Abstract
Background
Type 2 diabetes mellitus (T2DM) is a global epidemic associated with increased health expenditure, and low quality of life. Many non-genetic risk factors have been suggested, but their overall epidemiological credibility has not been assessed.
Methods
We searched PubMed to capture all meta-analyses and Mendelian randomization studies for risk factors of T2DM. For each association, we estimated the summary effect size, its 95% confidence and prediction interval, and the I2 metric. We examined the presence of small-study effects and excess significance bias. We assessed the epidemiological credibility through a set of predefined criteria.
Results
We captured 86 eligible papers (142 associations) covering a wide range of biomarkers, medical conditions, and dietary, lifestyle, environmental and psychosocial factors. Adiposity, low hip circumference, serum biomarkers (increased level of alanine aminotransferase, gamma-glutamyl transferase, uric acid and C-reactive protein, and decreased level of adiponectin and vitamin D), an unhealthy dietary pattern (increased consumption of processed meat and sugar-sweetened beverages, decreased intake of whole grains, coffee and heme iron, and low adherence to a healthy dietary pattern), low level of education and conscientiousness, decreased physical activity, high sedentary time and duration of television watching, low alcohol drinking, smoking, air pollution, and some medical conditions (high systolic blood pressure, late menarche age, gestational diabetes, metabolic syndrome, preterm birth) presented robust evidence for increased risk of T2DM.
Conclusions
A healthy lifestyle pattern could lead to decreased risk for T2DM. Future randomized clinical trials should focus on identifying efficient strategies to modify harmful daily habits and predisposing dietary patterns.
Background
Type 2 diabetes mellitus (T2DM) ranks highly on the international health agenda as a global pandemic and as a threat to human health and global economies. The number of people with T2DM worldwide has more than doubled during the past 20 years [1]. According to the International Diabetes Federation, 415 million people are living with T2DM in 2015, and by 2040 the number will be almost 642 million [2]. These estimates correspond to a global prevalence of 8.8% (95% confidence interval, 7.2–11.4%) in 2015, and a projected global prevalence of 10.4% (95% confidence interval, 8.5–13.5%) in 2040 [2]. Epidemiological data predict an inexorable and unsustainable increase in global health expenditure attributable to T2DM, so disease prevention should be given high priority.
T2DM results from an interaction between genetic and environmental factors [3]. Genes and the environment together are important determinants of insulin resistance and β-cell dysfunction [4]. Because changes in the gene pool cannot account for the rapid increase in prevalence of T2DM in recent decades, environmental changes are essential to the understanding of the epidemic.
Systematic reviews and meta-analyses of observational studies have indicated numerous risk factors for T2DM. However, the epidemiological credibility of these associations has not been appraised across the field. In the present work, we performed an umbrella review of the evidence across existing systematic reviews and meta-analyses of observational studies that examine any non-genetic risk factor for T2DM. We primarily aim to provide an overview of the range and validity of the reported associations of diverse environmental risk factors and biomarkers with T2DM. Furthermore, we assessed whether there is evidence for diverse biases and which of the previously studied associations have robust evidence.
Materials and methods
Search strategy and eligibility criteria
We conducted an umbrella review, i.e. a comprehensive and systematic collection and evaluation of systematic reviews and meta-analyses performed on a specific research topic using previously described and applied methodology [5–12].
We systematically searched PubMed from inception until February 10, 2016 to identify systematic reviews and meta-analyses of observational studies examining associations of non-genetic risk factors with T2DM. We used the following search strategy: diabetes AND (“systematic review” OR meta-analysis). Two independent investigators (VB, LB) retrieved and abstracted the full text of potentially eligible articles. We excluded meta-analyses that investigated the association between genetic polymorphisms and risk for T2DM; that included less than 3 component studies; that included studies with overlapping populations; that included studies using different units of comparison of the same exposure without transforming the effect estimates appropriately. We further excluded meta-analyses performing comparison between drug agents and subsequent risk for developing T2DM in population at high risk. When an association was covered by more than one meta-analyses, we kept the meta-analysis including the largest number of component studies and adequately presenting the study-specific effect estimates and sample sizes of component studies. We did not apply any language restrictions in our search strategy.
Finally, in order to assess the causality of the associations between the reported risk factors and T2DM, we conducted an additional systematic search on PubMed to capture mendelian randomization (MR) studies for T2DM. This search algorithm used the keywords: “mendelian randomization” OR “mendelian randomisation”. MR studies were eligible if they studied T2DM and examined the potentially causal effect of a risk factor that was also included in our umbrella review. We excluded studies focused on impaired glucose tolerance, impaired fasting glucose or insulin resistance as outcomes.
Data extraction
Two independent investigators (VB, LB) extracted the data, and in case of discrepancies consensus was reached. From each eligible article, we abstracted information on the first author, journal and year of publication, the examined risk factors and the number of studies considered. We also extracted the study-specific risk estimates (i.e. risk ratio, odds ratio, hazard ratio, standardized mean difference) along with their 95% confidence interval (CI) and the number of cases and controls in each study. If a meta-analysis included multiple effect estimates from the same observational study using the same control group, we included only the effect estimate that corresponded to the largest sample size.
From each eligible MR study, we extracted the first author and year of publication, the definition of outcome, the risk factor considered, the level of comparison for exposure, the genetic instrument used, the applied statistical approach, the sample size, the causal odds ratio and its 95% CI, the P-value for the association, and whether the authors claimed that a causal relationship exists. If an MR study used a genetic instrument based on a single variant and a genetic instrument based on polygenic risk score (PRS), we extracted the information from the PRS, as this approach is more powerful.
Statistical analysis
For each meta-analysis, we estimated the summary effect size and its 95% CI using both fixed-effect and random-effects models. [13,14] We also estimated the 95% prediction interval (PI), which accounts for the between-study heterogeneity and evaluates the uncertainty for the effect that would be expected in a new study addressing that same association. [15,16]
Between-study heterogeneity was quantified using the I2 metric. I2 ranges between 0% and 100% and quantifies the variability in effect estimates that is due to heterogeneity rather than sampling error. [17] Values exceeding 50% or 75% are considered to represent large or very large heterogeneity, respectively. This step is necessary to ensure that all results from each meta-analysis are available to assess the epidemiological credibility of the associations.
We assessed small-study effects using the Egger’s regression asymmetry test. [18,19] A P <0.10 combined with a more conservative effect in the largest study than in random-effects meta-analysis was judged to provide adequate evidence for small-study effects. We further applied the excess statistical significance test, which evaluates whether there is a relative excess of formally significant findings in the published literature due to any reason. [20] We used the effect size of the largest study (smallest standard error) in each meta-analysis to calculate the power of each study using a non-central t distribution. [21,22] Excess statistical significance was claimed at two-sided P <0.10. [21] In two meta-analyses (glycemic load as dichotomous exposure, and breastfeeding), the excess significance test was not performed, because the sample size was not reported in some of the component studies.
Assessment of epidemiological credibility
We identified associations that had the strongest evidence and no signals of large heterogeneity or bias. We considered as convincing the associations that fulfilled all the following criteria: statistical significance per random-effects model at P <10−6; based on >1,000 cases; without large between-study heterogeneity (I2<50%); 95% PI excluding the null value; and no evidence of small-study effects and excess significance bias. Associations with >1,000 cases, P <10−6 and largest study presenting a statistically significant effect were graded as highly suggestive. The associations supported by >1,000 cases and a significant effect at P <10−3 were considered as suggestive. The remaining nominally significant associations (P <0.05) were considered as having weak evidence.
For associations with convincing and highly suggestive evidence, we performed a sensitivity analysis limited to prospective cohort studies and nested case-control studies, and we examined whether there was a change in the level of epidemiological credibility. Also, we compared the findings from the meta-analyses of observational studies with the findings from MR studies.
The statistical analysis and the power calculations were done with STATA version 12.0 and RStudio version 1.0.44.
Results
Eligible studies
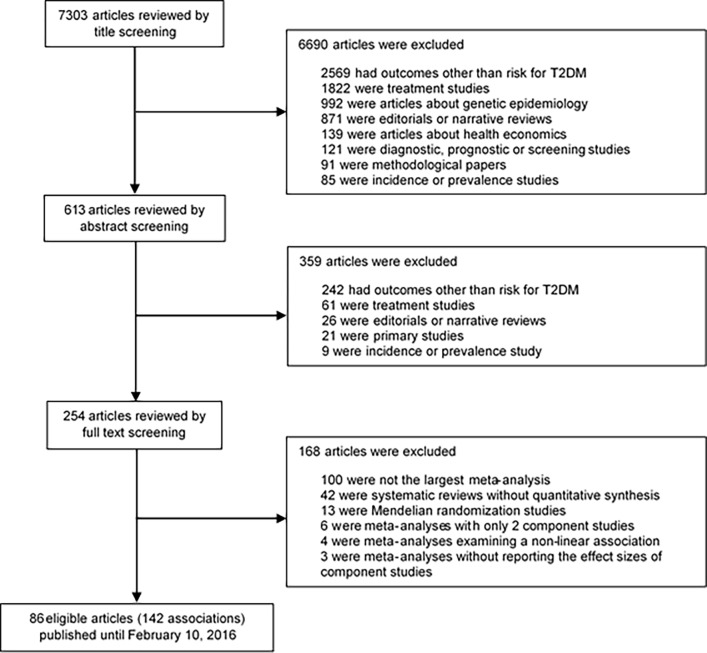
Our literature search yielded 7,303 papers, of which 86 papers met our inclusion criteria (Fig 1). Fourteen papers, including 16 associations (i.e., sedentary time, breakfast skipping, psoriasis, psoriatic arthritis, breastfeeding, adverse childhood experience, height, hip circumference, serum osteocalcin, spousal diabetes, osteoarthritis, polycystic ovary syndrome, schizophrenia, major depressive disorder, and bipolar disorder), combined cross-sectional studies with either cohort studies or case-control studies in their analysis.
Fig 1. Flow chart of literature search.
The 86 eligible papers examined 109 unique risk factors and 142 associations related to risk for developing T2DM. These associations covered a wide range of exposures: biomarkers (n = 25 associations), dietary factors (n = 53 associations), lifestyle factors and environmental exposures (n = 22 associations), medical history (n = 16 associations), metabolic factors and anthropometric traits (n = 15 associations), and psychosocial factors (n = 11 associations). The median number of cases per meta-analysis was 8,825 (IQR, 2,892–17,782), and the median number of datasets was 10 (IQR, 6–14). Only 7 meta-analyses included less than 1,000 T2DM cases.
Statistically significant associations, heterogeneity and biases
One hundred and sixteen of 142 associations (82%) presented a statistically significant effect at P <0.05 under the random-effects model, whereas 46 associations had a statistically significant effect at P <10−6 (Table 1). Fig 2 displays the distribution of the P-values in each category of associations. Only 33 of 142 associations (23%) had a 95% PI that excluded the null value and 26 of these also had a P <10−6.
Table 1. Characteristics of 142 associations between non-genetic risk factors and type 2 diabetes mellitus.
