Abstract
Previous research has revealed similarities in the neuropathology, clinical presentation, and risk factors between persons with Alzheimer’s disease from the general population (GP-AD) and those with Down syndrome (DS-AD). Less is known, however, about the extent of similarities and differences in the cognitive profiles of these two populations. Fifty-one moderate-to-severely demented GP-AD and 59 DS-AD individuals participated in this study which compared the cognitive profiles of these two populations on the Severe Impairment Battery (SIB), controlling for gender as well as level of functional ability using a modified version of the Bristol Activities of Daily Living Scale. Overall, the neuropsychological profiles of the higher-functioning individuals within the DS-AD and advanced GP-AD groups, as represented by mean difference scores on the SIB as a whole and across the nine separate cognitive domains, were very similar to one another after adjusting for gender and functional impairment. To our knowledge, this is the first study to directly compare the cognitive profiles of these two populations on the SIB. Findings suggest that the underlying dementia in GP-AD and DS-AD may have corresponding and parallel effects on cognition.
Keywords: Down syndrome, Alzheimer’s disease, Severe Impairment Battery, Bristol Activities of Daily Living Scale, Cognitive Profile
By the time individuals with Down syndrome (DS) reach age 40, the neuropathological changes of Alzheimer’s disease (AD), including extra-cellular β-amyloid neuritic plaques, intra-neural neurofibrillary tau tangles, β-amyloid deposition within vascular wall, inflammation, and oxidative damage, are present. These changes closely resemble those found in both sporadic and early onset AD within the general population.1 In addition to almost identical neuropathology, dementia in individuals with DS (DS-AD) and in the general population (GP-AD) share a common set of risk factors, the most important of which is advancing age.2 The average prevalence of DS-AD is about 15% at age 45 versus 12% in GP-AD at age 65. Thereafter the rate of dementia doubles with each 5-year interval in both groups.2 Additional risk factors common to both groups include hypercholesterolemia, estrogen deficiency, reduced cerebral reserve, and the presence of multiple medical problems.3 Patients with AD in both at-risk groups show a history of slowly progressive cognitive decline, typically involving problems in recent memory as well as one or more other cognitive domains, such as orientation, language, attention, visuospatial abilities, and executive functioning.
Although the first and most noticeable symptom in 9 out of 10 individuals who develop GP-AD, difficulties with recent episodic memory may or may not be the initial symptom of dementia in DS-AD.3,4 Some have argued that executive dysfunction, including problems with planning, attention, and articulation, and other frontal lobe symptoms, including apathy, depression, indifference, and uncooperativeness, are the earliest manifestations of DS-AD.5,6 Others emphasize memory problems over frontal lobe symptoms.3 In addition to cognitive deficits, individuals with DS-AD and GP-AD may experience progressive behavior and personality changes, especially apathy, irritability, agitation, anxiety, and sleep-related disturbances.7 In both populations, these cognitive and behavioral deficits represent a significant change from the individual’s premorbid level of functioning and interfere with his/her social and occupational responsibilities.
Despite commonalities between DS-AD and GP-AD in neuropathology, risk factors, and clinical presentation, the lack of appropriate tests and acceptable cutoff scores to identify dementia-related cognitive impairment in DS make it more difficult to diagnose AD in this than the general population. Commonly used instruments for assessing the neuropsychological functioning of individuals within the general population, such as the Mini-Mental State Examination (MMSE) 8 and the Alzheimer’s Disease Assessment Scale (ADAS-Cog) 9 cannot be directly applied to persons with DS due to their underlying intellectual disability. Designed to evaluate cognition in individuals with at least “average” levels of intelligence, these instruments typically produce “floor effects” in persons with DS. For example, Deb and Braganza found that almost half (i.e., 45%) of 62 adults with DS age 35–72 could not complete the MMSE.10 Of the tests available to assess the cognitive functioning in individuals with DS, such as the Cambridge Examination for Mental Disorders of Older People with Down Syndrome and Others with Intellectual Disabilities (CAMDEX-DS),11 Vineland Adaptive Behavior Scales (VABS),12 Brief Praxis Test (BPT),13 Down’s Syndrome Mental Status Examination (DSMSE),14 and the Severe Impairment Battery (SIB),15 only the SIB has been used to evaluate cognition in GP-AD. To our knowledge, no studies have directly compared the SIB total scores or cognitive profiles in these two populations.
