Abstract
Endocarditis is a rare, serious manifestation of Listeria monocytogenes (LM). The optimal treatment strategy for LM endocarditis has yet to be established. Current antibiotic strategies for listeriosis include penicillin G or ampicillin (AMP) monotherapy, or AMP + gentamicin combination therapy, which is often favored for endocarditis. The primary objective of our investigation was to assess the utility of AMP + ceftriaxone (CRO) and AMP + daptomycin (DAP) against LM, modeling less nephrotoxic antibiotic combinations traditionally used to manage resistant enterococcal endocarditis. Here we report a case of LM endocarditis, review the world literature, and evaluate alternative treatment strategies for listeriosis utilizing in vitro and ex vivo studies. The combinations AMP + CRO and AMP + DAP were each noted to have synergistic activity against an LM endocarditis isolate. In addition, pretreatment of the isolate with sub-lethal concentrations of antibiotics (AMP, CRO, DAP, AMP + CRO or AMP + DAP) sensitized the bacterium to whole blood killing while CRO and DAP further sensitized the bacterium to neutrophil killing. However, these effects did not reflect potentiation of antibiotic activity to human cathelicidin peptide LL-37, which is abundant in neutrophils and highly active against LM. Interestingly, AMP pretreatment of the LM endocarditis isolate resulted in increased DAP binding to the bacterium as assessed by fluorescence microscopy. The results of these in vitro and ex vivo studies warrant further investigation of combination therapy using AMP + CRO or AMP + DAP as an alternative treatment for LM infection.
Keywords: Listeria monocytogenes, Endocarditis, Literature review, Treatment, Antibiotic synergy
1. Introduction
1.1. Background
The Gram-positive facultative intracellular coccobacillus, Listeria monocytogenes (LM) is an important foodborne and opportunistic pathogen. Widely distributed in nature, LM is found in soil, vegetation, and stool of healthy mammals. It has also been isolated from many foods, including processed/delicatessen meats, soft cheeses, pâtés and cantaloupe. Listeria monocytogenes can survive many modern food processing technologies by tolerating high salt and low pH conditions, and can multiply even at refrigeration temperatures.
Human LM infection occurs through ingestion of contaminated foods, which results in an infection spectrum ranging from self-limiting febrile gastroenteritis in healthy individuals, to life–threatening bacteremia, meningitis, cerebritis, rhombencephalitis, and focal disease in high–risk groups, including neonates, the elderly, pregnant women, and immunosuppressed patients. Listeria monocytogenes has the third highest mortality rate (16%) among foodborne infections in the United States, after Vibrio vulnificus (35%) and Clostridium botulinum (17%), and accounts for 19% of foodborne–related deaths overall [1].
Endocarditis is a rare, serious complication affecting the native or prosthetic valves of around 8% of listeriosis patients, with frequent embolic complications and an associated mortality of 37–50% [2,3]. Listeria monocytogenes endocarditis was first reported by Hoeprich and Chernoff in 1955, and only 68 cases of LM endocarditis have since been described in the medical literature [4,5]. The scarcity of patient cases and absence of randomized controlled treatment studies means the optimal antibiotic strategy for LM endocarditis has yet to be established. Common treatment strategies include penicillin G (PCN) or ampicillin (AMP) monotherapy, or AMP + gentamicin (GENT) combination therapy. In vitro, PCN or AMP had a delayed bactericidal effect on LM following prolonged exposure. In contrast, AMP + GENT show rapid and potent synergistic bactericidal activity in vitro and in vivo; therefore, this combination is first–line therapy for LM in humans [6–8]. Of note, retrospective clinical studies have failed to show consistent in vivo AMP + GENT synergy or superior outcomes compared with simple AMP monotherapy [9].
As for LM, traditional standard of care for Enterococcus faecalis endocarditis has been a cell wall-active agent (e.g. AMP) + aminoglycoside (e.g. GENT). However, over time, alternative enterococcal therapeutic strategies have emerged. Dual β-lactam therapy with AMP + ceftriaxone (CRO) for enterococcal endocarditis (with or without high–level aminoglycoside resistance) yields clinical cure rates equivalent to AMP + GENT while avoiding renal and oto-toxicity potentially associated with aminoglycosides [10]. AMP with the cyclic lipopeptide antibiotic, daptomycin (DAP) may also show benefit in antibiotic-resistant or recalcitrant Enterococcus infections based on in vitro studies and limited clinical data [11].
Here we review 100 cases of native and prosthetic valve LM endocarditis identified in the literature, with anemphasis on therapeutic outcomes, and evaluate alternative antimicrobial strategies against LM. We assessed the efficacy of AMP + CRO and AMP + DAP, which are antibiotic combinations traditionally associated with resistant enterococcal infections, against LM strains from various food and clinical sources, including a contemporaneous clinical isolate from a patient with LM endocarditis using minimum inhibitory concentration (MIC) testing, checkerboard synergy assays, kinetic killing curves, and bacterial cytological profiling. In addition, ex vivo studies evaluating human whole blood and neutrophil killing were performed on LM exposed to different antibiotic concentrations.
1.2. Brief history of source case
A 79-year-old man with end–stage renal disease on hemodialysis via a tunneled left internal jugular catheter presented with fever, chills, generalized weakness, poor appetite and malaise. Hismedical history included hypertension, dyslipidemia, diabetes mellitus, systolic heart failure, aortic valve stenosis, regurgitation of all four major heart valves, pulmonary hypertension, atrial fibrillation, alcohol and tobacco dependence, anemia, gout and an episode of E. faecalis bacteremia one year earlier. Multiple peripheral and catheter–derived blood cultures and the removed catheter (tip) grew LM (hereafter isolate LM SDVA) (Supplementary Fig. S1). Trans–thoracic echocardiogram revealed an aortic valve mass of 1.25 cm × 1.0 cm (Supplementary Fig. S2), which resolved following 6 weeks of renally dosed AMP 2 g IV every 12 h. Unfortunately, the patient died 9 months later from complications of congestive heart failure.
1.3. Review of reported cases
A comprehensive search for all human cases of LM endocarditis was performed using the MEDLINE database (1946 to March 2017). The search terms used included ‘Listeria monocytogenes,’ ‘Listeria monocytogenes endocarditis,’ ‘Listeria endocarditis,’ ‘endocarditis,’ ‘Listeria prosthetic valve endocarditis,’ ‘prosthetic valve endocarditis,’ and ‘Listeria bacteremia.’ Two criteria were required for inclusion: (1) LM isolation from blood or tissue, and (2) diagnosis of endocarditis. Cases published in English, Spanish, French, German, Danish, Swedish, Portuguese and Japanese were included. Ultimately, this search revealed 100 cases of endocarditis due to LM in addition to the case presently reported (Table 1). The average age of the patients was 65 years, with a range from5 to 87 years. LM endocarditis occurred in 62 men and 37 women with a male–to–female ratio of 1.7 to 1. For the 98 cases with reported outcomes, overall mortality was 30% (29 deaths, 69 survivors), with mortality in cases reported since 2000 at a lower level of 16% (5 deaths, 26 survivors) (Table 1 and 2). Underlying cardiac and non-cardiac conditions were reported in 93 of 100 patients and are shown in Table 2. Overall, 71% of patients had an underlying cardiac condition, the most common being a prosthetic valve (43 patients) and underlying valvular disease—aortic, mitral and/or tricuspid (24 patients). The most common underlying non-cardiac conditions included diabetes mellitus (12 patients) and malignancy (11 patients).
Table 1.