| Reference | Risk factor | Level of comparison | Number of cases/controls | Number of datasets | Effect size metric | Random-effects summary effect size (95% CI) | P random | 95% prediction interval | I2 | Small-study effects/Excess significance bias | Grading |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomarkers | |||||||||||
| Aune, 2015 [57] | Resting heart rate | Per 10 bpm increase | 6217/106,601 | 9 | RR | 1.20 (1.07–1.35) | 1.74 × 10−3 | 0.80–1.79 | 93.4 | No/Yes | Weak |
| Chen, 2014 [58] | Serum leptin | Per 1 log ng/ml increase | 4084/22,367 | 17 | RR | 1.13 (1.01–1.27) | 0.038 | 0.74–1.73 | 76 | Yes/Yes | Weak |
| Emdin, 2015 [39] | Systolic blood pressure | Per 20 mmHg increase | 204,803/4,212,999 | 40 | RR | 1.75 (1.56–1.97) | 6.15 × 10−21 | 0.97–3.16 | 85.7 | No/No | Highly suggestive |
| Fraser, 2009 [59] | Serum ALT | Per 1 log unit increase | 2009/32,292 | 14 | HR | 1.85 (1.57–2.18) | 2.85 × 10−13 | 1.31–2.61 | 19.2 | No/No | Convincing |
| Fraser, 2009 [59] | Serum ALT | Highest vs. lowest category | 1087/22,729 | 10 | HR | 2.07 (1.54–2.79) | 1.52 × 10−6 | 1.07–4.02 | 27.3 | No/No | Suggestive |
| Fraser, 2009 [59] | Serum γGT | Highest vs. lowest category | 1352/20,955 | 10 | HR | 3.07 (2.22–4.23) | 1.02 × 10−11 | 1.60–5.86 | 19.9 | Yes/No | Highly suggestive |
| Fraser, 2009 [59] | Serum γGT | Per 1 log unit increase | 2742/60,173 | 18 | HR | 1.92 (1.66–2.21) | 1.58 × 10−19 | 1.20–3.07 | 54.8 | No/No | Highly suggestive |
| Jia, 2013 [60] | Serum uric acid | Highest vs. lowest category | 5115/43,693 | 11 | RR | 1.60 (1.44–1.78) | 4.60 × 10−18 | 1.39–1.85 | 3.4 | No/No | Convincing |
| Kodama, 2009 [61] | Serum uric acid | Per 1 mg/dl increase | 3305/39,529 | 14 | RR | 1.17 (1.09–1.25) | 1.15 × 10−5 | 0.92–1.48 | 74.8 | Yes/Yes | Suggestive |
| Kunutsor, 2013 [25] | Serum ferritin | Highest vs. lowest category | 3391/22,948 | 9 | RR | 1.73 (1.35–2.22) | 1.23 × 10−5 | 0.84–3.56 | 58.2 | No/No | Suggestive |
| Kunutsor, 2013 | Serum AST | Highest vs. lowest category | 5985/79,958 | 11 | RR | 1.26 (1.11–1.42) | 1.98 × 10−4 | 0.89–1.78 | 56.4 | Yes/Yes | Suggestive |
| Kunutsor, 2013 | Serum AST | Per 1 SD increase | 1828/20,290 | 7 | RR | 1.13 (1.02–1.25) | 0.021 | 0.85–1.49 | 52.5 | No/Yes | Weak |
| Kunutsor, 2015 [62] | Serum osteocalcin | Highest vs. lowest category | 1673/6963 | 9 | RR | 0.43 (0.29–0.65) | 5.56 × 10−5 | 0.12–1.52 | 87.8 | Yes/Yes | Suggestive |
| Lee, 2009 [63] | Serum CRP | Highest vs. lowest category | 3920/24,914 | 16 | RR | 1.79 (1.51–2.13) | 3.30 × 10−11 | 1.03–3.11 | 53.4 | No/No | Highly suggestive |
| Li, 2009 [64] | Serum adiponectin | Per 1 log μg/ml increase | 2623/11,986 | 14 | RR | 0.72 (0.67–0.78) | 4.51 × 10−16 | 0.59–0.89 | 42.4 | No/Yes | Highly suggestive |
| Sabanayagam, 2015 [65] | Central retinal arteriolar equivalent | Per 20 μm decrease | 2581/16,190 | 5 | HR | 0.95 (0.86–1.06) | 0.369 | 0.68–1.33 | 61.6 | No/No | Not significant |
| Sabanayagam, 2015 [65] | Central retinal venular retinal equivalent | Per 20 μm increase | 2581/16,190 | 5 | HR | 1.08 (1.02–1.15) | 7.80 × 10−3 | 0.93–1.26 | 30.7 | Yes/No | Weak |
| Sing, 2015 [66] | Serum calcium | Highest vs. lowest category | 1476/32,641 | 3 | HR | 1.40 (1.11–1.75) | 4.19 × 10−3 | 0.19–10.08 | 24.6 | Yes/No | Weak |
| Song, 2013 [44] | Serum vitamin D | Highest vs. lowest category | 5142/71,115 | 21 | RR | 0.62 (0.54–0.70) | 1.44 × 10−13 | 0.46–0.83 | 19.4 | No/No | Convincing |
| Wang, 2013 [67] | Serum CRP | Per 1 log pm/ml increase | 5750/35,097 | 22 | RR | 1.26 (1.16–1.37) | 5.79 × 10−8 | 0.92–1.71 | 63.9 | No/Yes | Highly suggestive |
| Wang, 2013 [67] | Serum IL-6 | Per 1 log pm/ml increase | 4480/15,229 | 11 | RR | 1.31 (1.17–1.46) | 3.40 × 10−6 | 0.97–1.75 | 42.5 | No/Yes | Suggestive |
| Wang, 2015 [68] | Resting heart rate | Highest vs. lowest category | 10,049/169,329 | 9 | HR | 1.57 (1.29–1.92) | 6.11 × 10−6 | 0.83–2.98 | 84.3 | No/No | Suggestive |
| Wu, 2012 [69] | Serum EPA and DHA | Per 3% of total fatty acids increase | 1581/8801 | 5 | RR | 0.94 (0.75–1.17) | 0.566 | 0.50–1.76 | 40.1 | No/No | Not significant |
| Wu, 2012 [69] | Serum ALA | Per 0.1% of total fatty acids increase | 1833/11,458 | 6 | RR | 0.89 (0.79–1.01) | 0.064 | 0.69–1.14 | 17.1 | Yes/Yes | Not significant |
| Yarmolinsky, 2016 [70] | Serum PAI-1 | Highest vs. lowest category | 980/8276 | 8 | OR | 1.67 (1.28–2.18) | 1.38 × 10−4 | 0.88–3.18 | 38.2 | No/Yes | Weak |
| Dietary factors | |||||||||||
| Afshin, 2014 [71] | Nuts consumption | Per 4 servings/week increase | 13,308/216,908 | 6 | RR | 0.87 (0.81–0.93) | 9.49 × 10−5 | 0.75–1.01 | 21.1 | No/No | Suggestive |
| Alhazmi, 2012 [72] | Total protein intake | Highest vs. lowest category | 6290/201,223 | 3 | HR | 1.02 (0.90–1.17) | 0.733 | 0.35–2.99 | 19 | No/No | Not significant |
| Aune, 2009 [23] | Processed meat consumption | Highest vs. lowest category | 9999/370,607 | 9 | RR | 1.41 (1.25–1.59) | 3.03 × 10−8 | 1.01–1.98 | 52.5 | No/No | Highly suggestive |
| Aune, 2009 [23] | Processed meat consumption | Per 50 g/day increase | 9,456/362,749 | 8 | RR | 1.57 (1.28–1.93) | 1.85 × 10−5 | 0.84–2.94 | 74.1 | No/No | Suggestive |
| Aune, 2009 [23] | Total meat consumption | Highest vs. lowest category | 6525/438,798 | 5 | RR | 1.17 (0.92–1.48) | 0.193 | 0.49–2.81 | 86.9 | No/No | Not significant |
| Aune, 2009 [23] | Total meat consumption | Per 120 g/day increase | 5579/174,626 | 4 | RR | 1.26 (0.84–1.88) | 0.259 | 0.21–7.61 | 90.8 | No/No | Not significant |
| Aune, 2009 [23] | Total red meat consumption | Highest vs. lowest category | 12,226/420,844 | 10 | RR | 1.21 (1.07–1.38) | 3.08 × 10−3 | 0.83–1.76 | 58.5 | No/No | Weak |
| Aune, 2009 [23] | Total red meat consumption | Per 120 g/day increase | 10,305/387,067 | 9 | RR | 1.20 (1.04–1.38) | 0.014 | 0.76–1.87 | 68.4 | No/No | Weak |
| Aune, 2013 [73] | Dairy products | Per 400g/day increase | 21,996/319,537 | 12 | RR | 0.93 (0.87–0.99) | 0.019 | 0.81–1.07 | 31.9 | No/No | Weak |
| Aune, 2013 [73] | Dairy products | Highest vs. lowest category | 26,966/399,089 | 14 | RR | 0.89 (0.82–0.96) | 3.24 × 10−3 | 0.72–1.10 | 42.2 | No/No | Weak |
| Aune, 2013 [26] | Refined grains | Highest vs. lowest category | 9547/248,531 | 6 | RR | 0.94 (0.82–1.09) | 0.444 | 0.61–1.47 | 63.8 | Yes/No | Not significant |
| Aune, 2013 [26] | Refined grains | Per 3 servings/day increase | 9547/248,531 | 6 | RR | 0.96 (0.88–1.04) | 0.320 | 0.75–1.22 | 52.6 | No/No | Not significant |
| Aune, 2013 [26] | Whole grains | Highest vs. lowest category | 19,107/364,443 | 9 | RR | 0.74 (0.70–0.78) | 5.45 × 10−30 | 0.70–0.79 | 0 | No/No | Convincing |
| Aune, 2013 [26] | Whole grains | Per 3 servings/day increase | 19,831/366,037 | 10 | RR | 0.68 (0.57–0.81) | 1.47 × 10−5 | 0.38–1.24 | 82.5 | No/Yes | Suggestive |
| Bhupathiraju, 2014 [74] | Glycemic index | Highest vs. lowest category | 36,562/400,485 | 20 | RR | 1.12 (1.03–1.21) | 8.98 × 10−3 | 0.82–1.52 | 68.5 | No/No | Weak |
| Bhupathiraju, 2014 [74] | Glycemic load | Highest vs. lowest category | NA/NA | 30 | RR | 1.12 (1.06–1.17) | 3.07 × 10−5 | 0.96–1.29 | 26.4 | No/NA | Suggestive |
| Bi, 2015 [75] | Breakfast skipping | Yes vs. no | 7419/99,516 | 8 | RR | 1.15 (1.04–1.27) | 6.35 × 10−3 | 0.90–1.47 | 50 | No/No | Weak |
| de Souza, 2015 [76] | Total saturated fat | Highest vs. lowest category | 8739/228,715 | 8 | RR | 0.95 (0.88–1.03) | 0.206 | 0.87–1.05 | 0 | No/No | Not significant |
| de Souza, 2015 [76] | Total saturated fatty acids | Highest vs. lowest category | 9758/234,788 | 10 | RR | 1.00 (0.90–1.12) | 0.945 | 0.76–1.33 | 41.6 | No/No | Not significant |
| de Souza, 2015 [76] | Total trans fat | Highest vs. lowest category | 8690/221,445 | 6 | RR | 1.10 (0.95–1.26) | 0.216 | 0.70–1.71 | 66 | No/No | Not significant |
| de Souza, 2015 [76] | Total trans unsaturated fat | Highest vs. lowest category | 9923/227,734 | 9 | RR | 0.98 (0.82–1.18) | 0.828 | 0.54–1.77 | 78.1 | No/No | Not significant |
| de Souza, 2015 [76] | Trans palmitoleic acid | Highest vs. lowest category | 1153/11,789 | 5 | RR | 0.58 (0.46–0.74) | 1.09 × 10−5 | 0.31–1.08 | 30.8 | No/No | Suggestive |
| Ding, 2014 [77] | Coffee consumption | Highest vs. lowest category | 50,273/1,046,597 | 32 | RR | 0.70 (0.65–0.75) | 1.52 × 10−25 | 0.54–0.90 | 50.3 | No/No | Highly suggestive |
| Djousse, 2016 [78] | Eggs consumption | Highest vs. lowest category | 8911/211,068 | 12 | RR | 1.06 (0.86–1.30) | 0.610 | 0.53–2.10 | 73.6 | No/No | Not significant |
| Dong, 2012 [79] | Dietary calcium intake | Highest vs. lowest category | 11,195/253,023 | 7 | RR | 0.85 (0.75–0.97) | 0.018 | 0.59–1.23 | 53.4 | No/No | Weak |
| Esposito, 2014 [28] | Healthy dietary pattern | Highest vs. lowest category | 15,574/350,610 | 18 | RR | 0.80 (0.76–0.84) | 4.86 × 10−17 | 0.73–0.88 | 8.6 | No/No | Convincing |
| Greenwood, 2013 [80] | Glycemic index | Per 5 units/day increase | 16,419/422,326 | 15 | RR | 1.08 (1.02–1.14) | 0.013 | 0.87–1.34 | 87.6 | No/Yes | Weak |
| Greenwood, 2013 [80] | Glycemic load | Per 20 units/day increase | 24,942/486,351 | 16 | RR | 1.03 (1.00–1.05) | 0.034 | 0.96–1.10 | 52.7 | No/Yes | Weak |
