Abstract
Metabolic syndrome (MetS) is known to be associated with elevated serum ferritin levels. The possible association with other iron markers has been less well studied. We aimed to investigate the cross-sectional association of soluble transferrin receptor (sTfR) and ferritin levels with MetS components, insulin resistance and HbA1C. The sample consisted of 725 adults, aged 19-93 years (284 men, 151 premenopausal and 290 postmenopausal women), from the Croatian island of Vis. Serum sTfR and ferritin levels were measured by immunoturbidometry and electrochemiluminescence assays, respectively. MetS was defined using modified international consensus criteria. Logistic and linear regression analyses were conducted to investigate the associations adjusting for age, fibrinogen, smoking, alcohol consumption and body mass index. Prevalence of MetS was 48.7%. Standardized values of ferritin were positively associated with all of the MetS components (except high blood pressure and waist circumference) in men(P<0.05). Ferritin was significantly associated with MetS in men(adjusted odds ratio 95% confidence interval:1.78[1.31-2.42]) and postmenopausal women(1.71[1.12-2.62]). Interestingly, sTfR was independently and positively associated with insulin resistance (HOMA-IR) in men(adjusted βeta=0.44[0.14 to 0.75],P=0.004) and postmenopausal women(adjusted βeta coefficient 95% confidence interval= 0.34[0.05 to 0.63],P=0.020). However, there was no significant relationship between serum sTfR levels and MetS or its components. Neither ferritin nor sTfR were significantly associated with HbA1C (P>0.05). sTfR levels could be spuriously elevated in subjects with insulin resistance and without association with MetS or its components. We conclude that different markers of iron metabolism are not consistently associated with cardiometabolic risk.
Keywords: soluble transferrin receptor, insulin resistance, metabolic syndrome, ferritin
Introduction
Associations between high levels of ferritin, the major iron storage protein that is also an acute phase protein, and type 2 diabetes have been described in cross-sectional and prospective studies (1, 2). Both iron deficiency and iron overload have been associated with cardiovascular disease (3). An association between body iron stores and metabolic syndrome (MetS), a cluster of vascular, metabolic and anthropometric abnormalities has also been identified (4). Deleterious effects on insulin signaling as a consequence of pro-oxidant effects has been suggested as a potential underlying mechanism (5). However, the nature of the relationship between iron status and cardio-metabolic status remains unclear and exploration of additional aspects of iron metabolism has been recommended (6).
There are other important proteins in iron metabolism in addition to ferritin, such as transferrin and transferrin receptors (7,8). Transferrin receptors are of particular interest since they act as a sensor for body iron demands. Iron transport in the plasma is carried out by transferrin, which donates iron to cells through its interaction with a specific membrane receptor, the transferrin receptor (TfR). A soluble form of the TfR has been identified in human serum. Soluble TfR is a truncated monomer of the tissue receptor, lacking its first 100 amino acids, which circulates in the form of a complex of transferrin and its receptor. The higher the body iron stores, the lower the transferrin receptors in cell membranes which in turn down-regulates of intestinal iron absorption (9). Increased numbers of transferrin receptors in cell membranes reflect body iron deprivation and intestinal iron absorption is up-regulated as compensatory mechanism. Levels of serum transferrin receptor (sTfR) are proportional to tissue concentrations (10). sTfR and ferritin are influenced by the acute phase response of inflammation and therefore their evaluation should include additional measurements of inflammatory markers (11).
Type 2 diabetes and cardiovascular disease are multi-factorial disorders and the exploration of potential new risk factors, such as iron metabolism, is necessary in order to obtain information about modification of cardiometabolic risk and a better understanding of underlying mechanisms in aetiology. The association between sTfR and cardio-metabolic risk factors has not been widely investigated. A recent meta-analysis reported conflicting results for the association between sTfR and type 2 diabetes in eight studies and described the limited power of the included studies due to small sample sizes (1). Only one study has described the association between sTfR and MetS (12) but did not adjust for BMI, and two studies have described the association between sTfR and some MetS components (13,14). Most of these studies reported no evidence of an association with MetS and very few associations with MetS components. Our primary aim was to investigate whether sTfR levels were associated with MetS, its components, insulin resistance and HbA1C in a relatively large well-characterised population. We also describe the association between ferritin with MetS, its components, insulin resistance and HbA1C.
Material and Methods
Participants
Participants were identified from the 10,001 Dalmatians research programme, originating from the sub-cohort recruited from the island of Vis in Croatia. A total of 1,029 subjects were initially recruited, aged 18–93 years, who were recruited from the villages of Vis and Komiza during 2003 and 2004 within a larger genetic epidemiology study (15,16,17). In the original study, the only eligibility criterion was being 18 years or older. sTfR and ferritin levels were measured in 774 subjects, of whom, 49 subjects with missing values for covariates (age, fibrinogen, glycosylated hemoglobin, smoking and alcohol consumption, body mass index and history of cardiometabolic disease) were excluded leaving a study population of 725 people. Distribution of exposure, adjustment variables and outcomes were similar in people with and without missing data with the exception of fibrinogen levels which were higher in people without missing data. Ethical approval was issued by Multi-centre research ethics committee for Scotland (MREC) under designation MREC 01/0/71, and all participants gave written informed consent.
Clinical and anthropometric measurements
Anthropometric measurements, included height measured using a stadiometer, weight and waist circumference measurement (measured half-way between the lowest rib and iliac crest). Blood pressure was measured in a seated position, after at least 5 minutes of rest. Two measurements were made, and only the second one recorded, in order to reduce the “white coat” effect. Menopausal status was defined on the basis of self-report with pre-menopausal status defined by continuing menstruation and post-menopausal status by lack of menstruation For physical activity it was used a self-perceived report on the physical activities at work and at leisure on a 4-point rating scale (sitting, light, moderate, and hard).