Reported cases of Listeria monocytogenes endocarditis.
| Case | Age, Sex | Underlying Condition (Including Cardiac Pathology) |
Antibiotic Regimen | Valve Type | Medical or Medical + Surgical Treatment |
Outcome | Referenceb |
|---|---|---|---|---|---|---|---|
| 1 | 42, M | Rheumatic Heart Disease, Periodontoclasia | Penicillin + Dihydrostreptomycin + Erythromycin | Native | Medical | Survived | [1] |
| 2 | Unknown, F | Severe Anemia | Ciprocinal | Native | Medical | Survived | [2] |
| 3 | 35, F | Chorea, Septic Abortion | Penicillin + Streptomycin + Erythromycin | Native | Medical | Died | [2] |
| 4 | 28, M | Rheumatic Heart Disease | Penicillin + Streptomycin | Native | Medical | Survived | [3] |
| 5 | 55, M | Alcoholism, Metastatic Pancreatic Carcinoma | None | Native | None | Died | [3] |
| 6 | 40, F | Rheumatic Heart Disease | Oxytetracycline + Streptomycin | Native | Medical | Survived | [4] |
| 7 | 22, F | Hodgkin’s Lymphoma, Adrenal Insufficiency, Steroid Treatment, Zoster | None | Native | None | Died | [5] |
| 8 | 49, M | Rheumatic Heart Disease, Diabetes Mellitus, Alcoholism | Penicillin + Streptomycin + Erythromycin (+Isoniazid) | Native | Medical | Died | [6] |
| 9 | 56, M | Alcoholism, Facial Basal Cell Carcinoma | Penicillin + Erythromycin | Native | Medical | Survived | [7] |
| 10 | 10, M | Kwashiorkor | Penicillin + Chloramphenicol | Native | Medical | Survived | [8] |
| 11 | 23, M | Aortic Stenosis | Penicillin + Streptomycin | Native | Medical + Surgical | Died | [9] |
| 12 | 31, M | Aortic Stenosis | Ampicillin + Streptomycin (Followed By Amphotericin B For Fungal Endocarditis) | Native | Medical + Surgical | Died | [10] |
| 13 | 64, M | Angina Pectoris | Penicillin + Gentamicin | Native | Medical | Died | [11] |
| 14 | 32, M | Heart Failure | Chloramphenicol + Oleandomycin + Erythromycin | Native | Medical | Survived | [12] |
| 15 | 54, M | Unknown | Penicillin + Streptomycin | Native | Unknown | Survived | [13] |
| 16 | 76, M | Hemolytic Anemia, Autoimmune Radiculitis, Steroid Therapy | None | Native | None | Died | [13] |
| 17 | 27, F | End Stage Renal Disease, Hemodialysis, Renal Transplant | Ampicillin + Gentamicin | Native | Medical | Survived | [14] |
| 18 | 69, M | End Stage Renal Disease, Hemodialysis, Renal Transplant, Heart Failure, Myocardial Infarction, Bicuspid Aortic Valve, Atherosclerosis, Hypertension, Angina Pectoris | Ampicillin + Oxacillin | Native | Medical | Died | [14] |
| 19 | 75, M | Atrial Fibrillation, Heart Failure | Penicillin | Native | Medical | Survived | [15] |
| 20 | 72, F | Aortic Stenosis, Valve Replacement, Heart Failure | Penicillin + Kanamycin | Prosthetic | Medical + Surgical | Died | [16] |
| 21 | 64, M | Angina Pectoris | Unknown | Unknown | Unknown | Died | [17] |
| 22 | 23, M | Aortic Stenosis | Unknown | Native | Unknown | Died | [17] |
| 23 | 67, F | Rheumatic Heart Disease, Streptococcal Endocarditis, Milroy Disease (Hereditary Lymphedema), Heart Failure, Cellulitis | Cephalothin (First Generation Cephalosporin) | Native | Medical | Died | [18] |
| 24 | 16, F | Rheumatic Heart Disease | Penicillin | Native | Medical | Survived | [19] |
| 25 | 55, F | Rheumatic Heart Disease | Penicillin | Native | Medical | Survived | [20] |
| 26 | 25, F | Pregnancy, Diabetes Mellitus | Ampicillin (For 4 Weeks Then Followed By Amoxicillin For 2 Weeks) | Native | Medical | Survived | [21] |
| 27 | 64, M | Heart Failure, Valve Replacement | Penicillin | Prosthetic | Medical | Died | [22] |
| 28 | 53, M | Hypertrophic Cardiomyopathy, Anemia | Penicillin | Native | Medical | Survived | [23] |
| 29 | 58, F | Rheumatic Heart Disease, Valve Replacement | Penicillin + Tobramycin | Prosthetic | Medical | Survived | [24] |
| 30 | 23, F | Septic Abortion | Penicillin + Cephalothin + Streptomycin | Native | Medical | Died | [25] |
| 31 | Unknown, M | Unknown | Unknown | Unknown | Medical + Surgical | Survived | [26] |
| 32 | 66, M | Chronic Lymphocytic Leukemia | Penicillin + Streptomycin | Native | Medical | Survived | [27] |
| 33 | 27, M | Marfan Syndrome, Valve Replacement, Started On Peritoneal Dialysis Over Hospital Course | Penicillin | Prosthetic | Medical | Died | [28] |
| 34 | 56, F | Mitral Valve Prolapse | Penicillin | Native | Medical | Survived | [29] |
| 35 | 63, M | Chronic Rectal Fistula, Mitral Stenosis, Valve Replacement | Penicillin + Streptomycin | Prosthetic | Medical | Survived | [30] |
| 36 | 69, F | Mitral Regurgitation, Valve Replacement | Ampicillin + Gentamicin | Prosthetic | Medical | Survived | [31] |
| 37 | 34, F | None | Penicillin | Native | Medical | Survived | [32] |
| 38 | 43, M | Redo Valve Replacement | Ampicillin + Amikacin | Prosthetic | Medical | Died | [33] |
| 39 | 74, M | Atrial Fibrillation | Ampicillin + Netilmicin (For 3–4 Weeks Followed by Amoxicillin For 4 Weeks) | Native | Medical | Died | [34] |
| 40 | 68, M | Diabetes Mellitus | Unknown | Unknown | Unknown | Survived | [35] |
| 41 | 72, F | Unknown | Unknown | Prosthetic | Unknown | Survived | [36] |
| 42 | 69, F | Myocardial Infarction, Diabetes Mellitus, Hypertension, Heart Failure, Right Iliofemoral Endarterectomy, Arterial Venous Graft, Bilateral Carotid Endarterectomies, Aortic Aneurysm Resection, Aortic Iliac Graft Placement, End Stage Renal Disease, Hemodialysis, Vascular Infection, Vineberg Procedure (Forerunner To CABG) | Vancomycin + Gentamicin | Native | Medical | Survived | [37] |
| 43 | 77, F | Unknown | Unknown | Unknown | Unknown | Survived | [38] |
| 44 | 55, M | Myocardial Infarction, Seizure Episode | Penicillin + Netilmicin | Native | Medical + Surgical | Survived | [39] |
| 45 | 58, M | Colon Adenocarcinoma | Ampicillin + Netilmicin | Native | Medical + Surgical | Died | [40] |
| 46 | 75, F | Aortic Stenosis, Streptococcal Endocarditis, Lung Adenocarcinoma (Diagnosed During Admission), Colon Adenocarcinoma, Chronic Anemia | Ampicillin + Gentamicin | Native | Medical | Died | [40] |
| 47 | 83, F | Colon Adenocarcinoma, Atherosclerosis | Ampicillin (Initially Then Pivampicillin) | Native | Medical | Died | [40] |
| 48 | 52, F | Cirrhosis | Ampicillin + Gentamicin | Native | Medical | Survived | [41] |
| 49 | 55, M | HIV, Ischemic Heart Disease, Seizures? | Penicillin + Netilmicin | Native | Medical + Surgical | Survived | [42] |
| 50 | 69, M | Valve Replacement, Rheumatoid Arthritis, Alcoholism | Ampicillin + Streptomycin | Prosthetic | Medical + Surgical | Survived | [43] |
| 51 | 70, F | Coronary Artery Bypass Graft, Atherosclerosis | Ampicillin + Gentamicin | Native | Medical | Survived | [44] |
| 52 | 73, F | Valve Replacement, Aortic Insufficiency | Ampicillin + Gentamicin | Prosthetic | Medical + Surgical | Survived | [45] |
| 53 | 46, M | Valve Replacement | Amoxicillin + Netilmicin | Prosthetic | Medical | Survived | [46] |
| 54 | 55, Unknown | HIV, Myocardial Infarction | Penicillin + Netilmicin | Native | Medical + Surgical | Survived | [47] |
| 55 | 35, M | HIV, Intravenous Drug Use, Candida endophthalmitis, Cirrhosis | Co-trimoxazole | Native | Medical | Died | [48] |
