Abstract
OBJECTIVE
We performed a secondary analysis to evaluate the effect of the Women’s Health Initiative dietary intervention on incident diabetes and diabetes treatment in postmenopausal women.
RESEARCH DESIGN AND METHODS
A total of 48,835 women were randomized to a comparison group or an intervention group that underwent a behavioral/nutritional modification program to decrease fat and increase vegetable, fruit, and grain intake for an average of 8.1 years. Ninety-three percent of participants completed the intervention, and 71% participated in active follow-up through 30 September 2015 (median 17.3 years). We measured time to development of treated diabetes and progression from oral antihyperglycemic agents to insulin. Serum glucose and insulin were measured in a subsample of women (N = 2,324) at baseline and years 1, 3, and 6.
RESULTS
During the trial, intervention group women had lower rates of initiation of insulin therapy (hazard ratio [HR] 0.74 [95% CI 0.59, 0.94]; P = 0.01). Moreover, women with baseline waist circumference ≥88 cm (P interaction = 0.01) and worse metabolic syndrome scores (P interaction = 0.02) had the greatest reduction in risk of initiating insulin therapy. The decreased risk from the intervention was present during the cumulative follow-up (HR 0.88 [95% CI 0.78, 0.99]; P = 0.04). In participants with measured biomarkers (5.8% subsample) who had baseline glucose <100 mg/dL, the intervention reduced the risk of developing glucose ≥100 mg/dL by 25% (odds ratio 0.75 [95% CI 0.61, 0.93]; P = 0.008). Adjustment for weight change did not alter the results.
CONCLUSIONS
In this secondary analysis, a dietary intervention in postmenopausal women aimed at reducing fat and increasing intake of vegetables, fruits, and grains did not increase risk of diabetes and may have slowed progression.
Introduction
Diabetes incidence in the U.S. and throughout the world is increasing markedly (1–4). Diabetes is associated with multiple comorbidities, increased health care costs, and premature mortality (1,5,6).
A lower-fat diet, in addition to increased physical activity, has been shown to lower diabetes incidence in individuals with impaired glucose tolerance (7–9). The Women’s Health Initiative (WHI) Dietary Modification Trial (DMT) is the only large (n = 48,835), long-term, randomized low-fat, non–weight-loss diet trial. It was designed to study the effect of decreased fat intake (20% of calories) and increased vegetable/fruit (five servings per day) and grain (six servings per day) consumption on incidence of breast and colorectal cancers (10–12). Cardiovascular disease was a secondary end point, and data on diabetes were collected by self-report.
After the 8.1-year trial, a nonsignificant reduction in diabetes incidence was observed (13) in intervention versus comparison group women. In the earlier analysis, however, the outcome was broadly defined as the time from randomization to first reported use of a glucose-lowering oral agent or injected insulin (13) and did not explore the intervention’s impact on individual components of diabetes treatment as indicators of disease progression. For the current analysis, we separately investigated types of treatment and also had access to longitudinal summaries of glucose and medication histories at baseline and three other time points.
The objectives of this secondary analysis were to evaluate the effect of the WHI DMT intervention on specific diabetes-related outcomes during the intervention (8.1 years) and to assess the longer-term effects of the intervention on these outcomes during the postintervention follow-up (median [SD] 9.8 [1.5] years), for a cumulative follow-up of 17.3 years.
Research Design and Methods
The WHI DMT methods from randomization through the end of the trial intervention period (31 March 2005) have been presented (14–17). All participants were postmenopausal. The intervention group (40%) received a nutrition education and behavioral change program, participating in 18 group sessions and 1 individual session during the first year, with quarterly sessions for the duration of the intervention. The comparison group (60%) received only printed health-related materials. Adherence to the intervention goals was assessed by periodic food frequency questionnaires and, in a 5.8% subsample of DMT women, blood measures of fasting glucose (years 1, 3, and 6) (18). In the comparison group, no changes were observed from baseline to year 8.1 in diet composition. In the intervention group, mean fat intake as a fraction of energy intake decreased by 8.2%, with relatively similar decreases in saturated (2.9% kcal), monounsaturated (3.3% kcal), and polyunsaturated (1.5% kcal) fat. A 1.1-serving/day increase in vegetable/fruit intake, a 0.5-serving/day increase in grains, and an 8.1% increase in total carbohydrate intake occurred.
Change in physical activity was not a goal of the intervention. Recreational physical activity was assessed using a self-reported questionnaire (19). However, changes in self-reported physical activity and energy intake were not consistent with the small changes in measured weights. Therefore, we were unable to assess whether changes in physical activity and total energy influenced the findings in the current report.
