Abstract
Introduction
Chronic itch has been drawing much attention due to its clinical significance and the complexity of its mechanisms. To facilitate the development of anti-itch strategies, it is necessary to investigate the key players in itch sensation under chronic itch conditions. Several members of the Mrgpr family were identified as itch receptors that detect cutaneous pruritogens in primary sensory neurons. However, the role of Mrgprs in chronic itch conditions has not been well described.
Methods
Scratching behaviors of WT and Mrgpr-clusterΔ−/− mice were examined in dry skin model and contact dermatitis model to examine the role of Mrgpr genes in mediating chronic itch sensation. Scratching behaviors of the mice were also examined in allergic itch model. Real-time PCR were performed to examine the expression level of MrgprA3 and MrgprC11 under naïve and dry skin conditions. The MrgprA3+ itch-sensing fibers were labeled by tdTomato fluorescence in Mrgpra3GFP-Cre; ROSA26tdTomato mice, and the morphology and density of those fibers in the epidermis were analyzed under dry skin condition.
Results
We showed that deleting a cluster of Mrgpr genes in mice reduced scratching behavior severely under two chronic itch conditions, namely dry skin and contact dermatitis, and the allergic itch condition. Moreover, the gene expressions of itch receptors MrgprA3 and MrgprC11 in dorsal root ganglia (DRG) were upregulated significantly under dry skin condition. Consistently, the percentage of MrgprA3+ itch-sensing neurons was increased as well. We also observed hyperinnervation of MrgprA3+ itch-sensing fibers in the epidermis of the skin under dry skin condition.
Discussion
We demonstrate that Mrgprs play important roles in mediating chronic itch and allergic itch. These findings enrich our knowledge of itch mechanism and may lead to the development of novel therapeutic approach to combat itch.
Keywords: Chronic itch, MrgprA3, MrgprC11, skin
Introduction
Chronic itch accompanying cutaneous disease or systemic disorders is a serious condition [1–3]. In older adults, xerosis (a.k.a. dry skin), a common skin problem, usually causes pruritus, and the scratching response results in a high risk of secondary infection in skin [4]. Patients with advanced renal disease and liver disease frequently report pruritus as a common symptom [2, 3]. The persistent itching significantly affects patients’ quality of life [5–7]. Despite its clinical importance, the molecular and cellular mechanisms of chronic itch are largely unknown.
Recent studies have identified several members of the Mrgpr (mas-related G-protein coupled receptor) family as new itch receptors [8–12]. The Mrgpr family consists of more than 20 functional members that are grouped into several subfamilies: MrgprA1-22, MrgprB1-13, MrgprC1-14, and MrgprD-G. Among them, MrgprA3, MrgprC11, and MrgprD have been identified as itch receptors that are specifically expressed in small-diameter sensory neurons and directly detect pruritogens in the skin [9, 11, 12]. MrgprA3 is the receptor for chloroquine, a drug used to treat malaria and generates itch as a side effect. MrgprC11 is the receptor for pruritogen bovine adrenal medulla 8–22 peptide (BAM8–22) and SLIGRL. β-alanine, a muscle building supplement that frequently elicits itch in human, acts through MrgprD. Moreover, the expression of MrgprA3 defines a subset of nociceptors that specifically mediate itch, but not pain [13]. These neurons express multiple itch receptors including MrgprA3, MrgprC11, and histamine receptor 1, and correspondingly respond to multiple pruritogens. Deletion of MrgprA3+ sensory neurons significantly inhibits itch behavior not only in acute itch conditions [14], but also in chronic itch conditions [13, 15], demonstrating that this neuronal population is crucial for mediating itch sensation. However, whether the chronic itch response requires the activation of Mrgprs in MrgprA3+ neurons is unknown.
Here we show that the scratching behaviors evoked under two chronic itch conditions, namely dry skin and contact dermatitis, were significantly suppressed in Mrgprs-deficient mice. We also found that allergen-induced scratching behaviors were reduced after the deletion of Mrgprs. Moreover, dry skin chronic itch condition increased the expression of itch receptors MrgprA3 and MrgprC11. We observed for the first time that dry skin condition induced the hyperinnervation of MrgprA3+ itch-sensing fibers in the epidermis. Taken together, we demonstrate that Mrgprs play a critical role in mediating chronic itch and allergic itch.
Experimental Procedures
Animals
All experiments were performed with approval from the Georgia Institute of Technology Animal Use and Care Committee. The generations of Mrgpr-clusterΔ−/− mice and Mrgpra3GFP-Cre mice were described previously [12, 13]. ROSA26tdTomato mouse line was purchased from Jackson Laboratory. All the mice used had been backcrossed to C57BL/6 mice for at least ten generations. 2- to 3-month old males (20 – 30g) were used for all experiments. Mice were housed in the vivarium with 12-hr-light/dark cycle, and all the behavioral tests were performed from 9 a.m. to 1 p.m. in the light cycle. The housing group was 5 at maximum with food and water ad libitum.
Scratch assay
All procedures were performed using protocols approved by the Animal Care and Use Committee of Georgia Institute of Technology. The experiments were performed as previously described [13]. Briefly, the day before the scratch assay, all the mice were acclimated for at least 30 min to their testing environment. During scratch assay, mice were kept in plastic cylinders individually and the behavioral responses were video recorded for 30–60 min. The video recording was subsequently played back in a slow motion and the number of bouts of scratching with the hindpaw were counted. All behavioral tests were performed by an experimenter blind to the genotypes of the mice.