| 56 | 67, M | Valve Replacement, Left Atrial Thrombus, Colon Adenocarcinoma, Mitral Insufficiency | Vancomycin + Gentamicin | Prosthetic | Medical | Survived | [49] |
| 57 | 78, M | Valve Replacement, Pacemaker, Mitral Regurgitation, Aortic Regurgitation | Ampicillin + Tobramycin | Prosthetic | Medical + Surgical | Survived | [50] |
| 58 | 56, F | Valve Replacement | Co-trimoxazole | Prosthetic | Medical + Surgical | Survived | [51] |
| 59 | 71, M | Valve Replacement, Vertebral Fracture, Spondylodiscitis | Ampicillin + Amikacin | Prosthetic | Medical + Surgical | Survived | [52] |
| 60 | 61, M | Rheumatic Heart Disease, Valve Replacement | Ampicillin + Gentamicin | Prosthetic | Medical | Survived | [53] |
| 61 | 69, M | Valve Replacement, Colon Adenocarcinoma, Multiple Bouts of Endocarditis (E. faecalis, S. sanguis, K. pneumoniae), Mitral Valvuloplasty | Vancomycin + Gentamicin | Prosthetic | Medical | Survived | [54] |
| 62 | 78, F | Valve Replacement | Ampicillin | Prosthetic | Medical | Survived | [55] |
| 63 | 65, M | Hemachromatosis, Duodenal Ulcer, Laparotomy | Ampicillin + Tobramycin | Native | Medical | Died | [56] |
| 64 | 67, M | Alcoholism, Valve Replacement | Ampicillin + Gentamicin | Prosthetic | Medical + Surgical | Survived | [57] |
| 65 | 76, F | Urinary Tract Infection, Valve Replacement | Ampicillin + Gentamicin | Prosthetic | Medical | Died | [58] |
| 66 | 41, F | Liver Transplant, Immunosuppressive Therapy, CMV Hepatitis, Hickman’s Catheter | Ampicillin (Followed By Penicillin) + Gentamicin | Native | Medical | Survived | [59] |
| 67 | 74, M | Varicose Vein Stripping, Valve Replacement | Amoxicillin + Gentamicin | Prosthetic | Medical + Surgical | Survived | [60] |
| 68 | 79, M | Hepatitis B Virus, Valve Replacement | Ampicillin + Gentamicin | Prosthetic | Medical + Surgical | Survived | [61] |
| 69 | 69, F | Valvuloplasty, Valve Replacement, Pacemaker | Ampicillin (For 3 Weeks Then Co-trimoxazole + Rifampicin + Teicoplanin For 4–5 Weeks) | Prosthetic | Medical | Survived | [62] |
| 70 | 42, M | HIV, Diabetes Mellitus, Chronic Renal Failure, Heart Failure, Redo Valve Replacement | Ampicillin + Gentamicin | Prosthetic | Medical | Survived | [63] |
| 71 | 81, M | Valve Replacement | Ampicillin + Gentamicin (Then Lifelong Suppressive Therapy With Amoxicillin) | Prosthetic | Medical + Surgical | Survived | [64] |
| 72 | 77, M | Rheumatic Heart Disease, Valve Replacement | Vancomycin + Gentamicin | Prosthetic | Medical + Surgical | Survived | [65] |
| 73 | 68, M | Rheumatic Heart Disease, Valve Replacement | Penicillin + Gentamicin | Prosthetic | Medical + Surgical | Survived | [65] |
| 74 | 70, M | Aortic Stenosis, Colon Polyp | Ampicillin + Gentamicin (Stopped After 7 Days Due To Renal Insufficiency) | Native | Medical + Surgical | Survived | [66] |
| 75 | 76, M | Valve Replacement, Defibrillator, Chronic Renal Insufficiency, Polymyalgia Rheumatica, Barrett’s Esophagus, GERD, Chronic Gastritis, Diverticulosis, Colon Polyp, Duodenal Ulcer, Iron Deficiency Anemia, Transient Ischemic Attack | Vancomycin (For 2 Weeks Then Linezolid For 4 Weeks) | Prosthetic | Medical + Surgical | Survived | [67] |
| 76 | 76, M | Hypertension, Hyperlipidemia, Coronary Artery Bypass Graft, Valve Replacement, Tobacco Use, Invasive Rectal Carcinoma | Unknown | Prosthetic | Medical + Surgical | Died | [68] |
| 77 | 74, M | Valve Replacement, Aortic Stenosis | Meropenem + Gentamicin | Prosthetic | Medical | Survived | [69] |
| 78 | 71, M | Hypertension, Atherosclerosis, Alcoholism, Alcoholic Hepatitis, Chronic Renal Insufficiency, Tobacco Use, Dental Procedure | Penicillin | Native | Medical | Survived | [70] |
| 79 | 80, F | Listeria Meningitis (1 Year Prior), Valve Replacements (Mitral + Aortic) | Ampicillin + Gentamicin | Prosthetic | Unknown | Unknown | [71] |
| 80 | 73, F | Diabetes Mellitus, Valve Replacement | Co-trimoxazole + Aminoglycoside (Type Unknown) | Prosthetic | Unknown | Unknown | [71] |
| 81 | 87, M | Valve Replacement, Atrial Fibrillation, Heart Failure, Prostate Cancer, Hemorrhoids | Ampicillin (6 Weeks) + Tobramycin (3 Weeks) (Followed By Amoxicillin For 9 Weeks) | Prosthetic | Medical | Survived | [72] |
| 82 | 67, M | Valve Replacement | Linezolid + Co-trimoxazole | Prosthetic | Medical + Surgical | Survived | [73] |
| 83 | 5, M | None | Levofloxacin + Rifampin | N/Aa | Medical + Surgical | Survived | [74] |
| 84 | 78, F | 3rd Degree Heart Block, Pacemaker, Heart Failure, Tricuspid Regurgitation, Coronary Artery Bypass Graft, Hypoglycemia | Ampicillin + Gentamicin | Native | Medical | Died | [75] |
| 85 | 42, F | Psoriatic Arthritis On Infliximab | Ampicillin | Native | Medical | Survived | [76] |
| 86 | 74, M | HIV | Unknown | N/Aa | Medical + Surgical | Survived | [77] |
| 87 | 74, M | Rheumatic Heart Disease, Atrial Fibrillation, Atrophic Gastritis | Ampicillin + Gentamicin | Native | Medical + Surgical | Died | [78] |
| 88 | 58, F | Rheumatic Heart Disease, Valvuloplasties, Valve Replacement, Atrial Fibrillation, Chronic Renal Insufficiency, Right Nephrectomy | Ampicillin + Gentamicin | Prosthetic | Medical + Surgical | Died | [78] |
| 89 | 44, F | Multiple Sclerosis | Amoxicillin + Gentamicin | N/Aa | Medical + Surgical | Survived | [79] |
| 90 | 77, M | Coarctation of the Aorta, Valve Replacements (Mitral + Aortic), Hypertension, Heart Failure, Hypothyroidism | Ampicillin + Gentamicin | Prosthetic | Medical | Survived | [80] |
| 91 | 73, F | Breast Cancer With Metastases, Diabetes Mellitus | Ampicillin + Gentamicin | N/Aa | Medical | Survived | [81] |
| 92 | 70, M | Valve Replacement, Aortic Stenosis, Atrial Fibrillation, Pacemaker, Diabetes Mellitus, Cerebrovascular Accident, Rheumatoid Arthritis on Methotrexate, Chronic Obstructive Pulmonary Disease | Meropenem + Ciprofloxacin | Native | Medical | Died | [82] |
| 93 | 54, F | None | Amoxicillin + Gentamicin + Co-trimoxazole | Native | Medical | Survived | [83] |
| 94 | 54, M | Congenitally Corrected Transposition of the Great Vessels, Valve Replacements (Mitral + Aortic) | Ampicillin + Gentamicin | Prosthetic | Medical | Survived | [84] |
| 95 | 36, F | Pregnancy, Bicuspid Aortic Valve | Ampicillin + Gentamicin | Native | Medical + Surgical | Survived | [85] |
| 96 | 84, M | Chronic Renal Failure, Diabetes Mellitus, Collagenopathy, Chronic Corticosteroid Use, Valve Replacement | Meropenem + Daptomycin | Prosthetic | Medical | Survived | [86] |
| 97 | 84, M | Valve Replacement | Ampicillin + Gentamicin | Prosthetic | Medical | Survived | [86] |
| 98 | 80, F | Diabetes Mellitus, Valve Replacement | Ampicillin + Gentamicin | Prosthetic | Medical | Survived | [86] |
| 99 | 79, M | Diabetes Mellitus, Valve Replacement | Ampicillin + Gentamicin | Native | Medical | Survived | [86] |
| 100 | 63, M | Hypertension, Diabetes Mellitus, Atrial Flutter, Valve Replacement | Penicillin + Rifampicin (For 4–6 Weeks Then Amoxicillin For 18 Weeks) | Prosthetic | Medical + Surgical | Survived | [87] |
Mass/Vegetation Identified Along Right Atrium, Left Atrium And/Or Left Ventricular Outflow Tract.