At enrollment, prevalent diabetes was defined as a positive response to the question, “Did a doctor ever say that you had sugar diabetes or high blood sugar when you were not pregnant?” For the current analysis of diabetes progression among the 48,835 DMT participants, 2,947 women were excluded for prevalent diabetes, and 309 additional women were excluded based on reported insulin use during follow-up without report of preceding or concurrent use of oral agents. This also excluded any incident type 1 diabetes. For the current analysis, we focused on the 45,579 women without prevalent diabetes and 1,444 women who had diabetes at baseline and were taking oral agents (total of 47,023 women).
Incident diabetes during the trial was ascertained twice annually using a medical update questionnaire, in which participants were asked to report any new use of “pills for diabetes” or “insulin shots for diabetes.” These self-reports agreed with baseline fasting glucose measures in the 5.8% subsample and with a medication inventory obtained 1 year after randomization (15). Self-report was concordant with the medication inventory in 79% of self-reports (20).
These diabetes outcome ascertainment procedures also were applied annually during the posttrial extensions for the 81.1 and 86.3% of intervention and 84.4 and 86.2% of comparison group women who consented to continued active follow-up through 30 September 2010 and through 30 September 2015, respectively. Baseline characteristics of participants who consented to extended follow-up remained balanced between the two groups as previously reported (21).
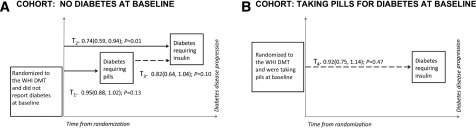
Our analyses focused on four time-to-response indices of diabetes development and/or progression (Fig. 1), none of which was a prespecified outcome of the DMT. The first three outcomes were applied to women without baseline diabetes, and the fourth was applied to women with baseline diabetes:
Figure 1.
Diabetes outcomes during the WHI DMT. A: Diabetes progression for the DMT participants, exclusive of women with diabetes at randomization. The reduced risk for insulin initiation, from randomization to first report of insulin, for intervention-group women (T2, HR 0.74) is shown in the component outcomes: T1, time from randomization to first report of pills for diabetes (HR 0.95), and T3, time from pills to first report of insulin (HR 0.82). B: Progression from oral agents to insulin for DMT participants with diabetes and taking oral agents at baseline.
T1. Time from randomization to first report of oral agents.
T2. Time from randomization to first report of insulin.
T3. Time from oral agent initiation to first report of insulin among women who reported first use of oral agents during the intervention.
T4. Time from randomization to first report of insulin among women with diabetes at baseline.
Statistical Analysis
With the exception of the T3 analyses, all analyses were intention-to-treat based on randomization groups. Cox regression models, stratified by baseline age and randomization status in the WHI hormone therapy trials, were used to evaluate the association between the dietary intervention and the four diabetes progression outcomes. For the T1, T2, and T4 analyses, all randomized participants were included, and participants lost to follow-up were censored at their last visit. Analyses over the cumulative intervention and postintervention phases were also stratified by study phase (time dependent). Results are reported as hazard ratios (HRs) and 95% CIs.
The first outcome (T1) (Fig. 1) was time from randomization to first report of oral agent use, with censoring at the end of the planned intervention (31 March 2005) or earlier if the participant died or was lost to follow-up.
The same procedures were used for the outcome of time from randomization to first report of insulin (T2) and for the outcome of time from first reported oral agent use to first reported insulin use (T3) (Fig. 1). However, the analysis for T3 included only the subcohort of women who began oral agents during the trial. Because the subcohort of women used to investigate the T3 outcome was not a randomized group, corresponding Cox models also were stratified by time from randomization to first use of oral agents (in quartiles) (Supplementary Table 1). A strategy similar to that for T1 and T2 was used to analyze time to first insulin use among women who reported using oral antidiabetic agents at baseline (T4).
For the longitudinal analysis of glucose, linear mixed-effects regression was used. All randomized participants in the random subsample in which glucose was measured were included in the analysis. For the longitudinal analysis of glucose as a binary variable (<100 vs. ≥100 mg/dL), general estimating equations were used, and all randomized participants in the subsample were included. Participants with missing follow-up measurements were included by fitting the model on all of the measurements that were observed.
Subsample analyses, defined according to prespecified baseline characteristics, including age, race/ethnicity, BMI, waist circumference, physical activity, baseline hypertension/cardiovascular disease (CVD), blood pressure, high cholesterol, and metabolic syndrome score (4,18), were performed. Statistical interactions between the dietary intervention and subgroup characteristics were tested using product terms of these factors in the models. All P values were two-sided (not corrected for multiple testing), and values of ≤0.05 were considered significant. For each outcome, the aforementioned subgroups were tested, so up to two (0.05 × 4 × 9) of the interactions were expected to be significant by chance alone. Statistical analyses were performed using SAS statistical software, version 9.3 (SAS Institute, Cary, NC).