The SIB was specifically developed to evaluate the cognitive functioning of moderate to severely impaired patients across domains typically assessed by standard psychometric batteries such as the ADAS-cog.15, 16 Unlike the ADAS-cog, which cannot be completed by patients with advanced dementia, the SIB is still able to gather useful information about the cognitive functioning of individuals who are typically considered “untestable” with standard psychometric tools. The SIB consists of 51 one-step questions and commands assessing nine domains: Social Interaction, Orientation, Visuospatial Ability, Constructional Ability, Language, Memory, Attention, Orientation to Name, and Praxis. Although the items are presented verbally, nonverbal responses are scorable, and many of the questions can be repeated and accompanied by gestural cues to facilitate comprehension. The SIB is scored from 0 to 100 points, with higher scores indicating less impairment. Although the 51-item SIB is relatively brief, taking only 20–30 minutes to administer, shorter versions of 26 (SIB-S) and 8 items have been developed to increase efficiency for clinical use.17,18 In general, research has shown that the SIB is most useful for assessing the cognitive functioning of moderately to severely impaired GP-AD individuals with MMSE scores of 12 or lower.19
The primary goal of this study was to compare the neuropsychological profiles of advanced GP-AD and DS-AD patients using the SIB. Identifying a way to equate participants from the two groups was essential because of differences in chronological age, level of education, and premorbid intelligence. Despite these differences, we expected that participants from the two groups would likely be comparable in level of everyday functioning as measured by scores on a modified version of the Bristol Activities of Daily Living Scale (BADLS).20 More than simply examining mean differences in composite total scores, we were interested in determining whether the two groups differed in their cognitive strengths and weaknesses. Given the similarities between AD in older adults from the general population and in persons with DS,2, 3 we assumed that the GP-AD and DS-AD groups would show comparable scores across the nine cognitive domains assessed by the SIB when matched on level of functional disability.
A secondary goal was to examine any differences in the language abilities of the two groups. As loss of language abilities in late-stage AD has a profound effect on the individual’s ability to communicate his/her needs and participate in customary relationships with family members and caregivers, the SIB places a special emphasis on assessing language, with half of the sub-items (i.e., 24 of the 51) tapping this cognitive domain. These items cover a broad range of linguistic functions, including reading and writing, word-finding or verbal fluency, object-naming ability, repetition, and verbal comprehension. 15, 16 Given the centrality of language in the functioning of persons with dementia and the SIB,21–23 we thought it worthwhile to examine any differences between the GP-AD and DS-AD groups in the various aspects of language assessed as well as the total domain score.
METHODS
Participants
One-hundred-and-ten adults participated in this study, comprising 59 individuals with DS-AD and 51 patients with advanced GP-AD. All subjects were recruited from the Alzheimer’s Disease Research Center (ADRC) at the University of California, Irvine (UCI) between September 2005 and May 2014. A comprehensive clinical evaluation was carried out on each individual that included a detailed medical history, neurological examination, and neuropsychological assessment. A study partner or knowledgeable informant who lived or had frequent face-to-face contact with the participant provided information about the individual’s functional abilities, current affective state, and behavioral symptoms via a standardized clinical interview. Typically, the informant for GP-AD participants was a spouse or a knowledgeable family member. For individuals with DS-AD, the informant was a family member or the primary paid caregiver at a residential facility. Exclusionary criteria included neuroimaging evidence of stroke, subcortical vascular disease, space occupying lesion, traumatic brain injury, or normal pressure hydrocephalus. Other exclusionary criteria were a history of alcohol/substance abuse, significant head injury, and established major neurological or psychiatric disorders other than those related to dementia. Potential subjects were also excluded if physical limitations or major sensory deficits in vision and hearing impaired the individual’s ability to complete the neuropsychological evaluation. Two teams of professionals representing the fields of neurology, neuropsychology, and nursing at the UCI ADRC diagnosed each participant according to the NINCDS-ADRDA clinical criteria for either probable or possible AD based on a consensus of their expert opinions.24 Depression as a cause of cognitive impairment in both groups was excluded based on clinical judgment and scores of ≥ 4 on the 9-item DSM-IV Structured Clinical Interview for Depression (SCID-I).25
A majority, that is 40 (78%) of the 51 GP-AD participants had completed the median of 4 or more (range 1–14) annual reassessments in the UCI ADRC prior to being administered the SIB. These participants demonstrated significant deficits in memory and two or more other cognitive domains with compromised activities of daily living as indicated by impaired scores on the Functional Activities Questionnaire (FAQ) 26 and the BADLS. Additionally, all GP-AD patients scored either at or below 13 out of 30 points on the MMSE and had a global Clinical Dementia Rating (CDR) 27 scale score of 2 or 3, suggesting a moderate-to-severe dementia when the SIB was first administered.
The diagnosis of dementia was established for the 59 DS-AD individuals according to DSM-IV criteria using information gathered directly from the participants as well as their informants. The performance-based measures administered to the DS participants included the SIB,15 BPT,13 and DSMSE.14 The informant-based measures were the Dementia Questionnaire for Mentally Retarded Persons (DMR),28 VABS,12 and BADLS.20 Scores on the SIB were not used in determining the diagnosis of dementia in either GP-AD or DS-AD participants.
The protocol and informed consent forms used with the GP-AD and DS-AD subjects were approved by independent ethics committees in the Institutional Review Board (IRB) at UCI.