| Greenwood, 2013 [80] | Carbohydrates consumption | Per 50 g/day increase | 11,976/285,117 | 8 | RR | 0.97 (0.90–1.06) | 0.514 | 0.75–1.26 | 75.5 | No/Yes | Not significant |
| Guo, 2015 [81] | Nuts consumption | Highest vs. lowest category | 11,580/251,083 | 6 | RR | 0.98 (0.84–1.15) | 0.827 | 0.61–1.58 | 67.7 | No/Yes | Not significant |
| Hu, 2012 [82] | Rice consumption | Highest vs. lowest category | 13,583/338,765 | 7 | RR | 1.27 (1.04–1.54) | 0.020 | 0.67–2.38 | 72 | No/No | Weak |
| Imamura, 2015 [24] | Artificially-sweetened beverages | Per 1 serving/day increase | 29,448/263,765 | 9 | RR | 1.07 (1.03–1.10) | 1.32 × 10−4 | 0.99–1.14 | 28.8 | No/Yes | Suggestive |
| Imamura, 2015 [24] | Fruit juice consumption | Per 1 serving/day increase | 33,172/363,805 | 12 | RR | 1.07 (1.01–1.14) | 0.031 | 0.90–1.27 | 50.9 | No/No | Weak |
| Imamura, 2015 [24] | Sugar-sweetened beverages | Per 1 serving/day increase | 38,253/426,684 | 17 | RR | 1.12 (1.06–1.20) | 2.47 × 10−4 | 0.90–1.40 | 77.2 | No/Yes | Suggestive |
| InterAct consortium, 2015 [83] | Total dietary fiber intake | Per 10 g/day increase | 57,407/326,028 | 15 | RR | 0.91 (0.87–0.96) | 3.43 × 10−4 | 0.81–1.03 | 31 | No/No | Suggestive |
| Koloverou, 2014 [84] | Mediterranean diet | Highest vs. lowest category | 19,663/115,923 | 10 | RR | 0.83 (0.74–0.93) | 2.03 × 10−3 | 0.60–1.15 | 59 | No/No | Weak |
| Kunutsor, 2013 [25] | Dietary heme iron | Highest vs. lowest category | 7708/151,415 | 3 | RR | 1.28 (1.16–1.41) | 3.35 × 10−7 | 0.69–2.37 | 0 | No/No | Highly suggestive |
| Larsson, 2007 [85] | Magnesium intake | Per 100 mg/day increase | 10,912/275,988 | 8 | RR | 0.85 (0.79–0.92) | 1.43 × 10−5 | 0.69–1.06 | 65.8 | No/Yes | Suggestive |
| Leermakers, 2016 [86] | Lutein intake | Highest vs. lowest category | 1661/33,581 | 5 | RR | 0.97 (0.77–1.22) | 0.783 | 0.50–1.89 | 48.8 | No/No | Not significant |
| Li, 2014 [87] | Vegetables consumption | Highest vs. lowest category | 20,933/269,994 | 9 | RR | 0.90 (0.80–1.01) | 0.068 | 0.64–1.27 | 66.5 | No/No | Not significant |
| Li, 2016 [88] | Alcohol consumption | Moderate drinkers vs. never drinkers | 30,436/647,388 | 25 | RR | 0.74 (0.67–0.82) | 4.86 × 10−9 | 0.49–1.10 | 74.4 | No/No | Highly suggestive |
| Liu, 2014 [89] | Flavonoids intake | Highest vs. lowest category | 18,146/266,460 | 6 | RR | 0.92 (0.87–0.98) | 6.68 × 10−3 | 0.81–1.05 | 25.8 | No/No | Weak |
| Tajima, 2014 [90] | Cholesterol intake | Highest vs. lowest category | 7589/196,314 | 6 | RR | 1.24 (1.10–1.40) | 4.93 × 10−4 | 0.91–1.68 | 41.4 | No/No | Suggestive |
| Tajima, 2014 [90] | Cholesterol intake | Per 100 mg/day increase | 6268/155,131 | 5 | RR | 1.09 (1.03–1.16) | 4.34 × 10−3 | 0.91–1.31 | 50.4 | No/No | Weak |
| Wang, 2015 [91] | Fruit consumption | Highest vs. lowest category | 33,987/474,591 | 13 | RR | 0.92 (0.87–0.97) | 1.92 × 10−3 | 0.83–1.01 | 11.2 | No/No | Weak |
| Wang, 2015 [92] | Sugar-sweetened beverages | Highest vs. lowest category | 30,005/347,941 | 9 | RR | 1.30 (1.21–1.41) | 2.31 × 10−12 | 1.14–1.49 | 12.6 | No/No | Convincing |
| Wu, 2012 [69] | Dietary ALA | Per 0.5 g/day increase | 7365/124,575 | 7 | RR | 0.93 (0.83–1.04) | 0.177 | 0.69–1.24 | 53 | No/No | Not significant |
| Wu, 2012 [69] | Dietary EPA and DHA | Per 250 mg/day increase | 23,739/500,199 | 16 | RR | 1.04 (0.97–1.10) | 0.274 | 0.82–1.31 | 81.3 | No/Yes | Not significant |
| Wu, 2012 [69] | Fish and seafood consumption | Per 100 g/day increase | 20,830/460,659 | 13 | RR | 1.12 (0.94–1.34) | 0.203 | 0.60–2.10 | 82.7 | No/No | Not significant |
| Xi, 2014 [93] | Fruit juice | Highest vs. lowest category | 19,986/355,275 | 8 | RR | 1.14 (1.03–1.27) | 0.010 | 0.89–1.47 | 43.5 | No/No | Weak |
| Yang, 2014 [94] | Tea consumption | Highest vs. lowest category | 15,488/364,344 | 12 | RR | 0.84 (0.73–0.97) | 0.014 | 0.57–1.23 | 42.5 | Yes/No | Weak |
| Yao, 2014 [95] | Total dietary fiber intake | Highest vs. lowest category | 14,973/355,422 | 12 | HR | 0.81 (0.73–0.90) | 1.04 × 10−4 | 0.60–1.09 | 53.6 | No/Yes | Suggestive |
| Zhao, 2014 [96] | Vitamin D intake | Highest vs. lowest category | 9456/178,096 | 5 | RR | 0.93 (0.85–1.01) | 0.067 | 0.81–1.06 | 0 | No/No | Not significant |
| Lifestyle and environmental factors | |||||||||||
| Aune, 2015 [97] | Leisure time physical activity | Highest vs. lowest category | 151,523/1,669,717 | 55 | RR | 0.75 (0.70–0.79) | 4.71 × 10−22 | 0.54–1.03 | 84 | Yes/Yes | Highly suggestive |
| Aune, 2015 [97] | Leisure time physical activity | Per 5 hours/week increase | 63,049/891,089 | 10 | RR | 0.75 (0.66–0.85) | 4.44 × 10−6 | 0.51–1.11 | 90 | Yes/Yes | Suggestive |
| Aune, 2015 [97] | Total physical activity | Highest vs. lowest category | 17,103/87,459 | 14 | RR | 0.65 (0.59–0.71) | 2.87 × 10−21 | 0.54–0.78 | 18.4 | Yes/No | Highly suggestive |
| Biswas, 2015 [98] | Sedentary time | Highest vs. lowest category | 6712/157,247 | 5 | HR | 1.91 (1.66–2.19) | 9.30 × 10−20 | 1.52–2.39 | 0 | No/No | Convincing |
| Capuccio, 2010 [99] | Difficulty in initiating sleep | Yes vs. no | 787/23,405 | 6 | RR | 1.57 (1.26–1.97) | 8.54 × 10−5 | 1.14–2.17 | 0 | No/No | Weak |
| Capuccio, 2010 [99] | Difficulty in maintaining sleep | Yes vs. no | 544/17,669 | 6 | RR | 1.84 (1.39–2.43) | 2.16 × 10−5 | 1.00–3.37 | 22.3 | No/No | Weak |
| Capuccio, 2010 [99] | Sleep duration | Long vs. normal | 2903/85,708 | 7 | RR | 1.48 (1.12–1.96) | 5.48 × 10−3 | 0.77–2.84 | 37.9 | No/No | Weak |
| Galling, 2016 [100] | Antipsychotics | Yes vs. no | 796/530,315 | 8 | RR | 3.02 (1.70–5.35) | 1.56 × 10−4 | 0.46–19.63 | 89.8 | No/No | Weak |
| Grontved, 2011 [101] | Television watching | Per 2 hours/day increase | 6428/169,510 | 4 | RR | 1.20 (1.14–1.27) | 5.66 × 10−11 | 0.98–1.47 | 50.3 | No/No | Highly suggestive |
| Holliday, 2013 [102] | Sleep duration | Short vs. normal | 17,660/429,464 | 12 | OR | 1.38 (1.18–1.60) | 3.23 × 10−5 | 0.96–1.97 | 33.2 | No/No | Suggestive |
| Leong, 2014 [103] | Spousal diabetes | Yes vs. no | 5689/69,809 | 4 | OR | 1.39 (1.04–1.87) | 0.026 | 0.44–4.47 | 59.6 | Yes/No | Weak |
| Pan, 2015 [52] | Passive smoking | Ever vs. never | 7843/148,596 | 7 | RR | 1.22 (1.10–1.35) | 1.21 × 10−4 | 0.97–1.54 | 31.8 | No/Yes | Suggestive |
| Pan, 2015 [52] | Smoking | Former vs. never smokers | 161,938/2,714,859 | 47 | RR | 1.14 (1.10–1.19) | 5.97 × 10−12 | 0.98–1.34 | 64 | Yes/No | Highly suggestive |
| Pan, 2015 [52] | Smoking | Current vs. never smokers | 270,705/5,580,157 | 88 | RR | 1.39 (1.33–1.44) | 6.10 × 10−65 | 1.10–1.74 | 70.2 | Yes/Yes | Highly suggestive |
| Pan, 2015 [52] | Smoking cessation | New quitters vs. never smokers | 49,457/1,046,789 | 13 | RR | 1.54 (1.36–1.75) | 2.13 × 10−11 | 0.99–2.40 | 82.5 | Yes/Yes | Highly suggestive |
| Pan, 2015 [52] | Smoking cessation | Middle-term quitters vs. never smokers | 39,130/1,033,615 | 11 | RR | 1.18 (1.07–1.29) | 5.24 × 10−4 | 0.92–1.50 | 55.8 | No/No | Suggestive |
| Pan, 2015 [52] | Smoking cessation | Long-term quitters vs. never smokers | 48,357/988,055 | 11 | RR | 1.11 (1.02–1.21) | 0.014 | 0.85–1.44 | 76.3 | Yes/No | Weak |
| Wang, 2014 [104] | NO2 | Per 10 μg/m3 increase | 5113/69,922 | 6 | RR | 1.11 (1.07–1.16) | 6.44 × 10−7 | 1.00–1.24 | 46.1 | No/Yes | Highly suggestive |
| Wang, 2014 [104] | PM10 | Per 10 μg/m3 increase | 4974/92,653 | 4 | RR | 1.34 (1.22–1.47) | 4.26 × 10−10 | 1.10–1.65 | 0 | No/No | Convincing |
| Wang, 2014 [104] | PM2.5 | Per 10 μg/m3 increase | 16,165/2,284,699 | 5 | RR | 1.39 (1.14–1.68) | 8.18 × 10−4 | 0.73–2.63 | 86.3 | No/No | Suggestive |
| Wu, 2013 [105] | Persistent organic pollutants | Highest vs. lowest category | 381/3672 | 8 | OR | 1.70 (1.23–2.35) | 1.24 × 10−3 | 0.93–3.13 | 16 | No/No | Weak |
| Zaccardi, 2015 [106] | Cardiorespiratory fitness | Per 1 metabolic equivalent increase | 8564/84,428 | 8 | HR | 0.95 (0.92–0.98) | 2.98 × 10−3 | 0.86–1.05 | 88.1 | No/Yes | Weak |
| Medical history | |||||||||||
| Aune, 2014 [107] | Breastfeeding* | Highest vs. lowest category | 10,842/263,119 | 6 | RR | 0.68 (0.57–0.82) | 3.75 × 10−5 | 0.38–1.22 | 74.7 | No/Yes | Suggestive |
| Aune, 2014 [107] | Breastfeeding* | Per 12 months increase | 10,306/261,523 | 4 | RR | 0.91 (0.86–0.96) | 7.24 × 10−4 | 0.72–1.16 | 81.1 | No/Yes | Suggestive |
| Bellamy, 2009 [37] | Gestational diabetes | Yes vs. no | 10,859/664,596 | 20 | RR | 7.43 (4.79–11.51) | 3.09 × 10−19 | 1.57–35.07 | 85.9 | No/No | Highly suggestive |
| Coto, 2013 [108] | Psoriasis | Yes vs. no | 255,203/5,393,406 | 38 | OR | 1.69 (1.50–1.89) | 1.60 × 10−19 | 0.88–3.24 | 98.1 | No/No | Highly suggestive |
| Coto, 2013 [108] | Psoriatic arthritis | Yes vs. no | 1420/15,494 | 3 | OR | 2.18 (1.36–3.48) | 1.20 × 10−3 | 0.01–395.32 | 77.2 | Yes/No | Weak |
| Ford, 2008 [38] | Metabolic syndrome | Yes vs. no | 2248/29,401 | 14 | HR | 3.35 (2.75–4.08) | 4.69 × 10−33 | 1.66–6.74 | 74.6 | Yes/No | Highly suggestive |
| Horta, 2015 [109] | Breastfeeding** | Ever vs. never | NA/NA | 11 | OR | 0.65 (0.49–0.86) | 2.66 × 10−3 | 0.31–1.37 | 52.6 | No/NA | Weak |
| Janghorbani, 2014 [110] | Age at menarche | Highest vs. lowest category | 21,095/294,333 | 12 | RR | 1.25 (1.15–1.35) | 5.77 × 10−8 | 0.99–1.58 | 66.6 | No/No | Highly suggestive |
