Abstract
Reductive transformation of toxic arsenic (As) species by As reducing bacteria (AsRB) is a key process in As-biogeochemical-cycling within the subsurface aquifer environment. In this study, we have characterized a Gram-stain-negative, non-spore-forming, rod-shaped As reducing bacterium designated KAs 5-3T, isolated from highly As-contaminated groundwater of India. Strain KAs 5-3T displayed high 16S rRNA gene sequence similarity to the members of the genus Pseudoxanthomonas, with P. mexicana AMX 26BT (99.25% similarity), P. japonensis 12-3T (98.9 0%), P. putridarboris WD-12T (98.02%), and P. indica P15T (97.27%) as closest phylogenetic neighbours. DNA-DNA hybridization study unambiguously indicated that strain KAs 5-3T represented a novel species that was separate from reference strains of P. mexicana AMX 26BT (35.7%), P. japonensis 12-3T (35.5%), P. suwonensis 4M1T (35.5%), P. wuyuanensis XC21-2T (35.0%), P. indica P15T (32.5%), P. daejeonensis TR6-08T (32.0%), and P. putridarboris WD12T (22.1%). The DNA G+C content of strain KAs 5-3T was 64.9 mol %. The predominant fatty acids were C15:0 (37.4%), C16:0 iso (12.6%), C17:1 iso ω9c (10.5%), C15:0 anteiso (9.5%), C11:0 iso 3-OH (8.5%), and C16:1 ω7c/ C16:1 ω6c (7.5%). The major polar lipids were diphosphatidylglycerol, phosphatidyldimethylethanolamine, phosphatidylcholine, and two unknown phospholipids (PL1, PL2). Ubiquinone 8 (Q8) was the predominant respiratory quinone and spermidine was the major polyamine of the strain KAs 5-3T. Cells of strain KAs 5-3T showed the ability to use O2, As5+, NO3-, NO2-, and Fe3+ as terminal electron acceptors as well as to reduce As5+ through the cytosolic process under aerobic incubations. Genes encoding arsenate reductase (arsC) for As-detoxification, nitrate- and nitrite reductase (narG and nirS) for denitrification were detected in the strain KAs 5-3T. Based on taxonomic and physiological data, strain KAs 5-3T is described as a new representative member of the genus Pseudoxanthomonas, for which the name Pseudoxanthomonas arseniciresistens sp. nov. is proposed. The type strain is KAs 5-3T (= LMG 29169T = MTCC 12116T = MCC 3121T).
Introduction
Taxonomic hierarchy of the genus Pseudoxanthomonas denotes its affiliation to the class Gammaproteobacteria, family Xanthomonadaceae of phylum Proteobacteria. Members of the genera Xanthomonas, Xyllela, and Stenotrophomonas are found to be the nearest phylogenetic neighbours of Pseudoxanthomonas [1]. Finkmann et al. [2] reported the first validly described species of Pseudoxanthomonas, P. broegbernensis isolated from an experimental biofilter. The taxon has been subsequently emended by Thierry et al. [3] and Lee et al. [4]. Members of this genus were described as Gram-stain-negative, non-spore forming rods, with iso C15:0 and anteiso C15:0 as major fatty acids, ubiquinone (Q8) as major respiratory quinone and capable of performing strict respiratory metabolism with O2 as preferred terminal electron acceptor [3]. The genus can be well differentiated from the two other related members Xanthomonas and Stenotrophomonas by the absence of fatty acid C13:0 3-OH and from genus Xylella by the presence of branched-chain fatty acids (as described in the Bergey’s Manual of Systematic Bacteriology, 2nd edition, Volume II, The Proteobacteria [4]). At the time of writing this manuscript, 17 validly described and two non-validly described (but effectively published) type species of the genus Pseudoxanthomonas were reported from varied environments [2–17]. The non-validly described members (but effectively published): P. kaohsiungensis and P. gei are isolated from an oil-polluted site and plant stem respectively [18, 19]. The members of this genus are ecologically important due to their ability to reduce both nitrite and nitrate; degrade a variety of hydrocarbons (including benzene, toluene, ethyl-benzene and o-, m-, p- xylene) [20–22]. Recently, the presence of Pseudoxanthomonas and other members of Xanthomonadaceae have been reported for As-contaminated groundwater of alluvial aquifers in West Bengal and Bangladesh [23–26]. However, neither the taxonomic identity of these strains nor their eco-physiology towards As-transformation has been adequately studied. As a result, the role of such organisms in biogeochemical-cycling of As in contaminated groundwater remained highly unexplored.
The present study was therefore undertaken to investigate the taxonomic and eco-physiological properties of an As-resistant and -reducing Pseudoxanthomonas strain previously isolated from As rich groundwater of West Bengal [23]. A polyphasic taxonomic approach was undertaken to characterize and delineate the taxonomic position of the strain KAs 5-3T. This strain was found to possess abilities for anaerobic As reduction and hydrocarbons utilization as well as several other traits potentially important for surviving in highly As-contaminated oligotrophic aquifer environment. To the best of our knowledge, till date no Pseudoxanthomonas type strain has been characterized from As-contaminated groundwater and capable of reducing toxic As5+ while assimilating hydrocarbons.
Materials and methods
Bacterial strains and culture conditions
The strain KAs 5-3T (LMG 29169T = MTCC 12116T = MCC 3121T) was originally isolated from an As-contaminated groundwater (total As of 500 μg/L, salinity of 0.4 parts per thousand) of West Bengal [23]. Type strains of Pseudoxanthomonas (P. mexicana AMX 26BT, P. japonensis 12-3T, P. indica P15T, P. suwonensis 4M1T, P. wuyuanensis XC21-2T, P. putridarboris WD12T, and P. daejeonensis TR6-08T) were obtained from various culture collections [Japan Collection of Microorganisms (JCM, Japan), Microbial Type Culture Collection (MTCC, India), Korean Type Culture Collection (KCTC), and Korean Agricultural Culture Collection (KACC, Korea)] and used as reference organisms in various experiments. Strain KAs 5-3T and the reference type strains were routinely sub-cultured and maintained on Luria-Bertani broth (g L-1; Casein enzymic hydrolysate, 10.0; yeast extract, 5.0; NaCl, 10.0; pH adjusted to 7.5) or minimal salt medium (MSM) (g L-1; Tris buffer, 6.0; NaCl, 5.0; KCl, 1.52; NH4Cl, 1.04; Na2SO4, 0.4; MgCl2.6H2O, 0.2; CaCl2.2H2O, 0.03; K2HPO4, 0.01; KH2PO4, 0.01, pH adjusted to 7.0). For MSM, either glucose (10 mM, v/v) or yeast extract (2.0%, v/v) was used as carbon source, as appropriate.
16S rRNA gene phylogeny and multi locus sequence typing
Nearly complete stretch of 16S rRNA gene was PCR amplified using 27F/1492R primers (Table A in S1 File); individual sequences were edited and assembled by BioEdit version 7.1.11 [27], subjected to similarity search in NCBI BLAST [28], RDP II [29], and against validly described members in the EzBioCloud database (http://www.ezbiocloud.net/eztaxon; [30]). Multiple alignments with 16S rRNA gene sequences of Pseudoxanthomonas type strains were performed using the CLUSTAL W package of the MEGA software version 7.0 [31]. All ambiguous positions were removed for each sequence pair and a total of 1492 positions were taken in the final dataset for construction of phylogenetic trees. Phylogenetic reconstruction and validation were performed using neighbour-joining (NJ) method [32] (Fig 1) based on bootstrap analysis with 1000 replications using Jukes-Cantor [33] distance model. Both maximum-likelihood (ML) [34] and minimum-evolution (ME) [35] methods were employed to test the robustness of the trees (Figure A in S1 File). Multi locus sequence analysis (MLSA) was performed using single copy genes which include gyrB (1200 bp), dnaJ (1000 bp), atpG (400 bp), and rpoB (1200 bp). PCR primers and conditions are given (Table A in S1 File). All PCR products were gel purified, cloned into pTZ57R/T vector and sequenced using vector specific primer set (M13F/M13R). Sequences obtained were searched for similarity level using BLASTN, concatenated, and phylogeny was inferred by constructing NJ tree with 1000 bootstrap resampling (Figure B in S1 File).
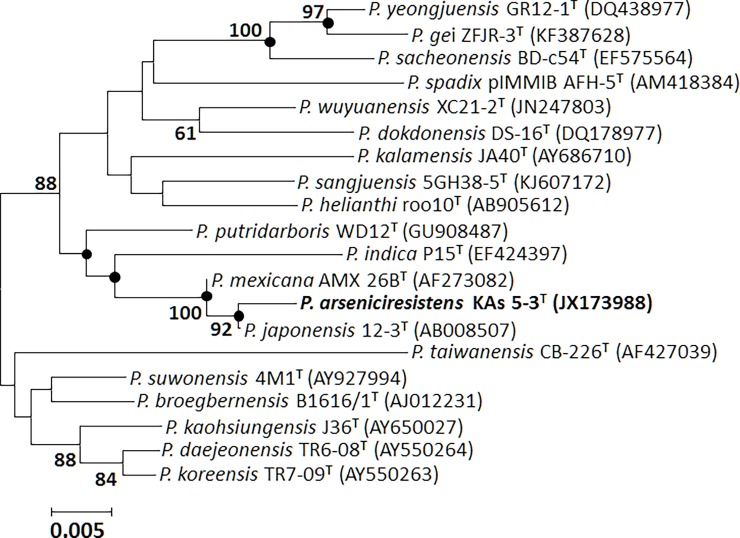
Fig 1. Neighbour-joining phylogenetic tree based on 16S rRNA gene sequence of KAs 5-3T with type strains of the genus Pseudoxanthomonas.
