Abstract
Being generally regarded as commensal bacteria, the pro-inflammatory capacity of Ureaplasma species has long been debated. Recently, we confirmed Ureaplasma–driven pro-inflammatory cytokine responses and a disturbance of cytokine equilibrium in primary human monocytes in vitro. The present study addressed the expression of CC chemokines and matrix metalloproteinase-9 (MMP-9) in purified term neonatal and adult monocytes stimulated with serovar 8 of Ureaplasma urealyticum (Uu) and serovar 3 of U. parvum (Up). Using qRT-PCR and multi-analyte immunoassay, we assessed mRNA and protein expression of the monocyte chemotactic proteins 1 and 3 (MCP-1/3), the macrophage inflammatory proteins 1α and 1β (MIP-1α/β) as well as MMP-9. For the most part, both isolates stimulated mRNA expression of all given chemokines and MMP-9 in cord blood and adult monocytes (p<0.05 and p<0.01). These results were paralleled by Uu and Up-induced secretion of MCP-1 protein in both cells (neonatal: p<0.01, adult: p<0.05 and p<0.01). Release of MCP-3, MIP-1α, MIP-1β and MMP-9 was enhanced upon exposure to Up (neonatal: p<0.05, p<0.01 and p<0.001, respectively; adult: p<0.05). Co-stimulation of LPS-primed monocytes with Up increased LPS-induced MCP-1 release in neonatal cells (p<0.05) and aggravated LPS-induced MMP-9 mRNA in both cell subsets (neonatal: p<0.05, adult: p<0.01). Our results document considerable expression of pro-inflammatory CC chemokines and MMP-9 in human monocytes in response to Ureaplasma isolates in vitro, adding to our previous data. Findings from co-stimulated cells indicate that Ureaplasma may modulate monocyte immune responses to a second stimulus.
Introduction
The genital mycoplasmas Ureaplasma urealyticum (serovars 2, 4, 5 and 7–13) and Ureaplasma parvum (serovars 1, 3, 6, 14) are among the smallest free living and self-replicating organisms. They lack a cell wall, have limited biosynthetic capacities and colonize mucosal surfaces of the genitourinary and respiratory tract [1]. Ureaplasma species (spp.) have been associated with vaginitis, cervicitis, urinary tract infection as well as female and male infertility [2,3]. Moreover, lower genital tract colonization has been described as an independent risk factor for intrauterine infection and preterm birth (PTB) (i.e., delivery < 37 weeks of gestation) [4–6]. Detection of Ureaplasma spp. in the amniotic fluid (AF) or placental tissues has been causally related to histologic chorioamnionitis, abortion and PTB, both at gestational ages less than 30 weeks [7,8] andin moderate preterm infants (i.e., birth between 32 and 36 weeks of gestation) [9,10]. In preterm and term neonates, Ureaplasma spp. may cause invasive diseases, such as pneumonia, sepsis and meningitis [11–13]. Moreover, epidemiologic studies indicate a role of prenatal and perinatal Ureaplasma infection in neonatal short and long-term morbidity, specifically in the development of bronchopulmonary dysplasia (BPD) [12,14].
Still, Ureaplasma isolates have not been conclusively proven as causative pathogens [13,15–18]. Given that Ureaplasma can be isolated in up to 40–80% of genitourinary tract specimen collected from adults [3,6,19], they are generally considered to be commensal bacteria and are regarded as being of low virulence in children and in adults [15,17,20]. Keeping in line with this, several studies do not support causal relationships between genital tract carriage of Ureaplasma spp. and PTB [15,21], between Ureaplasma upper genital tract colonization and adverse pregnancy outcome [18] or between neonatal respiratory tract colonization and BPD [22,23]. Interpretation of epidemiologic data may be hampered by several aspects, such as the detection of Ureaplasma spp. in the lower genital tract of mothers with PTB and those with full-term delivery [21,24], the bacterial colonization of placental tissues even in pregnancies delivering at term [16,25] and the often polymicrobial nature of chorioamnionitis [8]. Discrepant results have also been found regarding the intensity of Ureaplasma-driven host immune response. While some epidemiologic and experimental studies on intrauterine Ureaplasma infection reported severe choriodecidual and amniotic inflammation [9,26,27], others detected either moderate or little inflammatory response [28,29] or did not reveal any association with increased inflammatory parameters [7,30]. In preterm infants, respiratory tract colonization with Ureaplasma spp. has been implicated in bronchopulmonary inflammation and altered lung development [31,32], and invasive Ureaplasma infection has been associated with systemic inflammation and characteristic changes in cerebrospinal fluid profiles [11–13,33]. In other studies, however, endotracheal or systemic detection of Ureaplasma spp. did not correlate with neonatal systemic inflammation [22,23,30].
In vitro data on Ureaplasma-induced inflammation are limited, not least on account of special growth requirements. Recent data from our group demonstrated a significant induction of pro-inflammatory cytokines and the CXC chemokine IL-8 and a disturbance of pro- and anti-inflammatory cytokine equilibrium in Ureaplasma-stimulated monocytes [34]. The current study investigated Ureaplasma-induced CC chemokine responses in primary human monocytes and the expression of matrix metalloproteinase-9 (MMP-9), aiming to expand our understanding of Ureaplasma virulence.
Monocytes are a major source of chemokines and thereby trigger initial steps of innate immune response to microbial infection [35,36]. The monocyte chemotactic proteins 1 and 3 (MCP-1, MCP-3; synonym CCL2 and CCL7) and the macrophage inflammatory proteins 1α and 1β (MIP-1α, MIP-1β; synonym CCL3 and CCL4) recruit effector cells to the site of infection and activate immune cells by stimulating further release of inflammatory mediators [35,36]. Matrix metalloproteinases, such as MMP-9, play a crucial role in the degradation of extracellular matrix (ECM) components, the modulation of inflammatory mediators, the establishment of chemokine gradients, the generation of reactive oxygen species and the migration of effector cells [37]. Using real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) and multi-analyte immunoassay, we assessed MCP-1, MCP-3, MIP-1α and MIP-1β as well as MMP-9 in term neonatal and adult human monocytes stimulated with serovar 8 of U. urealyticum (Uu) and serovar 3 of U. parvum (Up) in the absence or presence of Escherichia coli (E. coli) lipopolysaccharide (LPS).
Materials and methods
Bacterial strains and culture conditions
Serovar 8 of U. urealyticum (ATCC #27618) and serovar 3 of U. parvum (#27815) were obtained from the American Tissue Culture Collection (ATCC). Both isolates were propagated in a liquid and yeast-free in-house medium (referred to as “broth”) containing 82% autoclaved PPLO medium (Becton, Dickinson & Company, USA), 10% heat-inactivated horse serum, 7% urea (20% aqueous solution) and 1% phenol red (0.2%) (each Sigma-Aldrich, USA) as described previously [38]. The medium was adjusted to pH 6.5 after passage through a 0.2 micron filter membrane. Mid-logarithmic-phase broth cultures of each isolate were frozen in 0.5 ml aliquots and stored at—80 °C until further use. For each experiment, aliquots of the same stock were inoculated 1: 10 in 5 ml “broth”. Adhering strictly to defined incubation times, 10-fold serial dilutions were incubated to obtain titers of 5 x 108 color-changing units (CCU)/ml. Determination of CCUs was performed in 96-well plates (Greiner, Frickenhausen, Germany) by 10-fold serial dilutions in 200 μl broth according to previous publications [39]. The number of CCUs was determined in duplicate. To confirm a consistent amount of Ureaplasma applied in each assay [40], corresponding amounts of Ureaplasma DNA were assessed in copy numbers at the Institute of Medical Microbiology and Hospital Hygiene Duesseldorf, Germany [41]. Viability of inoculated organisms was confirmed by re-culture of inoculums in “broth” and on selective agar plates (medco Diagnostika GmbH, Germany).
