Abstract
Nucleoside reverse transcriptase inhibitors (NRTIs) require intracellular phosphorylation to active triphosphate (TP) nucleotide metabolites before they can inhibit the HIV reverse transcriptase. However, monitoring these pharmacologically active TP metabolites is challenging due to their instability and their low concentrations at the pg/ml levels in blood and tissues. The combination of lamivudine (3TC) and abacavir (ABC) is one of the first lines for HIV therapy. Therefore, a sensitive, selective, accurate, and precise LC-MS/MS method was developed and validated for the simultaneous quantification of 3TC- and ABC-TP metabolites in mouse blood and tissues. Calibration curves were linear over the range of 10–100,000 pg/ml for 3TC-TP and 4– 40,000 pg/ml for carbovir-TP (CBV-TP; phosphorylated metabolite of ABC). This corresponds to 2.1– 21,322 fmol/106 cells for 3TC-TP and 0.8– 8,000 fmol/106 cells for CBV-TP. Accuracy and precision were less than 15% for all quality control sample (QCs), and absolute extraction recovery of were > 65 % for 3TC-TP and > 90 % for CVB-TP. The method was optimized to ensure stability of TP samples and standards during sample collection, preparation, analysis, and storage conditions. This method has enhanced sensitivity and requires smaller amounts of blood and tissue samples compared to previous LC-MS/MS methods for 3TC- and CBV-TP quantification. The developed method was successfully applied to characterize the pharmacokinetic profile of TP metabolites in mouse peripheral blood mononuclear cells (PBMCs), spleen, lymph nodes, and liver cells. In addition, another direct, simple, and high-throughput method for the quantification of TP standards was developed and used for the analysis of stability samples.
Keywords: Triphosphate Metabolites, LC-MS/MS, Nucleoside analogs, Mice
1. INTRODUCTION
The Department of Health and Human Services (DHHS) guidelines for antiretroviral drug administration in human immunodeficiency virus type one (HIV-1)-infected adults and adolescents recommends the use of two nucleoside reverse transcriptase inhibitors (NRTIs) in combination with a non-nucleoside reverse transcriptase (NNRTI), an integrase strand transfer (INSTI), or a protease inhibitor (PI). For the former, NRTIs are prodrugs that require intracellular phosphorylations to active triphosphate (TP) nucleotide metabolites, which are responsible for the inhibition of the viral reverse transcriptase [1, 2]. NRTIs are converted intracellularly to mono-, di-, and then tri- phosphates by nucleoside phosphate kinases. The rate determining step in this 3-step enzymatic conversion to TP metabolites is the first step of parent nucleoside phosphorylation into monophosphate metabolites [3, 4]. Lamivudine (3TC) and abacavir (ABC) are amongst the most commonly used NRTIs [5–7] and are commercially available as a combination in a single product.
Similar to all NRTIs, 3TC and ABC are metabolized intracellularly to their active TP metabolites. However, during phosphorylation, ABC is also deaminated into carbovir (CBV). ABC is converted to ABC-5’-monophosphate (ABC-MP) by an adenosine phosphotransferase and subsequently deaminated into carbovir 5’-monophosphate (CBV-MP) by a cytosolic deaminase. CBV-MP is then further phosphorylated in two successive steps to the active metabolite CBV-5’-triphosphate(CBV-TP) [1].
Monitoring NRTI intracellular TP metabolites, which exist at the pg/ml levels, is important to understand their pharmacological effects [8, 9]. Therefore, very sensitive and selective methods are required for their quantification. Mass spectrometry coupled with ultra-performance liquid chromatography (UPLC-MS/MS) is the gold standard technique for the quantification of NRTIs and their phosphorylated nucleotide metabolites.
Nucleotides contain 1–3 phosphate groups, deoxyribose or ribose sugar, and a purine or a pyrimidine nitrogen base [10]. Due to their hydrophilicity, chromatographic separation of nucleotides is challenging using traditional reverse phase chromatography. Therefore, alternative approaches are used for the chromatographic separation of nucleotide analytes, which can be grouped into two categories, direct and indirect approaches [8]. Direct liquid chromatography (LC)-MS methods rely on the direct quantification of the nucleotide metabolites under non-reverse phase LC conditions. Whereas, indirect methods rely on the quantification of the parent nucleosides resulting from the dephosphorylation of nucleotide metabolites during sample preparation, under reverse phase LC conditions. Both approaches have their advantages and disadvantages.
Several LC-MS/MS methods are available for the quantification of 3TC or ABC, and their triphosphate metabolites [11–20]. However, these methods were developed for the quantification of TPs in peripheral blood mononuclear cells (PBMCs) obtained from relatively large volumes of blood in humans. In this study, we developed, validated, and applied two sensitive and selective LC-MS/MS methods for quantification of these TP metabolites in PBMCs obtained from smaller blood volumes in mice. In addition, we preformed quantitation of TP metabolites in mouse immune cells obtained from spleen, liver, and lymph nodes. This method will be used to support the preclinical development of NRTIs as long-acting nanoformulated antiretroviral therapies.
2. MATERIALS AND METHODS
2.1. Materials
3TC-TP, CBV-TP, CBV, 15N213C-3TC, d4-ABC, and emtricitabine-TP (FTC-TP) were obtained from Toronto Research Chemicals (North York, Canada). 3TC and ABC were provided by GlaxoSmithKline Inc. (Research Triangle Park, NC). Type XA acid phosphatase (EC 3.1.3.2) from sweet potatoes, guanosine, guanosine monophosphate (GMP), guanosine diphosphate (GDP), guanosine triphosphate (GTP), cytidine, cytidine monophosphate (CMP), cytidine diphosphate (CDP), and cytidine triphosphate (CTP) were purchased from Sigma Chemical Co. (St. Louis, MO). Sep-Pak QMA anion-exchange cartridges and OASIS HLB reverse-phase cartridges were purchased from Waters Corp. (Milford, MA). Potassium chloride (KCl) was purchased from JT Baker Co. (Phillipsburg, NJ). LC-MS grade (Optima) water (H2O), acetonitrile (ACN), methanol (MeOH), acetic acid, ammonium hydroxide, and ammonium bicarbonate were purchased from Fisher chemicals (Fair Lawn, NJ). RPMI media 1640, phosphate buffered saline (PBS), Hanks’ Balanced salt solution (HBSS), and fetal bovine serum were purchased from Invitrogen (Gibco; Carlsbad, CA).
2.2. Instrumentation
2.2.1. LC-MS/MS conditions for the indirect quantification of TP metabolites
The LC-MS/MS system comprised of a Waters ACQUITY ultra-performance liquid chromatography (UPLC) system (Waters, Milford, MA) coupled to a triple quadrupole mass spectrometer with electrospray ionization (ESI) source (Waters Xevo TQ-XS). For the indirect quantification of TPs, chromatographic separation was performed with an ACQUITY UPLC using a CSH C18 analytical column (2.1×100 mm, 1.7µm; Waters) equipped with a guard column (Waters, Milford, MA). Mobile phase A consisted of ammonium bicarbonate (pH 7, 7.5 mM) and mobile phase B was methanol. The flow rate was 0.25 ml/min. The initial mobile phase composition was 12 % B for the first 2.5 min, gradually increased to 30 % B over 4 min, gradually increased again to 95 % B over 3.5 min, and then held constant for one min. Mobile phase B was then reset to 12 % over 0.25 min and the column was equilibrated for 2.75 min before the next injection. The total run time was 13 min. The mass spectrometer was operated in the positive ion mode using multiple reaction monitoring (MRM). The following transitions were monitored: m/z 230→112 for 3TC, m/z 248→152 for CBV, m/z 287→191 for ABC, m/z 233→115 for the internal standard (IS) 15N213C-3TC, and m/z 291→195 for IS d4-ABC. 3TC, CBV, ABC, 15N213C-3TC, and d4-ABC were detected at a cone voltage of 22, 2, 4, 12, and 2 V, respectively, and a collision energy of 12, 12, 20, 10, and 20 V, respectively.
2.2.2. LC-MS/MS conditions for direct quantification of TP metabolites
For direct quantitation of TP metabolites, the LC-MS/MS system comprised of a Waters ACQUITY-UPLC system coupled to a 4000 Q TRAP® quadrupole linear ion trap hybrid mass spectrometer with an electrospray ionization (ESI) source (Applied Biosystems, MDS Sciex, Foster City, CA). The chromatographic separation was performed with an anion-exchange column BioBasic-AX (150 mm × 2.1 mm, 5 µm; Thermo Fisher, Waltham, MA). Mobile phase A consisted of 40% ACN, 0.06 % acetic acid, and 10 mM ammonium formate in water, and mobile phase B comprised of 30% ACN, 0.3 % ammonium hydroxide, and 1 mM ammonium formate in deionized water. The flow rate was 0.45 ml/min. The initial mobile phase composition was 50 % B for the first min, gradually increased to 90 % B over two min, and then held constant for five min. Mobile phase B was then reset to 50 % over 0.25 min and the column was equilibrated for 1.75 min before the next injection. The total run time was 10 min. The mass spectrometer was operated in the positive ion mode using multiple reaction monitoring (MRM). The following transitions were monitored: m/z 470→112 for 3TC-TP, m/z 488→152 for CBV-TP, and m/z 488→130 for the IS (FTC-TP). 3TC-TP, CBV-TP, and FTC-TP were detected at a declustering potential (DP) of 66 V, 106 V, and 96 V, respectively, and a collision energy of 45, 43, and 25 V, respectively.