Results
The cohort without diabetes was ethnically diverse, with an average age of 62 years, and included a range of education and income levels (16,18,22); 3.2% reported a history of CVD (Table 1). No significant differences were found between the randomization groups for any baseline characteristics (Table 1).
Table 1.
Baseline characteristics of WHI DMT participants, stratified by randomization group and baseline diabetes status
| Baseline characteristics | No diabetes1 (N = 45,579) |
P value3 | Diabetes2 (N = 1,444) |
P value3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention (N = 18,250) |
Comparison (N = 27,329) |
Intervention (N = 564) |
Comparison (N = 880) |
|||||||
| Age | 62.1 | (6.9) | 62.2 | (6.9) | 0.68 | 63.8 | (6.5) | 63.7 | (6.8) | 0.67 |
| Race/ethnicity | 0.94 | 0.39 | ||||||||
| White | 15,040 | 82.4 | 22,547 | 82.5 | 349 | 61.9 | 590 | 67.0 | ||
| Black | 1,812 | 9.9 | 2,694 | 9.9 | 143 | 25.4 | 196 | 22.3 | ||
| Hispanic | 679 | 3.7 | 1,004 | 3.7 | 37 | 6.6 | 43 | 4.9 | ||
| American Indian | 79 | 0.4 | 103 | 0.4 | 3 | 0.5 | 7 | 0.8 | ||
| Asian/Pacific Islander | 397 | 2.2 | 616 | 2.3 | 21 | 3.7 | 30 | 3.4 | ||
| Unknown | 243 | 1.3 | 365 | 1.3 | 11 | 2.0 | 14 | 1.6 | ||
| Smoking | 0.34 | 0.58 | ||||||||
| Never | 9,280 | 51.4 | 14,018 | 51.8 | 296 | 53.6 | 458 | 52.6 | ||
| Past | 7,563 | 41.9 | 11,169 | 41.3 | 219 | 39.7 | 364 | 41.8 | ||
| Current | 1,197 | 6.6 | 1,855 | 6.9 | 37 | 6.7 | 49 | 5.6 | ||
| Family history of adult diabetes | 5,676 | 32.8 | 8,625 | 33.3 | 0.25 | 353 | 67.1 | 551 | 67.8 | 0.80 |
| Baseline waist circumference ≥88 cm | 8,599 | 47.3 | 13,038 | 47.8 | 0.23 | 470 | 83.6 | 730 | 83.1 | 0.81 |
| Baseline hypertension/CVD status | 0.48 | 0.21 | ||||||||
| Normotensive/no CVD | 9,046 | 54.0 | 13,391 | 53.4 | 144 | 27.0 | 191 | 22.8 | ||
| Hypertensive/no CVD | 7,167 | 42.8 | 10,875 | 43.4 | 340 | 63.7 | 563 | 67.1 | ||
| Prior CVD | 527 | 3.1 | 795 | 3.2 | 50 | 9.4 | 85 | 10.1 | ||
| Baseline BP/hypertension status | 0.23 | 0.23 | ||||||||
| BP <140 and 90 mmHg; never treated | 9,190 | 56.8 | 13,625 | 56.2 | 151 | 29.4 | 205 | 25.2 | ||
| BP ≥140 or 90 mmHg; never treated | 1,652 | 10.2 | 2,414 | 10.0 | 34 | 6.6 | 52 | 6.4 | ||
| Ever treated for hypertension | 5,338 | 33.0 | 8,184 | 33.8 | 329 | 64.0 | 555 | 68.3 | ||
| History of high cholesterol requiring medication | 1,953 | 10.7 | 2,993 | 11.0 | 0.40 | 138 | 24.5 | 219 | 24.9 | 0.86 |
| Metabolic syndrome score4 | 0.30 | 0.53 | ||||||||
| 0 | 4,294 | 25.1 | 6,313 | 24.7 | 11 | 2.0 | 24 | 2.8 | ||
| 1 | 6,471 | 37.9 | 9,668 | 37.8 | 123 | 22.7 | 170 | 19.9 | ||
| 2 | 5,524 | 32.3 | 8,322 | 32.5 | 314 | 57.8 | 509 | 59.6 | ||
| 3 | 804 | 4.7 | 1,296 | 5.1 | 95 | 17.5 | 151 | 17.7 | ||
| HT trial arm | ||||||||||
| CEE | 544 | 3.0 | 917 | 3.4 | 0.55 | 41 | 7.3 | 58 | 6.6 | 0.93 |
| CEE placebo | 593 | 3.2 | 956 | 3.5 | 33 | 5.9 | 48 | 5.5 | ||
| CEE+MPA | 922 | 5.1 | 1,357 | 5.0 | 0.51 | 23 | 4.1 | 46 | 5.2 | 0.17 |
| CEE+MPA placebo | 855 | 4.7 | 1,208 | 4.4 | 35 | 6.2 | 44 | 5.0 | ||
| Total energy expenditure/week from physical activity (MET-h) | 10.2 | (11.8) | 10.3 | (12.1) | 0.45 | 7.5 | (9.7) | 7.7 | (10.8) | 0.70 |
| BMI (kg/m2) | 28.9 | (5.8) | 28.9 | (5.8) | 0.61 | 32.8 | (6.0) | 33.1 | (6.2) | 0.30 |