Isolation of CD14+ monocytes from cord blood and peripheral blood mononuclear cells
Umbilical cord blood samples were taken from healthy term newborns (n = 6) delivered by elective caesarean section. Written parental consent had been obtained the day before. Exclusion criteria comprised clinical or laboratory evidence of chorioamnionitis and/or neonatal infection and congenital malformation. The study has been approved by the ethics committee of the Medical Faculty of Würzburg (approval #164/14) and was conducted in accordance with the World Medical Association Declaration of Helsinki. Cord blood was collected from the umbilical vein using a closed system (Maco Pharma International, France), and was processed within 2 h. Adult leukocyte concentrates were obtained from apheresis products from healthy adult donors (n = 6) at the Department of Immunohematology and Transfusion Medicine, University Hospital Würzburg. Due to randomization and anonymization donors’ individual consent was not required. As described before [34], cord blood and peripheral blood mononuclear cells were isolated by Ficoll-Paque gradient centrifugation (Linaris, Germany). Subsequent isolation of CD14+ monocytes was performed using CD14 MicroBeads®, the corresponding MidiMACS™ separator and LS type columns (Miltenyi Biotec, Germany). Purified CD14+ monocytes were resuspended in RPMI 1640 medium (Sigma-Aldrich) containing 10% fetal bovine serum (Thermo Fisher Scientific, Germany). The purity of CD14+ isolation was > 90%, as determined by flow cytometry.
Cell culture and stimulation assays
Suspensions of unpooled term neonatal and adult CD14+ monocytes were transferred to 24-well culture plates (Greiner) at a density of 1 × 106 cells/well. Cells rested for 2 h at 37 °C in a humidified atmosphere with 5% CO2. Then, aliquots of both Ureaplasma isolates were added at a concentration of 108 CCU viable organisms which corresponded to 200 μl per well and 1.3 x 106–1.8 x 107 copy numbers of Uu and Up/ml. For studies on LPS-primed monocytes, LPS from E. coli serotype 055:B5 (Sigma-Aldrich) was added 90 min prior to the infection of cells with Uu and Up. LPS dose and concentration of CCUs had been determined by preliminary dose-response experiments [34]. Preliminary experiments had further addressed expression kinetics of the given chemokines and MMP-9 (2, 4, 8, 14 and 40 h incubation), demonstrating peak expression of mRNA at 4 h and secreted protein at 24 h. Cell viability was ≥ 95% after 4 h and 24 h cell culture for native, LPS-primed and Ureaplasma-stimulated monocytes, as determined by flow cytometry.
RNA extraction and reverse transcription (RT)
For RNA extraction, neonatal and adult monocytes were harvested after 4 h of stimulation,separated by centrifugation and lysed by adding a lysis buffer containing guanidinium thiocyanate (Macherey-Nagel, Germany) and 2-mercaptoethanol (Sigma Aldrich). Total RNA was extracted using the NucleoSpin® RNA Kit (Macherey-Nagel) according to the manufacturer’s protocol,eluted into 60 μl nuclease-free water (Sigma-Aldrich) and stored at—80 °C until reverse transcription. For RNA quantitation, a Qubit® 2.0 Fluorometer (Thermo Fisher Scientific, Germany) was used. Amounts of 0.11 to 0.52 μg of total RNA of cord blood monocytes and 0.13 to 0.50 μg of total RNA of adult monocytes were reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). The reaction was terminated by heating at 70 °C for 10 min. First strand cDNA was stored at—80 °C until further processing.
Quantitative real-time RT-PCR (qRT-PCR)
For quantitative detection of MIP-1α, MIP-1β, MCP-1, MCP-3, and MMP-9 mRNA, cDNA was diluted 1: 10 in deionized, nuclease-free water and analyzed in duplicates of 25 μl using 12.5 μl iTaq™ Universal SYBR Green Supermix (Bio-Rad Laboratories, USA). Primers used for qRT-PCR are given in Table 1. Analysis was performed using a 7500 Real-Time PCR System (Applied Biosystems, USA) and a 2-step PCR protocol with 40 cycles of 95 °C for 15 s and 60 °C for 1 min following initial denaturation of DNA. A melt curve analysis was performed at the end of every run to verify single PCR products. Amplification was normalized to the reference gene PPIA (peptidyl prolyl isomerase A). Mean fold changes in mRNA expression were calculated using the ΔΔCT method [42].
Table 1. Primer sequences used for qRT-PCR.
| Gene symbol | Sequence accession # | Orientation | Sequence [5´to 3´] |
|---|---|---|---|
| MIP1α | NM_002983 | forward | TCATCTTCCTAACCAAGCGA |
| reverse | CATGTTCCCAAGGCTCAG | ||
| MIP1β | NM_002984 | forward | AGCCAGCTGTGGTATTCC |
| reverse | GGAGTCCTGAGTATGGAGGAG | ||
| MCP1 | NM_002982 | forward | GCTGTGATCTTCAAGACC |
| reverse | AAGTCTTCGGAGTTTGGG | ||
| MCP3 | NM_006273 | forward | CTGAGACCAAACCAGAAACC |
| reverse | TATTAATCCCAACTGGCTGAG | ||
| MMP9 | NM_004994 | forward | GCCACTACTGTGCCTTTGAG |
| reverse | AGAATCGCCAGTACTTCCCA | ||
| PPIA | NM_021130 | forward | CAGGGTTTATGTGTCAGGG |
| reverse | CCATCCAACCACTCAGTC |
Quantification of secreted cytokines
For cytokine measurements, supernatants of neonatal and adult monocytes were collected at 24 h incubation and stored at—80 °C until analysis. Concentrations of human MIP-1α, MIP-1β, MCP-1, MCP-3, and MMP-9 were measured by means of a multi-analyte immunoassay using Luminex® multiplex kits and the xPonent® software (Merck group, Germany). Samples of neonatal and adult monocytes were analyzed in duplicate. Concentration of each mediator was calculated from an individual standard curve. The lower detection limits of the assays were 4.75 pg/ml (MIP-1α), 3.17 pg/ml (MIP-1β), 4.07 pg/ml (MCP-1), 2.92 pg/ml (MCP-3), and 1.68 pg/ml (MMP-9).
Statistical analysis
Prism® 6 software (GraphPad Software, USA) was used for statistical analysis. Data report group means ± standard deviation (SD). Differences among groups were analyzed using the non-parametric Kruskal-Wallis test and Dunn’s multiple comparison post hoc-test. Mann-Whitney U-test was performed to compare stimulation intensities between corresponding neonatal and adult monocyte subsets. Statistical significance was defined as p < 0.05.
Results
Basal expression of CC chemokines and MMP-9 in neonatal and adult monocytes and characteristics upon exposure to E. coli LPS and Ureaplasma medium
In native term neonatal and adult monocytes, we detected hardly any endogenous expression of MCP-1, MCP-3, MIP-1α and MIP-1β as well as MMP-9. LPS treatment, on the contrary, resulted in a significant increase in mRNA and protein expression of the given chemokines and MMP-9 in both cell subsets (Figs 1–3). Compared to adult monocytes, LPS-stimulated cord blood monocytes showed less pronounced mRNA expression of MCP-1 (p < 0.001, vs. adult monocytes), MCP-3 (p < 0.001), MIP-1α (p < 0.05), MIP-1β (p < 0.01) and MMP-9 (p < 0.01). However, corresponding amounts of LPS-induced protein release did not significantly differ with exception of lower levels of MCP-3 protein in LPS-stimulated neonatal cells (p < 0.05, vs. adult monocytes). Monocyte exposure to the in-house, yeast-free Ureaplasma broth partly induced a slight, but non-significant increase in MCP-1, MCP-3 and MMP-9 mRNA as well as secreted MMP-9 (Figs 1A, 1B, 3A and 3B). Consequently, the stimulatory effects of Uu and Up were compared to broth control throughout this study, to adjust for potential confounding effects of the in-house medium.