| Li, 2014 [111] | Preterm birth | Preterm vs. normal term | 1898/29,580 | 5 | RR | 1.51 (1.33–1.72) | 4.54 × 10−10 | 1.22–1.87 | 0 | No/No | Convincing |
| Louati, 2015 [112] | Osteoarthritis | Yes vs. no | 130,457/909,718 | 20 | OR | 1.41 (1.21–1.65) | 1.36 × 10−5 | 0.81–2.47 | 95.2 | No/No | Suggestive |
| Moran, 2010 [113] | PCOS | Yes vs. no | 2337/66,727 | 13 | OR | 3.14 (1.86–5.31) | 1.80 × 10−5 | 0.86–11.49 | 55.5 | No/No | Suggestive |
| Stubbs, 2015 [114] | Schizophrenia | Yes vs. no | 131,675/2,147,884 | 26 | OR | 1.83 (1.53–2.18) | 2.63 × 10−11 | 0.79–4.20 | 98.1 | Yes/Yes | Suggestive |
| Ungprasert, 2015 [115] | Giant cell arteritis | Yes vs. no | 284/1683 | 5 | OR | 0.74 (0.57–0.96) | 0.025 | 0.49–1.13 | 0 | No/No | Weak |
| Vancampfort, 2015 [116] | Major depressive disorder | Yes vs. no | 128,807/2,123,622 | 10 | OR | 1.48 (1.28–1.71) | 8.11 × 10−8 | 0.95–2.33 | 87.2 | No/No | Highly suggestive |
| Vancampfort, 2015 [117] | Bipolar disorder | Yes vs. no | 87,168/702,464 | 5 | OR | 1.98 (1.62–2.41) | 1.14 × 10−11 | 1.01–3.86 | 76.8 | No/No | Highly suggestive |
| Wang, 2013 [118] | Obstructive sleep apnea | Yes vs. no | 422/5940 | 6 | RR | 1.63 (1.09–2.45) | 0.018 | 0.60–4.48 | 41.2 | No/Yes | Weak |
| Metabolic and anthropometric traits | |||||||||||
| Abdullah, 2010 [119] | BMI | Obese vs. lean | 16,109/574,142 | 18 | RR | 6.88 (5.39–8.78) | 4.20 × 10−54 | 2.39–19.81 | 91.1 | No/No | Highly suggestive |
| Abdullah, 2010 [119] | BMI | Overweight vs. lean | 15,796/419,466 | 17 | RR | 2.93 (2.33–3.68) | 2.80 × 10−20 | 1.11–7.76 | 90.6 | No/No | Highly suggestive |
| Bell, 2014 [120] | Metabolically healthy obesity | Metabolically healthy obese vs. metabolically healthy non-obese | 1285/26,196 | 10 | RR | 4.40 (2.83–6.84) | 4.97 × 10−11 | 1.29–14.95 | 47.8 | No/No | Convincing |
| Bell, 2014 [120] | Metabolically healthy obesity | Metabolically unhealthy obese vs. metabolically healthy non-obese | 1266/24,668 | 8 | RR | 9.50 (7.48–12.08) | 8.79 × 10−76 | 7.05–12.82 | 0 | Yes/No | Highly suggestive |
| Harder, 2007 [121] | Birth weight | >4,000 g vs. <4,000 g | 6005/108,400 | 9 | OR | 1.27 (1.01–1.59) | 0.044 | 0.62–2.58 | 68.2 | No/No | Weak |
| Harder, 2007 [121] | Birth weight | >2,500g vs. <2,500g | 5815/100,759 | 10 | OR | 1.32 (1.06–1.64) | 0.014 | 0.71–2.43 | 60.8 | No/No | Weak |
| Janghorbani, 2012 [122] | Height | Highest vs. lowest category | 2858/66,199 | 17 | OR | 0.85 (0.76–0.96) | 6.65 × 10−3 | 0.58–1.25 | 61.3 | Yes/Yes | Weak |
| Janghorbani, 2012 [122] | Hip circumference | Highest vs. lowest category | 5415/169,924 | 18 | OR | 0.57 (0.48–0.68) | 6.72 × 10−10 | 0.32–1.05 | 62.9 | No/No | Highly suggestive |
| Kodama, 2012 [123] | BMI | Per 1 SD increase | 10,043/132,442 | 15 | RR | 1.59 (1.40–1.80) | 3.99 × 10−13 | 0.95–2.65 | 94.3 | No/Yes | Highly suggestive |
| Kodama, 2012 [123] | Waist circumference | Per 1 SD increase | 10,043/132,442 | 15 | RR | 1.66 (1.47–1.88) | 1.14 × 10−15 | 1.00–2.76 | 94.5 | No/Yes | Highly suggestive |
| Kodama, 2012 [123] | Waist-height ratio | Per 1 SD increase | 10,043/132,442 | 15 | RR | 1.67 (1.46–1.90) | 3.68 × 10−14 | 0.97–2.87 | 94.2 | No/Yes | Highly suggestive |
| Kodama, 2012 [123] | Waist-to-hip ratio | Per 1 SD increase | 10,043/132,442 | 15 | RR | 1.54 (1.36–1.75) | 1.86 × 10−11 | 0.93–2.56 | 93.7 | No/Yes | Highly suggestive |
| Kodama, 2014 [124] | Weight gain in early adulthood | Per 5 kg/m2 increase | 15,701/327,002 | 10 | RR | 3.07 (2.49–3.80) | 1.92 × 10−25 | 1.39–6.78 | 98.2 | No/No | Highly suggestive |
| Kodama, 2014 [124] | Weight gain after the age of 25 years | Per 5 kg/m2 increase | 13,364/294,135 | 15 | RR | 2.12 (1.74–2.58) | 5.03 × 10−14 | 1.07–4.20 | 75.1 | Yes/No | Highly suggestive |
| Whincup, 2008 [125] | Birth weight | Per 1 kg increase | 6090/145,994 | 31 | OR | 0.80 (0.72–0.88) | 1.84 × 10−5 | 0.52–1.21 | 66.5 | No/No | Suggestive |
| Agardh, 2011 [36] | Educational status | Lowest vs. highest category | 20,649/234,796 | 23 | RR | 1.41 (1.28–1.55) | 1.01 × 10−12 | 1.02–1.96 | 65.5 | Yes/No | Highly suggestive |
| Agardh, 2011 [36] | Income level | Lowest vs. highest category | 1837/19,049 | 7 | RR | 1.40 (1.04–1.88) | 0.029 | 0.56–3.47 | 72 | Yes/Yes | Weak |
| Agardh, 2011 [36] | Occupation | Lowest vs. highest category | 2691/42,476 | 11 | RR | 1.31 (1.09–1.57) | 3.69 × 10−3 | 0.77–2.21 | 52.7 | No/No | Weak |
| Huang, 2015 [126] | Adverse childhood experience | Yes vs. no | 3481/83,770 | 7 | OR | 1.28 (1.05–1.55) | 0.014 | 0.76–2.16 | 60.9 | No/No | Weak |
| Jokela, 2014 [35] | Agreeableness | Per 1 SD increase in personality score | 1845/33,058 | 5 | OR | 1.05 (0.98–1.13) | 0.193 | 0.85–1.30 | 40.6 | No/No | Not significant |
| Jokela, 2014 [35] | Conscientiousness | Per 1 SD increase in personality score | 1845/33,058 | 5 | OR | 0.86 (0.82–0.91) | 9.94 × 10−8 | 0.79–0.94 | 0 | No/No | Convincing |
| Jokela, 2014 [35] | Extraversion | Per 1 SD increase in personality score | 1845/33,058 | 5 | OR | 1.01 (0.94–1.09) | 0.742 | 0.84–1.22 | 32.5 | No/No | Not significant |
| Jokela, 2014 [35] | Neuroticism | Per 1 SD increase in personality score | 1845/33,058 | 5 | OR | 1.06 (1.00–1.13) | 0.062 | 0.91–1.24 | 26.7 | No/No | Not significant |
| Jokela, 2014 [35] | Openness | Per 1 SD increase in personality score | 1845/33,058 | 5 | OR | 0.96 (0.85–1.08) | 0.453 | 0.62–1.46 | 77.7 | No/No | Not significant |
| Kivimaki, 2015 [127] | Working hours | Long vs. standard working hours | 4963/217,157 | 23 | RR | 1.09 (0.91–1.30) | 0.366 | 0.58–2.04 | 53.3 | No/No | Not significant |
| Nyberg, 2014 [128] | Job strain | Highest vs. lowest category | 3703/121,105 | 13 | HR | 1.15 (1.06–1.25) | 1.46 × 10−3 | 1.04–1.27 | 0 | No/No | Weak |
γGT: gamma-glutamyl transferase, ALA: α-linolenic acid, ALT: alanine aminotransferase, AST: aspartate aminotransferase, BMI: body mass index, CI: confidence interval, CRP: C-reactive protein, DHA: docosahexaenoic acid, EPA: eicosapentaenoic acid, HR: hazard ratio, IL-6: interleukin-6, NA: not available, NO2: nitrogen dioxide, OR: odds ratio, PAI-1: plasminogen activator inhibitor-1, PCOS: polycystic ovary syndrome, PM2,5: particulate matter with a diameter of 2,5 μm or less, PM10: particulate matter with a diameter between 2,5 and 10 μm, RR: risk ratio, SD: standard error
*maternal risk for T2DM
**offspring risk for T2DM
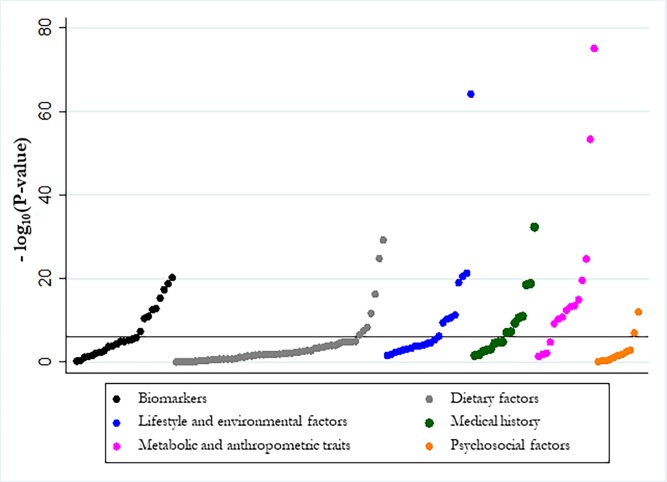
Fig 2. Manhattan plot for 142 associations between risk factors and T2DM.
The horizontal line corresponds to the significance threshold of P <10−6.
Thirty-eight associations (27%) were very heterogeneous (I2 >75%), and 50 associations (35%) had large heterogeneity estimates (I2 ≥50% and I2 ≤75%). The Egger’s test was statistically significant in 32 meta-analyses (23%), and 27 of them presented evidence for small-study effects. Thirty-nine meta-analyses (28%) had evidence for excess significance bias.
Assessment of epidemiological credibility
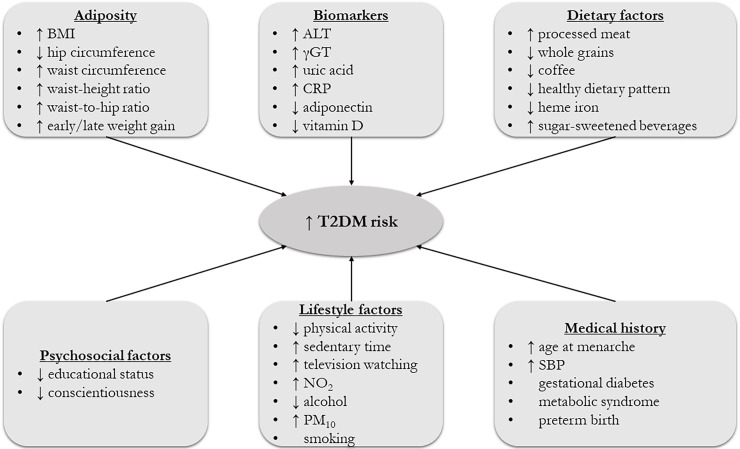
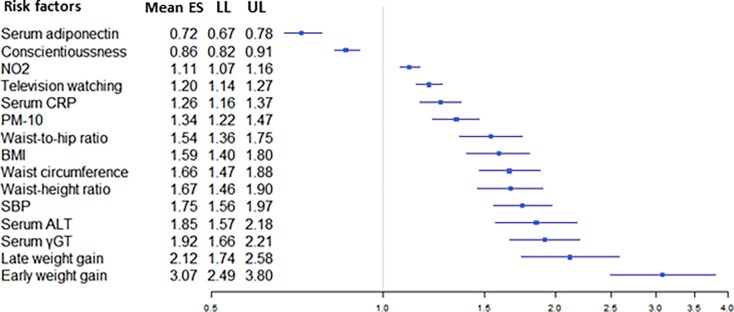
Eleven associations (8%) presented convincing evidence (>1,000 cases, P <10−6, not large between-study heterogeneity, 95% PI excluding the null value, no evidence for small-study effects and excess significance bias) for risk of T2DM. Low whole grains consumption, metabolically healthy obesity, increased sedentary time, low adherence to a healthy dietary pattern, high level of serum uric acid, low level of serum vitamin D, decreased conscientiousness, preterm birth, high consumption of sugar-sweetened beverages, high level of serum ALT, and exposure to high level of PM10 were associated with increased risk for T2DM and supported by convincing evidence.
Thirty-four associations (24%) were supported by highly suggestive evidence. The associations that were linked with a higher risk for T2DM and presented highly suggestive evidence were the following: high BMI (obese vs. lean, overweight vs. lean, and per 1 SD increase), low educational status, gestational diabetes, increased processed meat consumption, high level of total and leisure-time physical activity, metabolically unhealthy obesity, psoriasis, low coffee consumption, high systolic blood pressure, high level of serum gamma-glutamyl transferase (highest vs. lowest category, and per 1 log unit increase), metabolic syndrome, increased time of television watching, low hip circumference, late age at menarche, weight gain in early adulthood, weight gain after the age of 25 years, increased dietary heme iron intake, high level of serum C-reactive protein (highest vs. lowest category, and per 1 log pm/mL), low level of serum adiponectin (per 1 log μg/ml increase), low alcohol consumption, smoking (former vs. never smokers, and current vs. never smokers), smoking cessation (new quitters vs. never smokers), major depressive disorder, bipolar disorder, high waist-height ratio, high waist circumference, high waist-to-hip ratio, and exposure to high level of NO2 (per 10 μg/m3 increase). Twenty-nine associations had suggestive evidence (20%), and 42 associations had weak evidence (30%) for risk of T2DM.
