Abstract
Purpose
This study was conducted to compare iso-osmolar contrast medium, iodixanol, with low-osmolar contrast media (LOCM) for assessing contrast-induced nephropathy (CIN) incidence, exclusively in the diabetic population.
Method
A systematic search was conducted for full-text, prospective, randomized controlled trials (RCTs). The primary outcome was incidence of CIN. Medline, Cochrane Central Register of Controlled Trials, and other sources were searched until May 31, 2017.
Results
Twelve RCTs finally met the search criteria. Iodixanol did not significantly reduce the risk of CIN (risk ratio [RR]: 0.72, 95% confidence interval (CI): [0.49, 1.04], p = 0.08). However, there was significantly reduced risk of CIN when iodixanol was compared to a LOCM agent iohexol (RR: 0.32, 95% CI [0.12, 0.89]). There were no differences between iodixanol and the other non-iohexol LOCM (RR: 0.92, 95% CI [0.68, 1.25]).
Conclusion
In diabetic populations, iodixanol is not associated with a significant reduction of CIN risk. Iodixanol is associated with a reduced risk of CIN compared with iohexol, whereas no significant difference between iodixanol and other LOCM could be found.
Introduction
Contrast-induced nephropathy (CIN), is an acute impairment in renal function, and typically occurs within 3 days following the exposure of a contrast medium (CM) [1–3]. In the United States, CIN is one of the leading causes of acute kidney injury, accounting for 11–14.5% [4,5], and is associated with increased cost, hospital stay, and long-term morbidity and mortality [6–8].
Patients at highest risk for CIN include these with pre-existing renal injury, particularly when it is secondary to diabetic nephropathy (DN). It has been recognized that adults with diabetes have a higher risk of developing DN than those without this disease [9,10]. A milestone meta-analysis [11] mentioned the independent predictors of CIN included chronic kidney disease (CKD), CKD with DM, and use of LOCM. However, CKD with DM group had a much higher increase of maximum serum creatinine (SCr), than CKD without DM after contrast exposure. Also, paramount to say that this meta [11] extracted more data from internal database of GE Healthcare, the manufacturer of iso-osmolar contrast media iodixanol. Besides, some evidences investigated the diabetic patients may have some degree of reduced renal function despite having normal SCr levels [12–14]. And, a pilot small RCT [15] proved that in diabetic patients, iodixanol was associated with a lower incidence of CIN than low-osmolar ioversol. So far, it has no systemic reviews or meta-analyses to compare iodixanol with LOCM, exclusively in the diabetic population per se with or without renal insufficiency.
This meta-analysis will expand prior analyses by incorporating all fully-published, prospective RCTs. More specifically, our study will focus on the diabetic population with or without CKD to provide a comparison for nephrotoxicity between the iso-osmolar agent iodixanol and the LOCM.
Methods
Data sources and search strategy
A computerized search to identify eligible RCTs was conducted using the databases of Medline (via PubMed), Cochrane Central Register of Controlled Trials (CENTRAL, 1999–2017 John Wiley & Sons, Inc.), the internal database of GE Healthcare, the manuscript of iodixanol as well as references of retrieved articles and prior meta-analyses, using the following keywords: “Contrast-induced nephropathy,” “contrast-induced acute kidney injury,” “contrast medium,” “iodixanol,” “visipaque,” “iso-osmolar,” “low-osmolar,” “nephropathy,” “nephrotoxicity” and “renal dysfunction”.
Study selection
RCTs were included of comparing CIN events in diabetic patients who were given iso-osmolar iodixanol versus LOCM. Studies were eligible for inclusion if they prospectively randomized patients to either iodixanol or at least one kind of LOCM, and if data on renal function or CIN was routinely ascertained. The primary outcome was the incidence of CIN, defined as an absolute increase at least 0.5 mg/dL (44.2 μmol/L) or relative increase at least 25% from baseline value of SCr, based on current recommendations by the European Society of Urogenital Radiology (ESUR) in the year of 1999 [1] and 2011[2] as well as the Canadian Association of Radiologists [3]. Only fully-published, prospective, RCTs were included for quantitative assessment. Studies that were retrospective, non-randomized or those in which patients were not randomized to either iodixanol or LOCM were excluded strictly. Exclusion also applied to those without available data regarding the patients with diabetes mellitus. No restrictions were placed on languages or sample sizes.
Quality assessment
We used the Cochrane Collaboration’s recommended tool for assessing the risk of bias in included studies [16]. To determine an overall quality of included studies, we assessed trial’s quality by evaluating every element of study design (i.e., blinding description, randomization process, inclusion and exclusion criteria, concealed allocation, intention-to-treat analysis, and assessment of withdrawals and dropouts), together with Jadad scoring system. Risk for bias was assessed in duplicate, with disagreements resolved by consensus. Potential publication bias was further assessed by Begg’s funnel plot [17].