Please see supplementary data section for table references.
Table 2.
Underlying cardiac and non-cardiac conditions reported in 100 patients diagnosed with Listeria monocytogenes endocarditis.
| Variable |
Listeria monocytogenes Endocarditis (n = 100) |
|---|---|
| Age, Mean Years (Range) | 65 (5 to 87) |
| Ratio of Male-to-Female Patients | 1.7 |
| Underlying Cardiac Conditions (Top 10): | (No. of Patients) |
| Prosthetic Valve | 43 |
| Valvular Disease | 24 |
| Rheumatic Fever or Heart Disease | 13 |
| Heart Failure | 10 |
| Atrial Fibrillation/Flutter | 7 |
| Angina Pectoralis or Myocardial Infarction | 7 |
| Hypertension | 6 |
| Pacemaker/Defibrillator | 4 |
| Coronary Artery Bypass Graft or Vinberg | 4 |
| Atherosclerosis | 4 |
| Underlying Non-cardiac Conditions (Top 10): | (No. of Patients) |
| Diabetes Mellitus | 12 |
| Malignancy | 11 |
| Chronic Kidney Disease or End Stage Renal Disease | 7 |
| Alcoholism | 6 |
| Immunosuppressive Therapy | 5 |
| Anemia | 5 |
| Human Immunodeficiency Virus | 5 |
| Hemodialysis or Peritoneal Dialysis | 4 |
| Renal or Liver Transplant | 3 |
| Pregnancy or Septic Abortion | 3 |
| Medical or Medical + Surgical Therapy: | Proportion of Patients With Cure (% Survival) |
| Medical | 42/59 (71%) |
| Medical + Surgical | 23/30 (77%) |
| Overall Survival | 69/98 (70%) |
A comparison of the age, male–to–female ratio, site of cardiac involvement and outcome (based on the antibiotic regimen and medical vs. medical + surgical treatment) in patients with identified native valve and prosthetic valve endocarditis is illustrated in Table 3. The mean age in years of patients with native valve LM endocarditis was 51 (range 10 to 83; M:F 1.4 to 1) and with prosthetic valve LM endocarditis was 68 (range 27 to 87; M:F 2.2 to 1). For cases with a reported outcome, overall mortality for native valve LM endocarditis was 40% (20 deaths, 30 survivors) and for prosthetic valve LM endocarditis was 22% (9 deaths, 32 survivors). The 3 most common sites of valvular involvement for native valve LM endocarditis were aortic (14 patients), aortic + mitral (14 patients) and mitral (12 patients), and for prosthetic valve LM endocarditis were mitral (23 patients), aortic (14 patients) and aortic + mitral (2 patients).
Table 3.
Reported clinical characteristics and outcomes in patients with native and prosthetic valve endocarditis due to Listeria monocytogenes.
| Variable | Native Valve Endocarditis (n = 51) |
Prosthetic Valve Endocarditis (n = 41) |
|---|---|---|
| Age, Mean Years (Range) | 51 (10 to 83) | 68 (27 to 87) |
| Ratio of Male to Female Patients | 1.4 | 2.2 |
| Site of Cardiac Involvement: | (No. of Patients) | (No. of Patients) |
| Aortic | 14 | 14 |
| Mitral | 12 | 23 |
| Tricuspid | 3 | 1 |
| Aortic + Mitral | 14 | 2 |
| Aortic + Tricuspid | 1 | 0 |
| Mitral + Tricuspid | 0 | 1 |
| Unknown | 7 | 0 |
| Antibiotic Therapy: | Proportion of Patients With Cure (% Survival) |
Proportion of Patients With Cure (% Survival) |
|---|---|---|
| Penicillin or Ampicillin or Amoxicillin Only | 9/10 (90%) | 2/4 (50%) |
| Penicillin or Ampicillin or Amoxicillin + Aminoglycoside | 13/22 (59%) | 21/26 (77%) |
| Penicillin or Ampicillin or Amoxicillin Only and Penicillin or Ampicillin or Amoxicillin + Aminoglycoside | 22/32 (69%) | 23/30 (77%) |
| Medical or Medical + Surgical Therapy: | ||
| Medical | 24/37 (65%) | 17/22 (77%) |
| Medical + Surgical | 5/9 (56%) | 14/17 (82%) |
| Overall Survival | 30/50 (60%) | 32/41 (78%) |
A total of 59 patients received solely medical (antibiotic) treatment and 30 patients received medical (antibiotic) + surgical treatment. Outcomes were similar in the two groups. Forty-two of 59 patients (71%) who received medical treatment alone survived compared with 23 of 30 patients (77%) who received medical + surgical treatment. Survival rate for those who underwent medical treatment or medical + surgical treatment was higher among patients with prosthetic valve endocarditis compared with those with native valve endocarditis. Survival rates of 77% (5 deaths, 17 survivors) and 82% (3 deaths, 14 survivors) were noted for patients with prosthetic valve endocarditis who underwent medical treatment and medical + surgical treatment, respectively. Survival rates of 65% (13 deaths, 24 survivors) and 56% (4 death, 5 survivors) were noted for native valve endocarditis patients who underwent medical treatment or medical + surgical treatment, respectively. Increased survival in patients with prosthetic valves may reflect greater clinical suspicion for underlying endocarditis, rapid initiation of antibiotic therapy and increased use of surgery.