Separation of the Participants into ‘High’ and ‘Low’ Functioning Strata
While DS-AD and GP-AD participants differed sharply from one another in terms of multiple variables (e.g., chronological age, age at onset of dementia, premorbid intelligence, and overall level of development in social, cognitive, and functional skills), they could be matched in terms of current functional abilities using a modified version of the BADLS. Briefly, on the standard BADLS, a knowledgable informant rates the participant’s level of functioning during the past two weeks on 20 items that assess a variety of basic (e.g., eating, dressing, grooming, and toileting) and instrumental (e.g., shopping, meal preparation, using the telephone, and driving) activities of daily living (ADLs) as well as a small number of complex cognitive/social functions (e.g., orientation to time, spatial orientation, and communication skills) on a four-point scale from normal to totally dependent. The total score ranges from 0 (i.e., completely independent) to 60 points (i.e., completely dependent). Bucks et al. demonstrated that the BADLS has good validity and reliability, with total scores of 0 to 3 typical of most healthy older adults.20
While GP-AD participants were able to perform all activities assessed by the BADLS prior to dementia onset, the same would not be true of those with DS-AD who had never acquired certain higher-level instrumental skills. Consequently, to better equate the DS-AD and GP-AD participants on current functional abilities, we chose to eliminate those BADLS items that individuals with DS may never have been able to perform. The resulting “modified” version of the BADLS, henceforth referred to as the mBADLS, included the following 14 items: eating, drinking, dressing, personal hygiene/grooming, teeth cleaning, bathing/showering, toileting, transfer, mobility, housework/gardening, games/hobbies, communication, orientation to time, and orientation to place. Eliminating the instrumental ADLs reduced the total score from 60 to 42 points, with higher scores indicating more functional dependency. The average mBADLS total score for the combined DS-AD and GP-AD groups was 28.9 (SD = 9.8) points with a median score of 28. Using this information, the two groups were each subsequently separated into “high” and “low” functioning strata, above and below the median of 28 points, respectively, creating four subgroups.
Data Analyses
Preliminary examination of the SIB scores among the four subgroups revealed that the low-functioning DS-AD participants were markedly different from the other three groups, with a majority being essentially “untestable” (i.e., scoring 0 on the SIB). Hence, the resulting mean total SIB score of the low-functioning DS-AD participants fell at 28.5 (range 0.0 – 51.0) out of a possible 100 points, as compared to 62.0 (range 34.5 – 70.5) in the low-functioning GP-AD patients. Mean total SIB scores of the high-functioning DS-AD and GP-AD participants, however, were comparatively close, falling at 76.0 (range 57.0 – 88.0) and 71.0 (range 65 – 82.5) points, respectively. In addition to being more cognitively impaired, a majority of the low-functioning DS-AD participants were also highly dependent on caregivers for even the most basic ADLs, as shown by a mean mBADLS score of 37.8 (SD = 9.3) out of 42 points. As the low-functioning DS-AD participants were noticeably more cognitively and functionally impaired than those in the other three subgroups, we decided to limit this study to the high-functioning DS-AD and GP-AD individuals.
To accomplish our primary goal of effectively comparing the DS-AD and GP-AD neuropsychological profiles on the SIB, adjustments were made for gender as well as for differences in functional ability with the mBADLS. Gender differences have been reported in the DS population, with females having better cognitive abilities and more developed speech, as well as less challenging behavior than males.29 To examine differences on the SIB as a whole and its subscales, linear models were fit for the regression of the total SIB score and scores on each of the nine cognitive domains on indicators of the group (DS-AD vs GP-AD), gender, and the mBADLS score. Finally, the association between the SIB and mBADLS total scores in the two high-functioning groups was examined using regression equations and R-squared statistics.
RESULTS
Demographics of the 48 DS-AD and 20 GP-AD high-functioning participants (i.e., with a mBADLS score below the median of 28 points) are shown in Table 1. The two group differed in age by approximately 20 years, with GP-AD participants being older, as well in gender, with more female GP-AD (60%) than DS-AD (45.8%) participants. Ethnic backgrounds of participants were very similar across groups, with 85% being white. Most importantly, DS-AD and GP-AD participants were alike in terms of their current functional abilities, with nearly identical mBADLS scores of 21.92 (SD = 4.24) points for DS-AD and 21.95 (SD = 3.75) for GP-AD participants. DS-AD and GP-AD participants did not differ in regard to a clinical diagnosis of depression.
TABLE 1.
Demographics for the Higher-Functioning GP-AD and DS-AD Groups
| GP-AD | DS-AD | |||
|---|---|---|---|---|
|
|
||||
| N | 20 | 48 | ||
|
|
||||
| Gender (M/F) | 8/12 | 26/22 | ||
|
|
||||
| Mean | SD | Mean | SD | |
| Age (yrs.) | 72.42 | 8.97 | 51.11 | 6.71 |
| mBADLS Score | 21.95 | 3.75 | 21.92 | 4.24 |
|
| ||||
| Race | Number | % | Number | % |
|
| ||||
| Caucasian | 17 | 85.0 | 41 | 85.4 |
| Hispanic | 0 | 0 | 4 | 8.3 |
| Native American | 0 | 0 | 1 | 2.1 |
| Asian/Pacific Islander | 1 | 5.0 | 2 | 4.2 |
| African-American | 2 | 10.0 | 0 | 0 |
Abbreviations: M/F, male/female; GP-AD, individuals from the general population with Alzheimer’s disease; DS-AD, persons with Down syndrome and Alzheimer’s disease; mBADLS, modified Bristol Activities of Daily Living Scale.
