Abstract
OBJECTIVE
To assess the efficacy of a manualized occupational therapy (OT) intervention (Resilient, Empowered, Active Living with Diabetes [REAL Diabetes]) to improve glycemic control and psychosocial well-being among ethnically diverse young adults with low socioeconomic status (SES) who have type 1 or type 2 diabetes.
RESEARCH DESIGN AND METHODS
Eighty-one young adults (age 22.6 ± 3.5 years; hemoglobin A1c [HbA1c] = 10.8%/95 mmol/mol ± 1.9%/20.8 mmol/mol) were randomly assigned to the REAL Diabetes intervention group (IG) or an attention control group (CG) over 6 months. IG participants received biweekly sessions guided by a manual composed of seven content modules; CG participants received standardized educational materials and biweekly phone calls. Blinded assessors collected data at baseline and 6 months. The primary outcome was HbA1c; secondary outcomes included diabetes self-care, diabetes-related quality of life (QOL), diabetes distress, depressive symptoms, and life satisfaction. Change scores were analyzed using Wilcoxon rank sum tests.
RESULTS
Intent-to-treat analyses showed that IG participants showed significant improvement in HbA1c (−0.57%/6.2 mmol/mol vs. +0.36%/3.9 mmol/mol, P = 0.01), diabetes-related QOL (+0.7 vs. +0.15, P = 0.04), and habit strength for checking blood glucose (+3.9 vs. +1.7, P = 0.05) as compared with CG participants. There was no statistically significant effect modification by sex, ethnicity, diabetes type, recruitment site, or SES. No study-related serious adverse events were reported.
CONCLUSIONS
The REAL Diabetes intervention improved blood glucose control and diabetes-related QOL among a typically hard-to-reach population, thus providing evidence that a structured OT intervention may be beneficial in improving both clinical and psychosocial outcomes among individuals with diabetes.
Introduction
Young adulthood is a developmental stage with distinct challenges related to access to care, health behaviors, and health outcomes, yet this age-group has been largely overlooked with respect to self-management interventions (1). Young adults with diabetes are particularly vulnerable for several reasons, including the transition from pediatric to adult health care settings (2), the increasing complexity of diabetes care due to a high prevalence of mental health issues (3), the onset of medical comorbidities and diabetes complications (4), and the variability of their daily routines and social and physical environments. These self-management challenges are magnified among young adults from low socioeconomic status (SES) and/or underrepresented minority backgrounds, who often have limited finances and life stability (5); are disproportionately exposed to chronic stress (6); and experience more barriers to care, unsatisfactory health care encounters, and poor patient-provider relationships than more advantaged populations (5). Together, these issues pose barriers to self-management, which contribute to elevated hemoglobin A1c (HbA1c) levels and complication rates (7).
In diabetes, as with many chronic diseases, much of the potential to maintain health and prevent secondary complications stems from patients’ ability to consistently carry out self-management activities (e.g., dietary recommendations, self-monitoring, and medication adherence). These activities are often experienced as burdensome, and ongoing adherence is a challenge for many (8). In response, occupational therapy (OT) is increasingly being incorporated into intervention models for preventing and managing chronic diseases, including type 1 and type 2 diabetes (9–13). OT is a skilled health care profession that aims to maximize the ability of individuals and populations to participate in the daily life activities (occupations) they need or want to do. The core philosophical assumption of OT is that humans are occupational beings, for whom the ability to participate in desired and meaningful activities is central to health and well-being.
OT interventions center on activity analysis, which deconstructs the demands of an activity at the level of the individual (e.g., sensory, cognitive, and neuromuscular functions; motor, process, and social interaction skills; values and beliefs; and roles, habits, and routines), task (e.g., necessary tools and resources, physical space, social interaction, timing, and sequencing), and environment (physical, social, cultural, and temporal context). Occupational therapists identify barriers to the performance of a desired activity at one or more of these levels, to inform tailored interventions that facilitate task performance. For example, intervention strategies for someone who does not consistently take their insulin due to a fear of injections could include addressing pain hypersensitivity through sensory desensitization and relaxation strategies; adapting the task by using an injection port, applying an ice pack prior to injecting; and/or adapting the environment by performing the task in a calm, relaxing space (14). Although some intervention strategies used in OT are shared across disciplines, its overarching goal of promoting occupational participation and its focus on activities as the unit of analysis and intervention are unique within the diabetes care team. Thus, inclusion of OT may amplify the efficacy of diabetes treatment through enhancing performance of daily activities among individuals who struggle to carry them out consistently and correctly.
We conducted the Resilient, Empowered, Active Living with Diabetes (REAL Diabetes) study to examine the efficacy of an OT intervention to improve glycemic control and psychosocial well-being among ethnically diverse, low-SES young adults with type 1 or type 2 diabetes. We hypothesized that the REAL Diabetes intervention would improve diabetes self-management, and in turn HbA1c, by enhancing participants’ habit strength for performing diabetes self-care activities; satisfaction with daily activities; and diabetes-related self-efficacy, problem-solving, and knowledge. A secondary hypothesis was that the REAL Diabetes intervention would improve psychosocial well-being, as assessed via measures of diabetes-related distress and quality of life (QOL), depressive symptoms, and life satisfaction.
Research Design and Methods
Trial Design
The REAL Diabetes study methodology has been described in detail previously (15). The study was a two-arm, parallel-group, randomized, controlled trial in which participants were assigned in a 1:1 ratio to either the REAL Diabetes intervention group (IG) or an attention control group (CG).
Participants
Participants were initially recruited in person at one pediatric and one young adult diabetes clinic; recruitment efforts were later expanded to include mass mailings to clinic patients and social media advertisements. Trained graduate student assessors completed enrollment procedures and collected data at participants’ homes or community settings chosen by participants; participants received $25 at baseline and $50 at follow-up testing. Eligibility criteria were assessed via self-report, medical chart review, and point-of-care HbA1c testing and included the following: age 18–30 years old, diagnosis of type 1 or type 2 diabetes for ≥1 year, HbA1c ≥8.0%, low SES (as defined below), ability to communicate in English or Spanish, willingness to participate in study activities, and living in Los Angeles County. For two reasons, we felt it was appropriate to include individuals with type 1 or type 2 diabetes. First, our previous work with this population demonstrated that the rapid progression and limited treatment options available for youth-onset type 2 diabetes meant that many had similar self-management challenges as those with type 1 diabetes (e.g., insulin therapy and frequent self-monitoring of blood glucose [SMBG]). Second, the intervention manual was designed to be sufficiently flexible to address a range of self-management activities. Participants were excluded if they were pregnant or planned to become pregnant within the next 6 months, had a disability limiting life expectancy or functional participation in major life activities, had participated in a self-management intervention beyond usual care within the past year, or had participated in previous studies related to development of the REAL Diabetes intervention.
