Abstract
Cortisol synchrony is the degree to which mother-toddler cortisol levels are mutually regulated within a dyad. Synchrony’s impact on toddler development is not well understood, so this study investigated how synchronous cortisol levels (reactivity and total concentration) in mother-toddler dyads moderates the association between risk factors (i.e., maternal worry, toddler inhibition) and early internalizing symptoms. Seventy mothers and their 2-year-old toddlers provided interpretable saliva samples. Behavioral observations were made to assess the toddler’s temperament at age 2, and mothers reported on their toddler’s internalizing symptoms when toddlers were 2- and 3-years-old. Results suggest that mother-toddler synchrony in total cortisol concentration moderates the relation between risk factors and internalizing symptoms. Specifically, toddler inhibition and maternal worry were less associated with concurrent toddler internalizing symptoms when dyads demonstrated greater cortisol synchrony in total concentration. Further, synchrony in total cortisol levels marginally moderated the association between toddler inhibition and future internalizing symptoms, such that inhibited toddlers were less likely to demonstrate internalizing symptoms at age 3 when dyads demonstrated more cortisol synchrony. This suggests that cortisol synchrony may serve as an advantageous context that reduces the risk of developing of internalizing symptoms and augments the field’s understanding of the implications of shared physiological responses within mother-toddler dyads.
Keywords: cortisol synchrony, toddlers, inhibited temperament, maternal anxiety, internalizing symptoms
Behavioral synchrony within the caregiver-toddler dyad refers to the mutual and reciprocal regulation of affect and behavior. Such synchrony, also referred to as attunement, mutual regulation, or covariation, is associated with a host of positive socio-emotional developmental outcomes for children (Harrist & Waugh, 2002; Reyna & Pickler, 2009). Synchrony in the physiological systems of mothers and their toddlers may coincide with these synchronous behaviors and reflect increased maternal empathy and sensitivity, but physiological synchrony is a less understood phenomenon. This is likely because extant investigations have focused on factors that influence physiological synchrony as an outcome rather than investigating how synchrony can impact the association between risk factors and developmental outcomes. In order to provide novel information about synchrony’s role across early development, the current study examined cortisol synchrony within mother-toddler dyads as a moderator of the relation between risk factors in toddlerhood and concurrent and future internalizing symptoms. A nuanced understanding of how early emerging internalizing symptoms manifest is particularly important given that early development is an ideal period for interventions to reduce the numerous personal and societal costs associated with internalizing disorders (Rapee, Kennedy, Ingram, Edwards, & Sweeney, 2005).
Cortisol Synchrony
Cortisol synchrony is the degree to which adrenocortical levels covary together within a dyad (Sethre-Hofstad, Stansbury, & Rice, 2002). Cortisol is the hormonal end product that results from activation of the hypothalamic-pituitary-adrenocortical axis (HPA-axis; Gunnar & Quevedo, 2007). Cortisol is secreted throughout the day (i.e., total cortisol concentration) but cortisol is also released in response to stressors (i.e., reactivity) that are perceived as novel, unpredictable, and uncontrollable (Dickerson & Kemeny, 2004). There is also evidence to suggest that the HPA-axis functions in a pulsatile manner throughout the day, fluctuating between periods of activation and inhibition, which can influence how responsive the HPA-axis is to environmental stressors (Young, Abelson, & Lightman, 2004). Together, basal levels and fluctuations in the cortisol response constitute total cortisol concentration whereas the cortisol response to a stressor is captured by measures of cortisol reactivity. These measures of cortisol reflect different parameters of physiological function and are uniquely related to individual differences in mothers and their toddlers (Dickerson & Kemeny, 2004; Gunnar & Quevedo, 2007; Kagan, Reznick, & Snidman, 1987; Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). For example, it has been found that a mother’s total cortisol concentration is related to their parenting, whereas maternal cortisol reactivity was only related to parenting in the context of specific individual differences of the child (Merwin, Smith, & Dougherty, 2015). From such findings, it is clear that these measures of cortisol secretion may function differently to influence behavior and psychological processes.
Cortisol synchrony within mother-toddler dyads is important to consider given that this mutual regulation may facilitate the development of the toddler’s physiological regulatory capacities (Feldman, 2012; Pratt, Singer, Kanat-Maymon, & Feldman, 2015). Theoretical models, like the biobehavioral synchrony model, propose that through mother-toddler interactions, the autonomic, neurological, hormonal, and behavioral processes of both mothers and their toddlers coordinate across the early years to promote toddlers’ optimal emotional growth (Feldman, 2012). This gives rise to self-regulatory behavioral and physiological abilities. By toddlerhood, synchrony shifts from the parent adapting to their infant to more mutual regulation within the dyad. Also during toddlerhood, robust evidence suggests that individual cortisol levels in mothers and their toddlers meaningfully relate to parenting and toddler behaviors relevant for the prediction of future internalizing symptoms (e.g., El-Sheikh, Arsiwalla, Hinnat, & Erath, 2011; Merwin et al., 2015). The implications of individual cortisol levels on outcomes underscore the importance of understanding how shared dyadic cortisol functions to moderate risk for child internalizing outcomes longitudinally.
Extant research has focused separately on cortisol reactivity in response to stressors and daily cortisol levels to characterize caregiver-toddler dyads that are low or high in cortisol synchrony. In reaction to a challenge, dyads demonstrate more cortisol synchrony when mothers and their infants are physically together (Thompson & Trevathan, 2008) and when mothers are more sensitive and responsive to their toddlers (Atkinson et al., 2013; Sethre-Hofstad et al., 2002; van Bakel & Riksen-Walraven, 2008). Given research demonstrating that moderate levels of maternal sensitivity and positive attention are beneficial for toddlers at risk for anxiety (Mount, Crockenberg, Jò, & Wagar, 2010), findings like these suggest that cortisol synchrony may be adaptive for children at increased risk for internalizing symptoms. However, additional studies of cortisol synchrony in reactivity have found risk factors, like maternal depression (Laurent, Ablow, & Measelle, 2012) and family violence (Hibel, Granger, Blair, & Cox, 2009) to be associated with greater dyadic synchrony. Despite these inconsistencies, the bulk of the literature suggests synchrony in cortisol reactivity may be adaptive, particularly with non-clinical populations.
Synchrony in total cortisol levels is higher in families who spend more time together (Papp, Pendry, & Adam, 2009) but also in families characterized by greater stress (Ouellette et al., 2015) and negative affect (Williams et al., 2013). Synchrony in total cortisol concentration is associated with enhanced risk in the context of extreme cortisol responses or when working with clinical samples (Merwin, Smith, Kushner, Lemay, & Dougherty, 2017; Ouellette et al., 2015; Williams et al., 2013). It is possible that synchrony in moderate cortisol responses functions adaptively, but this has yet to be directly investigated. Given that moderate levels of maternal sensitivity are ideal for toddlers at risk for internalizing symptoms (Mount et al., 2010), perhaps cortisol synchrony in total concentration would be adaptive in typically developing samples expected to have more moderate cortisol levels.
Although these findings provide information regarding factors that may be associated with cortisol synchrony in mother-child dyads, a paucity of research exists suggesting how mother-toddler cortisol synchrony can influence early child development. Recent theory about biopsychosocial influences on development suggests that individual and family risk factors for maladjustment should be considered in the context of children’s biological functioning (Calkins, Propper, & Mills-Koonce, 2013). The child’s physiological functioning, however, is also shaped by their caregiver’s biological functioning during early development (Feldman, 2012). As such, cortisol synchrony in mother-toddler dyads could be an important moderator of the association between risk factors and maladaptive outcomes in early development.
Cortisol as a Moderator of Risk Factors and Future Internalizing Symptoms
Internalizing disorders are one of the most prevalent mental health problems in children and are predictive of poor outcomes throughout adolescence and adulthood (Bittner et al., 2007; Lewinsohn, Zinbarg, Seeley, Lewinsohn, & Sack, 1997). Symptoms of internalizing disorders include avoidance, withdrawal, and other anxious and depressive tendencies. Internalizing symptoms cause significant distress to children and their families as childhood internalizing symptoms are associated with school avoidance, peer problems, and academic difficulties (King & Bernstein, 2001; Reijntjes, Kamphuis, Prinzie, & Telch, 2010). Therefore, it is important to identify factors that contribute to the emergence of internalizing symptoms so that early interventions can mitigate the risk for clinical disorders.