Biochemical measurements
Blood samples were taken after overnight fasting. Classical biochemical analyses of the blood sample included triglycerides by using UV photometry with glycerolphosphate-oxidase (GPO PAP)(Olympus kit OSR60118); HDL cholesterol – homogeneous enzyme method with modified polyethylene glycol and acyclohexane-sulphate (Olympus kit OSR6195); glucose – UV hexokinase photometry; HbA1C (whole-blood sample) – cation exchange, immunochemistry electrophoresis and affinity linking, that is compatible with DCCT/UK PDS standard. The measurements of HbA1C and glucose were performed using Olympus kit OSR6192, OSR 6121 and OSR6221. Manufacturer’s reagents were used, with their internal quality control (ODC003 or ODC004 for glucose, and ODC022 for HbA1C). Fibrinogen was measured by the Clauss method using an MDA 180 coagulometer (Biomerieux, Marcy l’Etoile, France) with reagents from the manufacturer. The calibrant used was the 8th British Standard (NIBSC). Electrochemiluminescence immunoassay (Roche) was used to measure ferritin, Tina-quant® immunoturbidometry (Roche) for sTfR. The laboratory was ISO accredited and daily internal controls were performed (calibrator 66300). Repeated measurements of the sub-set of samples provided very high agreement (kappa 0.92 for the lowest pair of estimates). The coefficient of variation was less than 5% for each biochemical measurement. The biochemical assays were conducted by using an OLYMPUS AU400 chemistry immuno analyzer. Insulin resistance was estimated by homoeostatic model assessment using the formula: glucose levels [mmol/L] X insulin mU/L / 22.5 (18).
Metabolic syndrome
Cut- points from the international consensus definition for metabolic syndrome were used as follows(19): triglycerides ≥ 1.7 mmol/L, HDL-C <1.0 mmol/L in men and <1.3 in women, glucose ≥ 5.6 mmol/L (or drug treatment for elevated glucose), systolic blood pressure ≥ 130 mm/Hg and or/ diastolic blood pressure ≥ 85 mm/Hg (or antihypertensive drug treatment), and waist circumference ≥ 94cm in men and ≥ 80cmin women. Since information on lipid-lowering medications was not available to complement the component of high triglycerides, the MetS definition used in this study represents a modified definition of the international consensus’ criteria. Metabolic syndrome was defined as the presence of three or more variables meeting the definitions above.
Data analysis
The analyses were stratified by sex/menopausal status and by adjusting for group of sex/menopausal status (premenopausal women as reference/postmenopausal women/men) in analyses of the whole cohort. All continuous study variables were summarised as median (interquartile ranges) by sex/menopausal status and differences were tested using the Mann-Whitney U test. Logistic regression models were used to describe the associations between sTfR and ferritin as exposure variables with MetS and its components as outcome variables. sTfR and ferritin were used as continuous variables in terms of standard deviation units of their log-transformed levels (Z scores or standardized values) to facilitate interpretation of odds ratios. Odds of each outcome are therefore described for each standard deviation in log-transformed iron marker. Multivariable models with age, levels of fibrinogen, smoking (never smoker, ex-smoker, current smoker), alcohol consumption (no/yes), and body mass index (BMI) as covariates were used to investigate whether the associations were independent of these potential confounding factors. This set of confounding factors was chosen on the basis of possible influence of acute phase or subclinical inflammation in terms of fibrinogen levels (20,21), and general adiposity on levels of iron markers and/or outcome variables. In the case of the associations with waist circumference, BMI was included as covariate to investigate the role of central adiposity reflected by waist independently of the effect of general adiposity reflected by BMI. Relationships between measures of iron status and insulin resistance and HbA1C were described using Pearson correlation, and multiple linear regression analyses used to adjust for potential confounders as listed above with additional adjustment for treatment with insulin and/or hypoglycaemic drugs (yes/no). Evaluation of non-linear relationships with insulin resistance was performed using ANOVA and ANCOVA to describe the association between tertiles of iron markers and HOMA-IR values tests. The normality of distributions was assessed using histograms and Kolmogorov-Smirnov tests. For the linear regressions, Pearson correlation and ANCOVA analyses, transformed values of skewed variables were used as follows: logarithm of sTfR, ferritin, HOMA-IR values, body mass index and fibrinogen values; square of age and square root of glycosylated haemoglobin. The above set of arithmetic functions allowed the best approximation to normal distribution for each variable. Self-reported cardiovascular disease (heart attack, stroke) and diabetes, and self-reported physical activities at work and at leisure (sitting [reference], light, moderate, and hard), were additionally used as covariates in sensitivity analyses. In order to avoid collinearity, treatment with insulin and/or hypoglycemic drugs (yes/no) was not considered in the multivariable model when diabetes was used as covariate. A P value < 0.05 was considered statistically significant. Data were analyzed using Stata version 11.0 (StataCorp).
Results
The study encompassed a total of 725 subjects, stratified in three groups by sex/menopausal status (Table 1). Men had higher values of ferritin than women, while postmenopausal women had higher values of ferritin than premenopausal women. Postmenopausal women had significantly higher levels of sTfR than men, while the comparison with pre-menopausal women was not significant (Table 1). HOMA-IR was higher in men and postmenopausal women than among pre-menopausal women with a similar pattern observed for MetS components. Prevalence of high WC was above 60 % in all groups (Table 1). Prevalence of MetS was higher in post-menopausal women than in men and pre-menopausal women (Table 1).
Table 1.