Initial SES criteria were for participants to either be eligible for a means-tested social program such as MediCal (California’s Medicaid program) or have a self-reported household income ≤133% of the federal poverty level. Midway through recruitment, SES inclusion criteria were expanded to include participants whose self-reported household income was ≤250% of the federal poverty level or for whom, per self-report, neither parent had attained a bachelor’s degree or equivalent.
Interventions
The REAL Diabetes intervention is a manualized, individually tailored intervention, composed of seven content modules that are flexibly administered in accordance with participants’ intervention goals (16). Two licensed occupational therapists with training in motivational interviewing and diabetes self-management education delivered the intervention on an individual basis in participants’ homes and community settings over 6 months. Therapists were asked to provide a minimum of 10 h of treatment to each participant but had flexibility to extend the intervention to up to 16 h for individuals with more complex care needs who continued to make progress toward their goals. Sessions were conducted primarily on an individual basis, although some sessions engaged family members in therapist-facilitated family education, discussion, and problem-solving to address social support challenges identified by participants. An endocrinologist and a licensed clinical social worker were available for as-needed consultations with the therapists regarding medical and social issues outside the scope of the intervention.
The REAL Diabetes intervention is an adaptation of the Lifestyle Redesign OT intervention framework (17), which applies activity analysis to the health management tasks associated with preventing and managing chronic conditions. Lifestyle Redesign emphasizes client autonomy, narrative reasoning, and establishing health-promoting daily habits and routines. The content modules include the following: 1) assessment and goal setting, 2) living with diabetes (basic self-management knowledge and skills), 3) access and advocacy (accessing health care and self-advocacy in health care and community settings), 4) activity and health (establishing and maintaining health-promoting habits and routines), 5) social support (receiving desired support from family and friends and connecting to the diabetes community), 6) emotional well-being (managing stress and coping with diabetes-related burnout), and 7) long-term health (reflecting on progress and planning for the future). After an initial evaluation (module 1), therapists individually tailored the intervention by using content from the remaining modules that was relevant to clients’ individual goals, which were informed by a variety of factors, including their readiness to change, diabetes treatment regimen, and personal preferences. The manual was conceptualized as a “menu” of possible treatment goals and activities, organized thematically by module, rather than a fixed curriculum that every participant should complete. Among participants who received the intervention (n = 39), engagement in each module was as follows: module 1, 100%; module 2, 92%; module 3, 79%; module 4, 90%; module 5, 69%; module 6, 56%; and module 7, 62%. Motivational interviewing was used as a communication strategy with clients who expressed ambivalence regarding behavior change. Intervention fidelity was maintained through three strategies. First, therapists documented intervention dose, timing, and treatment activities in notes completed after each session. Second, ∼10% of sessions were observed by a second therapist trained in the intervention protocol, who completed a fidelity checklist and shared feedback with the treating therapist. Third, all team members trained in the intervention met weekly to facilitate problem-solving and prevent intervention drift.
An attention (rather than usual care) control condition was used to enhance retention by having more frequent contact with CG participants, and control for the Hawthorne effect of study participation. It included an initial home visit and 11 follow-up phone calls. At the home visit, a staff member delivered a standardized set of educational materials published by the National Diabetes Education Program and MyPlate.gov. Subsequently, a trained staff member called the participant biweekly and engaged in a scripted phone conversation to ask if the participant had read and had any questions about the materials.
Outcomes
All outcomes were prespecified and assessed at baseline and 6 months. The primary outcome was HbA1c, measured with the Axis-Shield Afinion point-of-care analyzer (18). For participants without a point-of-care assay (due to equipment malfunction or loss to follow-up), HbA1c values taken within ±6 weeks of the testing date were extracted from medical records when available. Secondary outcomes and process variables were also assessed. Secondary outcomes included diabetes self-care (Summary of Diabetes Self-Care Activities [SDSCA]) (19); diabetes-related QOL (Audit of Diabetes-Dependent QOL [ADDQOL], Cronbach α = 0.85) (20); diabetes distress (Problem Areas in Diabetes-Short Form [PAID-SF], Cronbach α = 0.83–0.86) (21); depressive symptoms (Patient Health Questionnaire-8 [PHQ-8], Cronbach α = 0.86–0.89) (22); and life satisfaction (Satisfaction with Life Scale [SWLS], Cronbach α = 0.87) (23). Process variables included diabetes self-efficacy (Diabetes Empowerment Scale-Short Form [DES-SF], Cronbach α = 0.85) (24); diabetes knowledge (Diabetes Knowledge Questionnaire [DKQ], Cronbach α = 0.78) (25); diabetes-related problem-solving (Diabetes Problem-Solving Inventory [DPSI], Cronbach α = 0.77) (26); habit strength for SMBG and taking insulin or diabetes-related medications (Self-Report Behavioral Automaticity Index [SRBAI], Cronbach α = 0.88) (27); and activity participation (Participation Objective, Participation Subjective [POPS], Cronbach α = 0.43 for objective participation, 0.70 for subjective participation) (28).
We analyzed two constructs from SDSCA: frequency of SMBG (using the single item “On how many of the last 7 days did you test your blood sugar the number of times recommended by your health care provider?”) and medication adherence (using an average of the following items, as appropriate: “On how many of the last 7 days did you take your recommended insulin injection/number of diabetes pills?”). For all other instruments, summary scores were calculated according to published guidelines. All instruments were available in English and Spanish, previously validated among young adults, and appropriate for both type 1 and type 2 diabetes. At baseline, participants provided demographic information, and medical charts were reviewed to obtain clinical and health care utilization data. All self-report instruments were administered by trained bilingual research assistants.
Sample Size
The study was powered on an intent-to-treat analysis of mean change in HbA1c at follow-up compared with baseline. A sample size of 80 was sufficient to afford 90% power to detect a between-group difference of 0.8% in HbA1c, assuming a pooled SD of 1%, two-sided α of 0.05, and 15% attrition. The study was not fully powered to examine secondary outcomes, process variables, and effect modification; such analyses were conducted on an exploratory basis to inform intervention refinements and power calculations for future studies.