Temperament is frequently investigated as it relates to child internalizing outcomes because it reflects a biological and dispositional tendency to respond to one’s environment that persists throughout development (Kagan, Reznick, Clark, Snidman, & Garcia-Coll, 1984). Toddlers with a behaviorally inhibited temperament respond to novel and unpredictable situations with fear, wariness, and avoidant behavior (Kagan et al., 1984). Although inhibited temperament is generally predictive of future internalizing symptoms, particularly anxiety, this is not always the case (Kiel & Buss, 2013; Rubin, Burgess, & Hastings, 2002; Rubin, Hastings, Stewart, Hendersen, & Chen, 1997). This has led to increased interest in determining the factors and contexts that increase or decrease the stability of inhibition over time, with investigations finding that both parenting and cortisol levels are relevant (Kiel & Kalomiris, 2016; Rubin et al., 2002). It is possible that the mutual regulation of cortisol within mother-toddler dyads reflects sensitive and empathetic parenting that can impact the consistency of inhibition over development, though investigations are needed to directly understand cortisol synchrony’s influence.
In addition to temperament, parental factors are often associated with the development of internalizing symptoms. Notably, maternal anxiety is risk factor for early emerging internalizing-spectrum symptoms in children (Beidel & Turner, 1997), and recent investigations revealed that parental worry might be the most potent aspect of anxiety that predicts this intergenerational transmission (Fisak, Holderfield, Douglas-Osborn, & Cartwright-Hatton, 2012). Several investigations have found that parenting behaviors such as overprotection, which serves to reinforce toddler anxiety, as well as genetic factors, are likely mechanisms of the transmission of anxiety from mothers to their children (Beidel & Turner, 1997; Bögels & Melick, 2004; Rubin et al., 1997; van der Bruggen, Stams, & Bögels, 2008). However, sometimes children of anxious parents do not develop any form of psychopathology, making it unclear when such risk factors reliably predict internalizing symptoms in children. Extant theory and research has provided empirical evidence to suggest that a child’s biology and physiological reactivity may interact with life stressors, like maternal psychopathology, to affect the child’s outcomes (Boyce & Ellis, 2005; Enoch, Steer, Newman, Gibson & Goldman, 2010). Additional research is needed to clarify the contexts, such as cortisol levels, that strengthen or weaken these associations between temperament or maternal anxiety and future child symptoms.
Although individual cortisol levels alone are not a reliable predictor of future psychopathological symptoms (Gunnar, 2001), recent work has found that individual cortisol levels function as an important moderator of the relation between risk factors and future maladjustment in children (Obradović, Bush, Stamperdahl, Adler, & Boyce, 2010; Rudolph, Troop-Gordon, & Granger, 2011). Davis and Buss (2012) found that shy toddlers only demonstrated maladaptive play behaviors in the context of poor, as opposed to better, cortisol regulation. When examining both reactivity and total concentration profiles, lower cortisol levels in preschoolers interacted with life stressors to predict increased future internalizing symptoms (Badanes, Watamura & Hankin, 2011). It is possible that these physiological responses within the child are accompanied by maladaptive behaviors that more physiologically synchronous parents are better at perceiving and helping their child to regulate to promote adaptive development (Pratt et al., 2015). Although the impact of individual cortisol levels on development has elicited significant research attention, no research to date has investigated cortisol synchrony within a mother-child dyad as a shared, contextual moderator of child development. Examining cortisol synchrony during this developmental period is crucial as parents serve an important regulatory role in shaping their child’s physiological functioning (Feldman, 2012). It is therefore necessary to more comprehensively understand how synchronous cortisol reactivity and total cortisol levels influence developmental outcomes.
The Current Study
This study sought to identify how mother-toddler cortisol synchrony moderates the relations between maternal worry or toddler inhibition and internalizing symptoms across toddlerhood. Given maternal sensitivity’s protective effects for internalizing outcomes and its association with greater cortisol synchrony in previous investigations (e.g., Sethre-Hofstad et al., 2002), we hypothesized that greater cortisol synchrony would reduce the association between these risk factors and internalizing symptoms. Additionally, given that previous investigations lack a clear-cut distinction regarding how cortisol synchrony in reactivity and total concentration function differently, we hypothesized that cortisol synchrony in both of these parameters will significantly moderate the relation between risk factors and future internalizing symptoms. Results from this investigation have the potential to reveal that shared physiological functioning within mothers and toddlers is an important contextual factor to consider when investigating the development of internalizing symptoms across early childhood.
Method
Participants
Participants were recruited from birth announcements in newspapers and from meetings at a Women, Infants, and Children program. The present study was conducted according to Declaration of Helsinki guidelines, with written informed consent obtained from a parent or guardian for each child before any assessment or data collection. The Campus Institutional Review Board at the University of Missouri approved all procedures.
Of the 117 mother-toddler dyads that participated, only the 70 dyads that provided interpretable saliva samples for both mothers and toddlers will be used for analyses. Mothers were 20.29 to 46.9 years old (M = 33.73), and toddlers were 23.97 to 27.00 months old (M = 24.71). A range of socioeconomic statuses (Hollingshead Index) was represented (Range = 17.00–66.00, M = 49.02). The ethnic composition of the sample was relatively homogenous; most mothers identified themselves and their toddlers as European American. The mother-toddler dyads’ ethnicities are detailed as follows: 87% of mothers and 79% of toddlers were European American, 3% of mothers and 9% of toddlers were African American, 5% of mothers and 8% of toddlers were Asian American, 1% of mothers and 1% of toddlers were American Indian, 0% of mothers and 2% of toddlers were biracial, 1% of mothers and 6% of toddlers were Hispanic/Latino, and 2% of mothers and 1% of toddlers identified as “other.”
Procedure
Mother-toddler dyads completed a 1 1/2 hour-long laboratory visit to assess both toddler inhibited temperament and toddler and mother cortisol reactivity and total cortisol concentration. Mothers completed questionnaires about their worry and their toddler’s internalizing behaviors. Several activities were completed (see Kiel & Buss, 2012 for larger study procedures) during this visit, but only the standardized Risk Room and Spider procedures (Buss & Goldsmith, 2000) are relevant to the current study. The Risk Room required the toddler to spend 3 minutes alone in a room with their mother and five novel objects before being prompted by a primary experimenter to interact with these stimuli (i.e., jump on a trampoline, crawl through a tunnel, walk across a balance beam, put their hand in a scary box, and touch a gorilla mask). During the Spider task, a large, remote-controlled plush spider approached the mother and toddler twice. The experimenter then invited the toddler to touch the spider. Although toddler behaviors during the spider episode are not relevant to this investigation, this is one of the most universally stressful laboratory procedures for toddlers (Buss, 2011) and was used to elicit a cortisol response. One year after this initial laboratory visit shortly following the toddler’s third birthdate (M = 346.67 days, SD = 10.02 days, Range = 328 – 365 days), mothers were invited to complete a follow-up packet of questionnaires. Interested mothers were mailed a new consent form, battery of questionnaires, and an envelope to mail the materials back to the laboratory.
Measures
Cortisol assessments
A baseline saliva sample was collected after a short acclimation period (i.e., 15–30 minutes) by asking mothers and their toddlers to saturate the end of cotton rolls with saliva. In order to best measure the peak cortisol response after a stressor, the postvisit saliva sample was collected 20-minutes after the most stressful novelty episode, the Spider episode (Buss, 2011; Gunnar & Quevedo, 2007). Mothers were also asked to collect a home saliva sample 20 minutes after leaving the laboratory and return it by mail in a provided preaddressed, prestamped envelope. This allowed for a measure of cortisol activity after leaving the laboratory and a better gauge of general daily cortisol levels. For the purpose of the current study, the home saliva sample was not directly used to calculate total cortisol concentration or cortisol reactivity. The home sample was only used as an auxiliary variable to inform imputation should participants be missing the baseline or postvisit saliva sample (Graham, 2009).