Three itch conditions
Dry skin pruritus [16]
The shaved back skin of a mouse was treated with an acetone/ether (1:1) mixture for 15s followed by distilled water (AEW) twice daily for 6 days to generate skin dehydration. On day 7, mice were recorded for 60 minutes to quantify the spontaneous scratching.
Contact dermatitis [17]
A mouse model of contact dermatitis is produced by treating the skin with an allergen squaric acid dibutylester (SADBE, 25ul, 0.5% in acetone). On day 1–3, SADBE was applied to the shaved abdomen once a day to initiate T lymphocyte sensitization. On day 8–10, SADBE was applied to the shaved back skin to induce dermal inflammation and spontaneous scratching. On day 12, the spontaneous scratching behavior was recorded for 60 minutes.
Allergic itch [18]
50 μg of ovalbumin dissolved in phosphate-buffered saline (PBS) was administered intraperitoneally together with 2 mg of Inject Alum twice at a 10-day interval. One week after the second sensitization, 50 μg of ovalbumin was injected subcutaneously into the nape of a mouse’ neck to induce acute allergic itch. The scratching behavior was recorded for 30 minutes.
Real-time RT-PCR
Dorsal root ganglia (DRG) innervating the dry skin treated area (C3-T10) were collected from the trunk region of mice and total RNA was extracted from the ganglia using the RNeasy Micro Kits (Qiagen) according to the manufacturer’s protocol. RNA was reverse-transcribed into cDNA using SuperScript® III First-Strand Synthesis System (Invitrogen). Quantitative real-time PCR was performed using a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific) with the SYBR Green detection method. The cycle threshold (CT) values were analyzed by the 2−ΔCt method to determine the normalized expression ratio of target genes. β-actin expression level was used as the internal control. The intron-spanning primers used are listed here: β-actin-F, AGCTGTGCTATGTTGCTCTAG, β-actin-R, AGGTCTTTACGGATGTCAACG, MrgprA3-F, ACACAAGCCAGCAAGCTACA, MrgprA3-R, ACTTCCAGGGATGGTTTCGT, MrgprC11-F, AGCATCCACAACCCCAGAAG, MrgprC11-R, TGGAGTGCAGTTGGGATGAC. TRPV1-F, ATCATCAACGAGGACCCAGG. TRPV1-R, TGCTATGCCTATCTCGAGTGC.
Quantification of itch-sensing neurons and fibers in the skin
After 6 days dry skin treatment, MrgprA3GFP-Cre; ROSA26tdTomato mice were anesthetized with pentobarbital and perfused with PBS (pH 7.4, 4ºC) followed with 4% paraformaldehyde (4ºC). DRG innervating the treated skin (C3-T10) were dissected from the perfused mice. DRG were postfixed in 4% paraformaldehyde at 4ºC for 30 minutes. Treated skin were dissected and fixed in 2% paraformaldehyde at 4 ºC overnight. All tissues were cryoprotected in 20% sucrose in PBS at 4 ºC for 24 hours, and frozen in OCT at −80 ºC. Tissues were sectioned at 20 μm with a Cryostar NX50 Cryostat (Thermo Fisher scientific). The sections on slides were dried at 37 ºC for 30 minutes, and fixed with 4% paraformaldehyde at room temperature for 10 minutes. The slides were washed with PBS, and the fluorescence labeled neurons or skin fibers were visualized and quantified under fluorescent microscope. Totally 3000 to 8000 sensory neurons from each mouse were counted to quantify the percentage of tdTomato labeled MrgprA3+ neurons. Cell nuclei in the skin sections were stained with DAPI to visualize the epidermis. 10–22 skin sections were analyzed to quantify the intraepidermal density of tdTomato labeled MrgprA3+ skin fibers. n = 3 for both naïve group and dry skin treated group.
Histological analysis
The treated skin were collected and fixed in 10% neutralized formalin for more than 24 hr. The skin samples were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E). The H&E stained images were taken using Nikon Ni-U microscopy and DS-Fi2 color camera. At least 10 sections per sample were analyzed. The area of each section to measure epidermal thickness was randomly picked. The skin thickness and epidermal thickness were analyzed using Image J.
Statistical analysis
Data are presented as means ± S.E.M. Statistical analysis was performed using Student’s two-tailed t-test to compare between groups. P-value less than 0.05 was considered statistically significant.