Randomization
A randomization list was electronically generated and securely maintained by the study’s statistician. Randomization was stratified by diabetes type using random block sizes. Randomization assignment was completed by the primary investigator or a PhD research assistant using the study’s Research Electronic Data Capture (REDCap) data management system (29).
Blinding
Data collectors were blinded to participants’ group assignment at baseline and follow-up testing. Additionally, the study’s interveners were blinded to the specific assessments used to collect outcome data.
Statistical Methods
All analyses were completed on an intent-to-treat basis, including all participants for whom data were available in their original assigned groups. We compared baseline values for demographic and outcome variables to see if those with and without follow-up values were equivalent at baseline. Change scores for each participant were calculated by subtracting baseline values from follow-up values. Wilcoxon rank sum tests were used to compare changes in outcome measures between the IG and the CG. Effect sizes were calculated as Cohen d values.
We explored effect modification of treatment effect on HbA1c by sex, ethnicity, recruitment site, diabetes type, and SES with separate regression models for each potential effect modifier. SES variables (Hollingshead Index and census tract data on neighborhood income and percentage below poverty) were dichotomized as below versus above median. Change in HbA1c rank was the dependent variable, and treatment group, the potential effect modifier, and an interaction term for treatment group and effect modifier were the independent variables. We investigated the association of amount of treatment with change in HbA1c and diabetes-related QOL within the IG with Spearman correlation. All data were analyzed using SAS for Windows, version 9.4 (SAS Institute, Cary, NC). All P values are two sided.
Results
Recruitment
Participants were recruited between October 2014 and December 2015. Follow-up testing was completed between April 2015 and July 2016. The trial ended after follow-up testing was completed for all participants and HbA1c data were extracted from all available medical charts for participants who were lost to follow-up.
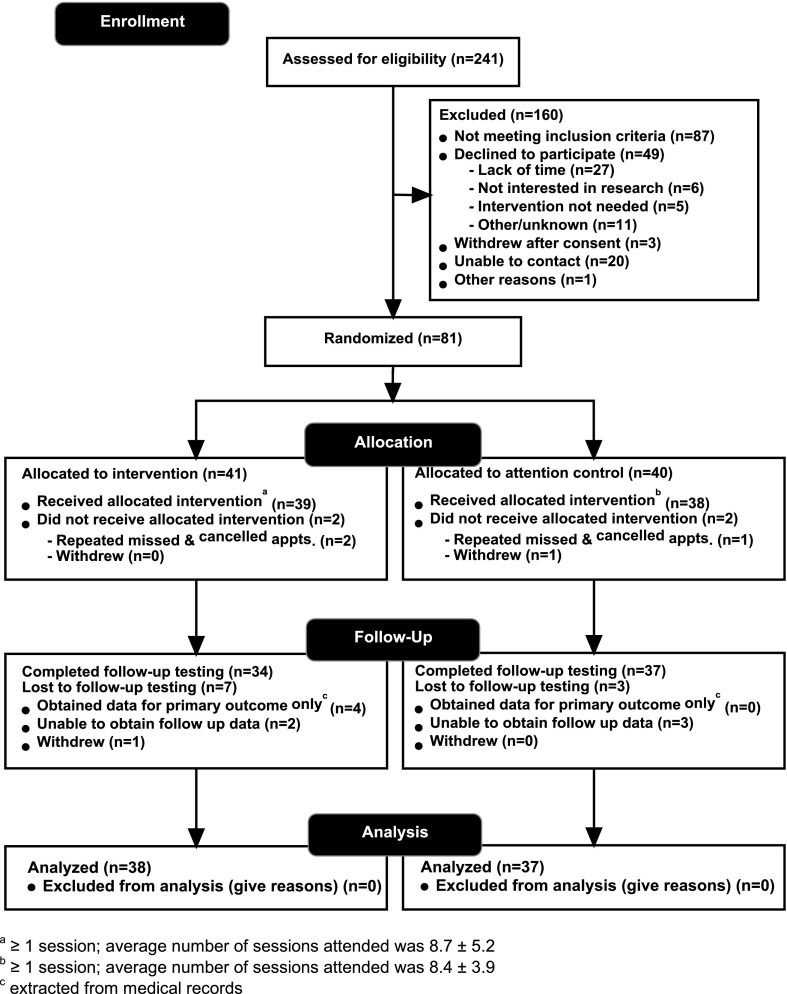
Participant flow is outlined in Fig. 1. Overall, of 81 randomized participants, 77 (95%) received their allocated intervention, 71 (88%) completed the follow-up assessment battery, and 75 (93%) had follow-up HbA1c data. Participants with and without follow-up assessment for the primary outcome did not significantly differ at baseline by any demographic or outcome variables. Among IG participants (n = 41), 39 (95%) attended at least 1 treatment session, 24 (59%) completed ≥10 sessions, and average treatment dose was 8.7 ± 5.2 sessions. Among CG participants (n = 40), 38 (95%) completed at least one visit/phone call, and the average number of visits/calls was 8.4 (±3.9). We found significant differences in treatment dose by sex among IG but not CG participants, with IG women completing fewer sessions than IG men (6.6 vs. 11.9, P = 0.002), whereas CG women and men completed a similar number of sessions (8.6 vs. 7.7, P = 0.54). No other baseline demographic variables were related to treatment dose.
Figure 1.
Study flow diagram.
Baseline Data
Participants’ baseline characteristics are presented in Table 1. Overall, participants were 22.6 ± 3.5 years old, 63% female, 78% Hispanic, and 75% had type 1 diabetes. Participants’ average HbA1c was 10.8 ± 1.9% (95 ± 20.8 mmol/mol). Data are presented for the sample as a whole and for IG and CG participants. The only significant difference between IG and CG participants was a stronger family history of diabetes among CG participants (92 vs. 68%, P = 0.01).
Table 1.
