Abstract
Background
Chronic thromboembolic pulmonary hypertension results from incomplete resolution of pulmonary emboli. Pulmonary endarterectomy (PEA) is potentially curative, but residual pulmonary hypertension following surgery is common and its impact on long-term outcome is poorly understood. We wanted to identify factors correlated with poor long-term outcome after surgery and specifically define clinically relevant residual pulmonary hypertension post-PEA.
Methods and Results
Eight hundred eighty consecutive patients (mean age, 57 years) underwent PEA for chronic thromboembolic pulmonary hypertension. Patients routinely underwent detailed reassessment with right heart catheterization and noninvasive testing at 3 to 6 months and annually thereafter with discharge if they were clinically stable at 3 to 5 years and did not require pulmonary vasodilator therapy. Cox regressions were used for survival (time-to-event) analyses. Overall survival was 86%, 84%, 79%, and 72% at 1, 3, 5, and 10 years for the whole cohort and 91% and 90% at 1 and 3 years for the recent half of the cohort. The majority of patient deaths after the perioperative period were not attributable to right ventricular failure (chronic thromboembolic pulmonary hypertension). At reassessment, a mean pulmonary artery pressure of ≥30 mm Hg correlated with the initiation of pulmonary vasodilator therapy post-PEA. A mean pulmonary artery pressure of ≥38 mm Hg and pulmonary vascular resistance ≥425 dynes·s–1·cm–5 at reassessment correlated with worse long-term survival.
Conclusions
Our data confirm excellent long-term survival and maintenance of good functional status post-PEA. Hemodynamic assessment 3 to 6 months and 12 months post-PEA allows stratification of patients at higher risk of dying of chronic thromboembolic pulmonary hypertension and identifies a level of residual pulmonary hypertension that may guide the long-term management of patients postsurgery.
Keywords: endarterectomy; hypertension, pulmonary; pulmonary embolism; survival
Chronic thromboembolic pulmonary hypertension (CTEPH) is a complication of acute pulmonary emboli with uncertain prevalence, ranging from 0.57% to 9.1%.1 The diagnosis is strongly associated with a history of acute venous thromboembolism.2 CTEPH results from incomplete resolution of pulmonary emboli that become organized into vessel walls and cause different degrees of obstruction to pulmonary blood flow. Increased pulmonary vascular resistance (PVR) leads to increased right ventricular pressure load and eventually to right ventricular failure. Historically, the long-term outlook was poor with 5-year survival rates as low as 10%.3 Pulmonary endarterectomy (PEA) is the treatment of choice and in selected patients is curative.4 It is recognized that there is a steep surgical and institutional learning curve at the start of a PEA program, but, in experienced centers, the operative mortality rate is <5%.5–7 A number of reports have confirmed improved short-term outcome in terms of hemodynamics, right ventricular function, quality of life, functional status, and exercise capacity after surgery.7–18 Fewer reports describe long-term outcome post-PEA, and those that have been published are mainly retrospective and either only had small numbers of patients19–26 or limited information of factors correlated with long-term outcome.6,27–31
CTEPH is described by a 2-compartmental model with proximal vessel obstruction that is surgically accessible and a small-vessel arteriopathy found in nonobstructed vessels, which is histologically similar to pulmonary arterial hypertension and cannot be removed by surgery.32,33 This model explains why residual pulmonary hypertension (PH) can occur despite an apparently successful surgical clearance. Depending on the definition used, between 11% and 35% of patients have residual PH following PEA.14,25,28–30 Evidence suggests that long-term outcomes are dependent on postoperative hemodynamics.6,7,12,17,23,34 Persistently increased PVR >500 dynes·s–1·cm–5 at the end of intensive care unit (ICU) stay is a significant risk factor for in-hospital death and mortality during the first year post-PEA.6,7,12,34 PVR measured between 3 and 12 months after PEA can predict longer-term outcome.17,23 In contrast, patients found to have a mean pulmonary artery pressure (mPAP) >30 mm Hg at follow-up had a smaller increase in exercise capacity, but no difference in survival at 4 years in comparison with patients with an mPAP <30 mm Hg after surgery.29 In addition, a recent trial has shown an improvement in exercise capacity in patients with CTEPH that are inoperable or have persistent PH post-PEA35; therefore, further information on the natural history of disease would be helpful in identifying a low-risk group that might not require drug therapy.
The understanding of post-PEA hemodynamics and the consequences for patient management are thus incomplete. There is no consensus on the definition of residual (ie, PH immediately after surgery or at early postoperative follow-up when the remodeling of the right ventricle is completed) or recurrent PH (ie, when there is no PH on first assessment, but then PH is identified subsequently). We present prospectively obtained long-term data from the United Kingdom (UK) national PEA cohort to identify the factors correlated with worse long-term outcome and specifically define clinically relevant PH, both residual and recurrent, after PEA.
Methods
Study Design
Eight designated PH specialist centers from the UK and Mater Misericordiae University Hospital, Dublin, Ireland, undertake the diagnosis and management of all CTEPH patients in these countries. All CTEPH patients are referred for consideration of PEA. Of importance, only designated PH centers can prescribe pulmonary vasodilator therapies. This system of care provides a unique opportunity to study the natural history of the disease following surgical intervention through the careful follow-up of a national cohort of all CTEPH patients. All data were entered prospectively into dedicated databases at Papworth Hospital and the other UK and Ireland specialist PH centers. This study was designed and conducted to describe current care; therefore, formal ethics approval was not required. The National Health Service Health Research Authority Confidentiality Group was fully informed regarding the use of patient data.
Patient Selection
Patient selection was as previously described.29 Consecutive patients who underwent PEA at Papworth Hospital from the first operation in January 1997 until December 31, 2012, were included in this study with the exception of patients who had chronic thromboembolic disease without PH (mPAP <25 mm Hg)36 and those with conditions mimicking CTEPH, eg, pulmonary artery vasculitis or sarcoma.
Surgical Technique
PEA was performed by using principles similar to those used by the University of California, San Diego group.6,34 All patients underwent surgical intervention with deep hypothermia, but complete arrest of the circulation was not used in all cases.37,38 Surgical specimens were classified into 4 types according to the Jamieson classification.39 Patients continued lifelong anticoagulation after surgery.
Clinical Assessment After Surgery and Long-Term Follow-Up
Pulmonary hemodynamic values were recorded at 7 am on the first day after surgery and were routinely monitored until patients were extubated. At the start of the program, in those who had poor preoperative hemodynamics and were in World Health Organization functional class III and IV, the decision to continue bridging pulmonary vasodilator therapy was made on reviewing the ICU hemodynamics. With institutional experience, bridging treatment is routinely stopped at the time of the surgery unless the preoperative hemodynamics is poor and there has been an unsatisfactory surgical clearance. Patients were followed up at Papworth Hospital at 3 to 6 months and 12 months post-PEA as previously described.29 After their 12-month post-PEA review, patients were solely followed up by their referring PH center. These teams managed the patients according to international guidelines including the need for additional right heart catheterization depending on clinical status and noninvasive tests. They also initiated pulmonary vasodilator therapy according to the UK national PH treatment guidelines, which allow off-license treatment of CTEPH patients in functional class (FC) II (only as part of a clinical trial), III, or IV. Patients were usually discharged from PH specialist center follow-up after 5 years if the patient had good surgical clearance, remained in good FC (I or II), and did not require pulmonary vasodilator therapy.
