Abstract
Purpose
Determine if intratherapy PSA (itPSA) changes during radiation (RT) predict prostate cancer outcomes.
Methods & Materials
We retrospectively identified patients treated with definitive external beam RT without hormones that had at least two itPSA measurements. We calculated the adjusted ratio of rise (ARR) in itPSA relative to pretreatment baseline PSA (bPSA) for each patient. This was defined as: ln(max(itPSA) + 1) / ln(bPSA + 1). We stratified patients based on ARR < vs. > than 1.1. This corresponds to less than vs. greater than ~30% increases in PSA during RT. Univariate and multivariate analyses (MVA) were carried out examining biochemical failure free survival (BFS) and overall survival (OS).
Results
With a median follow up of 74 months we identified 307 patients that met our criteria. Univariate analysis revealed that patients with an ARR<1.1 (n=182) had statistically significant inferior BFS and OS compared to those with ARR>1.1 (n=125). The median BFS and OS for these two groups were 51 months vs. 101 months (p=0.001) and 96 months vs. 128 months (p=0.01). On MVA the effect of ARR on the risk of BF for patients with ARR < 1.1 was significant (p=0.03) only during the first year post-RT. In contrast, the effect of ARR on the OS remained significant for a full 5 years (p=0.05).
Conclusions
ARR <1.1 predicts for inferior BFS and OS in patients treated with radiation alone. PSA measurements during RT are a novel clinical tool that could be used to identify patients that may warrant more aggressive therapeutic interventions.
Keywords: Prostatic neoplasms, prostate-specific antigen, radiotherapy, kinetics, statistics
Introduction
Public perception of localized prostate cancer is that it is a more indolent malignancy compared to other cancers due in part to the long median time interval (8 years) to metastasis after biochemical failure (BF). In addition, once metastatic disease develops, the median time to death is approximately 5 years1. However, despite curative intent treatment, prostate cancer mortality is a significant contributor of death in many prostate cancer patients. Thus there has been an interest in identifying or predicting which patients will do poorly despite curative intent treatment. One area of that has received a lot of attention is the kinetics of PSA changes either before or after treatment1–12. However, little attention has been focused on the predictive power of PSA changes during RT.
Therefore, we set out to examine if changes in intratherapy PSA measurements could predict treatment outcomes in prostate cancer patients treated with external beam RT. This information may help identify higher-risk patients during definitive treatment that warrant more aggressive therapeutic approaches and further study.
Methods and materials
Patient selection
Shortly after PSA testing became widely available and during the four year period between 1/1989 & 12/1993, it was customary for patients treated in the Department of Radiation Oncology at University of Michigan or its affiliates to receive itPSA measurements approximately every 2 weeks. With institutional review board approval, we retrospectively reviewed the medical records of patients treated for localized prostate cancer with three dimensional conformal RT (3D-CRT) with curative intent during that period. Required data for the inclusion in this study included documentation of: T stage, bPSA, and GS. In addition, patients required at least 2 recorded itPSA. Exclusion criteria included the presence of known lymphatic metastases, metastatic disease, the use of neoadjuvant or adjuvant androgen deprivation or chemotherapy, as well as history of previous prostatectomy, cryosurgery or brachytherapy. We identified 307 patients that met our criteria.
All patients had pretreatment evaluation that included history and physical, pretreatment PSA, prostate biopsy as well as bone scan and diagnostic CT if clinically indicated. Pretreatment risk groups were defined using the D’Amico scheme: low risk: T≤2a, GS<7, and PSA<10; high risk: >/= T2c and/or GS>7 and/or PSA>2013. All other patients were considered intermediate risk.
Patient treatment
All patients were treated with CT-based 3D-conformal treatment. Depending on their pretreatment risk group the pelvic lymph nodes and/or seminal vesicles were treated. Seventy three percent received pelvic nodal RT. The median prostate dose to the International Commission on Radiation Units and Measurements point was 69Gy.
Follow-up and end points
The median PSA follow-up was 74 months. Patients were seen at regular intervals, every 3 to 6 months for physical examinations, digital rectal examinations, and serial PSA measurements. Radiologic evaluation was performed if clinically indicated. We utilized a BF definition based on the Phoenix definition of current nadir +2 or the initiation of salvage hormonal therapy14. PFS was defined from the end of treatment without biochemical, clinical, or distant failure. The censoring date for patients free of progression or death was the last contact date.
Statistical analysis
Mean number of itPSA values was 3.7 and ranged from 2 to 6 per patient. bPSA was defined as last PSA measured before start of RT. Peak itPSA was defined as maximum itPSA recorded. The ARR was defined as the ln(max(itPSA) + 1) / ln(bPSA + 1). This equation measures the relative rise in PSA during RT by determining the ratio between maximum PSA during therapy and normalizing it to the baseline pre treatment PSA in the context of a log scale. An ARR of >1.1 corresponds approximately to a 30% or greater increase from bPSA to itPSA for patients with bPSA range of 4–20. An ARR of 1–1.1 corresponds to less than 30% increase while an ARR of ≤ 1.0 corresponds to no increase in PSA above the baseline value.
