Abstract
Background
Human Immunodeficiency Virus-Infected (HIV+) persons have elevated risks for various manifestations of cardiovascular disease (CVD). No studies to our knowledge have compared atrial fibrillation (AF) and atrial flutter (AFL) prevalence and associated characteristics for HIV+ persons and matched uninfected controls.
Methods and findings
Persons with diagnoses of HIV receiving care at a large urban academic medical center were frequency-matched 1:2 on age, sex, race, zip code, and clinic location with uninfected persons. Possible AF/AFL was screened for using administrative codes and diagnoses of AF/AFL were subsequently adjudicated using electrocardiography and physician notes; adjudication was performed given the inconsistent validity of administrative code-derived AF diagnoses found in previous studies. There were 101 confirmed AF/AFL cases (2.00%) among 5,052 HIV+ patients and 159 confirmed AF/AFL cases (1.57%) among 10,121 uninfected controls [Odds Ratio (OR) 1.27, 95% Confidence Interval (CI) 0.99–1.64; p = 0.056]. The association between HIV serostatus and AF/AFL was attenuated after adjustment for demographics and CVD risk factors. Among HIV+ persons, nadir CD4+ T cell count <200 cells/mm3 was associated with approximately twofold elevated odds of AF/AFL even after adjustment for demographics and CVD risk factors (Multivariable-adjusted OR 1.98, 95% CI 1.21–3.25). There was no significant association between log10 of peak HIV viral load and AF/AFL (Multivariable-adjusted OR 1.03, 95% CI 0.86–1.24). Older age, diabetes, hypertension, and chronic obstructive pulmonary disease were associated with similarly elevated odds of AF/AFL for HIV+ persons and uninfected controls.
Conclusion
HIV-related immunosuppression (nadir CD4 T cell count <200 cells/mm3) and traditional CVD risk factors are associated with significantly elevated odds of AF/AFL among HIV+ persons. Although atrial fibrillation and flutter was more common among HIV+ versus uninfected persons in this cohort, this difference was attenuated by adjustment for demographics and CVD risk factors.
Introduction
The longevity of human immunodeficiency virus-infected (HIV+) persons has increased due to effective and available antiretroviral therapy (ART) [1–8]. As the HIV+ population ages, HIV+ persons are experiencing an increasing burden of comorbidities, including cardiovascular diseases (CVDs) [9–11]. Myocardial infarction, arrhythmias, heart failure, and sudden cardiac death all appear to occur more frequently for HIV+ versus uninfected persons [10, 12–15]. However, although epidemiologic data suggest elevated risks for arrhythmias and sudden cardiac death among HIV+ persons, clinical characteristics and mechanisms associated with these risks are not well understood. A previous analysis of HIV+ persons in the Veterans Affairs (VA) HIV Clinical Case Registry (which is >97% male) used International Classification of Disease-9 (ICD-9) codes to identify likely AF/AFL diagnoses and found that high HIV viral load and low CD4+ T cell count (CD4 count) were associated with significantly elevated incidence of AF/AFL [14]. However, no previous studies to our knowledge have compared AF/AFL for HIV+ persons and uninfected controls. Similarly, although administrative codes may have a positive predictive value as low as 70% for identifying AF, no previous studies to our knowledge have adjudicated AF/AFL diagnoses among HIV+ persons [16].
In this study, we compared the prevalence of physician-adjudicated AF/AFL among HIV+ persons and matched uninfected controls in a large electronic cohort and evaluated factors associated with AF/AFL for HIV+ persons. We hypothesized that: 1) AF/AFL is more common among HIV+ persons compared with uninfected controls and 2) Traditional CVD risk factors, lower nadir CD4 count, and higher peak HIV viral load are associated with greater risk for AF/AFL among HIV+ persons.
Methods
Study population
We used a large electronic health record (EHR)-based cohort of HIV-infected persons and matched uninfected controls receiving care at a large urban academic center: the HIV Electronic Comprehensive Cohort of CVD Complications (HIVE-4CVD). During the period of observation from January 1, 2000 to July 12, 2016, we identified HIV+ adults aged 18 years and older using the following validated definition[17]: 1) positive HIV-1 antibody or serology, 2) positive (>0) HIV viral load, or 3) at least three orders of HIV viral load and a CD4 T cell count ordered on the same date. Uninfected controls were frequency-matched with HIV+ persons using a propensity score incorporating age, sex, race, zip-code, and clinic location. The HIVE-4CVD cohort creation and this protocol were approved by the Institutional Review Board at Northwestern University (Chicago, IL). A waiver of consent was applied by the Institutional Review Board due to the infeasibility of contacting patients in this retrospective analysis of already-collected data and chart review.