A vast array of antibiotic regimens with varying outcomes has been employed in the management of LM endocarditis (Table 4). The two most commonly utilized antibiotic strategies included PCN, AMP or amoxicillin (AMX) only (14 patients), and one of these β-lactam agents + aminoglycoside (49 patients). Survival rates for those treated with β–lactam alone vs. β-lactam + aminoglycoside were comparable at 79% and 73%, respectively. However, survival rate for those treated with β-lactam alone was 90% (1 death, 9 survivors) for native valve endocarditis and 50% (2 deaths, 2 survivors) for prosthetic valve endocarditis, whereas the survival rate for those treated with β–lactam + aminoglycoside was 59% (9 deaths, 13 survivors) for native valve endocarditis vs. 77% (5 deaths, 21 survivors) for prosthetic valve endocarditis (Table 3). Thus, improved outcomes using β-lactam alone were observed in patients with native valve endocarditis, whereas survival among patients with prosthetic valve endocarditis was better in those treated with the combination of a β-lactam and an aminoglycoside.
Table 4.
Antibiotic treatment regimens and survival.
| Antibiotic Regimen | Survived | Died | %Survival |
|---|---|---|---|
| Penicillin or Ampicillin or Amoxicillin Only | 11 | 3 | 79 |
| Penicillin or Ampicillin or Amoxicillin + Aminoglycoside | 36 | 13 | 73 |
| Penicillin or Ampicillin or Amoxicillin + Aminoglycoside + Other | 2 | 3 | 40 |
| Penicillin or Ampicillin or Amoxicillin + Non-aminoglycoside | 3 | 1 | 75 |
| Vancomycin + Aminoglycoside | 4 | 0 | 100 |
| Vancomycin or Linezolid Only | 1 | 0 | 100 |
| Co-trimoxazole ± Other | 2 | 1 | 67 |
| Meropenem + Other | 2 | 1 | 67 |
| Fluoroquinolone ± Other | 2 | 0 | 100 |
| Other Combination Regimens | 2 | 1 | 67 |
| Unknown Regimen | 4 | 3 | 57 |
| No Antibiotics | 0 | 3 | 0 |
2. Material and methods
2.1. Bacterial strains, media & antibiotics
The clinical isolate, LM SDVA was utilized in all experiments performed. Susceptibility and synergy testing was also performed on 4 additional strains: LM L028 (a virulent hemolytic carriage strain isolated from the feces of a healthy pregnant woman), LM 2203 (isolated during an outbreak affecting pregnant women and associated with intrauterine/cervical infections), LM 10403 (streptomycin–resistant strain isolated from a human skin lesion), and LM EGD-e (isolated from an epidemic involving laboratory animals and associated with foodborne illness). All isolates were stored in tryptic soy broth (TSB) containing 50% glycerol at −80 °C until use. AMP, CRO and DAP were purchased from Sigma Aldrich and cathelicidin LL-37 from the American Peptide Company. The medium, Mueller Hinton Broth (Spectrum Chemicals), was supplemented with cations (Ca2+ 20–25 mg/L and Mg2+ 10–12.5 mg/L [CA-MHB]) and 5% lysed horse blood (LHB).
2.2. Minimum inhibitory concentration, checkerboard and time kill assays
Broth microdilution antimicrobial susceptibility testing, checkerboard and time kill assays were performed in CA-MHB + 5% LHB and in accordance with CLSI guidelines [12,13]. Checkerboard synergy, additivity and antagonism were defined by the fractional inhibitory concentration index (FICI): FICI ≤0.5 defined synergy, >0.5 to ≤1 additivity, >1 to <4 indifference, and ≥4 antagonism. Bactericidal vs. bacteriostatic activity was determined by time kill assays using antibiotic combinations and concentrations identified to be synergistic by checkerboard assay. Time kill assay bactericidal activity was defined as a reduction in viable bacteria by ≥3 log10 colony–forming units (cfu)/mL, and bacteriostatic activity was defined as a reduction in viable bacteria by <3 log10 cfu/mL at 24 h compared with the starting inoculum.
2.3. Fluorescence microscopy
Fluorescence microscopy was performed as previously described with the following modifications [11]. LM VASD was grown overnight (14–16 h) in Luria broth (LB) at 37 °C in the presence or absence of ¼ MIC AMP (0.25 µg/mL). Overnight cultures were then diluted 1:100 into fresh LB in the presence or absence of ¼ MIC AMP (0.25 µg/mL). When an OD600 = 0.5 was reached, 4 µg/mL of Bodipy-fluorescein-labeled DAP (4× MIC, but a pharmacologically achievable concentration) was added to exponentially growing bacteria. Bacteria were then incubated with Bodipy-fluorescein-labeled DAP (Cubist Pharmaceuticals) for 40 min at room temperature before being washed with PBS × 3, counterstained with 2 µg/mL DAPI (nucleic acid) (Molecular Probes) and then transferred onto a 1.2% agarose pad containing 20% LB for microscopy and image analysis using ImageJ software v1.48f and CellProfiler 2.0.
2.4. Neutrophil isolation
Human neutrophils were isolated from healthy donors using the PolymorphPrep system (Axis-Shield) as per manufacturer’s instructions and under protocols approved by the UCSD Human Subjects Institutional Review Board.
2.5. Neutrophil killing assay
Neutrophil assays were performed as previously described with the following modifications [14]. Human neutrophils were resuspended in RPMI 1640 to 2 × 106 cells/mL and then used to seed the wells of a 96–well plate at 2 × 105 cells/well. Neutrophils were then infected at a multiplicity of infection (MOI) = 10 bacteria/neutrophil with untreated LM (control) and LM pretreated overnight with ¼ MIC antibiotic (AMP 0.25 µg/mL, CRO 4 µg/mL or DAP 1 µg/mL). Plates were centrifuged at 500 × g for 10 min then incubated for 45 min at 37 °C in 5% CO2. Serial dilutions in sterile PBS and Triton-X 100 (0.02%) were plated on trypticase soy agar (TSA) plates for bacterial enumeration. The percentage of surviving bacteria was calculated for comparison to the initial inoculum.
2.6. Whole blood assay
Stationary phase bacteria were washed twice, diluted to an inoculum of 104 cfu in 50 µL PBS and mixed with 300 µL heparinized human blood and 50 µL PBS with or without antibiotics in siliconized tubes. Final concentrations of tubes with ¼ MIC antibiotic concentrations were AMP 0.25 µg/mL, CRO 4 µg/mL, DAP 1 µg/mL, AMP/CRO 0.25/4 µg/mL and AMP/DAP 0.25/1 µg/mL. Tubes were incubated at 37 °C and rotated for 3 h. Serial dilutions performed using sterile PBS and Triton-X 100 (0.025%) were plated on TSA plates for bacterial enumeration. The survival index was defined as cfu enumerated at the end of the assay divided by cfu at time point 0 h.
2.7. Statistics
Statistical analyses were performed using GraphPad Prism 6.0f (GraphPad Software). One-way analysis of variance (ANOVA) or two-way ANOVA were utilized where appropriate. P values <0.05 were regarded to be statistically significant.