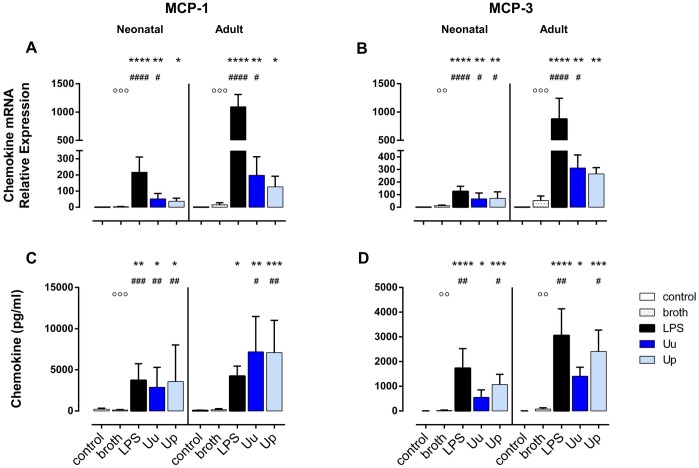
Fig 1. Uu and Up induce MCP-1 and MCP-3 in term neonatal and adult monocytes.
Relative quantification of chemokine mRNA (A, B) and chemokine concentration in the supernatant (C, D) are presented as mean ± SD. Unstimulated monocytes and monocytes exposed to in-house Ureaplasma medium (broth) served as negative controls. Monocytes stimulated with E. coli LPS served as positive control (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p< 0.0001, vs. unstimulated control; # p < 0.05, ## p < 0.01, ### p < 0.001, #### p< 0.0001, vs. broth control; ○○ p < 0.01, ○○○ p < 0.001, vs. LPS-stimulated monocytes).
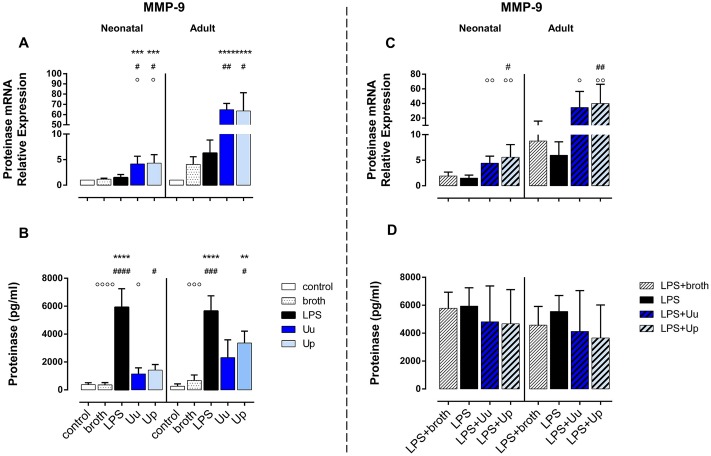
Fig 3. Ureaplasma isolates stimulate MMP-9 expression in human monocytes and partly modulate MMP-9 mRNA upon co-stimulation with E. coli LPS.
Relative quantification of mRNA and protein expression of MMP-9 are presented for monocytes exposed either to LPS or Uu and Up (A, B) and for monocytes co-stimulated with Ureaplasma isolates and LPS (C, D). Results are given as mean ± SD (** p < 0.01, *** p < 0.001, **** p< 0.0001, vs. unstimulated control; # p < 0.05, ## p < 0.01, ### p < 0.001, #### p< 0.0001, vs. broth control; ○ p < 0.05, ○○ p < 0.01, ○○○ p < 0.001, ○○○○ p < 0.001, vs. LPS-stimulated monocytes).
U. urealyticum and U. parvum-stimulated chemokine expression in neonatal and adult monocytes
Neonatal monocytes
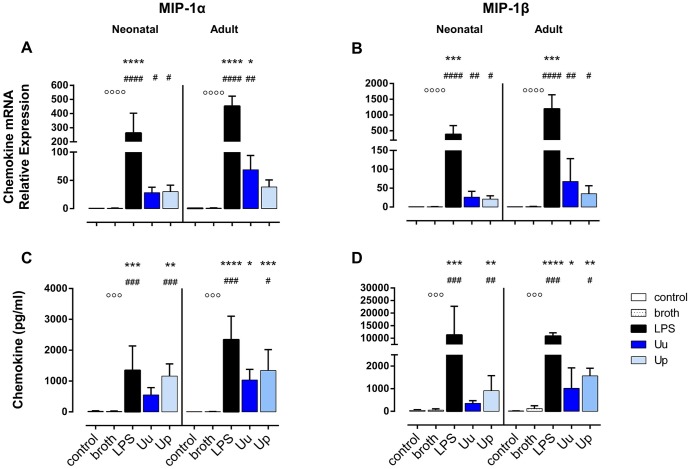
Uu and Up increased mRNA expression of MCP-3 (p < 0.05, vs. broth control), MIP-1α (p < 0.05) and MIP-1β (Uu: p < 0.01, Up3: p < 0.05) in neonatal monocytes (Figs 1B, 2A and 2B). Neonatal MCP-1 mRNA was enhanced upon exposure to Uu (p < 0.05, vs. broth) (Fig 1A). Both isolates amplified the levels of MCP-1 in the supernatant of neonatal cells (p < 0.01, vs. broth) (Fig 1C), while protein expression of MCP-3, MIP-1α and MIP-1β was increased only upon stimulation with Up (p < 0.05, p < 0.01 and p < 0.001, respectively, vs. broth) (Figs 1D, 2C and 2D). The stimulatory effects of Uu and Up were dose-dependent both at the level of transcription and translation, as determined in preliminary stimulation assays (S1 Fig, data given for mRNA expression). Ureaplasma-induced neonatal chemokines appeared to be less pronounced than LPS-induced expression, but these differences did not reach statistical significance.
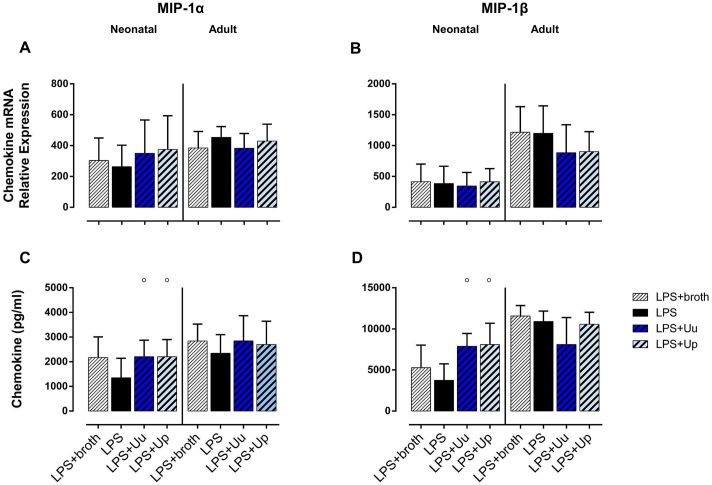
Fig 2. Neonatal and adult human monocytes display elevated levels of MIP-1α and MIP-1β mRNA and protein upon stimulation with Uu and Up.
Chemokine expression was assessed at the level of mRNA (A, B) and protein secretion (C, D). The values represent the means ± SD (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, versus unstimulated control; # p < 0.05, ## p < 0.01, ### p < 0.001, #### p< 0.0001, vs. broth control; ○○○ p < 0.001, ○○○○ p < 0.001, vs. LPS-stimulated monocytes).