Comparison of the Neuropsychological Profiles
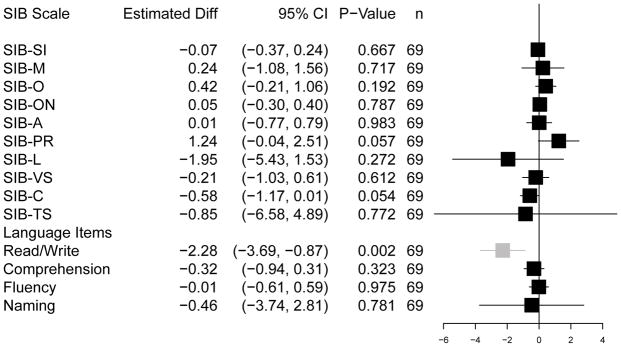
Analyses revealed that the neuropsychological profiles of the DS-AD and GP-AD participants were highly similar, as illustrated graphically in a forest plot (Figure 1) and shown by the data (Table 2). No differences in mean scores emerged between the GP-AD and the DS-AD groups on the SIB as a whole as well as on all nine cognitive subscales after adjusting for mBADLS and gender. The size of the difference in scores between the two groups on all of the major measures from the SIB were generally less than a single digit, with small standard errors and very narrow 95% confidence intervals. The only statistically significant difference between the DS-AD and GP-AD groups occurred in the secondary analysis performed on the SIB language items. As illustrated in Figure 1, the GP-AD patients had higher reading and writing scores than the DS-AD participants (i.e., estimated mean difference score = −2.28; 95% CI = −3.69, −0.87; p ≤ 0.002). The groups, however, scored similarly on the SIB measures of verbal comprehension, fluency or word-finding ability, and object naming as well as on the language domain as a whole.
Figure 1. The forest plot.
Estimated adjusted mean differences between the DS−AD minus GP−AD cohorts on the Severe Impairment Battery
Legend: Estimated mean differences between the DS-AD and the GP-AD cohorts on the Severe Impairment Battery. Mean differences were calculated for the total score (TS) and each cognitive domain score separately, including domains associated with specific language items. Error bars are for the 95% confidence intervals, and the box size was drawn proportional to the standard error. All differences were adjusted for scores on the modified BADLS. Mean differences were not significantly different from zero with the one exception being the read/write items in the language domain, where the GP-AD cohort scored higher than did the DS-AD cohort.
TABLE 2.
Median and Interquartile Range Scores for the GP-AD and DS-AD Groups on the SIB
|
|
||||||
|---|---|---|---|---|---|---|
| GP-AD | DS-AD | |||||
|
| ||||||
| SIB Domain | Label | Median | IQR | Median | IQR | Max. |
| Social Interaction | SIB-SI | 6.0 | 6.0 – 6.0 | 6.0 | 6.0 – 6.0 | 6.0 |
| Memory | SIB-M | 8.0 | 6.5 – 11.0 | 9.0 | 7.0 – 11.0 | 14.0 |
| Orientation | SIB-O | 3.0 | 2.0 – 4.0 | 3.0 | 2.0 – 5.0 | 6.0 |
| Orientation to Name | SIB-ON | 2.0 | 2.0 – 2.0 | 2.0 | 2.0 – 2.0 | 2.0 |
| Attention | SIB-A | 4.0 | 2.0 – 5.0 | 4.0 | 2.0 – 5.0 | 6.0 |
| Praxis | SIB-PR | 4.5 | 3.0 – 7.5 | 8.0 | 4.0 – 8.0 | 8.0 |
| Language | SIB-L | 36.0 | 32.5 – 38.5 | 36.0 | 26.0 – 40.0 | 46.0 |
| Visuospatial | SIB-VS | 6.5 | 5.5 – 8.0 | 7.0 | 5.0 – 8.0 | 8.0 |
| Construction | SIB-C | 4.0 | 3.0 – 4.0 | 4.0 | 2.0 – 4.0 | 4.0 |
|
| ||||||
| Total Score | SIB-TS | 71.00 | 65.0 – 82.5 | 76.00 | 57.0 – 88.0 | 100.0 |
|
| ||||||
| Language Components | ||||||
| Read/Write | 7.0 | 6.0 – 8.0 | 4.0 | 2.0 – 7.0 | 8.0 | |
| Comprehension | 6.0 | 5.0– 6.0 | 6.0 | 5.0 – 6.0 | 6.0 | |
| Fluency | 3.0 | 2.0 – 3.0 | 3.0 | 2.0 – 4.0 | 4.0 | |
| Naming | 22.0 | 18.0 – 24.0 | 22.0 | 18.0 – 25.0 | 28.0 | |
Abbreviations: GP-AD, individuals from the general population with Alzheimer’s disease; DS-AD, persons with Down syndrome and Alzheimer’s disease; IQR, interquartile range; SIB, Severe Impairment Battery.
Mean differences between the two groups estimated with and without adjustment for mBADLS scores and gender are shown in Table 3. While the mean differences were approximately the same with and without adjustment, the 95% confidence intervals after adjustment were on average 10% narrower. This finding suggests that the mBADLS score served the important role of a precision variable in the comparison between groups, a function that can be exploited fruitfully in the design and analysis of future studies comparing these two populations.
Table 3.