Data extraction
Data were independently extracted by two reviewers (HX, ZX), and disagreements were resolved by consensus with the third reviewer (LK). The primary outcome, incidence of CIN, was extracted. Baseline demographic, clinical, and procedural characteristics were recorded, including patients’ characteristics (i.e. age), sample sizes, type of LOCM (i.e. iohexol, S1 Table), iodine concentration (i.e. iohexol 300 mg I/mL) and time frame of CIN after exposure of contrast was also extracted.
Data analysis
Analyses were mainly performed with Review Manager Software (RevMan Analyses Version 5.3.Copenhagen; The Nordic Cochrane Center, The Cochrane Collaboration, 2014). For dichotomous variables, the risk ratio (RR) was measured along with a 95% confidence interval (CI). A 95% CI not including 1 or p<0.05 was considered to be statistically significant. The inter-study statistical heterogeneity was examined by both Chi-squared tests and I2 statistics. A random-effects model was performed to attain a more relative conservative result. To explore sources of heterogeneity, each study was removed one by one to detect its contribution. If removal of the studies using this process did not create a statistical significant change, the results would be considered robust. Potential publication bias and skewness were assessed graphically using funnel plots in RevMan, if the included studies were greater than or equal to 10.
Subgroup analyses were also conducted based on the formulations of LOCM, which were also divided as iohexol group and non-iohexol group. All CIN events were incorporated, in spite of variant definitions of CIN. Thus, 4 studies [18–21] supplied available data of both two CIN definitions. In this study, we named, i.e. Chen (1) 2012 that meant Chen 2012 [19] with the data of relative CIN criterion, while the Chen (2) 2012 meant the absolute criterion.
Results
Study selection
Medline (via PubMed), CENTRAL (search criterion in S2 Table) and other sources were searched from inception to May 31, 2017.
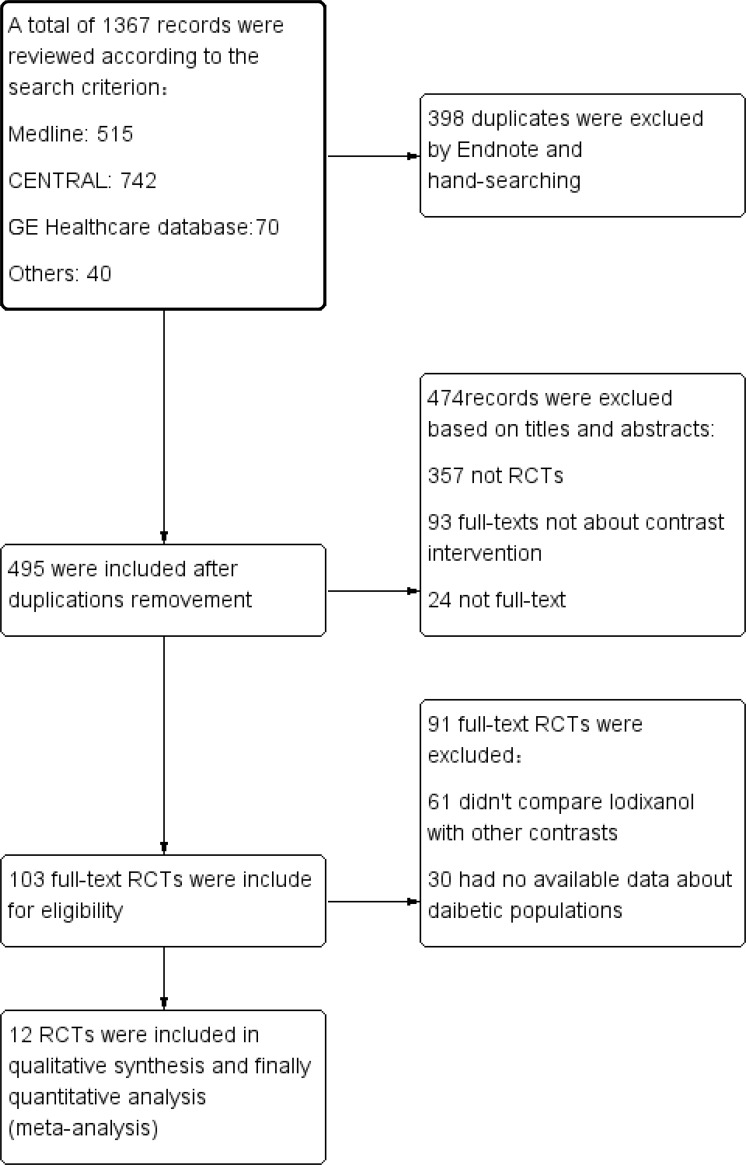
A total of 1367 potentially relevant studies were identified. Of these, 398 were duplicates and 474 had no association or available data about CIN based on review of title and abstract. Of the 103 remaining studies for full-text assessment, 61 didn’t compare iodixanol with LOCM and the other 30 were excluded as they had no available data individually about diabetic populations. Finally, 12 prospective full-text trials [15,18–28] were included in the process of qualitative analysis (Fig 1). Demographic and baseline characteristics of the 12 trials were summarized (Table 1). Elements of study quality were listed as blinding description, randomization process, inclusion and exclusion criteria, concealed allocation, intention-to-treat analysis and assessment of withdrawals and dropouts. And the total quality was assessed by Jadad scores (S3 Table). Among these studies, all were prospective, double-blind RCTs with a score greater than 3 scores, other than Hernandez 2009[15], a single-center, open-label but prospective, randomized study. The study was also consistent with the protocol of PRISMA [29] (Preferred Reporting Items for Systematic reviews and Meta-Analyses statement, S4 Table).