Demographics and clinical covariates
Data from the first clinical encounter for each patient were used to determine baseline age, sex, and race. Body-mass index (BMI) was defined as weight (kg) divided by the square of height (m2) at the first concurrent height and weight measurement for each patient recorded in the NMEDW. Smoking data were not available for HIV+ persons and inconsistently available for uninfected persons in our cohort and were therefore no able to be included in analyses. Hypertension was defined by ICD-9 (401–405) or ICD-10 (I10-I15) codes, and due to heterogeneity of inpatient and outpatient visits in this cohort with the potential for systematic differences in measurement, observed blood pressure values were not used in the definition of hypertension [12]. Diagnoses of diabetes were described as possible in an individual with ICD-9 or ICD-10 code (ICD-9-CM 249–250, ICD-10-CM E08-11, ICD-10-CM E13), and positive if an individual had an ICD-9 or ICD-10 code and (1) a hemoglobin A1C >6.4% or (2) was prescribed any diabetic medication [18]. Chronic obstructive pulmonary disease (COPD) diagnoses were identified using ICD9 codes (ICD-9-CM 491–492, 496) or ICD-10-CM codes (ICD-10-CM J43-J44). CHADS score was tabulated for both HIV+ and HIV- patients using these diagnosis codes as well as stroke diagnosis codes (ICD-9 (433–436) or ICD-10 (I60-I63)) [19, 20]. Hepatitis C virus infection was defined as a positive result for a hepatitis C virus antibody test or detectable viremia [21]. CD4 lymphocyte counts, HIV-1 RNA levels, and use of ART were also collected for HIV+ patients. We evaluated nadir CD4 count and peak HIV viral load, rather than baseline, most recent, or as a time-updated covariate, due to heterogeneity in the timing and setting of CD4 count and HIV viral load measurement in our EHR-based study.
Atrial fibrillation/atrial flutter screen and adjudication
Physician-adjudicated atrial fibrillation/atrial flutter was the primary outcome of interest in this study. In the initial screen, patients were considered to have possible AF/AFL based on the following criteria: 1) mention of AF/AFL in a physician note or problem list from inpatient or outpatient encounters, using “atrial fibrillation” and “atrial flutter” as search terms; 2) any ICD-9-CM or ICD-10-CM diagnosis code from inpatient or outpatient encounters consistent with atrial fibrillation (427.31, I48.0, I48.1, I48.2, I48.91) or atrial flutter (427.32; I18.3, I48.4, I48.92); and/or 3) any encounter with CPT procedural codes consistent with AF/AFL-related procedures (93653, 93655, 93656, 93657). Two independent investigators then performed chart review for each person with possible AF/AFL and identified persons with confirmed AF/AFL based on electrocardiographic evidence of AF/AFL and/or documentation in physician notes confirming AF/AFL.
Statistical analyses
We first compared demographics and clinical covariates of HIV+ persons and uninfected controls in our cohort. Next, we compared the prevalence of adjudicated AF/AFL for HIV+ persons and uninfected controls patients diagnosed with AF/AFL using multivariable-adjusted logistic regression. We then evaluated associations of HIV-specific exposures (nadir CD4 count <200 cells/mm3 and log-transformed peak HIV viral load) with AF/AFL for HIV+ persons using multivariable-adjusted logistic regression. Multivariate models were performed unadjusted (Model 1); adjusted for age, sex, and race (Model 2; persons of black, white, or Hispanic race were included due to incomplete data for other races in this cohort); and additionally adjusted for BMI, hypertension, diabetes, and COPD (Model 3). For a sensitivity analysis, we added duration of antiretroviral therapy (ART) as a covariate to Model 3; ART duration for each HIV+ person was approximated as the time between the earliest noted date of ART prescription and either most recent follow up or death.
Results
Demographics and clinical covariates of the HIVE-4CVD cohort are shown in Table 1. As expected in a frequency-matched cohort in which age and demographics were criteria for matching, the mean age in both HIV+ and HIV- groups was 48.2, and a similar proportion of patients in each group was male (82.5% and 82.8%, respectively). Patients diagnosed with HIV were more likely to have a lower BMI, high total cholesterol and low HDL cholesterol, hypertension, diabetes, CHD, MI, COPD, and stroke (p<0.01). Of the 5,052 HIV+ persons, 133 were identified as having possible AF/AFL from the primary screen, of which 101 (2.00%) were confirmed to have AF/AFL by physician adjudication. Among uninfected controls, 208 out of 10,121 persons were identified as having possible AF/AFL, of which 159 (1.57%) were confirmed to have AF/AFL by physician adjudication. This borderline-significant difference in AF/AFL prevalence for HIV+ versus uninfected persons (OR 1.28, 95% CI 0.996–1.65; p = 0.056) was attenuated after adjustment for demographics and CVD risk factors (Table 2).