Baseline demographic, clinical, and psychosocial characteristics of REAL Diabetes study participants
| Total, n = 81 | IG, n = 41 | CG, n = 40 | |
|---|---|---|---|
| Demographic | |||
| Age (years) | 22.6 ± 3.5 | 23.3 ± 3.6 | 21.9 ± 3.3 |
| Sex (% female) | 51 (63) | 22 (54) | 29 (72) |
| Generation* | |||
| 0 | 21 (26) | 10 (24) | 11 (28) |
| 1 | 35 (43) | 20 (49) | 15 (38) |
| 2 | 25 (31) | 11 (27) | 14 (35) |
| Race/ethnicity | |||
| White | 8 (10) | 3 (7) | 5 (12) |
| Black | 8 (10) | 3 (7) | 5 (12) |
| Hispanic/Latino | 63 (78) | 35 (85) | 28 (70) |
| Other | 2 (2) | 0 | 2 (5) |
| Hollingshead Index (n = 67) | 29.6 ± 13.1 | 27.3 ± 11.9 | 32.5 ± 14.1 |
| Neighborhood income ($K)† | 43.8 ± 16 | 42.6 ± 16.0 | 45.0 ± 16.0 |
| Neighborhood % below federal poverty level† | 23.8 ± 11.3 | 23.9 ± 11.0 | 23.8 ± 11.8 |
| Recruitment site (recruitment strategy) | |||
| County hospital (in person) | 39 (48) | 20 (49) | 19 (48) |
| Children’s hospital (in person/mass mailing) | 13 (16) | 8 (20) | 5 (13) |
| Other community settings (social media advertising) | 29 (36) | 13 (32) | 16 (40) |
| Diabetes care provider | |||
| Endocrinologist | 57 (70) | 30 (73) | 27 (68) |
| Primary care provider | 16 (20) | 8 (20) | 8 (20) |
| No regular source of care/unknown | 8 (10) | 3 (7) | 5 (13) |
| Clinical | |||
| Diabetes type | |||
| Type 1 diabetes | 61 (75) | 31 (76) | 30 (75) |
| Type 2 diabetes | 20 (25) | 10 (24) | 10 (25) |
| Diabetes duration (years) | 9.7 ± 5.8 | 10.0 ± 5.9 | 9.4 ± 5.8 |
| Family history of diabetes | 65 (80) | 28 (68) | 37 (92) |
| Treatment regimen | |||
| None | 3 (4) | 2 (5) | 1 (2) |
| Oral medication and/or noninsulin injectable only | 4 (5) | 3 (7) | 1 (2) |
| Insulin only | 63 (78) | 31 (76) | 32 (80) |
| Oral medication and/or noninsulin injectable + insulin | 11 (14) | 5 (12) | 6 (15) |
| Among those on insulin | |||
| Fixed regimen | 28 (38) | 12 (33) | 16 (42) |
| Intensive regimen: injections/pen | 35 (43) | 20 (49) | 15 (39) |
| Intensive regimen: insulin pump | 9 (11) | 5 (12) | 4 (11) |
| Unknown | 2 (2) | 1 (2) | 3 (8) |
| Health care utilization (12 months prior to baseline) | |||
| Number of routine diabetes visits (n = 77) | 3.2 ± 1.8 | 3.0 ± 1.9 | 3.5 ± 1.8 |
| ≥2 visits with HbA1c taken ≥3 months apart (n = 77) | 49 (64) | 24 (60) | 25 (68) |
| Proportion of participants reporting ≥1 diabetes-related hospitalization | 19 (23) | 9 (22) | 10 (25) |
| Psychosocial | |||
| Substance abuse (CAGE-AID; range 0–4) | 0.5 ± 1.0 | 0.6 ± 1.0 | 0.5 ± 1.0 |
| Stressful life events (range 0–24) | 5.0 ± 3.6 | 4.8 ± 3.6 | 5.1 ± 3.5 |
Data are mean ± SD or n (%). CAGE-AID, Cut down, Annoyed, Guilty, Eye-opener–Adapted to Include Drugs.
*0 = participant born outside U.S.; 1 = participant but neither parent born in U.S.; 2 = at least one parent born in the U.S.
†Using 2010 census tract data.
Main Outcomes
Changes in primary and secondary outcomes, and process variables, are presented in Table 2. For the primary outcome (change in HbA1c), data were available for 75 participants. Of these, 62 had Afinion HbA1c measurements at both baseline and follow-up, 7 had Afinion measurement at baseline and medical chart data at follow-up, and 6 had medical chart data at baseline and Afinion measurement at follow-up. We completed analyses among the 62 participants with study-administered Afinion HbA1c measurements and among participants with HbA1c measurements from any source, with similar findings. We found a significant improvement in HbA1c among IG participants as compared with CG participants (−0.57%/6.2 mmol/mol vs. +0.36%/3.9 mmol/mol, P = 0.01).
Table 2.