Bootstraps (1000 replications) of above 60% are shown at each branch points. A total of 1492 positions involving 20 nucleotide sequences were considered in the final dataset for construction of the tree. Filled circles indicate that the corresponding nodes were also recovered in trees generated with maximum-likelihood and minimum-evolution algorithm. Bar 0.005 indicates 0.5% substitution.
Genotypic characterization
Molar G+C content was (mol %) determined following the thermal denaturation method [36]. DNA-DNA hybridization was carried out between strain KAs 5-3T and reference type members (P. mexicana AMX 26BT, P. japonensis 12-3T, P. indica P15T, P. suwonensis 4M1T, P. wuyuanensis XC21-2T, P. putridarboris WD12T, and P. daejeonensis TR6-08T) using a thermal denaturation procedure involving SYBR green dye-DNA binding method [37]. Optimum renaturation temperature (TOR) was calculated and hybridization was performed as described by Mohapatra et al. [38]. DNA-DNA hybridization value < 70% or difference in Tm values of 5°C or higher was considered as the cut-off for distinct microbial species [39].
Phenotypic and chemotaxonomic characterization
Morphological, physiological, biochemical, and chemotaxonomic characterization of the strain KAs 5-3T and reference type strains (P. mexicana AMX 26BT, P. japonensis 12-3T, P. indica P15T, P. daejeonensis TR6-08T, P. suwonensis 4M1T, P. putridarboris WD12T, and P. wuyuanensis XC21-2T) were performed by routine cultivation on LB or MSM as appropriate at 30°C. Cell morphology was examined under bright-field (1000 X oil immersion, Olympus) and scanning electron microscopes (SEM-1400; JEOL). For SEM study, cells were fixed with 0.2% (v/v) glutaraldehyde (EM grade, Sigma) in 0.1 mM phosphate buffer saline (PBS), serially dehydrated with ethanol (30 to 100%) (v/v), placed on poly-L-lysine coated cover glass, and viewed under SEM after gold coating (Figure C in S1 File). Motility was tested by flagella staining protocol of Kodaka et al. [40]. Temperature sensitivity was assessed at 10–42°C (with increments of 5°C from 10–25°C and 2°C from 26–42°C). Sensitivity towards various pH (3.0–10.0, with increments of 1.0 pH unit) was investigated using appropriate buffer system [pH 3–5 (0.1 M citric acid/0.1 M sodium citrate), pH 6–8 (0.1 M KH2PO4/0.1 M NaOH, pH 9–10 (0.1 M NaHCO3/0.1 M Na2CO3)] in LB broth, where no significant pH change of the medium was noticed after autoclaving. NaCl tolerance [0–10% (w/v) with increments of 0.5%] was examined in LB broth, where appropriate volume of NaCl was added (from 0–5%) to the autoclaved medium from a sterile stock solution (20%, w/v). For > 5% of NaCl concentrations, the culture medium was prepared in the double strength (2 X) to avoid the dilution done with the addition of higher NaCl stock solution. For sensitivity towards temperature, pH, and NaCl concentrations, cellular growth was assessed by measuring absorbance (growth optical density, OD 600 nm) at 0, 12, 24, and 48 h. Tests for catalase, oxidase, nitrate reduction to N2, utilization of gelatin, esculin, citrate, and urea were performed following the standard procedures [41–43]. Other biochemical properties were studied using API 20NE kit (Bio-Merieux) at 30°C for 24–48 h and GEN-III microplate (Biolog) following the manufacturer’s instructions and are presented in Table 1. Gram-staining was performed using Gram staining kit (HiMedia). Susceptibility towards various antibiotics was tested following disc diffusion susceptibility method [44] involving commercially prepared paper antibiotic disks (HiMedia, India): cefixime (5 μg), ceftriaxone (30 μg), amikacin (30 μg), cefotaxime (30 μg), chloramphenicol (30 μg), ofloxacin (5 μg), polymyxin-B (300 units), tetracycline (30 μg), ciprofloxacin (5 μg), and erythromycin (15 μg). Freshly grown bacterial cultures (approximately 2×107 CFU/mL) were spreaded onto the surface of Mueller-Hinton (MH) agar plates and are incubated for 18–24 h at 30°C. The zones of growth inhibition around each antibiotic disks were correlated to the susceptibility of the isolate using the criteria published by the clinical and laboratory standards institute (CLSI, formerly the National Committee for Clinical Laboratory Standards or NCCLS) [45]. Minimum inhibitory concentration (MIC) of As and various heavy metals was evaluated by growing the cells in LB supplemented agar medium under aerobic condition by following the plate dilution protocol of Zhu et al. [46]. Increasing concentrations of As [0.1–200 mM] (As3+ as NaAsO2 and As5+ as Na2HAsO4) or heavy metals [0.1 to 30 mM] (Cd2+as CdCl2, Co2+ as CoCl2, Cu2+ as CuSO4, Fe3+as FeCl3, Hg2+ as HgCl2, Cr6+ as K2Cr2O7, Se6+ as Na2SeO4, Ni2+ as NiCl2, Zn2+as ZnCl2) were amended into the medium and medium without any heavy metal was treated as control. The lowest concentration of metals, which inhibited cellular growth completely, was considered for MIC evaluation (Table 2). Strains of Escherichia coli NCIM 2931T and Cupriavidus metallidurans DSM 2839T were used as negative and positive control respectively, as the strains are found to have the lowest and highest resistance respectively to the heavy metals tested.
Table 1. Phenotypic characteristics that differentiate strain KAs 5-3T from phylogenetically related type strains of Pseudoxanthomonas species.
Strains: 1, KAs 5-3T; 2, P. mexicana AMX 26BT; 3, P. japonensis 12-3T; 4, P. indica P15T; 5, P. daejeonensis TR6-08T; 6, P. suwonensis 4M1T; 7, P. wuyuanensis XC21-1T, 8, P. putridarboris WD12T. +; Positive, -; Negative, W; Weak, and ND; No data available. GW; groundwater, HCHD; hexachlorocyclohexane dumpsite, SAS; saline-alkali soil, RT; rotten tree.
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Habitat | GW | Sludge | Soil | HCHD | Soil | Compost | SAS | RT |
| Motility | - | + | + | + | + | - | + | + |
| Catalase | + | + | - | + | + | + | + | + |
| Oxidase | + | + | + | + | + | - | + | + |
| Growth | ||||||||
| Opt. (0C) | 30 | 28 | 28 | 28 | 30 | 30 | 35 | 37 |
| 10 0C | + | + | + | - | - | + | + | - |
| 40 0C | - | - | - | - | - | + | - | + |
| pH | 6–8 | 6–9 | 6–9.5 | 6–8 | 7–9 | ND | 6–7 | 6–8 |
| NaCl (%) | 0.5–5 | 0.5–4 | 0.5–3 | 1–4 | 1–5 | ND | 0.5–5 | 0–3 |
| Nitrate to N2 | + | - | - | - | - | - | - | - |
| Assimilation | ||||||||
| Esculin | + | + | + | + | - | + | + | + |
| Casein | + | + | + | - | + | - | + | + |
| Gelatin | + | + | + | - | W | + | + | + |
| Urea | + | - | - | - | - | - | - | - |
| Tween 80 | - | + | + | + | - | - | + | + |
| Arabinose | - | - | - | - | + | + | + | + |
| Mannose | - | + | - | + | - | - | - | - |
| NAG | + | - | + | - | + | + | + | + |
| Maltose | - | + | + | + | + | + | - | + |
| Gluconate | - | + | - | - | - | + | - | - |
| Caprate | - | - | - | - | - | - | - | - |
| Adipate | + | - | - | - | + | - | - | - |
| Malate | + | + | + | + | - | + | + | - |
| Citrate | + | + | + | - | + | - | - | - |
| β-galactosidase | + | + | + | - | + | + | + | - |
| β-glucosidase | + | - | - | - | + | - | - | + |
| G+C (mol %)* | 64.9 | 67.8±2 | 65.2±1 | 62.9±2 | 68.7±0.4 | 67.6±1 | 66.2 | 69.1 |
Table 2. Minimum inhibitory concentration (MIC) of As and other heavy metals tested for strain KAs 5-3T and reference type strains.