| Waist circumference (cm) | 88.3 | (13.5) | 88.3 | (13.4) | 0.86 | 100.2 | (13.0) | 100.8 | (13.5) | 0.43 |
| Systolic BP (mmHg) | 127.1 | (17.2) | 127.4 | (17.1) | 0.08 | 134.0 | (17.1) | 135.1 | (16.9) | 0.25 |
| Diastolic BP (mmHg) | 75.9 | (9.1) | 76.0 | (9.0) | 0.12 | 75.5 | (8.9) | 76.0 | (9.3) | 0.30 |
| LDL-C (mg/dL)5 | 133.2 | (35.3) | 134.9 | (36.2) | 0.26 | 132.6 | (33.9) | 127.1 | (33.6) | 0.40 |
| HDL-C (mg/dL)5 | 59.8 | (15.7) | 58.8 | (15.1) | 0.13 | 50.8 | (12.6) | 47.5 | (11.6) | 0.15 |
| Triglyceride (mg/dL), median (IQR)5 | 129.0 | (77.0) | 130.0 | (82.0) | 0.55 | 138.0 | (91.0) | 165.5 | (89.0) | 0.30 |
| Insulin (μIU/mL)5 | 10.0 | (6.9) | 10.0 | (7.1) | 0.53 | 13.9 | (11.9) | 15.0 | (9.2) | 0.66 |
| Glucose (mg/dL), median (IQR)5 | 93.0 | (15.0) | 93.0 | (12.0) | 0.59 | 174.5 | (65.0) | 168.5 | (79.0) | 0.67 |
Data are number and percent or mean (SD) unless otherwise indicated. BP, blood pressure; CEE, conjugated equine estrogen; HDL-C, HDL cholesterol; HT, hormone therapy; IQR, interquartile range; LDL-C, LDL cholesterol; MPA, medroxyprogesterone acetate.
1Full dietary trial cohort, exclusive of women with diabetes at baseline.
2Dietary trial participants with diabetes and taking oral agents at baseline.
3P value corresponds to χ2 tests (categorical variables), t tests (continuous variables; not skewed), or Wilcoxon rank tests (continuous variables; skewed); for skewed variables, median (IQR) is presented.
4Score ranges from 0 (best) to 3 (worst) and is a sum of these baseline indicators: waist >88 cm, high cholesterol requiring pills, or BP >130/85 mmHg (or ever treated for hypertension).
5Laboratory measurements based on a 5.8% subsample of trial participants; includes 2,469 participants without diabetes (966 vs. 1,503) and 112 participants with diabetes taking pills at baseline (46 vs. 66).
Figure 1 shows the four diabetes outcomes, stratified by baseline diabetes status during the intervention. Table 2 shows the results for the intervention period, as well as for the cumulative intervention plus the two postintervention extensions; summaries by each postintervention period are presented (Supplementary Table 2). Women in the intervention group without baseline diabetes were less likely to initiate insulin therapy (T2; HR 0.74 [95% CI 0.59, 0.94]; P = 0.01) during the trial. During the trial, data also suggested reduced rates of oral therapy initiation (T1; HR 0.95 [95% CI 0.88, 1.02]; P = 0.13) and progression from oral agents to insulin (T3; HR 0.82 [95% CI 0.64, 1.04]; P = 0.10). Among women using oral diabetes agents at baseline, the risk of insulin use was not reduced for women in the intervention versus comparison groups (T4; HR 0.92 [95% CI 0.75, 1.14]; P = 0.47); findings were similar during the trial and throughout the postintervention periods. In the subcohort with diabetes at randomization, analyses using the fasting blood glucose measures from the subsample and the medications inventory suggested that women in the intervention group who self-reported taking pills for diabetes tended to stop taking these medications more frequently than those in the comparison group, with significant evidence of differential changes in diabetes medication usage (P = 0.01 for cumulative years 1, 3, and 6).
Table 2.