Long-Term Survival Assessment
Survival after discharge from the hospital was calculated by using a censoring date of last review, transplant (n=1) or September 11, 2013, whichever was latest. The National Health Service summary care record tracking system was used for survival status (searched September 11, 2013). The causes of death were obtained from local databases and from the England or Scotland General Register Offices. The causes of death were independently classified into 4 groups by 3 independent adjudicators (J.E.C., M.T., and J.P.Z.). Discrepancies in the classification of death were discussed by the adjudicators to reach a consensus. The 4 groups were: (1) postoperative death, that is, related to surgery, eg, reperfusion pulmonary edema, multiorgan failure; (2) death caused by CTEPH (CTEPH direct), ie, right ventricular failure away from the operative period; (3) death related to CTEPH management (CTEPH related), eg, attributable to anticoagulation; and (4) unrelated to CTEPH, eg, malignancy.
Statistical Analysis
Statistical analyses were performed by using SAS (version 9.3, SAS institute, Cary, NC) and R (http://cran.r-project.org). Summary statistics (mean±standard deviation) were calculated, and paired t tests and Wilcoxon signed rank sum tests performed to test the differences in baseline and postsurgery (3–6 months) patient characteristics. Kaplan–Meier curves were created for overall survival by cohort and treatment initiation. Cumulative incidence functions of cause-specific mortality were estimated taking competing risks into account.40
Cox regression methods were used to analyze the time to treatment initiation after PEA and the survival time after hospital discharge (all causes). The time scale was the time since PEA surgery. Because of left truncation at hospital discharge for long-term survival analysis, the hospital length of stay was adjusted for in Cox models. Further hemodynamic, FC, and 6-minute walk distance measured at follow-up visits were included as time-varying covariates in Cox regression models to examine their temporal associations with time to treatment initiation and long-term survival. Proportional hazard assumptions were checked by Schoenfeld residuals in univariable analyses.41
To facilitate interpretations, we chose the best threshold values for mPAP, PVR, and cardiac index (at follow-up visits) based on the largest likelihood ratio test statistics obtained in separate Cox regressions for time to treatment initiation (with adjustment for sex, age, baseline body mass index, baseline history of atrial flutter or fibrillation or chronic obstructive pulmonary disease, bridging treatment presurgery, and intraoperative classification of disease) and CTEPH-direct deaths after hospital discharge (no adjustment was made because of the smaller number of CTEPH-direct deaths observed).42 In addition, graphical diagnostics on the functional forms of the relationships between log hazard ratios and mPAP, cardiac index, and PVR were performed to evaluate the plausibility of the chosen best threshold values. Results of these categorized mPAP, PVR, and cardiac index (based on best threshold values and clinical meaningful threshold values) were presented in the univariable and multivariable Cox regression analyses for long-term survival.
Results
Study Cohort
From 1997, until the end of 2012, a total of 880 consecutive patients with CTEPH underwent PEA at Papworth Hospital. The mean age was 57±15 years (range, 15–84) and 53% were male. The mean baseline mPAP was 47±11 mm Hg, PVR 830±382 dynes·s–1·cm–5, and 6-minute walk distance 260±126 m. Presurgery, 91% patients were in FC III or IV and 64% patients were taking at least 1 pulmonary vasodilator therapy as a bridge to surgery (Table 1). There was a history of malignancy or myeloproliferative disorder in 12.8%, antiphospholipid syndrome in 4.3%, and splenectomy in 2.8% of patients at the time of surgery (Table I in the online-only Data Supplement). There were significant improvements in hemodynamic variables, FC, and exercise capacity postsurgery (Table 1). Despite this, only 28% of patients had an mPAP ≤20 mm Hg, whereas 21% had mPAP 21 to 24 mm Hg and 51% had mPAP ≥25 mm Hg at the 3- to 6-month review. The mean follow-up of patients post-PEA was 4.3±3.6 years (range, 0–15.5 years).
Table 1. Baseline and Postsurgery Patient Characteristics.
| Preoperative | Postoperative | P Value | |
|---|---|---|---|
| Age, y | 57±15 | ||
| Sex (male:female), % | 53:47 | ||
| BMI, kg/m2 | 28.7±6.3 | ||
| FC 1/2/3/4, % | 0/9/68/23 | 38/47/15/0 | <0.0001* |
| mPAP, mm Hg | 47±11 | 27±10 | <0.0001* |
| PVR, dynes·s–1·cm–5 | 830±382 | 317±239 | <0.0001* |
| 6MWD, m | 260±126 | 353±118 | <0.0001* |
| TLCO, % predicted | 67 | ||
| Pulmonary vasodilator therapy, % | 64 | ||
| Mechanical ventilation length of time, median (mean±SD), days | 1 (2.5±4) | ||
| ICU length of stay, median (mean±SD), days | 3 (7±9) | ||
| Hospital length of stay, median (mean±SD), days | 16 (20±14) |
Baseline (n=880) and postoperative characteristics (n=748). Postoperative results are from 3- to 6-month post-PEA review. Values are mean±standard deviation. BMI indicates body mass index; FC, World Health Organization functional class; ICU, intensive care unit; mPAP, mean pulmonary artery pressure; 6MWD, 6-minute walk distance; PVR, pulmonary vascular resistance; SD, standard deviation; and TLCO, transfer coefficient of the lung for carbon monoxide.
Versus preoperative values from paired t tests (mPAP, PVR, 6MWD) and Wilcoxon signed rank test (for difference between preoperative and postoperative FC).
Initiation of Pulmonary Vasodilator Therapy During Long-Term Follow-Up
Post-PEA, 187 patients were treated with ≥1 pulmonary vasodilator therapies. Of these patients, 45 who were bridged continued therapy post-PEA long-term, whereas 142 patients were initiated on therapy after surgery, with the majority being started as the result of the first post-PEA review (Figure 1). In univariable Cox regressions, a number of demographic, functional, and hemodynamic factors correlated with increased risk of treatment initiation during follow-up (Table II in the online-only Data Supplement). In multivariable Cox regressions (Table 2), we reexamined the associations of treatment initiation for significant factors identified by univariable analyses. The associations of functional and hemodynamic variables at referral for PEA and over time post-PEA with treatment initiation remained statistically significant (P values <0.05 with the exception of cardiac index [over time] with a borderline P value of 0.067) in multivariable analyses (Table 2). Additional analyses using a binary outcome of mPAP ≥25 mm Hg at the first reassessment after hospital discharge had findings similar to those from the analyses for time to treatment initiation (Tables III and IV in the online-only Data Supplement).
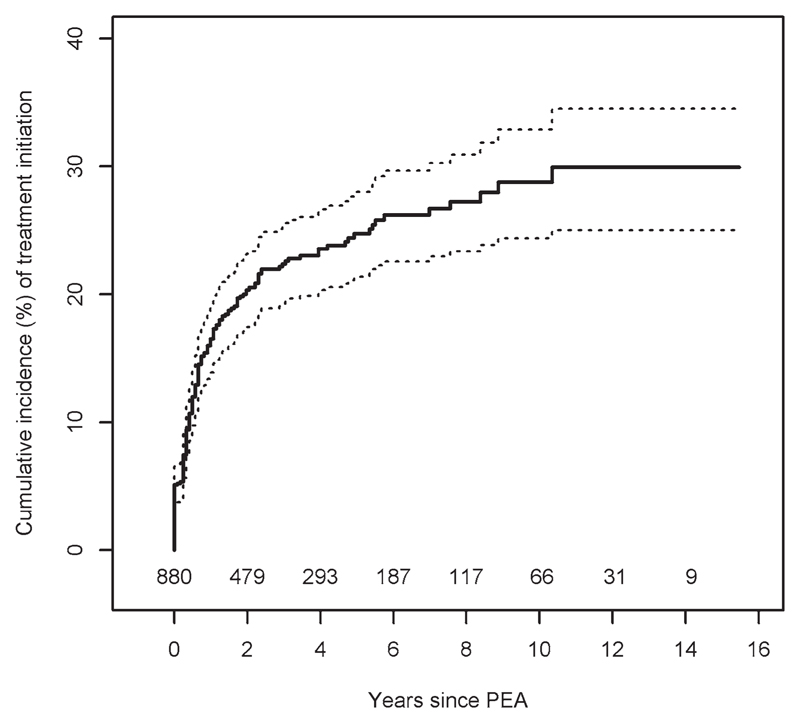
Figure 1.