Strains: 1, KAs 5-3T; 2, P. mexicana AMX 26BT; 3, P. japonensis 12-3T; 4, P. indica P15T; 5, P. daejeonensis TR6-08T; 6, P. suwonensis 4M1T; 7, P. wuyuanensis XC21-1T; 8, P. putridarboris WD12T; 9, E. coli NCIM 2931T; 10, C. metallidurans DSM 2839T.
| Heavy metals [mM] |
Bacterial strains | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Co2+ | 5.0 | 2.5 | 3 | 2.5 | 2.5 | 3.0 | 2.5 | 2.0 | 2.0 | 3.0 |
| Ni2+ | 3.0 | 2.5 | 2.5 | 2.5 | 2.5 | 3.0 | 2.5 | 2.0 | 2.0 | 2.5 |
| Cr6+ | 3.0 | 3.0 | 2.5 | 2.5 | 2.5 | 2.0 | 2.0 | 2.0 | 1.5 | 4.0 |
| Cu2+ | 5.0 | 2.5 | 2.5 | 2.0 | 2.0 | 2.0 | 2.0 | 5.0 | 2.5 | 3.5 |
| Se6+ | 10.0 | 2.5 | 3.0 | 2.0 | 2.0 | 2.0 | 2.5 | 3.0 | 4.0 | 15.0 |
| Hg2+ | 2.0 | 1.0 | 1.5 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 0.5 | 2.0 |
| Zn2+ | 3.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 3.0 |
| Cd2+ | 3.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.5 | 2.5 |
| As5+ | 150.0 | 1.5 | 2.0 | 1.5 | 1.5 | 2.0 | 1.0 | 1.0 | 3.0 | 10.0 |
| As3+ | 20.0 | 1.0 | 1.5 | 1.0 | 0.5 | 1.0 | 1.0 | 0.0 | 1.0 | 4.5 |
| Fe3+ | 20.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 2.5 | 5.0 | 10.0 | 20.0 |
The analysis of cellular fatty acid methyl esters (FAMEs) was performed after growth of bacterial strains (KAs 5-3T, P. mexicana AMX 26BT, P. japonensis 12-3T, P. indica P15T, P. suwonensis 4M1T, P. wuyuanensis XC21-2T, P. putridarboris WD12T, and P. daejeonensis TR6-08T) on Tryptic Soy agar (TSA) for 24 h at 30°C. One loopful of bacterial colony was harvested at exponential phase, subjected to saponification, methylation, and extraction. Fatty acids were determined by Microbial ID using the fully automated GC Sherlock Microbial Identification System (MIDI) using MIDI standard procedures [47]. Isoprenoid quinones were extracted from overnight grown culture following the procedure of Komagata & Suzuki [48] and analysed using high performance liquid chromatography (HPLC, Agilent 1100; column: Sorbax C18 reverse phase, Agilent), where methanol: isopropanol (2:1, v/v), was used as mobile phase with peak detection at 275 nm. The ubiquinone fractions were separated and identified by liquid chromatography-mass spectrometry (LC-MS, WATERS 2695) in a positive-mode electrospray analysis. Polar lipids were extracted and analyzed by two-dimensional TLC following protocol of Komagata & Suzuki [48] (Figure D in S1 File). Polyamines were extracted as described by Kumari et al., [13] and analysed by TLC (Silica gel 60 F254, 20×20 cm, Merck, Germany).
Utilization of carbon substrates, electron acceptors, and As-reductive growth
To test the utilization of different carbon substrates by strain KAs 5-3T, a range of hydrocarbon compounds (benzene, toluene, xylene, catechol, benzoic acid, naphthalene, phenanthrene, anthracene, pyrene, dodecane, pentadecane, hexadecane, nonadecane, docosane) were amended into MSM medium at a concentration of 500 μM. Freshly grown cell suspension (MSM culture medium) was centrifuged at 10,000 rpm for 5 min, washed twice with 0.85% saline, resuspended in the MSM (without any amendment), inoculated (1%, v/v) into the medium (OD600 0.03–0.05 at t0), and incubated for 72 h at 30°C. Growth was monitored at regular intervals by measuring colony forming unit (CFU)/mL, by plating 0.1 mL of the culture onto MSM plates supplemented with respective hydrocarbon sources. Utilization of various terminal electron acceptors (TEAs) was tested following anaerobic growth (OD at 600 nm) with As5+ (5 mM), Fe3+ (5 mM), NO3- (5 mM), NO2- (5 mM) or SO42- (5 mM) in MSM [37] as alternate electron acceptors following addition of either sugar substrates (glucose or lactate, 20 mM each) and hydrocarbons (pentadecane or naphthalene, 750 μM each) as the sole carbon/energy source (Fig 2). Medium with added TEAs and without any inoculum was used as abiotic control. The concentration of TEAs in growth medium was measured in duplicate at regular intervals using standard procedures [43, 49–51]. Cytosolic As5+ reduction was also checked by growing strain KAs 5-3T in MSM supplemented with carbon sources (as described above) and incubated at 30°C for 24 h. The growth parameters and rate of reduction of As5+ were calculated by checking growth OD (at 600 nm) and residual As5+ concentration in the medium by spectrophotometric method [52] and validated by atomic absorption spectrophotometer (AAS; PinAAcle900H, Perkin Elmer).
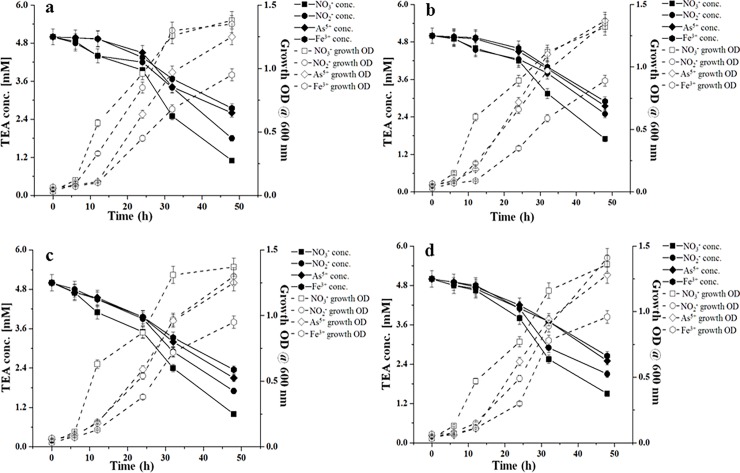
Fig 2.
Growth and reductive use of different electron acceptors (NO3-, NO2-, As5+, Fe3+) by strain KAs 5-3T in the presence of various sugar and hydrocarbon sources as principal carbon substrates: a) glucose, b) lactate, c) dodecane, and d) pentadecane.
Functional gene-based analysis
Genes responsible for cytosolic As5+ reduction (arsC), dissimilatory nitrate- (narG) and nitrite reduction (nirS) were also amplified through PCR based approach (Table A in S1 File). All PCR products were gel purified, cloned and sequenced (as described above for MLSA). Nucleotide sequences obtained were searched for similarity level using BLASTN. The corresponding nucleotides were translated to amino acids in ExPasy tool [53] using appropriate open reading frames (ORFs) and searched in BLASTP, (nr database) excluding options for uncultured/environmental sequences and including option for type material. Conserved domain was predicted through CDD database and phylogeny was inferred through neighbour-joining method (Figs 3 and 4) considering the translated amino acid sequence of strain KAs 5-3T and similar sequences (>90% similarity value). The nucleotide sequences were analyzed for GC content (mol %), GC % deviation from their respective genomes (Table B in S1 File) as well as p-distance calculations through MEGA 7.0. Phylogenetic network analysis was performed using SplitsTree software [54] (Figures E and F in S1 File).
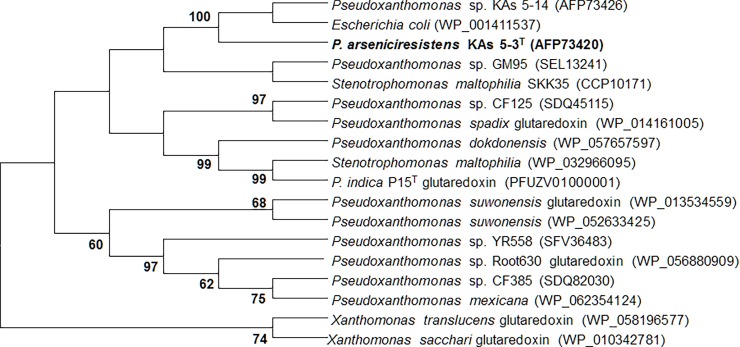
Fig 3. Neighbor-joining phylogenetic tree of genes encoding arsenate reductase (arsC) of KAs 5-3T with similar sequences (>90% identity) retrieved from NCBI database.
Bootstraps (1000 replications) of above 50% are shown at each branch points. The sequences obtained in this study are highlighted in bold, sequence accession numbers are in parentheses.