Saliva samples were stored in Salivette tubes (Sarstedt, INC) at −50°C before being shipped to Biochemisches Labor in Trier, Germany. Samples were centrifuged for 10 minutes at 2000g and were then each assayed twice using a competitive solid phase time-resolved fluorescence immunoassay with flouromeric end point detection (DELFIA). A minimum of 100 μl of saliva was required to complete each assay. Assay sensitivity ranged from .30–100 nmol/L and intra-assay variation ranged from 4.0%–6.7%.
Maternal worry
Mothers reported their general level of worry using the Penn State Worry Questionnaire (PSWQ; Meyer, Miller, Metzger, & Borkovec, 1990). This 16-item questionnaire is a widely used assessment of anxiety and demonstrates excellent reliability (α = .93; Meyer et al., 1990). Mothers responded to questions measuring the severity and frequency of their worry (e.g., “I worry all the time”) on a 5-point scale (1 = not at all typical to 5 = very typical). Items were averaged together (αcurrent study = .93) to form the final variable of maternal worry.
Toddler temperament
Observational data were coded from the Risk Room paradigm (Buss & Goldsmith, 2000). Trained coders obtained reliability with a master coder before coding independently (ICC = 0.78–0.98). Toddlers’ attempts to be held by their mother, proximity to their mother, and tentativeness of play were coded on a 0 (no display of behavior) to 3 (extreme display of behavior) scale across every 10-second epoch of the episode. The number of objects (0 to 5) with which the toddler interacted when prompted by the experimenter was recorded, as well as the latency in seconds from the start of the episode to the first intentional contact with an object. The number of objects with which the toddler interacted was reverse coded, and all variables were then standardized and averaged together because they demonstrated adequate interrelations (α = .84). Higher scores from this observational measure indicate more extreme inhibited temperament.
Mothers also reported on their toddlers’ temperament using the Toddler Behavior Assessment Questionnaire (TBAQ; Goldsmith, 1996), a 110-item questionnaire that assesses various domains of temperament. This is a commonly used instrument because of its adequate convergent validity and stability over development (Goldsmith, 1996). The 10-items of the Social Fear (α = .83) subscale were averaged together and used for this study (αcurrent study = .82). Using a 7-point scale (1 = never to 7 = always), mothers indicated the frequency of their toddlers’ withdrawal tendencies in novel social situations (e.g., “How often does your toddler show distress or cry when approached by a stranger?”).
To allow a multi-method assessment of temperament, a principal components analysis was used to aggregate the observational and maternal report data into one component that accounted for 65.15% of the variance.
Toddler internalizing symptoms at age 2 and 3
The Infant-Toddler Social Emotional Assessment – Revised (ITSEA; Carter & Briggs-Gowan, 2000) is a widely used maternal-report instrument used to assess behavioral symptoms in toddlers. The ITSEA has been previously shown to relate to independent raters’ perceptions of toddler behavior (Carter, Briggs-Gowan, Jones & Little, 2003). Mothers completed the questionnaire when toddlers were 2- and 3-years-old. The current study used the Internalizing Symptoms domain. Mothers reported on their toddler’s internalizing symptoms over the past month on a 3-point scale (0 = not true or rarely true, 1 = somewhat or sometimes true, 2 = very true or very often true). This 30-question subscale demonstrated adequate reliability (αage 2 = .79, αage 3 = .63) and contained questions assessing the toddler’s depression and withdrawal tendencies (e.g. “Does not make eye contact”), general anxiety (e.g., “Seems nervous, tense, or fearful”), separation distress (e.g., “Hangs on you and wants to be in your lap when with other people”), and level of inhibition when presented with novelty (e.g., “Is shy with new children”). An average of these questions was calculated with higher scores indicating more internalizing symptoms. This subscale was chosen as the age 3 outcome variable because it captures a wide range of internalizing symptoms.
Results
Creation of Synchrony Variables
Outliers (> 3 SDs from the mean) in raw cortisol values from the baseline, postvisit, and home samples were first truncated to the greatest value within 3 SDs of the mean, so no data points were deleted based on extreme values. Figure 1 displays the average cortisol values for each member of the dyad across the visit. Because significant skew was observed for both maternal cortisol values (skew range = 1.8 – 5.8) and toddler cortisol values (skew range = 3.68 – 6.90), all cortisol values were log transformed prior to any analyses. After the log transformation, all cortisol values demonstrated minimal skew (<|1.6|).
Figure 1.
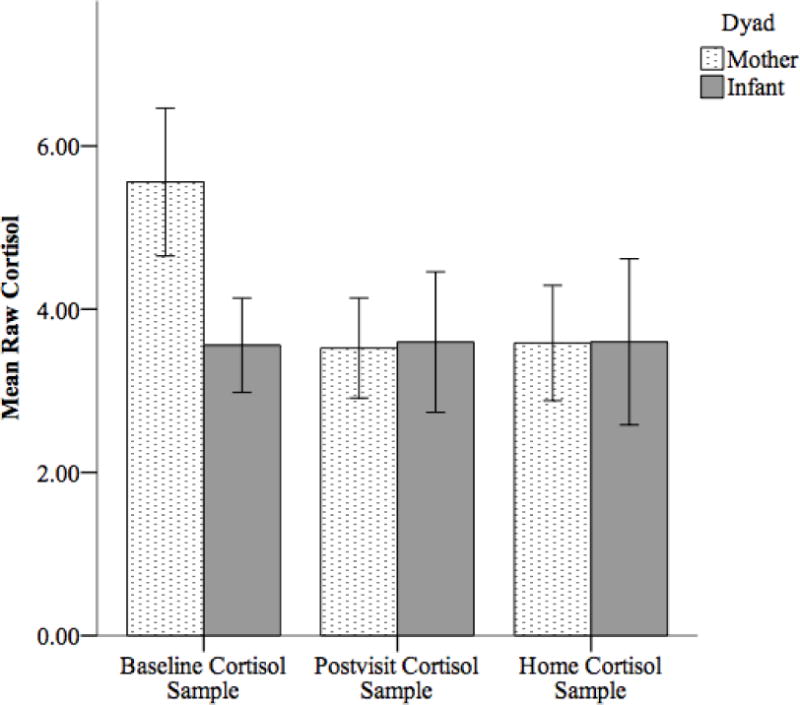
Average raw cortisol values for mothers and infants across three samples. All cortisol data were log transformed prior to analyses. Only the baseline and postvisit cortisol samples were used to calculate area under the curve. The home cortisol sample was used only to inform the imputation algorithm. Error bars represent 95% CI.
In order to calculate levels of cortisol across the visit, two formulas were used: area under the curve with respect to ground (AUCg) and area under the curve with respect to increase (AUCi). These measurements can uniquely relate to psychological assessments because they represent distinct parameters of endocrine function (Pruessner et al., 2003). AUCg reflects the total hormonal concentration over the course of the visit, while AUCi captures the reactivity (change) of the HPA-axis over the course of the visit (see Pruessner et al., 2003 for formulas) and is calculated using the time between samples (M = 1 hour and 12 minutes, SD = 12 minutes). AUC can be calculated from a minimum of two saliva samples (Nicolson, 2008; Weik & Deinzer, 2010; Young & Breslau, 2004), so AUCg and AUCi were calculated from the baseline and postvisit samples only. For clarity, AUCg will be referred to as the “total concentration” and AUCi will be referred to as “reactivity.”
In order to calculate the cortisol synchrony variables, total concentration and reactivity for toddlers were regressed on total concentration and reactivity for mothers, respectively. Individual cortisol values for mothers and toddlers did not differ based on use of any medication, though cortisol values differed based on time of day of the visit (M = 12:22 PM, SD = 3 hours 32 minutes) and the number of hours the toddler had been awake (M = 5 hours 12 minutes, SD = 3 hours 32 minutes), so these variables were included as covariates. Maternal total cortisol concentration was significantly associated with toddler total cortisol concentration (b = 0.47, SE = 0.14, t = 3.34, p < .01), but maternal cortisol reactivity was not significantly associated with toddler reactivity (b = 0.15, SE = 0.21, t = 0.72, p = .47). The unstandardized residuals from each of these regression equations yielded a continuous measure of cortisol synchrony in the mother-toddler dyad for both total concentration and reactivity. That is, higher scores indicate more cortisol synchrony than would be expected given the average level of responding across the sample and lower scores indicate less cortisol synchrony than would be expected.