Results
Mrgprs mediate chronic itch and allergic itch
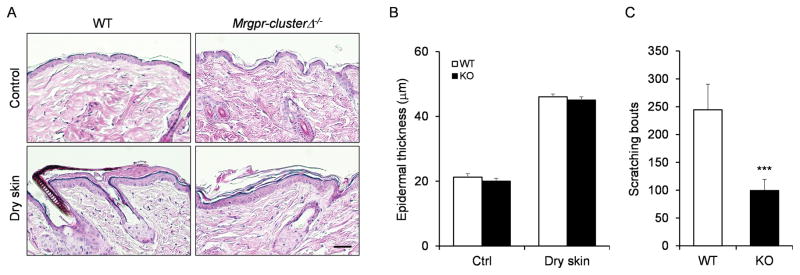
A straightforward way to determine the role of Mrgprs in chronic itch is to delete Mrgpr genes and see whether scratching behavior is affected. Since many Mrgpr genes in mice are clustered together, to avoid compensatory effects, Mrgpr-clusterΔ−/− mouse has been generated previously in which 12 Mrgpr genes including two identified itch receptors, MrgprA3 and MrgprC11, have been deleted in the mouse genome [12]. We compared the scratching behavior of wild-type and Mrgpr-clusterΔ−/− mice under two different chronic itch conditions: dry skin [16] and contact dermatitis [17]. Skin dehydration is always accompanied by persistent itchiness of the skin, especially in the elderly [4]. In the mouse model, the dry skin condition was generated by repetitive exposure of the skin to the mixture of acetone and ether [16]. The treatment induced pronounced epidermal hyperplasia and parakerotosis, but did not induce infiltration of the inflammatory cells [16]. Histological analysis demonstrated that the treated skin collected from WT and Mrgpr-clusterΔ−/− mice exhibited similar morphological changes (Fig. 1A) and comparable increase of the epidermal thickness (Fig. 1B). These results suggest that the deletion of Mrgpr genes did not affect the development of the dry skin model. Excitingly, Mrgpr-clusterΔ−/− mice exhibited significant decrease in scratching behavior compared to WT mice (Fig. 1C), suggesting that the activation of Mrgpr genes are required for dry skin itch.
Figure 1.
Mrgpr genes are required for dry skin itch. (A) Hematoxylin and eosin (H&E) staining of the skin sections collected from WT and Mrgprs clusterΔ−/− mice after dry skin treatment. (B) Dry skin treatment induced comparable increase of the epidermal thickness in WT and Mrgprs clusterΔ−/− mice (n ≥ 10 sections from 3–5 mice per group). (C) Mrgprs clusterΔ−/− mice exhibited reduced scratching behavior under dry skin condition. n ≥ 5 for each group. ***P < 0.005. Two tailed unpaired Student’s t test. Error bars represent s.e.m. Scale bar = 100 μm.
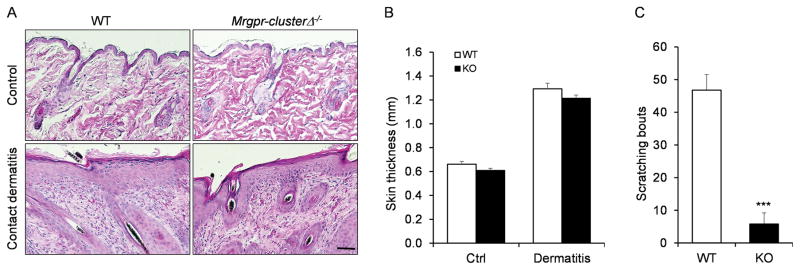
Contact dermatitis is a condition of skin inflammation produced by unwanted contact with irritant or allergic chemicals. The mouse model of contact dermatitis is generated by treating the skin with the allergen squaric acid dibutylester (SADBE). The treatment generated acanthosis (thickening of the skin), parakeratosis and dense inflammatory cells infiltration in both WT and Mrgpr-clusterΔ−/− mice (Fig. 2A). The skin thickness is comparable between the two groups after the treatment (Fig. 2B). However, Mrgpr-clusterΔ−/− mice showed a severe reduction in scratching behavior compare to WT mice suggesting that Mrgpr genes mediate itch sensation in the contact dermatitis model (Fig. 2C).
Figure 2.
Mrgpr genes are required for itch sensation in contact dermatitis model. (A) H&E stained skin sections collected from WT and Mrgprs clusterΔ−/− mice after contact dermatitis treatment. (B) WT and Mrgprs clusterΔ−/− mice showed similar increase of skin thickness after contact dermatitis treatment (n ≥ 10 sections from 3–5 mice per group). (D) Mrgprs clusterΔ−/− mice exhibited reduced scratching behavior in contact dermatitis model. n ≥ 5 for each group. ***P < 0.005. Two tailed unpaired Student’s t test. Error bars represent s.e.m. Scale bar = 100 μm.
We next examined whether Mrgprs are involved in acute allergen-induced itch sensation. In the allergic itch mouse model, the allergen ovalbumin was administered intraperitoneally twice, 10 days apart, to sensitize the mice. Injection of ovalbumin subcutaneously one week after the second sensitization induced acute allergic responses and scratching behavior directed to the injected site. We found that Mrgpr-clusterΔ−/− mice exhibited significantly less scratching behavior compared to WT littermates (Fig. 3) under the allergic itch condition. In summary, our data showed that Mrgprs play a critical role in mediating the itch sensation in three tested itch conditions.
Figure 3.