Cumulative incidence (%) of pulmonary vasodilator therapy initiation after pulmonary endarterectomy. Number of patients at risk of treatment initiation over follow-up as shown. PEA indicates pulmonary endarterectomy.
Table 2. Multivariable Analyses for Factors Correlated With Treatment Initiation.
| Variables | Hazard Ratio | 95% CI | P Value | N* | |
|---|---|---|---|---|---|
| Functional class (baseline) | |||||
| 1,2 | 0.0002 | 116 | |||
| 3 | 3.74 | 0.89 | 15.73 | ||
| 4 | 7.77 | 1.80 | 33.54 | ||
| 6MWD (baseline) | 0.65 | 0.52 | 0.80 | <0.0001 | 101 |
| mPAP (baseline) | |||||
| Linear effect | 1.44 | 1.12 | 1.84 | 0.0138 | 113 |
| Quadratic effect | 0.86 | 0.74 | 0.99 | ||
| RAP (baseline) | 1.29 | 1.12 | 1.50 | 0.0007 | 103 |
| Cardiac index (baseline) | 0.79 | 0.67 | 0.94 | 0.0070 | 111 |
| PVR (baseline) | |||||
| Linear effect | 1.27 | 1.09 | 1.48 | 0.0008 | 112 |
| Quadratic effect | 0.90 | 0.84 | 0.96 | ||
| Functional class (over time) | |||||
| 1 | <0.0001 | 95 | |||
| 2 | 3.94 | 1.73 | 8.97 | ||
| ≥3 | 22.84 | 9.83 | 53.05 | ||
| 6MWD (over time) | 0.52 | 0.40 | 0.68 | <0.0001 | 88 |
| mPAP (over time) | |||||
| Linear effect | 25.77 | 11.98 | 55.41 | <0.0001 | 92 |
| Quadratic effect | 0.52 | 0.41 | 0.67 | ||
| RAP (over time) | 1.84 | 1.48 | 2.27 | <0.0001 | 91 |
| Cardiac index (over time) | 0.81 | 0.65 | 1.02 | 0.067 | 92 |
| PVR (over time) | |||||
| Linear effect | 1.87 | 1.46 | 2.39 | <0.0001 | 92 |
| Quadratic effect | 0.75 | 0.69 | 0.83 | ||
| TLco (over time) | 0.44 | 0.33 | 0.60 | 0.0142 | 66 |
These analyses were adjusted for the following factors: sex, age, baseline BMI, baseline history of atrial flutter or fibrillation or COPD, bridging treatment presurgery, and intraoperative classification of disease. All continuous variables were standardized (cardiac index: x-2.5/0.5, mPAP: x-25/10, PVR: x-250/200). Quadratic terms for continuous variables are included if the formal tests on nonlinearity are significant at 5% level. CI indicates confidence interval; COPD, chronic obstructive pulmonary disease; mPAP, mean pulmonary artery pressure; 6MWD, 6-minute walk distance; PVR, pulmonary vascular resistance; RAP, right atrial pressure; and TLCO, transfer coefficient of the lung for carbon monoxide.
N is the number of events (pulmonary vasodilator therapy initiation) available for analyses.
The best threshold values for the initiation of pulmonary vasodilator therapy over long-term follow-up were identified for mPAP, cardiac index, and PVR as being 30 mm Hg, 2.14 L·min–1·m–2, and 318 dynes·s–1·cm–5, respectively. However, the graphical evidence in Figure I in the online-only Data Supplement indicates that there was a rapid increase in log hazard ratio for treatment initiation for mPAP between 25 and 30 mm Hg, and after 30 mm Hg the log hazard ratio started to plateau. This data analysis suggests that 30 mm Hg is a plausible threshold, and, therefore, patients with an mPAP ≥30 mm Hg were at risk of a deteriorating functional status, which triggered pulmonary vasodilator therapy initiation. We propose that this value represents clinically significant PH post-PEA and that these patients need close long-term follow-up. No apparent patterns were observed for cardiac index and PVR.
Recurrent PH and Pulmonary Emboli During Long-Term Observation Period
Only 5 of the whole observed cohort who had an mPAP <25 mm Hg at their 3- to 6-month reevaluation went onto develop PH (mPAP ≥25 mm Hg), had a clinical deterioration, and were started on pulmonary vasodilator therapy during follow-up (Table V in the online-only Data Supplement). Of these, only 2 patients had recurrent CTEPH without other causes, eg, recurrent pulmonary emboli or a different cause for PH. To investigate the frequency of recurrent thromboembolism as the cause of recurrent or worsening PH, we undertook an additional subanalysis of patients managed locally by Papworth Hospital in the long term. Six of 356 patients experienced recurrent pulmonary emboli. Of these patients, all had an inferior vena cava filter in situ and 4 had underlying antiphospholipid syndrome.
Survival
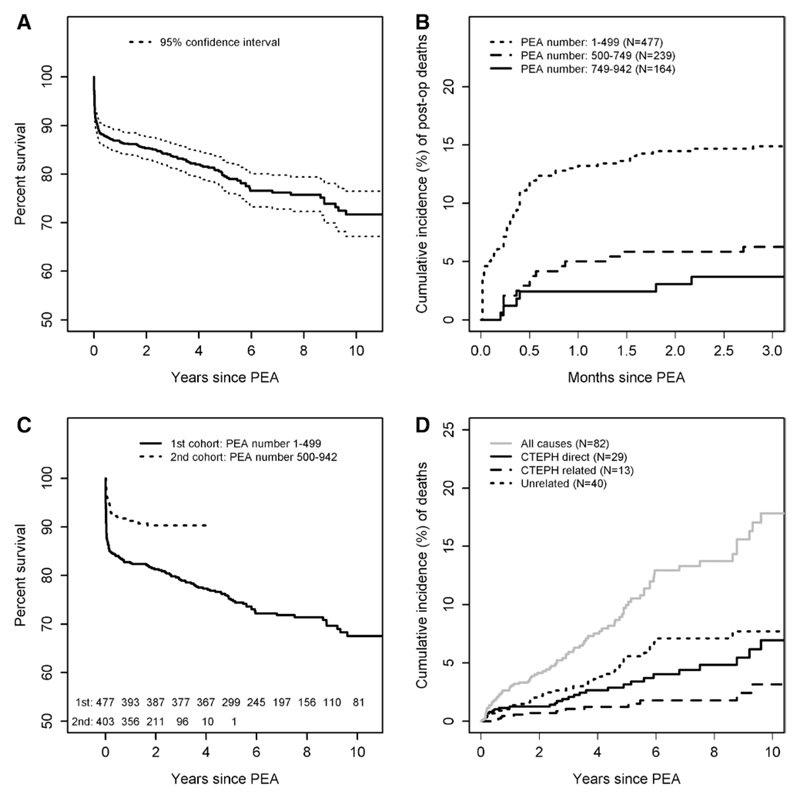
There were 174 deaths during a mean follow-up period of 4.3±3.6 years. The survival rates at 1, 3, 5, and 10 years were 86%, 84%, 79%, and 72%, respectively, for the entire cohort from 1997 (Figure 2A). Ninety-two of 174 (53%) of all deaths were classified as postoperative (Table VI in the online-only Data Supplement). The cumulative occurrence of 30-day mortality improved significantly over time from 13.2% (95% confidence interval [CI], 10.2%–16.3%) for the first half of our cohort to 2.4% (95% CI, 0.1%–4.8%) for the latest part of our cohort of patients undergoing PEA (Figure 2B). The overall survival rates at 1 and 3 years after surgery for the second half of our cohort were 91% and 90%, respectively (Figure 2C). This is despite the proportion of patients found to have the more distal bilateral type 3 thromboembolic disease at the time of surgery increasing from 8% in 2003 to 19% in 2012.