Changes in primary and secondary outcomes and process variables
| Overall | Intervention | Control | Between-group difference | P value* | Effect size (95% CI)† | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Primary outcome | Baseline n = 81 | Baseline n = 41 | Follow-up n = 38 | Change n = 38 | Baseline n = 40 | Follow-up n = 37 | Change n = 37 | |||
| HbA1c | 10.8 (1.9) | 11.0 (2.0) | 10.5 (2.4) | −0.6 (1.7) | 10.5 (1.7) | 10.8 (2.2) | 0.4 (1.6) | 0.9 | 0.01 | −0.5 (−0.9, −0.1) |
| Secondary outcomes | Baseline n = 41 | Follow-up n = 35 | Change n = 35 | Baseline n = 40 | Follow-up n = 37 | Change n = 37 | ||||
| Diabetes-related QOL (ADDQOL; range −9 to +1) | −2.6 (1.7) | −2.4 (1.8) | −1.8 (1.7) | 0.7 (1.1) | −2.8 (1.7) | −2.5 (1.6) | 0.2 (1.5) | 0.5 | 0.04 | 0.3 (−0.1, 0.7) |
| Glucose monitoring (days/week) (SDSCA; range 0–7) | 3.3 (2.7) | 3.1 (2.7) | 4.0 (2.6) | 0.6 (3.2) | 3.5 (2.7) | 3.6 (2.5) | −0.1 (2.7) | 0.7 | 0.37 | 0.3 (−0.2, 0.8) |
| Medication adherence (days/week) (SDSCA; range 0–7) | 5.9 (1.8) | 5.7 (2.2) | 6.3 (1.2) | 0.3 (1.8) | 6.0 (1.3) | 6.2 (1.5) | 0.1 (1.4) | 0.2 | 0.93 | 0.1 (−0.3, 0.5) |
| Diabetes distress (PAID-SF; range 0–20) | 9.6 (5.7) | 9.7 (5.2) | 7.4 (6.0) | −2.6 (4.3) | 9.4 (6.2) | 7.5 (4.9) | −1.7 (4.6) | 0.8 | 0.26 | −0.1 (−0.5, 0.2) |
| Life satisfaction (SWLS; range 5–35) | 20.5 (6.8) | 20.3 (6.1) | 23.0 (5.9) | 2.6 (5.2) | 20.7 (7.4) | 22.2 (7.3) | 1.4 (4.4) | 1.2 | 0.21 | 0.2 (−0.2, 0.5) |
| Depressive symptoms (PHQ-8; range 0–27) | 6.9 (5.0) | 6.6 (5.3) | 5.4 (5.0) | −0.9 (4.1) | 7.2 (4.7) | 6.9 (5.6) | −0.0 (4.5) | 0.9 | 0.42 | −0.2 (−0.6, 0.2) |
| Process variables | Baseline n = 41 | Follow-up n = 35 | Change n = 35 | Baseline n = 40 | Follow-up n = 37 | Change n = 37 | ||||
| Diabetes knowledge (DKQ; range 0–24) | 18.1 (3.2) | 18.2 (3.0) | 18.9 (2.4) | 0.6 (1.9) | 17.9 (3.5) | 18.2 (3.4) | 0.2 (1.6) | 0.3 | 0.50 | 0.1 (−0.2, 0.4) |
| Problem-solving (DPSI; range 1–5) | 3.6 (0.6) | 3.6 (0.8) | 3.7 (0.6) | 0.1 (0.6) | 3.6 (0.5) | 3.9 (0.5) | 0.3 (0.5) | 0.2 | 0.10 | −0.3 (−0.7, 0.1) |
| Diabetes self-efficacy (DES-SF; range 1–5) | 3.9 (0.7) | 3.9 (0.7) | 4.1 (0.8) | 0.2 (0.9) | 3.9 (0.7) | 3.9 (1.0) | 0.0 (0.9) | 0.2 | 0.27 | 0.2 (−0.4, 0.9) |
| Habit strength | ||||||||||
| Glucose monitoring (SRBAI; range 4–28) | 15.0 (6.9) | 13.9 (6.7) | 18.3 (6.2) | 3.9 (5.0) | 16.2 (6.9) | 18.1 (6.8) | 1.6 (5.1) | 2.3 | 0.05 | 0.3 (−0.2, −0.7) |
| Medication adherence (SRBAI; range 4–28) | 19.0 (6.3) | 17.9 (7.0) | 20.6 (6.2) | 2.1 (6.0) | 19.6 (5.7) | 20.9 (5.6) | 1.0 (6.8) | 1.2 | 0.32 | 0.2 (−0.3, 0.7) |
| Participation | ||||||||||
| Objective (POPS; range: weighted z scores −3 to 3) | −0.0 (0.2) | −0.0 (0.3) | −0.0 (0.3) | 0.0 (0.3) | −0.1 (0.2) | −0.1 (0.2) | −0.0 (0.2) | 0.0 | 0.56 | 0.1 (−0.3, 0.5) |
| Subjective (POPS; range −4 to +4) | −0.0 (0.1) | −0.1 (1.0) | 0.2 (1.0) | 0.3 (1.1) | −0.1 (0.8) | 0.3 (0.6) | 0.3 (0.7) | 0.0 | 0.95 | −0.0 (−0.6, 0.5) |
*Wilcoxon rank sum test for change difference between treatment groups.
†We provide Cohen d effect sizes as a well-recognized measure of the strength of intervention effects. It should be noted, however, that these effect sizes are based on the assumption of a normally distributed variable and are not necessarily fully consistent with the nonparametric Wilcoxon method that was used to calculate P values.
For analysis of secondary outcomes and process variables, data were available for 71 participants. IG participants had significant improvements in diabetes-related QOL as compared with CG participants (change in ADDQOL +0.7 vs. +0.15, P = 0.04). Furthermore, IG participants had greater improvement in habit strength for SMBG than CG participants (change in SRBAI +3.9 vs. +1.7, P = 0.05). No other between-group differences were statistically significant. With the exception of problem-solving, there were greater improvements in the IG as compared with the CG for all secondary outcomes and process variables; effect sizes for nonsignificant outcomes ranged from negligible (0.02) to medium (0.27).
Secondary Analyses
We examined whether there were differential intervention effects on HbA1c and diabetes-related QOL among key population subgroups: sex, ethnicity (Latino/non-Latino), diabetes type, recruitment strategy (in person vs. mailings/social media), and SES (30). These analyses did not suggest any effect modification by sex, ethnicity, diabetes type, or recruitment site (all P values >0.20). With respect to SES, the Hollingshead Index score approached significance as an effect modifier for change in HbA1c (P = 0.08).
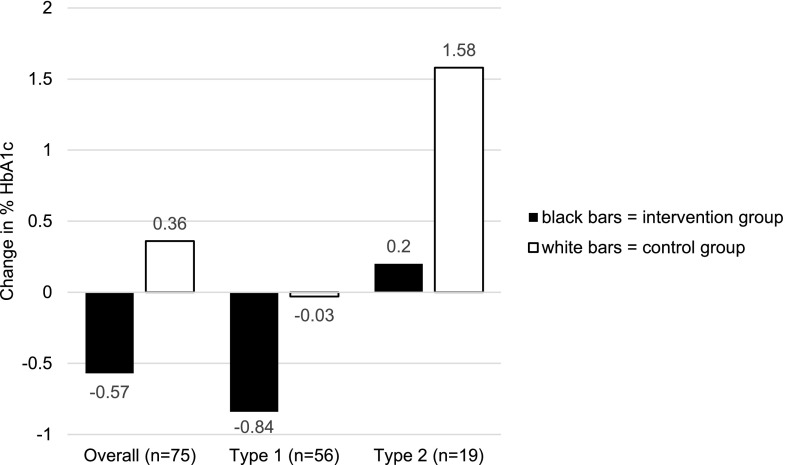
Although the intervention did not have differential effects according to diabetes type, in that IG participants with type 1 or type 2 diabetes had better HbA1c relative to their CG counterparts, there was a difference in the HbA1c trajectories of participants with type 1 diabetes as compared with those with type 2 diabetes. As shown in Fig. 2, IG participants with type 1 diabetes had a decrease in HbA1c (−0.84%/9.2 mmol/mol), whereas CG participants with type 1 diabetes had essentially no change in HbA1c (−0.03%/0.3 mmol/mol). In contrast, IG participants with type 2 diabetes had a modest increase in HbA1c (0.2%/2.2 mmol/mol), whereas CG participants with type 2 diabetes had a large increase in HbA1c (1.58%/17.3 mmol/mol).