Fig 1. Flow diagram of selection strategy.
Flow diagram depicting the selection strategy for trials used in this meta-analysis. 12 RCTs finally met our criteria. RCT = randomized clinical trials.
Table 1. Characteristics of trials.
| First Author | publish Year | Acronym | Patients’ charecteristic | Primary outcome* | LOCM† | Patients, n | Mean age‡ | Male, (%) | DM, (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Iodixanol | LOCM | Iodixanol | LOCM | Iodixanol | LOCM | Iodixanol | LOCM | ||||||
| Aspelin | 2003 | NEPHRIC | DM and CKD | peak increase of SCr between day 0 and 3 | iohexol, 350 | 129 | 64 | 65 | 71.1±6.0 | 70.6±8.6 | 64 | 53.8 | 100 | 100 |
| Jo | 2006 | RECOVER | CKD | SCr≥0.5 or ≥25% on day 1–2 | ioxaglate, NR | 275 | 140 | 135 | 66.1±8.6 | 68.7±7.5 | 56.4 | 55.6 | 34.3 | 36.3 |
| Feldkamp | 2006 | NR | normal renal function | SCr≥25% or CrCl <20% | iopromide, 300 | 221 | 105 | 116 | 60.6±10.0 | 62.1 ± 9.2 | 75.2 | 75.9 | 21.9 | 20.7 |
| Solomon | 2007 | CARE | CKD | SCr≥0.5 in 45–120 hrs | iopamidol, 370 | 414 | 210 | 204 | 70.5±9.9 | 72.4±9.0 | 60.5 | 67.6 | 43.8 | 38.2 |
| Rudnick | 2008 | VALOR | CKD | SCr>0.5 within 3d | ioversol, 320 | 299 | 156 | 143 | 71.1 ± 9.9 | 72.6 ± 10.2 | 68 | 74 | 52 | 52 |
| Hardiek | 2008 | NR | DM and CKD | SCr≥25% | iopamidol, 370 | 102 | 54 | 48 | 65±10 | 66±10 | 48 | 67 | 100 | 100 |
| Kuhn | 2008 | PREDICT | DM and CKD | SCr≥25% on day 2–3 | iopamidol, 370 | 248 | 123 | 125 | 68.3 ± 9.19 | 69.5 ±10.05 | 43.2 | 50.4 | 100 | 100 |
| Hernández | 2009 | NR | DM | SCr≥0.5 or 25% on day 3 | ioversol, 350 | 250 | 118 | 132 | 69.1±9.0 | 70.1±7.9 | 61.9 | 64.4 | 100 | 100 |
| Laskey | 2009 | NR | CKD, DM | SCr≥0.5 within 3d | iopamidol, 370 | 526 | 263 | 263 | 69.5±8.7 | 69.8±8.9 | 67 | 66 | 100 | 100 |
| Chuang | 2009 | NR | CKD with or with DM | SCr≥25% with 7 days | Iohexol, NR | 50 | 25 | 25 | 62.9 ± 13.7 | 53.0 ± 12.2 | 72 | 64 | 40 | 36 |
| Shin | 2011 | NR | CKD | SCr≥0.5 or 25% after the procedure | iopromide, 300 | 420 | 215 | 205 | 71.1±8.7 | 71.9±8.2 | 57 | 51 | 44 | 49 |
| Chen | 2012 | DIRECT | CKD | SCr≥50% on day 3 | iopromide, 370 | 562 | 284 | 278 | 70.0±9.25 | 69.0±10.5 | 66.9 | 67.6 | 32.7 | 27.7 |
*: mg/dL is used to calculate SCr; mL/min to CrCl
†: mg I/ml to LOCM
‡: Values reported as mean±SD
LOCM = low-osmolar contrast media; DM = diabetes mellitus; CKD = chronic kidney disease; SCr = serum creatinine; CrCl = creatinine clearance; NR = nor reported.