3. Results
3.1. In vitro susceptibilities of LM to the antibiotics AMP, CRO, DAP, AMP/CRO, AMP/DAP and AMP/LL-37
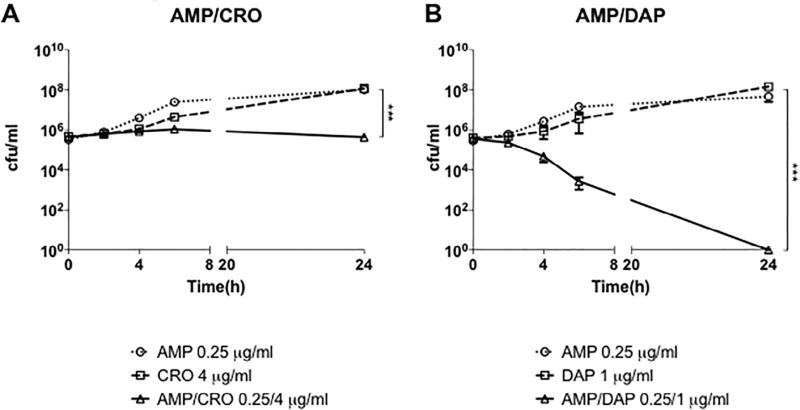
MIC testing, checkerboard assays and time kill assays were performed for AMP, CRO, DAP and combinations of AMP with CRO, DAP or LL-37 to assess antimicrobial activity against the clinical endocarditis isolate, LM SDVA (Table 5, Table 6 and Fig. 1). In addition, MIC testing and checkerboard assays were performed using 4 other strains of LM (L028, 2203, 10403S, and EDG-e) (Table 5 and Table 6). All 5 LM isolates exhibited susceptibility to AMP (MIC90 0.5–1 µg/mL), resistance to CRO (MIC90 8–16 µg/mL), resistance to DAP (MIC90 8–32 µg/mL) and susceptibility to LL-37 (MIC90 1–2 µM). Despite resistance to specific individual antibiotics, synergy defined as an FICI of ≤0.5 for the checkerboard assays was observed for LM SDVA treated with AMP+ CRO and with AMP + DAP. However, bactericidal activity, defined as a reduction in viable bacteria by ≥3 log10 cfu/mL, via time kill assays was observed only for LM SDVA treated with AMP + DAP, but not with AMP + CRO (Fig. 1). To summarize, synergy (FICI ≤0.5)was observed for 2/5 and 1/5 of the LM strains treated with AMP + CRO and AMP + DAP, respectively, and additivity (FICI >0.5 to ≤1) was observed for all other strains, except for AMP + CRO for LM 10403S (FICI = 1.25; indifference). No synergy or additivity was appreciated for any LM strains using the combination AMP + LL-37.
Table 5.
Antimicrobial susceptibility of Listeria monocytogenes strains to AMP and corresponding combinational therapy with CRO and DAP assessed by MIC and checkerboard assays.a
| MIC (µg/mL) | Checkerboard FICI (Interpretation) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Strain | AMP | CRO | DAP | AMP/CRO | AMP/DAP | FICIAMP-CRO | FICIAMP-DAP |
| L. monocytogenes | |||||||
| LM SDVA | 1 | 16 | 32 | 0.25/4 | 0.25/1 | 0.5(S) | 0.28125(S) |
| LM L028 | 1 | 8 | 8 | 0.5/2 | 0.5/2 | 0.75(A) | 0.75(A) |
| LM 2203 | 1 | 8 | 16 | 0.25/4 | 0.25/8 | 0.75(A) | 0.75(A) |
| LM 10403S | 0.5 | 8 | 32 | 0.5/1 | 0.25/16 | 1.25(I) | 1(A) |
| LM EDGE | 1 | 8 | 16 | 0.25/2 | 0.5/4 | 0.5(S) | 0.75(A) |
AMP, Ampicillin; CRO, Ceftriaxone; DAP, Daptomycin; FICI, Fractional Inhibitory Concentration Index; MIC, minimum inhibitory concentration; S, Synergy; A, Additivity; I, Indifference.
Table 6.
Antimicrobial susceptibility of Listeria monocytogenes strains to AMP and corresponding combinational therapy with LL-37 assessed by MIC and checkerboard assays.a
| MIC (µg/mL) | Checkerboard FICI (Interpretation) |
|||
|---|---|---|---|---|
|
|
|
|||
| Strain | AMP | LL-37 | AMP/LL-37 | FICIAMP-LL-37 |
| L. monocytogenes | ||||
| LM SDVA | 1 | 1 | 0.03125/1 | 1.03125(I) |
| LM L028 | 1 | 2 | 0.03125/2 | 1.03125(I) |
| LM 2203 | 1 | 2 | 0.03125/2 | 1.03125(I) |
| LM 10403S | 0.5 | 2 | 0.03125/2 | 1.0625(I) |
| LM EDGE | 1 | 2 | 0.03125/2 | 1.03125(I) |
AMP, Ampicillin; LL-37, Cathelicidin; FICI, Fractional Inhibitory Concentration Index; MIC, minimum inhibitory concentration; I, Indifference.
Fig. 1.
Time kill curve demonstrating (A) ampicillin + ceftriaxone and (B) ampicillin + daptomycin activity against Listeria monocytogenes SDVA in cation-adjusted Mueller Hinton broth supplemented with 5% lysed horse blood. Bactericidal synergy, defined as a ≥ 3 log10 decrease in cfu/mL for time kill assays, was observed only for ampicillin + daptomycin at ¼ MIC of each agent in combination. Data are plotted as mean ± SEM and represent the combination of three experiments performed in triplicate. *** P < 0.001 by two-way ANOVA. AMP, ampicillin; CRO, ceftriaxone; DAP, daptomycin.
3.2. LM pretreated with sub–lethal concentrations of AMP exhibits increased binding to bodipy-labeled DAP
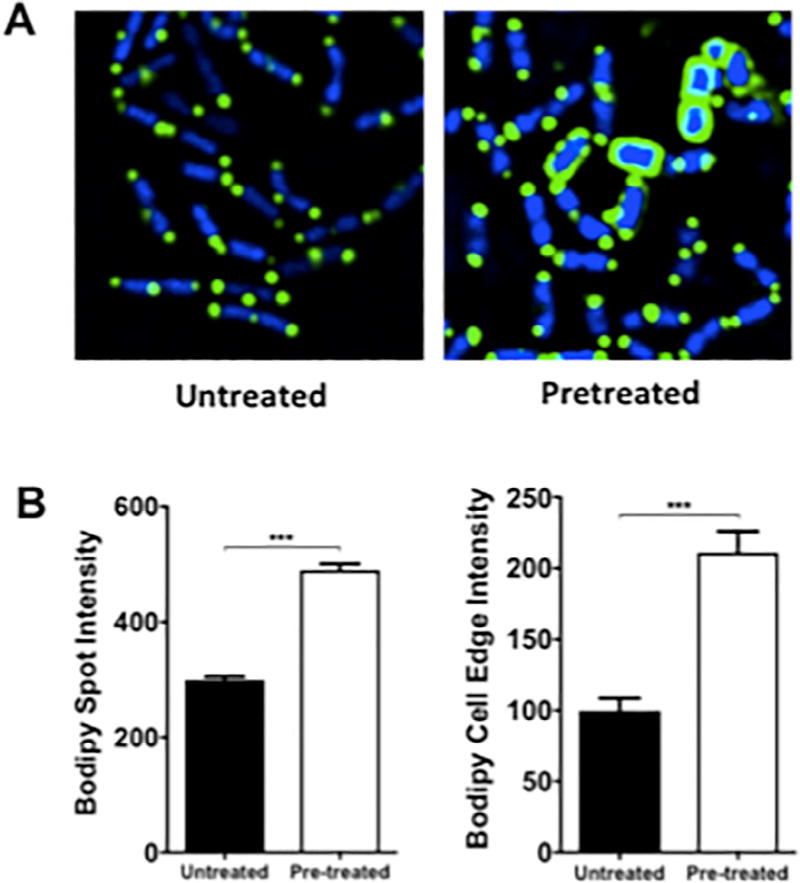
As a complement to the antimicrobial susceptibility assays illustrating synergy of AMP + DAP against LM SDVA, DAP membrane binding studies were performed using Bodipy-fluorescein-labeled DAP and LM SDVA grown in the presence vs. absence of ¼ MIC AMP (0.25 µg/mL). LM SDVA grown in AMP exhibited greater binding to labeled DAP compared with LM SDVA grown in antibiotic–free bacteriological medium when visualized by fluorescence microscopy, and measured by spot and cell edge intensity (Fig. 2).