Table 1. Demographic and clinical characteristics of HIV+ persons and frequency-matched controls.
| HIV+ (N = 5052) | Controls (N = 10121) | P-value | ||
|---|---|---|---|---|
| Age (years) | 48.2 ± 11.6 | 48.2 ± 11.4 | 0.68 | |
| 18–30, N (%) | 331 (6.6%) | 647 (6.4%) | 0.98 | |
| 31–40 | 921 (18.3%) | 1871 (18.5%) | ||
| 41–50 | 1451 (28.8%) | 2886 (28.5%) | ||
| 51–60 | 1615 (32.0%) | 3291 (32.5%) | ||
| 61–70 | 590 (11.7%) | 1159 (11.4%) | ||
| >70 | 133 (2.6%) | 267 (2.6%) | ||
| Race (N, %) | <0.01 | |||
| White | 1680 (34.5%) | 3207 (39.9%) | ||
| Black | 1573 (32.3%) | 2698 (33.5%) | ||
| Hispanic | 173 (3.6%) | 359 (4.5%) | ||
| Other/Unknown | 1438 (29.6%) | 1783 (22.2%) | ||
| Male (N, %) | 4167 (82.5%) | 8381 (82.8%) | 0.13 | |
| Body-Mass Index (kg/m2) | 25.9 ± 5.6 | 28.6 ± 6.5 | <0.01 | |
| Total cholesterol (mg/dl) | 202.2 ± 59.1 | 194.5 ± 50.6 | <0.01 | |
| HDL cholesterol (mg/dl) | 34.3 ± 13.1 | 42.9 ± 14.1 | <0.01 | |
| Hepatitis C Virus (N, %) | 425 (8.4%) | 90 (0.9%) | <0.01 | |
| Hypertension (N, %) | 1674 (33.1%) | 1893 (18.7%) | <0.01 | |
| Diabetes (N, %) | 463 (9.2%) | 603 (6.0%) | <0.01 | |
| Coronary Heart Disease (N, %) | 728 (14.4%) | 704 (7.0%) | <0.01 | |
| Myocardial Infarction (N, %) | 571 (11.3%) | 502 (5.0%) | <0.01 | |
| Chronic Obstructive Pulmonary Disease (N, %) | 305 (6.0%) | 223 (2.2%) | <0.01 | |
| Stroke, N (%) | 316 (6.3%) | 281 (2.8%) | <0.01 | |
| Atrial Fibrillation (N, %) | 101 (2.00%) | 159 (1.57%) | 0.056 | |
| CHADS Score (N, %) | <0.01 | |||
| 0 | 2915 (57.8%) | 7784 (76.9%) | ||
| 1 | 1213 (24.1%) | 1354 (13.4%) | ||
| 2 | 509 (10.1%) | 601 (5.9%) | ||
| 3 | 226 (4.5%) | 220 (2.2%) | ||
| 4 | 125 (2.5%) | 100 (1.0%) | ||
| 5 | 47 (0.9%) | 54 (0.5%) | ||
| 6 | 6 (0.1%) | 8 (0.1%) | ||
| CHADS Score <2 (N, %) | 4128 (81.9%) | 9138 (90.3%) | <0.01 | |
Note: Continuous variables compared with t-test or nonparametric equivalent; Categorical variables compared with chi-square or nonparametric equivalent; Total cholesterol and HDL cholesterol calculated as peak and nadir, respectively, given several measurements over time
Table 2. Odds ratios of atrial fibrillation/flutter by HIV status.