Data extraction
In the included studies, the incidence of CIN was reported. Among all studies, only five [15,18,20,23,28] recruited the diabetic populations. As for the remaining 7 studies [19,21,22,24–27], the available data of CIN in the diabetic patients were listed as a subgroup and disclosed in the main text. All data were extracted from the published articles directly.
CIN definition
The primary outcome of our study was the incidence of CIN, defined as a relative increase in the SCr of at least 25% or an absolute increase of at least 0.5 mg/dl from the baseline value after contrast medium exposure. Among all studies, 4 trials [20,26–28] defined CIN as SCr ≥25% as the primary clinical endpoint, 3 defines as SCr ≥0.5 mg/dl [21,23,24], and another 3 either [15,22,25]. Besides, 4 studies [18–21] also supplied other available data of CIN other than the primary outcome defined individually in each study.
Relative criterion of CIN
Available data from 7 studies [18–21,26–28] disclosed the incidence of CIN after contrast media exposure, which was defined as a relative increase in the SCr of at least 25% from the baseline.
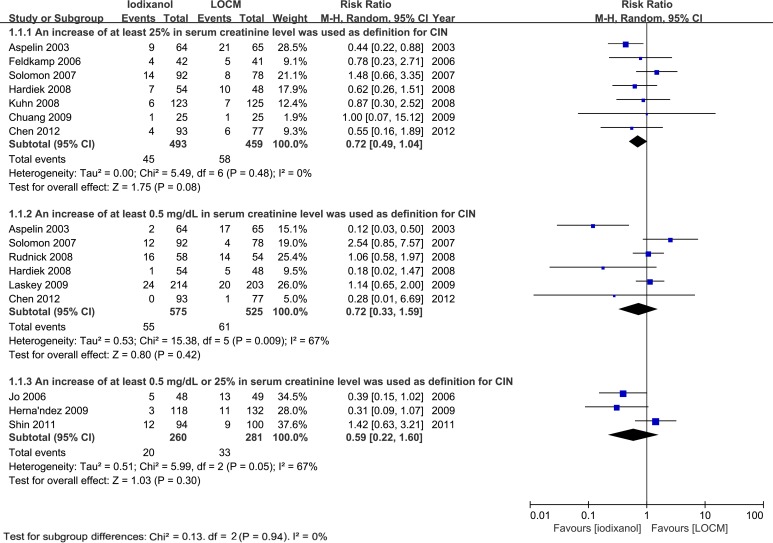
In total, CIN occurred among 45 out of 493 who received iodixanol, and 58 of 459 patients who received LOCM (n = 952, RR: 0.72, 95% confidence interval [CI]: 0.49–1.04, p = 0.08, I2 = 0%, Fig 2), indicating that there was slight reduction of incident CIN associated with iodixanol in comparison to LOCM, but insignificantly.
Fig 2. RR of CIN for comparison of iodixanol with LOCM in diabetic patients.
Forest plot of risk ratios (RR) for the incidence of contrast-induced nephropathy (CIN) for comparison of iodixanol with low-osmolar contrast media (LOCM). CIN was defined as an increase of more than 25%, or 0.5 mg/dL (44.2 μmol/L), or either, in serum creatinine level (SCr), respectively. LOCM low-osmolar contrast medium/media; CI confidence interval; M-H Mantel-Haenszel method.
However, the nonsignificant result wasn’t robust after removing the study of Solomon 2007[21] (RR: 0.59, 95% CI: 0.59–0.90, p = 0.01, I2 = 0%).
Absolute criterion of CIN
Separately, available data from 6 studies [18–21,23,24] uncovered the incident CIN defined as an absolute increase in the SCr of at least 0.5 mg/dl after given contrast media.
Totally, the events of CIN occurred among 55 out of 575 who received iodixanol, and 61 of 525 patients who received LOCM (n = 1100, RR: 0.72, 95% CI: 0.33–1.59, p = 0.42, Fig 2), indicating that there was no significant difference of incident CIN associated with iodixanol in comparison to LOCM, with a higher heterogeneity (p = 0.009, I2 = 67%).
Furthermore, the results were robust after removing each study one by one to detect its contribution without a statistically significant change.
Relative or absolute definition of CIN
Only 3 studies [15,22,25] defined the CIN as SCr≥25% or ≥0.5 mg/dl, without other available data separately. In total, CIN occurred among 20 out of 260 who received iodixanol, and 33 of 281 patients who received LOCM (RR: 0.59, 95% CI: 0.22–1.60, p = 0.30, Fig 2), indicating that there was no significant reduction of CIN rate associated with iodixanol in comparison to LOCM.