Diabetes outcomes1 and measures of fasting glucose, WHI DMT, by study cohort and randomization group
| Cohort and diabetes outcome | Intervention |
Comparison |
HR (95% CI) | P value | ||
|---|---|---|---|---|---|---|
| N2 | % | N | % | |||
| No diabetes at baseline3 | ||||||
| Time to first report of pills (T1) | ||||||
| Intervention phase | 1,228 | 0.83 | 1,951 | 0.88 | 0.95 (0.88, 1.02) | 0.13 |
| Cumulative: intervention plus postintervention phases | 2,565 | 1.00 | 4,093 | 1.05 | 0.96 (0.91, 1.00) | 0.07 |
| No diabetes at baseline3 | ||||||
| Time to first report of insulin (T2) | ||||||
| Intervention phase | 102 | 0.07 | 207 | 0.09 | 0.74 (0.59, 0.94) | 0.01 |
| Cumulative: intervention plus postintervention phases | 394 | 0.15 | 691 | 0.17 | 0.88 (0.78, 0.99) | 0.04 |
| No diabetes at baseline, but later self-report of taking pills during follow-up4 | ||||||
| Time from pills to first report of insulin (T3) | ||||||
| Intervention phase | 102 | 2.63 | 207 | 3.17 | 0.82 (0.64, 1.04) | 0.10 |
| Cumulative: intervention plus postintervention phases | 394 | 2.56 | 691 | 2.80 | 0.95 (0.84, 1.09) | 0.49 |
| Taking pills for diabetes at baseline5 | ||||||
| Time to first report of insulin (T4) | ||||||
| Intervention phase | 140 | 3.60 | 229 | 3.82 | 0.92 (0.75, 1.14) | 0.47 |
| Cumulative: intervention plus postintervention phases | 206 | 3.62 | 358 | 4.07 | 0.89 (0.75, 1.06) | 0.21 |
| Measures of fasting glucose in subsample | N6 | N | Ratio of geometric means7 (95% CI) | |||
|---|---|---|---|---|---|---|
| No diabetes at baseline: ratio of geometric means for fasting glucose during follow-up (intervention phase) | 916 | 1,408 | 0.98 (0.98, 0.99) | <0.001 | ||
| OR8 (95% CI) | ||||||
|---|---|---|---|---|---|---|
| No diabetes at baseline and fasting glucose <100 mg/dL at baseline: risk of developing elevated glucose (≥100 mg/dL) during follow-up (intervention phase) | 641 | 1,030 | 0.75 (0.61, 0.93) | 0.008 | ||
1Summary statistics include counts of self-reported outcomes (annualized incidence rates), HRs (95% CI), and P values. Proportional hazard models were stratified by baseline age-group and randomization arm of the WHI hormone therapy trials. The model for T3 was also stratified by time from randomization to pills, defined by quartiles specific to each study period. For T1, T2, and T4, the time scale started at randomization for the intervention period or the start of each extension period. For T3, the time scale started at self-report of pills.
2Number of events (annualized percentages).
3Full dietary trial cohort, exclusive of women with diabetes at randomization.
4Participants, among the full dietary trial cohort exclusive of women with diabetes at randomization, who later self-reported first use of oral agents during the follow-up.
5Dietary trial participants with diabetes and taking oral agents at baseline.
6Number of participants with a baseline glucose measurement and at least one postrandomization measurement.
7Average ratio of geometric means during follow-up computed from a repeated-measures model with an unstructured variance-covariance matrix and adjusted for age, hormone therapy randomization group, and baseline glucose.
8Average OR during the trial from generalized estimating equations with an unstructured log-OR matrix and adjusted for age, hormone therapy randomization group, and baseline glucose.
In the 5.8% subsample, women without diabetes at baseline who were randomized to the intervention group demonstrated a 2% lower mean glucose than women randomized to the comparison group (94.9 vs. 96.3 mg/dL averaged over years 1, 3, and 6; P < 0.001) (Table 2). Among participants with glucose <100 mg/dL at baseline, the intervention was associated with a 25% reduced risk of developing glucose >100 mg/dL during the trial (odds ratio [OR] 0.75 [95% CI 0.61, 0.93]; P = 0.008).