Estimated Mean Differences between the GP-AD and DS-AD Groups: Unadjusted and Adjusted for mBADLS Score and Gender.
| Unadjusted Difference | Adjusted Difference | ||||||
|---|---|---|---|---|---|---|---|
| SIB Domain | Label | Mean Diff. | (95% CI) | p-value | Mean Diff. | (95% CI) | p-value |
| Social Interaction | SIB-SI | −0.07 | (−0.41, 0.26) | 0.667 | −0.07 | (−0.37, 0.24) | 0.667 |
| Memory | SIB-M | 0.16 | (−1.35, 1.68) | 0.831 | 0.24 | (−1.08, 1.56) | 0.717 |
| Orientation | SIB-O | 0.51 | (−0.23, 1.24) | 0.176 | 0.42 | (−0.21, 1.06) | 0.192 |
| Orientation to Name | SIB-ON | 0.04 | (−0.33, 0.41) | 0.816 | 0.05 | (−0.30, 0.40) | 0.787 |
| Attention | SIB-A | 0.02 | (−0.81, 0.85) | 0.956 | 0.01 | (−0.77, 0.79) | 0.983 |
| Praxis | SIB-PR | 1.30 | (−0.04, 2.64) | 0.057 | 1.24 | (−0.04, 2.51) | 0.057 |
| Language | SIB-L | −1.97 | (−5.94, 2.01) | 0.333 | −1.95 | (−5.43, 1.53) | 0.272 |
| Visuospatial | SIB-VS | −0.21 | (−1.18, 0.77) | 0.679 | −0.21 | (−1.03, 0.61) | 0.612 |
| Construction | SIB-C | −0.59 | (−1.17, 0.00) | 0.050 | −0.58 | (−1.17, 0.01) | 0.054 |
| SIB Total Score | SIB-TS | −0.79 | (−8.14, 6.56) | 0.833 | −0.85 | (−6.58, 4.89) | 0.772 |
Abbreviations: GP-AD, individuals from the general population with Alzheimer’s disease; DS-AD, persons with Down syndrome and Alzheimer’s disease; CI, confidence interval; SIB, Severe Impairment Battery; mBADLS, modified Bristol Activities of Daily Living Scale.
An exploratory factor analysis was carried out to examine the similarity between the SIB factor structure in these two groups. Principal components analysis was performed for each group separately on scores from the items in the nine SIB cognitive domains. Principal components were derived by singular-value decomposition for its known numerical stability 30 and all analyses were completed in the graphical and programming environment R.31 The standard deviation, proportion of total variation, and cumulative proportion of total variation were examined for each factor. In the DS-AD group, the first factor (PC1) had a standard deviation of 11.26 points and was associated with 89.5% of the total variation. Factors PC2 and PC3 were associated with smaller standard deviations of 2.26 and 1.99 points, respectively, and only 4% and 3% of the variation. Nevertheless, the first three factors together accounted for a cumulative proportion of 95.8% of total variation. In the GP-AD group, the first factor (PC1) had a standard deviation of 6.47 points and was associated with 64.9% of the total variation. PC2 and PC3 had standard deviations of 3.33 and 2.48 points, respectively, and were associated with 17% and 10% of the variance. Overall, in this group, the first three factors accounted for a cumulative proportion of 91.6% of the total variation.
Factor loadings for the first three principal components are shown in Table 4 for the two groups. The individual loadings in each factor were used to assign weights to individual SIB items. Interpretation of the factors depends upon the relative loadings of the items and is based on the weights received by different items within a factor and on whether clusters of items appear with the same or opposite sign. A pair of items with the same sign will enter a factor like a mixture of these two components, while a pair of items with opposite signs will enter like a contrast or net difference between components. As expected, factor PC1 was heavily weighted in both the GP-AD and DS-AD groups primarily on the SIB language items. Examining factor PC2 in the DS-AD group, the praxis items received the heaviest weight but the factor may be interpreted as a contrast between performance on the praxis plus memory and visuospatial ability versus language and construction items. As PC2 was associated with so little variation in the DS-AD group, any associations should be interpreted with great caution. In comparison, factor PC2 in the GP-AD group was associated with a reasonable portion of variation and involved an equally weighted mixture of performance on praxis plus memory and visuospatial ability items. While the praxis items received the heaviest weight in factor PC3 for the GP-AD group, this factor could also be interpreted as a contrast or difference between performance on praxis versus memory.
Table 4.
