Abstract
OBJECTIVE
Cystic fibrosis–related diabetes (CFRD) is a common complication of cystic fibrosis (CF), increasing patient morbidity and mortality. Poor understanding of CFRD pathogenesis limits the development of targeted therapies to treat and/or prevent the disease. The aim of this study was to evaluate islet pathology, specifically, inflammation, amyloid deposition, and endocrine cell composition in subjects with CF with diabetes and with CF without diabetes.
RESEARCH DESIGN AND METHODS
A retrospective analysis of archived pancreas tissue collected at autopsy was conducted using pancreas tissue from subjects with CF and diabetes (CFRD) (n = 18) and CF without diabetes (CF-no DM) (n = 17). Two cohorts of control non-CF subjects were identified, each matched to CFRD and CF-no DM subjects for age, sex, and BMI (non-CF older, n = 20, and non-CF younger, n = 20), respectively. Immunohistochemistry was performed to assess interleukin-1β (IL-1β) and islet hormone (insulin, glucagon, somatostatin, and pancreatic polypeptide) immunoreactivity; histochemistry was performed to quantify amyloid deposition.
RESULTS
Islet IL-1β immunoreactivity was substantially increased in both CFRD and CF-no DM subjects compared with non-CF subjects and was common in young subjects with CF (≤10 years of age). In contrast, islet amyloid deposition was increased only in CFRD subjects. We also observe abnormal islet hormone immunoreactivity, characterized by increased glucagon immunoreactivity, in CF-no DM and CFRD subjects compared with non-CF subjects.
CONCLUSIONS
These findings reveal novel molecular pathways and therapeutic targets underlying islet pathology in CF subjects and may be important in developing new approaches to treat CFRD.
Introduction
Cystic fibrosis–related diabetes (CFRD) occurs in up to 50% of adults with cystic fibrosis (CF), with the highest incidence occurring during the second to third decade of life (1), although abnormal glucose tolerance is common even in very young children with the disease (2–4). CFRD is associated with increased morbidity and mortality in both males and females (5). Insulin therapy is the current standard of care for CFRD. Unlike type 1 diabetes, CFRD does not appear to be the result of islet autoimmunity; HLA susceptibility alleles are not observed with greater frequency, although studies differ with respect to the prevalence of islet autoantibodies (6,7). Physiologically, CFRD appears to have more in common with type 2 diabetes. Genetic susceptibility loci for type 2 diabetes appear to confer risk for CFRD (8–10), and, similar to type 2 diabetes, CFRD is characterized by a mixture of peripheral, particularly hepatic, insulin resistance and significant defects in β-cell function (4,11–13). However, the cause of β-cell dysfunction in CFRD is not known.
Work in various animal models, including the recently developed pig and ferret models, demonstrates abnormalities in glucose metabolism and insulin release, mostly in the newborn period (14–16). Human autopsy studies have been informative in describing islet cell histological changes with clinical manifestations. Some small studies have reported that subjects with CFRD have a lower density of endocrine (islet) tissue compared with patients with CF without diabetes (17) and/or loss of β-cells with no change in glucagon-producing α-cells or somatostatin-producing δ-cells compared with CF subjects without diabetes (18,19). However, one larger study found no difference in β-cell area among subjects with CF with or without diabetes (20).
As in type 2 diabetes, pancreatic islet amyloid deposition has been identified as a characteristic feature of CFRD in humans (19,20). Islet amyloid is a well-described pathological feature of type 2 diabetes, and consistent with the etiology of this form of diabetes, islet amyloid deposition is usually observed in older individuals (21–23). Islet amyloid deposition is toxic to β-cells, correlating with both reduced β-cell area and increased β-cell apoptosis in patients with type 2 diabetes (21–23). Recent data have linked islet amyloid deposition to islet inflammation, namely, showing that islet amyloid induces inflammatory cytokine production, chiefly interleukin-1β (IL-1β), by macrophages and dendritic cells (24,25). In a similar fashion, the systemic inflammatory process observed in CF subjects also seems to be skewed toward IL-1β–regulated cytokines such as IL-8 and IL-17 (26–28).
Given these previous observations of islet amyloidosis in CFRD (19,20), the inflammatory properties of islet amyloid (24,25), and the systemic inflammatory milieu of CF (26–28), we hypothesized that CFRD would be characterized not only by islet amyloid but also by islet expression of the inflammatory cytokine IL-1β. We conducted a histologic study to test this hypothesis. We observed greater IL-1β immunoreactivity in islets of subjects with CF with or without diabetes compared with control subjects without CF, while islet amyloid deposition was only increased in those individuals diagnosed with CFRD. Islet endocrine composition was also disturbed, with increased α-cell area observed in subjects with CF with and without diabetes. Based on these observations, we propose that CF is characterized by islet inflammation, which could predispose to β-cell failure, and by islet α-cell expansion, while islet amyloid formation is mainly restricted to CFRD.