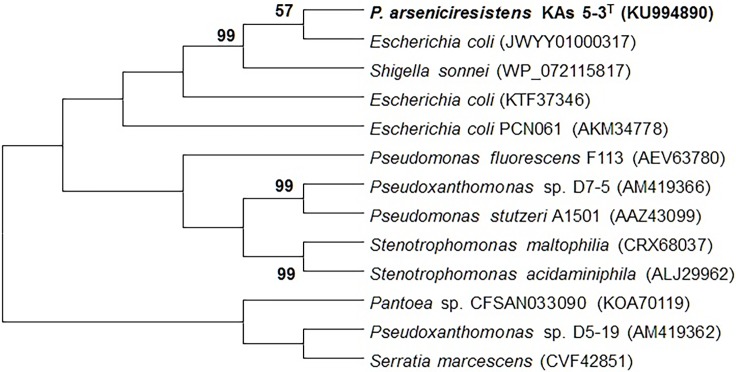
Fig 4. Neighbor-joining phylogenetic tree of genes encoding dissimilatory nitrate reductase (narG) of KAs 5-3T with similar sequences (>90% identity) retrieved from NCBI database.
Bootstraps (1000 replications) of above 50% are shown at each branch points. The sequences obtained in this study are highlighted in bold, sequence accession numbers are in parentheses.
The GenBank accession numbers for the 16S rRNA, gyrB, atpG, dnaJ, rpoB, arsC, narG, and nirS gene sequences of strain KAs 5-3T are JX173988, KX827793, KX827799, KX827796, KX880497, JX110552, KU994890 and KY563659 respectively.
Results and discussion
16S rRNA gene phylogeny and multi locus sequence typing
Comparison of nearly complete (1,495 nucleotides) 16S rRNA gene sequence indicated taxonomic affiliation of strain KAs 5-3T to the genus Pseudoxanthomonas, with highest sequence similarity to the type strains of P. mexicana AMX 26BT (99.25%), P. japonensis 12-3T (98.9%), followed by P. putridarboris WD-12T (98.02%), P. indica P15T (97.27%), P. wuyuanensis XC21-2T (97.12%), P. suwonensis 4M1T (97.0%), and P. daejeonensis TR6-08T (96.99%). The NJ phylogenetic analysis showed that strain KAs 5-3T formed a coherent cluster of monophyletic pattern with the type strains of P. mexicana AMX 26BT and P. japonensis 12-3T (bootstrap support of 100.0%) and claded to the type members of Pseudoxanthomonas (Fig 1). Both ML and ME phylogenetic reconstruction methods indicated a consistent tree topology cladding strain KAs 5-3T to the AMX 26BT, 12-3T, P15T, and WD12T as the nearest phylogenetic neighbours, while the clade comprising the near distant members of the strain KAs 5-3T was only supported by either of the methods (Figure A in S1 File). On the basis of high percentage of 16S rRNA gene sequence homology and coherent monophyletic cladding of strain KAs 5-3T, the type strains P. mexicana AMX 26BT,P. japonensis 12-3T, P. indica P15T, and P. putridarboris WD12T are inferred to be the closest phylogenetic neighbours. Multi locus sequence typing (MLST) involving various hose-keeping genes have been employed as a taxonomic marker for species level comparisons and clonal relationship [55, 56]. Sequence analysis of gyrB, dnaJ, rpoB, and atpG genes of strain KAs 5-3T showed > 92.0% sequence similarity to the type strains P. mexicana AMX 26BT and P. japonensis12-3T but formed a separate clade in the NJ phylogenetic reconstruction (Figure B in S1 File) indicating its non-clonal nature and species distinction from both the closest phylogenetic neighbours.
Genotypic characterization
The genomic G+C content of strain KAs 5-3T was found to be 64.9 mol %, this value is within the range for the genus Pseudoxanthomonas [2, 3]. It has been strongly emphasized that inter-species differentiation should be evaluated by using DNA–DNA hybridization (DDH) studies [56]. The levels of DNA-DNA relatedness of the strain KAs 5-3T with P. mexicana AMX 26BT, P. japonensis 12-3T, P. suwonensis 4M1T, P. wuyuanensis XC21-2T, P. indica P15T, P. daejeonensis TR6-08T, P. putridarboris WD12T were calculated to be 35.7%, 35.5%, 35.5%, 35.0%, 32.5%, 32.0%, and 22.1% respectively. Since DNA-DNA relatedness < 70% is considered to be the cut-off value for species delineation, KAs 5-3T is unambiguously proposed to be a novel species [57].
Phenotypic and chemotaxonomic characterization
Culture characteristics revealed that on LB agar plates, colonies of strain KAs 5-3T were creamy to pale yellow, circular, with entire margin and a diameter range of 1–2 mm after 24–48 h. Cells were Gram-stain-negative, rod-shaped, aerobic to facultative anaerobic, non-motile, catalase and oxidase positive, with a cell size of 1.2–1.5 μm length × 0.3–0.5 μm width (Figure C in S1 File). The strain was found to grow well at temperature range of 10–38°C (optimum at 28–32°C), pH range of 6.0–8.0 (optimum at 7.0) and over a broad spectrum of NaCl concentrations (0.5–5%; optimum of 1%) and growth did not occur without NaCl in the medium. The other details of phenotypic characteristics of the strain KAs 5-3T are presented in the species description and Table 1. Compared with other type members of the same genus (Pseudoxanthomonas), strain KAs 5-3T exhibited phenotypic differences (Table 1). The strain KAs 5-3T showed ability to reduce nitrate to N2, assimilate esculin, casein, gelatin, urea, adipate, malate, citrate, and N-acetyl glucosamine (NAG) and showed negative response for tween 80, arabinose, mannose, gluconate, and caprate. The catalase-, oxidase-positive, mesophilic, slightly alkalophilic and heterotrophic growth pattern confirmed relatedness of KAs 5-3T to the same genus [13]. The differential phenotypic properties viz., motility, assimilation of tween 80, urea, maltose, adipate, and production β-glucosidase confirmed the species level distinction of KAs 5-3T from the compared Pseudoxanthomonas members. In comparison with the phylogenetic neighbours, strain KAs 5-3T showed considerably higher resistance towards several metals Co2+, Cu2+, Se6+, Fe3+, As3+, and As5+ (Table 2). The strain’s ability to withstand Fe3+ was comparable to multi-metal resistant C. metallidurans and for As species, it was highest amongst all the strains tested.
The predominant quinone of the strain KAs 5-3T was found to be Q8. This seems to be a familiar character as prevalence of Q8 was previously reported as the major quinone in members of the genus Pseudoxanthomonas [3, 5, 13]. The overall FAME profile of the strain KAs 5-3T was found to be consistent to that of other type strains compared with some observed quantitative differences (Table 3). The major cellular fatty acids (> 5% of the total fatty acids) of strain KAs 5-3T consisted of C15:0 (37.4%), C16:0 iso (12.6%), C17:1 iso ω9c (10.5%), C15:0 anteiso (9.5%), C11:0 iso 3-OH (8.5%), and C16:1 ω7c/ C16:1 ω6c (7.5%). The overall FAME profile was similar with the type strains compared, but the differential presence of C11:0 anteiso, C16:0, as well as absence of C15:1 iso F and C16:1 iso H distinguished the strain KAs 5-3T from the reference type strains.
Table 3. Cellular fatty acid profiles of strain KAs 5-3T and related type members of the genus Pseudoxanthomonas.
Strains: 1, KAs 5-3T; 2, P. mexicana AMX 26BT; 3, P. japonensis 12-3T; 4, P. indica P15T; 5, P. daejeonensis TR6-08T; 6, P. suwonensis 4M1T; 7, P. wuyuanensis XC21-2T; 8, P. putridarboris WD12T.
| Fatty acids* | Strains | |||||||
|---|---|---|---|---|---|---|---|---|
| Saturated | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| C10:0 | - | - | - | 0.5 | - | 0.2 | - | 0.9 |
| C16:0 | 1.9 | 0.7 | 0.6 | 2.0 | 0.3 | 1.2 | 0.8 | 9.0 |
| Unsaturated | ||||||||
| C18:1 ω9c | 0.7 | 0.5 | 0.6 | 0.3 | 0.9 | - | - | 0.8 |
| Methyl branched | ||||||||
| C10:0 iso | 0.4 | - | - | 0.5 | - | 0.5 | - | - |
| C11:0 iso | 3.1 | 5.4 | 5.1 | 4.2 | 4.5 | 7.2 | 5.9 | 4.2 |
| C11:0 anteiso | 2.8 | - | - | 0.3 | - | 0.5 | 1.1 | 0.9 |
| C14:0 iso | 2.4 | 2.5 | 3.2 | 3.2 | 2.2 | 2.9 | 1.4 | 3.5 |
| C15:1 iso F | - | 1.3 | 1.6 | 1.9 | - | 1.5 | 0.7 | - |
| C15:0 iso | 37.4 | 39.2 | 35.6 | 27.9 | 40.5 | 31.7 | 28.5 | 14.5 |
| C15:0 anteiso | 9.5 | 2.6 | 3.8 | 5.6 | 6.5 | 12.8 | 3.6 | 6.2 |
| C16:1 iso H | - | 3.9 | 4.2 | 0.2 | - | 0.4 | 1.1 | 1.5 |
| C16:0 iso | 12.6 | 9.8 | 12.5 | 18.3 | 6.9 | 10.9 | 16.8 | 20.5 |
| C17:0 iso | 1.5 | 4.5 | 3.7 | 3.1 | 1.2 | 2.8 | 3.6 | - |
| C17:0 anteiso | - | 0.5 | 0.7 | 1.2 | 0.8 | 1.2 | 1.6 | 1.2 |
| Hydroxy | ||||||||
| C11:0 iso 3-OH | 8.5 | 6.4 | 4.9 | 6.6 | 4.5 | 7.2 | 5.5 | 4.5 |
| C12:0 iso 3-OH | 2.2 | 0.9 | 0.4 | 1.1 | - | 1.3 | 1.2 | 1.8 |
| Summed feature | ||||||||
| C16:1 ω7c/ C16:1 ω6c | 6.5 | 5.7 | 6.1 | 4.9 | 6.9 | 4.5 | 2.5 | 7.5 |
| C18:1 ω6c | - | 1.1 | 0.79 | 0.4 | 1.1 | 0.8 | - | 1.0 |
| C17:1 iso ω9c | 10.5 | 19.5 | 19.5 | 17.6 | 20.0 | 11.6 | 18.1 | 11.5 |
*All strains were cultured and grown under the same conditions. The values shown are percentages of total fatty acids.