Principal Factor Loadings on the Severe Impairment Battery (SIB) among Participants in the DS-AD and GP-AD Groups
| GP-AD | DS-AD | ||||||
|---|---|---|---|---|---|---|---|
| Principal Factor | Principal Factor | ||||||
| SIB Domain | Label | PC1 | PC2 | PC3 | PC1 | PC2 | PC3 |
| Social Interaction | SIB-SI | −0.01 | 0.04 | −0.00 | −0.04 | 0.04 | −0.01 |
| Memory | SIB-M | 0.03 | 0.68 | 0.60 | −0.26 | −0.26 | 0.78 |
| Orientation | SIB-O | 0.01 | 0.21 | −0.07 | −0.11 | 0.04 | −0.13 |
| Orientation to Name | SIB-ON | −0.00 | 0.11 | −0.09 | −0.02 | 0.01 | 0.09 |
| Attention | SIB-A | −0.06 | 0.13 | 0.15 | −0.13 | −0.05 | −0.06 |
| Praxis | SIB-PR | 0.09 | 0.50 | −0.77 | −0.14 | −0.87 | −0.40 |
| Language | SIB-L | 0.99 | −0.05 | 0.06 | −0.92 | 0.23 | −0.21 |
| Visuospatial | SIB-VS | −0.02 | 0.45 | −0.06 | −0.16 | −0.28 | 0.39 |
| Construction | SIB-C | 0.02 | 0.10 | 0.08 | −0.11 | 0.20 | 0.04 |
Loadings are reported by domain for the first three principal factors in order of importance based on the proportion of total variation. Signs of the loading are arbitrary. Of importance for interpretation are the relative weightings and differences of signs among the domains. Abbreviations: GP-AD, individuals from the general population with Alzheimer’s disease; DS-AD, persons with Down syndrome and Alzheimer’s disease; PC, Principal factor; SIB, Severe Impairment Battery.
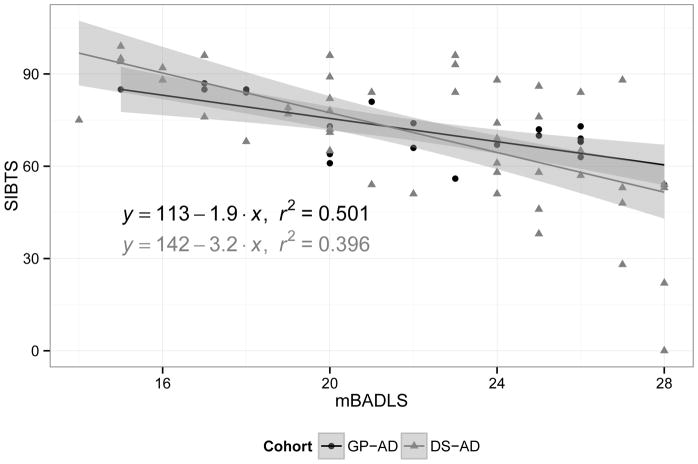
Finally, using linear regression and R-squared statistics (Figure 2), we found that higher mBADLS scores in both groups were associated with lower SIB scores, indicating that individuals with more disability and greater dependence on caregivers demonstrated lower cognitive functioning. Overall, each 1-point increase in the mBADLS was associated with a 2.91-point (CI −3.83, −1.99) decrease in the total SIB score (p < 0.001). The overall R-squared statistic was 38.9%.
Figure 2. Regression lines.
Linear regression of total SIB score based on the modified BADLS score.
A scatterplot was drawn for total SIB score (SIB-TS) versus the modified BADLS (mBADLS) score, and a linear regression of SIB-TS was based on mBADLS for each cohort, separately. The regression equations were reported with the respective r-squared statistic, while the shaded regions were bound by the 95% confidence bands. The association was similar across cohorts. Above average scores on the mBADLS were associated with below average scores on the SIB, while the association accounted for 40% to 50% of the overall variation among participants on the SIB.
DISCUSSION
The most important finding of the present study was that neuropsychological profiles of the DS-AD and GP-AD groups, as represented by mean difference scores on each of the nine SIB cognitive domains as well as the SIB as a whole, were very similar to one another after adjusting for gender and level of functional ability. The exploratory factor analysis confirmed the importance of (1) language items in differentiating among participants in both groups, and (2) the four domains of language, praxis, memory, and visuospatial ability in distinguishing participants within each group, although somewhat differently for the two groups. These findings reinforce the similarities seen between these two at-risk groups in other areas (e.g., risk factors, clinical presentation, and neuropathology) and additionally suggest that AD was impacting the brains of individuals with DS and non-learning disabled older adults in a comparable fashion.
Besides being the first study, to our knowledge, that directly compares the neuropsychological profiles of DS-AD and GP-AD participants on the SIB, this research has a number of strengths. Significant strengths include using well-described subjects with longitudinal follow-ups, employing a well-researched, reliable, and valid measure of cognitive abilities, and equating the DS-AD and GP-AD groups on level of functional abilities. First, all participants had undergone a comprehensive evaluation at the UCI ADRC and a majority of the GP-AD patients had completed a median of four annual evaluations prior to being administered the SIB. Secondly, use of the SIB to accurately assess the cognitive functioning of DS-AD and GP-AD participants is a strength given that this instrument has become the FDA approved “gold standard” for measuring treatment effects in clinical trials with patients with moderate-to-severe AD.32–34 Thirdly, equating the DS-AD and GP-AD groups on basic functional abilities with the mBADLS ensured that results of this study would not be skewed by the fact that many of the DS-AD participants were never capable of performing the instrumental ADLs in the full BADLs. Notably, the significant association found between SIB and mBADLS total scores indicates that the mBADLS could be successfully used as a matching variable in future comparative studies of these two clinical populations.