Research Design and Methods
Subjects
Forty-one patients with CF were identified by retrospective screening of autopsy records at the Seattle Children’s Hospital (SCH) and the University of Washington Medical Center for a CF diagnosis. We screened records from SCH between 1977 and 2012 and at University of Washington Medical Center between 1991 and 2013. Available clinical data (Table 1), including history of lung transplantation, were extracted from the medical record and managed using REDCap electronic data capture tools hosted at the University of Washington’s Institute of Translational Health Sciences. Three additional cases of CF were available from the JDRF-supported Network for Pancreatic Organ Donors with Diabetes (nPOD). Nine were excluded from further analysis based on the following criteria: incomplete medical records (n = 1), lack of availability or poor quality of pancreas tissue (n = 5), absence of pancreatic islets on the available pancreas sections (n = 2), or history of islet antigen 2 antibodies and zinc transporter 8 autoantibodies, suggestive of the presence of type 1 diabetes (n = 1). Of the remaining 35 subjects with CF, 18 were classified as “CFRD” based on presence of diabetes diagnosis by the treating physician and/or insulin use in the medical record; the other 17 were designated “CF-no DM.” Given the difference in age and BMI between the CFRD and CF-no DM cohorts, two groups of non-CF control subjects without diabetes (including 3 from the JDRF nPOD registry) were identified. These, designated “non-CF (younger)” (n = 20) and “non-CF (older)” (n = 21), were matched for age, sex, and BMI to CF-no DM and CFRD subjects, respectively, and to the extent possible, given that autopsies were performed over many years, these were also matched to the time of autopsy. One non-CF control subject [from the non-CF cohort (older)] was excluded from further analysis, as no pancreas specimen was available, bringing the sample size from the non-CF (older) group to 20. The study was approved by institutional review boards at the University of Washington and SCH.
Table 1.
Subject characteristics, transplant history, diabetes medications, and causes of death
| Non-CF (younger) | Non-CF (older) | CF-no DM | CFRD | |
|---|---|---|---|---|
| N | 20 | 20 | 17 | 18 |
| Age (years) | 13.1 ± 6.8 | 28.0 ± 5.5*# | 13.9 ± 8.3 | 28.6 ± 9.0*# |
| Sex (female/male) | 12/8 | 9/11 | 12/5 | 10/8 |
| BMI (kg/m2) | 19.8 ± 7.0 | 22.6 ± 5.5# | 16.5 ± 3.9 | 21.7 ± 3.3# |
| Diabetes duration (years) (n = 17) | 4.80 ± 5.16 | |||
| HbA1c (%) (n = 11) | 7.25 ± 1.67 | |||
| Lung transplant (yes/no/unknown) | 0/19/0 | 0/20/0 | 4/13/0 | 10/6/2 |
| Other transplant | 1 (heart) | 0 | 0 | 0 |
| Diabetes medications | ||||
| Oral agent | 1 | |||
| Insulin | 16 | |||
| Unknown | 2 | |||
| Steroid use (yes/no/unknown) | 4/13/3 | 0/15/5 | 4/13/0 | 11/5/2 |
| Pancreatic enzyme supplementation (yes/no/unknown) | 16/0/1 | 16/0/2 | ||
| Cause of death | ||||
| Respiratory | 5 | 5 | 14 | 14 |
| Cardiovascular | 4 | 4 | 2 | — |
| CNS | 3 | 6 | — | — |
| Infection | 4 | 3 | — | — |
| Malignancy | 2 | — | — | — |
| Multiorgan failure | 1 | 2 | — | 3 |
| Unknown | 1 | — | — | 1 |
Data are means ± SD or n. CNS, central nervous system.
*P < 0.05 vs. non-CF (younger).
#P < 0.05 vs. CF-no DM.
Pancreatic tissue was obtained during autopsies performed at the University of Washington and SCH or via the nPOD program. Histologic sampling from the body of the pancreas was routinely performed, although autopsy records did not always indicate the specific location of the sample. Specimens were included in the study only if they showed no or minimal autolysis, as assessed by CLF and RLH (University of Washington samples) or GHD and RLH (SCH samples). Pancreatic weight was not available; therefore, endocrine cell data are presented as relative area rather than mass.
Immunohistochemistry
Four-micron-thick sections of formalin-fixed, paraffin-embedded pancreas were subjected to immunohistochemistry (IHC) (LeicaBond Max; Leica Microsystems, Buffalo Grove, IL) as follows. The following primary antisera were used, for each of which the host species is denoted in square brackets: insulin (A0564 [guinea pig], 1:4,000; Dako, Carpenteria, CA), IL-1β (49-4960 [rabbit], 1:500; ProSci Incorporated, Poway, CA; and ab2105 [rabbit], 1:1,000; Abcam, Cambridge, MA), IL-1Ra (AF-280-NA [goat], 1:500; R&D Systems, Minneapolis, MN; and NBP1-32568 [rabbit], 2 µg/mL; Novus Biologicals, Littleton, CO), CD68 (Clone 514H12 [mouse], 0.34 µg/mL; Leica Biosystems/Novacastra), glucagon (EP3070 [rabbit], 1:10,000; Epitomics, Burlingame, CA), somatostatin (SC-7819 [goat], 1:200; Santa Cruz Biotechnology, Santa Cruz, CA), and pancreatic polypeptide (PP) (NB100-1793 [goat], 1:250; Novus). Isotype-matched irrelevant IgG-negative controls were used for all antisera (except those against islet hormones, which have been previously validated in pancreas).