Iodixanol versus LOCM
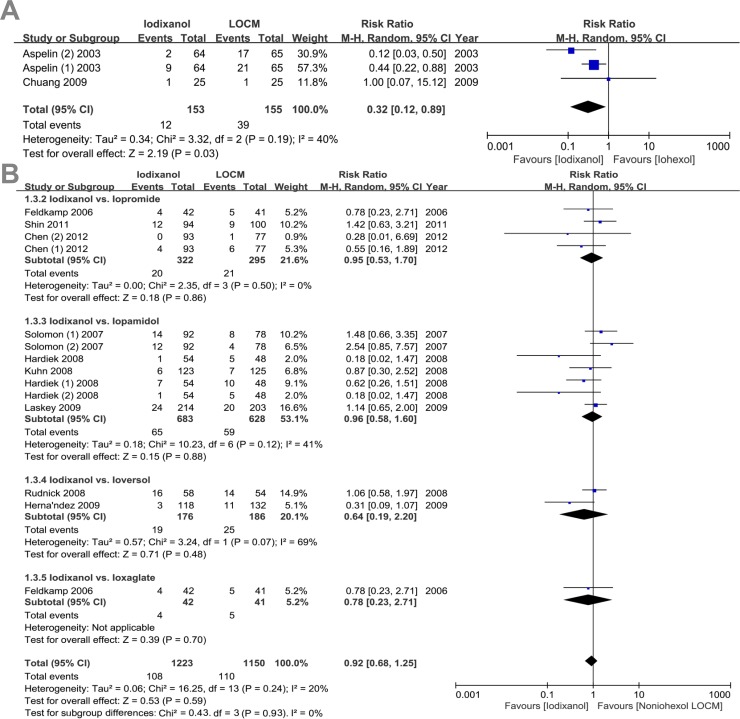
Subgroup analyses were conducted based on the formulations of contrast media (Fig 3). Patients receiving nonionic, monomeric LOCM were given iohexol (3 trials), iopamidol (7 trials), iopromide (4 trials), ioversol (2 trial), as well as ionic dimer, ioxaglate (1 trial).
Fig 3. RR of CIN for comparison of iodixanol with iohexol or other non-iohexol LOCM.
Funnel plot of studies for contrast-induced nephropathy (CIN) for comparison of iodixanol with iohexol or other non-iohexol LOCM in diabetic patients.
There was a significant reduction of CIN when iodixanol was compared to iohexol (n = 308, RR: 0.32, 95% CI: 0.12 to 0.89, p = 0.03; I2 = 40%), iopromide (n = 617, RR: 0.95, 95% CI: 0.53 to 1.70, p = 0.86) and iopamidol (n = 1,311, RR: 0.96, 95% CI: 0.58 to 1.60, p = 0.88). However, there was no significant difference between iodixanol and iopromide (n = 617, RR: 0.95, 95% CI: 0.53 to 1.70, p = 0.86) and iopamidol (n = 1,311, RR: 0.96, 95% CI: 0.58 to 1.60, p = 0.88). Furthermore, when iodixanol was compared to non-iohexol LOCMs, there was also no significant (n = 2,375, RR: 0.92, 95% CI: 0.68 to 1.25, p = 0.93).
Discussion
Major findings
From including 12 prospective full-text RCTs to compare iso-osmolar CM, iodixanol, with LOCM for assessing the incidence of CIN exclusively in the patients with diabetes mellitus, numerous findings were found as follows: 1) iodixanol isn’t superior to LOCM to reducing the risk of CIN; 2) If the CIN is defined as a relative increase in SCr of at least 25% from baseline, iodixanol showed slightly reduced CIN incidence non-significantly. However, the result seems not very robust; 3) iodixanol is superior to iohexol to reduce the risk of CIN; 5) No significant difference between iodixanol and other non-iohexol LOCMs could be found
Definitions of CIN
CIN was first defined in 1999 by ESUR [1] as an absolute increase in SCr of at least 0.5 mg/dl or a relative increase of >25% of baseline value. Although this definition was followed by some standard guidelines [2,3], a series of derivative definitions were still widely used in recent years, i.e. both absolute and relative increase, absolute increase solely, relative increase solely. Moreover, the upper limitations of SCr increase were not mandatory, which also deteriorated the consistency of criterion for CIN. For example, Chen 2012 [19] defined CIN as SCr increase ≥50%. Variant definitions brought inconsistency, even in the meta-analyses of RCTs. McCullough [11] firstly defined the CIN as SCr increase ≥0.5 mg/dl and found the iodixanol was associated with a reduced of CIN. While, From et al. [30] found that iodixanol was not associated with less CIN.
For avoiding the inconsistency of variant definitions, uniform criteria were conducted in this study. Also, the more widely-used definitions of SCr increase ≥25% or 0.5 mg/dl were adopted.