Adult monocytes
In adult monocytes, stimulation with Uu resulted in increased expression of MCP-1, MCP-3 and MIP-1α mRNA (p < 0.05 and p < 0.01, vs. broth control) (Figs 1A, 1B and 2A), and both isolates enhanced mRNA expression of MIP-1β (Uu: p < 0.01, Up3: p < 0.05, vs. broth) (Fig 2B). As far as protein expression was concerned, both Uu and Up enhanced MCP-1 synthesis (Uu: p < 0.05, Up: p < 0.01, vs. broth), and Up additionally stimulated the secretion of MCP-3, MIP-1α and MIP-1β into the supernatant (p < 0.05 each, vs. broth) (Figs 1 and 2). In accordance to neonatal monocytes, Ureaplasma-induced chemokine expression was proportional to the dose of Uu and Up applied (S1 Fig). Ureaplasma-induced levels of adult CC chemokines did not significantly differ from LPS-mediated levels.
Comparing neonatal and adult monocytes, Uu and Up-induced mRNA expression of MCP-1 (p < 0.01, vs. adult cells) and MCP-3 (p < 0.001) and, partly, MIP-1α and MIP-1β (Uu: p < 0.05 each) was less pronounced in neonatal cells. These differences were paralleled by lower levels of MCP-1 (Uu: p < 0.01, Up: p < 0.05, vs. adult cells) and MCP-3 protein release in neonatal monocytes (p < 0.01), while levels of secreted MIP-1α and MIP-1β did not differ.
Ureaplasma-induced expression of MMP-9 in primary human monocytes
Neonatal monocytes
For both isolates, we detected profound induction of MMP-9 mRNA in neonatal monocytes (p < 0.05, vs. broth control) (Fig 3A), exceeding LPS-stimulated mRNA expression (Uu and Up: p < 0.05, vs. LPS). Corresponding levels of secreted MMP-9 in neonatal monocytes were significantly increased upon stimulation with Up (p < 0.05, vs. broth) (Fig 3B). Compared to LPS, Ureaplasma-induced MMP-9 protein release was party less pronounced (Uu: p < 0.05, vs. LPS) (Fig 3B).
Adult monocytes
In accordance to neonatal cells, exposure of adult monocytes to Uu and Up resulted in a profound induction of MMP-9 mRNA (Uu: p < 0.01, Up: p < 0.05, vs. broth) (Fig 3A). Secretion of MMP-9 protein was enhanced upon Up-stimulation (p < 0.05, vs. broth) (Fig 3B).
Comparing neonatal and adult monocytes, Ureaplasma-induced MMP-9 mRNA was less pronounced in neonatal monocytes (Uu and Up: p < 0.001, vs. adult monocytes). The levels of corresponding protein release did not significantly differ.
Differential modulation of LPS-induced CC chemokine responses and MMP-9 expression in neonatal and adult monocytes co-stimulated with Ureaplasma spp. and E. coli LPS
Neonatal monocytes
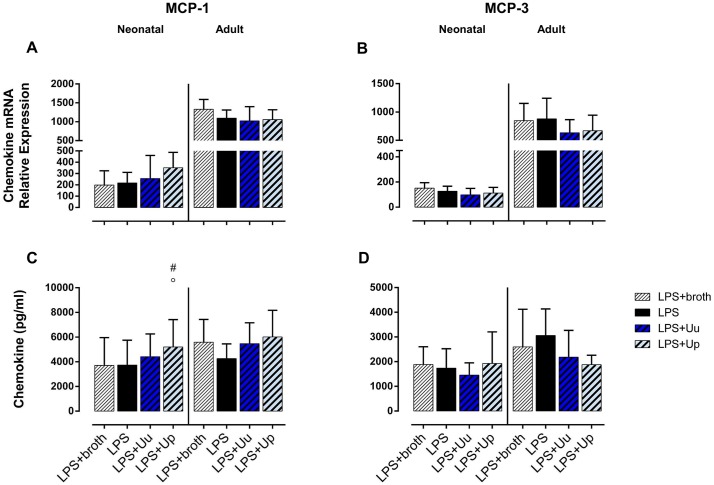
Stimulating LPS-primed neonatal monocytes with Ureaplasma isolates, we observed an increase in LPS-induced MCP-1 release (Up: p < 0.05; vs. LPS-stimulated cells) (Fig 4C) and augmented secretion of LPS-induced MIP-1α and MIP-1β (Uu and Up: p < 0.05) (Fig 5C and 5D). When adjusting for confounding effects of Ureaplasma broth, Up-mediated increase of LPS-induced MCP-1 secretion remained statistically significant (p < 0.05; vs. LPS-primed monocytes exposed to broth control) (Fig 4C). As far as MMP-9 was concerned, co-exposure of LPS-primed cells both to Uu and Up resulted in a significant increase in LPS-induced mRNA (Uu: p < 0.01, Up: p < 0.001; vs. LPS-stimulated cells) (Fig 3C). Comparing these effects to LPS-activated monocytes exposed to Ureaplasma broth, Ureaplasma-induced modulation remained partly significant (Up: p < 0.05, vs. broth control).
Fig 4. Impact of Ureaplasma co-stimulation on LPS-induced MCP-1 and MCP-3 responses in neonatal and adult monocytes.
Using qRT-PCR and multi-analyte immunoassay, we assessed mRNA expression (A, B) and protein secretion (C, D) in LPS-primed monocytes co-stimulated with Ureaplasma isolates. LPS-primed monocytes exposed to Ureaplasma broth served as negative control, LPS-activated cells served as positive control. Values represent the means ± SD (# p < 0.05, vs. LPS-primed monocytes exposed to broth control; ○ p < 0.05, vs. LPS-activated monocytes).
Fig 5. MIP-1α and MIP-1β expression in LPS-primed neonatal and adult monocytes following co-stimulation with Ureaplasma isolates.
Relative quantification of chemokine mRNA (A, B) and concentrations of secreted protein (C, D) are presented as mean ± SD (○ p < 0.05, vs. LPS-activated monocytes).
Adult monocytes
In LPS-primed adult monocytes, we observed a trend towards reduced release of LPS-stimulated MCP-3 upon co-exposure to Up (Up: p = 0.09; vs. LPS-primed monocytes) (Fig 5B and 5D). Moreover, co-stimulation with Uu and Up resulted in an increase in LPS-induced MMP-9 mRNA (Uu: p < 0.05, Up: p < 0.01; vs. LPS) (Fig 3C). Adjusting for the effects of Ureaplasma medium, the augmenting effect remained significant for Up (p < 0.01, vs. broth).
Discussion
In the current study, we demonstrate that Ureaplasma isolates stimulate the expression of pro-inflammatory MCP-1, MCP-3, MIP-1α and MIP-1β as well as MMP-9 in human monocytes in vitro. These features might link Ureaplasma infection with inflammatory responses underlying genital tract disorders, chorioamnionitis and neonatal morbidities, such as BPD [43–45]. Elevated concentrations of CC chemokines in the AF and uterine tissues have been related to microbial invasion of the amniotic cavity and intrauterine inflammation and have been associated with the onset of preterm labor and PTB [46–48]. In mechanically ventilated preterm infants, enhanced tracheal aspirate chemokines have been associated with the later development of BPD [49–51]. Early systemic expression of MCP-1, MIP-1α and MIP-1β seems to indicate an increased risk of chronic lung injury as well as microcephaly and retinopathy of prematurity in very immature preterm infants [52–54]. Early prolonged oxygen exposure, mechanical ventilation and histologic chorioamnionitis seem to be causally related to this enhanced pulmonary and systemic chemokine expression [51,55,56]. Of note, increased levels of MCP-1 and MIP-1α were found in the AF of pregnant women with intrauterine detection of Ureaplasma spp. and preterm premature rupture of membranes (PPROM) (i.e., rupture of membranes prior to 37 weeks of gestation) [57]. In mechanically ventilated preterm infants, Ureaplasma respiratory tract colonization correlated with higher levels of tracheal aspirate MCP-1 [51]. In a sheep model of intrauterine inflammation, intraamniotic administration of U. parvum induced increased expression of MCP-1 in fetal keratinocytes [58]. In line with these findings, our current data indicate that Ureaplasma spp. may play a role in CC chemokine expression in intrauterine and fetal or neonatal inflammation.