Univariate analyses to compare patients with high versus low ARR were carried out with chi-square tests for frequencies, t-tests for means, Wilcoxon rank sum tests for medians, and log-rank tests for survival analyses. Kaplan-Meier plots and Cox proportional hazards models were utilized with BFS, PFS, and OS as endpoints. Proportional hazards assumption was checked for all predictive factors for Cox models and when the assumption was found to fail, interactions with time were incorporated to handle the non-proportional hazards. Multivariate analyses were examined controlling for: age at RT, race, bPSA, RT dose, T-stage, GS, and pelvis nodal RT. Data was analyzed using the SAS system (version 9.1, SAS Institute, Cary, NC). A two sided p-value <0.05 was considered significant.
Results
Patient characteristics
We identified 450 patients that had at least one itPSA value. From this group 307 met our requirement of at least 2 recorded itPSA measurements as well as sufficient clinical and treatment information. The mean age of the group was 71 years. The median bPSA was 14.6 ng/mL. Forty two percent of patients had a GS of 7 or greater. Overall 16% were low risk, 29% were intermediate risk and 55% were high risk. Median RT dose was 69Gy. The median and mean ARR for the group were 1.06 and 1.12, respectively. We choose a cut point of 1.1 by rounding the median ARR of 1.06 to 1.1. We did not examine other cut points. When stratified by ARR, patients with ARR≤1.1 had significantly higher bPSA (22 ng/mL vs. 7.6 ng/mL, p<0.001), higher GS score (50% GS≥7 vs 30% GS≥7, p=0.003), and larger proportion of high risk patients (68% vs. 35%, p<0.001), compared to those with ARR >1.1. In addition, this group was more often treated with pelvic nodal RT (79% vs. 65%, p=0.004). The clinical and treatment characteristics for the cohort are shown in Table 1.
Table 1.
Clinical & Treatment Characteristics:
| Patient Characteristics | Overall | ARR > 1.1 | ARR < 1.1 | p-value* |
|---|---|---|---|---|
| Number of patients | 307 | 125 | 182 | |
| Mean age at start of RT (SD) | 70.8 (6.5) | 70.0 (5.8) | 71.4 (6.9) | 0.06 |
| Median bPSA (IQR) | 14.6 (7.1–27.4) | 7.6 (4.7–14.0) | 21.6 (11.8–33.8) | <.0001 |
| Median months from bPSA to RT (IQR) | 2.4 (1.5–3.4) | 2.7 (1.8–4.0) | 2.1 (1.3–3.3) | |
| Median treatment year (IQR) | 1991 (1991–1992) | 1991 (1991–1992) | 1991 (1991–1992) | |
| Median total dose (IQR) | 69 (67.0–69.0) | 67.8 (67.0–69.0) | 69 (67.6–69.0) | 0.004 |
| Whole Pelvis RT (%) | 225 (73.3) | 81 (64.8) | 144 (79.1) | 0.005 |
| Median Number of Post-RT PSAs (IQR) | 9 (5–14) | 9 (6–14) | 9 (5–14) | 0.76 |
| Race (%) | 0.67 | |||
| White | 257 (83.7) | 104 (83.2) | 153 (84.1) | |
| Black | 40 (13.0) | 18 (14.4) | 22 (12.1) | |
| Other/Unknown | 10 (3.3) | 3 (2.4) | 7 (3.8) | |
| T-stage (%) | 0.21 | |||
| T1 | 53 (17.3) | 24 (19.2) | 29 (15.9) | |
| T2 | 215 (70.0) | 90 (72.0) | 125 (68.7) | |
| T3/T4 | 39 (12.7) | 11 (8.8) | 28 (15.4) | |
| Gleason Score (%) | 0.003 | |||
| GS 2–6 | 178 (58.0) | 87 (69.6) | 91 (50.0) | |
| GS 7 | 98 (31.9) | 29 (23.2) | 69 (37.9) | |
| GS 8–10 | 31 (10.1) | 9 (7.2) | 22 (12.1) | |
| bPSA (%) | <.0001 | |||
| PSA < 10 | 111 (36.2) | 76 (60.8) | 35 (19.2) | |
| PSA 10–20 | 83 (27.0) | 31 (24.8) | 52 (28.6) | |
| PSA > 20 | 113 (36.8) | 18 (14.4) | 95 (52.2) | |
| Risk Group (%) | <.0001 | |||
| Low | 49 (16.0) | 36 (28.8) | 13 (7.1) | |
| Intermediate | 90 (29.3) | 45 (36.0) | 45 (24.7) | |
| High | 168 (54.7) | 44 (35.2) | 124 (68.1) | |
| Median Follow-up (Mo) | ||||
| PSA | 74.4 | 84.7 | 51.9 | |
| OS | 133.9 | 130.6 | 139.4 |
Abbreviations: ARR = Adjusted Ratio of Rise; RT = radiation therapy; bPSA = pretreatment PSA; Mo = months; OS = overall survival; SD = Standard Deviation; IQR = Interquartile range.
Comparisons between groups done with chi-square tests for frequencies, t-tests for means, and Wilcoxon rank sum tests for medians.