The incidence of CIN in diabetic populations
There is general agreement that CKD is the most significant risk factor for CIN and every multivariate analysis has shown that CKD is an independent risk factor for CIN [4,31–35]. A recently published meta-analysis reported a very closely related study, albeit examining chronic kidney disease patients rather than diabetic patients. Randomized trials comparing IOCM to LOCM in CKD stage 3 and above patients undergoing CA, and reporting incidence of CIN (defined by a rise in creatinine of 25% from baseline) were included in the analysis. In patients with CKD stage 3 and above undergoing coronary angiography, use of IOCM showed overall non-significant difference in incidence of CIN compared to LOCM. The difference was further attenuated when IOCM was compared with non-ionic LOCM [33]. Additionally, whether DM could be another independent risk factor for CIN is controversial. There is no conclusive evidence that diabetic patients weren’t at an increased risk of CIN if their renal function is normal [2]. The finding of this study indicated that iodixanol was not associated with a reduced risk of CIN, was based on the uniform definitions of CIN respectively, which indicated that DM per se wasn’t independent predictors of CIN.
Iodixanol versus LOCM
Three prior meta-analyses, Reed 2009[36], Heinrich 2009[37] and From 2010[38] also found that iodixanol was associated with a reduction in CIN compared to iohexol, although significant differences between iodixanol and non-iohexol nonionic LOCM were inconsistent. Reed [36] reported that iodixanol was superior to ioxaglate. However, Heinrich [37] and From [38] indicated that iodixanol was not associated with a reduction in CIN when compared to non-iohexol.
This study indicated that in patients with diabetes mellitus, iodixanol wasn’t associated with significant renal protection when compared to nonionic LOCM other than iohexol. This finding couldn’t be explained solely by osmolality [18,37,38], which are warranted for further exploration of other possible mechanisms.
Study strengths and limitations
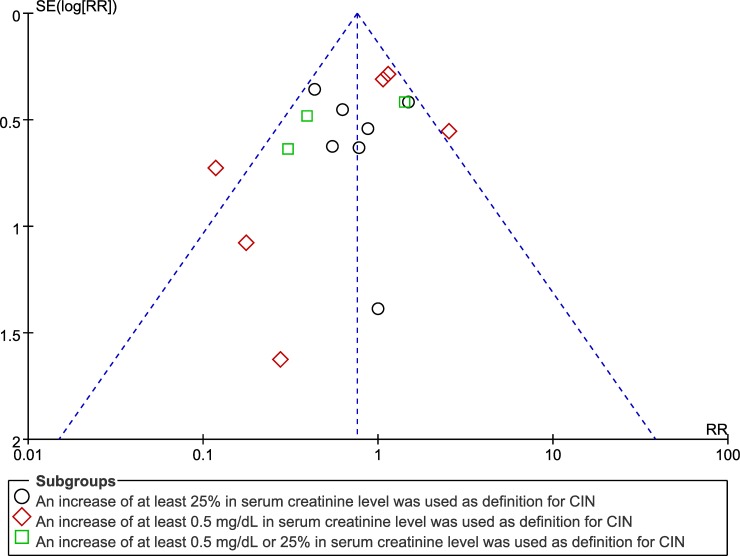
One limitation of our study is that the results are based on the comprehensive data of trials with heterogeneous RCTs. The included trials utilized different radiographic procedures, contrast loads, heterogeneous definitions of CIN, or different complications of diabetic patients (CKD or not). We have attempted to account for these differences using random-effects models to obtain more conservative results. A significant strength of our study is the inclusion of mainly relevant RCTs on CIN, divided by each definition. In addition, Begg’s funnel plot analysis results (Fig 4) showed negligible publication bias in our study under all three definitions of CIN, demonstrating that our conclusions are reliable.
Fig 4. Funnel plot for the association between contrast use and CIN under different definitions of CIN.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOC)
Acknowledgments
The authors gratefully acknowledged the assistance of Mrs. Chanjuan Hu, the product manager of GE Healthcare Core Imaging, for assistance in searching available studies.
Abbreviations
- CIN
Contrast-induced nephropathy
- CM
contrast medium/media
- CI
confidence interval
- CKD
chronic kidney disease
- DM
diabetes mellitus
- DN
diabetic nephropathy
- ESUR
European Society of Urogenital Radiology
- LOCM
low-osmolar contrast medium/media
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-analyses statement
- RCT
randomized controlled trials
- RR
risk ratio
- SCr
serum creatinine
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Anhui Provincial Natural Science Foundation, 1608085MH208.