Sections were deparaffinized followed by antigen retrieval (citrate buffer at 100°C for 20 min for IL-1β [both antisera]; EDTA at 100°C for 10–20 min for IL-1Ra [both antisera], CD68, and PP; and proteinase K for 15 min at 37°C for somatostatin). No antigen retrieval was used for insulin or glucagon IHC. Sections then underwent peroxide block followed by incubation in 10% (v/v) normal goat serum for 20 min at room temperature. Sections were then incubated in primary antisera. For primary antisera raised in mouse, goat, or guinea pig, linking IgGs (Leica Post Primary, rabbit anti-goat IgG, or rabbit anti-guinea pig IgG, respectively) were then applied (since the Leica polymer reagent recognizes rabbit IgG). Antibody binding was detected by poly–horseradish peroxidase polymerized secondary detection and Leica Bond Mixed Refine 3,3′-diaminobenzidene detection reagents (both DS9800; Leica Biosystems) followed by hematoxylin-eosin counterstaining and coverslipping.
Whole pancreas sections were then digitized (Nanozoomer Digital Pathology system; Hamamatsu Corporation, Bridgewater, NJ). Islets (an average of 53 per section) were classified as IL-1β positive or negative by manual counting. This was done by three independent observers, blinded to subject grouping (R.L.H., S.S., and Megan Larmore, a staff member from the University of Washington Cystic Fibrosis Research and Translation Center’s Host Response Core). For insulin, glucagon, somatostatin, and PP IHC, total pancreas tissue and hormone-positive areas were determined automatically based on pixel value and density (Visiopharm software, Hoersholm, Denmark) and verified by manual examination of segmented images, as we have done previously (29). Endocrine cell areas were expressed as percentage of total pancreatic tissue area. Individuals performing IHC and quantitative analysis thereof were blinded to the group assignment of specimens.
Additionally, sections underwent immunofluorescence labeling for insulin (I2018, clone K36AC10 [mouse] at 1:2,000; Sigma-Aldrich, St. Louis, MO) followed by Cy3-conjugated goat anti-mouse IgGs (diluted 1:200; Jackson ImmunoResearch, West Grove, PA) to aid the visualization of islets and counterstaining with thioflavin S (0.5% v/v) to visualize amyloid deposition. Islets were classified as thioflavin S positive or negative by manual counting of at least 50 islets per section, from which the proportion of amyloid-positive islets was determined for all subjects. Again, individuals performing (immuno)histochemistry and quantitative analysis thereof were blinded to the group assignment of specimens.
Data and Statistical Analysis
Comparisons between groups were done using one-way ANOVA for overall statistical differences; pairwise comparisons were conducted with a Tukey test to correct P values for multiple comparisons. A Fisher exact test was used to compare frequency of lung transplantation between groups, and a χ2 test was used to compare frequency of islet IL-1β and amyloid positivity among groups. In comparison of differences between lung transplant groups, two-way ANOVA was used to test for interactions between transplant and diabetes status on IL-1β and amyloid deposition. Linear regression was used to determine correlations between insulin and glucagon areas.
Results
Clinical Data
Subjects in the CFRD group were on average older than in the CF-no DM group (Table 1); however, by design the non-CF (older) and non-CF (younger) subject groups were matched for age and BMI to the CFRD and CF-no DM groups, respectively. Pancreatic insufficiency was documented in all but three CF case subjects (for whom data were not available), regardless of diabetes status. Subjects with CFRD had diabetes for ∼5 years, and all but two were insulin-treated. A history of lung transplantation was more common in the CFRD compared with CF-no DM group, and steroid use closely correlated with this parameter; only one non-CF subject had received organ (heart) transplantation.
Islet IL-1β Immunoreactivity Is a Common Feature of CF
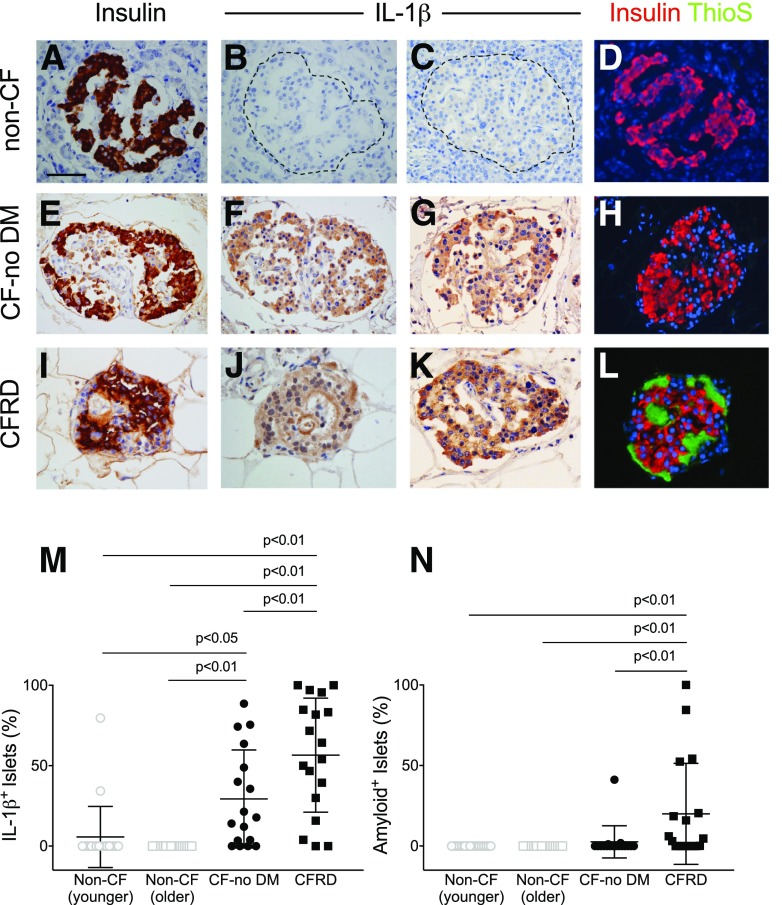
We first determined whether there was an increased IL-1β immunoreactivity in the islets of subjects with CF (ProSci antibody) (Fig. 1). Eighty-nine percent of CFRD subjects exhibited some degree of islet IL-1β positivity, with 57% of the islets on average from each subject being involved (Fig. 1M). Further, islet IL-1β was also observed in 76% of CF-no DM case subjects (P = 0.02 vs. CFRD), and this IL-1β positivity was observed in 29% of islets in this group. In contrast, only 10% of non-CF (younger) subjects exhibited any IL-1β–positive islets (P < 0.001 vs. CF-no DM), comprising only 6% of islets per subject. No IL-1β immunoreactivity was observed in the islets of non-CF (older) subjects (Fig. 1M). Validation of islet IL-1β immunoreactivity was performed using a second anti–IL-1β antibody (Abcam) in a subset of subjects (n = 10 [representative examples are shown in Supplementary Fig. 1]). These subjects were from both CF and non-CF groups and included subjects in whom IL-1β had been previously found to be positive or negative. The presence or absence of IL-1β was confirmed in 9 out of 10 subjects. The last subject was a non-CF (younger) subject who had been classified as IL-1β positive with the ProSci antibody but showed no as IL-1β immunoreactivity with the Abcam antibody. We believe the discrepancy in this finding is due to lower sensitivity of the latter antibody.