Covariates
Potential covariates were investigated using the pre-imputed dataset following the creation of the cortisol synchrony variables. SES was unrelated to any variable of interest (Table 1). Gender was related to age 3 internalizing symptoms (r = .24, p = .04), with females being more likely to demonstrate internalizing symptoms, so it was included in models investigating age 3 internalizing symptoms as a dependent variable. In addition, an auxiliary variable was examined that assesses the mother’s ability to accurately predict her child’s behaviors (i.e., maternal accuracy) because it is relevant for the development of anxiety symptoms in children (Kiel & Buss, 2012). Maternal accuracy was related to age 2 and age 3 internalizing symptoms (r = .41, p < .001, r = .39, p <.001, respectively), so it was controlled for in all analyses. It was confirmed that cosleeping (n = 6; total concentration: t(40) = 0.92, p = .36; reactivity: t(40) = −0.15, p = .88) and current breastfeeding (n = 2; total concentration: t(40) = 0.49, p = .63; reactivity: t(40) = −1.20, p = .24) were not related to cortisol synchrony.
Table 1.
Descriptive Statistics and Bivariate Relations Among the Variables
| Pooled Mean | SD | Range | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Synchrony in concentration | −0.92 | 12.31 | −21.74 – 39.37 | .14 | .07 | .35** | .12 | .11 | .11 | .19 |
| 2. Synchrony in reactivity | −1.02 | 9.76 | −22.54 – 28.11 | – | −.19 | .17 | −.10 | −.13 | .02 | .17 |
| 3. Maternal worry | 2.73 | 0.71 | 1.25 – 4.31 | – | .25† | .32* | .22 | −.02 | −.05 | |
| 4. Inhibited temperament | −0.10 | 0.95 | −1.60 – 2.66 | – | .60*** | .34* | −.03 | .44*** | ||
| 5. Age 2 internalizing | 0.54 | 0.24 | 0.00 – 1.04 | – | .49** | −.15 | .40** | |||
| 6. Age 3 internalizing | 0.49 | 0.20 | 0.11 – 0.91 | – | −.04 | .28* | ||||
| 7. SES | 47.85 | 13.39 | 17.00 – 66.00 | – | .14 | |||||
| 8. Maternal Accuracy | 0.08 | 0.04 | 0.01 – 0.18 | – |
Note. Synchrony represents the unstandardized residual from a regression equation in which maternal cortisol output predicted toddler cortisol output. Inhibited temperament is a composite of observed and maternal-reported inhibition.
p < .10,
p < .05,
p < .01,
p < .001.
Missing Data and Imputation
Of the total 117 participants, 62.39% (n=73) of mothers and 70.94% (n=83) of toddlers provided a baseline saliva sample, 59.83% mothers (n=70) and 74.36% (n=87) of toddlers provided a postvisit sample, and 70.09% (n=82) of mothers and 84.62% (n=99) of toddlers provided at least one of those samples. Across dyads, 59.82% (n=70) of mothers and toddlers provided at least one saliva sample. Generally, mothers and toddlers with missing cortisol values had samples that could not be analyzed due to failure to fully saturate the cotton roll or eating immediately before the laboratory visit. Only 2.6% (n=3) of mothers refused to provide saliva samples or have them taken from their toddlers.
Several analyses were conducted to determine if any variables related to missingness of individual saliva samples. Mothers were more likely to be missing saliva samples if they reported more anxiety (t[89] = 3.09, p = .003) or if they reported that their toddler demonstrated more withdrawal symptoms (t[107] = 2.08, p = .040) and fewer separation distress symptoms (t[107] = −2.07, p = .041). Female toddlers were also more likely than male toddlers to be missing saliva samples (t[115] = 2.67, p = .009). In addition, toddlers were more likely to be missing samples if their mothers reported they were more withdrawn and anxious at age 2 (t[107] = 2.03, p = .045, t[107] = 2.51, p = .013, respectively), reportedly demonstrated greater internalizing symptoms at age 2 and 3 (t[107] = 2.15, p = .034, t[74] = 2.27, p = .026, respectively), and demonstrated more behavioral inhibition in the laboratory (t[109] = 2.11, p = .037).
According to Little’s MCAR test, our data were consistent with the pattern of missing completely at random (X2[943] = 940.260, p = .537). Because this test measures patterns of missing data across the entire dataset and not only those variables being imputed, additional t-tests and correlations were used to determine the pattern of missing data among the cortisol synchrony and age 3 internalizing symptoms variables. Cortisol synchrony in total cortisol concentration was related to gender (r = .33, p < .05), observed inhibited temperament (r = .50, p < .01), and maternal accuracy (r = .31, p < .05), while cortisol synchrony in reactivity was unrelated to any variables of interest. Missingness of cortisol synchrony was only related to maternal worry (t[89] = 1.97, p = .053), with cortisol synchrony more likely to be missing when mothers reported greater symptoms of worry. Internalizing symptoms at age 3 was related to gender (r = .24, p < .05), observed inhibited temperament (r = .36, p < .01), and maternal accuracy (r = .39, p < .01), in addition to several subscales from the age 2 ITSEA relevant for internalizing symptoms (rs range from .21 to .47, p < .10). Missingness of maternal report of internalizing symptoms at age 3 was related to increased age 2 maternal report of toddler worry (t[106] = 3.38, p = .001), anxiety (t[106] = 2.13, p = .035) and internalizing symptoms (t[106] = 2.22, p = .030), greater observed behavioral inhibition (t[109] = 2.18, p = .031), having a female toddler (t[115] = 1.99, p = .049), increased maternal accuracy (t[114] = 2.14, p = .035), and lower socio-economic status (t[115] = −3.05, p = .003).
Contemporary multiple imputation techniques were used to replace missing cortisol synchrony in total concentration and reactivity and internalizing symptoms at age 3 from the original sample of 117 participants. Experts suggest that multiple imputation is acceptable when as much as 50% of the data is missing (Graham & Schafer, 1999) and because only 33% of age 3 internalizing symptoms and 40% of the cortisol synchrony variables were missing, this is well within this acceptable range for imputation. Additionally, cortisol synchrony in total concentration and reactivity were imputed following suggestions from Graham (2009) to impute variables in their most processed and final form to be used in analyses. All primary and auxiliary variables related to those being imputed or their missingness were included in the algorithm (see above; Collins, Schafer, & Kam, 2001; Graham, 2009). A total of 40 imputed datasets were generated (Graham, Olchowski, & Gilreath, 2007). Given the fundamental importance of the cortisol synchrony variable for our analyses, we felt that it was inappropriate to impute cortisol if each member of the dyad did not provide at least one saliva sample on which to base the imputation. As such, participants who did not provide at least a baseline or postvisit sample from each member of the dyad were removed for analyses, bringing the total number of participants to 70.
Descriptive statistics and bivariate relations
Table 1 lists the descriptive statistics and bivariate relations among the constructs of interest following imputation. Pooled means and correlations are reported. The reported standard deviations and ranges are from the pre-imputed dataset.
Data Analysis Plan
Synchrony in total cortisol concentration was tested as a moderator of the association between the age 2 independent variables (i.e., maternal worry, toddler inhibition) and internalizing symptoms at age 2, age 3, and age 3 controlling for age 2 (to assess for change) as separate dependent variables. The same analyses were completed with cortisol synchrony in reactivity as a moderator. All models controlled for maternal accuracy given its relation to both the age 2 and 3 outcome variables. Additionally, given gender’s significant relation to age 3 internalizing symptoms, gender was controlled for in all longitudinal models with age 3 internalizing symptoms as the outcome of interest. Moderations were tested by calculating the cross-product of the risk factor (maternal worry or toddler inhibition) and cortisol synchrony (total cortisol concentration or reactivity) and including this interaction term in a regression model along with the main effects. If the interaction term was significant, the interaction was probed by recentering the cortisol synchrony variable at ± 1 SD of the original, pre-imputed sample around the pooled mean and calculating the simple slopes of the independent variable at high (+1 SD), mean, and low (−1 SD) levels of cortisol synchrony (Aiken & West, 1991).