| Variable | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| HIV+ Status | 1.28 (0.996–1.65) | 1.06 (0.81–1.41) | 0.74 (0.55–1.004) |
| Age | 1.08 (1.07–1.09) | 1.05 (1.04–1.07) | |
| Male sex | 1.82 (1.14–2.90) | 2.03 (1.26–3.28) | |
| White | 1.0 (ref) | 1.0 (ref) | |
| Black | 1.16 (0.87–1.54) | 0.98 (0.72–1.34) | |
| Hispanic | 0.77 (0.43–1.38) | 0.67 (0.36–1.25) | |
| Body-Mass index | 1.02 (1.004–1.05) | ||
| Hypertension diagnosis | 2.74 (1.92–3.89) | ||
| Diabetes diagnosis | 1.95 (1.41–2.70) | ||
| COPD diagnosis | 2.52 (1.72–3.67) |
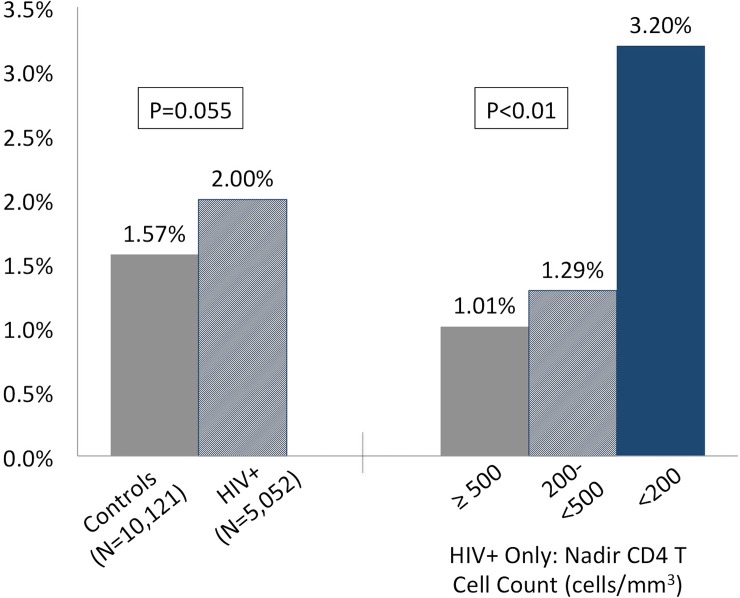
Among HIV+ persons, nadir CD4 count <200 cells/mm3 was associated with approximately two-fold greater odds of AF/AFL; this persisted even after adjustment for age, sex, race, BMI, diabetes, hypertension, smoking, and COPD (Table 3; adjusted OR 1.98, 95% CI 1.21–3.25). Meanwhile, peak HIV viral load was not associated with a significantly elevated odds of AF/AFL (Table 4); a sensitivity analysis comparing HIV+ persons with peak HIV viral load >100,000 copies/mL vs. <500 copies/mL similarly yielded no significant difference in AF/AFL odds (adjusted OR 1.35, 95% CI 0.83–2.20). Interestingly, whereas the proportion of HIV+ persons with CD4+ T cell count <200 with AF/AFL (3.2%) was more than twice that of uninfected controls (1.57%), the proportion of HIV+ persons with higher CD4+ T cell nadir with AF/AFL was actually lower than that of uninfected controls (Fig 1).
Table 3. Odds ratios of atrial Arrhythmias for HIV+ persons by CD4 nadir (N = 3355).
| Variable | Model 1 | Model 2 |
|---|---|---|
| CD4 nadir <200 cells/mm3 (vs. ≥200 cells/mm) | 1.99 (1.25–3.17) | 1.98 (1.21–3.25) |
| Age | 1.07 (1.05–1.09) | 1.04 (1.02–1.07) |
| Male sex | 1.57 (0.77–3.21) | 1.58 (0.75–3.30) |
| White | 1.0 (ref) | 1.0 (ref) |
| Black | 1.11 (0.69–1.80) | 0.89 (0.54–1.47) |
| Hispanic | 0.86 (0.38–1.97) | 0.66 (0.27–1.62) |
| Body-Mass Index (kg/m2) | 1.00 (0.96–1.05) | |
| Diabetes diagnosis | 2.22 (1.31–3.75) | |
| Hypertension diagnosis | 2.92 (1.65–5.17) | |
| COPD diagnosis | 2.03 (1.13–3.65) |
Table 4. Odds ratios of atrial Arrhythmias for HIV+ persons by peak HIV VL (N = 2913).
| Variable | Model 1 | Model 2 |
|---|---|---|
| Log10 of Peak HIV VL (copies/mL) | 1.04 (0.86–1.24) | 1.03 (0.86–1.24) |
| Age | 1.08 (1.05–1.10) | 1.05 (1.02–1.07) |
| Male sex | 1.23 (0.59–2.56) | 1.30 (0.61–2.78) |
| White | 1.0 (ref) | 1.0 (ref) |
| Black | 1.17 (0.69–1.98) | 0.91 (0.53–1.59) |
| Hispanic | 0.79 (0.30–2.06) | 0.52 (0.18–1.54) |
| Body-Mass Index (kg/m2) | 1.00 (0.95–1.04) | |
| Diabetes diagnosis | 2.51 (1.41–4.45) | |
| Hypertension diagnosis | 2.76 (1.50–5.08) | |
| COPD diagnosis | 2.02 (1.06–3.85) |
Fig 1. Atrial fibrillation and flutter prevalence for HIV-infected and uninfected persons in HIVE-4CVD.