The absence of prior studies comparing the cognitive profiles of DS-AD and GP-AD participants on the SIB is not surprising given the obvious differences between these two groups. Indeed, some might argue that comparing DS-AD to GP-AD individuals is similar to comparing “apples to oranges” as the two groups differ sharply from one another on age, dementia onset, premorbid intelligence, and level of development. Our results, however, demonstrate otherwise, showing that meaningful knowledge about cognition, like neuropathology, risk factors, and clinical presentation, in AD can be gained by exploring similarities and differences between affected individuals with DS and those from the general population. Additionally, it could be argued that the neuropsychological instruments (e.g., ADAS-Cog and MMSE) commonly used to assess the cognitive functioning of GP-AD patients would likely produce a “floor effect” in DS-AD individuals, and those instruments (e.g., CAMDEX-DS, VABs, BPT, and DSMSE) used to assess the mental abilities of individuals with DS would result in a “ceiling effect” when administered to many patients with AD from the general population. This argument, however, would only be valid if comparing the cognitive profiles of mildly impaired GP-AD patients to those of DS-AD individuals. When GP-AD individuals have advanced into the moderate-to-severe stages of dementia, as in the present study, their cognitive profiles as assessed by the SIB can be meaningfully compared to those of persons with DS-AD.
Two potential limitations of this study were the absence of (1) a comparison group of DS participants without AD, and (2) measures of executive functioning on the SIB. More specifically, we are unable to address whether the SIB profile of DS-AD participants would differ from that of DS individuals without dementia. Future studies should address whether the common pattern of SIB subdomain scores seen in the DS-AD and GP-AD groups generalizes to all DS individuals or is limited to only those with AD pathology.
Typically in late-onset AD, the medial temporal lobe is the first area of the brain affected, causing profound deficits in recent episodic memory. As the disease gradually spreads to the frontal and parietal lobes, deficits appear in other cognitive abilities, including reasoning, planning, attention, and working memory as well as language, spatial orientation, and visuospatial functioning. While many of these cognitive deficits are also seen in persons with DS, some investigators have argued that executive dysfunction, such as problems with planning, attention, articulation, reasoning, and mental flexibility, are the earliest manifestation of AD in this high-risk group. 5–7 Unfortunately, as executive functioning is not one of the nine cognitive domains assessed by the SIB, we were unable to determine if the DS-AD and GP-AD groups differed along this particular dimension. The absence of measures of executive functioning in the SIB is understandable as many AD patients will have bottomed out on traditional measures of executive skills, such as the Trail Making Tests and the Wisconsin Card Sorting Task, by the time they reach the moderate stage of dementia.35
In conclusion, this study adds to the literature on the similarities between AD in individuals with DS and in the general population by showing that their neuropsychological profiles, as measured using the SIB, follow a similar pattern. Additionally, recognizing a common pattern of cognitive strengths and weaknesses in these two at-risk groups increases appreciation for how AD compromises the ability of these individuals to relate to others and function in their daily lives.
Acknowledgments
This research was supported by the National Institute on Aging (NIA) [Grant P50 AG016573 to the University of California at Irvine, Alzheimer’s Disease Research Center, and Grant AG-21912 to the University of California, Irvine; PI: Ira T. Lott], the National Institute of Child Health and Human Development (NICHD) [Grant HD-065160 to the University of California; PI: Ira T. Lott], and the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences (NCATS) [Grant UL1 TR000153 to the University of California, Irvine, Institute for Clinical and Translational Science].
Footnotes
All of the authors declare that they have no conflicts of interest with respect to the present study.
References
- 1.Lott IT, Dierssen M. Cognitive deficits and associated neurological complications in individuals with Down’s syndrome. Lancet Neurol. 2010;9(6):623–633. doi: 10.1016/S1474-4422(10)70112-5. [DOI] [PubMed] [Google Scholar]
- 2.Nieuwenhuis-Mark RE. Diagnosing Alzheimer’s dementia in Down syndrome: Problems and possible solutions. Res Dev Disabil. 2009;30:827–838. doi: 10.1016/j.ridd.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Zigman WB, Devenny DA, Krinsky-McHale SJ, et al. Alzheimer’s disease in adults with Down syndrome. Int Rev Res Ment retard. 2008;36:103–145. doi: 10.1016/S0074-7750(08)00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 5.Ball SL, Holland AJ, Treppner P, et al. Executive dysfunction and its association with personality and behavioral changes in the development of Alzheimer’s disease in adults with Down syndrome and mild to moderate learning disabilities. Br J Clin Psychol. 2008;47:1–29. doi: 10.1348/014466507X230967. [DOI] [PubMed] [Google Scholar]
- 6.Lott IT, Head E. Down syndrome and Alzheimer’s disease: A link between development and aging. Ment Retard Dev Disabil Res Rev. 2001;7:172–178. doi: 10.1002/mrdd.1025. [DOI] [PubMed] [Google Scholar]