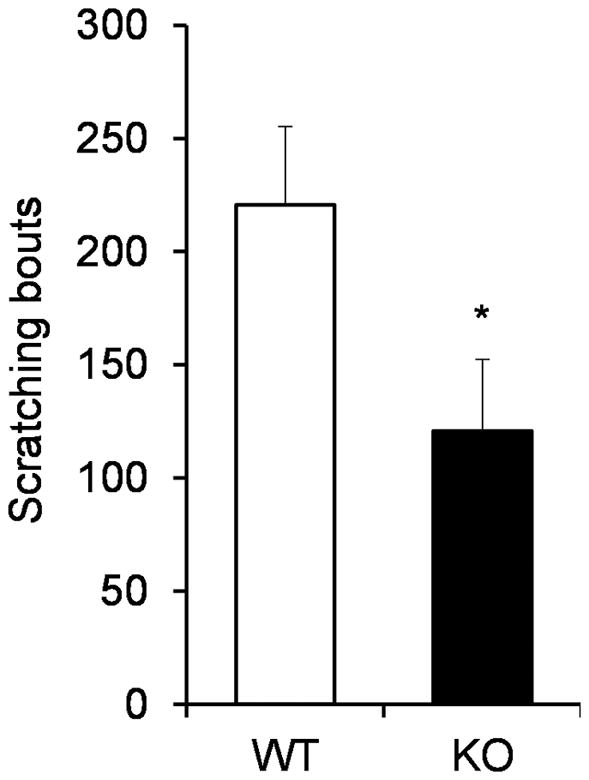
Mrgprs clusterΔ−/− mice exhibited reduced allergic itch behavior. n ≥ 5 for each group. *P < 0.05. Two tailed unpaired Student’s t test. Error bars represent s.e.m.
Dry skin condition upregulates the expression of Mrgprs
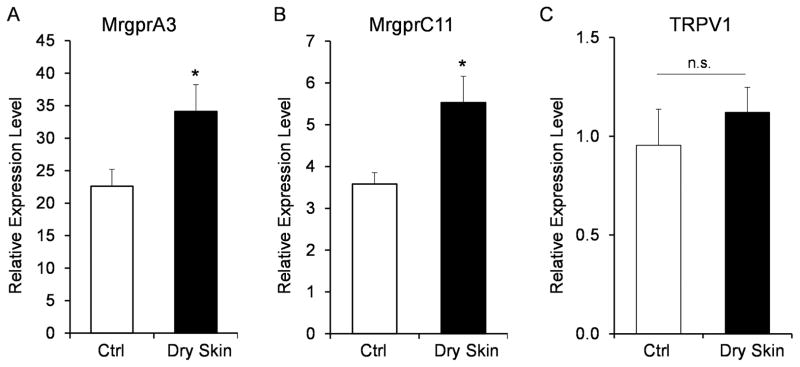
MrgprA3 and MrgprC11, two previously identified itch receptors, were among the 12 genes that were deleted in Mrgpr-clusterΔ−/− mice. Both of them are specifically expressed in the small-diameter sensory neurons [8, 12, 13]. MrgprA3 gene expression has been shown to be upregulated in sensory neurons in the dry skin model [19]. The percentage of sensory neurons responding to Chloroquine and SLIGRL were increased in the dry skin model [20, 21]. Consistently, we found that the mRNA levels of MrgprA3 and MrgprC11 were significantly increased after the dry skin treatment (Fig. 4A–B). In the contrary, the expression of TRPV1, a sensor for noxious heat and pain sensation [22, 23], was not changed in the dry skin model.
Figure 4.
Dry skin condition upregulates the expression of Mrgpr genes. Real-time PCR results showing that the expression of MrgprA3 (A) and MrgprC11 (B) were upregulated in the DRG under the dry skin condition. The expression of TRPV1 was not changed (C). n = 3 for MrgprA3 and TRPV1 and n = 6 for MrgprC11. *P < 0.05. Two tailed unpaired Student’s t test. Error bars represent s.e.m.
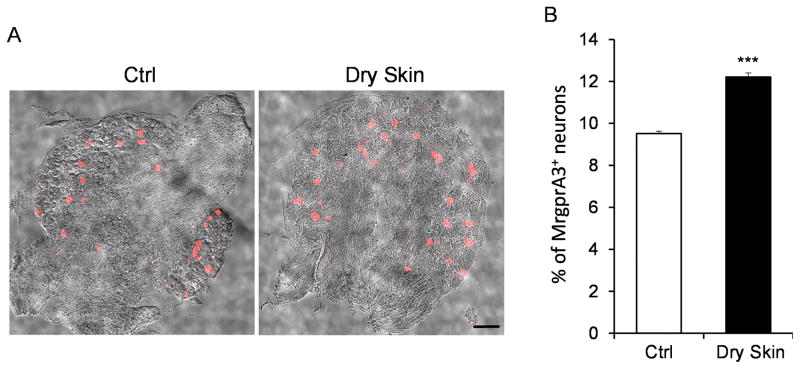
Next, we examined whether the percentage of MrgprA3+ neurons, the previously identified specific itch-sensing neurons [13], increased after the dry skin treatment. Our previous study generated a BAC transgenic mouse line Mrgpra3GFP-Cre to express GFP-Cre fusion protein in MrgprA3+ neurons [13]. MrgprA3+ neurons were labeled with tdTomato fluorescence by crossing the Mrgpra3GFP-Cre line with a Cre-dependent ROSA26tdTomato line (Fig. 5A). We collected the DRG sections from the Mrgpra3GFP-Cre; ROSA26tdTomato mice with or without dry skin treatment, and found that the percentage of tdTomato labeled MrgprA3+ neurons from dry skin treated mice was significantly higher than that from control animals (Fig. 5B). Collectively, our data demonstrated that the dry skin condition enhanced the expression of MrgprA3 and MrgprC11 and expanded the expression of MrgprA3 into a broader sensory neuron population.
Figure 5.