Distribution of iron status and cardiometabolic risk by sex and menopausal status
| Premenopausal women | Postmenopausal women | Men | P values | |||
|---|---|---|---|---|---|---|
| Premenopausal vs postmenopausal women | Men vs. premenopausal women | Men vs. postmenopausal women | ||||
| n | 151 | 290 | 284 | |||
| Age (years) | 40 (33-47) | 67(57-74) | 57 (46-68.7) | < 0.001 | < 0.001 | < 0.001 |
| BMI (kg/m2) | 26.6(21.6-27) | 28.3(25.4-30.9) | 27.6(25.2-29.6) | < 0.001 | < 0.001 | 0.013 |
| Ferritin(µg/L) | 25.5 (12-45.9) | 65.9 (38.6-102.2)) | 141.3 (90.5-233.7) | < 0.001 | < 0.001 | < 0.001 |
| sTfR (mg/L) | 3.19 (2.63-4.05) | 3.17 (2.73-3.90) | 3.07 (2.62-3.68) | 0.758 | 0.069 | 0.038 |
| Glucose (mmol/L) | 5.0(4.6-5.4) | 5.6(5.0-6.2) | 5.5(5.0-6.1) | < 0.001 | < 0.001 | 0.504 |
| TG (mmol/L) | 1.2(0.9-1.5) | 1.5(1.1-2.0) | 1.5(1.1-2.3) | < 0.001 | < 0.001 | 0.542 |
| HDL-C (mmol/L) | 1.16(1.09-1.25) | 1.14(0.98-1.23) | 1.13(0.96-1.22) | 0.011 | 0.001 | 0.410 |
| SBP (mmHg) | 118(108-128) | 145(130-161) | 136.7(122.6-150) | < 0.001 | < 0.001 | < 0.001 |
| DBP (mmHg) | 75(68-80) | 81.5(75-89) | 81.2(75-89) | < 0.001 | < 0.001 | 0.820 |
| WC (cm) | 83(76.9-91.5) | 99.2(91.7-105.4) | 98.3(92-105.1) | < 0.001 | < 0.001 | 0.403 |
| Insulin mU/L | 5.0(4.0-8.0) | 7.0(5.0-11) | 6.0(4.0-9.0) | < 0.001 | < 0.001 | 0.147 |
| HOMA-IR | 1.17(0.78-1.89) | 1.69(1.06-2.93) | 1.58(1.01-2.60) | < 0.001 | < 0.001 | 0.141 |
| HbA1C(%) | 5.1(4.9-5.4) | 5.5(5.2-5.7) | 5.2(5.0-5.6) | < 0.001 | < 0.001 | < 0.001 |
| Fibrinogen (g/L) | 3.5(2.9-4.0) | 3.9(3.4-4.5) | 3.5(2.9-4.1) | < 0.001 | 0.714 | < 0.001 |
| MetS and its components n(%) | ||||||
| High blood pressure¶ | 43(28.3) | 237(81.7) | 198(69.7) | < 0.001 | < 0.001 | 0.001 |
| High glucose † | 27(17.8) | 148(51.0) | 131(46.1) | < 0.001 | < 0.001 | 0.208 |
| Low HDL-C | 137(90.7) | 277(95.5) | 82(28.9) | 0.040 | <0.001 | <0.001 |
| High triglycerides | 25 (16.6) | 118(40.7) | 117(41.2) | < 0.001 | < 0.001 | 0.902 |
| High WC | 94 (62.3) | 278(95.9) | 191(67.3) | < 0.001 | 0.296 | < 0.001 |
| MetS | 51(33.8) | 257(88.6) | 144(50.7) | < 0.001 | 0.001 | <0.001 |
| Smoking n(%) | ||||||
| Yes | 64(42.4) | 47(16.2) | 85(29.9) | |||
| No | 58(38.4) | 184(63.4) | 73(25.7) | |||
| Ex-smoker | 29(19.2) | 59(20.3) | 126(44.4) | < 0.001 | < 0.001 | < 0.001 |
| Alcohol consumption n(%) | 79(52.3) | 108(37.2) | 230(81) | 0.002 | < 0.001 | < 0.001 |
| Cardiovascular disease n(%) | 12(7.9) | 150(51.7) | 102(35.9) | < 0.001 | < 0.001 | < 0.001 |
| Diabetes n(%) | 3(2.0) | 27(9.3) | 20(7.0) | 0.002 | 0.017 | 0.201 |
Data are median (interquartile range) or n(%). Comparison between groups by Mann-Whitney U and χ2 test. BMI, body mass index. sTfR, soluble transferrin receptor. TG, triglycerides. SBP, systolic blood pressure. DBP, diastolic blood pressure. WC, waist circumference. HDL-C, HDL cholesterol. MetS, metabolic syndrome. HOMA-IR, homeostatic model assessment insulin resistance.
Includes additionally individuals who reported current use of oral hypoglycemic medications or insulin regardless of fasting glucose values.
Includes additionally individuals who reported current use of antihypertensive medications regardless of blood pressure values.
The prevalence of MetS in the whole population was 48.7%, and 34.3% among individuals without cardiometabolic disease (cardiovascular diseases and/or diabetes) (data not shown).
There was no statistically significant association between standardized values of sTfR and MetS and its components in any of the sex/menopausal status groups (Table 2) or in the whole sample (Table 3). Since high prevalence of some components could limit the power of the study to detect an association with sTfR, we additionally conducted linear regression analysis between log-sTfR and log-transformed values of WC, HDL-C, glucose, triglycerides, SBP and DBP. The unadjusted linear regressions showed only association with log-glucose (βeta= 0.18 [0.002 to 0.37], P=0.047) and log-WC (βeta= 0.12 [0.02 to 0.22], P=0.015) in men, log-triglycerides in premenopausal women (βeta= 0.32 [0.01 to 0.63], P=0.041), and log-WC (βeta= 0.15 [0.05 to 0.25], P=0.002) in postmenopausal women, but after adjustments there were no significant associations (Supplementary table 1). Further adjustment for treatment with insulin and/or hypoglycemic drugs in associations with glucose, and for anti-hypertensive medication in associations with SBP and DBP, did not alter the significance of the above findings (data not shown).
Table 2.
Odds ratios(95% CI) for metabolic syndrome and its components per sex/menopausal-specific SD of the iron markers in the study subjects categorised by sex and menopausal status
| Z score log-sTfR | Z score log-ferritin | |||
|---|---|---|---|---|
| Non-adjusted | Adjusted* | Non-adjusted | Adjusted* | |
| Premenopausal women | ||||
| High glucose † | 1.35 (0.87-2.09) | 1.23 (0.77-1.95) | 0.89 (0.59-1.36) | 1.01 (0.65-1.58) |
| Low HDL-C | 1.18 (0.71-1.96) | 1.14 (0.68-1.90) | 0.95 (0.54-1.65) | 0.96 (0.51-1.79) |
| High TG | 1.35 (0.87-2.12) | 1.29 (0.80-2.08) | 1.51 (0.96-2.39) | 1.51 (0.94-2.44) |
| High BP¶ | 1.35 (0.91-1.99) | 1.29 (0.84-1.92) | 0.95 (0.66-1.36) | 0.96 (0.66-1.40) |
| High WC | 1.07 (0.77-1.49) | 1.01 (0.71-1.43) | 0.79 (0.56-1.11) | 0.79 (0.54-1.15) |
| MetS | 1.32 (0.90-1.92) | 1.21 (0.81-1.79) | 1.13 (0.80-1.59) | 1.24 (0.85-1.80) |
| Postmenopausal women | ||||
| High glucose † | 1.08 (0.86-1.37) | 1.009 (0.78-1.29) | 1.23 (0.97-1.56) | 1.20 (0.94-1.52) |
| Low HDL-C | 1.22 (0.66-2.28) | 1.45 (0.74-2.84) | 0.76 (0.41-1.40) | 0.81 (0.45-1.45) |
| High TG | 0.99 (0.78-1.25) | 0.97 (0.75-1.24) | 1.26 (0.98-1.61) | 1.28 (0.99-1.64) |
| High BP¶ | 1.13 (0.83-1.56) | 0.94 (0.66-1.34) | 1.14 (0.85-1.52) | 1.08 (0.77-1.51) |
| High WC | 1.10 (0.59-2.03) | 1.00 (0.50-1.97) | 1.10 (0.63-1.94) | 0.98 (0.51-1.90)Ψ |
| MetS | 0.99 (0.69-1.43) | 0.83 (0.54-1.26) | 1.65 (1.17-2.31) | 1.65 (1.11-2.46) |
| Men | ||||
| High glucose † | 1.20 (0.94-1.52) | 1.10 (0.85-1.41) | 1.37 (1.07-1.75) | 1.42 (1.10-1.84) |
| Low HDL-C | 0.93 (0.72-1.21) | 0.94 (0.72-1.24) | 1.61 (1.21-2.15) | 1.60 (1.19-2.15) |
| High TG | 1.03 (0.81-1.30) | 1.07 (0.84-1.38) | 1.69 (1.29-2.21) | 1.71 (1.29-2.26) |
| High BP¶ | 1.13 (0.87-1.46) | 0.97 (0.72-1.29) | 1.15 (0.89-1.48) | 1.21 (0.91-1.61) |
| High WC | 1.30 (1.00-1.70) | 1.16 (0.87-1.55) | 1.53 (1.18-1.98) | 1.62 (1.22-2.15) |
| MetS | 1.05 (0.83-1.33) | 0.95 (0.74-1.22) | 1.90 (1.44-2.50) | 2.02 (1.51-2.70) |
Age, fibrinogen levels, smoking status (yes/no/ex-smoker) and alcohol consumption (no/yes).