References
- 1.Morcos SK, Thomsen HS, Webb JA (1999) Contrast-media-induced nephrotoxicity: a consensus report. Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR). Eur Radiol 9: 1602–1613. [DOI] [PubMed] [Google Scholar]
- 2.Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, et al. (2011) Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol 21: 2527–2541. doi: 10.1007/s00330-011-2225-0 [DOI] [PubMed] [Google Scholar]
- 3.Benko A, Fraser-Hill M, Magner P, Capusten B. (2007) Canadian Association of Radiologists: consensus guidelines for the prevention of contrast-induced nephropathy. Can Assoc Radiol J 58: 79–87. [PubMed] [Google Scholar]
- 4.McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW (1997) Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med 103: 368–375. [DOI] [PubMed] [Google Scholar]
- 5.Nash K, Hafeez A, Hou S (2002) Hospital-acquired renal insufficiency. Am J Kidney Dis 39: 930–936. doi: 10.1053/ajkd.2002.32766 [DOI] [PubMed] [Google Scholar]
- 6.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, et al. (2002) Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 105: 2259–2264. [DOI] [PubMed] [Google Scholar]
- 7.Gupta R, Gurm HS, Bhatt DL, Chew DP, Ellis SG (2005) Renal failure after percutaneous coronary intervention is associated with high mortality. Catheter Cardiovasc Interv 64: 442–448. doi: 10.1002/ccd.20316 [DOI] [PubMed] [Google Scholar]
- 8.Liss P, Persson PB, Hansell P, Lagerqvist B (2006) Renal failure in 57 925 patients undergoing coronary procedures using iso-osmolar or low-osmolar contrast media. Kidney Int 70: 1811–1817. doi: 10.1038/sj.ki.5001887 [DOI] [PubMed] [Google Scholar]
- 9.Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297. [DOI] [PubMed] [Google Scholar]
- 10.Tan H, Yi H, Zhao W, Ma JX, Zhang Y, Zhou X. (2016) Intraglomerular crosstalk elaborately regulates podocyte injury and repair in diabetic patients: insights from a 3D multiscale modeling study. Oncotarget 7: 73130–73146. doi: 10.18632/oncotarget.12233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCullough PA, Bertrand ME, Brinker JA, Stacul F (2006) A meta-analysis of the renal safety of isosmolar iodixanol compared with low-osmolar contrast media. J Am Coll Cardiol 48: 692–699. doi: 10.1016/j.jacc.2006.02.073 [DOI] [PubMed] [Google Scholar]
- 12.Middleton RJ, Foley RN, Hegarty J, Cheung CM, McElduff P, Gibson JM, et al. (2006) The unrecognized prevalence of chronic kidney disease in diabetes. Nephrol Dial Transplant 21: 88–92. doi: 10.1093/ndt/gfi163 [DOI] [PubMed] [Google Scholar]
- 13.Bachorzewska-Gajewska H, Malyszko J, Malyszko JS, Musial W, Dobrzycki S (2006) Undiagnosed renal impairment in patients with and without diabetes with normal serum creatinine undergoing percutaneous coronary intervention. Nephrology (Carlton) 11: 549–554. [DOI] [PubMed] [Google Scholar]
- 14.New JP, Middleton RJ, Klebe B, Farmer CK, de Lusignan S, Stevens PE, et al. (2007) Assessing the prevalence, monitoring and management of chronic kidney disease in patients with diabetes compared with those without diabetes in general practice. Diabet Med 24: 364–369. doi: 10.1111/j.1464-5491.2007.02075.x [DOI] [PubMed] [Google Scholar]
- 15.Hernandez F, Mora L, Garcia-Tejada J, Velazquez M, Gomez-Blazquez I, Bastante T, et al. (2009) Comparison of iodixanol and ioversol for the prevention of contrast-induced nephropathy in diabetic patients after coronary angiography or angioplasty. Rev Esp Cardiol 62: 1373–1380. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JPT, Green S, Cochrane Collaboration. (2008) Cochrane handbook for systematic reviews of interventions Chichester, England; Hoboken, NJ: Wiley-Blackwell; xxi, 649 p. p. [Google Scholar]
- 17.Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 18.Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R, Berg KJ. (2003) Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med 348: 491–499. doi: 10.1056/NEJMoa021833 [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Hu S, Liu Y, Zhao R, Wang L, Fu G, et al. (2012) Renal tolerability of iopromide and iodixanol in 562 renally impaired patients undergoing cardiac catheterisation: the DIRECT study. EuroIntervention 8: 830–838. doi: 10.4244/EIJV8I7A126 [DOI] [PubMed] [Google Scholar]
- 20.Hardiek KJ, Katholi RE, Robbs RS, Katholi CE (2008) Renal effects of contrast media in diabetic patients undergoing diagnostic or interventional coronary angiography. Journal of Diabetes and Its Complications 22: 171–177. doi: 10.1016/j.jdiacomp.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 21.Solomon RJ, Natarajan MK, Doucet S, Sharma SK, Staniloae CS, Katholi RE, et al. (2007) Cardiac Angiography in Renally Impaired Patients (CARE) study: a randomized double-blind trial of contrast-induced nephropathy in patients with chronic kidney disease. Circulation 115: 3189–3196. doi: 10.1161/CIRCULATIONAHA.106.671644 [DOI] [PubMed] [Google Scholar]