Increased concentrations of MMP-9 have been related to microbial invasion of the amniotic cavity, intrauterine inflammation and preterm labor [59–61]. Imbalanced ratios of MMP-9 to its specific tissue inhibitors have been implicated in the pathogenesis of various lung diseases, including chronic lung injury in preterm infants [37,62], promotion of airway remodeling, disease exacerbation and the perturbance of alveolar development [37,63,64]. In very immature preterm infants, increased local and systemic expression of MMP-9 has also been associated with intraventricular hemorrhage, white matter brain injury and retinopathy of prematurity [62,65,66]. Early prolonged oxygen exposure and hyperoxia appear to stimulate such enhanced expression [55,64]. In vitro studies documented infection-induced MMP-9 expression in monocytes and human fetal membranes exposed to different pathogens and Toll-like receptor (TLR) agonists [67,68]. Data on Ureaplasma-induced MMP-9 expression originate from clinical studies and few experimental trials. In pregnant women with PPROM, the presence of Ureaplasma spp. in the AF was associated with increased levels of MMP-9 [57]. In animal models in Rhesus macaques and rats, intraamniotic inoculation of U. parvum and the related pathogen Mycoplasma pulmonis resulted in increased AF levels of MMP-9 [69,70]. Very recently, Ureaplasma-induced release of MMP-9 was demonstrated in human neutrophils [71]. Our current results support the hypothesis that colonization and/or infection with Ureaplasma spp. may interfere with MMP-9 activities and may trigger preterm labor and PTB as well as inflammation-related neonatal morbidities in preterm infants.
The molecular mechanisms underlying Ureaplasma-induced monocyte expression of CC chemokines and MMP-9 need to be addressed in further studies. LPS-induced expression of MCP-1 and MMP-9 in renal tubular epithelial cells was demonstrated to depend on nuclear transcription factor kappa B (NF-κB) signaling [72]. Pro-inflammatory TNF-α and IL-1β appear to contribute to LPS-induced MMP-9 expression in human fetal membranes through autocrine and paracrine signaling [73]. Against the background of Ureaplasma-induced TNF-α and IL-1β monocyte responses [34], similar mechanisms may underlie the induction of MMP-9 in Ureaplasma-stimulated cells. Of note, Ureaplasma-induced expression of MMP-9 mRNA exceeded the effects of E. coli LPS in the present study, while levels of monocyte MMP-9 in the supernatant showed a reverse trend. This observation may be due to particularities in MMP-9 secretion [37]. Ureaplasma spp. and E. coli LPS may differentially induce MMP-9 proenzyme release and its procession to active MMP-9.
Upon co-stimulation with E. coli LPS, Up enhanced the expression of MCP-1 protein in neonatal monocytes as well as MMP-9 mRNA in neonatal and adult cells. These data are partially in accordance with previous in vitro studies on monocyte cytokine responses performed by Viscardi et al. and by our group [34,74], which point to immunomodulatory features of Ureaplasma spp. in the context of a second pathogen. A recent in vitro study in fetal membranes found enhanced expression of pro-inflammatory cytokines upon co-stimulation with U. urealyticum and Gardnerella vaginalis compared to U. urealyticum stimulation alone [75]. In a sheep model of Ureaplasma chorioamnionitis, 7d intraamniotic infection with U. parvum mitigated LPS-induced cytokine expression in the AF [29], but amplified LPS-induced cytokine responses in fetal blood and lung monocytes [76]. Given the often polymicrobial nature of chorioamnionitis and pulmonary inflammation, in particular in early PTB [8], immunomodulatory effects of Ureaplasma spp. in the presence of a second stimulus may be of clinical relevance. Indeed, in women with preterm labor or PPROM, the detection of Ureaplasma isolates in combination with Mycoplasma hominis was associated with a significantly shorter interval to PTB and with significant increases in the incidence of PTB and histologic chorioamnionitis compared to the detection of Ureaplasma isolates alone [77].
In accordance with our previous study [34], we observed a dose-dependency of Uu and Up-induced expression of CC chemokines and MMP-9. In line with these in vitro findings, Abele-Horn et al. reported that high loads of Ureaplasma spp. in the lower genital tract of pregnant women correlated with adverse pregnancy outcome, while low colonization rates did not [4]. Epidemiologic data and data from a mouse model further suggest an impact of host genetic background on the clinical course of Ureaplasma respiratory tract colonization in preterm infants and Ureaplasma-induced fetal inflammation [78,79]. Moreover, developmental particularities of immune responses may contribute to a heightened susceptibility, as indicated by a higher prevalence of Ureaplasma-infected AF, a more severe intrauterine inflammation in Ureaplasma-associated chorioamnionitis and higher detection rates of Ureaplasma spp. in airway samples and cord blood cultures of preterm infants at lower gestational age [33,80,81]. Comparing neonatal and adult monocyte chemokine and MMP-9 responses in the present study, we observed less pronounced mRNA expression in Ureaplasma-stimulated cord blood monocytes. However, with the exception of smaller levels of Ureaplasma-induced MCP-1 and MCP-3 protein, corresponding protein secretion did not significantly differ among cord blood and adult monocytes, indicating a similar responsiveness of term neonatal and adult monocytes at the translational level and indicating a similar pro-inflammatory capacity of Ureaplasma spp. in both cells.
The strength of this study relates to the use of viable bacteria, contrasting with the use of heat-killed Ureaplasma and extracted or recombinant Ureaplasma outer membrane proteins in previous in vitro approaches [82,83]. A limitation of the study is that it was conducted using ATCC strains. Future studies shall include the use of clinical Ureaplasma isolates originated from chorioamnionitis or neonatal systemic infection in order to link clinical evidence of pathogenicity and in vitro immune responses.
Conclusion
To the best of our knowledge, this is the first study analyzing Ureaplasma-induced CC chemokine responses and MMP-expression in primary human monocytes. Our data indicate that Ureaplasma spp. may effectively trigger adverse inflammatory monocyte responses, may considerably interfere with MMP-9 activity and, as a result, provoke subsequent pathologies. It is most likely that in vivo, pathogen virulence [19,84], microbial load [4,9,81], the duration of exposure [76], polymicrobial interactions [77,85], host genetics [78] and gestational age [33,80,81] all shape the clinical course of infection.
Supporting information
Data are given for term neonatal and adult monocytes. Both isolates caused a dose-dependent induction of MCP-1 (A), MCP-3 (B), MIP-1α (C), MIP-1β (D) and MMP-9 mRNA (E) at 4 h assessment (n = 3).