Includes additionally individuals who reported current use of oral hypoglycemic medications or insulin regardless of fasting glucose values.
Includes additionally individuals who reported current use of antihypertensive medications regardless of blood pressure values. TG, triglycerides. BP, blood pressure.. WC, waist circumference.
59 cases were omitted because in the category of ex-smoker all of the subjects had high WC. HDL-C, HDL cholesterol. MetS, metabolic syndrome. Significant associations are show in bold (P<0.05).
Table 3.
Odds ratios(95% CI) for metabolic syndrome and its components per SD of iron markers in the whole sample
| Z score log-sTfR | Z score log-ferritin | Z score log-sTfR/ferritin ratio | ||||
|---|---|---|---|---|---|---|
| Non-adjusted | Adjusted* | Non-adjusted | Adjusted* | Non-adjusted | Adjusted* | |
| High glucose † | 1.11 (0.95-1.29) | 1.10 (0.92-1.30) | 1.48 (1.27-1.74) | 1.31 (1.06-1.61) | 0.73 (0.62-0.85) | 0.83 (0.68-1.02) |
| Low HDL-C | 1.16 (0.98-1.36) | 0.98 (0.76-1.25) | 0.46 (0.38-0.56) | 1.40 (1.06-1.84) | 2.07 (1.69-2.53) | 0.75 (0.57-0.98) |
| High TG | 1.02 (0.88-1.19) | 1.05 (0.89-1.24) | 1.71 (1.44-2.03) | 1.67 (1.34-2.09) | 0.63 (0.53-0.75) | 0.68 (0.55-0.83) |
| High BP¶ | 1.09 (0.93-1.28) | 1.05 (0.88-1.27) | 1.52 (1.30-1.78) | 1.14 (0.90-1.44) | 0.71 (0.61-0.84) | 0.92 (0.74-1.14) |
| High WC | 1.16 (0.97-1.39) | 1.02 (0.84-1.24) | 1.09 (0.91-1.29) | 1.30 (1.01-1.68) | 0.96 (0.81-1.15) | 0.82 (0.65-1.04) |
| MetS | 1.09 (0.94-1.27) | 0.97 (0.82-1.15) | 1.41 (1.21-1.65) | 1.92 (1.52-2.43) | 0.76 (0.65-0.88) | 0.60 (0.49-0.75) |
Age, fibrinogen levels, smoking status (yes/no/ex-smoker), alcohol consumption (no/yes), and sex/menopausal status (premenopausal women/postmenopausal women/men).
Includes additionally individuals who reported current use of oral hypoglycemic medications or insulin regardless of fasting glucose values.
Includes additionally individuals who reported current use of antihypertensive medications regardless of blood pressure values. TG, triglycerides. BP, blood pressure.. WC, waist circumference. HDL-C, HDL cholesterol. MetS, metabolic syndrome. Significant associations are show in bold (P<0.05).
Standardized values of ferritin were significantly associated with higher odds of having MetS components (except high blood pressure) in men, in unadjusted models and adjusting for age, fibrinogen levels, alcohol intake, and smoking (Table 2). Ferritin was significantly associated with MetS in men and postmenopausal women (Table 2). In the whole sample, the adjusted associations found for ferritin and MetS and its components were similar to those reported in men (Table 3).
In a separate analysis for women adjusting for menopausal status, sTfR was not associated with MetS or its components, and ferritin was independently associated with high triglycerides and MetS (Supplementary table 2).
We performed additional evaluation of associations between MetS and its components and standardized values of sTfR/ferritin ratio Supplementary table and 3). A lower ratio reflects higher iron status on the basis of increased iron stores regarding low iron demand in tissues. In general the associations for sTfR/ferritin ratio were similar to those described for ferritin (Table 2).