Moderation Analyses for Maternal Worry and Internalizing Symptoms
Synchrony in total cortisol concentration moderated the relation between maternal worry and internalizing symptoms at age 2 (Table 2; Figure 2, Panel A). Probing this interaction revealed that maternal worry related to concurrent toddler internalizing behaviors when the dyad was characterized by low (b = 0.19, SE = 0.06, t = 3.46, p = .001, 95% CI [0.08, 0.30], sr2 = .184) and mean (b = 0.09, SE = 0.04, t = 2.41, p = .016, CI [0.02, 0.17], sr2 = .086), but not high levels of cortisol synchrony (b = −0.01, SE = 0.07, t = −0.07, p = .941, CI [−0.13, 0.12], sr2 = .000). Synchrony in total cortisol concentration did not moderate the relation between maternal worry and internalizing symptoms at age 3 or change in internalizing from age 2 to age 3 (Table 2).
Table 2.
Moderations by mother-toddler cortisol synchrony
| Total Cortisol Concentration (AUCg) |
Reactivity (AUCi) |
|||||||
|---|---|---|---|---|---|---|---|---|
| b | SE | t | p | b | SE | t | p | |
| Maternal worry predicting toddler internalizing symptoms at age 2 | ||||||||
| Constant | 0.07 | 0.12 | 0.58 | .564 | 0.04 | 0.13 | 0.29 | .775 |
| Accuracy | 2.29 | 0.64 | 3.61 | .001 | 2.44 | 0.66 | 3.72 | .001 |
| Worry | 0.09 | 0.04 | 2.41 | .016 | 0.10 | 0.04 | 2.50 | .012 |
| Synchrony | 0.02 | 0.01 | 2.09 | .036 | −0.00 | 0.02 | −0.21 | .832 |
| Worry X synchrony | −0.01 | 0.00 | −2.14 | .033 | 0.00 | 0.01 | 0.05 | .961 |
| Maternal worry predicting toddler internalizing symptoms at age 3 | ||||||||
| Constant | 0.15 | 0.14 | 1.05 | .292 | 0.13 | 0.14 | 0.93 | .355 |
| Accuracy | 1.72 | 0.79 | 2.18 | .029 | 1.83 | 0.79 | 2.31 | .021 |
| Gender | 0.01 | 0.07 | 0.19 | .848 | 0.02 | 0.07 | 0.29 | .774 |
| Worry | 0.06 | 0.05 | 1.38 | .167 | 0.07 | 0.05 | 1.39 | .166 |
| Synchrony | 0.02 | 0.01 | 1.12 | .262 | −0.01 | 0.02 | −0.38 | .707 |
| Worry X synchrony | −0.01 | 0.01 | −1.10 | .274 | 0.00 | 0.01 | 0.19 | .852 |
| Maternal worry predicting change in toddler internalizing symptoms by age 3 | ||||||||
| Constant | 0.12 | 0.14 | 0.90 | .368 | 0.12 | 0.13 | 0.88 | .378 |
| Accuracy | 0.83 | 0.86 | 0.97 | .332 | 0.86 | 0.87 | 0.98 | .328 |
| Gender | 0.06 | 0.07 | 0.82 | .412 | 0.06 | 0.07 | 0.93 | .355 |
| Age 2 internalizing | 0.35 | 0.18 | 1.93 | .054 | 0.37 | 0.18 | 2.04 | .042 |
| Worry | 0.03 | 0.05 | 0.59 | .555 | 0.03 | 0.05 | 0.52 | .601 |
| Synchrony | 0.01 | 0.01 | 0.53 | .600 | −0.01 | 0.02 | −0.29 | .771 |
| Worry X synchrony | −0.00 | 0.01 | −0.50 | .619 | 0.00 | 0.01 | 0.14 | .892 |
| Toddler temperament predicting toddler internalizing symptoms at age 2 | ||||||||
| Constant | 0.48 | 0.05 | 8.90 | .001 | 0.47 | 0.06 | 8.23 | .001 |
| Accuracy | 1.24 | 0.61 | 2.05 | .041 | 1.05 | 0.65 | 1.62 | .104 |
| Temperament | 0.16 | 0.03 | 5.43 | .001 | 0.14 | 0.03 | 4.79 | .001 |
| Synchrony | −0.00 | 0.00 | −0.90 | .367 | −0.01 | 0.00 | −1.63 | .104 |
| Temp X Synchrony | −0.01 | 0.00 | −3.19 | .001 | −0.00 | 0.00 | −0.40 | .688 |
| Toddler temperament predicting toddler internalizing symptoms at age 3 | ||||||||
| Constant | 0.40 | 0.07 | 6.00 | .001 | 0.39 | 0.07 | 5.73 | .001 |
| Accuracy | 0.99 | 0.80 | 1.25 | .214 | 0.93 | 0.79 | 1.18 | .239 |
| Gender | 0.07 | 0.06 | 1.16 | .246 | 0.07 | 0.06 | 1.10 | .272 |
| Temperament | 0.07 | 0.04 | 2.01 | .045 | 0.07 | 0.04 | 1.95 | .052 |
| Synchrony | −0.00 | 0.00 | −0.23 | .819 | −0.01 | 0.00 | −1.40 | .163 |
| Temp X synchrony | −0.00 | 0.00 | −1.72 | .086 | −0.00 | 0.00 | −0.39 | .695 |
| Toddler temperament predicting change in toddler internalizing symptoms by age 3 | ||||||||
| Constant | 0.25 | 0.11 | 2.33 | .020 | 0.23 | 0.10 | 2.30 | .022 |
| Accuracy | 0.63 | 0.78 | 0.81 | .417 | 0.61 | 0.77 | 0.79 | .428 |
| Gender | 0.07 | 0.06 | 1.14 | .254 | 0.07 | 0.06 | 1.11 | .266 |
| Age 2 internalizing | 0.32 | 0.18 | 1.82 | .069 | 0.33 | 0.17 | 1.98 | .049 |
| Temperament | 0.03 | 0.04 | 0.62 | .534 | 0.02 | 0.04 | 0.56 | .576 |
| Synchrony | 0.00 | 0.00 | −0.06 | .954 | −0.00 | 0.00 | −0.96 | .338 |
| Temp X synchrony | −0.00 | 0.00 | −0.99 | .326 | −0.00 | 0.00 | −0.31 | .760 |
Note. Accuracy and gender were included in models where they were significantly related to the dependent variable.
Figure 2.
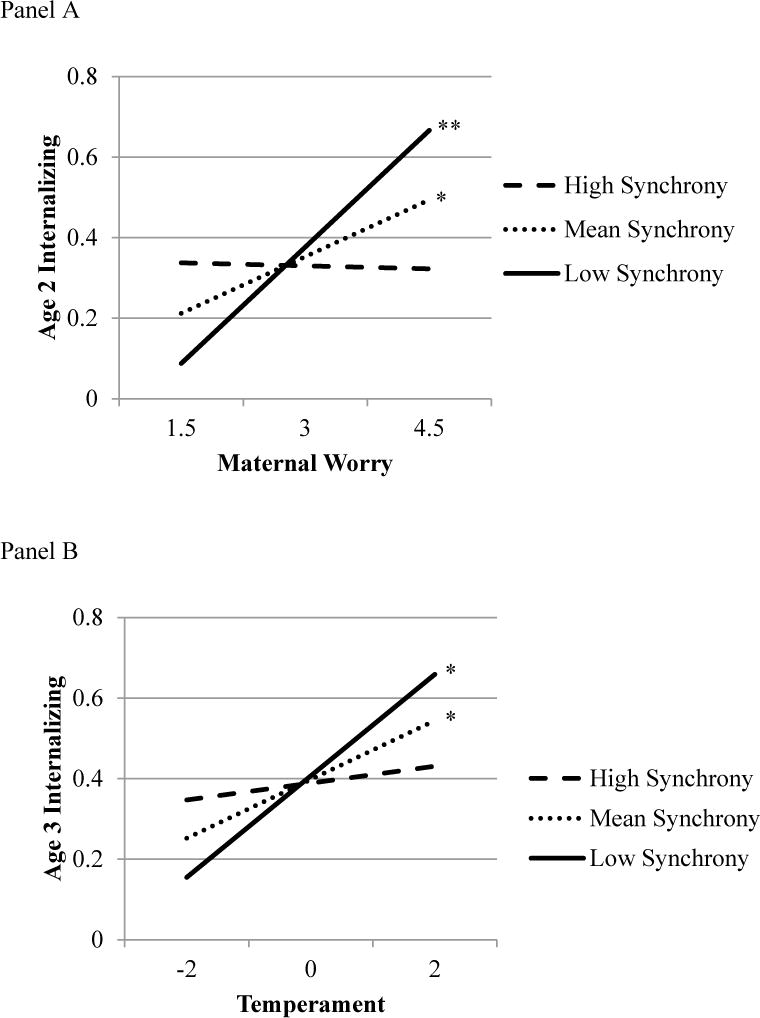
Panel A: Interaction between maternal worry and cortisol synchrony between mother-toddler dyads. Maternal worry significantly predicted increased internalizing symptoms at age 2 at low and mean levels of synchrony only. Panel B: Interaction between age 2 inhibited temperament and cortisol synchrony between mother-toddler dyads. Inhibited temperament at age 2 significantly predicted internalizing symptoms by age 3 at low levels and mean levels of synchrony only.