(TIFF)
Acknowledgments
We thank Brigitte Wollny, Silvia Seidenspinner and Mariola Dragan for excellent technical support and all cord blood donors for contributing to this study.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This project was supported by the Interdisciplinary Center for Clinical Research (IZKF) at the University of Würzburg, Germany. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Waites KB, Schelonka RL, Xiao L, Grigsby PL, Novy MJ (2009) Congenital and opportunistic infections: Ureaplasma species and Mycoplasma hominis. Semin Fetal Neonatal Med 14: 190–199. doi: 10.1016/j.siny.2008.11.009 [DOI] [PubMed] [Google Scholar]
- 2.Larsen B, Hwang J (2010) Mycoplasma, Ureaplasma, and adverse pregnancy outcomes: a fresh look. Infect Dis Obstet Gynecol 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasprzykowska U, Elias J, Elias M, Maczynska B, Sobieszczanska BM (2014) Colonization of the lower urogenital tract with Ureaplasma parvum can cause asymptomatic infection of the upper reproductive system in women: a preliminary study. Arch Gynecol Obstet 289: 1129–1134. doi: 10.1007/s00404-013-3102-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abele-Horn M, Scholz M, Wolff C, Kolben M (2000) High-density vaginal Ureaplasma urealyticum colonization as a risk factor for chorioamnionitis and preterm delivery. Acta Obstet Gynecol Scand 79: 973–978. [PubMed] [Google Scholar]
- 5.Kataoka S, Yamada T, Chou K, Nishida R, Morikawa M, Minami M, et al. (2006) Association between preterm birth and vaginal colonization by mycoplasmas in early pregnancy. J Clin Microbiol 44: 51–55. doi: 10.1128/JCM.44.1.51-55.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musilova I, Pliskova L, Kutova R, Hornychova H, Jacobsson B, Kacerovsky M (2016) Ureaplasma species and Mycoplasma hominis in cervical fluid of pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 29: 1–7. [DOI] [PubMed] [Google Scholar]
- 7.Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, et al. (2004) Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol 191: 1382–1386. doi: 10.1016/j.ajog.2004.05.070 [DOI] [PubMed] [Google Scholar]
- 8.DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, et al. (2008) Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One 3: e3056 doi: 10.1371/journal.pone.0003056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasper DC, Mechtler TP, Reischer GH, Witt A, Langgartner M, Pollak A, et al. (2010) The bacterial load of Ureaplasma parvum in amniotic fluid is correlated with an increased intrauterine inflammatory response. Diagn Microbiol Infect Dis 67: 117–121. doi: 10.1016/j.diagmicrobio.2009.12.023 [DOI] [PubMed] [Google Scholar]
- 10.Sweeney EL, Kallapur SG, Gisslen T, Lambers DS, Chougnet CA, Stephenson SA, et al. (2016) Placental Infection With Ureaplasma species Is Associated With Histologic Chorioamnionitis and Adverse Outcomes in Moderately Preterm and Late-Preterm Infants. J Infect Dis 213: 1340–1347. doi: 10.1093/infdis/jiv587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waites KB, Katz B, Schelonka RL (2005) Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev 18: 757–789. doi: 10.1128/CMR.18.4.757-789.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viscardi RM (2014) Ureaplasma species: role in neonatal morbidities and outcomes. Arch Dis Child Fetal Neonatal Ed 99: F87–92. doi: 10.1136/archdischild-2012-303351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glaser K, Speer CP (2015) Neonatal CNS infection and inflammation caused by Ureaplasma species: rare or relevant? Expert Rev Anti Infect Ther 13: 233–248. doi: 10.1586/14787210.2015.999670 [DOI] [PubMed] [Google Scholar]
- 14.Lowe J, Watkins WJ, Edwards MO, Spiller OB, Jacqz-Aigrain E, Kotecha SJ, et al. (2014) Association between pulmonary ureaplasma colonization and bronchopulmonary dysplasia in preterm infants: updated systematic review and meta-analysis. Pediatr Infect Dis J 33: 697–702. doi: 10.1097/INF.0000000000000239 [DOI] [PubMed] [Google Scholar]
- 15.Carey JC, Blackwelder WC, Nugent RP, Matteson MA, Rao AV, Eschenbach DA, et al. (1991) Antepartum cultures for Ureaplasma urealyticum are not useful in predicting pregnancy outcome. The Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol 164: 728–733. [DOI] [PubMed] [Google Scholar]
- 16.Steel JH, Malatos S, Kennea N, Edwards AD, Miles L, Duggan P, et al. (2005) Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res 57: 404–411. doi: 10.1203/01.PDR.0000153869.96337.90 [DOI] [PubMed] [Google Scholar]
- 17.Volgmann T, Ohlinger R, Panzig B (2005) Ureaplasma urealyticum-harmless commensal or underestimated enemy of human reproduction? A review. Arch Gynecol Obstet 273: 133–139. doi: 10.1007/s00404-005-0030-1 [DOI] [PubMed] [Google Scholar]
- 18.Mitchell CM, Haick A, Nkwopara E, Garcia R, Rendi M, Agnew K, et al. (2015) Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am J Obstet Gynecol 212: 611 e611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abele-Horn M, Wolff C, Dressel P, Pfaff F, Zimmermann A (1997) Association of Ureaplasma urealyticum biovars with clinical outcome for neonates, obstetric patients, and gynecological patients with pelvic inflammatory disease. J Clin Microbiol 35: 1199–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marovt M, Kese D, Kotar T, Kmet N, Miljkovic J, Soba B, et al. (2015) Ureaplasma parvum and Ureaplasma urealyticum detected with the same frequency among women with and without symptoms of urogenital tract infection. Eur J Clin Microbiol Infect Dis 34: 1237–1245. doi: 10.1007/s10096-015-2351-8 [DOI] [PubMed] [Google Scholar]
- 21.Lee SE, Romero R, Kim EC, Yoon BH (2009) A high Nugent score but not a positive culture for genital mycoplasmas is a risk factor for spontaneous preterm birth. J Matern Fetal Neonatal Med 22: 212–217. doi: 10.1080/14767050802616994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Payne MS, Goss KC, Connett GJ, Kollamparambil T, Legg JP, Thwaites R, et al. (2010) Molecular microbiological characterization of preterm neonates at risk of bronchopulmonary dysplasia. Pediatr Res 67: 412–418. doi: 10.1203/PDR.0b013e3181d026c3 [DOI] [PubMed] [Google Scholar]
- 23.Payne MS, Goss KC, Connett GJ, Legg JP, Bruce KD, Chalker V (2012) A quantitative analysis of Ureaplasma urealyticum and Ureaplasma parvum compared with host immune response in preterm neonates at risk of developing bronchopulmonary dysplasia. J Clin Microbiol 50: 909–914. doi: 10.1128/JCM.06625-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donders GGG, Ruban K, Bellen G, Petricevic L (2017) Mycoplasma/Ureaplasma infection in pregnancy: to screen or not to screen. J Perinat Med 45: 505–515. doi: 10.1515/jpm-2016-0111 [DOI] [PubMed] [Google Scholar]
- 25.Stout MJ, Conlon B, Landeau M, Lee I, Bower C, Zhao Q, et al. (2013) Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol 208: 226 e221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Namba F, Hasegawa T, Nakayama M, Hamanaka T, Yamashita T, Nakahira K, et al. (2010) Placental features of chorioamnionitis colonized with Ureaplasma species in preterm delivery. Pediatr Res 67: 166–172. doi: 10.1203/PDR.0b013e3181c6e58e [DOI] [PubMed] [Google Scholar]
- 27.Oh KJ, Lee KA, Sohn YK, Park CW, Hong JS, Romero R, et al. (2010) Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 203: 211 e211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menon R, Peltier MR, Eckardt J, Fortunato SJ (2009) Diversity in cytokine response to bacteria associated with preterm birth by fetal membranes. Am J Obstet Gynecol 201: 306 e301–306. [DOI] [PubMed] [Google Scholar]