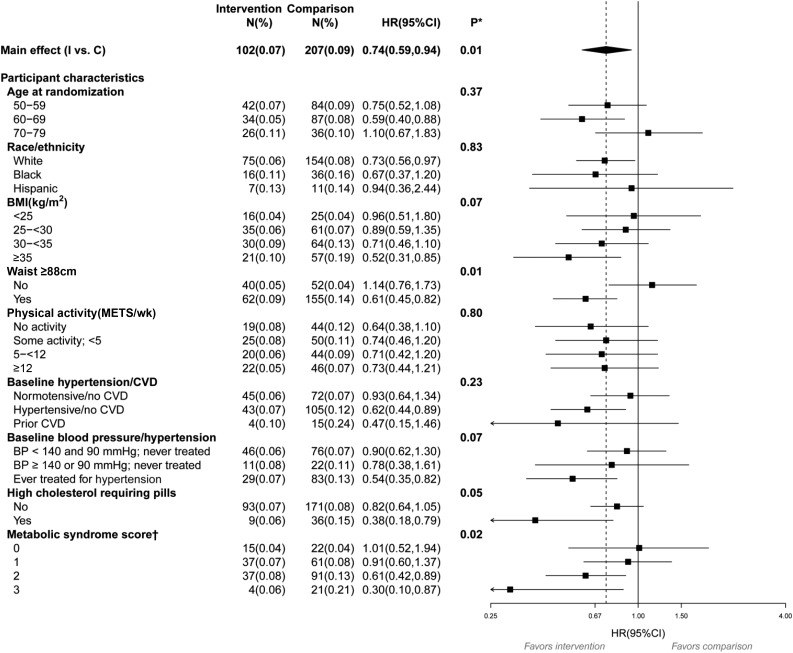
We analyzed the data on women without baseline diabetes by prespecified characteristics for each outcome, with the aim of hypothesis generation. For the first report of pills (T1) (Supplementary Fig. 1), no interactions were found. However, for the time from randomization to first report of insulin (T2) (Fig. 2), an interaction with waist circumference was observed, with the largest reduction occurring in those with a baseline value >88 cm (P = 0.01). An interaction also was observed with metabolic syndrome score, with each increase in the number of components at baseline associated with more improvement among those in the intervention group (P = 0.02). Of note, the above interactions observed for time from randomization to first report of insulin use (T2) were also observed for T3, time from first use of oral agents to first use of insulin among women who began taking pills during the trial (Supplementary Fig. 2).
Figure 2.
Subgroup analysis for the time from randomization to first report of insulin use (T2) during the WHI DMT in women without diabetes at randomization. BP, blood pressure; C, comparison; I, intervention; wk, week. *Statistical significance of subgroups is based on a test of the interaction between subgroup and randomization group. For the subgroups of age, BMI, physical activity, blood pressure/hypertension, and metabolic syndrome score, a 1-degree-of-freedom trend test of the interaction was used. †Score ranges from 0 (best) to 3 (worst) and is a sum of these binary components: waist circumference ≥88 cm, high cholesterol requiring pills, or blood pressure >130/85 mmHg (or ever treated for hypertension).
To determine whether the reduction in the number of women reporting first use of insulin in the intervention group (T2; HR 0.74 [95% CI 0.59, 0.94]; P = 0.01) could be attributed to weight loss associated with the intervention (16), we included baseline weight and postrandomization weight change (time-dependent variable) in our analyses of T2. Although higher baseline weight and weight gain were significant predictors of increased diabetes risk, the reduced risk seen at T2 for women in the intervention group slightly increased in significance with baseline weight and weight change in the model (HR 0.73 [95% CI 0.57, 0.93]; P = 0.009). We extended the above analyses to include weight change (time-dependent) in the analyses of the interaction with waist circumference. No appreciable changes were observed in HR estimates for waist circumference when weight change was added to the model (waist circumference <88 cm, HR 1.15 [95% CI 0.76, 1.74]; waist circumference ≥88 cm, HR 0.59 [95% CI 0.44, 0.79]; P < 0.01 for the interaction).
Conclusions
Among women without baseline diabetes participating in a dietary and behavioral modification aimed at consuming a low-fat diet with increased vegetables, fruits, and grains, we observed a decreased risk for initiating insulin therapy during 8.1 years of intervention. The effect was greatest in those with central obesity or higher metabolic syndrome score at baseline. Blood glucose data in a 5.8% subsample of women with measured values were consistent with these findings, with reduction in glucose among intervention-group women without diabetes at baseline and a 25% reduction in rate of conversion from normal glucose tolerance to impaired fasting glucose in intervention-group women during the trial. Overall, given multiple testing considerations, our results from this secondary analysis show that a low-fat diet does not increase diabetes risk and suggest that the dietary intervention improved glycemia and slowed diabetes progression.
Many short-term nutrition trials have been conducted among individuals with diabetes to explore the effects of various nutritional components and diet patterns on glucose control (23,24). However, to our knowledge, no previous long-term trial with a large number of participants has explored the effects of a dietary and behavioral modification intervention on development and progression of diabetes, as defined by changes in diabetes medications. Although this is a secondary analysis of a nonprespecified end point, our results support a hypothesis that a dietary and behavioral intervention aimed at reducing fat intake and promoting greater intake of vegetables, fruits, and grains may have a long-term positive effect on reducing the use of diabetes medications, especially in individuals with central obesity or insulin resistance, as reflected by the presence of metabolic syndrome components.