2.3. Sample preparation
2.3.1. Extraction of intracellular TP metabolite for the indirect TP method
Sep-Pak QMA cartridges (360 mg, 37–55 µm; Waters) were used to separate CBV-TP, and 3TC-TP from their mono- and di-phosphates counterparts. The QMA cartridges were conditioned with 10 ml of 500 mM KCl followed by 10 ml of 5 mM KCl. Cell lysate samples in 200 µl of 70% MeOH were loaded onto the cartridges and washed with 15 ml of 75 mM KCl. The triphosphate fraction was eluted with 3 ml of 500 mM KCl and collected for de-phosphorylation. The pH of the TP fraction was lowered to 4.25 by adding 15 µl ammonium acetate buffer (pH 4,10 mM) per ml eluate, and dephosphorylated by adding one unit of type XA sweet potato acid phosphatase per ml eluate and incubating at 37 °C for 30 min. The 15N213C-3TC internal standard was added at this point (10 µl of 5 ng/ml, final concentration of 0.5 ng/ml). Samples were then loaded onto Waters OASIS HLB cartridges (60 mg, 30 µm; Waters) pre-conditioned with 3 ml MeOH and 3 ml H2O, and washed with 3.5 ml H2O to remove salts. The nucleosides of interest were then eluted with 1.5 ml of MeOH and evaporated under vacuum. Once dry, the residue was reconstituted with a 100 µl of 25% MeOH and stored in the −20 °C freezer until the time of LC–MS/MS analyses.
Because of the unavailability of standards for the mono- and di-phosphate metabolites of 3TC and CBV and to confirm the separation of mono-, di-, and triphosphate metabolites of 3TC and CBV on the QMA SPE, we used surrogate mono-, di-, and tri-phosphates of their corresponding nitrogen bases, i.e. guanosine for CBV and cytidine for 3TC. The mono-, di-, and tri-phosphate metabolites of CBV and 3TC were extracted and separated under the same conditions described above but with continuous collection of one-ml fractions of the eluate, up to 18 ml. 250 µl of the collected fractions were transferred to quartz 96-well plates and UV absorbance was measured on a spectrophotometer at lambda of 268 nm for cytidine (C), C-MP, C--DP, CTP,3TC-TP, and lambda of 253 nm for guanosine (G), G-MP, G-DP, G-TP, and CBV-TP).
2.3.2. Preparation of stability samples for the direct TP method
The direct method for TP quantification was used only for the stability samples. Cell lysate samples for stability study samples were centrifuged at 17,000 × g for 10 minutes, and 50 µl of supernatants were collected and mixed with 40 µl of 70% MeOH and 10 µl of IS (FTC-TP at 100 ng/ml, final concentration of 10 ng/ml), and analyzed by LC-MS/MS. For stability samples stored in water, 25 µl aliquots of samples were mixed with 65 µl of MeOH and 10 µl IS. Finally, 45 µl samples were mixed with 5 µl of 2% NH4OH to a final concentration of 0.2% NH4OH, before LC-MS/MS analysis.
2.4. Calibration curves
2.4.1. Indirect TP method
Calibration curves were prepared over the range of 10–100,000 pg/ml for 3TC-TP, and 4– 40,000 pg/ml for CBV-TP. Each standard was prepared by spiking 1 × 106 blank PBMCs lysed in 200 µl of 70% MeOH with 10 µl of the appropriate spiking solutions of 3TC-TP and CBV-TP. Standards were then loaded onto the QMA cartridges, extracted, and analyzed as described in Section 2.3.1. Because concentrations from the in-vitro and in-vivo applications of this method are expressed in fmol or pmol/106 cells, pg/ml concentrations of the calibration curve standards were converted to fmol or pmol/106 cells bringing the calibration ranges to 2.1– 21,322 fmol/106 cells for 3TC-TP, and 0.8 –8,000 fmol/106 cells for CBV-TP. 15N213C-3TC was used as an internal standard (IS) at final concentration of 500 pg/ml. Calibration curves (area ratio of analyte/IS vs. nominal concentration) were fitted by least-squares linear regression using 1/x2 weighting scheme.
2.4.2. Direct TP method
Samples from the stability studies for 3TC-TP and CBV-TP were analyzed by the direct TP method. Calibration curves were prepared over the range of 1–500 ng/ml for both 3TC-TP and CBV-TP. Each standard was prepared by spiking 1 × 106 PBMCs lysed in 90 µl of 70% MeOH with 10 µl of the appropriate spiking solutions of 3TC-TP and CBV-TP, and extracted as discussed in section 2.3.2.
2.5. Method Validation
2.5.1. Indirect TP method
The method was validated using five QC points for each calibration curve and the concentrations of the QC points were 10, 30, 1500, 50,000, and 100,000 pg/ml for 3TC-TP, and 4, 12, 750, 20,000, and 40,000 pg/ml for CBV-TP. Accuracy and precision of the method were determined according to FDA guidelines by assaying five replicates of each QC point using freshly prepared calibration curves in three separate runs. Intra-day accuracy and precision were calculated from the % bias [% (measured− theoretical)/theoretical concentration] and relative standard deviation [% RSD = % standard deviation/mean], respectively, for the five replicates of each QC point. Inter-day precision was calculated similarly using the 15 replicates of each QC point from the three validation runs. Accuracy and precision were considered acceptable when they were less than 15%, except for the LLOQs, where 20% was acceptable.
The matrix effect and extraction recoveries of TP metabolites were determined at low, medium and high QC concentrations. The overall (absolute extraction) recovery, and recoveries of every step of sample extraction, i.e. anion-exchange SPE, enzymatic conversion (dephosphorylation), and reversed-phase SPE, were separately determined as follow:
Absolute overall recovery:
- Step 1: Recovery of the 1st anion-exchange SPE:
- Step 2: Recovery of enzymatic conversion (dephosphorylation):
- Step 3: Recovery of the 2nd reversed-phase SPE:
Matrix effect:
2.5.2. Direct TP method
The direct method for 3TC-TP and CBV-TP quantification in stability samples was validated using five QC points of 1, 3, 75, 375, and 500 ng/ml concentrations. The same validation criteria for the indirect method were followed.
2.6. Storage stability studies
3TC-TP and CBV-TP were subjected to various conditions in order to test analyte stability including freeze (−20 °C)-thaw, autosampler, room temperature (bench-top), and long-term storage (2 months at −20 °C) at 1 µg/ml concentration (n=2), in water and in PBMCs cell lysate. All storage stability samples were compared to freshly prepared samples.
2.7. Intra-cellular stability of TP metabolites in in-vitro and in-vivo samples
Intracellular stability of TP metabolites was tested in both in-vitro and in-vivo samples. For in-vitro samples, human monocyte-derived macrophages (MDM) prepared from PBMCs were incubated for 2 h with 100 µM nanoformulated 3TC and ABC for uptake as described previously [21, 22]. Synthesis and characterization of these nanoformulations have been reported previously [21, 22]. Adherent MDMs were washed and scraped with 7 ml PBS, centrifuged at 800 × g for 8 min, and the pellet was suspended in 1 ml PBS and cells counted using a hemocytometer. The volume of the cell suspension was adjusted to 10 × 106 cells/ml and the suspension was aliquoted into 100 µl volumes, and stored at 4° C for the stability study. At different time points, the above cell suspension aliquots were centrifuged at 800 × g for 8 min, cell pellets were lysed with 200 µl of 70 % MeOH, and samples were processed for indirect TP quantification as described in Section 2.3.1.
For in-vivo samples, six mice were dosed with 50 mg/kg equivalent doses of 3TC and ABC intramuscularly as native or nanoformulated drug, as described below in Section 2.9. Two hours after dose administration, mice were sacrificed, and spleens were collected. Spleens were chopped into small pieces, mixed, and divided into eight parts of around 50 mg each, and stored at 4° C in PBS for the stability study. The stored spleen aliquots were then processed for cell isolation and indirect TP quantification at 0, 6, 24, and 48 h.
2.8. Sample preparation and LC-MS/MS analysis of parent nucleosides in blood and tissues
Parent nucleosides were quantified in blood and tissues as we described previously [21, 22]. In short, freshly collected blood (20 µl) samples from mice were immediately mixed with 1 ml ACN, and spiked with 10 µl of IS (15N213C-3TC and d4-ABC at 10 ng/ml, final concentration of 1 ng/ml). Samples were then centrifuged at 17,000 × g for 10 min and supernatants were dried, reconstituted in 100 µl of 25% MeOH, and injected for LC-MS/MS analysis of 3TC, ABC, and CBV. For tissue sample preparation, 8–12 mg of spleen, 4–6 mg of lymph nodes, and 40–60 mg of liver were used for parent nucleoside analysis. Depending on the amount of tissues collected, liver, spleen, and lymph nodes were homogenized in 5–30 volumes of 90% MeOH and 50 µl of the homogenates were mixed with 40 µl of MeOH and 10 µl of IS. Samples were then centrifuged at 17,000 × g for 10 min and 30 µl of supernatants were collected and mixed with 50 µl H2O before LC-MS/MS analysis of parent nucleosides. Calibration curves were linear over the range of 0.1–1000 ng/ml in blood, 0.6–3000 ng/g in spleen, 1.2–12000 ng/g in lymph node, and 0.12–1200 ng/g in liver. The same LC-MS/MS system and conditions (Section 2.2.1.) that were used for the quantification of parent nucleosides resulting from dephosphorylation of TP metabolites, were used for the quantification of parent nucleosides in samples.