*p < .05, **p < .01.
Synchrony in cortisol reactivity did not moderate the relation between maternal worry and internalizing symptoms at age 2, age 3, or change in internalizing from age 2 to age 3 (Table 2).
Moderation Analyses for Toddler Inhibition and Internalizing Symptoms
Synchrony in the total concentration of cortisol moderated the relation between inhibition and internalizing symptoms at age 2 (Table 2). Although this association remained significant at all levels of cortisol synchrony, probing revealed that as cortisol synchrony increased, the association between temperament and internalizing symptoms weakened (low [−1 SD]: b = 0.23, SE = 0.04, t = 5.71, p = .001, 95% CI [0.15, 0.31], sr2 = .310; mean: b = 0.16 SE = 0.03, t = 5.43, p = .001, 95% CI [0.10, 0.21], sr2 = .272; high [+1 SD]: b = 0.08, SE = 0.03, t = 2.37, p = .018, 95% CI [0.01, 0.15], sr2 = .052).
Synchrony in total cortisol concentration also marginally moderated the relation between temperament at age 2 and internalizing symptoms at age 3 (Table 2; Figure 2, Panel B). The association between temperament and future internalizing symptoms was significant at low (b = 0.13, SE = 0.05, t = 2.44, p = .015, 95% CI [0.03, 0.23], sr2 = .104) and mean (b = 0.07, SE = 0.04, t = 2.01, p = .045, 95% CI [0.00, 0.15], sr2 = .071), but not high levels of cortisol synchrony (b = 0.02, SE = 0.04, t = 0.47, p = .636, 95% CI [−0.07, 0.11], sr2 = .004).
Synchrony in total cortisol concentration did not moderate the association between age 2 inhibited temperament and change in internalizing symptoms from age 2 to age 3 (Table 2).
Synchrony in cortisol reactivity did not moderate the relation between inhibition and internalizing symptoms at age 2, age 3, or change in internalizing from age 2 to age 3 (Table 2).
Discussion
Results from this investigation suggest that the extent of shared physiology within mother-toddler dyads should be considered as a context in which risk factors, like toddler temperament and maternal worry, relate to toddler internalizing symptoms. Specifically, mother-toddler cortisol synchrony in total concentration moderated the association between maternal worry and concurrent toddler internalizing symptoms at age 2, as well as the relation between toddler inhibition at age 2 and both concurrent and future toddler internalizing symptoms, albeit marginally. These moderations suggest that increased cortisol synchrony in total concentration is associated with less risk because the risk factors seemed to be operating solely or most strongly at lower and mean levels of synchrony. This provides novel information regarding the intergenerational transmission of internalizing symptoms and the stability of inhibited temperament to internalizing symptoms across time. These findings also contribute to existing models of biobehavioral synchrony and provide numerous avenues for future investigation.
It was surprising to find that only total cortisol concentration was related within the dyads, whereas cortisol reactivity in mothers was not significantly associated with cortisol responses in toddlers. This suggests that mother-toddler dyads are more attuned with the other’s total cortisol concentrations as opposed to each other’s stress response. Although it would have certainly been informative to understand how mothers’ and toddlers’ stress response systems mutually react, finding that total cortisol concentration is synchronous provides useful information about the dyad’s general cortisol levels across more than just stressful periods. This measure still captures reactivity of the HPA-axis but also accounts for baseline levels when the dyad entered the laboratory. Although the raw cortisol samples suggest that there was little cortisol reactivity across the sample (Figure 1), this is simply an average across all participants and not representative of the individual dyadic differences in reactivity targeted by this investigation. Our data suggest that it may be necessary to consider this baseline level when investigating cortisol synchrony within mother-toddler dyads. Several previous investigations, however, have found synchrony in the mother-toddler dyad’s cortisol responses to stressors (Atkinson et al., 2013; Sethre-Hofstad et al., 2002; van Bakel & Riksen-Walraven, 2008). Potentially, the methodology employed in this investigation did not capture synchrony in cortisol reactivity because the novelty episodes may have been differentially stressful for mothers and toddlers. Future research should employ a person-centered approach to examine the impact of different combinations of non-synchronous cortisol reactivity in mother-child dyads (e.g., mom high/child low, child high/mom low). Our investigation was also limited by having only two saliva samples from a small sample of participants, which provides a blunt measure of cortisol that may have sufficed to assess total cortisol concentration but it may not have captured the subtleties in reactivity required to assess cortisol synchrony. Perhaps, synchrony in cortisol reactivity should be assessed from a greater number of saliva samples and from a larger sample of participants in future investigations.
It was also surprising that individual differences in cortisol synchrony in reactivity did not moderate the relation between maternal worry or toddler temperament and toddler internalizing symptoms. This should not be taken to mean that cortisol synchrony in the mother-toddler stress response does not impact toddler outcomes. As detailed above, perhaps our blunt measure of cortisol reactivity was not nuanced enough to adequately assess the cortisol response to our laboratory-based stressor. It is also possible that because stressors occur with less frequency, cortisol reactivity provides fewer opportunities for synchrony to influence how risk factors influence early emerging internalizing symptoms. This speculation is consistent with our finding that synchrony in total cortisol concentrations exerts a significant influence over child outcomes because cortisol synchrony in this parameter happens with greater relative frequency.
Results from this investigation indicated that synchrony in total cortisol concentration moderated the association between maternal worry and toddler internalizing symptoms at age 2 only. This suggests that shared physiology is an important context to consider in understanding how internalizing symptoms are transmitted across generations (Beidel & Turner, 1997). This also supports the extant biobehavioral synchrony model, by suggesting that synchrony may be limited by maternal psychopathology (Feldman, 2012). Perhaps mothers who are more anxious are less affectively and behaviorally available to their child and this prevents the development synchronous physiological functioning that is adaptive for child development. Although understanding how cortisol synchrony is associated with maternal behaviors is beyond the scope of this investigation, anxious mothers typically demonstrate distinct parenting behaviors, like overprotection or overcontrol, that can predict the development of internalizing symptoms in their children (Bögels & Melick, 2004; Nicol-Harper, Harvey, & Stein, 2007). Although several parenting behaviors, like sensitivity, have been associated with cortisol synchrony in previous investigations (e.g., Sethre-Hofstad et al., 2002), it would be more comprehensive and contribute substantially more to the biobehavioral synchrony model to identify parenting behaviors associated with cortisol synchrony in a single model in order to understand the mechanisms that influence child outcomes. In addition, future research could elucidate how other symptoms of anxiety outside of worry (e.g., behavioral avoidance, panic) may interact differently with cortisol synchrony to influence outcomes.
Finding that toddler inhibition is related to internalizing symptoms at age 2 and at age 3 most strongly at low and mean levels of cortisol synchrony suggests that a lower degree of shared biology is an important contextual factor that can increase the consequences of inhibition across early development. Biobehavioral synchrony models suggest that synchrony in physiology and affect between mother-infant dyads contributes to the development of adaptive self-regulation (Feldman, 2012). Consistent with this theory, inhibited children who demonstrate more cortisol synchrony with their mothers may be protected from future internalizing symptoms because this synchrony coincides with the development of necessary child emotional and self-regulation abilities. Potentially, the mismatch in shared biology indicated by low cortisol synchrony also manifests in identifiable maternal behaviors that could be therapeutic targets during a particularly sensitive time for intervention (Rapee et al., 2005). Continued investigation is needed to understand the specific behavioral correlates of cortisol synchrony that provides the mechanism by which synchrony is associated with young children’s internalizing outcomes. Regardless, these results contribute to a recently growing area of the literature that seeks to determine factors that increase or decrease the consistency of inhibition across development.