Figure 2.
Survival and classification of causes of death for PEA cohort. A, Kaplan–Meier curve showing cohort survival. B, Cumulative incidence of postoperative deaths improves with center experience. PEA number is consecutive PEA operations including indications other than CTEPH. N indicates number of PEA operations for CTEPH. C, Kaplan–Meier curve comparing survival of first versus second half of cohort. D, Cumulative incidence of causes of death (see Methods for classification) for patients surviving postoperative period. The number of patients at risk over follow-up as shown bottom of A and C. CTEPH indicates chronic thromboembolic pulmonary hypertension; and PEA, pulmonary endarterectomy.
For those patients who survived surgery but died during the follow-up period, 29 of 82 (35%) of deaths were classified as directly caused by CTEPH, 13 (16%) were related to CTEPH, and 40 (49%) were attributable to unrelated causes (Figure 2D and Tables VI through VIII in the online-only Data Supplement).
Factors Correlated With Long-Term Survival
We wanted to understand the postoperative factors correlated with long-term survival. We, like others, have used day 1 post-PEA hemodynamic data as an indicator of hemodynamic response from PEA. There was only a moderate correlation between the day 1 and 3- to 6-month post-PEA mPAP (r=0.53) and PVR (r=0.57; Figure IIA and IIB in the online-only Data Supplement), but a stronger association between the 3- to 6-month and 12-month post-PEA mPAP (r=0.75) and PVR (r=0.79; Figure IIC and IID in the online-only Data Supplement). The 3- to 6-month hemodynamic measurements were therefore used for further analyses.
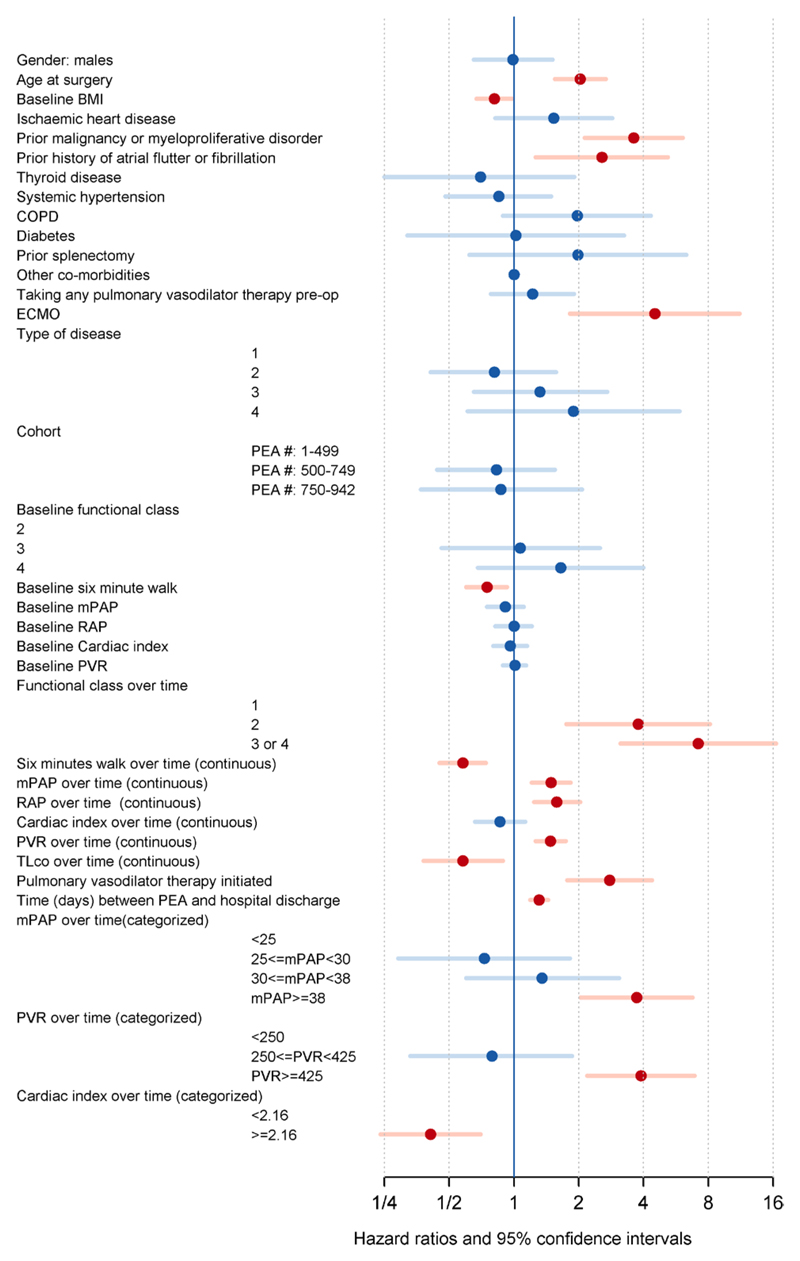
A number of demographic, functional, hemodynamic factors and comorbidities, including malignancy and atrial arrhythmias, were identified in univariable Cox regressions to be correlated with long-term survival (Figure 3 and Table IX in the online-only Data Supplement). In multivariable analyses (Table 3), we individually reexamined the associations of long-term survival for the significant factors identified in univariable analyses after adjusting for other important factors (sex, age, baseline body mass index, history of malignancy or myeloproliferative disorder, and hospital length of stay). Postoperative pulmonary vasodilator therapy initiation, worse FC, shorter 6-minute walk distance, higher mPAP, right atrial pressure, and PVR, and lower cardiac index remained negatively correlated with long-term survival in multivariable analyses. An mPAP ≥36 mm Hg and a PVR ≥416 dynes·s–1·cm–5 (as time-varying measures) were the optimal thresholds correlated with a higher risk of death from any cause, whereas an mPAP ≥38 mm Hg and a PVR ≥425 dynes·s–1·cm–5 identified those patients at higher risk of death because of CTEPH. The graphical diagnostics (Figure II in the online-only Data Supplement) confirmed the plausibility of mPAP ≥38 mm Hg as the threshold because there was a rapid increase in log hazard ratio between 35 and 38 mm Hg before the log hazard ratio plateaued.
Figure 3.
Variables correlated with long-term mortality by univariable analyses. All continuous variables were standardized (see Table 2 legend). Red circles/bars define significant factors (P<0.05) and blue circles/bars define the nonsignificant factors. BMI indicates body mass index; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; mPAP, mean pulmonary artery pressure PEA, pulmonary endarterectomy; PVR, pulmonary vascular resistance; and RAP, right atrial pressure.