2.9. Pharmacokinetics study
Eight-week-old male BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA). Sterilized 7012 Teklad diets (Harlan, Madison, WI) were used, and water was provided ad libitum. Mice were housed in the UNMC laboratory animal facility according to the American Animal Association Laboratory Animal Care guidance. All protocols and procedures were approved by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center. Mice were injected with native and nanoformulated 3TC, and ABC intramuscularly (IM) at 50 mg/kg equivalent doses of 3TC and ABC. Synthesis and characterization of these nanoformulations were reported previously [21, 22]. The dosing solution/suspension of native drug and nanoformulation was prepared at 25 mg/ml equivalent concentration of 3TC and ABC, and dose injected at 2 µl/g of body weight to each mouse. For native compounds, 3TC was dissolved in PBS, and ABC was in a mixture of DMSO-PG-ethanol-PEG 400-Cremophor EL-PBS (10-10-10-20-5-45 vol/vol), whereas nanoformulated 3TC and ABC were diluted in 10 mM HEPES and deionized water, respectively. Mice were sacrificed and 0.5–0.8 ml blood from each mouse was collected in tubes containing EDTA (1.5 mg/ml blood) by cardiac puncture. Liver, spleen, and lymph nodes were collected at 2 h, and 1, 3, and 7 days after drug administration.
2.9.1. Isolation of PBMCs
PBMCs were isolated from mice blood using Histopaque-1083 (Sigma Chemical Co. St. Louis, MO; Catalog # 1083-1) according to the manufacturer’s protocol. In summary, blood collected in EDTA tubes was layered on top of 1 mL Histopaque-1083 and centrifuged for 30 min at 400 × g at room temperature. PBMCs were then aspirated, washed twice with PBS, suspended in PBS, and counted with a hemocytometer. Further, PBMCs were centrifuged (400 × g for 10 min), lysed with cold 200 µl of 70% methanol for at least 15 minutes, and then cell lysates were stored in −20 °C until sample preparation for TP extraction.
2.9.2. Isolation of cells from spleen, lymph nodes, and liver
Spleens were collected in 60-mm culture (Petri) dishes, minced into small pieces using scalpel blades, and transferred to 70 µm nylon cell strainers (BD Falcon # 352350) inserted into the top of 50-ml conical tubes. One ml of HBSS was added and spleens were disrupted using the rubber end of the 3-ml syringe plunger, and the resulting cells were pushed through the cell strainer leaving behind some residual fibrotic tissues. The strainers were further washed with 4 ml of HBSS to collect any remaining cells through the strainer. Cell suspensions were transferred into 15-ml conical tubes, centrifuged at 200 × g for 10 minutes, and supernatants were discarded. The resulting cell pellets were resuspended in one ml of RBC lysing buffer (ACK buffer), incubated for two minutes at 37° C, neutralized with 14 ml of HBSS, centrifuged at 200 × g for 10 minutes, supernatants were discarded, and cell pellets underwent one more cycle of washing and centrifugation. The resulting cell pellet was resuspended in two ml PBS, and counted with a hemocytometer. Finally, cells were centrifuged at 400 × g for 10 minand cell pellet was lysed with 200 µl of cold 70% MeOH for TP sample extraction.
For lymph node lymphocyte preparation, freshly removed lymph nodes from all three mice were pooled (~15–18 lymph nodes) for every time-point, and cells were isolated similarly to splenocytes as described above, except that the RBC lysis step was not used.
Total non-parenchymal liver cells were isolated by a mechanical disruption method, using a modification of previously published methods [23, 24]. Livers were perfused with PBS as described [23], minced into small pieces, and forced gently through a 200 µm-gauge stainless steel mesh using a syringe plunger. The preparation obtained was suspended in 50 ml RPMI-1640 medium (containing GlutaMAX™-1, 25 mM HEPES and 10% FBS), centrifuged at 60 × g for one min, and the resulting supernatant (45 ml) was transferred to a new tube and centrifuged at 480 × g for eight min. RBCs were eliminated by suspending the cell pellet in 5 ml erythrocyte lysis buffer, incubated for 2–3 min at room temperature, neutralized with 25 ml cell suspension medium (RPMI-1640 with 5% FBS), transferred the suspension to a new 50-ml tube through a 70-µm sterile nylon gauze. Suspensions were then centrifuged at 450 × g for eight min, supernatants were discarded, and cell pellets were suspended in five ml cell suspension medium. At this stage, the suspension contains both parenchymal (hepatocytes) and non-parenchymal (polymorphonuclear cells, monocytes, macrophages, DCs, NK and NKT cells) liver cells. For the separation of non-parenchymal cells, eight ml Lymphoprep™ (Lucron Bioproducts) were added into a 15-ml tube, and liver suspension was slowly layered on top of it, and centrifuged at 800 × g for 25 min at 20 °C without acceleration or brake. The layer at interphase was carefully collected into a 15-ml tube. The tube was filled with MACS buffer (HBSS with 2% FBS and 3 mM EDTA), centrifuged at 800 × g for 7 min, and the supernatant was discarded. The resulting cell pellet was suspended in 0.2 ml of PBS and counted with a hemocytometer. Finally, cells were centrifuged (400 × g for 10 min) and cell pellet was lysed with 200 µl of cold 70% methanol for TP sample extraction.
3. RESULTS
3.1. Sample Preparation
The indirect approach of nucleotide metabolites quantification relies on the complete separation of the mono, di, and tri-phosphate metabolites during sample preparation prior to LC-MS/MS analyses. Because the standards of the mono- and di-phosphate 3TC and CBV were not available, we used mono-, di-, and tri-phosphates of the corresponding nitrogen bases. For example, we used guanosine and cytidine as surrogate standards, to develop the separation conditions of CBV and 3TC, respectively, during sample preparation. We used anion-exchange solid phase extraction (SPE) to achieve the separation.
Various types of SPE cartridges, buffers, pHs, counter-ions, and loading, washing, and elution conditions were investigated. Several strong anion-exchange (SAX) cartridges from different manufacturers were investigated and Sep-Pak QMA produced the highest recovery for both analytes. Two hundred µl of 70% MeOH was used for sample loading onto the SPE cartridge, we used. This sample solvent composition is compatible with cell lysis conditions required before SPE. Larger volumes and higher organic content in the sample solvent lead to some breakthrough and loss of analytes during sample loading.
For washing conditions, we used 15 ml of 75 mM KCl, which resulted in the retention of the TP metabolites, while the mono- and di-phosphate metabolites were washed away. The mono- and di-phosphates were not of interest for the current application, otherwise these metabolites could also be retained during washing with lower KCl concentrations (50 mM) and eluted sequentially with a gradient of KCl. Therefore, under our conditions, we avoided multiple-step washing [11–13, 25] and were able to optimize a one-step washing condition. For elution, three ml of 500 mM KCl was the optimum volume and counter-ion concentration for the complete elution of the TPs. Lower volumes and concentrations of KCl decreased recovery, while higher volumes and/ concentrations did not further increase recovery. Acidic, basic, and neutral KCl conditions were tested for washing, but the neutral KCl showed the highest recovery and separation of the TP metabolites.
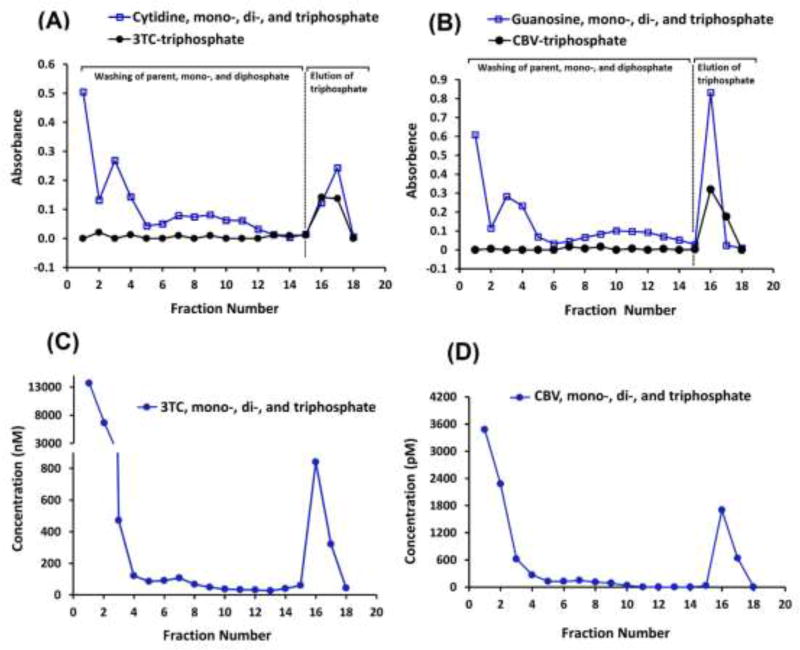
Fig. 1 A and B show the complete separation of guanosine- and cytidine-TPs, which were used as surrogates for CBV and 3TC, from their parent nucleosides and their mono- and di-phosphate counterparts. Similarly, Fig. 1 C and D show the complete separation of intracellular CBV- and 3TC-TP, formed by PBMCs in-vitro, from their parent nucleoside and mono- and di-phosphate metabolites.