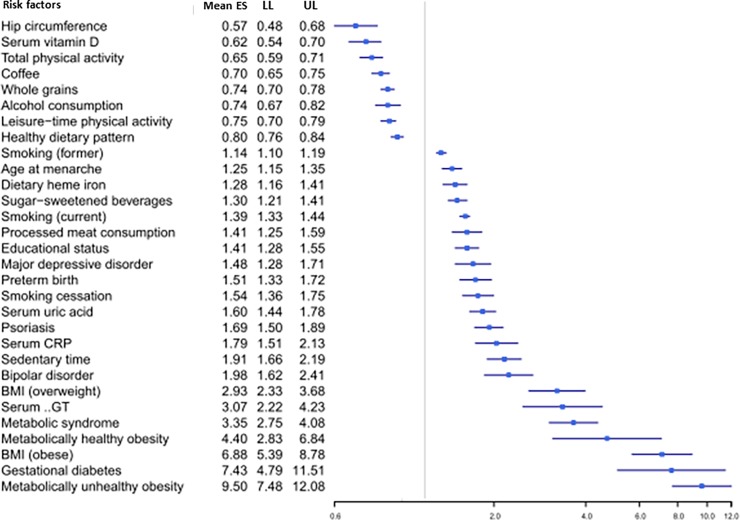
All but 6 associations with convincing or highly suggestive evidence were exclusively based on prospective cohort studies, case-cohort studies and/or nested case-control studies. The remaining six associations (i.e., sedentary time, psoriasis, hip circumference, age at menarche, bipolar disorder, and major depressive disorder) were based on a combination of cross-sectional studies and cohort studies. In a sensitivity analysis limited to prospective cohort studies, the associations for sedentary time, hip circumference, and age at menarche remained highly suggestive (Table 2). For psoriasis, the level of evidence became suggestive due to a P-value greater than 10−6 under random-effects model (Table 2). In the meta-analysis for bipolar disorder, no prospective cohort studies were included. In the meta-analysis for major depressive disorder, only 1 retrospective cohort study was included. All the risk factors with convincing and highly suggestive evidence are summarized in Fig 3, and they are graphically presented using forest plots in Figs 4 and 5.
Table 2. Sensitivity analysis of prospective cohort studies for associations with convincing or highly suggestive evidence that were based on a combination of cross-sectional and cohort studies.
| Reference | Risk factor | Level of comparison | Number of datasets | Number of cases/controls | Effect size metric | Random-effects summary effect size (95% CI) | P random | 95% prediction interval | I2 |
|---|---|---|---|---|---|---|---|---|---|
| Biswas, 2015 [98] | Sedentary time | Highest vs. lowest category | 4 | 6428/151,290 | HR | 1.88 (1.63–2.17) | 1.52 × 10−17 | 1.37–2.58 | 0 |
| Coto, 2013 [108] | Psoriasis | Yes vs. no | 8 | 49,064/1,564,468 | OR | 1.53 (1.29–1.81) | 1.15 × 10−6 | 0.83–2.80 | 96.7 |
| Janghorbani, 2012 [122] | Hip circumference | Highest vs. lowest category | 11 | 4460/137,666 | OR | 0.63 (0.53–0.75) | 3.76 × 10−7 | 0.39–1.01 | 50.4 |
| Janghorbani, 2014 [110] | Age at menarche | Highest vs. lowest category | 9 | 20,092/289,532 | RR | 1.26 (1.15–1.38) | 5.44 × 10−7 | 0.96–1.64 | 72.7 |
CI: confidence interval, HR: hazard ratio, OR: odds ratio, RR: risk ratio
Fig 3. Schematic representation of risk factors for T2DM with convincing or highly suggestive evidence.
The symbol ↑ denotes a higher exposure to a risk factor, and the symbol ↓ represents a lower exposure to a risk factor. For alcohol consumption, never drinkers presented a higher risk for T2DM than moderate drinkers.
Fig 4. Forest plot of risk factors (measured as continuous variables) for T2DM supported by convincing or highly suggestive evidence.
Fig 5. Forest plot of risk factors (measured as dichotomous variables) for T2DM supported by convincing or highly suggestive evidence.
Mendelian randomization studies
We identified 22 MR studies assessing the causal effect of a risk factor that was included in our umbrella review (Table 3). The median number of T2DM cases was 4,407 (IQR, 1,164–15,255). Two MR studies used a single SNP as instrumental variable and twenty MR studies constructed a polygenic risk score (PRS). In studies with PRS, the median number of variants was 5 (IQR, 3–8). The eligible MR studies assessed the following 13 exposures: alcohol intake, birth weight, BMI, coffee intake, milk intake, systolic blood pressure, serum adiponectin, serum CRP, serum ferritin, serum gamma-glutamyl transferase, serum uric acid, serum vitamin D, and waist circumference. Seven risk factors were examined by more than one MR study.
Table 3. Characteristics of mendelian randomization studies for type 2 diabetes mellitus.
| Reference | Exposure | Level of comparison | Genetic instrument | N of SNPs in instrument | N cases | Effect size metric | Causal effect size (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|
| Holmes, 2014 [29] | Alcohol intake | Per units/week increase | Single variant (rs1229984) | 1 | 14,549 | OR | 1.02 (0.95–1.09) | 0.627 |
| Wang, 2016 [54] | Birth weight | Per 1 SD decrease | PRS | 5 | 3627 | OR | 2.94 (1.70–5.16) | <0.001 |
| Afzal, 2014 [31] | BMI | Per 10 kg/m2 increase | PRS | 3 | 5037 | HR | 19.40 (6.40–59.10) | NR |
| Corbin, 2016 [32] | BMI | Per 1 kg/m2 increase | PRS | 96 | 12,171 | OR | 1.39 (1.14–1.68) | 0.002 |
| Fall, 2013 [33] | BMI | Per 1 kg/m2 increase | Single variant (rs9939609) | 1 | 1991 | OR | 1.35 (1.12–1.62) | 0.001 |
| Holmes, 2014 [34] | BMI | Per 1 kg/m2 increase | PRS | 14 | 4407 | OR | 1.27 (1.18–1.36) | 2.0 × 10−11 |
| Nordestgaard, 2015 [129] | Coffee intake | Per 1 cup/day | PRS | 5 | 26,632 | OR | 1.00 (0.99–1.01) | NR |
| Bergholdt, 2015 [130] | Milk intake | Per 1 glass/week increase | Single variant (rs4988235) | 1 | 951 | OR | 0.99 (0.93–1.06) | NR |
| Aikens, 2016 [131] | SBP | Per 1 mmHg increase | PRS | 13 | 37,293 | OR | 1.02 (1.01–1.03) | 9.1 × 10−5 |
| Marott, 2016 [132] | SBP | Per 1 mmHg increase | PRS | 6 | 2859 | OR | 0.97 (0.95–1.00) | 0.030 |
| Peters, 2013 [50] | Serum adiponectin | Per 1 SD decrease | PRS | 3 | 967 | OR | 0.86 (0.75–0.99) | 0.013 |
| Yaghootkar, 2013 [51] | Serum adiponectin | Per 1 SD decrease | PRS | 3 | 2777 | OR | 0.94 (0.75–1.19) | 0.610 |
| Yaghootkar, 2013 [51] | Serum adiponectin | Per 1 SD decrease | PRS | 3 | 15,960 | OR | 0.99 (0.95–1.04) | 0.770 |
| Prins, 2016 [40] | Serum CRP | Per 10-s% increase | PRS | 4 | 6698 | OR | 1.11 (0.94–1.32) | 0.230 |
| Prins, 2016 [40] | Serum CRP | Per 10-s% increase | PRS | 18 | 6698 | OR | 1.09 (0.95–1.24) | 0.210 |
| Gan, 2012 [133] | Serum ferritin | Per 1 ng/mL increase | Single variant (rs855791) | 1 | 272 | OR | 0.80 (0.65–0.98) | 0.031 |
| Gan, 2012 [133] | Serum ferritin | Per 1 ng/mL increase | Single variant (rs4820268) | 1 | 272 | OR | 0.80 (0.66–0.98) | 0.031 |
| Lee, 2016 [134] | Serum gamma-glutamyl transferase | Per 1 unit increase | PRS | 7 | 343 | OR | 1.05 (1.01–1.08) | NR |
| Kleber, 2015 [41] | Serum uric acid | Per 1 mg/dl increase | PRS | 8 | 1236 | OR | 0.83 (0.57–1.23) | 0.360 |
| Pfister, 2011 [42] | Serum uric acid | Per 1 mg/dl increase | PRS | 8 | 7504 | OR | 0.99 (0.94–1.04) | 0.620 |
| Slujis, 2015 [43] | Serum uric acid | Per 1 mg/dl increase | PRS | 24 | 41,508 | HR | 0.99 (0.92–1.06) | NR |
| Afzal, 2014 [31] | Serum vitamin D | Per 20 nmol/L decrease | PRS | 2 | 5037 | HR | 1.51 (0.98–2.33) | 0.240 |
| Afzal, 2014 [31] | Serum vitamin D | Per 20 nmol/L decrease | PRS | 2 | 5037 | HR | 1.02 (0.75–1.37) | 0.390 |
| Buijsse, 2013 [45] | Serum vitamin D | Per 5 nmol/L increase | PRS | 4 | 1572 | HR | 0.98 (0.89–1.08) | NR |
| Jorde, 2012 [46] | Serum vitamin D | Highest vs. lowest quartile | PRS | 5 | 1092 | HR | 1.01 (0.86–1.20) | NR |
| Leong, 2014 [47] | Serum vitamin D | Per 1 SD increase | Single variant (rs2282679) | 1 | 201 | OR | 0.99 (0.79–1.24) | 0.930 |
| Ye, 2015 [48] | Serum vitamin D | Per 1 SD increase | PRS | 4 | 28,144 | OR | 1.01 (0.75–1.36) | 0.940 |
| Marott, 2016 [132] | Waist circumference | Per 1 unit increase | PRS | 5 | 3762 | OR | 1.05 (1.01–1.10) | 0.020 |
BMI: body mass index, CI: confidence interval, CRP: C-reactive protein, HR: hazard ratio, NR: not reported, OR: odds ratio, PRS: polygenic risk score, SBP: systolic blood pressure, SD: standard deviation. SNPs: single nucleotide polymorphisms
A causal effect was claimed for 4 risk factors graded as highly suggestive in our umbrella review: BMI, systolic blood pressure, serum gamma-glutamyl transferase, and waist circumference. A causal association was also claimed for birth weight, but a relatively small number of T2DM cases was included in this analysis. The observed effects for alcohol intake, coffee intake, serum CRP, serum ferritin, serum uric acid and serum vitamin D were not causal. Milk intake presented weak evidence in our analysis and an MR study did not show a causal effect. Serum adiponectin was graded as highly suggestive in our analysis, but the findings from MR studies were conflicting, and the largest MR study indicated absence of a causal effect.
Discussion
We performed a mapping of environmental factors and biomarkers examined for an association with T2DM in systematic reviews and meta-analyses. Overall, more than 100 associations were considered. We identified eleven associations supported by convincing evidence and thirty-four additional associations having highly suggestive evidence for risk of T2DM. These associations mainly pertained to comorbid medical conditions, lifestyle and dietary factors, as well as serum biomarkers.
Even though more than one third of the associations examined various dietary factors, only six of them showed convincing or highly suggestive relationship with T2DM and the demonstrated effect sizes were modest. These factors were processed meat, whole grain products, healthy dietary pattern, sugar-sweetened beverages and dietary heme iron. Increased processed meat and sugar-sweetened beverages consumption are linked with other unhealthy lifestyle factors which showed highly significant association with T2DM, such as physical inactivity, increased BMI, smoking and unhealthy dietary patterns. [23,24] The association between dietary heme iron and T2DM could be explained by the fact that red meat is the main dietary source of heme iron. [25] The observed protective effect of whole grain products is independent of BMI as almost all observational studies have adjusted for its effect. Whole grain products have high concentration of fibers, which delay gastric emptying, therefore slowing glucose release in circulation. This results in reduced postprandial insulin response and could improve insulin sensitivity [26,27] The aforementioned associations are also supported by the observed protective effect of healthy dietary pattern against developing T2DM. Although the term “healthy dietary pattern” includes a variety of diets, the same principles apply: reduced red and processed meat consumption, moderate alcohol drinking, low intake of sugar-sweetened beverages and increased consumption of whole grain products. [28]
Moderate alcohol consumption has a protective effect against developing T2DM. This relationship could be explained by increased insulin sensitivity, lower fasting insulin resistance and lower glycated hemoglobin concentrations, which are induced by moderate amounts of alcohol. Moreover, moderate amount of alcohol drinking is a common feature of healthy diet pattern, who also lowered the risk for developing T2DM. Furthermore, coffee consumption lowers the risk for T2DM, which is attributed to the reduction of insulin resistance and the improvement of glucose metabolism. However, it is unclear whether this association is causal, given the findings of a recently published MR study [29].
Most of the associations yielded from our analyses were proxies of obesity and include body mass index (BMI), weight gain, and anthropometric characteristics (i.e., hip circumference, waist-height ratio, waist-hip ratio, waist circumference). The observed association between BMI and T2DM demonstrated a large effect size and was highly significant (RR = 6.88, P = 4.2 × 10−54). Increased BMI, waist-height ratio, waist-hip ratio and waist circumference express the presence of increased intra-abdominal visceral fat, which disrupts insulin metabolism through release of serum free fatty acids. [30] Not surprisingly, findings from MR studies further support a causal role of BMI in the pathogenesis of T2DM. [31–34] However, not all obese have the same risk for developing T2DM; it seems that the risk is affected by their metabolic profile. Metabolically unhealthy obese carry an about 10-fold risk for T2DM, whereas metabolically healthy obese have an about 4.5-fold risk for T2DM. Moreover, weight gain during early adulthood was more harmful than weight gain after the age of 25. On the contrary, peripheral fat accumulation has been linked to a better metabolic profile, which is depicted in the observed protective effect of larger hip circumference on T2DM.