Table 3. Multivariable Analyses for Long-Term Mortality.
| Variables | Hazard Ratio | 95% CI | P Value | N* | |
|---|---|---|---|---|---|
| Functional class (over time) | |||||
| 1 | <0.0001 | 52 | |||
| 2 | 6.37 | 2.23 | 18.26 | ||
| ≥3 | 12.88 | 4.11 | 40.41 | ||
| 6MWD (over time) | 0.48 | 0.35 | 0.68 | <0.0001 | 48 |
| mPAP (over time, continuous) | 1.57 | 1.20 | 2.04 | 0.0008 | 49 |
| mPAP (over time, categorized) | |||||
| <25 | 0.0003† | 49 | |||
| [25,30) | 0.60 | 0.22 | 1.65 | ||
| [30,38) | 1.57 | 0.66 | 3.75 | ||
| 38+ | 3.85 | 1.87 | 7.92 | ||
| RAP (over time) | 1.95 | 1.46 | 2.60 | <0.0001 | 49 |
| Cardiac index(over time, continuous) | 0.82 | 0.61 | 1.12 | 0.2149 | 48 |
| Cardiac index (over time, categorized) | |||||
| <2.16 | 0.0246† | 48 | |||
| ≥2.16 | 0.51 | 0.28 | 0.92 | ||
| PVR (over time, continuous) | 1.62 | 1.29 | 2.03 | <0.0001 | 47 |
| PVR (over time, categorized) | |||||
| <250 | <0.0001† | 47 | |||
| [250,425) | 0.62 | 0.24 | 1.61 | ||
| 425+ | 4.69 | 2.25 | 9.78 | ||
| TLCO (over time) | 0.71 | 0.43 | 1.15 | 0.1615 | 24 |
| Pulmonary vasodilator therapy initiated | |||||
| Yes | 2.59 | 1.48 | 4.53 | 0.0009 | 68 |
| ECMO | |||||
| Yes | 2.91 | 0.97 | 8.69 | 0.0562 | 68 |
Factors were adjusted for sex, age, baseline BMI, history of malignancy or myeloproliferative disorder, and hospital length of stay. All continuous variables were standardized (see Table 2 footnote). CI indicates confidence interval; ECMO, extracorporeal membrane oxygenation; mPAP, mean pulmonary artery pressure; 6MWD, 6-minute walk distance; PVR, pulmonary vascular resistance; RAP, right atrial pressure; and TLco, transfer coefficient of the lung for carbon monoxide.
N is the number of events (death) available for analyses.
These P values are not corrected for bias induced by selecting the largest likelihood ratio test statistics and cannot be used for inference.42
Discussion
This is the largest prospective national cohort study describing long-term outcomes post-PEA using a planned invasive hemodynamic evaluation and systematic long-term follow-up. Our study has a number of important messages: (1) risk stratification of patients using invasive hemodynamic measurements at 3 to 6 and 12 months post-PEA can guide which patients need close long-term follow-up to identify clinical deterioration and the need for additional treatment; (2) the majority of deaths following the immediate postoperative period were not attributable to right ventricular failure (CTEPH); and (3) longterm survival post-PEA is excellent, and most patients maintain a good functional status. It was previously described that PEA results in excellent long-term survival and our data confirm this despite an older or comparable age at the time of surgery in our cohort than in other series.4,23,31,43 Despite significant functional improvement following surgery with 85% of patients in either FC I or II, only 28% of patients had an mPAP ≤20 mm Hg, whereas 51% had an mPAP ≥25 mm Hg when measured by right heart catheterization at 3 to 6 months post-PEA. The majority of patients in our cohort maintained a good functional status in the long term despite residual PH by definition.
The Role of Post-PEA Hemodynamics in Long-Term Patient Management
There is no consensus on the definition of clinically significant residual PH post-PEA. Different centers apply a different definition depending on the local patient pathway. Our planned, systematic invasive hemodynamic evaluation in all UK patients over the first year postsurgery contrasts with others who base their postoperative outcome assessment on hemodynamics obtained immediately after PEA, while the patient is in the ICU.6,12 We only found a moderate correlation between the day 1 ICU hemodynamics and the 3- to 6-month measurements. A much stronger correlation existed between the 3- to 6-month and 12-month measurements. In our unit, we only record the day 1 ICU hemodynamics because stable patients have their Swan-Ganz catheter removed soon after, preventing later routine measurements in all patients. In comparison, the Vienna group (n=110)23 showed that postoperative hemodynamics measured 4 days (mean) post-PEA, when patients were off inotropes, were very similar to those assessed at 1-year post-PEA.
Pulmonary Vasodilator Therapy for Residual PH After PEA
This is the first study to report on factors correlated with the initiation of pulmonary vasodilator therapy post-PEA. One hundred eighty-seven patients in our cohort were treated with pulmonary vasodilator therapy during a mean follow-up period of 4.3 years. The majority were started soon after their 3- to 6-month or 12-month post-PEA evaluations. A number of factors were independently correlated with their initiation including worse FC and 6-minute walk distance and higher mPAP and right atrial pressure. Postoperative transfer coefficient of the lung for carbon monoxide also negatively correlated with the need to start pulmonary vasodilator therapy.
An arbitrary mPAP threshold of 30 mm Hg, based on historical survival data,3,44 was previously used by our group to analyze conditional long-term survival.29 In the current study, we also propose clinically significant PH post-PEA as an mPAP ≥30 mm Hg. In contrast to our previous study, this threshold is the result of an analysis of systematic clinical and hemodynamic data collected during the long-term follow-up of our large cohort of consecutive patients. This discussion of postoperative hemodynamics is currently specifically relevant because the first licensed therapy for the treatment of residual/recurrent PH post-PEA, riociguat, is now available. In the Study to Evaluate Efficacy and Safety of Oral BAY63-2521 in Patients With CTEPH (Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase–Stimulator Trial 1 [CHEST-1]) trial, 28% of patients included had residual/recurrent PH after PEA.35 Although the inclusion criteria included an mPAP ≥25 mm Hg and PVR >300 dynes·s–1·cm–5, the mean hemodynamics for the subgroup of patients with recurrent/residual PH post-PEA was 39 mm Hg and PVR 595 dynes·s–1·cm–5.45 In our cohort, 397 patients would have met the entry criteria for the CHEST-1 trial, but only 174 were treated offlicense with other pulmonary vasodilator therapy, whereas the other 223 patients have remained in a good FC (I or II) during careful follow-up by specialist PH physicians. Our data suggest that patients tolerated this hemodynamics well without evidence of deterioration. Our data analysis suggested that an mPAP of ≥30 mm Hg was a plausible threshold that identified patients in this study likely to be started on pulmonary vasodilator therapy because of clinical deterioration during long-term follow-up. Other hemodynamic variables including PVR and cardiac index could be used to define clinically significant PH post-PEA. The evidence from our data only suggests a likely change in log-hazard ratios for treatment initiation at an mPAP ≥30 mm Hg (Figure II in the online-only Data Supplement). We therefore propose that an mPAP ≥30 mm Hg represents clinically significant residual PH post-PEA and should guide clinicians which patients need close long-term follow-up after surgery.
Our approach to start pulmonary vasodilator therapy post-PEA was conservative because of the limited evidence of effectiveness in this group of patients during the study. Because of the observational nature of our study and the lack of a control group, we were unable to determine whether pulmonary vasodilator therapy had any effect on survival. Pulmonary vasodilator therapy initiation was however independently correlated with poorer long-term survival. Similar results have recently been reported from an international CTEPH registry.31 A potential bias with the factors correlated with the initiation of pulmonary vasodilator therapy post-PEA is that there were no fixed initiation criteria. However, in the UK, pulmonary vasodilator therapy prescribing in CTEPH is limited to patients in FC III or IV (unless the patient is enrolled in a clinical trial) according to the UK National PH prescribing policy.
Residual/Recurrent CTEPH
Only 5 patients developed recurrent PH (mPAP ≥25 mm Hg) during follow-up. There were only 2 patients who had true recurrent CTEPH without new thromboembolic disease or other cause for PH. These patients presented 9 and 10 years postsurgery, and, in both patients, there was evidence of ongoing mosaic changes seen on computed tomography at their first reevaluation post-PEA. This would suggest an ongoing disease process involving distal pulmonary arteries, which we hypothesize progressed over time, leading to recurrent CTEPH. The length of time after surgery that these patients represented is important because our mean follow-up was 4.3 years, and only 81 patients had a 10-year follow-up. Therefore, it will be important to continue close follow-up of the 223 patients in our cohort who have mild residual PH post-PEA, who currently remain in FC I or II without pulmonary vasodilator therapy.