Dry skin condition increases the percentage of MrgprA3+ itch-sensing neurons in the DRG. (A) DRG sections of Mrgpra3GFP-Cre; ROSA26tdTomato mice showing the MrgprA3+ neurons labeled by tdTomato fluorescence under control or dry skin condition. tdTomato fluorescent images were merged with phase contrast images to show the morphology of the DRG sections. (B) The percentage of MrgprA3+ neurons in the DRG under control or dry skin condition. n = 3 for each experiment. ***P < 0.005. Two tailed unpaired Student’s t test. Error bars represent s.e.m. Scale bar = 100 μm.
Dry skin condition induces hyperinnervation of MrgprA3+ itch-sensing fibers in the epidermis
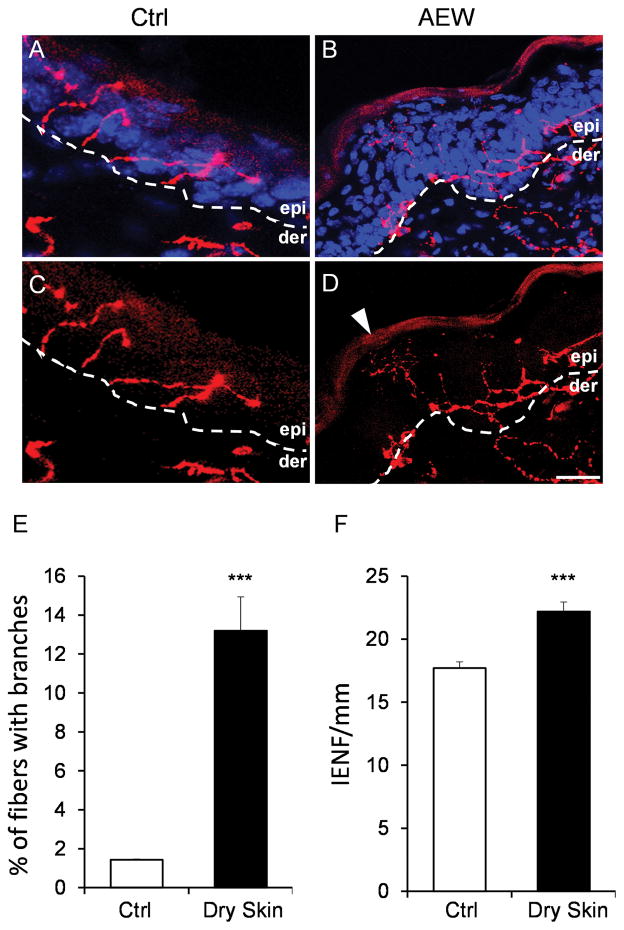
Pruritic skin is often accompanied by alteration of the sensory innervations in the skin. Skin biopsies from atopic dermatitis patients showed enhanced intraepidermal fiber density labeled by PGP9.5 [24, 25]. Dry skin pruritus has been shown to be associated with an increase of Ret-positive intraepidermal fiber density [20]. MrgprA3+ sensory neurons, which constitute about 8% of sensory neurons and exclusively innervate the epidermis of the skin, are required for mediating itch sensation induced by multiple pruritogens [13]. Whether and how the MrgprA3+ itch-sensing fibers are changed in the skin by the chronic itch conditions have not yet been investigated. With the tdTomato fluorescence labeling in the neuron, we were able to visualize the periphery projection of MrgprA3+ itch sensing fibers in the skin (Fig. 6A–D). Epidermis in the skin sections was identified by the morphology of the keratinocytes labeled by DAPI. Consistent with previous results, MrgprA3+ fibers were free nerve endings in the epidermis and terminated in the stratum granulosum, the superficial layer of the epidermis [13] (Fig. 6A–D). Epidermal hyperplasia induced by the dry skin treatment can be observed in dry skin sections but not in control sections (Fig. 6B, D). We found that the morphology of MrgprA3+ fibers was different under the chronic itch condition compared to the naïve condition. The fibers from the naïve condition normally penetrated the epidermis to reach the stratum granulosum and did not show fiber branches in the epidermis. However, about 13% of the MrgprA3+ fibers under chronic itch condition generated branches in the epidermis (Fig. 6A–E). Moreover, MrgprA3+ intraepidermal fiber density increased significantly under dry skin conditions (Fig. 6F). These data collectively demonstrate that the dry skin condition induced hyperinnervation of the MrgprA3+ itch sensing fibers in the epidermis.
Figure 6.
Dry skin condition induces hyperinnervation of itch-sensing fibers in the epidermis. (A–D) Skin sections of Mrgpra3GFP-Cre; ROSA26tdTomato mice with (A–B) and without (C–D) DAPI staining showing the MrgprA3+ fibers labeled by tdTomato fluorescence in the epidermis. The dashed line labels the boundary between dermis and epidermis. Arrow point to a branched fiber in the epidemis of a dry skin section. (E) The percentage of MrgprA3+ fibers with branches in the epidermis under control and dry skin conditions. (F) The MrgprA3+ intraepidermal nerve fiber (IENF) density under control and dry skin conditions. n = 3 for each experiment. ***P < 0.005. Two tailed unpaired Student’s t test. Error bars represent s.e.m. Scale bar = 20 μm.