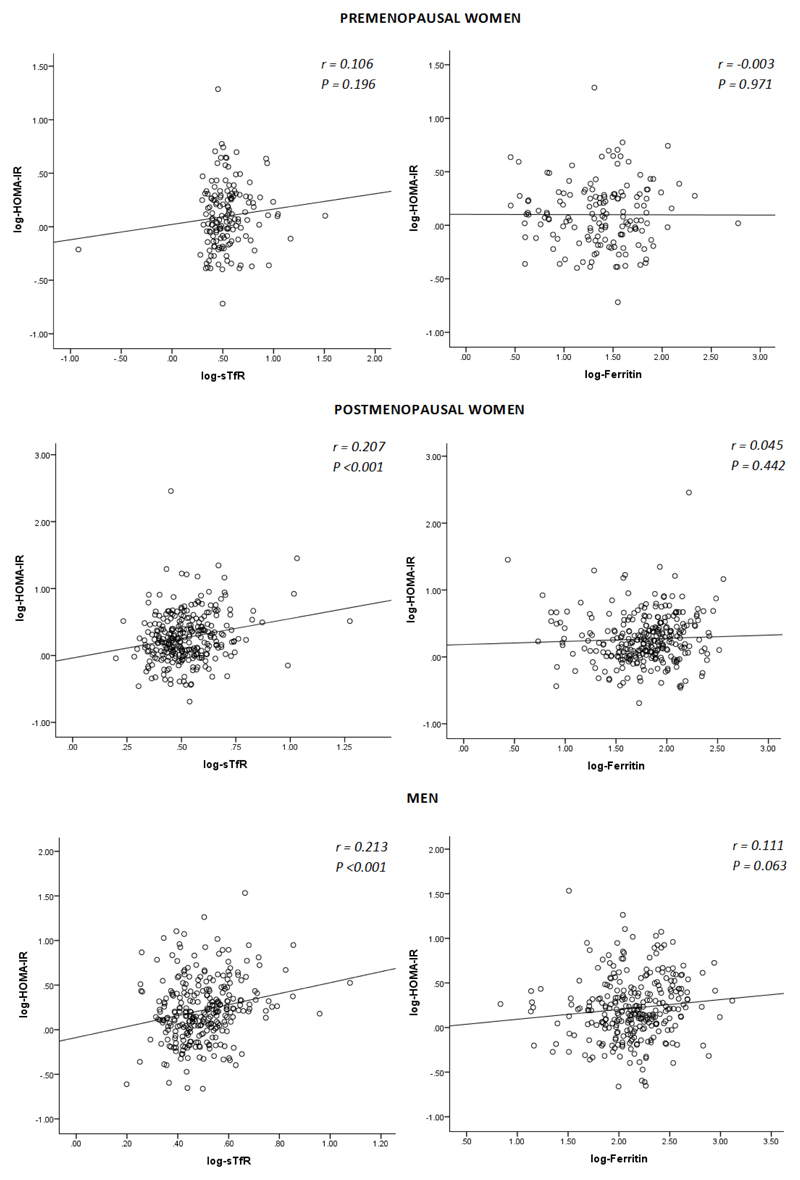
STfR levels correlated positively with insulin resistance in postmenopausal women and men, and this relationship remained statistically significant in linear regression analyses (Figure 1) with associations in two of the three strata remaining significant after adjusting for covariates: postmenopausal women (βeta= 0.34 [0.05 to 0.63], P=0.020) and men (βeta= 0.44 [0.14 to 0.75], P=0.004) (see also Supplementary table 4). On the other hand there was a borderline statistically significant correlation between ferritin and HOMA-IR in men (Figure 1) which did not persist after adjustments (Supplementary table 4). In the whole sample, sTfR levels were associated with insulin resistance after adjustment for covariates including sex/menopausal status (P<0.05), but no association was observed for ferritin in similar analyses (Supplementary table 4). The relationship between sTfR and HOMA-IR was driven by the relationship between sTfR and insulin levels which were similarly significant (Supplementary table 4), whereas adjusted associations with glucose levels were not statistically significant (Supplementary table 1).
Figure 1. Pearson correlations between insulin resistance and iron markers.
We also evaluated potential non-linear associations between ferritin and insulin resistance and found that mean HOMA-IR in the highest tertile of ferritin was significantly higher than for the lowest tertile even after adjustment for covariates (Supplementary figure 1).
In the unadjusted analysis, HbA1C, a marker of longer-term glucose metabolism, was significantly associated with sTfR in men and with ferritin in the whole sample, but after adjustments there were no significant independent associations (Supplementary table 5).
We additionally used diabetes, cardiovascular disease and categories of physical activity as covariates in the adjustment models, but the estimates of the associations described above between the iron markers, MetS, insulin resistance and HbA1C and the statistical significance did not change substantially (Supplementary table 6).
Supplementary tables 7 and 8 show the effect of additional adjustment for BMI on the association between iron markers and MetS and its components. All of the initial significant associations remained significant after adjustment for BMI, with the exception of the associations between ferritin and increased waist circumference in men and the whole sample.
Discussion
In this study of a population with high prevalence of metabolic syndrome we report three key findings. First, sTfR levels were associated with insulin resistance but not with MetS. Secondly, the positive association between sTfR and HOMA-IR was independent of covariates. Third, iron stores (measured as serum ferritin) were non-linearly associated with insulin resistance. This set of findings suggests that different iron related proteins are involved in cardiometabolic risk by separate underlying mechanisms.
The lack of association between sTfR levels and MetS is consistent with findings in different studies of different sizes ranging from 155 to 1,969 subjects (supplementary table 9). A Finnish study of middle-aged subjects from the general population also found no significant association when controlling for confounding factors (supplementary table 9) (12). In the same study, levels of sTfR levels were significantly higher in adjusted analyses (12). A small study reported lower levels of sTfR in subjects with MetS in a sex-stratified analysis but no additional adjustments were conducted (22)(supplementary table 9). A significant positive age-sex adjusted correlation of sTfR with waist circumference but no evidence of a relationship with HDL-C and triglyceride levels in participants from the EPIC-Postdam study have also been described (13) (supplementary Table 9). Diastolic blood pressure and triglycerides increased across quartiles of sTfR but no association was found with waist circumference, LDL-C, HDL-C, systolic blood pressure and fasting glucose levels in 1,262 women after adjustment for covariates (14) (supplementary table 9). Different adjustments, statistical approaches and discrepancies in methods measuring sTfR concentrations could contribute to the heterogeneity of results from different studies. Various commercial sTfR assays give disparate values because of the lack of an international standard. For instance, Hamalainen et al. reported similar sTfR levels (mean 2.9 mg/L) and prevalence of MetS (48% in men and 52% in women) to those we report (12). Very high sTfR levels (mean 9.09 mg/L) with lower prevalence of some MetS components (high WC 31%, low HDL 43%) were described by Aderibigbe et al (14). Meanwhile, Montonen et al. reported lower median sTfR levels across ferritin quintiles between 1.0 and 1.9 mg/L (13). In addition, it is important to note that the above studies included diverse populations in Europe and South Africa which may have influenced differences in association patterns, sTfR levels and prevalence of MetS components.