Figure 1.
Separation of TP metabolites (fractions # 16–18) from their parent, mono-, and di-phosphate metabolites (fractions # 1–15) by anion-exchange SPE for: (A) Cytidine-TP (surrogate marker for 3TC-TP) separation from parent cytidine, cytidine-MP, and -DP, absorbance was monitored at 268 nm after spiking 1× 106 blank PBMCs with standards; (B) Guanosine-TP (surrogate for CBV-TP) separation from parent guanosine, guanosine-MP, and -DP, absorbance was monitored at 253 nm absorbance monitored at 268 nm after spiking 1× 106 blank PBMCs with standards; (C, D) Concentrations of 3TC-TP and CBV-TP metabolites in an in-vitro PBMC sample after 2-hr incubation with nanoformulated 3TC and ABC at 100 µM. Concentrations were measured by the indirect LC-MS/MS method.
For the direct TP method, samples from the storage stability study were prepared by a one-step MeOH-protein precipitation as described in Section 2.3.2. Also, for the parent nucleosides quantification, samples were prepared by MeOH-protein precipitation as described in Section 2.8., and as we described previously [21, 22].
3.2. LC-MS/MS methods development and validation
3.2.1. Indirect TP method
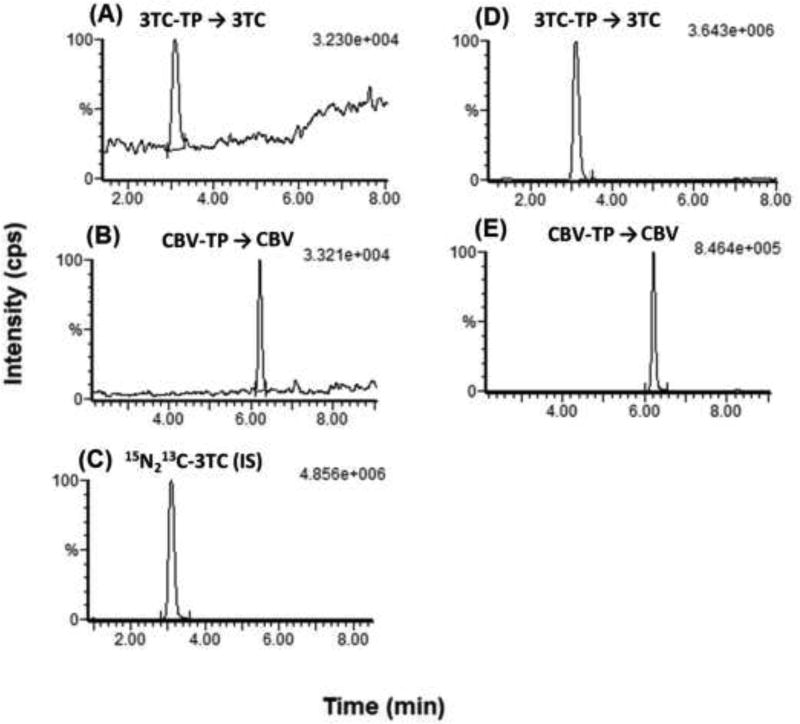
Samples were prepared differently for parent nucleoside and TP metabolites extraction, as described above, but they were analyzed by the same LC-MS/MS method. LC-MS/MS conditions were developed, optimized, and validated for 3TC, ABC, and CBV as we described previously [21, 22]. We applied the same conditions for the parent nucleosides resulting from the dephosphorylation of TP metabolites during sample preparation. These LC-MS/MS conditions resulted in complete separation and high sensitivity of all analytes (3TC, ABC, and CBV) within a 13-min run time. Representative chromatograms of 3TC-TP, CBV-TP at their LLOQs and the internal standard (IS) are shown in Fig. 2.
Figure 2.
Representative chromatograms of the indirect TP method using 1× 106 blank PBMCs spiked with (A) 3TC-TP at 10 pg/ml, (B) CBV-TP at 4 pg/ml, (C) IS at 500 pg/ml standards. (D, E) 3TC-TP, and CBV-TP in PBMC samples obtained from mice, two hours after dose administration of ABC and 3TC at 50 mg/kg. All chromatograms show the parent 3TC (230>112 m/z) and CBV (248>152 m/z) after dephosphorylation of their corresponding TP metabolites as described in Section 2.3.1.
Selectivity, sensitivity, linearity, accuracy, precision, and stability were determined as the parameters for method validation and ruggedness. Calibration curves were linear over a wide dynamic range of 10,000 with a correlation coefficient > 0.998. Calibration curves ranged from 10–100,000 pg/ml for 3TC-TP, and 4– 40,000 pg/ml for CBV-TP. This corresponds to 2.1– 21,322 fmol/106 cells for 3TC-TP and 0.8– 8,000 fmol/106 cells for CBV-TP. Inter-day and intra-day accuracy and precision data for both 3TC-TP and CBV-TP are summarized in Table 1. All accuracy and precision values were less than 15% for all QCs including the LLOQs. Method selectivity was verified by the lack of any interfering peaks that co-elute with the analytes or IS in blank matrices. Carry-over effect was also examined and found to be < 20% of the LLOQ.
Table 1.
Accuracy and precision of 3TC-TP and CBV-TP assay in PBMCs.
| Nominal conc. in injection (pg/ml) | 10 | 30 | 15,00 | 50,000 | 100,000 | ||
|
| |||||||
| Conc. in fmol/million cells | 2.1 | 6.4 | 320 | 10,661 | 21,322 | ||
|
| |||||||
| 3TC-TP | Day-1 | Mean conc. (n=5) | 10.0 | 29.3 | 1,484 | 46,654 | 94,601 |
| Accuracy (%) | 0.1 | −2.3 | −1.1 | −6.7 | −1.7 | ||
| Precision (%) | 6.6 | 5.0 | 5.2 | 6.1 | 2.5 | ||
|
| |||||||
| Day-2 | Mean conc. (n=5) | 9.7 | 29.2 | 1,551 | 49,679 | 96,225 | |
| Accuracy (%) | −2.9 | −2.6 | 3.4 | −0.6 | −3.8 | ||
| Precision (%) | 8.1 | 4.0 | 6.8 | 3.7 | 1.5 | ||
|
| |||||||
| Day-3 | Mean conc. (n=5) | 9.8 | 30.1 | 1,522 | 48,433 | 101,596 | |
| Accuracy (%) | −2.5 | 0.3 | 1.5 | −3.1 | 1.6 | ||
| Precision (%) | 7.6 | 4.7 | 6.1 | 2.1 | 6.5 | ||
|
| |||||||
| Inter-day | Mean conc. (n=15) | 9.8 | 29.5 | 1,519 | 48,255 | 97,474 | |
| Accuracy (%) | −1.8 | −1.5 | 1.3 | −3.5 | −1.3 | ||
| Precision (%) | 7.4 | 4.6 | 6.0 | 4.0 | 3.5 | ||
|
| |||||||
| Nominal conc. in injection (pg/ml) | 4.0 | 12.0 | 750 | 20,000 | 40,000 | ||
|
| |||||||
| Conc. in fmol/million cells | 0.82 | 2.5 | 154 | 4,107 | 8,214 | ||
|
| |||||||
| CBV-TP | Day-1 | Mean conc. (n=5) | 4.0 | 12.3 | 771 | 20,160 | 41,501 |
| Accuracy (%) | 0.4 | 3.1 | 2.7 | 0.8 | 3.8 | ||
| Precision (%) | 10.7 | 7.0 | 4.7 | 7.3 | 2.7 | ||
|
| |||||||
| Day-2 | Mean conc. (n=5) | 4.0 | 11.9 | 766 | 19,950 | 42,212 | |
| Accuracy (%) | −0.5 | −0.5 | 2.2 | −0.2 | 5.5 | ||
| Precision (%) | 8.1 | 10.8 | 6.5 | 3.3 | 4.8 | ||
|
| |||||||
| Day-3 | Mean conc. (n=5) | 3.9 | 13.1 | 776 | 20,600 | 39,742 | |
| Accuracy (%) | −2.0 | 8.5 | 3.5 | 3.0 | −0.6 | ||
| Precision (%) | 11.5 | 3.3 | 5.1 | 4.4 | 1.2 | ||
|
| |||||||
| Inter-day | Mean conc. (n=15) | 4.0 | 12.4 | 771 | 20,237 | 41,152 | |
| Accuracy (%) | −0.7 | 3.7 | 2.8 | 1.2 | 2.9 | ||
| Precision (%) | 10.1 | 7.0 | 5.4 | 5.0 | 2.9 | ||
The absolute extraction recovery of TP metabolites in PBMCs were ~ 65 % for 3TC-TP and ~ 90 % for CVB-TP. Recoveries of TP metabolites at the individual steps involved in the extraction, i.e. anion-exchange first SPE, dephosphorylation, and reversed-phase second SPE, are shown in the Table 2. Our results show that analyte losses during extraction were primarily associated with the first SPE step, whereas recoveries from second SPE, dephosphorylation, and matrix effect were near 100%. The extraction recovery was also consistent throughout the dynamic range.