The polar lipid profile of the strain KAs 5-3T was found to be consisting of diphosphatidylglycerol (DPG), phosphatidyldimethylethanolamine (PDE), phosphatidylcholine (PC), and unknown phospholipids (PL1, PL2, PL3). The presence of DPG, PDE and PL1 was found to be consistent in all the compared members (except P. indica P15T), indicating the affiliation of strain KAs 5-3T to the members of the genus Pseudoxanthomonas. The appearance of spot corresponding to PC and absence of PE, unknown lipids (UL1, UL2) uniquely distinguished the strain KAs 5-3T from all the compared members (Figure D in S1 File).
Utilization of carbon substrates, electron acceptors, and As-reductive growth
Cells of the strain KAs 5-3T were found to utilize catechol, naphthalene, dodecane, and pentadecane as sole carbon sources. Among various tested electron acceptors, strain KAs 5-3T showed growth on As5+, NO3-, NO2-, and Fe3+, while no growth was observed in SO42-. But, the preferential pattern [net reduction of each added TEA (mM) vs time] was found to be NO3- > NO2- > As5+ > Fe3+. The growth of the strain while growing under these preferred electron acceptors showed that after 48 h, it reduced NO3- preferably (5 mM to avg. of 1 mM) followed by NO2- (5 mM to avg. of 2.0 mM), As5+ (5 mM to avg. of 2.5 mM), and Fe3+ (5 mM to avg. of 2.8 mM) (Fig 2). Substantial growth [with a maximum growth OD of 1.2–1.3, μ = 0.11 h-1] along with the formation of As3+ in the aqueous medium, confirmed its reductive transformation ability. Cells of strain KAs 5-3T were also found to reduce As5+ (from 1 mM to 0.2 mM) within 30 h of aerobic growth with the concomitant release of As3+ in the supernatant, indicating its potential of cytosolic reduction of As5+. The ability of Pseudoxanthomonas members to metabolize alkyl and aromatic hydrocarbons (BTEX, chrysene, and phenanthrene) and degrade pollutants has been recently studied [20, 21, 58–60]. The As-rich groundwater of Bengal basin harbours low amount of petroleum-derived hydrocarbons (that naturally seeps into the groundwater from deeper mature sediments), presence and hydrocarbon metabolizing activity of Pseudoxanthomonas strains is highly justified [26, 61, 62]. Except for P. kausinghensis and P. dokdonensis, Pseudoxanthomonas type members have been known to reduce nitrite. Thus, the ability of strain KAs 5-3T to preferentially utilize NO3- over NO2- is considered to be a unique metabolic character, distinguishing the strain from its closest relatives. Strain’s ability in utilizing diverse electron acceptor sources, thus corroborates its potential to dwell at the interface of aerobic-anaerobic zones of groundwater [26, 62–65].
Functional gene-based analysis
The presence of cytosolic As5+ reductase (arsC; 118 AA), nitrate reductase (narG; 214 AA) and nitrite reductase (nirS; 146 AA) were noted for the strains KAs 5-3T but not for the other closest related strains. BLASTP search showed highest identity (100%) of arsC and narG genes to the same genes from Escherichia coli followed by several Pseudoxanthomonas and other Xanthomonas, while the sequence of nirS showed highest similarity with Pseudoxanthomonas helianthi roo 10. Elaborate phylogenetic analysis was conducted for the arsC and narG genes. Phylogenetic analysis (Figs 3 and 4), p-distance matrix based net amino acid substitution (Figures E and F in S1 File), and phylogenetic neighbour network (Figures E and F in S1 File) showed a close phylogenetic proximity among KAs 5-3T and E. coli with respect to both of these genes. The data further indicated presence of similar mutational (insertion/deletion) events in these genes from the organisms, thus suggesting their possible transfer through horizontal gene transfer events. So, the observed phylogenetic incongruence between these functional genes and 16S rRNA gene was further studied with respect to GC mol %. Measure of unrelated GC mol % of the functional genes in the genome of organisms is considered to be the possible site of gene transfer events [24, 66, 67]. Hence, GC content (mol % and mol % deviation) of both the genes was compared with the genomic GC mol % for Pseudoxanthomonas reference genomes (Table B in S1 File). The GC mol % of both arsC and narG of strain KAs 5-3T were close to the genomic GC content of E.coli genomes, but not to the genomes of any of the nearest Pseudoxanthomonas members, further supporting the possibility of horizontal gene transfer events [68, 69]. Unlike, nitrite reduction, a universal property for the genus Pseudoxanthomonas; nitrate reduction by strain KAs 5-3T, is a unique trait.
The abilities to utilize multiple hydrocarbons, different electron acceptors with As5+ reduction abilities and genetic validation of this potential clearly demonstrated the metabolic flexibility of the strain. Alluvial aquifer of West Bengal is oligotrophic in nature with low dissolved carbon, low oxygen tension, fluctuating availability of electron donors and acceptors, with a low concentration of naturally derived hydrocarbons [26, 38, 61, 62]. Considering the overall hydrogeochemistry of West Bengal groundwater, the metabolic versatility of the strain KAs 5-3T seems highly justified for its competitive niche adaptation.
Emended description of the genus Pseudoxanthomonas Finkmann et al. 2000 emend. Lee et al. 2008
As per the descriptions of Pseudoxanthomonas by Finkmann et al., emended by Lee et al. (2008) and properties tested in this study, an emended description of the genus Pseudoxanthomonas is provided. Type strains of all Pseudoxanthomonas species except P. kaohsiungensis, P. dokdonensis, and P. arseniciresistens have no nitrate reduction (to N2) ability.
Description of Pseudoxanthomonas arseniciresistens sp. nov.
Pseudoxanthomonas arseniciresistens (L. n. arsenicum, arsenic; L. part. adj. resistens, resisting; N.L. part. adj. arseniciresistens, arsenic resisting, referring to the high arsenic resistance of the type strain).
Colonies are creamy to yellow, smooth and circular (1–2 mm on LB agar after 24–48 h at 30 oC). Cells are Gram-stain-negative, and facultative anaerobic rods (~1.5 × 0.5 mm). It grows well at 28–32 oC, pH 6–8 and NaCl concentrations of 0.5–5% (optimum of 1%). Cells are catalase- and oxidase-positive, highly As-resistant and able to reduce arsenate, nitrate as well as nitrite. Cells are positive for hydrolyses of ONPG (beta-galactosidase), beta-glucosidase, esculin, gelatin, casein, utilization of adipate, malate, citrate, N-acetyl glucosamine (NAG), and urea but negative for tween 80, arabinose, mannose, mannitol, maltose, gluconate, and caprate. Among various sugars, it assimilates α-D glucose, D-turanose, D-raffinose, D-sorbitol, D-galactose, sucrose, myo-inositol, and dextrin but does not assimilate α-D lactose, D-maltose, D-trehalose, D-cellobiose, D-fucose, D-mannose, D-salicin, gentiobiose, inosine, tween 40, and 3-methyl glucose. Among sugar acids, it waspositive for the assimilation of α-keto glutaric acid, D-gluconic acid, D-glucuronic acid, D-galacturonic acid, D-lactic acid, D-aspartic acid, D-malic acid, L-malic acid, L-aspartic acid, L-glutamic acid, acetic acid, citric acid, mucic acid, propionic acid, fusidic acid, sodium lactate, amino butyric acid, β-hydroxy butyric acid but negative for α-hydroxy butyric acid, α-keto butyric acid, L-galactonic acid, aceto acetic acid, phenyl acetic acid, and N-acetyl neuraminic acid. Among N-containing compounds, it uses L-glycyl proline, L-alanine, L-serine, D-serine, but unable to use D-glycyl proline, L-arginine, and L-histidine. On Biolog plates, cells of the strain KAs 5-3T shows ability to use glucuronamide, guanidine-HCl, tetrazolium violet, tetrazolium blue, lithium chloride, and potassium tellurite and inability to use sodium bromate. The cells are resistant to erythromycin, but, susceptible to ceftriaxone, cefixime, amikacin, cefotaxime, chloramphenicol, ofloxacin, polymyxin-B, tetracycline, ciprofloxacin, troleandomycin, rifamycin SV, minocycline, lincomycin, vancomycin, nalidixic acid and aztreonam. Cells are able to use hydrocarbons and reduce arsenate through cytosolic reduction. The major cellular fatty acids are C15:0, C16:0 iso, C17:1 iso ω9c, C15:0 anteiso, C11:0 iso 3-OH and C16:1 ω7c/ C16:1 ω6c and Q8 as the major isoprenoid quinone. Polar lipids include diphosphatidylglycerol, phosphatidyldimethylethanolamine, phosphatidylcholine, and three unknown phospholipids. Spermidine is the predominant polyamine. The molar G+C content is 64.9 mol %.The type strain, KAs 5-3T (= LMG 29169T = MTCC 12116T = MCC 3121T), was isolated from highly As-rich groundwater of Kolsur village, North 24 Pargana of West Bengal, India.