Discussion
Itch sensation is initiated by the activation of sensory nerves in the skin. Itch receptors expressed in the nerve endings are responsible for first sensing the pruritogens. Among all the itch receptors, Mrgprs are relatively new but play a critical role in itch sensation. In this study, we showed that deleting 12 Mrgpr genes in mice suppressed scratching behavior in two chronic itch conditions and allergic itch condition. We further demonstrate that dry skin chronic itch condition triggered the active expression of itch receptors MrgprA3 and MrgprC11 in the DRG sensory neurons and the morphological expansion of itch-sensing nerves in the skin. Our data highlight the critical role of Mrgprs in mediating chronic itch and allergic itch.
Clinically, itch is categorized into 4 groups: dermatologic, systemic, neurologic, and psychiatric [26]. Currently, only a handful of mouse models for itch conditions have been established [14]. We have employed three representative dermatologic mouse itch models in this study, including a non-allergic chronic itch model (AEW-dry skin), an allergic chronic itch model (SADBE-contact dermatitis) and an allergic acute itch model (OVA-allergic itch). These three models involve distinct signaling pathways. Dry skin treatment decreases stratum corneum hydration, induced epidermal hyperplasia and parakeratosis, but does not induce skin inflammation [16] (Fig. 1A–B). SADBE-contact dermatitis model represents the allergic skin inflammation condition. The challenged skin is infiltrated with inflammatory cells and soaked in an inflammatory soup of cytokines and chemokines [27]. OVA-allergic itch model evokes IgE-dependent mast cell mediated acute allergic response. OVA challenge induces the secretion of a variety of mast cell mediators in the skin to evoke cutaneous anaphylactic symptoms including itch sensation [28, 29]. It is intriguing that our data suggest that Mrgprs can be activated in three different models. Since 12 Mrgpr genes were deleted in the Mrgpr-clusterΔ−/− mice, it is possible that different Mrgpr genes mediate itch sensation in different conditions. Alternatively, a certain Mrgpr gene may be activated by different pruritogens produced in different conditions. For example, MrgprC11 has multiple identified agonists such as NPFF, Cathepsin S, and Der p1 [12, 30–32], all of which can be good candidates for evoking itch. NPFF (Neuropeptide FF) is a neuropeptide that can be released from mouse bone-marrow derived mast cells [30]. It is possible that NPFF is released by the skin mast cells in the OVA-allergic itch condition and activate MrgprC11+ nerves. Cathepsin S is associated with inflammatory processes and chronic itch conditions. The expression of cathepsin S is upregulated in psoriatic keratinocytes [33]. Mice carrying a transgene expressing cathepsin S spontaneously develop atopic dermatitis [34]. Der p1 is a prominent allergen from house dust mite and has been involved in the allergic inflammatory processes in both skin [35, 36] and the respiratory system [37]. It will be interesting to examine whether cathepsin S and Der p1 are endogenous agonists for MrgprC11 under the itch conditions we tested.
Patients with chronic itch often display heightened sensitivity to itchy stimuli, which can be partly attributed to peripheral sensitization of sensory neurons [38–41]. MrgprA3+ sensory neurons are a major sensory neuron population mediating itch sensation [13]. Consistently, MrgprA3+ neurons exhibit enhanced excitability in a mouse model of inflammatory itch [15]. Here, we employed the dry skin chronic itch model to examine how chronic itch condition change the MrgprA3+ itch-sensing neurons. We showed that the dry skin treatment increased the gene expression of MrgprA3 and MrgprC11, and increased the percentage of MrgprA3+ neurons in DRG (Fig. 2). The dry skin treatment mainly generates skin barrier disruption and stratum corneum dehydration. It is very likely that the mediators secreted by keratinocytes during the treatment promote upregulation of itch receptors. In addition, neuronal activity-dependent transcription regulation is a common mechanism employed by many neuron types including DRG sensory neurons for proper development and adaptation during adulthood [42, 43]. It is also possible that the continuous activation of MrgprA3+ itch sensing neurons under dry skin condition induced the activity-dependent transcription of itch receptors. These hypotheses need to be tested with further studies.
We have analyzed the morphology and density of itch-sensing nerve fibers in the epidermis under dry skin condition. MrgprA3+ itch-sensing neurons only constitute a small percentage of DRG sensory neurons. Their intraepidermal nerve density is high enough to sense the potential stimuli in every spot in the skin, but not too high to prevent the clear visualization and quantification of the nerve fibers and the fiber branches. Using Mrgpra3GFP-Cre; ROSA26tdTomato mouse, we were able to observe the hyperinnervation of the MrgprA3+ itch-sensing fibers under dry skin conditions (Fig. 6). Notably, this is the first time that the morphological changes of MrgprA3+ itch specific fibers under chronic itch condition have been observed. Both upregulation of itch receptors and hyperinnervation of itch-sensing fibers might contribute to the hypersensitivity of itchy skin to pruritogens.
Taken together, our data suggest that Mrgprs play an important role in mediating itch sensation in multiple conditions. Human MrgprX1 is the functional receptor for both CQ and Bam8–22 and is the functional orthologue for MrgprA3 and MrgprC11 [12, 44]. Further studies are required to investigate the involvement of hMrgprX1 in chronic itch conditions in human patients and to test the possibility to antagonize hMrgprX1 for anti-itch treatment.
Acknowledgments
Assistance with the study: we would like to thank Dr. Xinzhong Dong for his comments on the manuscript.
Financial support and sponsorship: the research was supported by NINDS R00NS08708 and Georgia Institute of Technology Startup fund.
Conflicts of interest: none.
Presentations: the data were presented at the 7th World Congress on Itch meeting.