Figure 1.
IHC for insulin, IL-1β, and amyloid in subjects with and without CF. Representative staining of pancreas specimens from subjects in non-CF control (A–D), CF-no DM (E–H), and CFRD (I–L) groups, identifying islet β-cells using IHC for insulin (brown in A, E, and I and red in D, H, and L), IL-1β (brown; images shown from two subjects per group in B and C, F and G, and J and K), and amyloid (visualized by thioflavin S [ThioS] histochemistry; green in D, H, and L). Quantitation of the proportion of islets positive for IL-1β (M) and amyloid (N). Islet IL-1β positivity was increased in CF-no DM and CFRD subjects compared with age-matched non-CF control subjects, whereas islet amyloid deposition was only increased in CFRD subjects. Scale bar = 50 µm.
Islet IL-1β Immunoreactivity Is Seen Even in Very Young Subjects With CF
We next analyzed islet IL-1β immunoreactivity (using the ProSci antibody) in the subset of pediatric CF subjects <10 years of age, the currently recommended age to begin screening CF patients for CFRD with oral glucose tolerance tests (5). All six subjects with CF in this age range were in the CF-no DM group. Five of these showed evidence of IL-1β immunoreactivity affecting 24% of islets per subject and thus were reflective of the CF-no DM group as a whole.
Islet Amyloid Occurs Predominantly in CFRD Subjects
Islet amyloid was present in 61% of CFRD subjects, involving 20% of islets (Fig. 1N), while islet amyloid was present in 18% of CF-no DM subjects (P < 0.001 vs. CFRD), with only 3% of islets affected. Islet amyloid was not observed in either group of non-CF control subjects (Fig. 1N). Of note, none of the six young CF-no DM subjects <10 years of age described above exhibited amyloid deposition.
Effect of Prior Lung Transplantation on Islet IL-1β and Amyloid Deposition
Given the use of lung transplantation in advanced CF disease management, CF-no DM and CFRD subjects were subdivided based on lung transplant status and the presence of IL-1β and amyloid was analyzed. There was no statistically significant interaction between transplant status and diabetes status in relation to islet IL-1β immunoreactivity or amyloid deposition (Table 2). Of note, a history of steroid use closely mirrored that of lung transplantation. Accordingly, subdivision of subjects based on steroid usage did not reveal differences in islet IL-1β immunoreactivity or amyloid deposition (data not shown).
Table 2.
Subject characteristics and islet morphometric analyses in individuals with CF, subdivided according to diabetes and lung transplant status
| CF-no DM |
CFRD |
||||
|---|---|---|---|---|---|
| No lung transplant | Lung transplant | No lung transplant | Lung transplant | ||
| N | 13 | 4 | 6 | 10 | |
| Age (years) | 11.9 ± 2.4 | 20.3 ± 1.0 | 22.8 ± 3.4 | 31.4 ± 2.8 | |
| Sex (female/male) | 11/2 | 1/3 | 3/3 | 6/4 | |
| BMI (kg/m2) | 15.2 ± 0.6 | 20.3 ± 2.8 | 21.9 ± 1.3 | 22.0 ± 1.2 | |
| Diabetes duration (years) | — | — | 6.6 ± 3.1 | 4.2 ± 1.0 | |
| HbA1c (%) (n = 4–6) | — | — | 7.8 ± 0.9 | 6.5 ± 0.4 | |
| Subjects with islet IL-1β, n (%) | 11 (91) | 2 (50) | 6 (100) | 8 (80) | |
| IL-1β–positive islets (%) | 28.8 ± 8.5 | 31.1 ± 18.8 | 63.5 ± 9.5 | 61.8 ± 12.3 | |
| Subjects with islet amyloid, n (%) | 2 (11) | 1 (25) | 3 (50) | 7 (70) | |
| Amyloid-positive islets (%) | 3.3 ± 3.2 | 0.3 ± 0.3 | 5.2 ± 3.2 | 31.0 ± 12.2 | |
| Insulin-positive area (%) | 1.5 ± 0.3 | 2.8 ± 0.9 | 1.1 ± 0.4 | 1.3 ± 0.2 | |
| Glucagon-positive area (%) | 1.2 ± 0.3 | 3.5 ± 0.6 | 1.5 ± 0.5 | 2.5 ± 0.7 | |
| Somatostatin-positive area (%) (n = 8, 3, 4, and 5, respectively) | 0.5 ± 0.1 | 0.6 ± 0.2 | 0.2 ± 0.1 | 0.7 ± 0.3 | |
| PP-positive area (%) (n = 8, 3, 4, and 5, respectively) | 0.6 ± 0.1 | 0.1 ± 0.1* | 0.2 ± 0.1 | 0.2 ± 0.1 | |
Data are mean ± SEM or n unless otherwise indicated.