In addition to low nadir CD4 count, older age, diabetes, hypertension, and COPD were all associated with elevated odds of AF/AFL among HIV+ persons (Tables 3 and 4). Traditional CVD risk factors (age, diabetes, hypertension, and COPD) were also associated with AF/AFL for uninfected controls (S1 Table). Interestingly, whereas elevated BMI was associated with significantly elevated odds of AF/AFL for uninfected controls (S1 Table; OR 1.04, 95% CI 1.01–1.06 for every 1 kg/m2 increment in BMI), there was essentially no association between BMI and AF/AFL for HIV+ persons (Tables 3 and 4). In sensitivity analyses in which ART duration was added to multivariable models, each additional year of ART was associated with a slightly lower odds of AF/AFL (S2 Table and S3 Table).
Discussion
In this study, we found that HIV-associated immune dysfunction (as indicated by nadir CD4+ T cell count <200 cells/mm3) was associated with more than twofold greater likelihood of having AF/AFL even after adjustment for demographics and CVD risk factors. We also found that HIV+ patients had elevated odds of AF/AFL when compared to uninfected controls, but that this was attenuated after adjustment for demographic factors and comorbidities and appeared largely driven by HIV+ persons with nadir CD4+ T cell count <200 cells/mm3. Old age, hypertension, diabetes, and COPD all were associated with similarly elevated odds of AF/AFL for HIV+ patients and uninfected controls.
We found that HIV-related immune dysfunction (as represented by nadir CD4+ T cell count <200 cells/mm3) is associated with more than twofold greater odds of physician-adjudicated AF/AFL among HIV+ persons even after adjustment for demographics and CVD risk factors. Peak HIV viral load, meanwhile, was not associated with AF/AFL among HIV+ persons in this study. Whereas the finding regarding HIV-related immune dysfunction and AF/AFL corroborates a similar finding from the only previous study to our knowledge evaluating AF/AFL among HIV+ persons (conducted in the VA HIV Clinical Case Registry [14], our null finding regarding the HIV viral load and AF/AFL is not in agreement with the VA study.
Our study benefited from the inclusion of an uninfected control group, having a substantial minority of female participants (>17% compared with <3% in the VA study), and using adjudicated AF/AFL endpoints. Thus, it is possible that our different findings regarding HIV viral load and AF/AFL relate to cohort effects or differences in outcome ascertainment. Nevertheless, given the consistent and strong association we found between low nadir CD4+ T cell count and AF/AFL, it may be that immune dysregulation and dysfunction, rather than viremia itself, contributes most to AF/AFL in HIV+ patients.
Atrial remodeling and fibrosis are well-characterized causes of atrial fibrillation [22], and more recent studies have shown that four general types of remodeling lead to the development of AF: electrical, structural, neural, and calcium-related [23]. Systemic diseases with widespread effects (such as diabetes, hypertension, and COPD) and more localized disease processes (such as CAD and MI) are associated with chronic inflammation, myocardial remodeling, and ultimately AF [24, 25]. In HIV, chronic immune dysregulation (as marked by low CD4+ T cell count, among other factors) can lead to chronic inflammation and subclinical CVD [26–29]. Persons with HIV appear to have higher ambulatory blood pressure than uninfected persons and attenuated physiological blood pressure variation, particularly at lower CD4+ T cell counts [30, 31]. Furthermore, diastolic dysfunction and myocardial fibrosis are substantially more common and extensive among HIV-infected persons and may be associated with blood pressure dysregulation, chronic inflammation, immune dysregulation, and/or maladaptive reaction to vascular injury [32–34]. However, given the relative paucity of CVD event data from well-phenotyped HIV cohorts with available biospecimens, precise mechanisms by which HIV-related immune dysfunction and inflammation may lead to CVD remain uncertain. In light of our findings, it is plausible that HIV-related immune dysfunction (as reflected in low nadir CD4+ T cell count) and inflammation increase susceptibility to AF/AFL, but longitudinal studies incorporating biomarker data would be instrumental in further elucidating these mechanisms. Furthermore, studies with adequate power to differentiate between AF and AFL, as well as subtypes of AFL (e.g., right atrial versus left atrial origin) would be useful.