Across both models investigating maternal worry and inhibited temperament as independent variables, cortisol synchrony emerged as an important moderator of concurrent and stable levels of internalizing symptoms rather than change. Although the age 2 and age 3 internalizing symptoms scales were correlated, this correlation was modest and suggests that meaningful variance in internalizing symptoms persists throughout time and is associated with early observed inhibited temperament. Previous investigations have found similar patterns when assessing change in internalizing symptoms in this developmental period (Brooker et al., 2014). Importantly, results imply that early interventions to address cortisol synchrony and inhibited temperament are essential because these internalizing symptoms seem to persist across the preschool period.
Existing investigations have demonstrated that more extreme cortisol levels are associated with cortisol synchrony, potentially suggesting that physiological dysregulation in one member of the dyad is problematic (Laurent et al., 2012; Ouellette et al., 2015; Williams et al., 2013). Although understanding the effect of cortisol level on synchrony is beyond the scope of this investigation, future research should investigate how individual cortisol levels of mothers and their toddlers moderate the impact cortisol synchrony has on outcomes.
There are several limitations of the current project that should be considered. First, evidence suggests that genetics influence HPA-axis activity and glucocorticoid receptors, though cortisol synchrony’s genetic underpinnings were not directly investigated by the current project (Schatzberg et al., 2014). Future work should consider this to more holistically understand how the shared genetic material in mother-toddler dyads contributes to cortisol synchrony and child risk for internalizing symptoms. This project was conducted with a primarily Caucasian, middle-class sample so future investigations should determine if these results generalize to other socioeconomic and ethnic groups. Physiology can motivate different behaviors in cultures outsides of North America, so research should determine how cortisol synchrony might function differently in other countries (Grabell et al., 2015). Additionally, the outcome variable was assessed via maternal-report of toddler symptoms, and observational measures may have yielded different results. All analyses were also limited by a small sample size; a larger sample would have allowed simultaneous analysis of all independent variables to determine the relative strength of moderators and their association with internalizing symptoms. Replication is also warranted given our small sample size and extent of missing data. Finally, it would be more holistic to investigate how synchrony in other physiological parameters (e.g., cardiovascular activity, neurological responses) affects development.
Conclusions
In conclusion, the results suggest one way in which cortisol synchrony in mother-toddler dyads impacts how risk factors function across development. This provides novel information about the shared physiological conditions under which toddlers are less likely to develop future internalizing symptoms.
Acknowledgments
The project from which these data were derived was supported, in part, by a National Research Service Award from the National Institute of Mental Health (F31 MH077385-01) awarded to the second author and by a grant to Dr. Kristin Buss from the National Institute of Mental Health (R01 MH075750). The authors express appreciation to the families and toddlers who participated in this project.
Footnotes
The authors declare no conflicts of interest with regard to the funding source for this study.
References
- Aiken LS, West SG. Multiple regression testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Atkinson L, Gonzalez A, Kashy DA, Santo Basile V, Masellis M, Pereira J, Levitan R. Maternal sensitivity and infant and mother adrenocortical function across challenges. Psychoneuroendocrinology. 2013;38(12):2943–2951. doi: 10.1016/j.psyneuen.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Badanes LS, Watamura SE, Hankin BL. Hypocortisolism as a potential marker of allostatic load in children: Associations with family risk and internalizing disorders. Development and Psychopathology. 2011;23(03):881–896. doi: 10.1017/S095457941100037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beidel DC, Turner SM. At risk for anxiety: I. Psychopathology in the offspring of anxious parents. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):918–924. doi: 10.1097/00004583-199707000-00013. [DOI] [PubMed] [Google Scholar]
- Bittner A, Egger HL, Erkanli A, Costello EJ, Foley DL, Angold A. What do childhood anxiety disorders predict? Journal of Child Psychology and Psychiatry. 2007;48(12):1174–1183. doi: 10.1111/j.1469-7610.2007.01812.x. [DOI] [PubMed] [Google Scholar]
- Bögels SM, Melick MV. The relationship between child-report, parent self-report, and partner report of perceived parental rearing behaviors and anxiety in children and parents. Personality and Individual Differences. 2004;37(8):1583–1596. [Google Scholar]
- Brooker RJ, Neiderhiser JM, Ganiban JM, Leve LD, Shaw DS, Reiss D. Birth and adoptive parent anxiety symptoms moderate the link between infant attention control and internalizing problems in toddlerhood. Development and Psychopathology. 2014;26:347–359. doi: 10.1017/S095457941300103X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17(02):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Buss KA. Which fearful toddlers should we worry about? Context, fear regulation, and anxiety risk. Developmental Psychology. 2011;47(3):804–819. doi: 10.1037/a0023227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, Goldsmith HH. Manual and normative data for the Laboratory Temperament Assessment Battery–Toddler version. Madison, WI: Psychology Department Technical Report, University of Wisconsin; 2000. [Google Scholar]
- Calkins SD, Propper C, Mills-Koonce WR. A biopsychosocial perspective on parenting and developmental psychopathology. Development and Psychopathology. 2013;25(4):1399–1414. doi: 10.1017/S0954579413000680. [DOI] [PubMed] [Google Scholar]
- Carter AS, Briggs-Gowan MJ. Infant–Toddler Social and Emotional Assessment–Revised (ITSEA) New Haven, CT: Yale University, Department of Psychology; 2000. [Google Scholar]
- Carter AS, Briggs-Gowan MJ, Jones SM, Little TD. The infant–toddler social and emotional assessment (ITSEA): Factor structure, reliability, and validity. Journal of Abnormal Child Psychology. 2003;31(5):495–514. doi: 10.1023/a:1025449031360. [DOI] [PubMed] [Google Scholar]
- Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychological Methods. 2001;6(4):330–351. [PubMed] [Google Scholar]
- Davis EL, Buss KA. Moderators of the relation between shyness and behavior with peers: Cortisol dysregulation and maternal emotion socialization. Social Development. 2012;21(4):801–820. doi: 10.1111/j.1467-9507.2011.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Arsiwalla DD, Hinnant JB, Erath SA. Children’s internalizing symptoms: The role of interactions between cortisol and respiratory sinus arrhythmia. Physiology & Behavior. 2011;103(2):225–232. doi: 10.1016/j.physbeh.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Steer CD, Newman TK, Gibson N, Goldman D. Early life stress, MAOA, and gene‐environment interactions predict behavioral disinhibition in children. Genes, Brain and Behavior. 2010;9(1):65–74. doi: 10.1111/j.1601-183X.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. Parent–infant synchrony: A biobehavioral model of mutual influences in the formation of affiliative bonds. Monographs of the Society for Research in Child Development. 2012;77(2):42–51. [Google Scholar]
- Fisak B, Holderfield KG, Douglas-Osborn E, Cartwright-Hatton S. What do parents worry about? Examination of the construct of parent worry and the relation to parent and child anxiety. Behavioural and Cognitive Psychotherapy. 2012;40(5):542–557. doi: 10.1017/S1352465812000410. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH. Studying temperament via construction of the Toddler Behavior Assessment Questionnaire. Child Development. 1996;67(1):218–235. [PubMed] [Google Scholar]
- Grabell AS, Olson SL, Miller AL, Kessler DA, Felt B, Kaciroti N, Tardif T. The impact of culture on physiological processes of emotion regulation: A comparison of US and Chinese preschoolers. Developmental Science. 2015;18(3):420–435. doi: 10.1111/desc.12227. [DOI] [PubMed] [Google Scholar]
- Graham JW. Missing data analysis: Making it work in the real world. Annual Review of Psychology. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prevention Science. 2007;8(3):206–213. doi: 10.1007/s11121-007-0070-9. [DOI] [PubMed] [Google Scholar]