Notably, the women with baseline diabetes did not show significant improvements, but the medication data showed that women with baseline diabetes randomized to the intervention group decreased their use of diabetes medications compared with women with baseline diabetes randomized to the comparison group. This observation, which also was noted for statin use among women with baseline CVD who were randomized to the DMT intervention group (25), underscores an important message for those managing patients with chronic diseases: as they initiate diet therapy, even if weight loss occurs, the need to continue current medications should be evaluated, and regular monitoring of glycemic status should be stressed.
The WHI dietary intervention was multifaceted, resulting in changes in intake of most fatty acids and increased vegetable, fruit, and fiber intake. The observed results may be due to any or some combination of these changes. The major changes, however, were a reduction by ∼8% of total calories in fat intake and a corresponding increase by ∼8% of calories in carbohydrate intake. Our analyses suggest that this dietary change over a sustained time period did not increase, and may have reduced, diabetes incidence and progression.
The WHI DMT intervention has been demonstrated to contribute to small, but significant, weight loss (1.9 kg loss in the intervention group compared with the control group at year 1, diminishing to 0.4 kg at the end of the intervention) (16). In this analysis of progression of glucose intolerance, adjustment for weight loss had no impact on the findings in the overall group or in the subgroup analyses of women with or without central obesity, as measured by waist circumference. This observation suggests that the dietary intervention may have affected the sequence of events stemming from obesity and/or insulin resistance. Short-term studies could further explore this observation, especially if specific nutrients or diet patterns are identified as being associated with slowing glycemia progression.
This study has some important limitations. The principal limitation arises from the fact that the diabetes outcomes examined were not among the designated trial outcomes, which focused on cancers and coronary heart disease. Because multiple clinical outcomes were considered in a range of WHI papers, it is possible that some nominally significant association could emerge based on chance alone. Thus, the significance of trial comparisons should be cautiously interpreted.
Moreover, glucose measures were available only for a subset of participants, and medication inventories did not include detailed assessment of diabetes medication or frequency of physician monitoring or screening for diabetes. Prevailing diabetes treatments changed dramatically during the intervention period and do not represent current pharmacologic practices. For example, in women taking oral agents at baseline, metformin use tripled from baseline (17.5%) to year 6 (54.0%), thiazolidinedione use reached only 15% by year 6, and there was no reported usage of incretins or glucagon-like peptide 1 agonists. Finally, participants included only women aged 50–79 years at baseline, and although ethnically diverse, the majority were Caucasian. However, few data suggest that outcomes of nutritional interventions in adults with diabetes vary by age or sex.
This study has strengths, however, that make analysis of the available diabetes-related data valuable. The DMT followed >48,000 women for a median of 17.3 years. The intervention, although performed in a setting that can be translated to clinical practice, involved trained personnel with standardized intervention materials and systematic follow-up of diet intake and outcomes. Adherence to the diet, albeit through self-report, was demonstrated (15). All outcomes and physical measures were assessed using standardized techniques with rigorous quality control.
In summary, in this secondary analysis of a trial in postmenopausal women, randomization to a behavioral intervention that promoted a low-fat diet (including higher vegetable, fruit, and grain intake) did not increase risk of diabetes. Rather, it may have been associated with reductions in glycemia and with the rate of diabetes therapy initiation and progression. More long-term diet intervention trials would be valuable to confirm the findings from this secondary analysis and to explore related mechanisms.
Supplementary Material
Article Information
Acknowledgments. The authors thank Rachel Schaperow, MedStar Health Research Institute, for editing the manuscript.
Funding. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services, through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. Funding was also received from National Cancer Institute grants P01-CA-53996 and R01-CA-210921. Research reported in this work was supported in part by the National Center for Advancing Translational Science of the National Institutes of Health under award number UL1-TR001409.