Treatment Outcomes
Out of 307 patients 129 (42%) had a PSA failure, and 6 (2%) more were considered biochemical failure due to the start of salvage ADT. An additional 4 (1.3%) patients had clinical progression and a total of 175 (57%) of patients died during the 74 month median biochemical and 134 month median clinical follow up period.
Intra-treatment PSA Changes
One hundred patients (33%) had a ARR ≤ 1.0 (a drop in PSA during RT), 82 patients (27%) had a ARR of 1–1.1 (<30% increase in PSA) and 125 patients (41%) had an ARR>1.1 (greater than 30% increase in PSA). Sample PSA trajectories and calculations are depicted in Figure 1. When stratified by ARR >1.1 vs. ≤ 1.1 there was no difference in the mean number of itPSA values (3.7) or the median number of days from first to last itPSA measurement (41 days). However, the median peak itPSA was marginally higher in patients with ARR≤1.1 vs. those with ARR>1.1 (18 ng/mL vs 15 ng/mL, p=0.05). Table 2 summarizes the characteristics of itPSA for this cohort.
Figure 1.
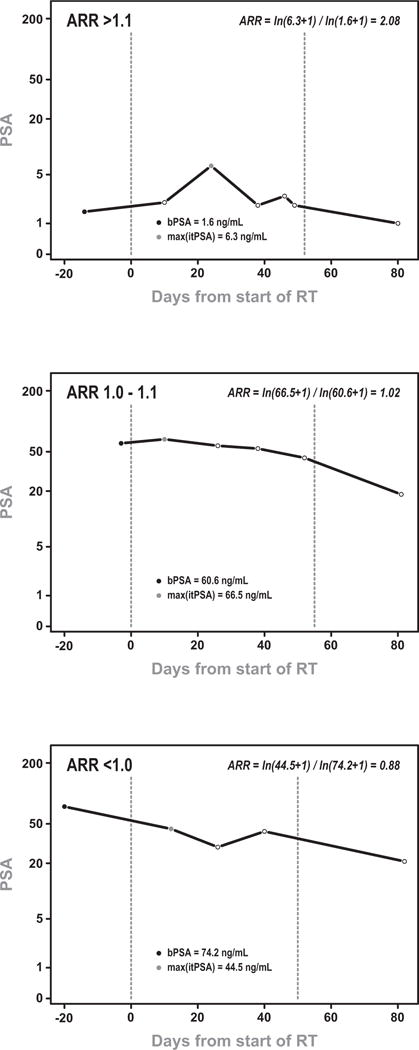
Sample PSA trajectories for patients with ARR: (a) >1.1, (b) 1.0–1.1, (c) <1.0. Adjusted Ratio of Rise (ARR) was calculated as ln(max(itPSA) + 1) / ln(bPSA + 1) and are depicted in each figures.
Table 2.
Intra-Therapy PSA:
| Overall | ARR > 1.1 | ARR < 1.1 | p-value | |
|---|---|---|---|---|
| Number of patients | 307 | 125 | 182 | |
| Mean Number of itPSAs (SD) | 3.7 (0.7) | 3.7 (0.6) | 3.6 (0.8) | 0.17 |
| Number of itPSAs (%) | ||||
| 2 | 23 (7.5) | 5 (4.0) | 18 (9.9) | |
| 3 | 77 (25.1) | 28 (22.4) | 49 (26.9) | |
| 4 | 189 (61.6) | 87 (69.6) | 102 (56.0) | |
| 5 | 15 (4.9) | 5 (4.0) | 10 (5.5) | |
| 6 | 3 (1.0) | 0 (0.0) | 3 (1.6) | |
| Median Days First to Last itPSA (IQR) | 41 (34–42) | 41 (35–42) | 41 (29–42) | 0.33 |
| Median Peak itPSA (IQR) | 16.2 (9.1–30.1) | 14.7 (8.6–25.9) | 18.2 (10.2–36.3) | 0.05 |
Abbreviations: ARR = Adjusted Ratio of Rise; itPSA = intra-therapy PSA; SD = Standard Deviation.
Survival Analyses
Univariate analysis revealed that patients with a ARR ≤ 1.1 had statistically significant inferior BFS (Figure 2), PFS (data not shown) and OS (Figure 3) compared to those with ARR>1.1. The median BFS, PFS, and OS were 51 months vs. 101 months (p=0.001), 44 months vs. 101 months (p=0.0003), and 96 months vs. 128 months (p=0.01). Treatment outcomes are depicted in Table 3. Further analysis revealed that the effect of ARR was strongest immediately following radiation therapy and decreased over time for all three endpoints. Therefore all Cox models included an interaction between ARR and time.
Fig 2.