- Graham JW, Schafer JL. On the performance of multiple imputation for multivariate data with small sample size. Statistical Strategies for Small Sample Research. 1999;50:1–27. [Google Scholar]
- Gunnar MR. The role of glucocorticoids in anxiety disorders: A critical analysis. In: Vasey MW, Dadds MR, editors. Developmental psychopathology of anxiety. New York, NY: Oxford University Press; 2001. pp. 143–159. [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Harrist AW, Waugh RM. Dyadic synchrony: Its structure and function in children’s development. Developmental Review. 2002;22(4):555–592. [Google Scholar]
- Hibel LC, Granger DA, Blair C, Cox MJ. Intimate partner violence moderates the association between mother–infant adrenocortical activity across an emotional challenge. Journal of Family Psychology. 2009;23(5):615–625. doi: 10.1037/a0016323. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Clarke C, Snidman N, Garcia-Coll C. Behavioral inhibition to the unfamiliar. Child Development. 1984;55(6):2212–2225. [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Development. 1987;58:1459–1473. [PubMed] [Google Scholar]
- Kiel EJ, Buss KA. Associations among context‐specific maternal protective behavior, toddlers’ fearful temperament, and maternal accuracy and goals. Social Development. 2012;21(4):742–760. doi: 10.1111/j.1467-9507.2011.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel EJ, Buss KA. Toddler inhibited temperament, maternal cortisol reactivity and embarrassment, and intrusive parenting. Journal of Family Psychology. 2013;27(3):512–517. doi: 10.1037/a0032892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel EJ, Kalomiris AE. Correlates and consequences of toddler cortisol reactivity to fear. Journal of Experimental Child Psychology. 2016;142:400–413. doi: 10.1016/j.jecp.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King NJ, Bernstein GA. School refusal in children and adolescents: A review of the past 10 years. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40(2):197–205. doi: 10.1097/00004583-200102000-00014. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC, Measelle J. Taking stress response out of the box: Stability, discontinuity, and temperament effects on HPA and SNS across social stressors in mother–infant dyads. Developmental Psychology. 2012;48(1):35–45. doi: 10.1037/a0025518. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Zinbarg R, Seeley JR, Lewinsohn M, Sack WH. Lifetime comorbidity among anxiety disorders and between anxiety disorders and other mental disorders in adolescents. Journal of Anxiety Disorders. 1997;11(4):377–394. doi: 10.1016/s0887-6185(97)00017-0. [DOI] [PubMed] [Google Scholar]
- Merwin SM, Smith VC, Dougherty LR. “It takes two”: The interaction between parenting and child temperament on parents’ stress physiology. Developmental Psychobiology. 2015;57(3):336–348. doi: 10.1002/dev.21301. [DOI] [PubMed] [Google Scholar]
- Merwin SM, Smith VC, Kushner M, Lemay EP, Dougherty LR. Parent-child adrenocortical concordance in early childhood: The moderating role of parental depression and child temperament. Biological Psychology. 2017;124:100–110. doi: 10.1016/j.biopsycho.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 1990;28(6):487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Mount KS, Crockenberg SC, Jó PSB, Wagar JL. Maternal and child correlates of anxiety in 2½-year-old children. Infant Behavior and Development. 2010;33(4):567–578. doi: 10.1016/j.infbeh.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Nicol-Harper R, Harvey AG, Stein A. Interactions between mothers and infants: Impact of maternal anxiety. Infant Behavior and Development. 2007;30(1):161–167. doi: 10.1016/j.infbeh.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson NA. Measurement of cortisol. In: Luecken JL, Gallo LC, editors. Handbook of physiological research methods in health psychology. Thousand Oaks, CA: Sage Publications; 2008. pp. 37–74. [Google Scholar]
- Obradović J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development. 2010;81(1):270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette SJ, Russell E, Kryski KR, Sheikh HI, Singh SM, Koren G, Hayden EP. Hair cortisol concentrations in higher- and lower-stress mother–daughter dyads: A pilot study of associations and moderators. Developmental Psychobiology. 2015;57(5):519–534. doi: 10.1002/dev.21302. [DOI] [PubMed] [Google Scholar]
- Papp LM, Pendry P, Adam EK. Mother-adolescent physiological synchrony in naturalistic settings: Within-family cortisol associations and moderators. Journal of Family Psychology. 2009;23(6):882–894. doi: 10.1037/a0017147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt M, Singer M, Kanat-Maymon Y, Feldman R. Infant negative reactivity defines the effects of parent–child synchrony on physiological and behavioral regulation of social stress. Development and Psychopathology. 2015;27(4):1191–1204. doi: 10.1017/S0954579415000760. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Kennedy S, Ingram M, Edwards S, Sweeney L. Prevention and early intervention of anxiety disorders in inhibited preschool children. Journal of Consulting and Clinical Psychology. 2005;73(3):488–497. doi: 10.1037/0022-006X.73.3.488. [DOI] [PubMed] [Google Scholar]
- Reijntjes A, Kamphuis JH, Prinzie P, Telch MJ. Peer victimization and internalizing problems in children: A meta-analysis of longitudinal studies. Child Abuse & Neglect. 2010;34(4):244–252. doi: 10.1016/j.chiabu.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Reyna BA, Pickler RH. Mother- infant synchrony. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2009;38(4):470–477. doi: 10.1111/j.1552-6909.2009.01044.x. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Burgess KB, Hastings PD. Stability and social–behavioral consequences of toddlers’ inhibited temperament and parenting behaviors. Child Development. 2002;73(2):483–495. doi: 10.1111/1467-8624.00419. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Hastings PD, Stewart SL, Henderson HA, Chen X. The consistency and concomitants of inhibition: Some of the children, all of the time. Child Development. 1997;68(3):467–483. [PubMed] [Google Scholar]
- Rudolph KD, Troop-Gordon W, Granger DA. Individual differences in biological stress responses moderate the contribution of early peer victimization to subsequent depressive symptoms. Psychopharmacology. 2011;214(1):209–219. doi: 10.1007/s00213-010-1879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzberg AF, Keller J, Tennakoon L, Lembke A, Williams G, Kraemer FB, Murphy GM. HPA axis genetic variation, cortisol and psychosis in major depression. Molecular Psychiatry. 2014;19(2):220–227. doi: 10.1038/mp.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethre-Hofstad L, Stansbury K, Rice MA. Attunement of maternal and child adrenocortical response to child challenge. Psychoneuroendocrinology. 2002;27(6):731–747. doi: 10.1016/s0306-4530(01)00077-4. [DOI] [PubMed] [Google Scholar]
- Thompson LA, Trevathan WR. Cortisol reactivity, maternal sensitivity, and learning in 3-month-old infants. Infant Behavior and Development. 2008;31(1):92–106. doi: 10.1016/j.infbeh.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bakel HJ, Riksen-Walraven JM. Adrenocortical and behavioral attunement in parents with 1-year-old infants. Developmental Psychobiology. 2008;50(2):196–201. doi: 10.1002/dev.20281. [DOI] [PubMed] [Google Scholar]
- van der Bruggen CO, Stams GJJ, Bögels SM. Research Review: The relation between child and parent anxiety and parental control: A meta‐analytic review. Journal of Child Psychology and Psychiatry. 2008;49(12):1257–1269. doi: 10.1111/j.1469-7610.2008.01898.x. [DOI] [PubMed] [Google Scholar]
- Weik U, Deinzer R. Alterations of postawakening cortisol parameters during a prolonged stress period: Results of a prospective controlled study. Hormones and Behavior. 2010;58(3):405–409. doi: 10.1016/j.yhbeh.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Williams SR, Cash E, Daup M, Geronimi EM, Sephton SE, Woodruff-Borden J. Exploring patterns in cortisol synchrony among anxious and nonanxious mother and child dyads: A preliminary study. Biological Psychology. 2013;93(2):287–295. doi: 10.1016/j.biopsycho.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson J, Lightman SL. Cortisol pulsatility and its role in stress regulation and health. Frontiers in Neuroendocrinology. 2004;25(2):69–76. doi: 10.1016/j.yfrne.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Young EA, Breslau N. Saliva cortisol in posttraumatic stress disorder: A community epidemiologic study. Biological Psychiatry. 2004;56(3):205–209. doi: 10.1016/j.biopsych.2004.05.011. [DOI] [PubMed] [Google Scholar]