Our finding of a positive association between sTfR and insulin resistance in postmenopausal women and men in our study is consistent with two previous studies by Fernandez-Real et al. (23) and Huth et al.(24). Fernandez-Real et al. described an inverse association between sTfR levels and insulin sensitivity estimated by minimal modeling in 221 Spanish individuals (97 non-obese with normal glucose tolerance, 36 with impaired glucose tolerance and 88 with type 2 diabetes)(23). There was no evidence of associations between sTfR and fasting glucose or insulin but positive correlations of sTfR with values of glucose and insulin during an oral glucose tolerance test were reported. We report similar findings to Huth et al who found that sTfR levels were significantly and positively correlated with HOMA-IR in 2893 participants of the population-based Cooperative Health Research in the Region of Augsburg (KORA) F4 study (Germany)(24). In contrast, Arija et al. did not find significant correlation between sTfR and HOMA-IR adjusting for sex, age and BMI either in Spanish non-diabetic individuals (n=302) as in a mixed group of those non-diabetic plus non-diabetic subjects who later developed diabetes (n=153)(25). The relationship between sTfR and glucose metabolism might be easier to identify in the postprandial than in fasting state since Fernandez-Real reported correlation with glucose concentrations after an oral glucose test tolerance test (23). In addition an insulin-sensitizing intervention of dietary change combined with exercise was associated with decreasing sTfR levels in obese individuals (26). We found that additional adjustment for physical activity did not substantially affect the non-significant associations of sTfR with MetS and its components but acknowledge that our measurement of physical activity is imperfect. A significant association between presence of transferrin receptor gene polymorphisms (rs3817672, 210AG, S142G) and type 2 diabetes has been described (27). In addition, individuals with 210A--G TfR gene polymorphism showed higher sTfR levels which correlated positively with glucose levels whereas in non-carriers there was no relationship between those markers (27). These associations with polymorphisms were not confirmed in genetic consortia databases (28). However, other studies have found other SNPS linked to both type 2 diabetes and sTfRs. For instance, significant associations have been observed for loci in TPMRSS6 with sTfR (P = 3.47×10(-6)) and type 2 diabetes risk (29). These findings imply that a common third factor might influence both circulating sTfR levels and diabetes susceptibility.
Although the finding of a positive association between sTfR and insulin resistance could suggest that low iron status, in terms of high sTfR, may also be associated with cardiometabolic risk, it appears unlikely given the absence of concomitant association with MetS or its components in this cross-sectional study. A potential explanation is the effect of insulin on sTfR levels. Insulin upregulates erythropoiesis (30), of which sTfR is a surrogate. sTfR represents a valuable quantitative assay of marrow erythropoietic activity as well as a marker of tissue iron deficiency (31). Marrow erythropoietic activity appears to be the most important determinant of sTfR levels, causing variations up to 8 times below and up to 20 times above average normal values (31). The erythroblasts rather than reticulocytes are the main source of serum sTfR. Soluble TfR levels are decreased when erythropoietic activity is low, and are increased in situations of hemolysis or ineffective erythropoiesis (31). As insulin has been described to upregulate erythropoiesis (30), it could be that this action of insulin remains sensitive in contrast to peripheral insulin resistance in the liver or in the muscle (the classical insulin sensitive tissues). If this is the case, sTfR might appear to be spuriously elevated and would not reflect the insulin sensitivity in other tissues. In addition, upregulation in the expression of transferrin receptors by insulin via a hypoxia inducible factor, as observed in human hepatic cells (HepG2)(32) is an alternative explanation.
We identified differences by sex and menopausal status in the relationship of ferritin with MetS and its components since significant associations were found in men and postmenopausal women, but not in premenopausal women in our study showed. Theoretically, in men and postmenopausal women the relationship between ferritin and cardiometabolic risk might be more obvious due to higher iron accumulation than in women who lose iron during menstruation. This is in line with studies describing no association between ferritin and MetS in premenopausal women (33, 34). However, several studies have also reported significant relationship between ferritin and MetS in premenopausal women (35,36,37,38). Threshold effects do not appear to contribute to the discrepancy in relationship since studies had comparable ferritin concentrations and MetS prevalence (~ 10%) among pre-menopausal women regardless of whether or not an association was described. Statistical power could explain the discrepant findings because most of the studies describing association had larger sample sizes for premenopausal women than those with no association. The relationship between insulin resistance and ferritin appeared to demonstrate a threshold effect when comparing the highest levels of ferritin vs. the lower (tertiles 1 and 2) in the whole group of subjects.
Neither ferritin nor sTfR were associated with HbA1c whether or not diabetes was included as a covariate in the models. Our finding is consistent with the lack of association between ferritin and HbA1C reported in the 3876 participants of NHANES III (1988-1994)(39). Previously, Fernandez-Real et al.(21) and Rajpathak et al. (40) described significant weak correlations (r=0.14 and r=0.12) between sTfR and HbA1C in 221 men and 560 overweight individuals respectively. However, in the first study the correlation was unadjusted, and the second only adjusted for age and sex, and it is unknown if metabolic and adiposity covariates might have attenuated the relationships. It is of note that the associations between sTfR and HbA1C do not appear to have been evaluated using robust multivariate analyses in the existing literature. Therefore, further population-based studies are needed to confirm absence of association between HbA1C and markers of iron metabolism after multivariate adjustment in general populations.
In the present study we presented adjusted associations between the iron markers, MetS and its components with and without BMI as covariate. MetS is common in overweight and obese individuals (41) and BMI might confound or mediate the relationship between iron metabolism and MetS We found that only the association between ferritin and waist circumference was markedly attenuated after adjustment for BMI. Although this might have been expected given the high correlation between waist circumference and BMI and other study reported similar attenuation (42), another study reported a significant association between ferritin and increased waist circumference independently of BMI adjustment (43). This latter study had a larger sample size with higher ferritin levels. In our study, the significant associations between ferritin, MetS (in men and premenopausal women), high glucose and triglycerides and low HDL-C in men appear to be independent of BMI and other covariates.
To the best of our knowledge, the present study is the first to investigate the association between sTfR and both MetS and insulin resistance by using a robust multivariable analysis. We also have extended the finding by Hamalainen et al. describing the absence of significant relationship between STfR and MetS by performing additional adjustments for BMI, alcohol consumption, cardiovascular disease and diabetes. In addition previous studies of the association between ferritin and sTfR and cardiometabolic risk may have provided biased results as a consequence of not adjusting for prevalent cardiometabolic disease. Concomitant chronic disease can influence iron status and reverse causality might lead to overestimation of the association between iron markers and MetS. Key limitations of our study include of the relatively small number of pre-menopausal women and the inability to adjust for hepatic dysfunction since markers such as transaminases were not measured in the original study. In the original project there were no specific questions about use of lipid-lowering medications and therefore associations with components of low HDL-C and high triglyceride could be underestimated since these components did not include individuals with prescribed medication that might affect these values. The cross-sectional design of the study means that it is not possible to provide evidence of a causal relationship between iron status and cardiometabolic risk factors. In addition, no adjustment for multiple testing was performed and some findings may be due to chance. For the present study, we used the sample of subjects with available measurements of exposure, outcome and adjustment variables and have not performed a power calculation. However, our sample as a whole is relatively large sample in comparison with previous studies but may have had limited power to detect small effects.