Fig. 2.
Binding of fluorescently–labeled daptomycin to Listeria monocytogenes (LM) SDVA pretreated without or with ampicillin. (A) Fluorescence microscopy was performed using log-phase LM SDVA grown in the presence or absence of ¼ MIC (0.25 µg/mL) ampicillin, and following exposure to Bodipy-fluorescein-labeled daptomycin (green) and nucleic acid counterstaining with DAPI (blue). (B) Bar graphs generated from software analysis of multiple random fluorescent microscopy fields of cells exposed to Bodipy-fluorescein-labeled daptomycin. Bodipy spot and cell edge intensity were noted to be >1.5× and >2× higher for cells grown in the presence of antibiotic than cells grown in the absence of antibiotic. Data are plotted as mean ± SEM and represent the combination of three experiments performed in triplicate. *** P < 0.001 by one-way ANOVA.
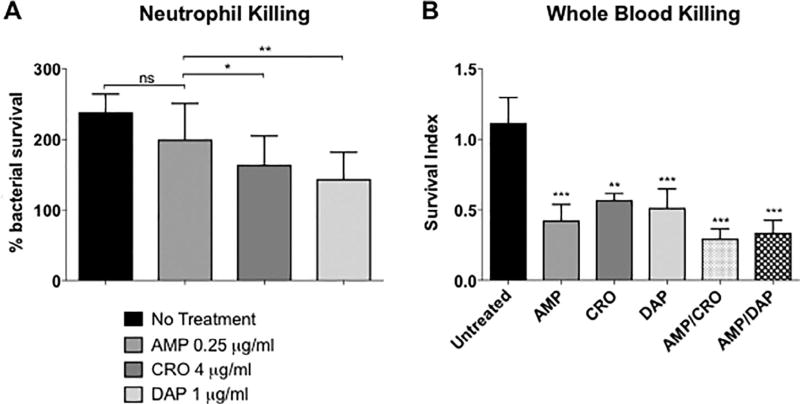
3.3. AMP, CRO & DAP enhance whole blood mediated killing of LM SDVA
Overnight pretreatment of LM SDVA with sub-bacteriostatic concentrations (¼ MIC) of CRO or DAP sensitized the bacterium to human neutrophil killing, whereas pretreatment with ¼ MIC AMP had no effect (Fig. 3A). However, enhanced whole blood killing of LM SDVA was observed in the presence of all antibiotics at ¼ MIC (AMP 0.25 µg/mL, CRO 4 µg/mL, DAP 1 µg/mL, AMP/CRO 0.25/4 µg/mL or AMP/DAP 0.25/1 µg/mL) compared with LM SDVA in the absence of antibiotic (Fig. 3B).
Fig. 3.
Neutrophil and whole blood killing assays for Listeria monocytogenes SDVA. (A) Percentage survival of untreated bacteria vs. bacteria pretreated overnight with ¼ MIC ampicillin, ceftriaxone or daptomycin in a neutrophil killing assay. (B) Survival of bacteria following co-incubation with human whole blood and antibiotics (¼ MIC ampicillin, ceftriaxone, daptomycin, ampicillin + ceftriaxone or ampicillin + daptomycin) or no antibiotics in a whole blood killing assay. Survival index = (cfu at end of assay)/(cfu at time 0). Data are plotted as mean ± SEM and represent the combination of three experiments performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001 or n.s., no statistical significance by two-way ANOVA comparing pretreated or treated bacteria to untreated bacteria. AMP, ampicillin; CRO, ceftriaxone; DAP, daptomycin.
4. Discussion
Prospective and randomized clinical studies of LM endocarditis identifying optimal antibiotic therapy are lacking because of the scarcity of cases. In vitro, LM appears susceptible to nearly all antibiotics. However, traditional cell–free in vitro susceptibility testing does not always translate to clinical efficacy for intracellular pathogens and often fails to adequately account for interactions with the innate immune system. In vivo, intracellular LM concealed from the extracellular environment may be protected from potent antibiotics with poor intracellular penetration and activity. The concentration of antibiotic at remote sites of infection like endocarditis or intracellularly may remain consistently below the MIC over the course of human antimicrobial therapy. Greater knowledge and insight of sub-inhibitory antibiotic concentrations, simultaneous interactions of two groups of antibiotics (potential synergy) and interactions with components of the innate immune system, such as endogenous antimicrobial peptides, serum and phagocytic cells, may provide guidance toward identifying optimal therapeutic strategies against LM beyond AMP + GENT.
Potentiation of killing E. faecalis, a Gram-positive opportunistic pathogen like LM, by dual β-lactam or β-lactam + lipopeptide combinations for treatment of endocarditis has emerged as a successful alternative therapeutic strategy to AMP + GENT, but without the associated adverse aminoglycoside toxicity [10]. However, a review of the world literature reveals its application has yet to be evaluated against Listeria. We hypothesized the alternative anti-enterococcal therapeutic strategies, AMP + CRO and AMP + DAP, may also be effective against LM.
In LM there are five penicillin–binding proteins (PBP1–5), which are essential enzymes for bacterial cell wall synthesis. However, unlike other PBPs only PBP3 is identical in all Listeria species [7]. Inhibition of PBP3, an enzyme involved in the final stages of peptidoglycan synthesis, has lethal consequences for LM cells and is a major target of β-lactam antibiotics, such as PCN and AMP [7]. PCN and AMP bind to all LM PBP, but have the greatest affinity for PBP3 and PBP5. The third generation cephalosporin, CRO, exhibits weak activity against LM due to a lack of affinity for PBP3 and is clinically ineffective [7]. However, CRO is known to be a good inhibitor of PBP1, PBP2 and PBP4 [15]. Therefore, we reasoned that combination antimicrobial therapy with AMP + CRO targeting complementary PBPs with inhibition of 5 out of 5 key LM PBPs would enhance killing to a greater extent than each agent alone. In our current investigation utilizing 5 CRO–resistant LM isolates, we indeed observed AMP + CRO synergy or additivity against 80% (4/5) of LM isolates via checkerboard assays.
The lipopeptide antibiotic, DAP alone exhibits potent activity against certain drug–resistant Gram-positive bacteria, including methicillin–resistant Staphylococcus aureus (MRSA) and vancomycin–resistant Enterococcus spp. (VRE), but is further enhanced in combination with AMP [11,16]. DAP irreversibly binds to the cytoplasmic cell membrane and oligomerizes to form a pore or ion channel resulting in membrane depolarization and cell death. However, in vitro activity against LM using standard MIC testing is variable and clinical experience is lacking. We studied 5 DAP–resistant LM isolates and confirmed in vitro AMP + DAP bactericidal synergy against one isolate of LM associated with endocarditis (LM SDVA) via checkerboard and time kill assays, and additivity against the other 4 LM isolates studied via checkerboard. Bactericidal killing of LM SDVA was observed at clinically–relevant, but sub-MIC, levels of AMP + DAP, even though ¼ MIC AMP and ¼ MIC DAP alone had no measurable effect on inhibiting bacterial growth. In addition, LM SDVA grown in ¼ MIC AMP bound Bodipy–labeled DAP to a greater extent than LM SDVA grown in antibiotic–free bacteriological medium when visualized by fluorescence microscopy. These findings indicate that AMP enhances target membrane binding by DAP.
The present findings are consistent with previous studies demonstrating favorable interactions between β-lactam antibiotics (AMP, oxacillin, nafcillin, ceftaroline, etc.) and the lipopeptide antibiotic, DAP against Gram-positive bacteria. Staphylococcal and enterococcal pre-exposure to AMP has been shown to reduce bacterial net positive surface charge and increase susceptibility to killing by cationic antimicrobials, such as DAP [11,16]. We propose two possible mechanisms for AMP enhancement of DAP activity. First, and as suggested previously, AMP may release lipoteichoic acid from the cell surface, which could then translate to either enhanced cell wall autolysin activity or reduced substrates for D-alanylation as a means of bacterial reduction in net negative surface charge [17]. Second, sub-lethal AMP concentrations may result in a reduction in cell–wall cross–linking, thereby enabling greater DAP access to bacterial membrane targets.