*P < 0.05 vs. CF-no DM, no transplant group.
CF Is Not Characterized by Islet IL-1Ra or CD68 Immunoreactivity
We next sought to determine whether differences in the balance of IL-1β and its antagonist, IL-1Ra, exist between subjects with CF with and without diabetes. Based on data from older subjects with and without type 2 diabetes (30), the expectation is that high IL-1Ra levels would be present in CF-no DM subjects, with lower levels being seen in CFRD subjects. Contrary to this expectation, however, little to no IL-1Ra immunoreactivity was observed in islets from any group despite the use of two well-validated antibodies. With the first antiserum (AF-280-NA), IL-1Ra staining was indistinguishable from that seen with an isotype-matched irrelevant IgG (data not shown). With the second (NBP1-32568), only sparse islet staining was detected, with no difference in IL-1Ra immunoreactivity being observed between subjects with and subjects without diabetes (Supplementary Fig. 2).
Pancreas sections were also stained for CD68 to investigate whether macrophage infiltration could explain the presence of islet IL-1β. While CD68-positive cells were clearly present in exocrine pancreas of subjects with CF-no DM and CFRD, no infiltration of macrophages was observed within islets, regardless of IL-1β positivity in all cases but one (Supplementary Fig. 3), suggesting that the observed islet IL-1β immunoreactivity did not derive from macrophages. Only in one case, an infant (in the CF-no DM group), was intra-islet CD68 immunoreactivity shown (Supplementary Fig. 3E), suggesting that islet macrophages may be present during early stages of CF.
CF and CFRD Are Characterized by Abnormal Islet Hormone Cell Composition
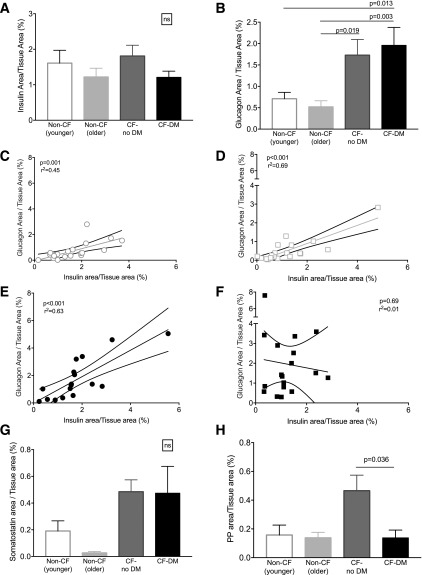
We next analyzed islet endocrine cell composition in non-CF (younger and older), CF-no DM, and CFRD subjects. When normalized to the total pancreatic tissue area, there was no significant difference in insulin-positive area between the groups (Fig. 2A). Insulin-positive area was also computed as a proportion of islet area in a subset of subjects (n = 5–10 per group), yielding the same result (β-cell area/islet area was 39 ± 0.6% in CF-no DM, 27 ± 0.2% in CFRD, and 39 ± 0.4% in non-CF control subject; P = 0.13 for the comparison of CF-no DM with CFRD and P = 0.95 for CF-no DM vs. non-CF control). However, a significant increase in glucagon immunoreactivity was observed in CF-no DM and CFRD subjects compared with non-CF control subjects (Fig. 2B). A significant correlation existed between insulin- and glucagon-positive areas in non-CF subjects (younger and older cohorts) and in CF-no DM subjects but not in CFRD subjects (Fig. 2C–F). In a subset of patients, pancreas sections were also stained for somatostatin and PP. While no significant difference between groups was observed for somatostatin immunoreactivity, CF-no DM subjects exhibited statistically greater pancreatic polypeptide area compared with CFRD subjects. (Fig. 2G and H). As with IL-1β and amyloid, we detected no significant interaction between transplant status (or steroid use) and diabetes status in predicting islet hormone immunoreactivity in CF subjects (Table 2 and data not shown).
Figure 2.
Quantitation of islet endocrine cell types in subjects with and subjects without CF. Insulin area (A) and glucagon area (B) normalized to total tissue area. Linear regression analysis for the relationship between relative insulin and glucagon areas in non-CF (younger) control subjects (C), non-CF (older) control subjects (D), and CF-no DM (E) and CFRD (F) subjects. In a subset of subjects (n = 10 per group), somatostatin area (G) and pancreatic polypeptide area (H) normalized to total tissue area.