Fibrinogen levels were higher in the selected subjects for this analysis than in the non-selected participants from the Croatia/Vis study, and this was the only difference in the study variables. It is not feasible to determine if this difference might have influenced the findings since an analysis of data for the excluded subjects would not be reliable given the small sample size and a large proportion of missing values. The difference in fibrinogen levels may also be a finding by chance due to the multiple testing. The nature of multivariable modelling means that people with missing data are excluded. For clearer and more coherent comparisons, the present study provides unadjusted and adjusted estimates using the subgroup with complete data.
In conclusion, our study found that sTfR levels are associated with insulin resistance but not with MetS, independently of age, subclinical/chronic inflammation, smoking and alcohol habits, glycosylated hemoglobin, and cardiometabolic disease, in a population with a high prevalence of MetS and abdominal obesity. It is possible that sTfR levels are a poor marker of erythropoiesis or iron metabolism in subjects with insulin resistance or hyperinsulinemia, and are spuriously elevated and therefore not associated with MetS or its components. We conclude that there is a complex relationship between markers of iron status and cardiometabolic risk, with inconsistent associations with different markers.
Supplementary Material
Acknowledgments
The original study was supported by MRC and the Croatian Ministry of Science, Education and Sport. We would like to acknowledge the staff of several institutions in Croatia that supported the field work, including but not limited to The University of Split and Zagreb Medical Schools, Institute for Anthropological Research in Zagreb and Croatian Institute for Public Health. The SNP genotyping for the CROATIA_Vis cohort was performed in the core genotyping laboratory of the Wellcome Trust Clinical Research Facility at the Western General Hospital, Edinburgh, Scotland
Abbreviations
- sTfR
soluble transferrin receptor
- HFG
high fasting glucose
- MetS
metabolic syndrome
- HDL-C
HDL cholesterol
- WC
waist circumference
- HOMA-IR
homeostatic model assessment insulin resistance
Footnotes
Author contributions
M.F.S.O conceived the study design, analysed data, wrote the manuscript. S.H.W, S.M and J.M.F.R supervised the analysis, reviewed/edited the manuscript and contributed to the discussion. C.H researched data and edited manuscript. OP was the coordinator of the project, researched data and reviewed/edited the manuscript and contributed to the discussion.
Conflict of interest
No potential conflicts of interest relevant to this article exist.
References
- 1.Orban E, Schwab S, Thorand B, Huth C. Association of iron indices and type 2 diabetes: a meta-analysis of observational studies. Diabetes Metab Res Rev. 2014;30(5):372–94. doi: 10.1002/dmrr.2506. [DOI] [PubMed] [Google Scholar]
- 2.Bao W, Rong Y, Rong S, Liu L. Dietary iron intake, body iron stores, and the risk of T2D: a systematic review and meta-analysis. BMC Medicine. 2012;10:119. doi: 10.1186/1741-7015-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lapice E, Masulli M, Vaccaro O. Iron deficiency and cardiovascular disease: an updated review of the evidence. Current Atherosclerosis Reports. 2013;15(10):358. doi: 10.1007/s11883-013-0358-0. [DOI] [PubMed] [Google Scholar]
- 4.Abril-Ulloa V, Flores-Mateo G, Solà-Alberich R, et al. Ferritin levels and risk of metabolic syndrome: meta-analysis of observational studies. BMC Public Health. 2014;14:483. doi: 10.1186/1471-2458-14-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Real JM, Lopez-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes. 2002;51(8):2348–54. doi: 10.2337/diabetes.51.8.2348. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Z, Li S, Liu G, et al. Body iron stores and heme-iron intake in relation to risk of T2D: A systematic review and meta-analysis. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0041641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aisen P, Wessling-Resnick M, Leibold EA. Iron metabolism. Curr Opin Chem Biol. 1999;3(2):200–6. doi: 10.1016/S1367-5931(99)80033-7. [DOI] [PubMed] [Google Scholar]
- 8.Trinder D, Baker E. Transferrin receptor 2: a new molecule in iron metabolism. Int J Biochem Cell Biol. 2003;35(3):292–6. doi: 10.1016/s1357-2725(02)00258-3. [DOI] [PubMed] [Google Scholar]
- 9.Ganz T, Nemeth E. Iron imports. IV. Hepcidin and regulation of body iron metabolism. Am J Physiol Gastrointest Liver Physiol. 2006;290:G199–203. doi: 10.1152/ajpgi.00412.2005. [DOI] [PubMed] [Google Scholar]
- 10.Cook JD, Skikne BS, Baynes RD. Serum transferrin receptor. Annu Rev Med. 1993;44:63–74. doi: 10.1146/annurev.me.44.020193.000431. [DOI] [PubMed] [Google Scholar]
- 11.Kæstel P, Aaby P, Ritz C, et al. Markers of iron status are associated with stage of pregnancy and acute-phase response, but not with parity among pregnant women in Guinea-Bissau. Br J Nutr. 2015;114(7):1072–9. doi: 10.1017/S0007114515001993. [DOI] [PubMed] [Google Scholar]
- 12.Hamalainen P, Saltevo J, Kautiainen H, et al. Erythropoietin, ferritin, haptoglobin, hemoglobin and transferrin receptor in metabolic syndrome: a case control study. Cardiovas Diabetol. 2012;11(116) doi: 10.1186/1475-2840-11-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montonen J, Boeing H, Steffen A, et al. Body iron stores and risk of T2D: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Diabetologia. 2012;55(10):2613–21. doi: 10.1007/s00125-012-2633-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aderibigbe OR, Pisa PT, Mamabolo RL, et al. Iron status and cardiovascular disease risk in black South African women: The PURE study. South African Journal of Clinical Nutrition. 2011;24(4):179–85. [Google Scholar]
- 15.Vitart V, Rudan I, Hayward C, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008;40(4):437–42. doi: 10.1038/ng.106. [DOI] [PubMed] [Google Scholar]