Postoperative Long-Term Mortality
In our cohort, over half of the deaths were attributable to recognized complications in the postoperative period, with the highest proportion in the early part of the series. The 30-day mortality rate in the latest part of our cohort was 2.4%, which is comparable to other expert centers around the world.5–7 The majority of patient deaths during long-term follow-up were from conditions other than progressive right ventricular failure (CTEPH-direct deaths). Ideally, it would have been better to investigate the combined factors correlated with CTEPH-direct deaths rather than all-cause mortality after surviving the operative period, but because of the small numbers this was not possible.
Independent variables negatively correlated with long-term survival included postoperative pulmonary vasodilator therapy, worse FC, higher mPAP, right atrial pressure, and PVR, and lower cardiac index. Our best-fit mPAP values of 36 mm Hg for all-cause deaths and 38 mm Hg for CTEPH direct deaths were similar to those reported in 2 smaller series where a postoperative mPAP of 34 mm Hg and 37 mm Hg correlated with poorer long-term outcome.19,26 In comparison, Skoro-Sajer and colleagues23 did not find postoperative mPAP to be correlated with poorer outcome, but this finding may be attributable to the small sample size in their study. Like mPAP, PVR has been reported as critical for post-PEA outcome and different thresholds suggested.7,12,19,23 Patients with a PVR >500 dynes·s–1·cm–5 immediately post-PEA have a poorer perioperative and 1-year survival.7,12 In comparison, data from Vienna suggest that a PVR >590 dynes·s–1·cm–5 was correlated with poor longer-term outcome.23 Our results show a slightly lower threshold with 416 dynes·s–1·cm–5 for all-cause mortality and 425 dynes·s–1·cm–5 for CTEPH-direct deaths.
Strengths and Limitations
Our baseline and follow-up demographic and hemodynamic data are very similar to previously published retrospective and prospective series from both single and multiple centers.6–31 This allows any conclusions from this current study to be transferrable to other centers outside the UK if the reassessment of patients post-PEA is similar. Our study has a number of strengths including the prospective data entry, a large sample size, and careful systematic long-term follow-up by specialist PH physicians at designated PH centers. We were also able to confirm the exact cause of death for all but 1 patient who died overseas, by obtaining death certificates for all patients. This allowed the adjudication of the cause of death by 3 independent observers. Our study also has a number of limitations. Although the data were entered prospectively, they were not 100% complete, particularly for the patients in the early part of the cohort. For classification of residual PH, ideally, it would also be important to know whether the surgical clearance was completed at surgery, but at present there is no consensus about how this should be defined Residual PH could be attributable to purely a small-vessel arteriopathy or mixed residual proximal disease and arteriopathy. This could contribute to a better description and prediction of those patients who may do worse or respond differently to treatment. Another factor that may alter the threshold for starting pulmonary vasodilator therapy post-PEA may be how well the right ventricle has remodeled post-PEA. Interestingly, cardiac index was not found to independently predict long-term outcome or the initiation of the pulmonary vasodilator therapy by multivariable analyses. In addition, the methods for assessing right ventricular function by echocardiography have changed significantly over the study period, making data analysis difficult.
Conclusion
Long-term survival post-PEA is excellent and the majority of patient deaths after the operative period were not attributable to right ventricular failure (CTEPH). Our data allow clinicians to better understand the natural history of CTEPH post-PEA and provide a description of patients at higher risk of long-term postoperative morbidity and mortality. Hemodynamic values obtained 3 to 6 and 12 months after PEA allow stratification of patients at higher risk of dying of CTEPH and identify a level of residual PH that may help in the selection of patients who need to be monitored closely and considered for other treatment options.
Supplementary Material
Clinical Perspective.
Chronic thromboembolic pulmonary hypertension results from incomplete resolution of pulmonary emboli that become organized into vessel walls. Pulmonary endarterectomy (PEA) is the treatment of choice and, in selected patients, is curative, but residual chronic thromboembolic pulmonary hypertension following surgery is common. Its impact on long-term outcome after PEA is poorly understood. We have analyzed the long-term outcome of 880 patients after PEA from the United Kingdom national PEA cohort. These patients underwent detailed reassessment with right heart catheterization and noninvasive testing at 3 to 6 months after surgery and annually thereafter in specialist pulmonary hypertension centers. If clinically stable and not requiring pulmonary vasodilator therapy, they were discharged after 3 to 5 years of follow-up. The overall survival was 86%, 84%, 79%, and 72% at 1, 3, 5, and 10 years for the whole cohort and 91% and 90% at 1 and 3 years for the recent half of the cohort. The majority of deaths after the perioperative period were not attributable to right ventricular failure (chronic thromboembolic pulmonary hypertension) despite 51% of patients having a mean pulmonary artery pressure of ≥25 mm Hg at the 3- to 6-month review. At reassessment, a mean pulmonary artery pressure of ≥30 mm Hg correlated with pulmonary vasodilator therapy initiation at some point during follow-up, and a mean pulmonary artery pressure ≥38 mm Hg and pulmonary vascular resistance ≥425 dynes·s−1·cm−5 were correlated with worse survival. Our data allow clinicians to stratify patient follow-up after PEA surgery and to identify those in need of close long-term follow-up because of the increased risk of deterioration or death from chronic thromboembolic pulmonary hypertension.
Acknowledgments
We thank all the medical and nursing staff at each UK and Ireland PH center who helped to manage this cohort of patients and specifically Prof. Nicholas Morrell, Marius Berman, Maureen Rootes, Iain Armstrong, Paul Sephton, Jayne Jones, Jane Wilkinson, Sian Garrad, Sheila Forshaw, Rachel Crackett, Dr Jay Sunthararlingham, Sally Reddecliffe, Adele Gallimore, Prof. Michael Gatzoulis, Carl Harries, and Lisa Parfitt. We also acknowledge the helpful comments of the reviewers.
Sources of Funding
The National Institute of Health Research Cambridge Biomedical Research Center provided research infrastructure. Dr Su was supported by the Medical Research Council (Unit Program number U105261167).
Footnotes
Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document.
Disclosures
Dr Cannon has received speaker fees and honoraria from Actelion and GSK. Dr Kiely has received speaker fees, honoraria and has been on advisory boards for Actelion, Bayer, GSK, Pfizer and United Therapeutics and has received research grants from Actelion, Pfizer and Bayer. Dr Toshner has received speaker fees and honoraria from Actelion, GSK and Bayer and research grants from Bayer. Dr Condliffe has received speaker fees and honoraria from Actelion and GSK, research grants from Actelion, Pfizer and Bayer, and is on advisory boards for Actelion and Bayer. Dr Sheares has received honoraria from Actelion, GSK, Bayer and Pfizer. Dr Taboada has received honoraria from Actelion, Bayer, Pfizer, Lilly and GSK. Dr Elliot has received honoraria from Actelion and Bayer, has been on advisory boards for Actelion and GSK, and has received research grants from Actelion, Bayer and Pfizer. Dr Gibbs has received speaker fees and honoraria from Actelion, Bayer, GSK, Lilly, Pfizer and United Therapeutics, has received research grants from Actelion and United Therapeutics and is on advisory boards for Actelion, Gilead, Lilly and Novartis. Dr Howard has received speaker fees and honoraria from Actelion, GSK and Pfizer and is on advisory boards for Actelion, Bayer, GSK, Pfizer and Novartis. Dr Lordan has received speaker fees and honoraria from Bayer, Pfizer and Eli Lilly, and is on advisory boards for Bayer. Dr Corris has received research grants from Actelion, Pfizer, and GSK, and is on advisory boards for Actelion, Pfizer, Bayer, GSK, Novartis, and Lilly. Dr Johnson has received speaker fees from Actelion and GSK and honoraria from Actelion, Bayer, GSK, and Pfizer. Dr Peacock has received speaker fees, honoraria, and research grants from Actelion, Pfizer, and GSK, and is on advisory boards for Actelion, Bayer, GSK, Pfizer and Lilly. Dr Schreiber has received honoraria from Actelion, GSK, and Pfizer. Dr Coghlan has received honoraria for lecturing from Actelion, GSK, Pfizer, and Lilly, and is on advisory boards for Actelion, Bayer, and Pfizer. Dr Dimopoulos has received speaker fees from Actelion, honoraria from Actelion and GSK, and research grants from Actelion, GSK, and Pfizer. Dr Wort has received speaker fees and honoraria from Actelion and Pfizer, has received research grants from Actelion, Pfizer, and Bayer, and is on advisory boards for Pfizer, GSK, Novartis, and Bayer. Dr Gaine has received speaker fees and honoraria and is on advisory boards for Actelion, GSK, and Pfizer. Dr Moledina has received speaker fees and honoraria from Actelion. Dr Jenkins has received honoraria for lecturing and consultancy from Actelion, Bayer, and GSK. Dr Pepke-Zaba has received honoraria for lecturing and consulting from Bayer, Actelion, and GSK, is on advisory boards for Actelion, Pfizer, Bayer, GSK, and United Therapeutics, and her institution has received educational and research grants from Actelion, Bayer, and GSK. The other authors report no conflicts.