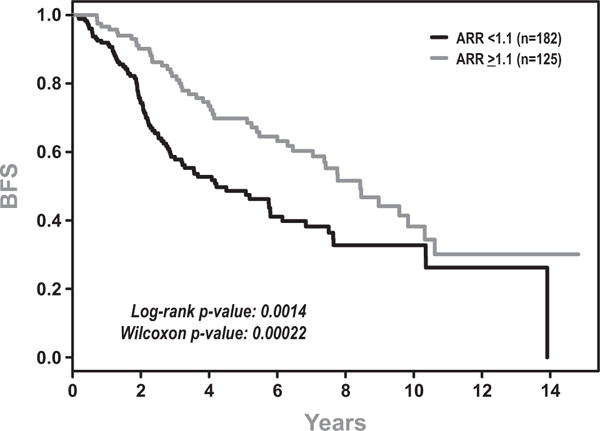
Biochemical Failure Free Survival (BFS) for patients with Adjusted Ratio of Rise (ARR) >1.1 vs <1.1.
Fig 3.
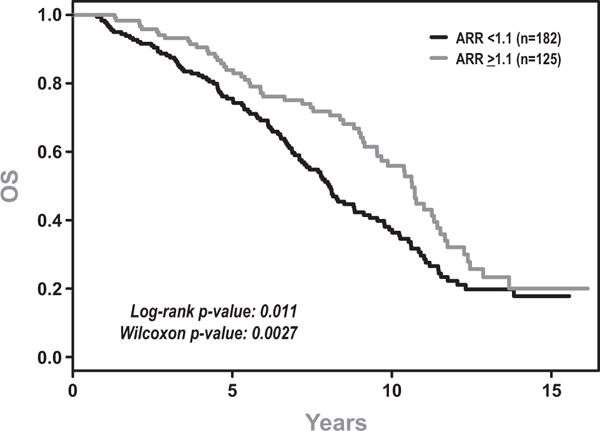
Overall Survival (OS) for patients with Adjusted Ratio of Rise (ARR) >1.1 vs <1.1.
Table 3.
Treatment Outcomes:
| Patient Characteristics | Overall | ARR > 1.1 | ARR < 1.1 | p-value* |
|---|---|---|---|---|
| Number of patients | 307 | 125 | 182 | |
| Median Survival (Mo) (95% CI) | ||||
| BFS | 75.7 (61.4, 93.2) | 101.3 (84.6, 124.0) | 50.6 (36.0, 73.8) | 0.001 |
| PFS | 69.1 (49.8, 90.1) | 101.3 (75.7, 127.5) | 44.1 (34.0, 69.1) | 0.0003 |
| OS | 109.5 (99.3, 122.8) | 127.6 (114.5, 137.2) | 96.4 (85.3, 109.5) | 0.01 |
| 5-Year Survival (95% CI) | ||||
| BFS | 58% (51%, 64%) | 70% (61%, 79%) | 49% (40%, 57%) | |
| PFS | 55% (48%, 61%) | 68% (58%, 77%) | 45% (36%, 54%) | |
| OS | 79% (74%, 84%) | 84% (77%, 91%) | 76% (69%, 82%) |
Abbreviations: ARR = Adjusted Ratio of Rise; BFS = Biochemical Failure Free Survival; PFS = Progression Free Survival; OS = overall survival, Mo = months.
Log-rank tests.
In an unadjusted analysis of the effect of ARR on BFS the risk of failure immediately following RT was 5.8 times higher for subjects with ARR < 1.1 (95% CI: 2.1–16, p=0.001) and decreased with time since therapy. The risk remained statistically significant until 4 years post-therapy. Controlling for clinical characteristics in a multivariate analysis (MVA) diminished the effect of ARR; in an adjusted analysis the risk of failure for patients with ARR < 1.1 was 3.4 times higher initially (95% CI: 1.1–10, p=0.03) but by just one year post-RT the hazard fell to 1.7 and was statistically non-significant, result shown in Figure 4a. In the MVA bPSA (p<0.0001), Gleason (GS) (p=0.01), and RT dose (p=0.04) were significant of BFS. Table 4 displays the results of both the unadjusted and adjusted analyses. Results for PFS were similar to BFS, data not shown.
Figure 4.
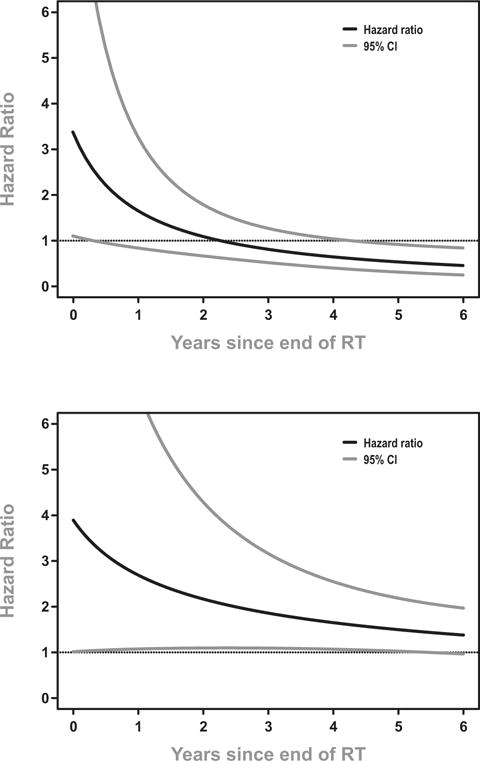
Relative hazards for two outcomes following radiation therapy, Adjusted Ratio of Rise (ARR) < 1.1 versus ARR > 1.1. Results from multivariate Cox models adjusting for additional clinical characteristics such as Gleason, T-stage, bPSA. Dotted lines indicate the 95% confidence interval. A hazard ratio of 1 denotes no elevated risk of failure.