Table 2.
Overall recovery and recoveries of the individual steps involved in the sample preparation of 3TC-TP and CBV-TP in PBMCs.
| Recovery | 3TC-TP | CBV-TP |
|---|---|---|
|
| ||
| Overall | 60–65% | 85–90% |
| Step-1 extraction# | 70–75% | 85–90% |
| Enzymatic Conversion (dephosphorylation) | 95–100% | 95–100% |
| Step-2 extraction* | 90–100% | 90–100 % |
| Matrix effect | 95–100% | 95–100% |
Separation of TPs by anion-exchange SPE using QMA cartridge
Desalting by reversed-phase SPE using OASIS HLB cartridges.
In addition, due to the variability of the number of cells obtained from study samples, we have compared accuracy, precision, and recoveries of QC samples prepared in various number of black PBMCs (0.1, 1.0, and 10 × 106 cells). None of these parameters were affected by the number of blank cells in the matrix (data not shown).
3.2.2. Direct TP method
Because of the large number of samples used in the stability study, and because of the time-consuming sample preparation associated with the indirect TP method, stability samples were analyzed by the direct TP method.
We investigated several LC approaches to retain the TP analytes, including hydrophilic interaction liquid chromatography (HILIC), normal phase chromatography, ion-pairing chromatography with the reagents added to the mobile phase and/or sample solvent, as well as anion-exchange chromatography. Anion-exchange chromatography with the BioBasic AX resulted in the retention and separation of 3TC-TP, CBV-TP, and IS (emtricitabine-TP; FTC-TP) within 8 minutes. For the mobile phase composition, acetic acid in mobile phase A was required for analytes retention, while NH4OH in mobile phase B was required for analytes elution.
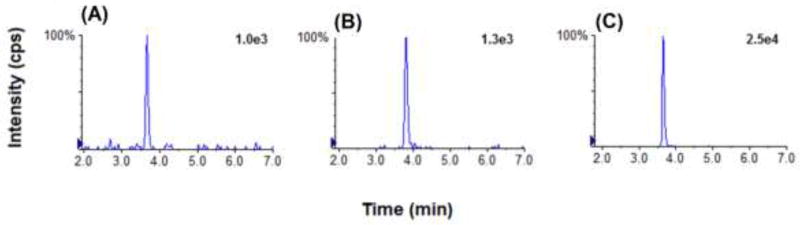
TP metabolites produce a MS signal in both the positive and negative ionization modes due to the ionization of their nitrogen base or phosphate groups in the MS source, respectively [10]. For this method, we selected the positive ionization mode because it produced a higher signal than the negative ionization mode. In addition, we observed a 6–8-fold increase in sensitivity by using 0.2% NH4OH in the sample solvent. Representative chromatograms of 3TC-TP and CBV-TP at the final direct method conditions are shown in Fig. 3.
Figure 3.
Representative chromatograms of the direct TP method using 1× 106 blank PBMCs spiked with (A) 3TC-TP at 5 ng/ml, (B) CBV-TP at 5 ng/ml, (C) IS at 50 ng/ml standards.
For the direct TP method, calibration curves were linear over a dynamic range of 1–500 ng/ml with a correlation coefficient > 0.998. All accuracy and precision values were less than 15% for all QCs (3, 75, 375, 500 ng/ml), and were less than 20% for the LLOQs (1 ng/ml) (data not shown).
3.3. Storage stability
Nucleotides are highly susceptible to enzymatic and non-enzymatic degradation of their labile phosphate bonds. Therefore, evaluation of stability was carried out to ensure that none of the steps taken during sample preparation, analysis, or storage conditions, caused analyte degradation.
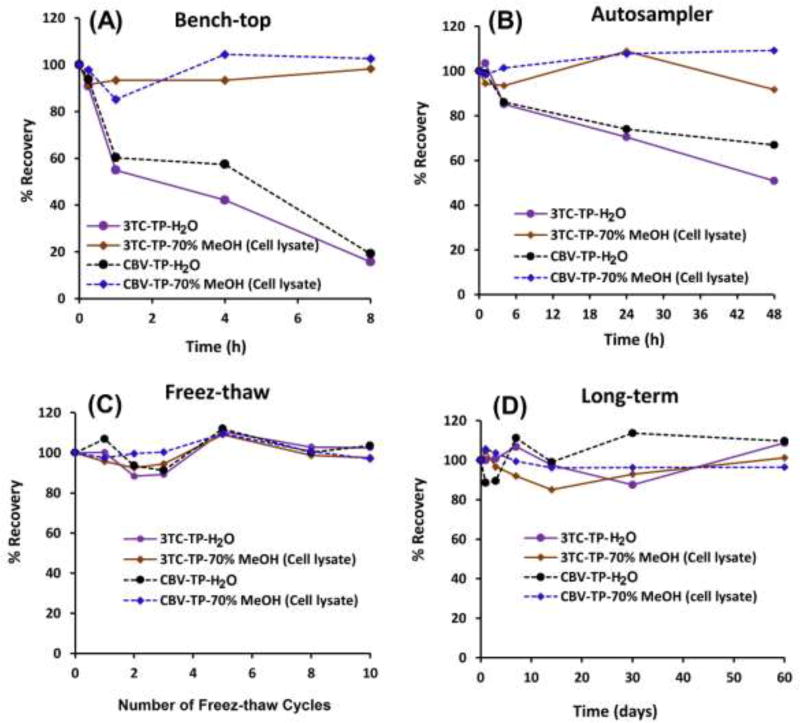
3TC-TP and CBV-TP QC samples were subjected to various storage conditions including freeze-thaw, autosampler, bench-top, and long-term storage. Fig. 4 summarizes stability data, which are expressed as percentages of analyte concentrations at different time points compared to freshly prepared samples under the various conditions. In bench-top stability, both TPs were found stable (>95%) in PBMC cell lysate up to 8 h. However, TPs were not stable in water with less than 20 % recovery by 8 h. In autosampler stability studies, samples stored for 48 hours in the autosampler at 4 °C, were found stable in cell lysate with more than 92 % recovery, while they were not stable in water (50–70% recovery by 48 h). In freeze-thaw stability study, both TPs were found stable in both water and cell lysate, and recoveries were more than 95 % in samples, which underwent 10 freeze-thaw cycles. In long-term (−20 °C) storage, both TPs were found stable in both water and cell lysate for two months with more than 95% recovery.
Figure 4.
Stability studies of 3TC-TP and CBV-TP under various storage conditions in cell lysate (70% MeOH) and in H2O matrices under various conditions: (A) Bench-top, (B) Autosampler, (C) Freeze-thaw cycles, (D) Long-term storage at −20°.
Stability data in the cell-lysate matrix inform about stability of study samples during sample preparation and analysis (bench top and autosampler) as well as sample storage before analysis or for re-analysis (long-term and freeze-thaw). Next we optimized sample solvent composition to minimize TP hydrolysis in the stocks during storage. Typically, water had been used as a solvent for TP stocks, because of the high solubility of these hydrophilic compounds in water. We confirmed that TP stocks were 100% stable in water for at least two months at both −20 and −80 °C, and they withstood at least 10 freeze-thaw cycles (Fig. 4 C and D). However, TPs started degrading immediately and gradually at room temperature (bench-top) leaving only 20% of the original concentration by 8 h. In addition, TPs were not stable at 4 °C in the autosampler, but degraded at a slower rate compared to room temperature, with 50–70 % recovery by 48 h. In contrast, adding an organic (MeOH or ACN) to the stock solvent, stabilized TPs and prevented their degradation at 4 °C and at room temperature. By varying the percentage of organic solvent, we found that at least 50% (data shown for 70% organic in Fig. 4) organic is needed for a 100% recovery on the bench for 8 h and in the autosampler for 48 h (Fig. 4 A and B). Therefore, stock solutions are stable in water in the freezer but they have to be diluted with organic solvent to at least 50% to be stable outside the freezer.
In summary, these data demonstrate the stability of both 3TC- and CBV-TP during sample preparation, LC-MS/MS analyses, and storage conditions. In addition, these data demonstrate the stability of the stock solutions and standards during their working and storage conditions.