Conclusions
In this study, we identified islet inflammation, detected by IL-1β immunoreactivity, in more than half of subjects with CF without diabetes, and in the vast majority of subjects with CFRD, whereas islet IL-1β immunoreactivity was essentially absent from age-matched subjects without CF. In line with previous studies (19,20), islet amyloid was observed in subjects with CFRD. However, islet IL-1β was far more frequently observed than islet amyloid in CF subjects, both those with and those without diabetes, and therefore likely does not occur as a consequence of islet amyloid deposition. This increase in islet IL-1β in CFRD versus CF-no DM could have occurred as a result of the increased age (and possibly age-related changes such as fibrosis) in the former cohort rather than the presence/absence of diabetes. Importantly, however, we observed islet IL-1β immunoreactivity in pediatric subjects <10 years of age, the currently recommended age to begin screening CF patients for CFRD with oral glucose tolerance tests (5). The prevalence of islet IL-1β immunoreactivity in this young cohort was similar to that in the CF-no DM group as a whole, suggesting that islet inflammation could begin very early in CF patients, consistent with clinical studies showing derangements in glucose tolerance and β-cell function in very young subjects with CF (2–4). Given that IL-1β is known to contribute to impaired islet function and viability (31), increased production of IL-1β in the islets of CF subjects may contribute to impaired β-cell function that characterizes CFRD.
Unexpectedly, in contrast to some previous studies, we did not observe a decrease in relative β-cell area in subjects with CFRD relative to subjects with CF without diabetes (17–19) or control subjects without CF (20). However, the latter study used an older cohort of control subjects without CF (mean age 65 years) as a comparison group. This could explain the discrepant findings; specifically, older subjects are likely to have a higher BMI than younger subjects (especially those with CF), and increased BMI is associated with increased islet β-cell area (32). For this reason, we were careful to age, sex, and BMI match our comparison groups without CF and without diabetes to avoid this potential confounder. Those previous studies that reported decreased β-cell area between subjects with CF, with and without diabetes, included small numbers of subjects with CFRD (n = 6–7) (17,18), whereas a larger study (n = 16 subjects with CFRD) also failed to find a significant difference in β-cell area between subjects with CF with and subjects with CF without diabetes (20). It should be noted that without pancreatic mass data (which has been shown to be decreased in subjects with CF [33]), it is not possible to definitively determine whether β-cell mass is preserved in CF/CFRD. However, our present data, in agreement with those of Couce et al. (20), suggest that a predominantly functional defect underlies the impaired insulin secretion seen in subjects with CFRD, which would be supported by animal data showing that CF pigs exhibit reduced insulin release despite preservation of islet mass (16).
We observed a striking increase in islet α-cell area in both CF-no DM and CFRD groups compared with non-CF control subjects. The source of these new α-cells is unknown but could arise because of proliferation of existing α-cells, formation of new α-cells from ductal or other precursors, or transdifferentiation of β-cells, the latter having been proposed as a source of increased α-cell mass in other forms of diabetes or models thereof (34). These data are intriguing, given that subjects with CF exhibit impaired glucagon responses to stimuli such as hypoglycemia (35), suggesting that α-cell expansion may occur as an attempt to compensate for decreased α-cell function. Conversely, these morphometric data correlate with previous clinical studies that have documented impaired suppression of glucagon responses to oral glucose tolerance tests in CFRD subjects (13,36) and in vitro data showing that cystic fibrosis transmembrane conductance regulator (CFTR) is a negative regulator of glucagon release (37), situations where glucagon levels would be increased under conditions of CF. Interestingly, in subjects with CF without diabetes, glucagon area was correlated with insulin area, suggesting that in the face of α-cell expansion, islet endocrine cell composition was still somewhat preserved in this group. In contrast, there was no such relationship between glucagon and insulin areas in subjects with CFRD, suggesting that abnormal islet composition characterizes diabetes in CF subjects.
The finding of IL-1β in the islets of CF and CFRD subjects suggests an activation of the NLRP3 inflammasome in islet cells (likely β-cells). The NLRP3 inflammasome is an intracellular protein complex that responds to a variety of intracellular and extracellular signals by triggering inflammatory cascades, resulting in IL-1β expression (38,39). While we did observe immune cell infiltrates in exocrine pancreas of subjects with CF, these were not present within islets aside from one very young subject, suggesting that they were not the source of the intra-islet IL-1β immunoreactivity. Our current data do not reveal the mechanism of activation of inflammasome activation in CF subjects. One hypothesis could be that mutations in the CFTR could generate intracellular stress in β-cells, resulting in inflammasome activation. Another possibility is that extensive exocrine tissue autolysis in CF subjects could prime and initiate inflammasome-mediated IL-1β production. Finally, systemic inflammatory processes in subjects with CF appear skewed to a program of IL-1β–regulated cytokines (e.g., IL-8, IL-17 [26–28]), suggesting that circulating levels of IL-1β itself may also be increased. This could in turn result in autostimulation of IL-1β expression in islets, a phenomenon that has been documented in cultured human islets (40). The islet in CF is therefore also likely exposed to increased IL-1β from both local and systemic sources, making it especially vulnerable to the effects of this proinflammatory cytokine. Additional studies are needed to investigate the mechanisms underlying islet IL-1β production in CF/CFRD.