Like DAP, the endogenous cationic host defense antimicrobial peptide, cathelicidin LL-37 forms pores to disrupt bacterial membranes. LL-37 is abundantly produced by epithelial cells and neutrophils, is known to exert antimicrobial activity within the neutrophil phagolysosome, and likely plays a significant role in neutrophil intracellular bacterial killing [18]. All 5 LM isolates were noted to be highly sensitive to LL-37 (MIC 1–2 µM). However, the presence of LL-37 did not potentiate the killing of any of the 5 LM isolates by AMP in combination (FICI >1; indifference) nor did pretreatment of LM SDVA with ¼ MIC AMP sensitize LM SDVA to neutrophil killing (Table 6 and Fig. 3A). In contrast, pretreatment of LM SDVA with sub-lethal CRO or DAP (at ¼ MIC) enhanced neutrophil killing of the bacterial strain (Fig. 3A). However, exposure to ¼ MIC of AMP, CRO, DAP, AMP/CRO or AMP/DAP sensitized LM SDVA to whole blood killing where neutrophils, serum and platelets can all contribute to innate immune bacterial clearance (Fig. 3A) [19]. In addition, a trend toward reduced bacterial survival with combinations of AMP + CRO or AMP + DAP compared with AMP, CRO or DAP alone was noted with whole blood killing, but did not reach statistical significance.
In conclusion, these findings illustrate potentially beneficial interactions between LM, different antibiotic combinations, and components of host innate immunity. Certain antibiotics deemed ineffective by conventional antimicrobial susceptibility testing may nevertheless exhibit favorable properties in combination therapy or in conjunction with host defense. Limitations of our study include the modest number of LM isolates tested (including only one endocarditis isolate), and a lack of extension to in vivo models (e.g. mice, rat, guinea pig, rabbit, etc.) and clinical experience comparing or contrasting the efficacy of AMP + CRO or AMP + DAP therapy to AMP + GENT or AMP monotherapy in patients. Nevertheless, these results indicate that alternative therapeutic strategies employed against sensitive and resistant Enterococcus spp. may also have clinical utility against LM infections. In addition, AMP, CRO and DAP may be beneficial beyond their direct antimicrobial properties in combination and enhance bacterial clearance by components of the innate immune system. Future prospective clinical trials in humans to evaluate and compare the efficacy of antibiotic combinations, such as AMP + CRO, AMP + DAP, AMP + GENT and AMP monotherapy, would be required to definitively determine the optimal therapeutic strategy for LM endocarditis, but will likely never be achieved given the paucity and sporadic nature of these serious infections.
Supplementary Material
Acknowledgments
We thank Dr. Eric G. Pamer (Memorial Sloan Kettering Cancer Center) and Dr. John-Demian Sauer (University of Wisconsin, Madison) for providing bacterial isolates.
Funding: This work was supported by National Institutes of Health grants 1U01AI124316-01 (to M. K., G.S, J.P. and V.N), HD071600 (to G. S. and V. N.), AI052453 and AR052728 (to V. N.), and AI113295 (to J. P.).
Footnotes
Competing interests: GS has received speaking honoraria from Allergan, Sunovion, and Theravance as well as honoraria and consulting fees from The Medicines Company.
Ethical approval: Not required.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ijantimicag.2017.12.032.
References
- 1.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spyrou N, Anderson M, Foale R. Listeria endocarditis: current management and patient outcome–world literature review. Heart. 1997;77:380–3. doi: 10.1136/hrt.77.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 7. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010. [Google Scholar]
- 4.Hoeprich PD, Chernoff HM. Subacute bacterial endocarditis due to Listeria monocytogenes. Am J Med. 1955;19:488–94. doi: 10.1016/0002-9343(55)90132-7. [DOI] [PubMed] [Google Scholar]
- 5.Antolin J, Gutierrez A, Segoviano R, Lopez R, Ciguenza R. Endocarditis due to Listeria: description of two cases and review of the literature. Eur J Intern Med. 2008;19:295–6. doi: 10.1016/j.ejim.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Lorber B. Listeriosis. Clin Infect Dis. 1997;24:1–9. doi: 10.1093/clinids/24.1.1. quiz 10–1. [DOI] [PubMed] [Google Scholar]
- 7.Hof H, Nichterlein T, Kretschmar M. Management of listeriosis. Clin Microbiol Rev. 1997;10:345–57. doi: 10.1128/cmr.10.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moellering RC, Jr, Medoff G, Leech I, Wennersten C, Kunz LJ. Antibiotic synergism against Listeria monocytogenes. Antimicrob Agents Chemother. 1972;1:30–4. doi: 10.1128/aac.1.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez Guerrero ML, Rivas P, Rabago R, Nunez A, de Gorgolas M, Martinell J. Prosthetic valve endocarditis due to Listeria monocytogenes. Report of two cases and reviews. Int J Infect Dis. 2004;8:97–102. doi: 10.1016/j.ijid.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Hidalgo N, Almirante B, Gavalda J, Gurgui M, Pena C, de Alarcon A, et al. Ampicillin plus ceftriaxone is as effective as ampicillin plus gentamicin for treating enterococcus faecalis infective endocarditis. Clin Infect Dis. 2013;56:1261–8. doi: 10.1093/cid/cit052. [DOI] [PubMed] [Google Scholar]
- 11.Sakoulas G, Bayer AS, Pogliano J, Tsuji BT, Yang SJ, Mishra NN, et al. Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin- and vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother. 2012;56:838–44. doi: 10.1128/AAC.05551-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute CaLS. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-fifth Informational Supplement M100-S25. Wayne, PA, USA: 2015. [Google Scholar]
- 13.Sopirala MM, Mangino JE, Gebreyes WA, Biller B, Bannerman T, Balada-Llasat JM, et al. Synergy testing by Etest, microdilution checkerboard, and time-kill methods for pan-drug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2010;54:4678–83. doi: 10.1128/AAC.00497-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumaraswamy M, Lin L, Olson J, Sun CF, Nonejuie P, Corriden R, et al. Standard susceptibility testing overlooks potent azithromycin activity and cationic peptide synergy against MDR Stenotrophomonas maltophilia. J Antimicrob Chemother. 2016;71:1264–9. doi: 10.1093/jac/dkv487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vicente MF, Perez-Daz JC, Baquero F, Angel de Pedro M, Berenguer J. Penicillin-binding protein 3 of Listeria monocytogenes as the primary lethal target for beta-lactams. Antimicrob Agents Chemother. 1990;34:539–42. doi: 10.1128/aac.34.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhand A, Bayer AS, Pogliano J, Yang SJ, Bolaris M, Nizet V, et al. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin Infect Dis. 2011;53:158–63. doi: 10.1093/cid/cir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.al-Obeid S, Gutmann L, Williamson R. Correlation of penicillin-induced lysis of Enterococcus faecium with saturation of essential penicillin-binding proteins and release of lipoteichoic acid. Antimicrob Agents Chemother. 1990;34:1901–7. doi: 10.1128/aac.34.10.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehrer RI, Ganz T. Cathelicidins: a family of endogenous antimicrobial peptides. Curr Opin Hematol. 2002;9:18–22. doi: 10.1097/00062752-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Asensi V, Fierer J. Synergistic effect of human lysozyme plus ampicillin or beta-lysin on the killing of Listeria monocytogenes. J Infect Dis. 1991;163:574–8. doi: 10.1093/infdis/163.3.574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.