3.4. Intra-cellular stability of TP metabolites in in-vitro and in-vivo samples
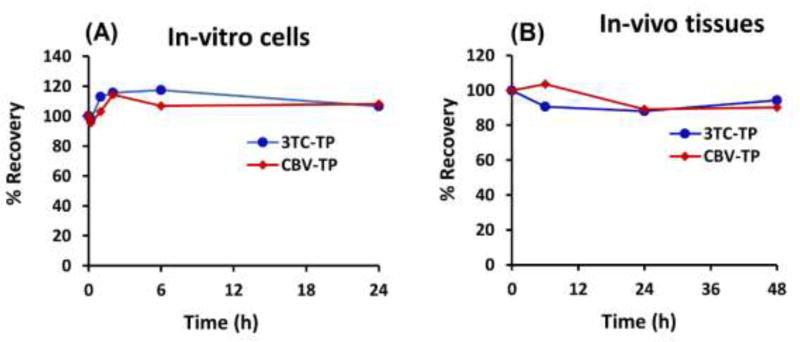
Isolation of cells from in-vitro and in-vivo samples is a complex and multi-step process, and the time it takes from sample collection to cell lysis can vary markedly based on the operator, experiment conditions, and number of samples. Therefore, and given that TP metabolites are stable in cell lysates (as shown above), it is important to characterize the stability of intracellular TP metabolites from the time samples are collected to the time cells are lysed. This information will reveal any constraints or limitations on the maximum time allowed between sample collection until cells are isolated and lysed. For the in-vitro stability study, cells were treated (loaded) with 3TC and ABC for two hours, removed, and stored at 4 °C for various time points before cell lysis. Fig. 5 A shows that intracellular concentrations of 3TC-TP and CBV-TP were constant up to 24 h. This means that PBMCs obtained from in-vitro experiments can be stored up to 24 h at 4 °C before cell lysis and sample preparation for TPs extraction.
Figure 5.
Intra-cellular stability of 3TC-TP and CBV-TP in (A) in-vitro conditions where monocyte derived macrophages were stored up to 24 h at 4 °C, and (B) in-vivo conditions where spleen tissues were stored up to 48 h at 4 °C.
For the in-vivo stability experiment, we collected, chopped into small pieces, and pooled spleens from six mice and stored them in PBS at 4 °C for 48 h. At various time points, splenocytes were isolated and TPs extracted from the pooled spleen tissue. Fig. 5 B shows that intracellular concentrations of 3TC-TP and CBV-TP were constant in spleen tissues stored at 4 °C up to 48 h. This means, tissues collected from in-vivo experiments could be stored up to 48 h before cell isolation and TP extraction.
3.5. Pharmacokinetics of parent nucleosides and TP metabolites
The indirect method for simultaneous quantification of 3TC-TP and CBV-TP was applied in an in-vivo mouse PK study. The concentrations of TP metabolites were quantified in PBMCs, splenocytes, lymph node lymphocytes, and liver immune cells obtained from mice after intramuscular administration of nanoformulated and native 3TC and ABC at 50 mg/kg. The concentrations of TP metabolites and parent nucleosides from the mouse PK study are shown in Table 3. 3TC-TP levels in PBMCs and splenocytes were detected for at least 7 days after both nanoformulation and native drug administration. The CBV-TP levels in PBMCs were detected at least up to day seven, while in splenocytes they were detected only up to 2 h after both nanoformulation and native drug administration. Two hours after dose administration, concentrations of 3TC-TP and CBV-TP in PBMCs were 6–8 fold higher after administration of native drug solution compared to the nanoformulation. However, by day seven, 3TC-TP and CBV-TP concentrations were 4–5 fold higher after administration of the nanoformulation compared to native drug solution. Similar to PBMCs, both TP metabolites were initially higher in splenocytes after native drug vs. nanoformulation administration, which was reversed with time, where TP levels in splenocytes after nanoformulation administration were 4-fold higher than those after native drug administration by day 7.
Table 3.
3TC-TP and CBV-TP concentrations in mouse PBMCs, splenocytes, lymph node lymphocytes, and liver immune cells; and parent 3TC, ABC, and CBV concentrations in blood, spleen, lymph node, and liver tissues after intramuscular administration of native and nanoformulated 3TC and ABC at 50 mg/kg dose in mice (n=3).
| Time | 3TC-TP | 3TC | ||||||||||
|
|
||||||||||||
| NP | Native | NP | Native | |||||||||
| Mean* | SEM | Mean* | SEM | Mean# | SEM | Mean# | SEM | |||||
|
|
||||||||||||
| PBMCs | Blood | |||||||||||
|
|
||||||||||||
| 2 h | 248.4 | 62.2 | 1405.2 | 404.0 | 915.8 | 222.3 | 2270.4 | 300.5 | ||||
| 1 day | 156.4 | 16.9 | 49.7 | 23.2 | 338.5 | 23.8 | 3.6 | 0.8 | ||||
| 3 day | 133.5 | 35.9 | 115.4 | 55.8 | 99.1 | 9.2 | 1.1 | 0.4 | ||||
| 7 day | 41.5 | 13.2 | 9.6 | 3.2 | 34.3 | 6.4 | 0.2 | 0.0 | ||||
| Splenocytes | Spleen | |||||||||||
|
|
||||||||||||
| 2 h | 274.6 | 226.1 | 485.8 | 45.7 | 781.9 | 98.6 | 6967.7 | 1853.3 | ||||
| 1 day | 24.9 | 4.2 | 2.6 | 0.2 | 444.2 | 10.2 | 13.2 | 3.0 | ||||
| 3 day | 8.4 | 2.9 | 2.4 | 0.8 | 97.0 | 14.9 | 3.2 | 1.1 | ||||
| 7 day | 4.8 | 1.0 | 1.1 | 0.1 | 51.0 | 5.7 | 1.5 | 0.8 | ||||
| Lymph node lymphocytes | Lymph node | |||||||||||
|
|
||||||||||||
| 2 h | 5.2 | 99.7 | 1920.2 | 23684.9 | ||||||||
| 1 day | 5.0 | 0.4 | 994.9 | 177.6 | ||||||||
| 3 day | 0.4 | nd | 104.1 | 8.6 | ||||||||
| 7 day | 0.5 | nd | 45.8 | 2.5 | ||||||||
| Liver immune cells | Liver | |||||||||||
|
|
||||||||||||
| 2 h | nd | 136.7 | 23.8 | 1001.0 | 178.9 | 4877.2 | 2033.8 | |||||
| 1 day | nd | nd | 384.3 | 41.4 | 8.1 | 0.6 | ||||||
| 3 day | nd | nd | 87.8 | 15.5 | 2.2 | 0.6 | ||||||
| 7 day | nd | nd | 41.2 | 5.7 | 2.1 | 1.4 | ||||||
|
| ||||||||||||
| Time | CVB-TP | ABC | CBV | |||||||||
|
| ||||||||||||
| NP | Native | NP | Native | NP | Native | |||||||
|
| ||||||||||||
| Mean* | SEM | Mean* | SEM | Mean# | SEM | Mean# | SEM | Mean# | SEM | Mean# | SEM | |
|
| ||||||||||||
| PBMCs | Blood | Blood | ||||||||||
|
| ||||||||||||
| 2 h | 11.3 | 9.7 | 98.8 | 20.0 | 117.2 | 13.2 | 3241.8 | 586.8 | 1.3 | 0.1 | 17.0 | 0.3 |
| 1 day | 2.8 | 0.6 | 13.4 | 1.5 | 76.7 | 5.4 | 0.6 | 0.2 | 0.7 | 0.1 | 0.1 | 0.0 |
| 3 day | 1.3 | 0.1 | 1.3 | 0.3 | 33.4 | 3.9 | 0.1 | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 |
| 7 day | 2.9 | 1.1 | 0.5 | 0.1 | 27.8 | 0.5 | nd | 0.4 | 0.0 | nd | ||
| Splenocytes | Spleen | Spleen | ||||||||||
|
| ||||||||||||
| 2 h | 0.9 | 0.7 | 2.3 | 0.2 | 117.0 | 10.8 | 3311.5 | 940.9 | 6.7 | 0.5 | 187.2 | 9.1 |
| 1 day | nd | nd | 87.0 | 3.2 | 1.6 | 0.4 | 6.6 | 1.0 | 0.7 | 0.1 | ||
| 3 day | nd | nd | 30.6 | 3.2 | 2.0 | 0.9 | 3.4 | 0.5 | 0.3 | 0.2 | ||
| 7 day | nd | nd | 26.0 | 1.7 | 0.5 | 0.1 | 3.2 | 0.4 | nd | |||
| Lymph node lymphocytes | Lymph node | Lymph node | ||||||||||
|
| ||||||||||||
| 2 h | nd | 0.8 | 101.1 | 3999.0 | 12.2 | 278.5 | ||||||
| 1 day | nd | nd | 121.9 | 60.9 | 9.6 | 1.1 | ||||||
| 3 day | nd | nd | 45.0 | 0.2 | 4.0 | 0.2 | ||||||
| 7 day | nd | nd | 19.1 | 0.2 | 1.4 | 0.0 | ||||||
| Liver immune cells | Liver | Liver | ||||||||||
|
| ||||||||||||
| 2 h | nd | 7.8 | 3.7 | 314.2 | 3.4 | 6136.5 | 2298.9 | 58.6 | 5.2 | 455.9 | 13.9 | |
| 1 day | nd | nd | 135.7 | 9.4 | 1.3 | 0.6 | 15.4 | 1.7 | 3.7 | 0.5 | ||
| 3 day | nd | nd | 59.7 | 4.5 | 0.3 | 0.0 | 7.9 | 0.4 | 0.5 | 0.1 | ||
| 7 day | nd | nd | 38.3 | 1.1 | 0.2 | 0.1 | 6.7 | 0.5 | 0.1 | 0.0 | ||
TP levels are in fmol/million cells;
Parent levels in ng/ml in blood, and ng/g of tissue in spleen, liver, and lymph nodes;
NP: nanoformulation; nd: not detected.