The toxic actions of IL-1β can be attenuated by its endogenous antagonist, IL-1Ra. In the context of type 2 diabetes, it has been suggested that individuals without diabetes express high levels of IL-1Ra in islets, whereas subjects with diabetes exhibit a loss of IL-1Ra, further exacerbating the proinflammatory effects of IL-1β (30). In the current study, we were able to detect only small numbers of IL-1Ra–positive cells in islets from any subject, regardless of CF or diabetes status. This excludes the possibility that any effects of islet IL-1β production (for example, in CF subjects without diabetes) may be offset by increased islet IL-1Ra and suggests the possibility of a targeted intervention with clinically available IL-Ra antagonists.
Our study has some limitations. First, premortem oral glucose tolerance test data were not available for the vast majority of subjects, precluding the determination of glucose tolerance status, particularly in the CF-no diabetes group. Further, our analysis of inflammatory cytokine immunoreactivity was limited to IL-1β, and our investigation into islet hormone immunoreactivity in some cases was limited to 10 subjects per group. In addition, given the limited number of subjects, we could not adequately control for potentially confounding factors such as concomitant medication usage. However, despite the presence of these potential confounders we were still able to identify islet IL-1β immunoreactivity as a common feature of CF and exclude islet amyloid as a likely cause of its presence. Finally, the cross-sectional nature of the analysis of postmortem specimens limits our ability to definitively characterize a sequential process in which IL-1β production occurs before islet amyloidosis. However, the development of more refined animal models and the use of IL-1–related biomarkers may be useful in answering these questions both in the laboratory and at the bedside.
In conclusion, we provide a histologic analysis of human CF pancreas specimens, in subjects with or without diabetes, in which we identified islet IL-1β immunoreactivity as well as increased glucagon immunoreactivity as key abnormalities in islet morphology. These findings may help explain islet failure and observed metabolic abnormalities in patients with CF. Future studies should focus on a better understanding of the physiologic effects of CFTR on β-cell biology as well as a broader exploration of inflammasome activation in the islet of subjects with CF. In addition, the development of IL-1β–related peripheral biomarkers may be an important tool to help in the early detection of CFRD. These findings also suggest a possible role for anti-inflammatory drugs in preventing the development of islet pathology in CF.
Supplementary Material
Article Information
Acknowledgments. The authors thank Sonya Heltshe (Clinical Core, University of Washington Cystic Fibrosis Research and Translation Center) for biostatistical consultation and Brian Johnson and Megan Larmore (Host Response Core, University of Washington Cystic Fibrosis Research and Translation Center) for expert assistance with IHC and morphometric analyses.
Funding. This research was performed with the support of nPOD, a collaborative type 1 diabetes research project sponsored by JDRF. Organ procurement organizations partnering with nPOD to provide research resources are listed at www.jdrfnpod.org/for-partners/npod-partners/. This work was supported by the University of Washington Cystic Fibrosis Research and Translation Center (National Institute of Diabetes and Digestive and Kidney Diseases grant P30-DK-089507), through a Pilot and Feasibility Award (to R.L.H. and S.S.) and through work performed in the Host Response and Clinical Cores. This work was also supported by the University of Washington’s Institute of Translational Health Sciences, which receives support from the National Center for Advancing Translational Sciences (UL1 TR000423) and Cystic Fibrosis Foundation Research Development Program awards (R565CR11, SINGH15R0, and VERKMA15R0).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.L.H., R.L.G., C.W.F., B.W.R., and S.S. conceived the study and designed the experiments. R.L.H., R.L.G., S.M., G.H.D., C.L.F., C.W.F., and S.S. collected and analyzed data. R.L.H. and S.S. performed data analysis and wrote the manuscript. R.L.G., G.H.D., C.L.F., C.W.F., and B.W.R. revised the manuscript. All authors approved the final version of the manuscript. R.L.H. and S.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-1387/-/DC1.
References
- 1.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care 2009;32:1626–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ode KL, Frohnert B, Laguna T, et al. . Oral glucose tolerance testing in children with cystic fibrosis. Pediatr Diabetes 2010;11:487–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ode KL, Moran A. New insights into cystic fibrosis-related diabetes in children. Lancet Diabetes Endocrinol 2013;1:52–58 [DOI] [PubMed] [Google Scholar]
- 4.Yi Y, Norris AW, Wang K, et al. . Abnormal glucose tolerance in infants and young children with cystic fibrosis. Am J Respir Crit Care Med 2016;194:974–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moran A, Brunzell C, Cohen RC, et al.; CFRD Guidelines Committee . Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010;33:2697–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanng S, Thorsteinsson B, Pociot F, et al. . Diabetes mellitus in cystic fibrosis: genetic and immunological markers. Acta Paediatr 1993;82:150–154 [DOI] [PubMed] [Google Scholar]
- 7.Konrad K, Kapellen T, Lilienthal E, et al.; DPV Initiative and the Competence Network Diabetes Mellitus . Does β-cell autoimmunity play a role in cystic fibrosis-related diabetes? Analysis based on the German/Austrian Diabetes Patienten Verlaufsdokumentation Registry. Diabetes Care 2016;39:1338–1344 [DOI] [PubMed] [Google Scholar]