Abbreviations
- CQ
chloroquine
- DRG
dorsal root ganglia
- IENF
intraepidermal nerve fiber
- Mrgprs
mas-related G-protein coupled receptors
- SADBE
squaric acid dibutylester
References
- 1.Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625–1634. doi: 10.1056/NEJMcp1208814. [DOI] [PubMed] [Google Scholar]
- 2.Bolier AR, Peri S, Oude Elferink RP, Beuers U. The challenge of cholestatic pruritus. Acta Gastroenterol Belg. 2012;75:399–404. [PubMed] [Google Scholar]
- 3.Kfoury LW, Jurdi MA. Uremic pruritus. J Nephrol. 2012;25:644–652. doi: 10.5301/jn.5000039. [DOI] [PubMed] [Google Scholar]
- 4.White-Chu EF, Reddy M. Dry skin in the elderly: complexities of a common problem. Clin Dermatol. 2011;29:37–42. doi: 10.1016/j.clindermatol.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto Y, Yamazaki S, Hayashino Y, Takahashi O, Tokuda Y, Shimbo T, Fukui T, Hinohara S, Miyachi Y, Fukuhara S. Association between frequency of pruritic symptoms and perceived psychological stress: a Japanese population-based study. Arch Dermatol. 2009;145:1384–1388. doi: 10.1001/archdermatol.2009.290. [DOI] [PubMed] [Google Scholar]
- 6.Dalgard F, Stern R, Lien L, Hauser S. Itch, stress and self-efficacy among 18-year-old boys and girls: a Norwegian population-based cross-sectional study. Acta Derm Venereol. 2012;92:547–552. doi: 10.2340/00015555-1309. [DOI] [PubMed] [Google Scholar]
- 7.Kini SP, DeLong LK, Veledar E, McKenzie-Brown AM, Schaufele M, Chen SC. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147:1153–1156. doi: 10.1001/archdermatol.2011.178. [DOI] [PubMed] [Google Scholar]
- 8.Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte RH, Dong X. Mechanisms of itch evoked by beta-alanine. J Neurosci. 2012;32:14532–14537. doi: 10.1523/JNEUROSCI.3509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sikand P, Dong X, LaMotte RH. BAM8–22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J Neurosci. 2011;31:7563–7567. doi: 10.1523/JNEUROSCI.1192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q, Weng HJ, Patel KN, Tang Z, Bai H, Steinhoff M, Dong X. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci Signal. 2011;4:ra45. doi: 10.1126/scisignal.2001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han L, Dong X. Itch mechanisms and circuits. Annu Rev Biophys. 2014;43:331–355. doi: 10.1146/annurev-biophys-051013-022826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qu L, Fan N, Ma C, Wang T, Han L, Fu K, Wang Y, Shimada SG, Dong X, LaMotte RH. Enhanced excitability of MRGPRA3- and MRGPRD-positive nociceptors in a model of inflammatory itch and pain. Brain. 2014;137:1039–1050. doi: 10.1093/brain/awu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyamoto T, Nojima H, Shinkado T, Nakahashi T, Kuraishi Y. Itch-associated response induced by experimental dry skin in mice. Jpn J Pharmacol. 2002;88:285–292. doi: 10.1254/jjp.88.285. [DOI] [PubMed] [Google Scholar]
- 17.Scott AE, Kashon ML, Yucesoy B, Luster MI, Tinkle SS. Insights into the quantitative relationship between sensitization and challenge for allergic contact dermatitis reactions. Toxicol Appl Pharmacol. 2002;183:66–70. doi: 10.1006/taap.2002.9469. [DOI] [PubMed] [Google Scholar]
- 18.Patel KN, Liu Q, Meeker S, Undem BJ, Dong X. Pirt, a TRPV1 modulator, is required for histamine-dependent and -independent itch. PLoS One. 2011;6:e20559. doi: 10.1371/journal.pone.0020559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, Lumpkin EA, Bautista DM. The ion channel TRPA1 is required for chronic itch. J Neurosci. 2013;33:9283–9294. doi: 10.1523/JNEUROSCI.5318-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valtcheva MV, Samineni VK, Golden JP, Gereau RWt, Davidson S. Enhanced nonpeptidergic intraepidermal fiber density and an expanded subset of chloroquine-responsive trigeminal neurons in a mouse model of dry skin itch. J Pain. 2015;16:346–356. doi: 10.1016/j.jpain.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010;151:378–383. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 23.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 24.Foster EL, Simpson EL, Fredrikson LJ, Lee JJ, Lee NA, Fryer AD, Jacoby DB. Eosinophils increase neuron branching in human and murine skin and in vitro. PLoS One. 2011;6:e22029. doi: 10.1371/journal.pone.0022029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tobin D, Nabarro G, Baart de la Faille H, van Vloten WA, van der Putte SC, Schuurman HJ. Increased number of immunoreactive nerve fibers in atopic dermatitis. J Allergy Clin Immunol. 1992;90:613–622. doi: 10.1016/0091-6749(92)90134-n. [DOI] [PubMed] [Google Scholar]