Conclusion
The phylogenetic, chemotaxonomic and phenotypic analysis supported the affiliation of strain KAs 5-3T to the genus Pseudoxanthomonas. The strain KAs 5-3T showed distinguishing physiological, phenotypic as well as molecular characteristic. Multi locus sequence analysis involving four house-keeping genes and DNA–DNA relatedness unambiguously demarcated the species novelty. Dissimilatory reduction of nitrate and nitrite as well as ability to metabolize hydrocarbons and reduce As5+ through cytosolic processes highlighted the unique properties of the strain KAs 5-3T, which are of ecological significance. On the basis of phenotypic and physiological characteristics, chemotaxonomic analysis, multi locus sequence analysis, and DNA–DNA relatedness data, the isolate represents a novel species of the genus Pseudoxanthomonas, therefore, the name Pseudoxanthomonas arseniciresistens sp. nov. is proposed.
Supporting information
Table A, Details of PCR primers used for 16S rRNA, MLSA, and functional gene analysis. Table B, GC mol % and dGC mol % (deviation) from their respective genomic GC content of arsC and narG sequences (phylogenetically closest) as a measure of horizontal gene transfer event. Figure A, Phylogenetic tree involving 16S rRNA gene sequences of strain KAs 5-3T and type members of Pseudoxanthomonas species obtained through (a) maximum likelihood (b) and minimum evolution methods. Bootstraps (1000 resampling) of above 60% are shown at each branch. Genbank accession numbers are presented in parentheses. Bar 0.005 indicates 0.5% substitution. Figure B, Neighbor-joining phylogenetic tree based on Multi Locus Sequence Alignment (MLSA) of four concatenated housekeeping genes: gyrB (1200 bp), dnaJ (1000 bp), atpG (400 bp), and rpoB (1200 bp) of KAs 5-3T with the Pseudoxanthomonas type members. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. The evolutionary distances were computed and are in the units of the number of base substitutions per site. All ambiguous positions were removed and all codon positions were included for construction of the tree in the final dataset through MEGA 7.0. GenBank accession numbers for the genes of strain KAs 5-3T are: KX827793 (gyrB), KX827796 (dnaJ), KX827799 (atpG), and KX880497 (rpoB). Figure C, Scanning electron micrograph of cells of the strain KAs 5-3Tafter growth on LB agar plate for 18 h at 30°C. Figure D, Polar lipid profile of the strain KAs 5-3T and reference type strain members of Pseudoxanthomonas as shown on TLC plate, developed after spraying with 5% ethanolic molybdophosphoric acid lipid detection solvents; a) KAs 5-3T, b) P. mexicana AMX 26BT, c) P. japonensis 12-3T, d) P. daejeonensis TR6-08T, e) P. indica P15T, f) P. suwonensis 4M1T, g) P. wuyuanensis XC21-2T, h) P. putridarboris WD12T. Figure E, Analysis of gene encoding arsenate (As5+) reductase (arsC) a) distance matrix for aligned sequence of KAs 5-3T with related sequences, b) NeighborNet phylogenetic network of arsC gene of KAs 5-3T with related sequences obtained through SplitsTree software. Colour codes indicate the p-distance value against the specified sequences. Intensity in each branch indicates the similar evolutionary events. Bar, 0.1 indicates extent of evolution (10%) at amino acid level. Figure F, Analysis of gene encoding nitrate (NO3-) reductase (narG) a) distance matrix for aligned sequence of KAs 5-3T with related sequences, b) NeighborNet phylogenetic network of nitrate reductase (narG) of KAs 5-3T with related sequences obtained through SplitsTree software. Colour codes indicate the p-distance value against the specified sequences. Intensity in each branch indicates the similar evolutionary events. Bar, 0.1 indicates extent of evolution (10%) at amino acid level.
(PDF)
A, Microbial culture collection (MCC) deposition certificate of strain KAs 5-3T. B, Microbial type culture collection (MTCC) deposition certificate of strain KAs 5-3T. C, Belgian coordinated culture collection (BCCM) deposition certificate of strain KAs 5-3T.
(PDF)
Acknowledgments
Authors acknowledge kind help of Dr. Takashi Iizuka, Central Research Laboratories, Ajinomoto Co. Inc. 1–1, Suzuki-Cho, Kawasaki-ku, Kawasaki-shi, 210–8681, Japan, Dr. Herve Macarie, Laboratoire de Microbiologie IRD, IFR-BAIM, Universite´ s de Provence et de la Me´diterrane, ESIL case 925, 163 avenue de Luminy, 13288 Marseille cedex 9, France, Korean Collection for Type Culture (KCTC), Korea, and Korean Agricultural Culture Collection (KACC), Korea for providing the necessary type strains of Pseudoxanthomonas. We also thank Dr. A. Oren and Dr. A. C. Parte for suggesting species epithet and etymology. Authors also acknowledge the help of Microbial Culture Collection (MCC), National Centre for Cell Sciences (NCCS), Pune, India for analysing fatty acids through MIDI Sherlock identification system. The authors would like to thank the editor and the anonymous reviewers for critically reading and providing necessary suggestions to improve the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work was financially supported by the grant from Department of Science and Technology, Govt. of India (grant no. DST/TM/WTI/2K15/182) to PS and SKK, and the Indian Institute of Technology Kharagpur (grant no. IIT/SRIC/BT/ODM/2015-16/141) to PS. BM is a recipient of INSPIRE fellowship (doctoral research) of Department of Science and Technology (DST), Govt. of India, DST/INSPIRE Fellowship/2012/763, fellowship number IF 120832. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Garrity GM, Holt JG. The roadmap to the Manual In: Garrity GM, Boone DR, Castenholz RW, editors. Bergey’s Manual of Systematic Bacteriology, New York: Springer; 2001. pp. 119–166. [Google Scholar]
- 2.Finkmann W, Altendorf K, Stackebrandt E, Lipski A. Characterization of N O-producing Xanthomonas-like isolates from biofilters as Stenotrophomonas nitritireducens sp. nov., Luteimonas mephitis gen. nov., sp. nov. and Pseudoxanthomonas broegbernensis gen. nov., sp. nov. Int J Syst Evol Microbiol. 2000; 50: 273–282. doi: 10.1099/00207713-50-1-273 [DOI] [PubMed] [Google Scholar]
- 3.Thierry S, Macarie H, Iizuka T, Geißdorfer W, Assih EA., Spanevello M, et al. Pseudoxanthomonas mexicana sp. nov. and Pseudoxanthomonas japonensis sp. nov., isolated from diverse environments, and emended descriptions of the genus Pseudoxanthomonas Finkmann et al., 2000 and of its type species. Int J Syst Evol Microbiol. 2004; 54: 2245–2255. doi: 10.1099/ijs.0.02810-0 [DOI] [PubMed] [Google Scholar]
- 4.George MG, Julia AB, Timothy GL. Bergey’s manual of systematic bacteriology In: Volume 2, The Proteobacteria, Springer-Verlag, Berlin; 2005. pp. 735–769. [Google Scholar]
- 5.Lee DS, Ryu SH, Hwang HW, Kim YJ, Park M, Lee JR, et al. Pseudoxanthomonas sacheonensis sp. nov., isolated from BTEX-contaminated soil in Korea, transfer of Stenotrophomonas dokdonensis Yoon et al., 2006 to the genus Pseudoxanthomonas as Pseudoxanthomonas dokdonensis comb. nov. and emended description of the genus Pseudoxanthomonas. Int J Syst Evol Microbiol. 2008; 58: 2235–2240. doi: 10.1099/ijs.0.65678-0 [DOI] [PubMed] [Google Scholar]
- 6.Chen MY, Tsay SS, Chen KY, Shi YC, Lin YT, Lin GH. Pseudoxanthomonas taiwanensis sp. nov., a novel thermophilic, N2O-producing species isolated from hot springs. Int J Syst Evol Microbiol. 2002; 52: 2155–2161 doi: 10.1099/00207713-52-6-2155 [DOI] [PubMed] [Google Scholar]
- 7.Yang DC, Im WT, Kim MK, Lee ST. Pseudoxanthomonas koreensis sp. nov. and Pseudoxanthomonas daejeonensis sp. nov. Int J Syst Evol Microbiol. 2005; 55: 787–791. doi: 10.1099/ijs.0.63210-0 [DOI] [PubMed] [Google Scholar]
- 8.Weon HY, Kim BY, Kim JS, Lee SY, Cho YH, Go SJ, et al. Pseudoxanthomonas suwonensis sp. nov., isolated from cotton waste composts. Int J Syst Evol Microbiol. 2006; 56: 659–662. doi: 10.1099/ijs.0.63749-0 [DOI] [PubMed] [Google Scholar]