- 16.Polasek O. Future of biobanks - bigger, longer, and more dimensional. Croat Med J. 2013;54(5):496–500. doi: 10.3325/cmj.2013.54.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudan I, Marusić A, Janković S, et al. "10001 Dalmatians:" Croatia launches its national biobank. Croat Med J. 2009;50(1):4–6. doi: 10.3325/cmj.2009.50.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 20.Bardini G, Dicembrini I, Cresci B, Rotella CM. Inflammation markers and metabolic characteristics of subjects with 1-h plasma glucose levels. Diabetes Care. 2010;33(2):411–3. doi: 10.2337/dc09-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Temelkova-Kurktschiev T, Siegert G, Bergmann S, Henkel E, Koehler C, Jaross W, Hanefeld M. Subclinical inflammation is strongly related to insulin resistance but not to impaired insulin secretion in a high risk population for diabetes. Metabolism. 2002;51(6):743–9. doi: 10.1053/meta.2002.32804. [DOI] [PubMed] [Google Scholar]
- 22.Leiva E, Mujica V, Sepúlveda P, et al. High levels of iron status and oxidative stress in patients with metabolic syndrome. Biol Trace Elem Res. 2013 J;151(1):1–8. doi: 10.1007/s12011-012-9525-3. [DOI] [PubMed] [Google Scholar]
- 23.Fernández-Real JM, Moreno JM, López-Bermejo A, et al. Circulating soluble transferrin receptor according to glucose tolerance status and insulin sensitivity. Diabetes Care. 2007;30:604–8. doi: 10.2337/dc06-1138. [DOI] [PubMed] [Google Scholar]
- 24.Huth C, Beuerle S, Zierer A, et al. Biomarkers of iron metabolism are independently associated with impaired glucose metabolism and type 2 diabetes: the KORA F4 study. Eur J Endocrinol. 2015;173(5):643–53. doi: 10.1530/EJE-15-0631. [DOI] [PubMed] [Google Scholar]
- 25.Arija V, Fernández-Cao JC, Basora J, et al. Excess body iron and the risk of type 2 diabetes mellitus: a nested case-control in the PREDIMED (PREvention with MEDiterranean Diet) study. Br J Nutr. 2014;112(11):1896–904. doi: 10.1017/S0007114514002852. [DOI] [PubMed] [Google Scholar]
- 26.Fernández-Real JM, Izquierdo M, Moreno-Navarrete JM, et al. Circulating soluble transferrin receptor concentration decreases after exercise-induced improvement of insulin sensitivity in obese individuals. Int J Obes (Lond) 2009;33:768–74. doi: 10.1038/ijo.2009.99. [DOI] [PubMed] [Google Scholar]
- 27.Fernández-Real JM, Mercader JM, Ortega FJ, et al. Transferrin receptor-1 gene polymorphisms are associated with type 2 diabetes. Eur J Clin Invest. 2010;40(7):600–7. doi: 10.1111/j.1365-2362.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- 28.Morris AP, Voight BF, Teslovich TM, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44(9):981–90. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He M, Workalemahu T, Manson JE, et al. Genetic determinants for body iron store and type 2 diabetes risk in US men and women. PLoS One. 2012;7(7):e40919. doi: 10.1371/journal.pone.0040919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratajczak J, Zhang Q, Pertusini E, et al. The role of insulin (INS) and insulin-like growth factor-I (IGF-I) in regulating human erythropoiesis. Studies in vitro under serum-free conditions--comparison to other cytokines and growth factors. Leukemia. 1998;12(3):371–81. doi: 10.1038/sj.leu.2400927. [DOI] [PubMed] [Google Scholar]
- 31.Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta. 2003;329:9–22. doi: 10.1016/s0009-8981(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 32.Biswas S, Tapryal N, Mukherjee R, et al. Insulin promotes iron uptake in human hepatic cell by regulating transferrin receptor-1 transcription mediated by hypoxia inducible factor-1. Biochim Biophys Acta. 2013;1832(2):293–301. doi: 10.1016/j.bbadis.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Cho GJ, Shin JH, Yi KW, et al. Serum ferritin levels are associated with metabolic syndrome in postmenopausal women but not in premenopausal women. Menopause. 2011;18(10):1120–4. doi: 10.1097/gme.0b013e318217e172. [DOI] [PubMed] [Google Scholar]
- 34.Yoon JH, Linton JA, Koh SB, et al. Serum ferritin concentrations predict incidence of metabolic syndrome in rural Korean adults. Clin Chem Lab Med. 2012;50(11):2057–9. doi: 10.1515/cclm-2011-0928. [DOI] [PubMed] [Google Scholar]
- 35.Jehn M, Clark JM, Guallar E. Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes care. 2004;27(10):2422–8. doi: 10.2337/diacare.27.10.2422. [DOI] [PubMed] [Google Scholar]
- 36.Vari IS, Balkau B, Kettaneh A, et al. Ferritin and transferrin are associated with metabolic syndrome abnormalities and their change over time in a general population: Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR) Diabetes care. 2007;30(7):1795–801. doi: 10.2337/dc06-2312. [DOI] [PubMed] [Google Scholar]
- 37.Lee BK, Kim Y, Kim YI. Association of serum ferritin with metabolic syndrome and diabetes mellitus in the South Korean general population according to the Korean National Health and Nutrition Examination Survey 2008. Metabolism. 2011;60(10):1416–24. doi: 10.1016/j.metabol.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Yoo KD, Ko SH, Park JE, et al. High serum ferritin levels are associated with metabolic risk factors in non-obese Korean young adults: Korean National Health and Nutrition Examination Survey (KNHANES) IV. Clin Endocrinol (Oxf) 2012;77(2):233–40. doi: 10.1111/j.1365-2265.2011.04248.x. [DOI] [PubMed] [Google Scholar]
- 39.Cheung CL, Cheung TT, Lam KS, et al. High ferritin and low transferrin saturation are associated with pre-diabetes among a national representative sample of U.S. adults. Clin Nutr. 2013;32(6):1055–60. doi: 10.1016/j.clnu.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 40.Rajpathak SN, Wylie-Rosett J, Gunter MJ, et al. Biomarkers of body iron stores and risk of developing type 2 diabetes. Diabetes Obes Metab. 2009;11(5):472–9. doi: 10.1111/j.1463-1326.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meigs JB. The Role of Obesity in Insulin Resistance. In: Hansen BC, Bray GA, editors. The Metabolic Syndrome:: Epidemiology, Clinical Treatment, and Underlying Mechanisms. Totowa, New Jersey: Humana Press, a part of Springer Science & Business Media; 2008. [Google Scholar]
- 42.Jehn M, Clark JM, Guallar E. Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care. 2004;27(10):2422–8. doi: 10.2337/diacare.27.10.2422. [DOI] [PubMed] [Google Scholar]
- 43.Tang Q, Liu Z, Tang Y, et al. High serum ferritin level is an independent risk factor for metabolic syndrome in a Chinese male cohort population. Diabetology & metabolic syndrome. 2015;7:11. doi: 10.1186/s13098-015-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.