- 29.Snyder CC, Wolfe KB, Gisslen T, Knox CL, Kemp MW, Kramer BW, et al. (2013) Modulation of lipopolysaccharide-induced chorioamnionitis by Ureaplasma parvum in sheep. Am J Obstet Gynecol 208: 399 e391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kacerovsky M, Pliskova L, Menon R, Kutova R, Musilova I, Maly J, et al. (2014) Microbial load of umbilical cord blood Ureaplasma species and Mycoplasma hominis in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 27: 1627–1632. doi: 10.3109/14767058.2014.887068 [DOI] [PubMed] [Google Scholar]
- 31.Patterson AM, Taciak V, Lovchik J, Fox RE, Campbell AB, Viscardi RM (1998) Ureaplasma urealyticum respiratory tract colonization is associated with an increase in interleukin 1-beta and tumor necrosis factor alpha relative to interleukin 6 in tracheal aspirates of preterm infants. Pediatr Infect Dis J 17: 321–328. [DOI] [PubMed] [Google Scholar]
- 32.Viscardi RM, Manimtim WM, Sun CC, Duffy L, Cassell GH (2002) Lung pathology in premature infants with Ureaplasma urealyticum infection. Pediatr Dev Pathol 5: 141–150. [DOI] [PubMed] [Google Scholar]
- 33.Goldenberg RL, Andrews WW, Goepfert AR, Faye-Petersen O, Cliver SP, Carlo WA, et al. (2008) The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol 198: 43 e41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glaser K, Silwedel C, Fehrholz M, Waaga-Gasser A-M, Henrich B, Claus H, et al. (2017) Ureaplasma species differentially modulate pro- and anti-inflammatory cytokine responses in newborn and adult human monocytes pushing the state towards pro-inflammation Frontiers in cellular and infection microbiology accepted manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arango Duque G, Descoteaux A (2014) Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 5: 491 doi: 10.3389/fimmu.2014.00491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner MD, Nedjai B, Hurst T, Pennington DJ (2014) Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta 1843: 2563–2582. doi: 10.1016/j.bbamcr.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 37.Atkinson JJ, Senior RM (2003) Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol 28: 12–24. doi: 10.1165/rcmb.2002-0166TR [DOI] [PubMed] [Google Scholar]
- 38.Glaser K, Silwedel C, Fehrholz M, Waaga-Gasser AM, Henrich B, Claus H, et al. (2017) Ureaplasma Species Differentially Modulate Pro- and Anti-Inflammatory Cytokine Responses in Newborn and Adult Human Monocytes Pushing the State Toward Pro-Inflammation. Front Cell Infect Microbiol 7: 484 doi: 10.3389/fcimb.2017.00484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purcell RH, Taylor-Robinson D, Wong D, Chanock RM (1966) Color test for the measurement of antibody to T-strain mycoplasmas. J Bacteriol 92: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stemke GW, Robertson JA (1982) Comparison of two methods for enumeration of mycoplasmas. J Clin Microbiol 16: 959–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mobius N, Brenneisen W, Schaeffer A, Henrich B (2012) Protocol for the rapid detection of the urogenital tract mollicutes and Chlamydia with concomitant LGV-(sub)typing. Methods Mol Biol 903: 235–253. doi: 10.1007/978-1-61779-937-2_15 [DOI] [PubMed] [Google Scholar]
- 42.Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 43.Speer CP (2006) Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med 11: 354–362. doi: 10.1016/j.siny.2006.03.004 [DOI] [PubMed] [Google Scholar]
- 44.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S (2007) The role of inflammation and infection in preterm birth. Semin Reprod Med 25: 21–39. doi: 10.1055/s-2006-956773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss G, Goldsmith LT, Taylor RN, Bellet D, Taylor HS (2009) Inflammation in reproductive disorders. Reprod Sci 16: 216–229. doi: 10.1177/1933719108330087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon BH, et al. (1994) Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol 32: 108–113. [DOI] [PubMed] [Google Scholar]
- 47.Jacobsson B, Holst RM, Wennerholm UB, Andersson B, Lilja H, Hagberg H (2003) Monocyte chemotactic protein-1 in cervical and amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation, and preterm delivery. Am J Obstet Gynecol 189: 1161–1167. [DOI] [PubMed] [Google Scholar]
- 48.Keeler SM, Kiefer DG, Rust OA, Vintzileos A, Atlas RO, Bornstein E, et al. (2009) Comprehensive amniotic fluid cytokine profile evaluation in women with a short cervix: which cytokine(s) correlates best with outcome? Am J Obstet Gynecol 201: 276 e271–276. [DOI] [PubMed] [Google Scholar]
- 49.Groneck P, Gotze-Speer B, Oppermann M, Eiffert H, Speer CP (1994) Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high-risk preterm neonates. Pediatrics 93: 712–718. [PubMed] [Google Scholar]
- 50.Murch SH, Costeloe K, Klein NJ, MacDonald TT (1996) Early production of macrophage inflammatory protein-1 alpha occurs in respiratory distress syndrome and is associated with poor outcome. Pediatr Res 40: 490–497. doi: 10.1203/00006450-199609000-00020 [DOI] [PubMed] [Google Scholar]
- 51.Baier RJ, Majid A, Parupia H, Loggins J, Kruger TE (2004) CC chemokine concentrations increase in respiratory distress syndrome and correlate with development of bronchopulmonary dysplasia. Pediatr Pulmonol 37: 137–148. doi: 10.1002/ppul.10417 [DOI] [PubMed] [Google Scholar]
- 52.Bose C, Laughon M, Allred EN, Van Marter LJ, O’Shea TM, Ehrenkranz RA, et al. (2011) Blood protein concentrations in the first two postnatal weeks that predict bronchopulmonary dysplasia among infants born before the 28th week of gestation. Pediatr Res 69: 347–353. doi: 10.1203/PDR.0b013e31820a58f3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leviton A, Kuban KC, Allred EN, Fichorova RN, O’Shea TM, Paneth N, et al. (2011) Early postnatal blood concentrations of inflammation-related proteins and microcephaly two years later in infants born before the 28th post-menstrual week. Early Hum Dev 87: 325–330. doi: 10.1016/j.earlhumdev.2011.01.043 [DOI] [PubMed] [Google Scholar]
- 54.Yu H, Yuan L, Zou Y, Peng L, Wang Y, Li T, et al. (2014) Serum concentrations of cytokines in infants with retinopathy of prematurity. APMIS 122: 818–823. doi: 10.1111/apm.12223 [DOI] [PubMed] [Google Scholar]
- 55.Natarajan G, Shankaran S, McDonald SA, Das A, Stoll BJ, Higgins RD, et al. (2010) Circulating beta chemokine and MMP 9 as markers of oxidative injury in extremely low birth weight infants. Pediatr Res 67: 77–82. doi: 10.1203/PDR.0b013e3181c0b16c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aghai ZH, Camacho J, Saslow JG, Mody K, Eydelman R, Bhat V, et al. (2012) Impact of histological chorioamnionitis on tracheal aspirate cytokines in premature infants. Am J Perinatol 29: 567–572. doi: 10.1055/s-0032-1311980 [DOI] [PubMed] [Google Scholar]
- 57.Kacerovsky M, Celec P, Vlkova B, Skogstrand K, Hougaard DM, Cobo T, et al. (2013) Amniotic fluid protein profiles of intraamniotic inflammatory response to Ureaplasma spp. and other bacteria. PLoS One 8: e60399 doi: 10.1371/journal.pone.0060399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kemp MW, Saito M, Kallapur SG, Jobe AH, Keelan JA, Li S, et al. (2011) Inflammation of the fetal ovine skin following in utero exposure to Ureaplasma parvum. Reprod Sci 18: 1128–1137. doi: 10.1177/1933719111408114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Athayde N, Edwin SS, Romero R, Gomez R, Maymon E, Pacora P, et al. (1998) A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol 179: 1248–1253. [DOI] [PubMed] [Google Scholar]