Several lifestyle factors presented either convincing or highly suggestive evidence. Total and leisure-time physical activity lowered the relative risk for T2DM. High sedentary time and TV watching are inter-correlated, and they are surrogates of physical inactivity, which is a common feature in people with high BMI. Additionally, the convincing association of low conscientiousness with increased risk for T2DM could be explained by the correlation of this personality trait with physical inactivity and high risk for obesity. [35] Our analysis also indicated that there is highly suggestive evidence for the association of lower educational attainment and higher risk for T2DM. Educational level constitutes a component of socioeconomic status. Lower socioeconomic status is associated with higher stress levels, leading to disruption in endocrine function through perturbations in the neuroendocrine system. [36] Also, people with low socioeconomic status are more prone to an unhealthy lifestyle pattern and they have limited access to healthcare care facilities. [36]
Several medical conditions have been traditionally linked to increased risk for T2DM. Patients with metabolic syndrome and gestational diabetes presented higher risk for T2DM. The seven-fold increase of risk for developing T2DM in women with gestational diabetes could be attributed to common underlying genetic and environmental risk factors between the two conditions. [37] Metabolic syndrome is considered a predictor of T2DM and has a stronger association with T2DM than its components. [38] Furthermore, higher systolic blood pressure was associated with increased risk for T2DM, but this association might not be causal. Some antihypertensive drugs have been associated with an increased risk, whereas the use of antihypertensive drugs inhibiting the renin-angiotensin system showed a protective effect. In turn, increased activity of renin-angiotensin system induces systemic inflammation processes that may exert a diabetogenic effect. [39]
Our analysis showed that a set of serum biomarkers is highly associated with the risk for T2DM. These biomarkers pertained to serum level of alanine aminotransferase (ALT), gamma-glutamyl transferase, C-reactive protein (CRP), uric acid, adiponectin, and vitamin D. High serum ALT and gamma-glutamyl transferase in patients with T2DM could be a manifestation of ongoing low-grade hepatic inflammation or hepatocellular damage, which is common in T2DM and metabolic syndrome. Among hepatic enzymes, ALT is the most specific indicator of hepatic pathology in non-alcoholic fatty liver disease and most closely related to liver fat accumulation. The presence of systemic inflammation is linked to β-cell dysfunction, leading to impaired glucose metabolism and the development of T2DM. [4] Both CRP and uric acid are inflammatory markers associated with systemic inflammation. Also, meat consumption is directly associated with serum uric acid level, and, as we have already shown, processed meat consumption is linked to higher risk for T2DM. However, MR studies for serum CRP [40], and serum uric acid [41–43] suggested that the associations with T2DM might not be causal.
Furthermore, our results indicated an inverse association between vitamin D level and risk for T2DM. It is unclear if this is a true association or the effect of adiposity as a potential confounder or intermediate factor. Obesity leads to storage of vitamin D in adipose tissue and to less sun exposure, on the grounds of limited mobility and accumulation of subcutaneous fat [44]. All the former result in low circulating level of vitamin D in obese individuals. Also, vitamin D may directly affect adiposity and other metabolic parameters, such as dyslipidemia, hypertension, and systemic inflammation, that mediate the pathway from vitamin D status to T2DM. Adiponectin is another serum biomarker that expresses the body composition. It affects the glucose metabolism, and higher serum level of adiponectin are associated with higher insulin sensitivity. However, MR studies examining the role of serum vitamin D indicated a non-causal association that might be explained by confounding factors [31,45–48], whereas the evidence on the causal role of serum adiponectin are contradictory [49–51].
The association between smoking and T2DM has biological foundation because smoking is associated with central obesity and increased oxidative stress and inflammation, and eventually leads to insulin resistance and hyperglycaemia. However, residual confounding can be the case since smoking is often linked to other unhealthy lifestyle factors (e.g., poor diet, physical inactivity) and comorbidities. The increased risk of T2DM associated with smoking cessation in new quitters could be mediated by weight gain or be due to reverse causation because people who try to quit smoking are more likely to have preclinical conditions or high cumulative smoking exposure [52].
Based on our assessment, adults delivered preterm presented a larger risk for development of T2DM during adulthood than adults delivered full-term. According to the “fetal origin of disease” hypothesis, the biological mechanisms that mediate this association could be explained through intrauterine growth restriction. Preterm newborns have low birth weight and they are prone to disrupted glucose metabolism in later life [53], which in turn predisposes to an increased risk of T2DM. Although the association between birth weight and T2DM had weak epidemiological credibility [12], an MR study indicated that there is a potential causal association between birth weight and risk for T2DM [54].
Two components of ambient air pollution, PM10 and NO2, were found to have robust association with risk for T2DM. It has been suggested that air pollution causes elevated systemic inflammation and oxidative stress, whereas it increases the insulin resistance leading to abnormal glucose metabolism and elevated fasting glucose. [55]
Furthermore, older age at menarche was associated with risk for T2DM. However, there are doubts whether it constitutes a genuine association. Observational studies found that this association is attenuated after adjustment for BMI in adulthood, suggesting that adult adiposity may mediate this association. The inverse association between age at menarche and BMI in adulthood could explain this finding. [56]
We presented an exposure-wide mapping of the meta-analyses on non-genetic risk factors for T2DM. Our umbrella review indicated that a very wide range of risk factors has been considered for T2DM. Compared to previously published umbrella reviews [6–10], there is tremendous amount of meta-analyses for risk factors of T2DM. Also, the majority of these associations were examined in large prospective cohort studies. The increasing incidence and large burden of T2DM could explain the observed interest in the field of non-genetic and modifiable risk factors for T2DM.
Our study has some caveats. First, the statistical test for small-study effects should be interpreted with caution in case of large between-study heterogeneity. Second, the observational studies did not often clearly report the sample sizes for the statistical analyses. Thus, the power calculations might be conservative, and the extent of excess significance bias is probably conservative. Furthermore, genetic instruments of the MR studies were not assessed, and power calculations for the MR studies could not be performed, because the percentage of variance explained often was not available. Consequently, the claims of MR studies should be interpreted with caution.
Conclusions
Our paper identified several robust risk factors for T2DM. Our findings indicate specific strategies for public health interventions to reduce the future incidence of T2DM. Interventions for the promotion of physical activity and a healthy lifestyle and dietary pattern combined with interventions against the increased incidence of obesity could alleviate the projections for an increase of T2DM incidence in near future. However, these findings are based on observational data and should be interpreted with caution. Even though MR studies may support or not causality, the power of those studies could not be assessed. Therefore, randomized clinical trials and additional well-designed MR studies are needed to clarify which of these observations are causal associations. Also, these findings should be replicated by large-scale environment-wide association studies in various ethnic groups, and they could be used for the development of reliable risk prediction models in combination with known genetic polymorphisms.
Supporting information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. lancet Diabetes Endocrinol. 2014;2: 56–64. doi: 10.1016/S2213-8587(13)70112-8 [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetes Atlas. 7th ed. Brussels; 2015.
- 3.Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L. The many faces of diabetes: a disease with increasing heterogeneity. Lancet (London, England). 2014;383: 1084–94. doi: 10.1016/S0140-6736(13)62219-9 [DOI] [PubMed] [Google Scholar]
- 4.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet (London, England). 2014;383: 1068–83. doi: 10.1016/S0140-6736(13)62154-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ioannidis JPA. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ. 2009;181: 488–93. doi: 10.1503/cmaj.081086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belbasis L, Bellou V, Evangelou E, Ioannidis JPA, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14: 263–73. doi: 10.1016/S1474-4422(14)70267-4 [DOI] [PubMed] [Google Scholar]
- 7.Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Ioannidis JPA. Environmental risk factors and Parkinson’s disease: An umbrella review of meta-analyses. Parkinsonism Relat Disord. 2016;23: 1–9. doi: 10.1016/j.parkreldis.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 8.Belbasis L, Bellou V, Evangelou E. Environmental Risk Factors and Amyotrophic Lateral Sclerosis: An Umbrella Review and Critical Assessment of Current Evidence from Systematic Reviews and Meta-Analyses of Observational Studies. Neuroepidemiology. 2016;46: 96–105. doi: 10.1159/000443146 [DOI] [PubMed] [Google Scholar]
- 9.Bellou V, Belbasis L, Tzoulaki I, Middleton LT, Ioannidis JPA, Evangelou E. Systematic evaluation of the associations between environmental risk factors and dementia: An umbrella review of systematic reviews and meta-analyses. Alzheimers Dement. 2016; doi: 10.1016/j.jalz.2016.07.152 [DOI] [PubMed] [Google Scholar]
- 10.Belbasis L, Stefanaki I, Stratigos AJ, Evangelou E. Non-genetic risk factors for cutaneous melanoma and keratinocyte skin cancers: An umbrella review of meta-analyses. J Dermatol Sci. 2016; doi: 10.1016/j.jdermsci.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 11.Belbasis L, Köhler CA, Stefanis N, Stubbs B, van Os J, Vieta E, et al. Risk factors and peripheral biomarkers for schizophrenia spectrum disorders: an umbrella review of meta-analyses. Acta Psychiatr Scand. 2018;137: 88–97. doi: 10.1111/acps.12847 [DOI] [PubMed] [Google Scholar]
- 12.Belbasis L, Savvidou MD, Kanu C, Evangelou E, Tzoulaki I. Birth weight in relation to health and disease in later life: an umbrella review of systematic reviews and meta-analyses. BMC Med. 2016;14: 147 doi: 10.1186/s12916-016-0692-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7: 177–88. [DOI] [PubMed] [Google Scholar]
- 14.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127: 820–6. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172: 137–159. doi: 10.1111/j.1467-985X.2008.00552.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37: 1158–60. doi: 10.1093/ije/dyn204 [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21: 1539–58. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 18.Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343: d4002 doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ioannidis JPA, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. 2007;4: 245–53. doi: 10.1177/1740774507079441 [DOI] [PubMed] [Google Scholar]
- 21.Ioannidis JPA. Clarifications on the application and interpretation of the test for excess significance and its extensions. J Math Psychol. 2013;57: 184–187. doi: 10.1016/j.jmp.2013.03.002 [Google Scholar]
- 22.Lubin JH, Gail MH. On power and sample size for studying features of the relative odds of disease. Am J Epidemiol. 1990;131: 552–66. [DOI] [PubMed] [Google Scholar]
- 23.Aune D, Ursin G, Veierød MB. Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Diabetologia. 2009;52: 2277–87. doi: 10.1007/s00125-009-1481-x [DOI] [PubMed] [Google Scholar]
- 24.Imamura F, O’Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351: h3576 Available: http://www.ncbi.nlm.nih.gov/pubmed/26199070 doi: 10.1136/bmj.h3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunutsor SK, Apekey TA, Walley J, Kain K. Ferritin levels and risk of type 2 diabetes mellitus: an updated systematic review and meta-analysis of prospective evidence. Diabetes Metab Res Rev. 2013;29: 308–318. doi: 10.1002/dmrr.2394 [DOI] [PubMed] [Google Scholar]