Figure 2.
Change in HbA1c by diabetes type.
We investigated the extent to which, within the IG, changes in HbA1c and diabetes-related QOL were associated with demographic characteristics or with intervention dose. With respect to demographic characteristics, we found that census tract–level SES, but not individual-level SES, was associated with change in HbA1c. Specifically, median neighborhood income and a lower proportion of residents below the poverty level were associated with change in HbA1c (r = −0.46, P = 0.002 and r = 0.42, P = 0.03, respectively). However, Hollingshead Index scores were not associated with change in HbA1c (r = −0.06, P value = 0.71). With respect to intervention dose, findings were in the expected direction but were not statistically significant, with a stronger association between dose and change in diabetes-related QOL (r = 0.31, P = 0.07) than between dose and change in HbA1c (r = −0.08, P = 0.62).
Intervention Implementation
Fidelity monitoring and process evaluation data indicated that therapists had 96% adherence to the intervention’s key components and that participants were satisfied with the intervention. All serious adverse events reported to study personnel were evaluated by an independent medical monitor to determine whether they were study related. Eleven events were reported in total, five among CG participants and six among IG participants, of which none were determined to be study related. Of the 11 events, 7 were diabetes-related hospitalizations (for gastroparesis, diabetic ketoacidosis, or severe hyperglycemia) and 4 were hospitalizations for unrelated medical conditions.
Conclusions
In the REAL Diabetes study, a manualized, individually tailored diabetes management intervention delivered by occupational therapists improved both HbA1c and diabetes-related QOL among low-SES, ethnically diverse young adults with diabetes. Although OT interventions to support chronic disease management have shown promise in previous studies, methodological limitations such as small sample sizes and lack of randomization have limited the strength of this evidence (9–13). This study provides additional evidence of the potential for OT to improve clinical and psychosocial outcomes among individuals with diabetes.
Meta-analyses of behavioral interventions to support diabetes self-management have demonstrated modest improvements in HbA1c among adults with type 1 diabetes (−0.44%/4.8 mmol/mol vs. active control) (31) and type 2 diabetes (−0.35%/3.8 mmol/mol) (32), but not improved QOL. Among transition and self-management interventions for young adults with diabetes specifically, improvements in HbA1c ranging from 0.3% to 0.7% (3.3 to 7.7 mmol/mol) have been reported (33–36); two of these studies also reported improved psychosocial well-being (34,35). Thus, the impact of the REAL Diabetes intervention on HbA1c and QOL is in line with the modest but clinically significant benefits of other behavioral interventions for diabetes self-management.
The REAL Diabetes study’s enrollment and treatment adherence rates are also comparable to those in other behavioral interventions in this population, supporting the feasibility and acceptability of the REAL Diabetes intervention. Enrollment rates ranging from 20% (37) to 66% (38) of eligible participants have been reported in previous diabetes management interventions, in line with our 53% enrollment rate. Treatment adherence (averaging 8.7 of 10 planned sessions; 59% completed ≥10 sessions) is also in line with that reported in previous research, such as the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study lifestyle intervention, which reported 60% overall adherence to planned sessions (39) and a young adult support group in which 80% of participants attended three of five sessions and 53% attended four of five sessions (35). Although our enrollment and adherence rates are in line with similar interventions conducted among young adults with diabetes, higher rates (indicating greater acceptability and potential for reach) would be desirable. The significant sex difference in treatment dose within the IG also suggests that the REAL Diabetes intervention may require further refinements to facilitate greater treatment adherence among women. To work toward this goal, we plan to use telehealth as a delivery modality, which has demonstrated strong acceptability and potential for reach among this population (40), and greater stakeholder engagement (e.g., an advisory committee of young adults with diabetes) to enhance enrollment and treatment adherence.
Although this study lacked sufficient statistical power to rigorously evaluate the mechanisms underlying the REAL Diabetes intervention’s effects, we did assess process variables hypothesized to influence intervention outcomes. Of these, we found that habit strength for SMBG significantly improved. Developing habits and routines is a central focus of OT interventions in chronic disease management and is a key mechanism by which health behaviors are sustained over time (41). Thus, we are encouraged that the intervention had a positive effect on habit strength and will seek to further enhance its focus on developing healthy habits. Furthermore, self-efficacy and habit strength for taking medications had effect sizes of 0.24 and 0.18, which, although modest, may indicate that they played a role in the intervention’s effects. In contrast, problem-solving had a small to moderate effect size (0.30) in favor of the CG and was the only variable for which greater improvements were observed in the CG as compared with the IG. Further research is needed to determine whether this was a chance finding or if the REAL Diabetes intervention undermines the development of problem-solving skills and requires refinements to address this limitation.
Overall, we did not observe evidence of effect modification related to demographic characteristics, although such analyses were underpowered and should be interpreted with caution. However, we did find that both individual-level and neighborhood-level SES may be related to changes in HbA1c, which is plausible and consistent with previous research (42). Individual-level SES was the only variable to approach statistical significance as an effect modifier for HbA1c (P = 0.08). This suggests the possibility that although the intervention targeted a low-SES population overall, it may have been more effective for those at the higher end of the included SES range. Additionally, within the IG, there was a correlation between neighborhood-level SES and change in HbA1c. This finding is consistent with research indicating that aspects of the physical and social environment in low-SES communities, such as the limited availability of healthy food outlets and recreational facilities and poor access to health care, often pose barriers to well-being for residents of these communities (42). Collectively, these results suggest that the intervention may benefit from further refinements to better support very low-SES populations.