(a) Biochemical Failure
(b) Death (all causes)
Table 4.
Effect of ARR on biochemical failure-free survival. Results from Cox models (a) unadjusted and (b) adjusted for clinical characteristics.
| BFS | (a) Unadjusted Model | (b) Adjusted Model | ||||
|---|---|---|---|---|---|---|
| Variable | Years since RT | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | p-value |
| ARR < 1.1 vs. ARR > 1.1 | 0 | 5.8 | (2.0, 16) | 3.4 | (1.10, 10.34) | |
| 1 | 3.2 | (1.7, 5.9) | 1.7 | (0.84, 3.27) | ||
| 2 | 2.3 | (1.5, 3.5) | 1.1 | (0.67, 1.79) | ||
| 3 | 1.8 | (1.2, 2.6) | 0.81 | (0.52, 1.27) | ||
| 4 | 1.5 | (0.98, 2.2) | 0.65 | (0.40, 1.04) | ||
| ln(bPSA + 1)+ | 2.11 | (1.6, 2.7) | <.0001 | |||
| Gleason*: GS 7 vs. GS 2–6 | 1.69 | (1.1, 2.5) | 0.01 | |||
| Gleason: GS 8-10 vs. GS 2–6 | 1.92 | (1.1, 3.4) | 0.03 | |||
| T-stage**: T2 vs. T1 | 1.42 | (0.82, 2.5) | 0.21 | |||
| T-stage: T3/T4 vs. T1 | 1.69 | (0.84, 3.4) | 0.14 | |||
| Total Dose+ | 0.97 | (0.94, 1.0) | 0.04 | |||
| Age at RT+ | 0.99 | (0.96, 1.02) | 0.41 | |||
| Whole Pelvis | 1.4 | (0.90, 2.3) | 0.13 | |||
| White vs. Non-White | 0.68 | (0.42, 1.08) | 0.10 | |||
Abbreviations: ARR = Adjusted Ratio of Rise; bPSA = pretreatment PSA; RT = radiation therapy. Model stratified by hospital.
Hazard ratios for a 1-unit increase.
p-value for overall Gleason Score effect = 0.02.
p-value for overall T-stage effect = 0.31
The interaction of time with ARR for an OS endpoint was also examined and found to be significant. In an unadjusted analysis, ARR was found to be a significant predictor for OS for a full 6 years of follow up, p=0.03 (Table 5). In addition, when other factors were included that were significant, the predictive value of ARR on OS remained for a full 5 yrs after follow up (Figure 4b). Other factors predictive of OS in the MVA included age at RT (p=0.001) and race (p=0.002). However, in our OS analysis bPSA, GS, T stage and RT dose were not significant (Table 5).
Table 5.
Effect of ARR on overall survival. Results from Cox models (a) unadjusted and (b) adjusted for clinical characteristics.
| OS | (a) Unadjusted Model | (b) Adjusted Model | ||||
|---|---|---|---|---|---|---|
| Variable | Years since RT | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | p-value |
| ARR > 1.1 vs. ARR < 1.1 | 0 | 4.4 | (1.2, 16.8) | 3.9 | (1.01, 15) | |
| 1 | 3.0 | (1.2, 7.4) | 2.7 | (1.1, 6.7) | ||
| 2 | 2.4 | (1.2, 4.7) | 2.2 | (1.1, 4.3) | ||
| 3 | 2.1 | (1.2, 3.4) | 1.9 | (1.1, 3.2) | ||
| 4 | 1.8 | (1.2, 2.7) | 1.6 | (1.1, 2.5) | ||
| 5 | 1.6 | (1.2, 2.3) | 1.5 | (1.03, 2.2) | ||
| 6 | 1.5 | (1.1, 2.1) | 1.4 | (0.97, 2.0) | ||
| ln(bPSA + 1)+ | 0.91 | (0.74, 1.1) | 0.33 | |||
| Gleason*: GS 7 vs. GS 2–6 | 1.28 | (0.89, 1.8) | 0.19 | |||
| Gleason: GS 8–10 vs. GS 2–6 | 1.37 | (0.81, 2.3) | 0.25 | |||
| T-stage**: T2 vs. T1 | 1.05 | (0.70, 1.6) | 0.81 | |||
| T-stage: T3/T4 vs. T1 | 0.83 | (0.45, 1.6) | 0.57 | |||
| Total Dose+ | 1.02 | (0.98, 1.06) | 0.31 | |||
| Age at RT+ | 1.05 | (1.02, 1.07) | 0.001 | |||
| Whole Pelvis | 1.4 | (0.93, 2.1) | 0.10 | |||
| White vs. Non-White | 0.50 | (0.33, 0.78) | 0.002 | |||
Abbreviations: ARR = Adjusted Ratio of Rise; bPSA = pretreatment PSA; RT = radiation therapy. Model Model stratified by hospital.
