PA. Detection versus estimation in event-related fMRI: Choosing the optimal stimulus timing. NeuroImage. 2002;15(1):252–264. doi: 10.1006/nimg.2001.0964. https://doi.org/10.1006/nimg.2001.0964. [DOI] [PubMed] [Google Scholar]
- Booth JR, Wood L, Lu D, Houk JC, Bitan T. The role of the basal ganglia and cerebellum in language processing. Brain Research. 2007;1133:136–144. doi: 10.1016/j.brainres.2006.11.074. https://doi.org/10.1016/j.brainres.2006.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y-HA, Kemmotsu N, Leyden KM, Kucukboyaci NE, Iragui VJ, Tecoma ES, Kansal L, Norman MA, Compton R, Ehrlich TJ, Uttarwar VS, Reyes A, Paul BM, McDonald CR. Multimodal imaging of language reorganization in patients with left temporal lobe epilepsy. Brain and Language. 2017;170:82–92. doi: 10.1016/j.bandl.2017.03.012. https://doi.org/10.1016/j.bandl.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MWL, Venkatraman V, Westphal C, Siong SC. Comparison of block and event-related fMRI designs in evaluating the word-frequency effect. Human Brain Mapping. 2003;18(3):186–193. doi: 10.1002/hbm.10092. https://doi.org/10.1002/hbm.10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SHA, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. NeuroImage. 2005a;24(2):332–338. doi: 10.1016/j.neuroimage.2004.08.032. https://doi.org/10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Chen SHA, Desmond JE. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005b;43(9):1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. https://doi.org/10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Costafreda SG. Pooling fMRI data: Meta-analysis, mega-analysis and multi-center studies. Frontiers in Neuroinformatics. 2009;3 doi: 10.3389/neuro.11.033.2009. https://doi.org/10.3389/neuro.11.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. https://doi.org/10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. https://doi.org/10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. Journal of Cognitive Neuroscience. 1993;5(2):162–176. doi: 10.1162/jocn.1993.5.2.162. https://doi.org/10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends in Cognitive Sciences. 2011;15(6):254–262. doi: 10.1016/j.tics.2011.04.003. https://doi.org/10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system (D-KEFS) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Desmond JE, Sum JM, Wagner AD, Demb JB, Shear PK, Glover GH, Gabrieli JDE, Morrell MJ. Functional MRI measurement of language lateralization in Wada-tested patients. Brain. 1995;118(6):1411–1419. doi: 10.1093/brain/118.6.1411. https://doi.org/10.1093/brain/118.6.1411. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Zarahn E, Aguirre GK. Event-related functional MRI: Implications for cognitive psychology. Psychological Bulletin. 1999;125(1):155–164. doi: 10.1037/0033-2909.125.1.155. http://dx.doi.org/10.1037/0033-2909.125.1.155. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. https://doi.org/10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson DI, Buckner RL. Effective paradigm design. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An introduction to methods. Oxford: Oxford University Press; 2001. pp. 177–196. [Google Scholar]
- Francis W, Kucera H. Frequency analysis of English usage: Lexicon and grammar. Boston: Houghton Mifflin Company; 1982. [Google Scholar]
- Friston KJ, Zarahn E, Josephs O, Henson RNA, Dale AM. Stochastic designs in event-related fMRI. NeuroImage. 1999;10(5):607–619. doi: 10.1006/nimg.1999.0498. https://doi.org/10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT. Auditory and visual naming tests: Normative and patient data for accuracy, response time, and tip-of-the-tongue. Journal of the International Neuropsychological Society. 2003;9(3):479–489. doi: 10.1017/s135561770393013x. https://doi.org/10.1017/s135561770393013x. [DOI] [PubMed] [Google Scholar]
- Holland D, Kuperman JM, Dale AM. Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. NeuroImage. 2010;50(1):175–183. doi: 10.1016/j.neuroimage.2009.11.044. https://doi.org/10.1016/j.neuroimage.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- Kulynych JJ, Vladar K, Jones DW, Weinberger DR. Gender differences in the normal lateralization of the supratemporal cortex: MRI surface-rendering morphometry of Heschl’s gyrus and the planum temporale. Cerebral Cortex (New York, N.Y.:1991) 1994;4(2):107–118. doi: 10.1093/cercor/4.2.107. [DOI] [PubMed] [Google Scholar]
- Levy J. Possible basis for the evolution of lateral specialization of the human brain. Nature. 1969;224(5219):614–615. doi: 10.1038/224614a0. https://doi.org/10.1038/224614a0. [DOI] [PubMed] [Google Scholar]