Another finding that merits further investigation is the different response to the intervention observed among participants with type 1 diabetes versus type 2 diabetes. IG participants with type 1 diabetes had a 0.84% (8.7 mmol/mol) reduction in HbA1c, well above the threshold of 0.5% (5.5 mmol/mol) that is considered clinically significant. However, IG participants with type 2 diabetes had a slight deterioration in HbA1c at follow-up, although substantially less than CG participants with type 2 diabetes. Given the small number of participants with type 2 diabetes overall, this finding has a high level of uncertainty. It is consistent, however, with literature indicating that youth-onset type 2 diabetes is particularly aggressive compared with other forms of diabetes (4,43). This is perhaps especially true for participants in our study, given our inclusion criteria of HbA1c ≥8%. Indeed, no participants attained the recommended target HbA1c ≤7.0% (53 mmol/mol); it is likely that ongoing intervention at multiple levels, addressing individual, family, environmental, and health system barriers to health and well-being, would be necessary to enable this high-risk population to achieve glycemic targets.
The design and implementation of the REAL Diabetes study were bolstered by several strengths that enhance confidence in its findings. First, we successfully recruited a population typically conceived of as “hard to reach” (ethnically diverse young adults with low SES). A sizeable proportion of participants were recruited from community settings rather than from specialized medical centers, strengthening the generalizability of the results. Furthermore, a high level of retention decreases the likelihood that the findings were influenced by attrition bias. Finally, aspects of the study design, including randomization, blinding of data collectors, and fidelity monitoring of the intervention, further enhance the validity of the findings.
Despite these strengths, the study has several limitations. First, the study’s sample size was relatively small and lacked statistical power to examine mediation or effect modification. Furthermore, the sample was not representative of young adults with diabetes as a whole, as it represents a higher-risk group than is typical of the population overall. Finally, the study did not incorporate long-term follow-up; given that intervention effects often attenuate during a no-treatment follow-up period, future research should investigate the maintenance of improvements that were observed in this study.
In conclusion, this study provides evidence that the REAL Diabetes intervention improves both blood glucose control and diabetes-related QOL among ethnically diverse, low-SES young adults with diabetes. Larger-scale translational studies evaluating this approach among various populations in real-world settings should be conducted to assess the potential impact of including OTs on diabetes care teams. Given the increasing prevalence of diabetes, workforce shortages among frontline diabetes care providers, and the shift toward multidisciplinary team-based approaches to chronic care management, OTs may merit consideration as an untapped resource to address the growing burden of diabetes in the U.S.
Article Information
Acknowledgments. The authors gratefully acknowledge study staff members Alexandra Gonzalez, Cindy Culp, Daniella Floríndez, Emily Friedberg, Eva Ortega, Jennie Lam, Laura Cox, Laura Guzman, Maria Gonzalez, Nancy Dominguez, Veronica Gomez, and Grace Cho (Chan Division of Occupational Science and Occupational Therapy) for their contributions to this project.
Funding. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (1K01 DK099202-01A1).
The content is solely the responsibility of the authors and does not represent the official view of the National Institutes of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. E.A.P., K.C., C.L.P.V., and J.B. researched data, contributed to the discussion, and wrote and edited the manuscript. J.D. and A.C.-C. contributed to the discussion and reviewed and edited the manuscript. P.A.S., J.R.W., R.W., D.S.-M., and A.L.P. reviewed and edited the manuscript. E.A.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 77th Scientific Sessions of the American Diabetes Association, San Diego, CA, 9–13 June 2017.
Footnotes
Clinical trial reg. no. NCT02214641, clinicaltrials.gov.
References
- 1.Hendricks M, Monaghan M, Soutor S, Chen R, Holmes CS. A profile of self-care behaviors in emerging adults with type 1 diabetes. Diabetes Educ 2013;39:195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters A, Laffel L; American Diabetes Association Transitions Working Group . Diabetes care for emerging adults: recommendations for transition from pediatric to adult diabetes care systems: a position statement of the American Diabetes Association, with representation by the American College of Osteopathic Family Physicians, the American Academy of Pediatrics, the American Association of Clinical Endocrinologists, the American Osteopathic Association, the Centers for Disease Control and Prevention, Children with Diabetes, The Endocrine Society, the International Society for Pediatric and Adolescent Diabetes, Juvenile Diabetes Research Foundation International, the National Diabetes Education Program, and the Pediatric Endocrine Society (formerly Lawson Wilkins Pediatric Endocrine Society). Diabetes Care 2011;34:2477–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson B, Elliott J, Scott A, Heller S, Eiser C. Medical and psychological outcomes for young adults with type 1 diabetes: no improvement despite recent advances in diabetes care. Diabet Med 2014;31:227–231 [DOI] [PubMed] [Google Scholar]
- 4.Dabelea D, Stafford JM, Mayer-Davis EJ, et al.; SEARCH for Diabetes in Youth Research Group . Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA 2017;317:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kibbey KJ, Speight J, Wong JL, Smith LA, Teede HJ. Diabetes care provision: barriers, enablers and service needs of young adults with type 1 diabetes from a region of social disadvantage. Diabet Med 2013;30:878–884 [DOI] [PubMed] [Google Scholar]
- 6.Peyrot M, McMurry JF Jr., Kruger DF. A biopsychosocial model of glycemic control in diabetes: stress, coping and regimen adherence. J Health Soc Behav 1999;40:141–158 [PubMed] [Google Scholar]
- 7.Lado JJ, Lipman TH. Racial and ethnic disparities in the incidence, treatment, and outcomes of youth with type 1 diabetes. Endocrinol Metab Clin North Am 2016;45:453–461 [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez JS, Tanenbaum ML, Commissariat PV. Psychosocial factors in medication adherence and diabetes self-management: implications for research and practice. Am Psychol 2016;71:539–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haltiwanger EP. Effect of a group adherence intervention for Mexican-American older adults with type 2 diabetes. Am J Occup Ther 2012;66:447–454 [DOI] [PubMed] [Google Scholar]