In this study, we also found that AF/AFL was somewhat more prevalent among HIV+ persons compared with frequency-matched controls, but that this difference was attenuated to statistical non-significance after adjustment for demographics and CVD risk factors. This may be due in part to a greater burden of co-morbidity among HIV+ persons compared with uninfected controls, as reflected in the generally sicker nature of the HIV+ persons in our cohort compared with uninfected controls frequency-matched on demographics and zip code. Nevertheless, our finding that greater progression of HIV was associated with a substantially greater likelihood of AF/AFL among HIV+ persons suggests that HIV disease-related factors may also play a role in AF/AFL.
Strengths and limitations
In our relatively large cohort of HIV+ persons and uninfected matched controls in clinical care, we had the opportunity to review comprehensive clinical data and adjudicate AF/AFL diagnoses. This is the first study to our knowledge to (1) evaluate AF/AFL among HIV+ persons and matched uninfected controls, and (2) analyze adjudicated AF/AFL for HIV+ persons (rather than an administrative code-based endpoint of AF/AFL). These types of analyses are particularly timely given the increasing size and age of the HIV+ population as well as its increasingly apparent elevated risks for various CVD manifestations. Thus, these observational data in a real-life clinical cohort represent a necessary early step to ultimately improving CVD diagnosis, treatment, and prevention among HIV+ persons.
There are several limitations that must be acknowledged. First, as with any electronic health record cohort analysis we are limited by the quality of the data available in the EHR. Although our initial screen included the gold standard ICD-9 and ICD-10 codes, in addition to CPT and word search terms, individual physician documentation is variable, and it is likely that some patients with inadequate documentation of AF/AFL events (particularly out-of-network events) were excluded. However, these limitations were largely unavoidable given the nature of our EHR cohort analyses, and we attempted to account for some of the misclassification through physician adjudication of AF/AFL cases. Second, these analyses were cross-sectional and observational and thus we could not draw causal inferences from our conclusions; we were unable to evaluate time-updated variables or reliable incidence rates of AF/AFL given the structure of our limited electronic dataset. Third, smoking was not included as a covariate in multivariable modeling due to inconsistent availability of these data in our cohort. Given the mildly elevated risk for AF among smokers [35], it is possible that additional adjustment for smoking would have further lowered the multivariable-adjusted odds ratio AF/AFL for HIV+ persons compared with uninfected controls. We were also unable to evaluate alcohol, a known risk factor for AF, as a clinical covariate because these data were not available in our cohort and could not be feasibly collected due to the retrospective nature of this study. Finally, the observation period of 16 years in our study could affect the strength of correlation between HIV status and AF/AFL, as patients may have not received comparable treatments for both HIV and other comorbid conditions.
Conclusion
We found in a cohort of HIV+ persons and matched, uninfected controls that greater HIV-related immunosuppression is associated with substantially greater odds of AF/AFL among HIV+ persons. Although HIV+ persons had a greater prevalence of AF/AFL than uninfected controls in unadjusted analyses, this was attenuated to non-significance by adjustment for demographics and CVD risk factors and was driven largely by HIV+ persons with nadir CD4+ T cell count <200; HIV+ persons with higher nadir CD4+ T cell counts were no more likely than uninfected controls to have AF/AFL. These findings suggest a potential role of advanced HIV infection and related immunosuppression as a factor associated with AF/AFL for HIV+ patients. Future longitudinal analyses in cohorts with available biorepositories should evaluate potential mechanisms by which HIV-related immune dysfunction may be associated with myocardial fibrosis, dysfunction, and related CVD outcomes including but not limited to atrial arrhythmias.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Data Availability
Data are available upon request. There are legal and ethical restrictions on making the source data for this paper publicly available; data contain potentially identifying information and access is governed by our institutional Data Security and Access policies (http://www.feinberg.northwestern.edu/it/policies/information-security/index.html). The contact information for the data access committee is: FSMIT-Policy@northwestern.edu.