- Liu TT, Frank LR, Wong EC, Buxton RB. Detection power, estimation efficiency, and predictability in event-related fMRI. NeuroImage. 2001;13(4):759–773. doi: 10.1006/nimg.2000.0728. https://doi.org/10.1006/nimg.2000.0728. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D, Iacoboni M, Schormann T, Amunts K, Palomero-Gallagher N, Geyer S, Parsons L, Narr K, Kabani N, Le Goualher G, Boomsma D, Cannon T, Kawashima R, Mazoyer B. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2001;356(1412):1293–1322. doi: 10.1098/rstb.2001.0915. https://doi.org/10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Thesen T, Carlson C, Blumberg M, Girard HM, Trongnetrpunya A, Sherfey J, Devinsky O, Kuzniecky R, Dolye WK, Cash SS, Leonard MK, Hagler DJ, Jr, Dale AM, Halgren E. Multimodal imaging of repetition priming: Using fMRI, MEG, and intracranial EEG to reveal spatiotemporal profiles of word processing. NeuroImage. 2010;53(2):707–717. doi: 10.1016/j.neuroimage.2010.06.069. https://doi.org/10.1016/j.neuroimage.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Thesen T, Hagler DJ, Jr, Carlson C, Devinksy O, Kuzniecky R, Barr W, Gharapetian L, Trongnetrpunya A, Dale AM, Halgren E. Distributed source modeling of language with magnetoencephalography: Application to patients with intractable epilepsy. Epilepsia. 2009;50(10):2256–2266. doi: 10.1111/j.1528-1167.2009.02172.x. https://doi.org/10.1111/j.1528-1167.2009.02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro A, Tettamanti M, Perani D, Donati C, Cappa SF, Fazio F. Syntax and the brain: Disentangling grammar by selective anomalies. NeuroImage. 2001;13(1):110–118. doi: 10.1006/nimg.2000.0668. https://doi.org/10.1006/nimg.2000.0668. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. https://doi.org/10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Krams M, Passingham RE. The attentional role of the left parietal cortex: The distinct lateralization and localization of motor attention in the human brain. Journal of Cognitive Neuroscience. 2001;13(5):698–710. doi: 10.1162/089892901750363244. https://doi.org/10.1162/089892901750363244. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Nixon PD, Renowden S, Wade DT, Passingham RE. The left parietal cortex and motor attention. Neuropsychologia. 1997;35(9):1261–1273. doi: 10.1016/s0028-3932(97)00050-x. https://doi.org/10.1016/S0028-3932(97)00050-X. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Reynolds RC. SUMA. NeuroImage. 2012;62(2):768–773. doi: 10.1016/j.neuroimage.2011.09.016. https://doi.org/10.1016/j.neuroimage.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovlund E, Fenstad GU. Should we always choose a nonparametric test when comparing two apparently nonnormal distributions? Journal of Clinical Epidemiology. 2001;54(1):86–92. doi: 10.1016/s0895-4356(00)00264-x. https://doi.org/10.1016/S0895-4356(00)00264-X. [DOI] [PubMed] [Google Scholar]
- Sommer IEC, Aleman A, Bouma A, Kahn RS. Do women really have more bilateral language representation than men? A meta-analysis of functional imaging studies. Brain. 2004;127(8):1845–1852. doi: 10.1093/brain/awh207. https://doi.org/10.1093/brain/awh207. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behavior Research Methods, Instruments, & Computers. 1999;31(1):137–149. doi: 10.3758/bf03207704. https://doi.org/10.3758/BF03207704. [DOI] [PubMed] [Google Scholar]
- Stippich C, Rapps N, Dreyhaupt J, Durst A, Kress B, Nennig E, Tronnier VM, Sartor K. Localizing and lateralizing language in patients with brain tumors: feasibility of routine preoperative functional MR imaging in 81 consecutive patients. Radiology. 2007;243(3):828–836. doi: 10.1148/radiol.2433060068. https://doi.org/10.1148/radiol.2433060068. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. An fMRI study of language lateralization in children and adults. Human Brain Mapping. 2006;27(3):202–212. doi: 10.1002/hbm.20177. https://doi.org/10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettamanti M, Moro A, Messa C, Moresco RM, Rizzo G, Carpinelli A, Matarrese M, Fazio F, Perani D. Basal ganglia and language: phonology modulates dopaminergic release. Neuroreport. 2005;16(4):397–401. doi: 10.1097/00001756-200503150-00018. [DOI] [PubMed] [Google Scholar]
- Thesen T, McDonald CR, Carlson C, Doyle W, Cash S, Sherfey J, Felsovalyi O, Girard H, Barr W, Devinsky O, Kuzniecky R, Halgren E. Sequential then interactive processing of letters and words in the left fusiform gyrus. Nature Communications. 2012;3:1284. doi: 10.1038/ncomms2220. https://doi.org/10.1038/ncomms2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie Y, Suarez RO, Whalen S, Radmanesh A, Norton IH, Golby AJ. Comparison of blocked and event-related fMRI designs for pre-surgical language mapping. NeuroImage. 2009;47(Suppl 2):T107–T115. doi: 10.1016/j.neuroimage.2008.11.020. https://doi.org/10.1016/j.neuroimage.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woermann FG, Jokeit H, Luerding R, Freitag H, Schulz R, Guertler S, Okujava M, Wolf P, Tuxhorn I, Ebner A. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61(5):699–701. doi: 10.1212/01.wnl.0000078815.03224.57. https://doi.org/10.1212/01.WNL.0000078815.03224.57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.