In lymphocytes prepared from lymph nodes, 3TC-TP levels were detected at least up to day 7 after nanoformulation administration, while they were only detected up to day 1 after native drug administration. In contrast CBV-TP was detected in lymph node lymphocytes only at 2 h after native dose administration. Also in liver immune cells, 3TC-TP and CBV-TP levels were detected only at two hours after native drug administration.
Parent drug concentrations were detected in PBMCs for the entire period of the study at levels 20–60,000 times higher than their TP metabolites (Table 3). The parent nucleosides followed a similar pattern to their TP metabolites by demonstrating 5–350 fold higher concentrations in blood and tissues in mice, after nanoformulated vs. native solution administration.
In summary, higher and sustained concentrations of 3TC and ABC and their TP metabolites were detected in blood and tissues in mice, after nanoformulated vs. native drug administration.
4. DISCUSSION
Nucleotide metabolites are quantified by direct or indirect approaches. In the direct LC-MS methods, chromatographic retention of the nucleotides is accomplished by ion-exchange, ion-pairing, hydrophilic interaction (HILIC), or porous graphitic carbon (PGC) chromatography [8]. In the indirect LC-MS methods, parent nucleosides are reverse generated from their nucleotide metabolites during sample preparation using enzymatic dephosphorylation. Generally, direct methods put the pressure on the LC-MS end by using relatively incompatible and irreproducible LC conditions, whereas, indirect methods put the pressure on the sample preparation end by using laborious sample purification and extraction processes. In contrast, direct methods have the advantage of simple sample preparation, while indirect methods are more rugged and sensitive because they quantify the parent nucleosides rather than the nucleotide metabolites [26].
Here we developed an indirect LC-MS/MS method with improved sensitivity at LLOQ of 10 pg/ml and 4 pg/ml for the quantification of 3TC-TP and CBV-TP, respectively, in mouse tissues and fluids. This method was developed to support the development of long-acting nanoformulations of anti-HIV therapies including NRTIs. These efforts start at the preclinical stages using mouse as the main animal model. Working with the mouse model imparts more challenges to monitoring TP intracellular metabolites in fluids and tissues in addition to the high sensitivity requirements, mentioned earlier. These challenges include: (i) simultaneous measurement of more than one analyte, i.e. 3TC-TP and CBV-TP; (ii) limited sample size of both blood and tissues; and (iii) the very low yield of isolation of the immune cells of interest from these small-size samples.
Several LC-MS/MS methods are available for monitoring NRTI-TP metabolites including 3TC and ABC [11–20], which are summarized in Table 4. These methods were primarily developed for monitoring TPs in human samples particularly in plasma. Typically, millions of PBMCs are obtained from such large-volume (>5ml) samples. Therefore, we sought to improve upon these methods to satisfy the limited sample size and the even lower-sensitivity requirements associated with TP analysis in mouse tissues and fluids. In our method, LLOQs were 10 pg/ml for 3TC-TP and 4 pg/ml for CBV-TP, which corresponds to 2.1 fmol/106 cells for 3TC-TP, and 0.8 fmol/106 cells for CBV-TP. This is 12.5- to 50-fold more sensitive than the previous direct and indirect LC-MS/MS methods for 3TC- and CBV-TP (Table 4).
Table 4.
Summary of previously reported 3TC- and CBV-TP LC-MS/MS methods
| Compound | LLOQ (pg/ml) |
LLOQ (fmol/ 106cells) |
Number of cells$ |
Sample size (blood ml) |
Validation | References | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 3TC-TP | 2500 | 100 fmol/million | 10 × 106 | 10 | Yes (partial for TP) | [12] |
| CBV-TP | 50 | 2 fmol/million | |||||
|
| |||||||
| 2 | 3TC-TP | * | 400 fmol / million | 10 × 106 | * | Partial | [16] |
|
| |||||||
| 3 | 3TC-TP | 10,000 | * | * | 6–8 | no | [17] |
|
| |||||||
| 4 | CBV-TP | 50 | * | * | 16 | no | [13] |
|
| |||||||
| 5 | 3TC-TP | 1000 | 59 fmol/million | 36 × 106 | 18 | Partial | [20] |
|
| |||||||
| 6 | 3TC-TP | 470 | 100 fmol/million | * | 8 | yes | [11] |
|
| |||||||
| 7 | 3TC-TP | 470–4700 | 10–40 fmol/million | 5–25 × 106 | In-vitro | no | [18] |
| CBV-TP | |||||||
| 470–4700 | |||||||
|
| |||||||
| 8 | 3TC-TP | * | 24 fmol/on column | 1–10 × 106/ in injection | 16 | no | [15] |
| CBV-TP | |||||||
| 76 fmol/on column | |||||||
|
| |||||||
| 9 | CBV-TP | 10,000 | * | 50 × 106/ ml | * | Partial | [19] |
|
| |||||||
| 10 | 3TC-TP | 870 | * | 5.7 × 106 | * | no | [14] |
|
| |||||||
| This Assay | 3TC-TP | 10 | 2.1 fmol/million | 0.3– 0.6 × 106 | 0.5–0.8 | Yes | |
| CBV-TP | 4 | 0.82 fmol/million | |||||
Data not provided;
number of cells used for LC-MS/MS injections in study samples.
Previously published methods have used large blood volumes (6–18 ml) and isolated large number of PBMCs (2–20 million) [11–13, 15, 17, 20]. However, we were able to quantify both 3TC-TP and CBV-TP levels using much smaller blood volumes (~0.5 ml) and lower amounts of tissues (12 mg lymph node and 50 mg spleen tissues). This smaller-sized blood samples only produced <0.3 × 106 cells, however, with the sensitivity of this assay we were able to further lower the sample size and number of PBMCs.
The first step of TP metabolites isolation by anion-exchange SPE is the most time consuming step in indirect quantification methods of nucleotides. In this method, we optimized a single-step wash for isolation of TP metabolites rather than the previously used multiple-step gradient washing for removal of mono-and di-phosphates [11–13, 25], which increased the throughput and reproducibility of sample preparation.
Because of the high cost of TP standards, special storage requirements of TP standards, and the time- and labor-intensive ion-exchange SPE, fraction collection, and dephosphorylation steps, we tried to bypass these steps by building a calibration curve using parent nucleosides spiked to cell lysates before the second SPE step, as suggested previously [11, 12]. However, when these “short-cut” curves were validated with QC samples made with TP standards that underwent the entire sample preparation process, they showed low accuracy (data not shown). This was due to the fact that most of the analytes losses during sample preparation (60–90% recovery) were associated with the first step (Table 2). For this approach to work properly, TPs recovery from the bypassed steps should be ~100%. Therefore, calibration curves were prepared using TP metabolites that underwent the same entire sample preparation process to that of the study samples.
For the same reasons, storage stability studies were of concern due to the large number of samples analyzed in this type of long-term studies. Therefore, we developed a direct method for the quantification of TPs in stability samples, which did not require the sample extraction, fractionation, and dephosphorylation associated with the indirect sample preparation. This direct method involved one-step MeOH-protein precipitation and direct LC-MS/MS analysis of the TPs with ion-exchange chromatography. However, the down-side of this method is its low sensitivity compared to the indirect method (>100-fold), which did not allow its use to analyze biological samples, but it provided the required sensitivity for the storage stability studies.
Other advantages for our approach include the optimization of storage conditions to ensure stability of the TP standards under all working and short- and long-term storage conditions (Fig. 4). This was critical because TPs are known to be unstable due to the hydrolysis of their phosphate bond(s). Importantly, we also optimized the conditions of biological tissue and fluid collection and cell isolation to ensure TP stability in in-vivo samples from the time they are collected until the time cells are lysed, extracted, and stabilized (Fig. 5). The time it takes to collect in-vivo samples and process them for cell isolation can vary markedly depending on study design and operators. Therefore, characterizing TP stability during that time and defining the time limits for TPs stability is critical.
Finally, this method was used in a preliminary in-vivo mouse study to compare the effect of nanoformulations of 3TC and ABC on the PK properties of these compounds. We have previously characterized the PK behavior of these nanoformulated nucleosides in mice [21, 22]. In this study, we extended these PK studies to include intracellular TP metabolites in blood and tissues. Similarly to the parent nucleosides, these nanoformulations resulted in higher and sustained concentrations of 3TC and ABC TP metabolites in mouse blood and tissues (Table 3).
In conclusion, we developed a sensitive, selective, accurate, and precise LC-MS/MS method for the simultaneous quantification of intracellular 3TC-TP and CBV-TP in mouse blood and tissues. Conditions were optimized to ensure stability of TP samples and standards under sample collection, preparation, analysis, and storage. The developed method was successfully applied to characterize the PK profile of TP metabolites in mouse blood and tissues.