References
- 1.Cannon JE, Pepke-Zaba J. Is distal chronic thromboembolic pulmonary hypertension treatable with PAH targeted drugs? Semin Respir Crit Care Med. 2013;34:620–626. doi: 10.1055/s-0033-1355442. [DOI] [PubMed] [Google Scholar]
- 2.Pepke-Zaba J, Delcroix M, Lang I, Mayer E, Jansa P, Ambroz D, Treacy C, D’Armini AM, Morsolini M, Snijder R, Bresser P, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124:1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008. [DOI] [PubMed] [Google Scholar]
- 3.Riedel M, Stanek V, Widimsky J, Prerovsky I. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest. 1982;81:151–158. doi: 10.1378/chest.81.2.151. [DOI] [PubMed] [Google Scholar]
- 4.Kim NH, Delcroix M, Jenkins DP, Channick R, Dartevelle P, Jansa P, Lang I, Madani MM, Ogino H, Pengo V, Mayer E. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D92–D99. doi: 10.1016/j.jacc.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins DP, Madani M, Mayer E, Kerr K, Kim N, Klepetko W, Morsolini M, Dartevelle P. Surgical treatment of chronic thromboembolic pulmonary hypertension. Eur Respir J. 2013;41:735–742. doi: 10.1183/09031936.00058112. [DOI] [PubMed] [Google Scholar]
- 6.Madani MM, Auger WR, Pretorius V, Sakakibara N, Kerr KM, Kim NH, Fedullo PF, Jamieson SW. Pulmonary endarterectomy: recent changes in a single institution’s experience of more than 2,700 patients. Ann Thorac Surg. 2012;94:97–103. doi: 10.1016/j.athoracsur.2012.04.004. discussion 103. [DOI] [PubMed] [Google Scholar]
- 7.Mayer E, Jenkins D, Lindner J, D’Armini A, Kloek J, Meyns B, Ilkjaer LB, Klepetko W, Delcroix M, Lang I, Pepke-Zaba J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141:702–710. doi: 10.1016/j.jtcvs.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 8.Berman M, Gopalan D, Sharples L, Screaton N, Maccan C, Sheares K, Pepke-Zaba J, Dunning J, Tsui S, Jenkins DP. Right ventricular reverse remodeling after pulmonary endarterectomy: magnetic resonance imaging and clinical and right heart catheterization assessment. Pulm Circ. 2014;4:36–44. doi: 10.1086/674884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coronel ML, Chamorro N, Blanco I, Amado V, Del Pozo R, Pomar JL, Badia JR, Rovira I, Matute P, Argemí G, Castellà M, et al. Medical and surgical management for chronic thromboembolic pulmonary hypertension: a single center experience. Arch Bronconeumol. 2014;50:521–527. doi: 10.1016/j.arbres.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 10.D’Armini AM, Zanotti G, Ghio S, Magrini G, Pozzi M, Scelsi L, Meloni G, Klersy C, Viganò M. Reverse right ventricular remodeling after pulmonary endarterectomy. J Thorac Cardiovasc Surg. 2007;133:162–168. doi: 10.1016/j.jtcvs.2006.08.059. [DOI] [PubMed] [Google Scholar]
- 11.Freed DH, Thomson BM, Tsui SS, Dunning JJ, Sheares KK, Pepke-Zaba J, Jenkins DP. Functional and haemodynamic outcome 1 year after pulmonary thromboendarterectomy. Eur J Cardiothorac Surg. 2008;34:525–529. doi: 10.1016/j.ejcts.2008.04.018. discussion 529. [DOI] [PubMed] [Google Scholar]
- 12.Jamieson SW, Kapelanski DP, Sakakibara N, Manecke GR, Thistlethwaite PA, Kerr KM, Channick RN, Fedullo PF, Auger WR. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003;76:1457–1462. doi: 10.1016/s0003-4975(03)00828-2. discussion 1462. [DOI] [PubMed] [Google Scholar]
- 13.Maliyasena VA, Hopkins PM, Thomson BM, Dunning J, Wall DA, Ng BJ, McNeil KD, Mullany D, Kermeen FD. An Australian tertiary referral center experience of the management of chronic thromboembolic pulmonary hypertension. Pulm Circ. 2012;2:359–364. doi: 10.4103/2045-8932.101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogino H, Ando M, Matsuda H, Minatoya K, Sasaki H, Nakanishi N, Kyotani S, Imanaka H, Kitamura S. Japanese single-center experience of surgery for chronic thromboembolic pulmonary hypertension. Ann Thorac Surg. 2006;82:630–636. doi: 10.1016/j.athoracsur.2006.03.121. [DOI] [PubMed] [Google Scholar]
- 15.Rubens FD, Bourke M, Hynes M, Nicholson D, Kotrec M, Boodhwani M, Ruel M, Dennie CJ, Mesana T. Surgery for chronic thromboembolic pulmonary hypertension–inclusive experience from a national referral center. Ann Thorac Surg. 2007;83:1075–1081. doi: 10.1016/j.athoracsur.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Sato M, Ando M, Kaneko K, Higuchi Y, Kondo H, Akita K, Ishida M, Takagi Y. Respiratory and hemodynamic changes in patients with chronic thromboembolic pulmonary hypertension 1 year after pulmonary endarterectomy. Ann Vasc Dis. 2013;6:578–582. doi: 10.3400/avd.oa.13-00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tscholl D, Langer F, Wendler O, Wilkens H, Georg T, Schäfers HJ. Pulmonary thromboendarterectomy–risk factors for early survival and hemodynamic improvement. Eur J Cardiothorac Surg. 2001;19:771–776. doi: 10.1016/s1010-7940(01)00686-8. [DOI] [PubMed] [Google Scholar]
- 18.Yıldızeli B, Taş S, Yanartaş M, Kaymaz C, Mutlu B, Karakurt S, Altınay E, Eldem B, Ermerak NO, Batırel HF, Koçak T, et al. Pulmonary endarterectomy for chronic thrombo-embolic pulmonary hypertension: an institutional experience. Eur J Cardiothorac Surg. 2013;44:e219–e227. doi: 10.1093/ejcts/ezt293. discussion e227. [DOI] [PubMed] [Google Scholar]
- 19.Corsico AG, D’Armini AM, Cerveri I, Klersy C, Ansaldo E, Niniano R, Gatto E, Monterosso C, Morsolini M, Nicolardi S, Tramontin C, et al. Long-term outcome after pulmonary endarterectomy. Am J Respir Crit Care Med. 2008;178:419–424. doi: 10.1164/rccm.200801-101OC. [DOI] [PubMed] [Google Scholar]
- 20.Mellemkjaer S, Ilkjaer LB, Klaaborg KE, Christiansen CL, Severinsen IK, Nielsen-Kudsk JE, Allermand H, Egeblad M, Kristensen BO. Pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Ten years experience in Denmark. Scand Cardiovasc J. 2006;40:49–53. doi: 10.1080/14017430500338513. [DOI] [PubMed] [Google Scholar]
- 21.Saouti N, Morshuis WJ, Heijmen RH, Snijder RJ. Long-term outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension: a single institution experience. Eur J Cardiothorac Surg. 2009;35:947–952. doi: 10.1016/j.ejcts.2009.01.023. discussion 952. [DOI] [PubMed] [Google Scholar]