Funding Statement
Research reported in this publication was supported, in part, by the National Institutes of Health's National Center for Advancing Translational Sciences (Grant Number UL1TR001422) and the Northwestern Medicine Enterprise Data Warehouse (NMEDW); the National Institutes of Health P30AI117943; and the American Heart Association Fellow-to-Faculty Transition Award 16FTF31200010. URL for the National Institutes of Health: https://www.nih.gov/. URL for the American Heart Association: http://www.heart.org/HEARTORG/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Palella FJ Jr., Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–60. doi: 10.1056/NEJM199803263381301 [DOI] [PubMed] [Google Scholar]
- 2.Detels R, Tarwater P, Phair JP, Margolick J, Riddler SA, Munoz A, et al. Effectiveness of potent antiretroviral therapies on the incidence of opportunistic infections before and after AIDS diagnosis. AIDS. 2001;15(3):347–55. [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ Jr, Chmiel JS, Moorman AC, Holmberg SD, Investigators HIVOS. Durability and predictors of success of highly active antiretroviral therapy for ambulatory HIV-infected patients. AIDS. 2002;16(12):1617–26. [DOI] [PubMed] [Google Scholar]
- 4.Moore RD, Chaisson RE. Natural history of HIV infection in the era of combination antiretroviral therapy. AIDS. 1999;13(14):1933–42. [DOI] [PubMed] [Google Scholar]
- 5.Vittinghoff E, Scheer S, O'Malley P, Colfax G, Holmberg SD, Buchbinder SP. Combination antiretroviral therapy and recent declines in AIDS incidence and mortality. J Infect Dis. 1999;179(3):717–20. doi: 10.1086/314623 [DOI] [PubMed] [Google Scholar]
- 6.Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998;352(9142):1725–30. [DOI] [PubMed] [Google Scholar]
- 7.Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, d'Arminio Monforte A, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362(9377):22–9. [DOI] [PubMed] [Google Scholar]
- 8.Sterne JA, Hernan MA, Ledergerber B, Tilling K, Weber R, Sendi P, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366(9483):378–84. doi: 10.1016/S0140-6736(05)67022-5 [DOI] [PubMed] [Google Scholar]
- 9.Palella FJ, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era—Changing causes of death and disease in the HIV outpatient study. Jaids-J Acq Imm Def. 2006;43(1):27–34. [DOI] [PubMed] [Google Scholar]
- 10.Feinstein MJ, Bahiru E, Achenbach C, Longenecker CT, Hsue P, So-Armah K, et al. Patterns of Cardiovascular Mortality for HIV-Infected Adults in the United States: 1999 to 2013. The American journal of cardiology. 2016;117(2):214–20. doi: 10.1016/j.amjcard.2015.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trickey A, May MT, Vehreschild J, Obel N, Gill MJ, Crane H, et al. Cause-Specific Mortality in HIV-Positive Patients Who Survived Ten Years after Starting Antiretroviral Therapy. PLoS One. 2016;11(8):e0160460 doi: 10.1371/journal.pone.0160460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butt AA, Chang CC, Kuller L, Goetz MB, Leaf D, Rimland D, et al. Risk of Heart Failure With Human Immunodeficiency Virus in the Absence of Prior Diagnosis of Coronary Heart Disease. Arch Intern Med. 2011;171(8):737–43. doi: 10.1001/archinternmed.2011.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paisible AL, Chang CC, So-Armah KA, Butt AA, Leaf DA, Budoff M, et al. HIV Infection, Cardiovascular Disease Risk Factor Profile, and Risk for Acute Myocardial Infarction. Journal of acquired immune deficiency syndromes. 2015;68(2):209–16. doi: 10.1097/QAI.0000000000000419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu JC, Li Y, Marcus GM, Hsue PY, Scherzer R, Grunfeld C, et al. Atrial fibrillation and atrial flutter in human immunodeficiency virus-infected persons: incidence, risk factors, and association with markers of HIV disease severity. Journal of the American College of Cardiology. 2013;61(22):2288–95. doi: 10.1016/j.jacc.2013.03.022 [DOI] [PubMed] [Google Scholar]
- 15.Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, et al. Sudden Cardiac Death in Patients With Human Immunodeficiency Virus Infection. J Am Coll Cardiol. 2012;59(21):1891–6. doi: 10.1016/j.jacc.2012.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21 Suppl 1:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felsen UR, Bellin EY, Cunningham CO, Zingman BS. Development of an electronic medical record-based algorithm to identify patients with unknown HIV status. AIDS care. 2014;26(10):1318–25. doi: 10.1080/09540121.2014.911813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilke RA, Berg RL, Peissig P, Kitchner T, Sijercic B, McCarty CA, et al. Use of an electronic medical record for the identification of research subjects with diabetes mellitus. Clin Med Res. 2007;5(1):1–7. doi: 10.3121/cmr.2007.726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348(8):702–10. doi: 10.1056/NEJMoa022048 [DOI] [PubMed] [Google Scholar]