Highlights.
Simultaneous quantification of intracellular 3TC- and ABC-TP metabolites in mouse blood and tissues.
Conditions were optimized for stability of TP samples during analysis, and storage.
This method has enhanced sensitivity compared to previous LC-MS/MS methods.
This method requires a very small amount of sample compared to previous method.
Method was applied to characterize PK profile of TP metabolites in mouse.
Acknowledgments
This work was supported by the National Institutes of Health [P01 DA028555].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balzarini J, Aquaro S, Hassan-Abdallah A, Daluge SM, Perno CF, McGuigan C. Improved antiviral activity of the aryloxymethoxyalaninyl phosphoramidate (APA) prodrug of abacavir (ABC) is due to the formation of markedly increased carbovir 5'-triphosphate metabolite levels. FEBS Lett. 2004;573(1–3):38–44. doi: 10.1016/j.febslet.2004.07.049. [DOI] [PubMed] [Google Scholar]
- 2.Coulier L, Gerritsen H, van Kampen JJ, Reedijk ML, Luider TM, Osterhaus AD, Gruters RA, Brull L. Comprehensive analysis of the intracellular metabolism of antiretroviral nucleosides and nucleotides using liquid chromatography-tandem mass spectrometry and method improvement by using ultra performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(26):2772–82. doi: 10.1016/j.jchromb.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 3.Van Rompay AR, Johansson M, Karlsson A. Phosphorylation of nucleosides and nucleoside analogs by mammalian nucleoside monophosphate kinases. Pharmacol Ther. 2000;87(2–3):189–98. doi: 10.1016/s0163-7258(00)00048-6. [DOI] [PubMed] [Google Scholar]
- 4.Jordheim LP, Durantel D, Zoulim F, Dumontet C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat Rev Drug Discov. 2013;12(6):447–64. doi: 10.1038/nrd4010. [DOI] [PubMed] [Google Scholar]
- 5.Rathbun RC, Lockhart SM, Stephens JR. Current HIV treatment guidelines--an overview. Curr Pharm Des. 2006;12(9):1045–63. doi: 10.2174/138161206776055840. [DOI] [PubMed] [Google Scholar]
- 6.Richardson ET, Grant PM, Zolopa AR. Evolution of HIV treatment guidelines in high- and low-income countries: converging recommendations. Antiviral Res. 2014;103:88–93. doi: 10.1016/j.antiviral.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sivasubramanian G, Frempong-Manso E, Macarthur RD. Abacavir/lamivudine combination in the treatment of HIV: a review. Ther Clin Risk Manag. 2010;6:83–94. doi: 10.2147/tcrm.s1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen RS, Rosing H, Schellens JH, Beijnen JH. Mass spectrometry in the quantitative analysis of therapeutic intracellular nucleotide analogs. Mass Spectrom Rev. 2011;30(2):321–43. doi: 10.1002/mas.20280. [DOI] [PubMed] [Google Scholar]
- 9.Thomas D, Herold N, Keppler OT, Geisslinger G, Ferreiros N. Quantitation of endogenous nucleoside triphosphates and nucleosides in human cells by liquid chromatography tandem mass spectrometry. Anal Bioanal Chem. 2015;407(13):3693–704. doi: 10.1007/s00216-015-8588-3. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Zhang Y, Wiegand R, Wang J, Bepler G, Li J. Quantitative analysis of intracellular nucleoside triphosphates and other polar metabolites using ion pair reversedphase liquid chromatography coupled with tandem mass spectrometry. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2015;1006:167–78. doi: 10.1016/j.jchromb.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bushman LR, Kiser JJ, Rower JE, Klein B, Zheng JH, Ray ML, Anderson PL. Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J Pharm Biomed Anal. 2011;56(2):390–401. doi: 10.1016/j.jpba.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins BL, Poston PA, Neal EF, Slaughter C, Rodman JH. Simultaneous measurement of intracellular triphosphate metabolites of zidovudine, lamivudine and abacavir (carbovir) in human peripheral blood mononuclear cells by combined anion exchange solid phase extraction and LC-MS/MS. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2007;850(1–2):310–7. doi: 10.1016/j.jchromb.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Moyle G, Boffito M, Fletcher C, Higgs C, Hay PE, Song IH, Lou Y, Yuen GJ, Min SS, Guerini EM. Steady-state pharmacokinetics of abacavir in plasma and intracellular carbovir triphosphate following administration of abacavir at 600 milligrams once daily and 300 milligrams twice daily in human immunodeficiency virus-infected subjects. Antimicrobial agents and chemotherapy. 2009;53(4):1532–8. doi: 10.1128/AAC.01000-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuklenyik Z, Martin A, Pau CP, Holder A, Youngpairoj AS, Zheng Q, Cong ME, Garcia-Lerma JG, Heneine W, Pirkle JL, Barr JR. On-line coupling of anion exchange and ion-pair chromatography for measurement of intracellular triphosphate metabolites of reverse transcriptase inhibitors. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(29):3659–66. doi: 10.1016/j.jchromb.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins T, Veikley W, St Claire RL, 3rd, Guyer B, Clark N, Kearney BP. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J Acquir Immune Defic Syndr. 2005;39(4):406–11. doi: 10.1097/01.qai.0000167155.44980.e8. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez JF, Rodriguez JL, Santana J, Garcia H, Rosario O. Simultaneous quantitation of intracellular zidovudine and lamivudine triphosphates in human immunodeficiency virus-infected individuals. Antimicrob Agents Chemother. 2000;44(11):3097–100. doi: 10.1128/aac.44.11.3097-3100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuen GJ, Lou Y, Bumgarner NF, Bishop JP, Smith GA, Otto VR, Hoelscher DD. Equivalent steady-state pharmacokinetics of lamivudine in plasma and lamivudine triphosphate within cells following administration of lamivudine at 300 milligrams once daily and 150 milligrams twice daily. Antimicrob Agents Chemother. 2004;48(1):176–82. doi: 10.1128/AAC.48.1.176-182.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray AS, Myrick F, Vela JE, Olson LY, Eisenberg EJ, Borroto-Esodo K, Miller MD, Fridland A. Lack of a metabolic and antiviral drug interaction between tenofovir, abacavir and lamivudine. Antivir Ther. 2005;10(3):451–7. [PubMed] [Google Scholar]
- 19.Fung EN, Cai Z, Burnette TC, Sinhababu AK. Simultaneous determination of Ziagen and its phosphorylated metabolites by ion-pairing high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Biomed Sci Appl. 2001;754(2):285–95. doi: 10.1016/s0378-4347(00)00619-8. [DOI] [PubMed] [Google Scholar]
- 20.Holdich T, Shiveley LA, Sawyer J. Effect of Lamivudine on the plasma and intracellular pharmacokinetics of apricitabine, a novel nucleoside reverse transcriptase inhibitor, in healthy volunteers. Antimicrob Agents Chemother. 2007;51(8):2943–7. doi: 10.1128/AAC.01013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh D, McMillan J, Hilaire J, Gautam N, Palandri D, Alnouti Y, Gendelman HE, Edagwa B. Development and characterization of a long-acting nanoformulated abacavir prodrug. Nanomedicine (Lond) 2016;11(15):1913–27. doi: 10.2217/nnm-2016-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo D, Zhou T, Arainga M, Palandri D, Gautam N, Bronich T, Alnouti Y, McMillan J, Edagwa B, Gendelman HE. Creation of a Long-Acting Nanoformulated 2',3'-Dideoxy-3'-Thiacytidine. J Acquir Immune Defic Syndr. 2017;74(3):e75–e83. doi: 10.1097/QAI.0000000000001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blom KG, Qazi MR, Matos JB, Nelson BD, DePierre JW, Abedi-Valugerdi M. Isolation of murine intrahepatic immune cells employing a modified procedure for mechanical disruption and functional characterization of the B, T and natural killer T cells obtained. Clin Exp Immunol. 2009;155(2):320–9. doi: 10.1111/j.1365-2249.2008.03815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohar I, Brempelis KJ, Murray SA, Ebrahimkhani MR, Crispe IN. Isolation of Non-parenchymal Cells from the Mouse Liver. Methods Mol Biol. 2015;1325:3–17. doi: 10.1007/978-1-4939-2815-6_1. [DOI] [PubMed] [Google Scholar]
- 25.Robbins BL, Waibel BH, Fridland A. Quantitation of intracellular zidovudine phosphates by use of combined cartridge-radioimmunoassay methodology. Antimicrobial agents and chemotherapy. 1996;40(11):2651–4. doi: 10.1128/aac.40.11.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King T, Bushman L, Anderson PL, Delahunty T, Ray M, Fletcher CV. Quantitation of zidovudine triphosphate concentrations from human peripheral blood mononuclear cells by anion exchange solid phase extraction and liquid chromatographytandem mass spectroscopy; an indirect quantitation methodology. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;831(1–2):248–57. doi: 10.1016/j.jchromb.2005.12.033. [DOI] [PubMed] [Google Scholar]