Hazard ratios for a 1-unit increase.
p-value for overall Gleason Score effect = 0.32.
p-value for overall T-stage effect = 0.69
Discussion
PSA is widely recognized as an important tool in screening, risk stratification and monitoring for disease recurrence in patients with prostate cancer. However, a variety of non malignant factors have been shown to alter serum PSA levels including: digital manipulation15, biopsy, transurethral prostate resection16, cycling17, racial ethnicity18, BMI19, inflammatory process20–22, and RT23, 24.
Two series have described the incidental increase in PSA in patients receiving pelvic radiation for non prostatic malignancies. The University of Chicago reported increases up to 6½ fold23. Other groups have noted similar findings with up to 3½ fold increases in PSA levels during RT24. The reason for this incidental increase in PSA in non-prostate cancer patients has been proposed to be due to prostate acinar cell death, inflammation, as well as disruption of the capillary basement membrane with subsequent release of PSA into the circulation23.
A number of limited studies have examined PSA changes during RT in prostate cancer patients. Ben-Josef et al reported on a series of 158 patients with up to 2.7 years of follow-up. They examined the interaction of clinical factors as well as pre, post and intra PSA values on predicting BF. In their, initial univariate analysis a greater difference between bPSA and itPSA was significantly associated with a lower risk of failure. However, in the final multivariable model this was no longer significant25. A 2nd series 64 prostate cancer patients described PSA changes during RT but did not examine treatment outcomes23.
In the salvage RT setting two series have been reported. A published series 42 patients measured PSA at 30 and 45Gy. In their multivariate analysis, an increasing PSA level at 45Gy was a significant predictor of subsequent BF26. Similar findings were observed by Wiegel27. However, we caution that these post prostatectomy series may be examples of extraprostatic PSA sources such as regional lymph node or metastatic disease that would not have been addressed by local salvage. In addition, since these patients were post prostatectomy, they should have near zero residual normal prostate tissue. Thus these patterns of itPSA changes should not be considered when evaluating intact prostate RT induced itPSA changes.
In our series, we examined changes in PSA during treatment by examining utilizing a novel parameter known as ARR. This was defined as the ln(max(itPSA) + 1) / ln(bPSA + 1). An ARR of 1.1 corresponds approximately to a 30% increase from bPSA to itPSA. We utilized this PSA kinetics parameter as it would allow us to compare the relative magnitude of rise in PSA during treatment by controlling for there bPSA. In our series we dichotomized patients into those with greater than vs. less than 30% increases in PSA above baseline.
We observed that patients with an itPSA increase of less than 30% (ARR≤1.1) had a significantly larger proportion of high risk patients 68% vs. 35%, compared to those with a greater than 30% increase in itPSA (ARR >1.1). However, these patients were more often treated with more aggressive therapy that included regional nodal RT (Table 1). Fortunately, our series were well balanced in regards to the number and temporal spacing of itPSA values.
In our analysis we observed that an ARR≤1.1 had statistically significant inferior BFS, PFS, and OS (Figure 3) compared to those with ARR>1.1. For all of these endpoints we found an interaction between ARR and time. Therefore a Cox models with time and ARR were evaluated. For example, for BFS a significant impact of ARR during the first 3 years of follow up after RT. However, in the MVA, ARR was significant for BF only during the 1st year after RT (p=0.03) (Table 4). Interestingly, ARR was found to be a significant predictor for OS for the full 6 years of follow up (Table 5). Furthermore, the predictive value of ARR on OS remained for a full 5yrs after follow up. To our knowledge, this is the first time intratherapy PSA rise has been found to predict for both a biochemical and overall survival endpoint.
While the mechanism for these findings is unknown, we hypothesize that rise in itPSA may represent the normal prostate tissue response to RT insult. This is supported by the observation that prostate manipulation, inflammatory process and even incidental RT in non prostate cancer patients can cause PSA elevations15, 17,20–24. This response would be abrogated in prostate cancer tissue. This is supported by the observation, in the University of Chicago prostate cancer series that only 5% of patients with bPSA >10 ng/ml had an increasing PSA during RT. In contrast, 48% of patients with bPSA ≤ 10ng/ml had an increasing PSA during RT23. Thus ARR may be another indicator of prostate disease burden. For patients with a low percentage of prostate involvement the normal prostate tissue response to RT would predominate and thus a large relative itPSA increase above bPSA would occur (ARR would be >1.1). As tumor burden (indicated by bPSA) within the gland predominates, there would be less normal prostate tissue available to produce an increase in itPSA and thus the ARR would be ≤1.1. In our series, 78% of patients with bPSA<10 (N=111) and 61% of patients with bPSA>10 (N=196) showed a rise in itPSA, consistent with the hypothesis that with less disease burden, there is more of a rise in itPSA. Finally, the ARR appears to be independent predictor compared to bPSA.