- 60.Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon BH, Edwin S, et al. (2002) Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol 187: 1125–1130. [DOI] [PubMed] [Google Scholar]
- 61.Tency I, Verstraelen H, Kroes I, Holtappels G, Verhasselt B, Vaneechoutte M, et al. (2012) Imbalances between matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) in maternal serum during preterm labor. PLoS One 7: e49042 doi: 10.1371/journal.pone.0049042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cockle JV, Gopichandran N, Walker JJ, Levene MI, Orsi NM (2007) Matrix metalloproteinases and their tissue inhibitors in preterm perinatal complications. Reprod Sci 14: 629–645. doi: 10.1177/1933719107304563 [DOI] [PubMed] [Google Scholar]
- 63.Speer CP, Ruess D, Harms K, Herting E, Gefeller O (1993) Neutrophil elastase and acute pulmonary damage in neonates with severe respiratory distress syndrome. Pediatrics 91: 794–799. [PubMed] [Google Scholar]
- 64.Chetty A, Cao GJ, Severgnini M, Simon A, Warburton R, Nielsen HC (2008) Role of matrix metalloprotease-9 in hyperoxic injury in developing lung. Am J Physiol Lung Cell Mol Physiol 295: L584–592. doi: 10.1152/ajplung.00441.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Das A, McLamore A, Song W, McGuire PG (1999) Retinal neovascularization is suppressed with a matrix metalloproteinase inhibitor. Arch Ophthalmol 117: 498–503. [DOI] [PubMed] [Google Scholar]
- 66.Girard S, Sebire G, Kadhim H (2010) Proinflammatory orientation of the interleukin 1 system and downstream induction of matrix metalloproteinase 9 in the pathophysiology of human perinatal white matter damage. J Neuropathol Exp Neurol 69: 1116–1129. doi: 10.1097/NEN.0b013e3181f971e4 [DOI] [PubMed] [Google Scholar]
- 67.Straat K, de Klark R, Gredmark-Russ S, Eriksson P, Soderberg-Naucler C (2009) Infection with human cytomegalovirus alters the MMP-9/TIMP-1 balance in human macrophages. J Virol 83: 830–835. doi: 10.1128/JVI.01363-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoang M, Potter JA, Gysler SM, Han CS, Guller S, Norwitz ER, et al. (2014) Human fetal membranes generate distinct cytokine profiles in response to bacterial Toll-like receptor and nod-like receptor agonists. Biol Reprod 90: 39 doi: 10.1095/biolreprod.113.115428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peltier MR, Barney BM, Brown MB (2007) Effect of experimental genital mycoplasmosis on production of matrix metalloproteinases in membranes and amniotic fluid of Sprague-Dawley rats. Am J Reprod Immunol 57: 116–121. doi: 10.1111/j.1600-0897.2006.00449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, et al. (2009) Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci 16: 56–70. doi: 10.1177/1933719108325508 [DOI] [PubMed] [Google Scholar]
- 71.Lal CV, Xu X, Jackson P, Atkinson TP, Faye-Petersen OM, Kandasamy J, et al. (2016) Ureaplasma infection-mediated release of matrix metalloproteinase-9 and PGP: a novel mechanism of preterm rupture of membranes and chorioamnionitis. Pediatr Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsuboi N, Yoshikai Y, Matsuo S, Kikuchi T, Iwami K, Nagai Y, et al. (2002) Roles of toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J Immunol 169: 2026–2033. [DOI] [PubMed] [Google Scholar]
- 73.Arechavaleta-Velasco F, Ogando D, Parry S, Vadillo-Ortega F (2002) Production of matrix metalloproteinase-9 in lipopolysaccharide-stimulated human amnion occurs through an autocrine and paracrine proinflammatory cytokine-dependent system. Biol Reprod 67: 1952–1958. [DOI] [PubMed] [Google Scholar]
- 74.Manimtim WM, Hasday JD, Hester L, Fairchild KD, Lovchik JC, Viscardi RM (2001) Ureaplasma urealyticum modulates endotoxin-induced cytokine release by human monocytes derived from preterm and term newborns and adults. Infect Immun 69: 3906–3915. doi: 10.1128/IAI.69.6.3906-3915.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noda-Nicolau NM, Polettini J, Peltier MR, da Silva MG, Menon R (2016) Combinations and loads of bacteria affect the cytokine production by fetal membranes: An in vitro study. Am J Reprod Immunol 76: 504–511. doi: 10.1111/aji.12596 [DOI] [PubMed] [Google Scholar]
- 76.Kallapur SG, Kramer BW, Knox CL, Berry CA, Collins JJ, Kemp MW, et al. (2011) Chronic fetal exposure to Ureaplasma parvum suppresses innate immune responses in sheep. J Immunol 187: 2688–2695. doi: 10.4049/jimmunol.1100779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwak DW, Hwang HS, Kwon JY, Park YW, Kim YH (2014) Co-infection with vaginal Ureaplasma urealyticum and Mycoplasma hominis increases adverse pregnancy outcomes in patients with preterm labor or preterm premature rupture of membranes. J Matern Fetal Neonatal Med 27: 333–337. doi: 10.3109/14767058.2013.818124 [DOI] [PubMed] [Google Scholar]
- 78.von Chamier M, Allam A, Brown MB, Reinhard MK, Reyes L (2012) Host genetic background impacts disease outcome during intrauterine infection with Ureaplasma parvum. PLoS One 7: e44047 doi: 10.1371/journal.pone.0044047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Winters AH, Levan TD, Vogel SN, Chesko KL, Pollin TI, Viscardi RM (2013) Single nucleotide polymorphism in toll-like receptor 6 is associated with a decreased risk for ureaplasma respiratory tract colonization and bronchopulmonary dysplasia in preterm infants. Pediatr Infect Dis J 32: 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sung TJ, Xiao L, Duffy L, Waites KB, Chesko KL, Viscardi RM (2011) Frequency of ureaplasma serovars in respiratory secretions of preterm infants at risk for bronchopulmonary dysplasia. Pediatr Infect Dis J 30: 379–383. doi: 10.1097/INF.0b013e318202ac3a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kacerovsky M, Pliskova L, Bolehovska R, Skogstrand K, Hougaard DM, Tsiartas P, et al. (2012) The impact of the microbial load of genital mycoplasmas and gestational age on the intensity of intraamniotic inflammation. Am J Obstet Gynecol 206: 342 e341–348. [DOI] [PubMed] [Google Scholar]
- 82.Shimizu T, Kida Y, Kuwano K (2008) Ureaplasma parvum lipoproteins, including MB antigen, activate NF-{kappa}B through TLR1, TLR2 and TLR6. Microbiology 154: 1318–1325. doi: 10.1099/mic.0.2007/016212-0 [DOI] [PubMed] [Google Scholar]
- 83.Friedland YD, Lee-Pullen TF, Nathan E, Watts R, Keelan JA, Payne MS, et al. (2015) Whole blood flow cytometric analysis of Ureaplasma-stimulated monocytes from pregnant women. J Reprod Immunol 109: 84–88. doi: 10.1016/j.jri.2014.12.008 [DOI] [PubMed] [Google Scholar]
- 84.Cobo T, Vives I, Rodriguez-Trujillo A, Murillo C, Angeles MA, Bosch J, et al. (2017) Impact of microbial invasion of amniotic cavity and the type of microorganisms on short-term neonatal outcome in women with preterm labor and intact membranes. Acta Obstet Gynecol Scand 96: 570–579. doi: 10.1111/aogs.13095 [DOI] [PubMed] [Google Scholar]
- 85.Gussenhoven R, Ophelders D, Kemp MW, Payne MS, Spiller OB, Beeton ML, et al. (2017) The Paradoxical Effects of Chronic Intra-Amniotic Ureaplasma parvum Exposure on Ovine Fetal Brain Development. Dev Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data are given for term neonatal and adult monocytes. Both isolates caused a dose-dependent induction of MCP-1 (A), MCP-3 (B), MIP-1α (C), MIP-1β (D) and MMP-9 mRNA (E) at 4 h assessment (n = 3).
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.