- 9.Harada RM, Campbell S, Li QX. Pseudoxanthomonas kalamensis sp. nov., a novel gammaproteobacterium isolated from Johnston Atoll, North Pacific Ocean. Int J Syst Evol Microbiol. 2006; 56:1103–1107. doi: 10.1099/ijs.0.63556-0 [DOI] [PubMed] [Google Scholar]
- 10.Yoo SH, Weon HY, Kim BY, Kim JH, Baek YK, Kwon SW, Go SJ, Stackebrandt E. Pseudoxanthomonas yeongjuensis sp. nov., isolated from soil cultivated with Korean ginseng. Int J Syst Evol Microbiol. 2007; 57:646–649. doi: 10.1099/ijs.0.64427-0 [DOI] [PubMed] [Google Scholar]
- 11.Young CC, Ho MJ, Arun AB, Chen WM, Lai WA, Shen FT, Rekha PD, Yassin AF. Pseudoxanthomonas spadix sp. nov., isolated from oil-contaminated soil. Int J Syst Evol Microbiol. 2007; 57:1823–1827. doi: 10.1099/ijs.0.65053-0 [DOI] [PubMed] [Google Scholar]
- 12.Yoon JH, Kang SJ, Oh HW, Oh TK. Stenotrophomonas dokdonensis sp. nov., isolated from soil. Int J Syst Evol Microbiol. 2006; 56: 1363–1367. doi: 10.1099/ijs.0.64091-0 [DOI] [PubMed] [Google Scholar]
- 13.Kumari K, Sharma P, Tyagi K, Lal R. Pseudoxanthomonas indica sp. nov., isolated from a hexachlorocyclohexane dumpsite. Int J Syst Evol Microbiol. 2011; 61: 2107–2111. doi: 10.1099/ijs.0.017624-0 [DOI] [PubMed] [Google Scholar]
- 14.Li D, Pang H, Sun L, Fan J, Li Y, Zhang J. Pseudoxanthomonas wuyuanensis sp. nov., isolated from saline-alkali soil. Int J Syst Evol Microbiol. 2014; 64: 799–804. doi: 10.1099/ijs.0.056796-0 [DOI] [PubMed] [Google Scholar]
- 15.Kim SJ, Ahn JH, Weon HY, Lim JM, Kim SG, Kwon SW. Pseudoxanthomonas sangjuensis sp. nov., isolated from greenhouse soil. Int J Syst Evol Microbiol. 2015; 65: 3170–3174. doi: 10.1099/ijsem.0.000395 [DOI] [PubMed] [Google Scholar]
- 16.Kittiwongwattana C, Thawai C. Pseudoxanthomonas helianthi sp. nov., isolated from roots of Jerusalem artichoke (Helianthus tuberosus). Int J Syst Evol Microbiol. 2016; 66: 5034–5038. doi: 10.1099/ijsem.0.001465 [DOI] [PubMed] [Google Scholar]
- 17.Lee JK, Oh JS, Cho WD, Roh DH. Pseudoxanthomonas putridarboris sp. nov. isolated from rotten tree. Int J Syst Evol Microbiol. 2017; 67: 1807–1812. doi: 10.1099/ijsem.0.001867 [DOI] [PubMed] [Google Scholar]
- 18.Chang JS, Chou CL, Lin GH, Sheu SY, Chen WM. Pseudoxanthomonas kaohsiungensis sp. nov., a novel bacterium isolated from oil-polluted site produces extracellular surface activity. Syst Appl Microbiol. 2005; 28: 137–144. doi: 10.1016/j.syapm.2004.11.003 [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Wei L, Zhu L, Li C, Wang Y, Shen X. Pseudoxanthomonas gei sp. nov., a novel endophytic bacterium isolated from the stem of Geum aleppicum. Antonie Leeuwenhoek. 2014; 105: 653–661. doi: 10.1007/s10482-014-0119-2 [DOI] [PubMed] [Google Scholar]
- 20.Nayak AS, Sanjeev KS, Santosh KM, Anjaneya O, Karegoudar TB. A catabolic pathway for the degradation of chrysene by Pseudoxanthomonas sp. PNK-04. FEMS Microbiol Lett. 2011; 320: 128–134. doi: 10.1111/j.1574-6968.2011.02301.x [DOI] [PubMed] [Google Scholar]
- 21.Patel V, Cheturvedula S, Madamwar D. Phenanthrene degradation by Pseudoxanthomonas sp. DMVP2 isolated from hydrocarbon contaminated sediment of Amlakhadi canal, Gujarat, India. J Hazard Mater. 2012; 201: 43–51. doi: 10.1016/j.jhazmat.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 22.Xu M, Zhang Q, Xia C, Zhang Y, Sun G, Guo J. Elevated nitrate enriches microbial functional genes for potential bioremediation of complexly contaminated sediments. ISME J. 2014; 8: 1932–1944. doi: 10.1038/ismej.2014.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarkar A, Kazy SK., Sar P. Characterization of arsenic resistant bacteria from arsenic rich groundwater of West Bengal, India. Ecotoxicol. 2013; 22: 363–376. [DOI] [PubMed] [Google Scholar]
- 24.Sarkar A, Kazy SK., Sar P. Studies on arsenic transforming groundwater bacteria and their role in arsenic release from subsurface sediment. Environ Sci Pollut Res. 2014; 21: 8645–8662. [DOI] [PubMed] [Google Scholar]
- 25.Kibria M. Hydrogeochemistry and microbial geochemistry of different depth aquifer sediments from Matlab Bangladesh: relation to arsenic contamination in groundwaters (Doctoral dissertation, Kansas State University), 2014.
- 26.Paul D, Kazy SK, Gupta AK., Pal T, Sar P. Diversity, metabolic properties and arsenic mobilization potential of indigenous bacteria in arsenic contaminated groundwater of West Bengal, India. Plos One. 2015; 10 (3): e0118735 doi: 10.1371/journal.pone.0118735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999; 41: 95–98. [Google Scholar]
- 28.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990; 215: 403–410. doi: 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 29.Maidak BL, Cole JR, Lilburn TG, Parker CT, Saxman PR, Farris RJ, et al. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 2001; 29: 173–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA and whole genome assemblies. Int J Syst Evol Microbiol. 2017; 67: 1613–1617. doi: 10.1099/ijsem.0.001755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016; 33: 1870–1874. doi: 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987; 4: 406–425. doi: 10.1093/oxfordjournals.molbev.a040454 [DOI] [PubMed] [Google Scholar]
- 33.Jukes T, Cantor CR. Evolution of protein molecules In Munro HN, editor. Mammalian Protein Metabolism. New York: Academic Press, 1969. pp. 132. [Google Scholar]
- 34.Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981; 17: 368–376. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K, Nei M. Efficiencies of fast algorithms of phylogenetic inference under the criteria of maximum parsimony, minimum evolution, and maximum likelihood when a large number of sequences are used. Mol Biol Evol. 2000; 17: 1251–1258. doi: 10.1093/oxfordjournals.molbev.a026408 [DOI] [PubMed] [Google Scholar]
- 36.De Ley J, Cattoir H, Reynaerts A. The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem. 1970; 12: 133–142. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez JM, Saiz-Jimenez C. A simple fluorimetric method for the estimation of DNA–DNA relatedness between closely related microorganisms by thermal denaturation temperatures. Extremophiles. 2005; 9: 75–79. doi: 10.1007/s00792-004-0417-0 [DOI] [PubMed] [Google Scholar]
- 38.Mohapatra B., Sarkar A., Joshi S., Chatterjee A., Kazy S. K., Maiti M. K., Satyanarayana T., Sar P. (2016) An arsenate-reducing and alkane-metabolizing novel bacterium, Rhizobium arsenicireducens sp. nov., isolated from arsenic-rich groundwater. Arch Microbiol 198, 1–11. doi: 10.1007/s00203-015-1148-6 [DOI] [PubMed] [Google Scholar]
- 39.Rosselló-Mora R, Amann R The species concept for prokaryotes. FEMS Microbiol Rev. 2001; 25: 39–67. [DOI] [PubMed] [Google Scholar]
- 40.Kodaka H, Armfield AY, Lombard GL, Dowell VR. Practical procedure for demonstrating bacterial flagella. J Clin Microbiol. 1982; 16: 948–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly A, Fulton M. Use of triphenyl tetrazolium in motility test medium. Am J Clin Pathol. 1953; 23: 512 [DOI] [PubMed] [Google Scholar]
- 42.Cowan ST, Steel KJ. Manual For The Identification Of Medical Bacteria. London, Cambridge University Press; 1965. [Google Scholar]
- 43.Smibert RM, Krieg NR. Phenotypic characterization In: Gerhardt P, Murray RGE, Wood WA, Krieg NR, editors. Methods for General and Molecular Bacteriology. Washington DC: American Society for Microbiology, 1994. pp. 607–654. [Google Scholar]
- 44.Bauer AW, Kirby WMM, Sherris JC, Turk M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966; 45: 493–496. [PubMed] [Google Scholar]
- 45.Clinical and Laboratory Standards Institute, Performance standards for antimicrobial disk susceptibility tests Approved standard M2-A10, 2009. Wayne, PA, Clinical and Laboratory Standards Institute. [Google Scholar]