Future work could focus on validating these clinical findings, establishing pathophyisology behind this observation, as well as examining if ARR could be used as true surrogate for OS. In order to establish this one would have to meet all four of Prentice’s criteria. Previous, analysis of other PSA kinetics variables such as PSA DT have failed to meet all of the Prentice requirements28. Should ARR meet the all parameters required it would be the first validated surrogate for survival in prostate cancer.
Our study did have several limitations. First our study was retrospective in nature and thus carries any inherent biases in such a study design. As such we cannot exclude that there may be unaccounted for confounding patient, tumor, or treatment factors that have impacted our results. Secondly, our sample size of 307 patients is small. Thus, these findings should be considered preliminary and in need of validation in a larger patient group. A third concern, is that our median dose of 69Gy does not reflect the current escalated doses being employed. Furthermore, patients with intermediate and high risk features may be treated with ADT depending on there co-morbities. ADT patients were excluded as PSA kinetics would be significantly affected by hormonal therapy. While lower RT doses do compromise the applicability of these findings to a more modern cohort we believe it’s reasonable to expect that the observed phenomena would not differ in patients treated at the current standard dose for high risk patients of 78Gy, but without ADT. However, these observations clearly require validation in a modern patient cohort. In addition, these results cannot be extrapolated to patients receiving brachytherapy or chemotherapy as part of their treatment. These treatment factors could alter the kinetics of itPSA.
We acknowledge that a clinical scenario in which patients are informed that there PSA has increased during therapy could cause anxiety if discussed without adequate background information. However, for those patients with small increases in PSA (ARR<1.1), an opportunity to potentially augment their therapy can be identified and may potentially improve their treatment outcomes with additional adjuvant care. We hypothesize that ARR <1.1 may be a surrogate for significant prostate disease burden. This might then predict for subclinical distant or regional disease. For those patients with observed early BFs, local curative treatment would not address extraprostatic disease. Whether an early systemic therapy intervention would alter there treatment outcomes is not known but would be an interesting area of future study. Surveyed cancer patients have been noted to accept systemic therapy for an only 1% gain in cure rates (29), so we believe that patients would value this additional information.
Conclusion
This is the first report providing evidence that itPSA is a valuable tool in predicting BF and OS after definitive external beam RT. These effects were found to be independent of bPSA, GS, T-stage, RT dose, age, and race. If these findings are validated in larger patient populations they may become a pivotal tool in a new form of patient risk stratification that could occur during treatment. Such schemes may be employed in order to identify patients that may warrant more aggressive therapeutic interventions as part of there definitive therapy. In addition, these patients could potentially be considered candidates for protocols that improve local and or systemic control.
Acknowledgments
We are indebted to Steven Kronenberg for assistance with the generation of figures.
Acknowledgment of Grant or other financial support: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Notification: None. No conflicts of interest exist.
References
- 1.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. Jama. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 2.Hanks GE, Hanlon AL, Lee WR, et al. Pretreatment prostate-specific antigen doubling times: clinical utility of this predictor of prostate cancer behavior. Int J Radiat Oncol Biol Phys. 1996;34:549–553. doi: 10.1016/0360-3016(95)02154-x. [DOI] [PubMed] [Google Scholar]
- 3.D’Amico AV, Moul J, Carroll PR, et al. Prostate specific antigen doubling time as a surrogate end point for prostate cancer specific mortality following radical prostatectomy or radiation therapy. J Urol. 2004;172:S42–46. doi: 10.1097/01.ju.0000141845.99899.12. discussion S46–47. [DOI] [PubMed] [Google Scholar]
- 4.D’Amico AV, Renshaw AA, Sussman B, et al. Pretreatment PSA velocity and risk of death from prostate cancer following external beam radiation therapy. Jama. 2005;294:440–447. doi: 10.1001/jama.294.4.440. [DOI] [PubMed] [Google Scholar]
- 5.Sengupta S, Myers RP, Slezak JM, et al. Preoperative prostate specific antigen doubling time and velocity are strong and independent predictors of outcomes following radical prostatectomy. J Urol. 2005;174:2191–2196. doi: 10.1097/01.ju.0000181209.37013.99. [DOI] [PubMed] [Google Scholar]
- 6.Zagars GK, Pollack A. Kinetics of serum prostate-specific antigen after external beam radiation for clinically localized prostate cancer. Radiother Oncol. 1997;44:213–221. doi: 10.1016/s0167-8140(97)00123-0. [DOI] [PubMed] [Google Scholar]
- 7.Zhou P, Chen MH, McLeod D, et al. Predictors of prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Clin Oncol. 2005;23:6992–6998. doi: 10.1200/JCO.2005.01.2906. [DOI] [PubMed] [Google Scholar]