- 26.Aune D, Norat T, Romundstad P, Vatten LJ. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol. 2013;28: 845–58. doi: 10.1007/s10654-013-9852-5 [DOI] [PubMed] [Google Scholar]
- 27.Fung TT, Hu FB, Pereira MA, Liu S, Stampfer MJ, Colditz GA, et al. Whole-grain intake and the risk of type 2 diabetes: a prospective study in men. Am J Clin Nutr. 2002;76: 535–40. Available: http://www.ncbi.nlm.nih.gov/pubmed/12197996 [DOI] [PubMed] [Google Scholar]
- 28.Esposito K, Chiodini P, Maiorino MI, Bellastella G, Panagiotakos D, Giugliano D. Which diet for prevention of type 2 diabetes? A meta-analysis of prospective studies. Endocrine. 2014;47: 107–116. doi: 10.1007/s12020-014-0264-4 [DOI] [PubMed] [Google Scholar]
- 29.Holmes M V, Dale CE, Zuccolo L, Silverwood RJ, Guo Y, Ye Z, et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ. 2014;349: g4164 Available: http://www.ncbi.nlm.nih.gov/pubmed/25011450 doi: 10.1136/bmj.g4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106: 473–81. doi: 10.1172/JCI10842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Afzal S, Brøndum-Jacobsen P, Bojesen SE, Nordestgaard BG. Vitamin D concentration, obesity, and risk of diabetes: a mendelian randomisation study. lancet Diabetes Endocrinol. 2014;2: 298–306. doi: 10.1016/S2213-8587(13)70200-6 [DOI] [PubMed] [Google Scholar]
- 32.Corbin LJ, Richmond RC, Wade KH, Burgess S, Bowden J, Smith GD, et al. BMI as a Modifiable Risk Factor for Type 2 Diabetes: Refining and Understanding Causal Estimates Using Mendelian Randomization. Diabetes. 2016;65: 3002–7. doi: 10.2337/db16-0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fall T, Hägg S, Mägi R, Ploner A, Fischer K, Horikoshi M, et al. The role of adiposity in cardiometabolic traits: a Mendelian randomization analysis. PLoS Med. 2013;10: e1001474 doi: 10.1371/journal.pmed.1001474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmes M V, Lange LA, Palmer T, Lanktree MB, North KE, Almoguera B, et al. Causal effects of body mass index on cardiometabolic traits and events: a Mendelian randomization analysis. Am J Hum Genet. 2014;94: 198–208. doi: 10.1016/j.ajhg.2013.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jokela M, Elovainio M, Nyberg ST, Tabák AG, Hintsa T, Batty GD, et al. Personality and risk of diabetes in adults: Pooled analysis of 5 cohort studies. Heal Psychol. 2014;33: 1618–1621. doi: 10.1037/hea0000003 [DOI] [PubMed] [Google Scholar]
- 36.Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol. 2011;40: 804–18. doi: 10.1093/ije/dyr029 [DOI] [PubMed] [Google Scholar]
- 37.Bellamy L, Casas J-P, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373: 1773–1779. doi: 10.1016/S0140-6736(09)60731-5 [DOI] [PubMed] [Google Scholar]
- 38.Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care. 2008;31: 1898–904. doi: 10.2337/dc08-0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emdin CA, Anderson SG, Woodward M, Rahimi K. Usual Blood Pressure and Risk of New-Onset Diabetes: Evidence From 4.1 Million Adults and a Meta-Analysis of Prospective Studies. J Am Coll Cardiol. 2015;66: 1552–62. doi: 10.1016/j.jacc.2015.07.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prins BP, Abbasi A, Wong A, Vaez A, Nolte I, Franceschini N, et al. Investigating the Causal Relationship of C-Reactive Protein with 32 Complex Somatic and Psychiatric Outcomes: A Large-Scale Cross-Consortium Mendelian Randomization Study. PLoS Med. 2016;13: e1001976 doi: 10.1371/journal.pmed.1001976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleber ME, Delgado G, Grammer TB, Silbernagel G, Huang J, Krämer BK, et al. Uric Acid and Cardiovascular Events: A Mendelian Randomization Study. J Am Soc Nephrol. 2015;26: 2831–8. doi: 10.1681/ASN.2014070660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfister R, Barnes D, Luben R, Forouhi NG, Bochud M, Khaw K-T, et al. No evidence for a causal link between uric acid and type 2 diabetes: a Mendelian randomisation approach. Diabetologia. 2011;54: 2561–9. doi: 10.1007/s00125-011-2235-0 [DOI] [PubMed] [Google Scholar]
- 43.Sluijs I, Holmes M V, van der Schouw YT, Beulens JWJ, Asselbergs FW, Huerta JM, et al. A Mendelian Randomization Study of Circulating Uric Acid and Type 2 Diabetes. Diabetes. 2015;64: 3028–36. doi: 10.2337/db14-0742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36: 1422–8. doi: 10.2337/dc12-0962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buijsse B, Boeing H, Hirche F, Weikert C, Schulze MB, Gottschald M, et al. Plasma 25-hydroxyvitamin D and its genetic determinants in relation to incident type 2 diabetes: a prospective case-cohort study. Eur J Epidemiol. 2013;28: 743–52. doi: 10.1007/s10654-013-9844-5 [DOI] [PubMed] [Google Scholar]
- 46.Jorde R, Schirmer H, Wilsgaard T, Joakimsen RM, Mathiesen EB, Njølstad I, et al. Polymorphisms related to the serum 25-hydroxyvitamin D level and risk of myocardial infarction, diabetes, cancer and mortality. The Tromsø Study. PLoS One. 2012;7: e37295 doi: 10.1371/journal.pone.0037295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leong A, Rehman W, Dastani Z, Greenwood C, Timpson N, Langsetmo L, et al. The causal effect of vitamin D binding protein (DBP) levels on calcemic and cardiometabolic diseases: a Mendelian randomization study. PLoS Med. 2014;11: e1001751 doi: 10.1371/journal.pmed.1001751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye Z, Sharp SJ, Burgess S, Scott RA, Imamura F, Langenberg C, et al. Association between circulating 25-hydroxyvitamin D and incident type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2015;3: 35–42. doi: 10.1016/S2213-8587(14)70184-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dastani Z, Hivert M-F, Timpson N, Perry JRB, Yuan X, Scott RA, et al. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012;8: e1002607 doi: 10.1371/journal.pgen.1002607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peters KE, Beilby J, Cadby G, Warrington NM, Bruce DG, Davis WA, et al. A comprehensive investigation of variants in genes encoding adiponectin (ADIPOQ) and its receptors (ADIPOR1/R2), and their association with serum adiponectin, type 2 diabetes, insulin resistance and the metabolic syndrome. BMC Med Genet. 2013;14: 15 doi: 10.1186/1471-2350-14-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yaghootkar H, Lamina C, Scott RA, Dastani Z, Hivert M-F, Warren LL, et al. Mendelian randomization studies do not support a causal role for reduced circulating adiponectin levels in insulin resistance and type 2 diabetes. Diabetes. 2013;62: 3589–98. doi: 10.2337/db13-0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. lancet Diabetes Endocrinol. 2015;3: 958–67. doi: 10.1016/S2213-8587(15)00316-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mericq V, Martinez-Aguayo A, Uauy R, Iñiguez G, Van der Steen M, Hokken-Koelega A. Long-term metabolic risk among children born premature or small for gestational age. Nat Rev Endocrinol. 2017;13: 50–62. doi: 10.1038/nrendo.2016.127 [DOI] [PubMed] [Google Scholar]
- 54.Wang T, Huang T, Li Y, Zheng Y, Manson JE, Hu FB, et al. Low birthweight and risk of type 2 diabetes: a Mendelian randomisation study. Diabetologia. 2016;59: 1920–7. doi: 10.1007/s00125-016-4019-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thiering E, Heinrich J. Epidemiology of air pollution and diabetes. Trends Endocrinol Metab. 2015;26: 384–394. doi: 10.1016/j.tem.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 56.He C, Zhang C, Hunter DJ, Hankinson SE, Buck Louis GM, Hediger ML, et al. Age at menarche and risk of type 2 diabetes: results from 2 large prospective cohort studies. Am J Epidemiol. 2010;171: 334–44. doi: 10.1093/aje/kwp372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aune D, Hartaigh B, Vatten LJ. Resting heart rate and the risk of type 2 diabetes: A systematic review and dose—response meta-analysis of cohort studies. Nutr Metab Cardiovasc Dis. 2015;25: 526–34. doi: 10.1016/j.numecd.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 58.Chen G-C, Qin L-Q, Ye J-K. Leptin levels and risk of type 2 diabetes: gender-specific meta-analysis. Obes Rev. 2014;15: 134–42. doi: 10.1111/obr.12088 [DOI] [PubMed] [Google Scholar]
- 59.Fraser A, Harris R, Sattar N, Ebrahim S, Davey Smith G, Lawlor DA. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women’s Heart and Health Study and meta-analysis. Diabetes Care. 2009;32: 741–50. doi: 10.2337/dc08-1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jia Z, Zhang X, Kang S, Wu Y. Serum uric acid levels and incidence of impaired fasting glucose and type 2 diabetes mellitus: a meta-analysis of cohort studies. Diabetes Res Clin Pract. 2013;101: 88–96. doi: 10.1016/j.diabres.2013.03.026 [DOI] [PubMed] [Google Scholar]
- 61.Kodama S, Saito K, Yachi Y, Asumi M, Sugawara A, Totsuka K, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32: 1737–42. doi: 10.2337/dc09-0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kunutsor SK, Apekey TA, Laukkanen JA. Association of serum total osteocalcin with type 2 diabetes and intermediate metabolic phenotypes: systematic review and meta-analysis of observational evidence. Eur J Epidemiol. 2015;30: 599–614. doi: 10.1007/s10654-015-0058-x [DOI] [PubMed] [Google Scholar]
- 63.Lee CC, Adler AI, Sandhu MS, Sharp SJ, Forouhi NG, Erqou S, et al. Association of C-reactive protein with type 2 diabetes: prospective analysis and meta-analysis. Diabetologia. 2009;52: 1040–7. doi: 10.1007/s00125-009-1338-3 [DOI] [PubMed] [Google Scholar]
- 64.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302: 179–88. doi: 10.1001/jama.2009.976 [DOI] [PubMed] [Google Scholar]
- 65.Sabanayagam C, Lye WK, Klein R, Klein BEK, Cotch MF, Wang JJ, et al. Retinal microvascular calibre and risk of diabetes mellitus: a systematic review and participant-level meta-analysis. Diabetologia. 2015;58: 2476–85. doi: 10.1007/s00125-015-3717-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sing CW, Cheng VKF, Ho DKC, Kung AWC, Cheung BMY, Wong ICK, et al. Serum calcium and incident diabetes: an observational study and meta-analysis. Osteoporos Int. 2016;27: 1747–54. doi: 10.1007/s00198-015-3444-z [DOI] [PubMed] [Google Scholar]
- 67.Wang X, Bao W, Liu J, Ouyang Y-Y, Wang D, Rong S, et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36: 166–75. doi: 10.2337/dc12-0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L, Cui L, Wang Y, Vaidya A, Chen S, Zhang C, et al. Resting heart rate and the risk of developing impaired fasting glucose and diabetes: the Kailuan prospective study. Int J Epidemiol. 2015;44: 689–99. doi: 10.1093/ije/dyv079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu JHY, Micha R, Imamura F, Pan A, Biggs ML, Ajaz O, et al. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr. 2012;107: S214–S227. doi: 10.1017/S0007114512001602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yarmolinsky J, Bordin Barbieri N, Weinmann T, Ziegelmann PK, Duncan BB, Inês Schmidt M. Plasminogen activator inhibitor-1 and type 2 diabetes: a systematic review and meta-analysis of observational studies. Sci Rep. 2016;6: 17714 doi: 10.1038/srep17714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Afshin A, Micha R, Khatibzadeh S, Mozaffarian D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100: 278–88. doi: 10.3945/ajcn.113.076901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alhazmi A, Stojanovski E, McEvoy M, Garg ML. Macronutrient intakes and development of type 2 diabetes: a systematic review and meta-analysis of cohort studies. J Am Coll Nutr. 2012;31: 243–58. Available: http://www.ncbi.nlm.nih.gov/pubmed/23378452 [DOI] [PubMed] [Google Scholar]
- 73.Aune D, Norat T, Romundstad P, Vatten LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr. 2013;98: 1066–83. doi: 10.3945/ajcn.113.059030 [DOI] [PubMed] [Google Scholar]
- 74.Bhupathiraju SN, Tobias DK, Malik VS, Pan A, Hruby A, Manson JE, et al. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr. 2014;100: 218–32. doi: 10.3945/ajcn.113.079533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bi H, Gan Y, Yang C, Chen Y, Tong X, Lu Z. Breakfast skipping and the risk of type 2 diabetes: a meta-analysis of observational studies. Public Health Nutr. 2015;18: 3013–9. doi: 10.1017/S1368980015000257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015;351: h3978 Available: http://www.ncbi.nlm.nih.gov/pubmed/26268692 doi: 10.1136/bmj.h3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ding M, Bhupathiraju SN, Chen M, van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care. 2014;37: 569–86. doi: 10.2337/dc13-1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Djoussé L, Khawaja OA, Gaziano JM. Egg consumption and risk of type 2 diabetes: a meta-analysis of prospective studies. Am J Clin Nutr. 2016;103: 474–80. doi: 10.3945/ajcn.115.119933 [DOI] [PubMed] [Google Scholar]
- 79.Dong J-Y, Qin L-Q. Dietary calcium intake and risk of type 2 diabetes: possible confounding by magnesium. Eur J Clin Nutr. 2012;66: 408–10. doi: 10.1038/ejcn.2012.5 [DOI] [PubMed] [Google Scholar]