- 8.Blackman SM, Commander CW, Watson C, et al. . Genetic modifiers of cystic fibrosis-related diabetes. Diabetes 2013;62:3627–3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackman SM, Hsu S, Ritter SE, et al. . A susceptibility gene for type 2 diabetes confers substantial risk for diabetes complicating cystic fibrosis. Diabetologia 2009;52:1858–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furgeri DT, Marson FA, Ribeiro AF, Bertuzzo CS. Association between the IVS4G>T mutation in the TCF7L2 gene and susceptibility to diabetes in cystic fibrosis patients. BMC Res Notes 2012;5:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Austin A, Kalhan SC, Orenstein D, Nixon P, Arslanian S. Roles of insulin resistance and beta-cell dysfunction in the pathogenesis of glucose intolerance in cystic fibrosis. J Clin Endocrinol Metab 1994;79:80–85 [DOI] [PubMed] [Google Scholar]
- 12.Elder DA, Wooldridge JL, Dolan LM, D’Alessio DA. Glucose tolerance, insulin secretion, and insulin sensitivity in children and adolescents with cystic fibrosis and no prior history of diabetes. J Pediatr 2007;151:653–658 [DOI] [PubMed] [Google Scholar]
- 13.Lanng S, Thorsteinsson B, Røder ME, Nerup J, Koch C. Insulin sensitivity and insulin clearance in cystic fibrosis patients with normal and diabetic glucose tolerance. Clin Endocrinol (Oxf) 1994;41:217–223 [DOI] [PubMed] [Google Scholar]
- 14.Olivier AK, Yi Y, Sun X, et al. . Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest 2012;122:3755–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers CS, Stoltz DA, Meyerholz DK, et al. . Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 2008;321:1837–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uc A, Olivier AK, Griffin MA, et al. . Glycaemic regulation and insulin secretion are abnormal in cystic fibrosis pigs despite sparing of islet cell mass. Clin Sci (Lond) 2015;128:131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soejima K, Landing BH. Pancreatic islets in older patients with cystic fibrosis with and without diabetes mellitus: morphometric and immunocytologic studies. Pediatr Pathol 1986;6:25–46 [DOI] [PubMed] [Google Scholar]
- 18.Abdul-Karim FW, Dahms BB, Velasco ME, Rodman HM. Islets of Langerhans in adolescents and adults with cystic fibrosis. A quantitative study. Arch Pathol Lab Med 1986;110:602–606 [PubMed] [Google Scholar]
- 19.Iannucci A, Mukai K, Johnson D, Burke B. Endocrine pancreas in cystic fibrosis: an immunohistochemical study. Hum Pathol 1984;15:278–284 [DOI] [PubMed] [Google Scholar]
- 20.Couce M, O’Brien TD, Moran A, Roche PC, Butler PC. Diabetes mellitus in cystic fibrosis is characterized by islet amyloidosis. J Clin Endocrinol Metab 1996;81:1267–1272 [DOI] [PubMed] [Google Scholar]
- 21.Clark A, Wells CA, Buley ID, et al. . Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res 1988;9:151–159 [PubMed] [Google Scholar]
- 22.Jurgens CA, Toukatly MN, Fligner CL, et al. . β-Cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol 2011;178:2632–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westermark P. Quantitative studies on amyloid in the islets of Langerhans. Ups J Med Sci 1972;77:91–94 [DOI] [PubMed] [Google Scholar]
- 24.Masters SL, Dunne A, Subramanian SL, et al. . Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol 2010;11:897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westwell-Roper C, Dai DL, Soukhatcheva G, et al. . IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol 2011;187:2755–2765 [DOI] [PubMed] [Google Scholar]
- 26.Mulcahy EM, Hudson JB, Beggs SA, Reid DW, Roddam LF, Cooley MA. High peripheral blood th17 percent associated with poor lung function in cystic fibrosis. PLoS One 2015;10:e0120912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan HL, Regamey N, Brown S, Bush A, Lloyd CM, Davies JC. The Th17 pathway in cystic fibrosis lung disease. Am J Respir Crit Care Med 2011;184:252–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiringer K, Treis A, Fucik P, et al. . A Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 2013;187:621–629 [DOI] [PubMed] [Google Scholar]
- 29.Rountree AM, Reed BJ, Cummings BP, et al. . Loss of coupling between calcium influx, energy consumption and insulin secretion associated with development of hyperglycaemia in the UCD-T2DM rat model of type 2 diabetes. Diabetologia 2013;56:803–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maedler K, Sergeev P, Ehses JA, et al. . Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proc Natl Acad Sci U S A 2004;101:8138–8143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 2005;54(Suppl. 2):S97–S107 [DOI] [PubMed] [Google Scholar]
- 32.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 33.Sequeiros IM, Hester K, Callaway M, et al.; Bristol Cystic Fibrosis Diabetes Group . MRI appearance of the pancreas in patients with cystic fibrosis: a comparison of pancreas volume in diabetic and non-diabetic patients. Br J Radiol 2010;83:921–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 2012;150:1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moran A, Diem P, Klein DJ, Levitt MD, Robertson RP. Pancreatic endocrine function in cystic fibrosis. J Pediatr 1991;118:715–723 [DOI] [PubMed] [Google Scholar]
- 36.Hinds A, Sheehan AG, Machida H, Parsons HG. Postprandial hyperglycemia and pancreatic function in cystic fibrosis patients. Diabetes Res 1991;18:69–78 [PubMed] [Google Scholar]
- 37.Edlund A, Pedersen MG, Lindqvist A, Wierup N, Flodström-Tullberg M, Eliasson L. CFTR is involved in the regulation of glucagon secretion in human and rodent alpha cells. Sci Rep 2017;7:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanaja SK, Rathinam VA, Fitzgerald KA. Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol 2015;25:308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015;21:677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Böni-Schnetzler M, Thorne J, Parnaud G, et al. . Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J Clin Endocrinol Metab 2008;93:4065–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.