- 46.Zhu H, Guo J, Chen M, Feng G, Yao Q. Burkholderia dabaoshanensis sp. nov., a heavy-metal-tolerant bacteria isolated from Dabaoshan mining area soil in China. PloS one. 2012; 7(12):e50225 doi: 10.1371/journal.pone.0050225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasser M. Identification of bacteria by gas chromatography of cellular fatty acids MIDI Technical Note 101. Newark, DE: MIDI Inc; 1990. [Google Scholar]
- 48.Komagata K, Suzuki K. Lipid and cell wall analysis in bacterial systematics. Methods Microbiol. 1987; 19: 161–206. [Google Scholar]
- 49.Johnson DL. Simultaneous determination of arsenate and phosphate in natural waters. Environ Sci Technol. 1971; 5: 411–414. [Google Scholar]
- 50.Cataldo DA, Maroon M, Schrader LE, Youngs VL. Rapid colorimetric determination of nitrate in plant-tissue by nitration of salicyclic acid. Commun Soil Sci Plan. 1975; 6: 71–80. [Google Scholar]
- 51.Lovely DR, Phillips EJ. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl Environ Microbiol. 1986; 152, 751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Cummings, Caccavo F, Fendorf S, Rosenzweig RF. Arsenic Mobilization by the Dissimilatory Fe(III)-Reducing Bacterium Shewanella alga BrY. Environ Sci Technol. 1999; 33: 723–729. [Google Scholar]
- 53.Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003; 31: 3784–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huson DH, Bryant D. Application of Phylogenetic Networks in Evolutionary Studies. Mol Bio Evol. 2006; 23: 254–267. [DOI] [PubMed] [Google Scholar]
- 55.Stackebrandt E, Frederiksen W, Garrity GM, Grimont PA, Kämpfer P, Maiden MC, et al. Report of the ad hoc committee for the re-evaluation of the species definition in bacteriology. Int J Syst Evol Microbiol. 2002; 52: 1043–1047. doi: 10.1099/00207713-52-3-1043 [DOI] [PubMed] [Google Scholar]
- 56.Glaeser SP, Kämpfer P. Multilocus sequence analysis (MLSA) in prokaryotic taxonomy. Syst Appl Microbiol. 2015; 38: 237–245. doi: 10.1016/j.syapm.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 57.Chun J, Rainey FA. Integrating genomics into the taxonomy and systematics of the Bacteria and Archaea. Int J Syst Evol Microbiol. 2014; 64: 316–324. doi: 10.1099/ijs.0.054171-0 [DOI] [PubMed] [Google Scholar]
- 58.Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, et al. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 2012; 62: 716–721. doi: 10.1099/ijs.0.038075-0 [DOI] [PubMed] [Google Scholar]
- 59.Olsen RH, Kukor JJ, Kaphammer B. A novel toluene-3-monooxygenase pathway cloned from Pseudomonas pickettii PKO1. J Bacteriol. 1994; 176: 3749–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jahn MK, Haderlein SB, Meckenstock RU. Anaerobic degradation of benzene, toluene, ethylbenzene, and o-xylene in sediment-free iron-reducing enrichment cultures. Appl Environ Microbiol. 2005; 71: 3355–3358. doi: 10.1128/AEM.71.6.3355-3358.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McArthur JM, Banerjee DM, Hudson-Edwards KA, Mishra R, Purohit R. Natural organic matter in sedimentary basins and its relation to arsenic in anoxic ground water: the example of West Bengal and its worldwide implications. Appl Geochem. 2004; 19: 1255–1293. [Google Scholar]
- 62.Hery M, Van Dongen BE, Gill F, Mondal D, Vaughan DJ, Pancost RD, et al. Arsenic release and attenuation in low organic carbon aquifer sediments from West Bengal. Geobiol. 2010; 8: 155–168. [DOI] [PubMed] [Google Scholar]
- 63.Sultana M, Härtig C, Planer-Friedrich B, Seifert J, Schlömann M. Bacterial communities in Bangladesh aquifers differing in aqueous arsenic concentration. Geomicrobiol J. 1990; 28: 198–211. [Google Scholar]
- 64.Leahy JG, Colwell RR. Microbial degradation of hydrocarbons in the environment. Microbiological reviews. 1990; 54: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kostka JE, Prakash O, Overholt WA, Green SJ, Freyer G, Canion A, Huettel M. Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the Deepwater Horizon oil spill. Appl environ microbial. 2011; 77: 7962–7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia-Vallvé S, Romeu A, Palau J. Horizontal gene transfer in bacterial and archaeal complete genomes. Genome Res. 2000; 10: 1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hayek N. Lateral transfer and GC content of bacterial resistance genes. Front Microbiol. 2013; 4: 41 doi: 10.3389/fmicb.2013.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silver S, Phung LT. Genes and enzymes involved in bacterial oxidation and reduction of inorganic arsenic. Appl Environ Microbiol. 2005; 71: 599–608. doi: 10.1128/AEM.71.2.599-608.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Achour-Rokbani A, Bauda P, Billard P. Diversity of arsenite transporter genes from arsenic-resistant soil bacteria. Res Microbiol. 2007; 158: 128–137. doi: 10.1016/j.resmic.2006.11.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A, Details of PCR primers used for 16S rRNA, MLSA, and functional gene analysis. Table B, GC mol % and dGC mol % (deviation) from their respective genomic GC content of arsC and narG sequences (phylogenetically closest) as a measure of horizontal gene transfer event. Figure A, Phylogenetic tree involving 16S rRNA gene sequences of strain KAs 5-3T and type members of Pseudoxanthomonas species obtained through (a) maximum likelihood (b) and minimum evolution methods. Bootstraps (1000 resampling) of above 60% are shown at each branch. Genbank accession numbers are presented in parentheses. Bar 0.005 indicates 0.5% substitution. Figure B, Neighbor-joining phylogenetic tree based on Multi Locus Sequence Alignment (MLSA) of four concatenated housekeeping genes: gyrB (1200 bp), dnaJ (1000 bp), atpG (400 bp), and rpoB (1200 bp) of KAs 5-3T with the Pseudoxanthomonas type members. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. The evolutionary distances were computed and are in the units of the number of base substitutions per site. All ambiguous positions were removed and all codon positions were included for construction of the tree in the final dataset through MEGA 7.0. GenBank accession numbers for the genes of strain KAs 5-3T are: KX827793 (gyrB), KX827796 (dnaJ), KX827799 (atpG), and KX880497 (rpoB). Figure C, Scanning electron micrograph of cells of the strain KAs 5-3Tafter growth on LB agar plate for 18 h at 30°C. Figure D, Polar lipid profile of the strain KAs 5-3T and reference type strain members of Pseudoxanthomonas as shown on TLC plate, developed after spraying with 5% ethanolic molybdophosphoric acid lipid detection solvents; a) KAs 5-3T, b) P. mexicana AMX 26BT, c) P. japonensis 12-3T, d) P. daejeonensis TR6-08T, e) P. indica P15T, f) P. suwonensis 4M1T, g) P. wuyuanensis XC21-2T, h) P. putridarboris WD12T. Figure E, Analysis of gene encoding arsenate (As5+) reductase (arsC) a) distance matrix for aligned sequence of KAs 5-3T with related sequences, b) NeighborNet phylogenetic network of arsC gene of KAs 5-3T with related sequences obtained through SplitsTree software. Colour codes indicate the p-distance value against the specified sequences. Intensity in each branch indicates the similar evolutionary events. Bar, 0.1 indicates extent of evolution (10%) at amino acid level. Figure F, Analysis of gene encoding nitrate (NO3-) reductase (narG) a) distance matrix for aligned sequence of KAs 5-3T with related sequences, b) NeighborNet phylogenetic network of nitrate reductase (narG) of KAs 5-3T with related sequences obtained through SplitsTree software. Colour codes indicate the p-distance value against the specified sequences. Intensity in each branch indicates the similar evolutionary events. Bar, 0.1 indicates extent of evolution (10%) at amino acid level.
(PDF)
A, Microbial culture collection (MCC) deposition certificate of strain KAs 5-3T. B, Microbial type culture collection (MTCC) deposition certificate of strain KAs 5-3T. C, Belgian coordinated culture collection (BCCM) deposition certificate of strain KAs 5-3T.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.