- 22.Shin DH, Choi DJ, Youn TJ, Yoon CH, Suh JW, Kim KI, et al. (2011) Comparison of contrast-induced nephrotoxicity of iodixanol and iopromide in patients with renal insufficiency undergoing coronary angiography. Am J Cardiol 108: 189–194. doi: 10.1016/j.amjcard.2011.03.019 [DOI] [PubMed] [Google Scholar]
- 23.Laskey W, Aspelin P, Davidson C, Rudnick M, Aubry P, Kumar S, et al. (2009) Nephrotoxicity of iodixanol versus iopamidol in patients with chronic kidney disease and diabetes mellitus undergoing coronary angiographic procedures. Am Heart J 158: 822–828 e823. doi: 10.1016/j.ahj.2009.08.016 [DOI] [PubMed] [Google Scholar]
- 24.Rudnick MR, Davidson C, Laskey W, Stafford JL, Sherwin PF (2008) Nephrotoxicity of iodixanol versus ioversol in patients with chronic kidney disease: the Visipaque Angiography/Interventions with Laboratory Outcomes in Renal Insufficiency (VALOR) Trial. Am Heart J 156: 776–782. [DOI] [PubMed] [Google Scholar]
- 25.Jo SH, Youn TJ, Koo BK, Park JS, Kang HJ, Cho YS, et al. (2006) Renal toxicity evaluation and comparison between visipaque (iodixanol) and hexabrix (ioxaglate) in patients with renal insufficiency undergoing coronary angiography: the RECOVER study: a randomized controlled trial. J Am Coll Cardiol 48: 924–930. doi: 10.1016/j.jacc.2006.06.047 [DOI] [PubMed] [Google Scholar]
- 26.Chuang FR, Chen TC, Wang IK, Chuang CH, Chang HW, Ting-Yu Chiou T, et al. (2009) Comparison of iodixanol and iohexol in patients undergoing intravenous pyelography: a prospective controlled study. Ren Fail 31: 181–188. doi: 10.1080/08860220802669636 [DOI] [PubMed] [Google Scholar]
- 27.Feldkamp T, Baumgart D, Elsner M, Herget-Rosenthal S, Pietruck F, Erbel R, et al. (2006) Nephrotoxicity of iso-osmolar versus low-osmolar contrast media is equal in low risk patients. Clin Nephrol 66: 322–330. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn MJ, Chen N, Sahani DV, Reimer D, van Beek EJ, Heiken JP, et al. (2008) The PREDICT study: a randomized double-blind comparison of contrast-induced nephropathy after low- or isoosmolar contrast agent exposure. AJR Am J Roentgenol 191: 151–157. doi: 10.2214/AJR.07.3370 [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339: b2535 doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20. doi: 10.1016/j.cell.2004.12.035 [DOI] [PubMed] [Google Scholar]
- 31.Bartholomew BA, Harjai KJ, Dukkipati S, Boura JA, Yerkey MW, Glazier S, et al. (2004) Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. American Journal of Cardiology 93: 1515–1519. doi: 10.1016/j.amjcard.2004.03.008 [DOI] [PubMed] [Google Scholar]
- 32.Brown JR, DeVries JT, Piper WD, Robb JF, Hearne MJ, Ver Lee PM, et al. (2008) Serious renal dysfunction after percutaneous coronary interventions can be predicted. American Heart Journal 155: 260–266. doi: 10.1016/j.ahj.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 33.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. (2004) A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention—Development and initial validation. Journal of the American College of Cardiology 44: 1393–1399. doi: 10.1016/j.jacc.2004.06.068 [DOI] [PubMed] [Google Scholar]
- 34.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, et al. (2002) Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 105: 2259–2264. [DOI] [PubMed] [Google Scholar]
- 35.Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, et al. (2005) Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. American Journal of Cardiology 95: 13–19. doi: 10.1016/j.amjcard.2004.08.056 [DOI] [PubMed] [Google Scholar]
- 36.Reed M, Meier P, Tamhane UU, Welch KB, Moscucci M, Gurm HS. (2009) The relative renal safety of iodixanol compared with low-osmolar contrast media: a meta-analysis of randomized controlled trials. JACC Cardiovasc Interv 2: 645–654. doi: 10.1016/j.jcin.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 37.Heinrich MC, Haberle L, Muller V, Bautz W, Uder M (2009) Nephrotoxicity of iso-osmolar iodixanol compared with nonionic low-osmolar contrast media: meta-analysis of randomized controlled trials. Radiology 250: 68–86. doi: 10.1148/radiol.2501080833 [DOI] [PubMed] [Google Scholar]
- 38.From AM, Al Badarin FJ, McDonald FS, Bartholmai BJ, Cha SS, Rihal CS. (2010) Iodixanol versus low-osmolar contrast media for prevention of contrast induced nephropathy: meta-analysis of randomized, controlled trials. Circ Cardiovasc Interv 3: 351–358. doi: 10.1161/CIRCINTERVENTIONS.109.917070 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.