- 7.Ball SL, Holland AJ, Hon J, et al. Personality and behaviour changes mark the early stages of Alzheimer’s disease in adults with Down syndrome: Findings from a prospective population-based study. Int J Geriatr Psychiatry. 2006;21:661–673. doi: 10.1002/gps.1545. [DOI] [PubMed] [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 9.Rosen WG, Mohs RC, Davis KL, et al. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 10.Deb S, Braganza J. Comparison of rating scales for the diagnosis of dementia in adults with Down’s syndrome. J Intellect Disabil Res. 1999;43:400–407. doi: 10.1046/j.1365-2788.1999.043005400.x. [DOI] [PubMed] [Google Scholar]
- 11.Ball SL, Holland AJ, Huppert FA, et al. The modified CAMDEX informant interview is a valid and reliable tool for use in the diagnosis of dementia in adults with Down’s syndrome. J Intellect Disabil Res. 2004;48:611–620. doi: 10.1111/j.1365-2788.2004.00630.x. [DOI] [PubMed] [Google Scholar]
- 12.Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales: Interview Edition – Survey Form. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- 13.Dalton A, Fedor B. DYSPRAXIA scale for adults with Down Syndrome. Staten Island, NY: NYS Institute for Basic Research in Developmental Disabilities; 1997. [Google Scholar]
- 14.Haxby JV. Neuropsychological evaluation of adults with Down syndrome: Patterns of selective impairment in non-demented old adults. J Ment Deficiency. 1989;33:193–210. doi: 10.1111/j.1365-2788.1989.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 15.Saxton J, McGonigle KL, Swihart AA, et al. The severe impairment battery. Thames Valley Test Company; 1993. [Google Scholar]
- 16.Saxton J, McGonigle-Gibson K, Swihart A, et al. Assessment of the severely impaired patient: description and validation of a new neuropsychological test battery. Psych Assess. 1990;2:298–303. [Google Scholar]
- 17.de Jonghe JFM, Wetzels RB, Mulders A, et al. Validity of the severe impairment battery short version. J Neurol Neurosurg Psychiatry. 2009;80:954–959. doi: 10.1136/jnnp.2008.163220. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt FA, Saxton J, Ferris SH, et al. Evaluation of an 8-item Severe Impairment Battery (SIB-8) vs. the full SIB in moderate to severe Alzheimer’s disease patients participating in a donepezil study. In J Clin Pract. 2013;67(10):1050–1056. doi: 10.1111/ijcp.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt FA, Ashford W, Ernesto C, et al. The severe impairment battery: Concurrent validity and the assessment of longitudinal change in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(2 Suppl):51–65. [PubMed] [Google Scholar]
- 20.Bucks R, Ashworth D, Wilcock G, et al. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age Ageing. 1996;25:113–120. doi: 10.1093/ageing/25.2.113. [DOI] [PubMed] [Google Scholar]
- 21.Ferris S, Ihl R, Robert P, et al. Severe Impairment Battery Language Scale: A language-assessment tool for Alzheimer’s disease patients. Alzheimer’s & Dementia. 2009;5(5):375–379. doi: 10.1016/j.jalz.2009.04.1236. [DOI] [PubMed] [Google Scholar]
- 22.Ferris S, Mackell J, Bai Z, et al. Effect of donepezil 23 mg/day on language function in patients with moderate-to-severe Alzheimer’s disease (AD): Subgroup analysis of United States (U.S.) – based patients. Alzheimer’s & Dementia. 2012;8(4) supplement:591–592. [Google Scholar]
- 23.Ferris S, Schmitt FA, Saxton J, et al. Analyzing the impact of 23 mg/day donepezil on language dysfunction in moderate to severe Alzheimer’s disease. Alzheimer’s Research & Therapy. 2011;3(22) doi: 10.1186/alzrt84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and human Services task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 25.Teng E, Ringman JM, Ross LK, et al. Diagnosing depression in Alzheimer’s disease with the National Institute of Mental Health provisional criteria. Am J Geriatr Psychiatry. 2008;16(6):469–477. doi: 10.1097/JGP.0b013e318165dbae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeffer R, Kurosaki T, Harrah C, et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 27.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 28.Evenhuis HM. Evaluation of a screening instrument for dementia in aging mentally retarded persons. J Intellect Disabil Res. 1992;36:337–347. doi: 10.1111/j.1365-2788.1992.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 29.Määttä T, Trevo- Määttä T, Taanila A, et al. Mental health, behavior and intellectual abilities of people with Down syndrome. Downs Syndr Res Pract. 2006;11:37–43. doi: 10.3104/reports.313. [DOI] [PubMed] [Google Scholar]
- 30.Venables WN, Ripley BD. Modern Applied Statistics with S. 4. New York: Springer; 2002. [Google Scholar]
- 31.R Core Team. R: A language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. URL: http://www.R-project.org/ [Google Scholar]
- 32.Black SE, Doody R, Li H, et al. Donepezil preserves cognition and global function in patients with severe Alzheimer’s disease. Neurology. 2007;69:459–469. doi: 10.1212/01.wnl.0000266627.96040.5a. [DOI] [PubMed] [Google Scholar]
- 33.Winblad B, Kilander L, Eriksson S, et al. Donepezil in patients with severe Alzheimer’s disease: double-blind, parallel-group, placebo-controlled study. Lancet. 2006;367:1057–1065. doi: 10.1016/S0140-6736(06)68350-5. [DOI] [PubMed] [Google Scholar]
- 34.Reisberg B, Doody R, Stoffler A, et al. Memantine Study Group: Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 35.Zakzanis KK, Leach L, Kaplan E. Neuropsychological Differential Diagnosis. Lisse, the Netherlands: Swets & Zeitlinger; 1999. [Google Scholar]