- 80.Greenwood DC, Threapleton DE, Evans CEL, Cleghorn CL, Nykjaer C, Woodhead C, et al. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Diabetes Care. 2013;36: 4166–71. doi: 10.2337/dc13-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo K, Zhou Z, Jiang Y, Li W, Li Y. Meta-analysis of prospective studies on the effects of nut consumption on hypertension and type 2 diabetes mellitus. J Diabetes. 2015;7: 202–12. doi: 10.1111/1753-0407.12173 [DOI] [PubMed] [Google Scholar]
- 82.Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ. 2012;344: e1454 Available: http://www.ncbi.nlm.nih.gov/pubmed/22422870 doi: 10.1136/bmj.e1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Consortium InterAct. Dietary fibre and incidence of type 2 diabetes in eight European countries: the EPIC-InterAct Study and a meta-analysis of prospective studies. Diabetologia. 2015;58: 1394–408. doi: 10.1007/s00125-015-3585-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koloverou E, Esposito K, Giugliano D, Panagiotakos D. The effect of Mediterranean diet on the development of type 2 diabetes mellitus: a meta-analysis of 10 prospective studies and 136,846 participants. Metabolism. 2014;63: 903–11. doi: 10.1016/j.metabol.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 85.Larsson SC, Wolk A. Magnesium intake and risk of type 2 diabetes: a meta-analysis. J Intern Med. 2007;262: 208–14. doi: 10.1111/j.1365-2796.2007.01840.x [DOI] [PubMed] [Google Scholar]
- 86.Leermakers ET, Darweesh SK, Baena CP, Moreira EM, Melo van Lent D, Tielemans MJ, et al. The effects of lutein on cardiometabolic health across the life course: a systematic review and meta-analysis. Am J Clin Nutr. 2016;103: 481–94. doi: 10.3945/ajcn.115.120931 [DOI] [PubMed] [Google Scholar]
- 87.Li M, Fan Y, Zhang X, Hou W, Tang Z. Fruit and vegetable intake and risk of type 2 diabetes mellitus: meta-analysis of prospective cohort studies. BMJ Open. 2014;4: e005497 doi: 10.1136/bmjopen-2014-005497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li X-H, Yu F-F, Zhou Y-H, He J. Association between alcohol consumption and the risk of incident type 2 diabetes: a systematic review and dose-response meta-analysis. Am J Clin Nutr. 2016;103: 818–29. doi: 10.3945/ajcn.115.114389 [DOI] [PubMed] [Google Scholar]
- 89.Liu Y-J, Zhan J, Liu X-L, Wang Y, Ji J, He Q-Q. Dietary flavonoids intake and risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Clin Nutr. 2014;33: 59–63. doi: 10.1016/j.clnu.2013.03.011 [DOI] [PubMed] [Google Scholar]
- 90.Tajima R, Kodama S, Hirata M, Horikawa C, Fujihara K, Yachi Y, et al. High cholesterol intake is associated with elevated risk of type 2 diabetes mellitus—a meta-analysis. Clin Nutr. 2014;33: 946–50. doi: 10.1016/j.clnu.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 91.Wang P-Y, Fang J-C, Gao Z-H, Zhang C, Xie S-Y. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. J Diabetes Investig. 2016;7: 56–69. doi: 10.1111/jdi.12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang M, Yu M, Fang L, Hu R-Y. Association between sugar-sweetened beverages and type 2 diabetes: A meta-analysis. J Diabetes Investig. 2015;6: 360–6. doi: 10.1111/jdi.12309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xi B, Li S, Liu Z, Tian H, Yin X, Huai P, et al. Intake of fruit juice and incidence of type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2014;9: e93471 doi: 10.1371/journal.pone.0093471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang J, Mao Q-X, Xu H-X, Ma X, Zeng C-Y. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open. 2014;4: e005632 doi: 10.1136/bmjopen-2014-005632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yao B, Fang H, Xu W, Yan Y, Xu H, Liu Y, et al. Dietary fiber intake and risk of type 2 diabetes: a dose-response analysis of prospective studies. Eur J Epidemiol. 2014;29: 79–88. doi: 10.1007/s10654-013-9876-x [DOI] [PubMed] [Google Scholar]
- 96.Zhao L, Tian X, Ge J, Xu Y. Vitamin D intake and type 2 diabetes risk: a meta-analysis of prospective cohort studies. Afr Health Sci. 2013;13: 1130–8. doi: 10.4314/ahs.v13i4.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol. 2015;30: 529–42. doi: 10.1007/s10654-015-0056-z [DOI] [PubMed] [Google Scholar]
- 98.Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162: 123–32. doi: 10.7326/M14-1651 [DOI] [PubMed] [Google Scholar]
- 99.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33: 414–20. doi: 10.2337/dc09-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Galling B, Roldán A, Nielsen RE, Nielsen J, Gerhard T, Carbon M, et al. Type 2 Diabetes Mellitus in Youth Exposed to Antipsychotics: A Systematic Review and Meta-analysis. JAMA psychiatry. 2016;73: 247–59. doi: 10.1001/jamapsychiatry.2015.2923 [DOI] [PubMed] [Google Scholar]
- 101.Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. 2011;305: 2448–55. doi: 10.1001/jama.2011.812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Holliday EG, Magee CA, Kritharides L, Banks E, Attia J. Short sleep duration is associated with risk of future diabetes but not cardiovascular disease: a prospective study and meta-analysis. PLoS One. 2013;8: e82305 doi: 10.1371/journal.pone.0082305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leong A, Rahme E, Dasgupta K. Spousal diabetes as a diabetes risk factor: a systematic review and meta-analysis. BMC Med. 2014;12: 12 doi: 10.1186/1741-7015-12-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang B, Xu D, Jing Z, Liu D, Yan S, Wang Y. Effect of long-term exposure to air pollution on type 2 diabetes mellitus risk: a systemic review and meta-analysis of cohort studies. Eur J Endocrinol. 2014;171: R173–82. doi: 10.1530/EJE-14-0365 [DOI] [PubMed] [Google Scholar]
- 105.Wu H, Bertrand KA, Choi AL, Hu FB, Laden F, Grandjean P, et al. Persistent organic pollutants and type 2 diabetes: a prospective analysis in the nurses’ health study and meta-analysis. Environ Health Perspect. 2013;121: 153–61. doi: 10.1289/ehp.1205248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zaccardi F, O’Donovan G, Webb DR, Yates T, Kurl S, Khunti K, et al. Cardiorespiratory fitness and risk of type 2 diabetes mellitus: A 23-year cohort study and a meta-analysis of prospective studies. Atherosclerosis. 2015;243: 131–7. doi: 10.1016/j.atherosclerosis.2015.09.016 [DOI] [PubMed] [Google Scholar]
- 107.Aune D, Norat T, Romundstad P, Vatten LJ. Breastfeeding and the maternal risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Nutr Metab Cardiovasc Dis. 2014;24: 107–15. doi: 10.1016/j.numecd.2013.10.028 [DOI] [PubMed] [Google Scholar]
- 108.Coto-Segura P, Eiris-Salvado N, González-Lara L, Queiro-Silva R, Martinez-Camblor P, Maldonado-Seral C, et al. Psoriasis, psoriatic arthritis and type 2 diabetes mellitus: a systematic review and meta-analysis. Br J Dermatol. 2013;169: 783–93. doi: 10.1111/bjd.12473 [DOI] [PubMed] [Google Scholar]
- 109.Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015;104: 30–7. doi: 10.1111/apa.13133 [DOI] [PubMed] [Google Scholar]
- 110.Janghorbani M, Mansourian M, Hosseini E. Systematic review and meta-analysis of age at menarche and risk of type 2 diabetes. Acta Diabetol. 2014;51: 519–28. doi: 10.1007/s00592-014-0579-x [DOI] [PubMed] [Google Scholar]
- 111.Li S, Zhang M, Tian H, Liu Z, Yin X, Xi B. Preterm birth and risk of type 1 and type 2 diabetes: systematic review and meta-analysis. Obes Rev. 2014;15: 804–11. doi: 10.1111/obr.12214 [DOI] [PubMed] [Google Scholar]
- 112.Louati K, Vidal C, Berenbaum F, Sellam J. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD open. 2015;1: e000077 doi: 10.1136/rmdopen-2015-000077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16: 347–363. doi: 10.1093/humupd/dmq001 [DOI] [PubMed] [Google Scholar]
- 114.Stubbs B, Vancampfort D, De Hert M, Mitchell AJ. The prevalence and predictors of type two diabetes mellitus in people with schizophrenia: a systematic review and comparative meta-analysis. Acta Psychiatr Scand. 2015;132: 144–57. doi: 10.1111/acps.12439 [DOI] [PubMed] [Google Scholar]
- 115.Ungprasert P, Upala S, Sanguankeo A, Warrington KJ. Patients with giant cell arteritis have a lower prevalence of diabetes mellitus: A systematic review and meta-analysis. Mod Rheumatol. 2016;26: 410–4. doi: 10.3109/14397595.2015.1081722 [DOI] [PubMed] [Google Scholar]
- 116.Vancampfort D, Mitchell AJ, De Hert M, Sienaert P, Probst M, Buys R, et al. TYPE 2 DIABETES IN PATIENTS WITH MAJOR DEPRESSIVE DISORDER: A META-ANALYSIS OF PREVALENCE ESTIMATES AND PREDICTORS. Depress Anxiety. 2015;32: 763–73. doi: 10.1002/da.22387 [DOI] [PubMed] [Google Scholar]
- 117.Vancampfort D, Mitchell AJ, De Hert M, Sienaert P, Probst M, Buys R, et al. Prevalence and predictors of type 2 diabetes mellitus in people with bipolar disorder: a systematic review and meta-analysis. J Clin Psychiatry. 2015;76: 1490–9. doi: 10.4088/JCP.14r09635 [DOI] [PubMed] [Google Scholar]
- 118.Wang X, Bi Y, Zhang Q, Pan F. Obstructive sleep apnoea and the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Respirology. 2013;18: 140–6. doi: 10.1111/j.1440-1843.2012.02267.x [DOI] [PubMed] [Google Scholar]
- 119.Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89: 309–19. doi: 10.1016/j.diabres.2010.04.012 [DOI] [PubMed] [Google Scholar]
- 120.Bell JA, Kivimaki M, Hamer M. Metabolically healthy obesity and risk of incident type 2 diabetes: a meta-analysis of prospective cohort studies. Obes Rev. 2014;15: 504–15. doi: 10.1111/obr.12157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol. 2007;165: 849–57. doi: 10.1093/aje/kwk071 [DOI] [PubMed] [Google Scholar]
- 122.Janghorbani M, Momeni F, Dehghani M. Hip circumference, height and risk of type 2 diabetes: systematic review and meta-analysis. Obes Rev. 2012;13: 1172–81. doi: 10.1111/j.1467-789X.2012.01030.x [DOI] [PubMed] [Google Scholar]
- 123.Kodama S, Horikawa C, Fujihara K, Heianza Y, Hirasawa R, Yachi Y, et al. Comparisons of the strength of associations with future type 2 diabetes risk among anthropometric obesity indicators, including waist-to-height ratio: a meta-analysis. Am J Epidemiol. 2012;176: 959–69. doi: 10.1093/aje/kws172 [DOI] [PubMed] [Google Scholar]
- 124.Kodama S, Horikawa C, Fujihara K, Yoshizawa S, Yachi Y, Tanaka S, et al. Quantitative relationship between body weight gain in adulthood and incident type 2 diabetes: a meta-analysis. Obes Rev. 2014;15: 202–14. doi: 10.1111/obr.12129 [DOI] [PubMed] [Google Scholar]
- 125.Whincup P, Kaye S, Owen CG, Al E. Birth Weight and Risk of Type 2 Diabetes. JAMA. 2008;300: 2886 doi: 10.1001/jama.2008.886 [DOI] [PubMed] [Google Scholar]
- 126.Huang H, Yan P, Shan Z, Chen S, Li M, Luo C, et al. Adverse childhood experiences and risk of type 2 diabetes: A systematic review and meta-analysis. Metabolism. 2015;64: 1408–18. doi: 10.1016/j.metabol.2015.08.019 [DOI] [PubMed] [Google Scholar]
- 127.Kivimäki M, Virtanen M, Kawachi I, Nyberg ST, Alfredsson L, Batty GD, et al. Long working hours, socioeconomic status, and the risk of incident type 2 diabetes: a meta-analysis of published and unpublished data from 222 120 individuals. lancet Diabetes Endocrinol. 2015;3: 27–34. doi: 10.1016/S2213-8587(14)70178-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nyberg ST, Fransson EI, Heikkilä K, Ahola K, Alfredsson L, Bjorner JB, et al. Job strain as a risk factor for type 2 diabetes: a pooled analysis of 124,808 men and women. Diabetes Care. 2014;37: 2268–75. doi: 10.2337/dc13-2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nordestgaard AT, Thomsen M, Nordestgaard BG. Coffee intake and risk of obesity, metabolic syndrome and type 2 diabetes: a Mendelian randomization study. Int J Epidemiol. 2015;44: 551–65. doi: 10.1093/ije/dyv083 [DOI] [PubMed] [Google Scholar]
- 130.Bergholdt HKM, Nordestgaard BG, Ellervik C. Milk intake is not associated with low risk of diabetes or overweight-obesity: a Mendelian randomization study in 97,811 Danish individuals. Am J Clin Nutr. 2015;102: 487–96. doi: 10.3945/ajcn.114.105049 [DOI] [PubMed] [Google Scholar]
- 131.Aikens RC, Zhao W, Saleheen D, Reilly MP, Epstein SE, Tikkanen E, et al. Systolic Blood Pressure and Risk of Type 2 Diabetes: a Mendelian Randomization Study. Diabetes. 2016; db160868 doi: 10.2337/db16-0868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Marott SCW, Nordestgaard BG, Tybjærg-Hansen A, Benn M. Components of the Metabolic Syndrome and Risk of Type 2 Diabetes. J Clin Endocrinol Metab. 2016;101: 3212–21. doi: 10.1210/jc.2015-3777 [DOI] [PubMed] [Google Scholar]
- 133.Gan W, Guan Y, Wu Q, An P, Zhu J, Lu L, et al. Association of TMPRSS6 polymorphisms with ferritin, hemoglobin, and type 2 diabetes risk in a Chinese Han population. Am J Clin Nutr. 2012;95: 626–32. doi: 10.3945/ajcn.111.025684 [DOI] [PubMed] [Google Scholar]
- 134.Lee YS, Cho Y, Burgess S, Davey Smith G, Relton CL, Shin S-Y, et al. Serum gamma-glutamyl transferase and risk of type 2 diabetes in the general Korean population: a Mendelian randomization study. Hum Mol Genet. 2016; ddw226 doi: 10.1093/hmg/ddw226 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.