- 8.D’Amico AV, Kantoff P, Loffredo M, et al. Predictors of mortality after prostate-specific antigen failure. Int J Radiat Oncol Biol Phys. 2006;65:656–660. doi: 10.1016/j.ijrobp.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 9.Kim-Sing C, Pickles T. Intervention after PSA failure: examination of intervention time and subsequent outcomes from a prospective patient database. Int J Radiat Oncol Biol Phys. 2004;60:463–469. doi: 10.1016/j.ijrobp.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Freedland SJ, Humphreys EB, Mangold LA, et al. Death in patients with recurrent prostate cancer after radical prostatectomy: prostate-specific antigen doubling time subgroups and their associated contributions to all-cause mortality. J Clin Oncol. 2007;25:1765–1771. doi: 10.1200/JCO.2006.08.0572. [DOI] [PubMed] [Google Scholar]
- 11.Sandler HM, Dunn RL, McLaughlin PW, et al. Overall survival after prostate-specific-antigen-detected recurrence following conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2000;48:629–633. doi: 10.1016/s0360-3016(00)00717-3. [DOI] [PubMed] [Google Scholar]
- 12.Cheung R, Tucker SL, Kuban DA. First-year PSA kinetics and minima after prostate cancer radiotherapy are predictive of overall survival. Int J Radiat Oncol Biol Phys. 2006;66:20–24. doi: 10.1016/j.ijrobp.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 13.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. Jama. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 14.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Tarhan F, Orcun A, Kucukercan I, et al. Effect of prostatic massage on serum complexed prostate-specific antigen levels. Urology. 2005;66:1234–1238. doi: 10.1016/j.urology.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 16.Oesterling JE, Rice DC, Glenski WJ, et al. Effect of cystoscopy, prostate biopsy, and transurethral resection of prostate on serum prostate-specific antigen concentration. Urology. 1993;42:276–282. doi: 10.1016/0090-4295(93)90616-i. [DOI] [PubMed] [Google Scholar]
- 17.Rana A, Chisholm GD. He sold his bike for a low prostate specific antigen. J Urol. 1994;151:700. doi: 10.1016/s0022-5347(17)35053-x. [DOI] [PubMed] [Google Scholar]
- 18.Morgan TO, Jacobsen SJ, McCarthy WF, et al. Age-specific reference ranges for prostate-specific antigen in black men. N Engl J Med. 1996;335:304–310. doi: 10.1056/NEJM199608013350502. [DOI] [PubMed] [Google Scholar]
- 19.Baillargeon J, Pollock BH, Kristal AR, et al. The association of body mass index and prostate-specific antigen in a population-based study. Cancer. 2005;103:1092–1095. doi: 10.1002/cncr.20856. [DOI] [PubMed] [Google Scholar]
- 20.Nadler RB, Humphrey PA, Smith DS, et al. Effect of inflammation and benign prostatic hyperplasia on elevated serum prostate specific antigen levels. J Urol. 1995;154:407–413. doi: 10.1097/00005392-199508000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Simardi LH, Tobias-MacHado M, Kappaz GT, et al. Influence of asymptomatic histologic prostatitis on serum prostate-specific antigen: a prospective study. Urology. 2004;64:1098–1101. doi: 10.1016/j.urology.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 22.Dalton DL. Elevated serum prostate-specific antigen due to acute bacterial prostatitis. Urology. 1989;33:465. doi: 10.1016/0090-4295(89)90131-3. [DOI] [PubMed] [Google Scholar]
- 23.Vijayakumar S, Quadri SF, Sen S, et al. Measurement of weekly prostate specific antigen levels in patients receiving pelvic radiotherapy for nonprostatic malignancies. Int J Radiat Oncol Biol Phys. 1995;32:189–195. doi: 10.1016/0360-3016(94)00460-3. [DOI] [PubMed] [Google Scholar]
- 24.Gripp S, Haller JC, Metz J, et al. Prostate-specific antigen: effect of pelvic irradiation. Radiology. 2000;215:757–760. doi: 10.1148/radiology.215.3.r00jn09757. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Josef E, Shamsa F, Forman JD. Predicting the outcome of radiotherapy for prostate carcinoma: a model-building strategy. Cancer. 1998;82:1334–1342. doi: 10.1002/(sici)1097-0142(19980401)82:7<1334::aid-cncr17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Do T, Dave G, Parker R, et al. Serum PSA evaluations during salvage radiotherapy for post-prostatectomy biochemical failures as prognosticators for treatment outcomes. Int J Radiat Oncol Biol Phys. 2001;50:1220–1225. doi: 10.1016/s0360-3016(01)01558-9. [DOI] [PubMed] [Google Scholar]
- 27.Wiegel T, Bottke D, Bandlow P, et al. The value of PSA measurements at 30 Gy, 50 Gy and 60 Gy for dose limitation in patients with radiotherapy for PSA increase after radical prostatectomy. Strahlenther Onkol. 2002;178:422–425. doi: 10.1007/s00066-002-0917-8. [DOI] [PubMed] [Google Scholar]
- 28.Valicenti RK, Desilvio M, Hanks GE, et al. Posttreatment prostatic-specific antigen doubling time as a surrogate endpoint for prostate cancer-specific survival: An analysis of Radiation Therapy Oncology Group Protocol 92-02. Int J Radiat Oncol Biol Phys. 2006;66:1064–1071. doi: 10.1016/j.ijrobp.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Slevin ML, Stubbs L, Plant HJ, et al. Attitudes to chemotherapy: comparing views of patients with cancer with those of doctors, nurses, and general public. Bmj. 1990;300:1458–1460. doi: 10.1136/bmj.300.6737.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]