- 26.Stander S, Weisshaar E, Mettang T, Szepietowski JC, Carstens E, Ikoma A, Bergasa NV, Gieler U, Misery L, Wallengren J, Darsow U, Streit M, Metze D, Luger TA, Greaves MW, Schmelz M, Yosipovitch G, Bernhard JD. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007;87:291–294. doi: 10.2340/00015555-0305. [DOI] [PubMed] [Google Scholar]
- 27.Christensen AD, Haase C. Immunological mechanisms of contact hypersensitivity in mice. APMIS. 2012;120:1–27. doi: 10.1111/j.1600-0463.2011.02832.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang LF, Lin JY, Hsieh KH, Lin RH. Epicutaneous exposure of protein antigen induces a predominant Th2-like response with high IgE production in mice. J Immunol. 1996;156:4077–4082. [PubMed] [Google Scholar]
- 29.Yatsuzuka R, Inoue T, Jiang S, Nakano Y, Kamei C. Development of new atopic dermatitis models characterized by not only itching but also inflammatory skin in mice. Eur J Pharmacol. 2007;565:225–231. doi: 10.1016/j.ejphar.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 30.Lee MG, Dong X, Liu Q, Patel KN, Choi OH, Vonakis B, Undem BJ. Agonists of the MAS-related gene (Mrgs) orphan receptors as novel mediators of mast cell-sensory nerve interactions. J Immunol. 2008;180:2251–2255. doi: 10.4049/jimmunol.180.4.2251. [DOI] [PubMed] [Google Scholar]
- 31.Reddy VB, Sun S, Azimi E, Elmariah SB, Dong X, Lerner EA. Redefining the concept of protease-activated receptors: cathepsin S evokes itch via activation of Mrgprs. Nat Commun. 2015;6:7864. doi: 10.1038/ncomms8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy VB, Lerner EA. Activation of mas-related G-protein coupled receptors by the house dust mite cysteine protease Der p1 provides a new mechanism linking allergy and inflammation. J Biol Chem. 2017 doi: 10.1074/jbc.M117.787887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schonefuss A, Wendt W, Schattling B, Schulten R, Hoffmann K, Stuecker M, Tigges C, Lubbert H, Stichel C. Upregulation of cathepsin S in psoriatic keratinocytes. Exp Dermatol. 2010;19:e80–88. doi: 10.1111/j.1600-0625.2009.00990.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim N, Bae KB, Kim MO, Yu DH, Kim HJ, Yuh HS, Ji YR, Park SJ, Kim S, Son KH, Park SJ, Yoon D, Lee DS, Lee S, Lee HS, Kim TY, Ryoo ZY. Overexpression of cathepsin S induces chronic atopic dermatitis in mice. J Invest Dermatol. 2012;132:1169–1176. doi: 10.1038/jid.2011.404. [DOI] [PubMed] [Google Scholar]
- 35.Roesner LM, Heratizadeh A, Begemann G, Kienlin P, Hradetzky S, Niebuhr M, Eiz-Vesper B, Hennig C, Hansen G, Baron-Bodo V, Moingeon P, Werfel T. Der p1 and Der p2-Specific T Cells Display a Th2, Th17, and Th2/Th17 Phenotype in Atopic Dermatitis. J Invest Dermatol. 2015;135:2324–2327. doi: 10.1038/jid.2015.162. [DOI] [PubMed] [Google Scholar]
- 36.Baris S, Ozen A, Akdeniz T, Karakoc-Aydiner E, Aydin O, Ercan H, Ogulur I, Camcioglu Y, Cengizlier R, Demirkesen C, Yucelten D, Demirel G, Barlan IB. House Dust Mites Confer a Distinct Immunological Feature among Dermatitis. Iran J Allergy Asthma Immunol. 2016;15:264–274. [PubMed] [Google Scholar]
- 37.Kauffman HF, Tamm M, Timmerman JA, Borger P. House dust mite major allergens Der p 1 and Der p 5 activate human airway-derived epithelial cells by protease-dependent and protease-independent mechanisms. Clin Mol Allergy. 2006;4:5. doi: 10.1186/1476-7961-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Laarhoven AI, Kraaimaat FW, Wilder-Smith OH, van Riel PL, van de Kerkhof PC, Evers AW. Sensitivity to itch and pain in patients with psoriasis and rheumatoid arthritis. Exp Dermatol. 2013;22:530–534. doi: 10.1111/exd.12189. [DOI] [PubMed] [Google Scholar]
- 39.Ozawa M, Tsuchiyama K, Gomi R, Kurosaki F, Kawamoto Y, Aiba S. Neuroselective transcutaneous electrical stimulation reveals neuronal sensitization in atopic dermatitis. J Am Acad Dermatol. 2009;60:609–614. doi: 10.1016/j.jaad.2008.11.900. [DOI] [PubMed] [Google Scholar]
- 40.Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain. 2006;126:16–23. doi: 10.1016/j.pain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Ikoma A, Handwerker H, Miyachi Y, Schmelz M. Electrically evoked itch in humans. Pain. 2005;113:148–154. doi: 10.1016/j.pain.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Jung H, Miller RJ. Activation of the nuclear factor of activated T-cells (NFAT) mediates upregulation of CCR2 chemokine receptors in dorsal root ganglion (DRG) neurons: a possible mechanism for activity-dependent transcription in DRG neurons in association with neuropathic pain. Mol Cell Neurosci. 2008;37:170–177. doi: 10.1016/j.mcn.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Q, Dong X. The role of the Mrgpr receptor family in itch. Handb Exp Pharmacol. 2015;226:71–88. doi: 10.1007/978-3-662-44605-8_5. [DOI] [PubMed] [Google Scholar]