- 10.O’Toole L, Connolly D, Smith S. Impact of an occupation-based self-management programme on chronic disease management. Aust Occup Ther J 2013;60:30–38 [DOI] [PubMed] [Google Scholar]
- 11.Schwartz JK, Smith RO. Intervention promoting medication adherence: a randomized, phase I, small-N study. Am J Occup Ther 2016;70(6):7006240010p1–7006240010p11 [DOI] [PubMed] [Google Scholar]
- 12.Garvey J, Connolly D, Boland F, Smith SM. OPTIMAL, an occupational therapy led self-management support programme for people with multimorbidity in primary care: a randomized controlled trial. BMC Fam Pract 2015;16:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uyeshiro Simon A, Collins CER. Lifestyle Redesign for chronic pain management: a retrospective clinical efficacy study. Am J Occup Ther 2017;71:7104190040p1–7104190040p7 [DOI] [PubMed] [Google Scholar]
- 14.American Occupational Therapy Association AOTA Fact Sheet: The Role of Occupational Therapy in Chronic Disease Management. Bethesda, MD, AOTA, 2015 [Google Scholar]
- 15.Pyatak EA, Carandang K, Vigen C, et al. . Resilient, Empowered, Active Living with Diabetes (REAL Diabetes) study: methodology and baseline characteristics of a randomized controlled trial evaluating an occupation-based diabetes management intervention for young adults. Contemp Clin Trials 2017;54:8–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pyatak EA, Carandang K, Davis S. Developing a manualized occupational therapy diabetes management intervention: Resilient, Empowered, Active Living with Diabetes. OTJR (Thorofare, NJ) 2015;35:187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson J, Carlson M, Mandel D, Zemke R, Clark F. Occupation in Lifestyle Redesign: the Well Elderly Study occupational therapy program. Am J Occup Ther 1998;52:326–336 [DOI] [PubMed] [Google Scholar]
- 18.Wood JR, Kaminski BM, Kollman C, et al. . Accuracy and precision of the Axis-Shield Afinion hemoglobin A1c measurement device. J Diabetes Sci Technol 2012;6:380–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000;23:943–950 [DOI] [PubMed] [Google Scholar]
- 20.Bradley C, Todd C, Gorton T, Symonds E, Martin A, Plowright R. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: the ADDQoL. Qual Life Res 1999;8:79–91 [DOI] [PubMed] [Google Scholar]
- 21.McGuire BE, Morrison TG, Hermanns N, et al. . Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes scale (PAID)-5 and PAID-1. Diabetologia 2010;53:66–69 [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–173 [DOI] [PubMed] [Google Scholar]
- 23.Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess 1985;49:71–75 [DOI] [PubMed] [Google Scholar]
- 24.Anderson RM, Fitzgerald JT, Gruppen LD, Funnell MM, Oh MS. The Diabetes Empowerment Scale-Short Form (DES-SF). Diabetes Care 2003;26:1641–1642 [DOI] [PubMed] [Google Scholar]
- 25.Garcia AA, Villagomez ET, Brown SA, Kouzekanani K, Hanis CL. The Starr County Diabetes Education study: development of the Spanish-language diabetes knowledge questionnaire. Diabetes Care 2001;24:16–21 [DOI] [PubMed] [Google Scholar]
- 26.Glasgow RE, Toobert DJ, Barrera M Jr, Strycker LA. Assessment of problem-solving: a key to successful diabetes self-management. J Behav Med 2004;27:477–490 [DOI] [PubMed] [Google Scholar]
- 27.Gardner B, Abraham C, Lally P, de Bruijn GJ. Towards parsimony in habit measurement: testing the convergent and predictive validity of an automaticity subscale of the Self-Report Habit Index. Int J Behav Nutr Phys Act 2012;9:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown M, Dijkers MPJM, Gordon WA, Ashman T, Charatz H, Cheng Z. Participation objective, participation subjective: a measure of participation combining outsider and insider perspectives. J Head Trauma Rehabil 2004;19:459–481 [DOI] [PubMed] [Google Scholar]
- 29.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollingshead AdB. Four Factor Index of Social Status. New Haven, CT, Yale University, Department of Sociology, 1975 [Google Scholar]
- 31.Pillay J, Armstrong MJ, Butalia S, et al. . Behavioral programs for type 1 diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2015;163:836–847 [DOI] [PubMed] [Google Scholar]
- 32.Pillay J, Armstrong MJ, Butalia S, et al. . Behavioral programs for type 2 diabetes mellitus: a systematic review and network meta-analysis. Ann Intern Med 2015;163:848–860 [DOI] [PubMed] [Google Scholar]
- 33.Holmes-Walker DJ, Llewellyn AC, Farrell K. A transition care programme which improves diabetes control and reduces hospital admission rates in young adults with type 1 diabetes aged 15-25 years. Diabet Med 2007;24:764–769 [DOI] [PubMed] [Google Scholar]
- 34.Sequeira PA, Pyatak EA, Weigensberg MJ, et al. . Let’s Empower and Prepare (LEAP): evaluation of a structured transition program for young adults with type 1 diabetes. Diabetes Care 2015;38:1412–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markowitz JT, Laffel LM. Transitions in care: support group for young adults with type 1 diabetes. Diabet Med 2012;29:522–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal S, Raymond JK, Schutta MH, Cardillo S, Miller VA, Long JA. An adult health care-based pediatric to adult transition program for emerging adults with type 1 diabetes. Diabetes Educ 2017;43:87–96 [DOI] [PubMed] [Google Scholar]
- 37.Steinbeck KS, Shrewsbury VA, Harvey V, et al. . A pilot randomized controlled trial of a post-discharge program to support emerging adults with type 1 diabetes mellitus transition from pediatric to adult care. Pediatr Diabetes 2015;16:634–639 [DOI] [PubMed] [Google Scholar]
- 38.Markowitz JT, Cousineau T, Franko DL, et al. . Text messaging intervention for teens and young adults with diabetes. J Diabetes Sci Technol 2014;8:1029–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berkowitz RI, Marcus MD, Anderson BJ, et al.; TODAY Study Group . Adherence to a lifestyle program for youth with type 2 diabetes and its association with treatment outcome in the TODAY clinical trial. Pediatr Diabetes 30 June 2017. [Epub ahead of print]. 10.1111/pedi.12555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raymond JK, Berget CL, Driscoll KA, Ketchum K, Cain C, Fred Thomas JF. CoYoT1 Clinic: innovative telemedicine care model for young adults with type 1 diabetes. Diabetes Technol Ther 2016;18:385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood W, Neal DT. Healthy through habit: interventions for initiating & maintaining health behavior change. Behavioral Sci Policy 2016;2:71–83 [Google Scholar]
- 42.Smalls BL, Gregory CM, Zoller JS, Egede LE. Assessing the relationship between neighborhood factors and diabetes related health outcomes and self-care behaviors. BMC Health Serv Res 2015;15:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhodes ET, Prosser LA, Hoerger TJ, Lieu T, Ludwig DS, Laffel LM. Estimated morbidity and mortality in adolescents and young adults diagnosed with type 2 diabetes mellitus. Diabet Med 2012;29:453–463 [DOI] [PubMed] [Google Scholar]