The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. B.V.H. contributed to the data collection and wrote the manuscript. A.K.A. contributed to the data analysis and reviewed and edited the manuscript. L.F.T. contributed to the data collection, reviewed and edited the manuscript, and contributed to the discussion. M.A. contributed to the collection of data and reviewed and edited the manuscript. M.D.H. reviewed and edited the manuscript. K.C.J. contributed to the data collection and reviewed and edited the manuscript. J.E.M. contributed to the data collection, reviewed and edited the manuscript, and contributed to the discussion. A.H.S. reviewed and edited the manuscript. J.M.S. reviewed and edited the manuscript. L.G.S. reviewed and edited the manuscript. C.A.T. contributed to the data collection, reviewed and edited the manuscript, and contributed to the discussion. O.Z. reviewed and edited the manuscript. R.L.P. contributed to the data collection, helped formulate the analytical approach, and reviewed and edited the manuscript. B.V.H. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00000611, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-0534/-/DC1.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetes Atlas, 8th ed [Internet], 2013. Available from http://www.idf.org/diabetesatlas. Accessed 5 February 2018
- 3.Centers for Disease Control and Prevention. National Diabetes Statistics Report [Internet], 2017. Available from https://www.cdc.gov/diabetes/data/statistics/statistics-report.html. Accessed 5 February 2018
- 4.World Health Organization. Diabetes [Internet]. Available from http://www.who.int/diabetes/en/. Accessed 18 December 2017
- 5.Centers for Disease Control and Prevention Diabetes Report Card 2012. Atlanta, GA, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, 2012 [Google Scholar]
- 6.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med 2007;147:149–155 [DOI] [PubMed] [Google Scholar]
- 7.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 8.Tuomilehto J, Lindström J, Eriksson JG, et al.; Finnish Diabetes Prevention Study Group . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 9.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Women’s Health Initiative Study Group Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials 1998;19:61–109 [DOI] [PubMed] [Google Scholar]
- 11.Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA 1991;266:93–98 [PubMed] [Google Scholar]
- 12.Tinker LF, Burrows ER, Henry H, Patterson R, Rupp L, Van Horn L. The Women’s Health Initiative: overview of the nutrition components. In Nutrition in Women’s Health. Krummel D, Kris-Etherton P, Eds. Gaithersburg, MD, Aspen Publishers, 1996, p. 510–542 [Google Scholar]
- 13.Tinker LF, Bonds DE, Margolis KL, et al.; Women’s Health Initiative . Low-fat dietary pattern and risk of treated diabetes mellitus in postmenopausal women: the Women’s Health Initiative randomized controlled Dietary Modification Trial. Arch Intern Med 2008;168:1500–1511 [DOI] [PubMed] [Google Scholar]
- 14.Ritenbaugh C, Patterson RE, Chlebowski RT, et al. The Women’s Health Initiative Dietary Modification trial: overview and baseline characteristics of participants. Ann Epidemiol 2003;13(Suppl.):S87–S97 [DOI] [PubMed] [Google Scholar]
- 15.Patterson RE, Kristal A, Rodabough R, et al. Changes in food sources of dietary fat in response to an intensive low-fat dietary intervention: early results from the Women’s Health Initiative. J Am Diet Assoc 2003;103:454–460 [DOI] [PubMed] [Google Scholar]
- 16.Howard BV, Manson JE, Stefanick ML, et al. Low-fat dietary pattern and weight change over 7 years: the Women’s Health Initiative Dietary Modification Trial. JAMA 2006;295:39–49 [DOI] [PubMed] [Google Scholar]
- 17.Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295:629–642 [DOI] [PubMed] [Google Scholar]
- 18.Howard BV, Van Horn L, Hsia J, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295:655–666 [DOI] [PubMed] [Google Scholar]
- 19.Meyer AM, Evenson KR, Morimoto L, Siscovick D, White E. Test-retest reliability of the Women’s Health Initiative physical activity questionnaire. Med Sci Sports Exerc 2009;41:530–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margolis KL, Lihong Qi, Brzyski R, et al.; Women’s Health Initiative Investigators . Validity of diabetes self-reports in the Women’s Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials 2008;5:240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomson CA, Van Horn L, Caan BJ, et al. Cancer incidence and mortality during the intervention and postintervention periods of the Women’s Health Initiative dietary modification trial. Cancer Epidemiol Biomarkers Prev 2014;23:2924–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beresford SAA, Johnson KC, Ritenbaugh C, et al. Low-fat dietary pattern and risk of colorectal cancer: the Women’s Health Initiative randomized controlled Dietary Modification Trial. JAMA 2006;295:643–654 [DOI] [PubMed] [Google Scholar]
- 23.Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2014;37(Suppl. 1):S120–S143 [DOI] [PubMed] [Google Scholar]
- 24.Feinglos MN, Totten SE. Are you what you eat, or how much you eat? The case of type 2 diabetes mellitus. Arch Intern Med 2008;168:1485–1486 DOI: 10.1001/archinte.168.14.1485 [DOI] [PubMed] [Google Scholar]
- 25.Prentice RL, Aragaki AK, Van Horn L, et al. Low-fat dietary pattern and cardiovascular disease: results from the Women’s Health Initiative randomized controlled trial. Am J Clin Nutr 2017;106:35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.