- 20.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278–85. [DOI] [PubMed] [Google Scholar]
- 21.Moorman AC, Gordon SC, Rupp LB, Spradling PR, Teshale EH, Lu M, et al. Baseline characteristics and mortality among people in care for chronic viral hepatitis: the chronic hepatitis cohort study. Clin Infect Dis. 2013;56(1):40–50. doi: 10.1093/cid/cis815 [DOI] [PubMed] [Google Scholar]
- 22.Sanfilippo AJ, Abascal VM, Sheehan M, Oertel LB, Harrigan P, Hughes RA, et al. Atrial Enlargement as a Consequence of Atrial-Fibrillation—a Prospective Echocardiographic Study. Circulation. 1990;82(3):792–7. [DOI] [PubMed] [Google Scholar]
- 23.Nattel S, Harada M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol. 2014;63(22):2335–45. doi: 10.1016/j.jacc.2014.02.555 [DOI] [PubMed] [Google Scholar]
- 24.Nattel S, Burstein B, Dobrev D. Atrial Remodeling and Atrial Fibrillation Mechanisms and Implications. Circ-Arrhythmia Elec. 2008;1(1):62–73. [DOI] [PubMed] [Google Scholar]
- 25.Wakili R, Voigt N, Kaab S, Dobrev D, Nattell S. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest. 2011;121(8):2955–68. doi: 10.1172/JCI46315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. The Journal of infectious diseases. 2012;205 Suppl 3:S375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsue PY, Scherzer R, Hunt PW, Schnell A, Bolger AF, Kalapus SC, et al. Carotid Intima-Media Thickness Progression in HIV-Infected Adults Occurs Preferentially at the Carotid Bifurcation and Is Predicted by Inflammation. J Am Heart Assoc. 2012;1(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein JH, Hsue PY. Inflammation, Immune Activation, and CVD Risk in Individuals With HIV Infection. Jama-J Am Med Assoc. 2012;308(4):405–6. [DOI] [PubMed] [Google Scholar]
- 29.Triant VA, Meigs JB, Grinspoon SK. Association of C-Reactive Protein and HIV Infection With Acute Myocardial Infarction. Jaids-J Acq Imm Def. 2009;51(3):268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Socio GV, Bonfanti P, Martinelli C, Ricci E, Pucci G, Marinoni M, et al. Negative influence of HIV infection on day-night blood pressure variability. Journal of acquired immune deficiency syndromes. 2010;55(3):356–60. doi: 10.1097/QAI.0b013e3181e46456 [DOI] [PubMed] [Google Scholar]
- 31.Schillaci G, Maggi P, Madeddu G, Pucci G, Mazzotta E, Penco G, et al. Symmetric ambulatory arterial stiffness index and 24-h pulse pressure in HIV infection: results of a nationwide cross-sectional study. J Hypertens. 2013;31(3):560–7; discussion 7. doi: 10.1097/HJH.0b013e32835ca949 [DOI] [PubMed] [Google Scholar]
- 32.Holloway CJ, Ntusi N, Suttie J, Mahmod M, Wainwright E, Clutton G, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128(8):814–22. doi: 10.1161/CIRCULATIONAHA.113.001719 [DOI] [PubMed] [Google Scholar]
- 33.Feinstein MJ, Mitter SS, Yadlapati A, Achenbach CJ, Palella FJ Jr., Gonzalez PE, et al. HIV-Related Myocardial Vulnerability to Infarction and Coronary Artery Disease. J Am Coll Cardiol. 2016;68(18):2026–7. doi: 10.1016/j.jacc.2016.07.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsue PY, Hunt PW, Ho JE, Farah HH, Schnell A, Hoh R, et al. Impact of HIV infection on diastolic function and left ventricular mass. Circulation Heart failure. 2010;3(1):132–9. doi: 10.1161/CIRCHEARTFAILURE.109.854943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu W, Yuan P, Shen Y, Wan R, Hong K. Association of smoking with the risk of incident atrial fibrillation: A meta-analysis of prospective studies. International journal of cardiology. 2016;218:259–66. doi: 10.1016/j.ijcard.2016.05.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Data are available upon request. There are legal and ethical restrictions on making the source data for this paper publicly available; data contain potentially identifying information and access is governed by our institutional Data Security and Access policies (http://www.feinberg.northwestern.edu/it/policies/information-security/index.html). The contact information for the data access committee is: FSMIT-Policy@northwestern.edu.