- 22.Schölzel B, Snijder R, Morshuis W, Saouti N, Plokker T, Post M. Clinical worsening after pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension. Neth Heart J. 2011;19:498–503. doi: 10.1007/s12471-011-0203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skoro-Sajer N, Marta G, Gerges C, Hlavin G, Nierlich P, Taghavi S, Sadushi-Kolici R, Klepetko W, Lang IM. Surgical specimens, haemodynamics and long-term outcomes after pulmonary endarterectomy. Thorax. 2014;69:116–122. doi: 10.1136/thoraxjnl-2013-203746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuda H, Ogino H, Minatoya K, Sasaki H, Nakanishi N, Kyotani S, Kobayashi J, Yagihara T, Kitamura S. Long-term recovery of exercise ability after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Ann Thorac Surg. 2006;82:1338–1343. doi: 10.1016/j.athoracsur.2006.03.105. discussion 1343. [DOI] [PubMed] [Google Scholar]
- 25.Bonderman D, Skoro-Sajer N, Jakowitsch J, Adlbrecht C, Dunkler D, Taghavi S, Klepetko W, Kneussl M, Lang IM. Predictors of outcome in chronic thromboembolic pulmonary hypertension. Circulation. 2007;115:2153–2158. doi: 10.1161/CIRCULATIONAHA.106.661041. [DOI] [PubMed] [Google Scholar]
- 26.Ishida K, Masuda M, Tanabe N, Matsumiya G, Tatsumi K, Nakajima N. Long-term outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2012;144:321–326. doi: 10.1016/j.jtcvs.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Archibald CJ, Auger WR, Fedullo PF, Channick RN, Kerr KM, Jamieson SW, Kapelanski DP, Watt CN, Moser KM. Long-term outcome after pulmonary thromboendarterectomy. Am J Respir Crit Care Med. 1999;160:523–528. doi: 10.1164/ajrccm.160.2.9808109. [DOI] [PubMed] [Google Scholar]
- 28.Condliffe R, Kiely DG, Gibbs JS, Corris PA, Peacock AJ, Jenkins DP, Hodgkins D, Goldsmith K, Hughes RJ, Sheares K, Tsui SS, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:1122–1127. doi: 10.1164/rccm.200712-1841OC. [DOI] [PubMed] [Google Scholar]
- 29.Freed DH, Thomson BM, Berman M, Tsui SS, Dunning J, Sheares KK, Pepke-Zaba J, Jenkins DP. Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg. 2011;141:383–387. doi: 10.1016/j.jtcvs.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 30.van der Plas MN, Surie S, Reesink HJ, van Steenwijk RP, Kloek JJ, Bresser P. Longitudinal follow-up of six-minute walk distance after pulmonary endarterectomy. Ann Thorac Surg. 2011;91:1094–1099. doi: 10.1016/j.athoracsur.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 31.Delcroix M, Lang I, Pepke-Zaba J, Jansa P, D’Armini AM, Snijder R, Bresser P, Torbicki A, Mellemkjaer S, Lewczuk J, Simkova I, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Circulation. 2016;133:859–871. doi: 10.1161/CIRCULATIONAHA.115.016522. [DOI] [PubMed] [Google Scholar]
- 32.Dartevelle P, Fadel E, Mussot S, Chapelier A, Hervé P, de Perrot M, Cerrina J, Ladurie FL, Lehouerou D, Humbert M, Sitbon O, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2004;23:637–648. doi: 10.1183/09031936.04.00079704. [DOI] [PubMed] [Google Scholar]
- 33.Moser KM, Braunwald NS. Successful surgical intervention in severe chronic thromboembolic pulmonary hypertension. Chest. 1973;64:29–35. doi: 10.1378/chest.64.1.29. [DOI] [PubMed] [Google Scholar]
- 34.Thistlethwaite PA, Kaneko K, Madani MM, Jamieson SW. Technique and outcomes of pulmonary endarterectomy surgery. Ann Thorac Cardiovasc Surg. 2008;14:274–282. [PubMed] [Google Scholar]
- 35.Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 36.Taboada D, Pepke-Zaba J, Jenkins DP, Berman M, Treacy CM, Cannon JE, Toshner M, Dunning JJ, Ng C, Tsui SS, Sheares KK. Outcome of pulmonary endarterectomy in symptomatic chronic thromboembolic disease. Eur Respir J. 2014;44:1635–1645. doi: 10.1183/09031936.00050114. [DOI] [PubMed] [Google Scholar]
- 37.Thomson B, Tsui SS, Dunning J, Goodwin A, Vuylsteke A, Latimer R, Pepke-Zaba J, Jenkins DP. Pulmonary endarterectomy is possible and effective without the use of complete circulatory arrest–the UK experience in over 150 patients. Eur J Cardiothorac Surg. 2008;33:157–163. doi: 10.1016/j.ejcts.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Vuylsteke A, Sharples L, Charman G, Kneeshaw J, Tsui S, Dunning J, Wheaton E, Klein A, Arrowsmith J, Hall R, Jenkins D. Circulatory arrest versus cerebral perfusion during pulmonary endarterectomy surgery (PEACOG): a randomised controlled trial. Lancet. 2011;378:1379–1387. doi: 10.1016/S0140-6736(11)61144-6. [DOI] [PubMed] [Google Scholar]
- 39.Thistlethwaite PA, Mo M, Madani MM, Deutsch R, Blanchard D, Kapelanski DP, Jamieson SW. Operative classification of thromboembolic disease determines outcome after pulmonary endarterectomy. J Thorac Cardiovasc Surg. 2002;124:1203–1211. doi: 10.1067/mtc.2002.127313. [DOI] [PubMed] [Google Scholar]
- 40.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 41.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 42.Klein JP, Wu JT. Discretizing a continuous covariate in survival studies. Handbook of Statistics. 2004;23:27–42. [Google Scholar]
- 43.Rahnavardi M, Yan TD, Cao C, Vallely MP, Bannon PG, Wilson MK. Pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension: a systematic review. Ann Thorac Cardiovasc Surg. 2011;17:435–445. doi: 10.5761/atcs.oa.10.01653. [DOI] [PubMed] [Google Scholar]
- 44.Lewczuk J, Piszko P, Jagas J, Porada A, Wójciak S, Sobkowicz B, Wrabec K. Prognostic factors in medically treated patients with chronic pulmonary embolism. Chest. 2001;119:818–823. doi: 10.1378/chest.119.3.818. [DOI] [PubMed] [Google Scholar]
- 45.Jansa P, Ghofrani H-A, Hoeper MM, Kim NH, Meyer E, Neurohr C, Simonneau G, Fritsch A, Davie N, Wilkins MR. Comparison of haemodynamic parameters in patients with inoperable and persistent/recurrent chronic thromboembolic pulmonary hypertension in the Phase III CHEST-1 study. Eur Heart J. 2013;34(suppl 1):1066. 187. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.