Abstract
OBJECTIVE
To compare the effect of Roux-en-Y gastric bypass (RYGB) surgery versus intensive medical diabetes and weight management (IMWM) on clinical and patient-reported outcomes in obese patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
We prospectively randomized 38 obese patients with type 2 diabetes (15 male and 23 female, with mean ± SD weight 104 ± 16 kg, BMI 36.3 ± 3.4 kg/m2, age 52 ± 6 years, and HbA1c 8.5 ± 1.3% [69 ± 14 mmol/mol]) to laparoscopic RYGB (n = 19) or IMWM (n = 19). Changes in weight, HbA1c, cardiovascular risk factors (UKPDS risk engine), and self-reported health status (the 36-Item Short-Form [SF-36] survey, Impact of Weight on Quality of Life [IWQOL] instrument, and Problem Areas in Diabetes Survey [PAID]) were assessed.
RESULTS
After 3 years, the RYGB group had greater weight loss (mean −24.9 kg [95% CI −29.5, −20.4] vs. −5.2 [−10.3, −0.2]; P < 0.001) and lowering of HbA1c (−1.79% [−2.38, −1.20] vs. −0.39% [−1.06, 0.28] [−19.6 mmol/mol {95% CI −26.0, −13.1} vs. −4.3 {−11.6, 3.1}]; P < 0.001) compared with the IMWM group. Changes in cardiometabolic risk for coronary heart disease and stroke were all more favorable in RYGB versus IMWM (P < 0.05 to P < 0.01). IWQOL improved more after RYGB (P < 0.001), primarily due to subscales of physical function, self-esteem, and work performance. SF-36 and PAID scores improved in both groups, with no difference between treatments. A structural equation model demonstrated that improvement in overall quality of life was more strongly associated with weight loss than with improved HbA1c and was manifest by greater improvements in IWQOL than with either SF-36 or PAID.
CONCLUSIONS
Three years after randomization to RYGB versus IMWM, surgery produced greater weight loss, lower HbA1c, reduced cardiovascular risk, and improvements in obesity-related quality of life in obese patients with type 2 diabetes.
Introduction
Weight loss in obese patients with type 2 diabetes often results in improved glycemic control and a reduction in cardiovascular risk factors such as hypertension and dyslipidemia. However, <10% of patients are able to maintain substantial weight loss using traditional dietary and lifestyle modification methods (1–3).
In addition to these adverse metabolic and clinical outcomes, both obesity and type 2 diabetes adversely affect patients’ self-reported health status and quality of life. Overweight and obese individuals report more physical and psychological distress that is typically associated with the severity of obesity. In type 2 diabetes, reduced health status and quality of life are often associated with the symptoms of poor glycemic control, glycemic variability, insulin use, and the presence of diabetes complications. Both conditions are associated with reduced activity, depressive symptoms, increased pain, impaired sexual function, poor sleep, and reduced work performance and social interaction (4–6).
Recent clinical trials and cohort studies have demonstrated that bariatric surgery is highly effective in promoting sustained weight loss, improving glycemic control, and reducing cardiometabolic risk factors (7–12). Results are particularly impressive after Roux-en-Y gastric bypass (RYGB) surgery, although benefits are also observed after the gastric sleeve, laparoscopic adjustable gastric band, and other procedures. However, few prospective randomized studies have compared patient-reported outcomes after randomization to bariatric surgery versus an intensive medical management program to determine whether the approach to improve metabolic health is an important determinant of changes in self-reported health status, quality of life, and problems associated with managing diabetes.
Here, we report the 3-year results of the Surgery or Lifestyle With Intensive Medical Management in the Treatment of Type 2 Diabetes (SLIMM-T2D) study—a prospective, randomized, controlled clinical trial designed to assess the comparative effectiveness of bariatric surgery with medical management to improve glycemic control, reduce cardiovascular risk, and enhance patient-reported health status and quality of life in obese patients with type 2 diabetes (10). We compared RYGB with an intensive multidisciplinary medical diabetes- and weight-management program that incorporates diabetes medication adjustment to facilitate weight loss, structured modified dietary intervention, an individualized exercise program, cognitive behavioral intervention, and group education. Both interventions are clinically available at our institution and comparable at many others, suggesting that the improvements in the outcomes that we observed could be readily incorporated into standard medical practice.
Research Design and Methods
Study Overview
The rationale, design, and methods, including recruitment, inclusion/exclusion criteria, randomization, intervention, and assessments through 1 year, have previously been published (10). In brief, the SLIMM-T2D study was a randomized, parallel-group clinical trial to assess the feasibility of methods to conduct a larger multisite trial comparing the long-term effect of bariatric surgery versus medical management to improve glycemic control, cardiometabolic risk, and health outcomes in obese patients with type 2 diabetes (clinicaltrials.gov identifier NCT01073020). The study conformed to the Consolidated Standards of Reporting Trials (CONSORT) statement, and a diagram showing enrollment, randomization, and retention of the study participants is presented in Supplementary Data. The protocol was approved by the Partners HealthCare institutional review board, and an independent data-monitoring committee reviewed patient safety.
Major eligibility criteria included: 1) age 21–65 years for male or female sex; 2) diagnosis of type 2 diabetes for at least 1 year; 3) BMI of 30–42 kg/m2; 4) HbA1c ≥7.0% (53 mmol/mol) regardless of ongoing treatment, or ≥6.5% (48 mmol/mol) while receiving either two oral antihyperglycemic agents, at greater than or equal to half-maximal dose, or insulin, with a stable medication regimen for >8 weeks; and 5) no clinical or symptomatic evidence of significant cardiovascular or other diseases prohibiting safely exercising or undergoing a RYGB. Individuals were excluded if they had detectable levels of anti-GAD antibodies, a history of diabetic ketoacidosis, uncontrolled type 2 diabetes (HbA1c >12% [>108 mmol/mol]), gastrointestinal disease, malignant disease within 5 years, significant cardiopulmonary or renal disease, an active eating disorder, impaired mental status, weight loss >3% within the previous 3 months, abused drugs/alcohol, participated in another weight-reduction program, or were using weight-reducing medications and/or supplements. Participants had to be nonsmoking for >2 months. Additional information on the full inclusion and exclusion criteria has previously been published (10).
Patients with a preference for a bariatric procedure other than RYGB were not enrolled. The RYGB procedure was performed at Brigham and Women’s Hospital using standard operative protocols (10).
Participants randomized to the medical arm of the study enrolled in the Why WAIT (Weight Achievement and Intensive Treatment) program, which is designed for clinical practice and conducted quarterly at the Joslin Diabetes Center for groups of 10–15 patients (13). The multidisciplinary approach includes an endocrinologist, registered dietitian, exercise physiologist, mental health provider, and certified diabetes nurse educator. Two-hour group sessions are conducted weekly during a 12-week initiation phase in which patients receive individual medication adjustments and participate in supervised group exercise and didactic sessions. Key aspects of Why WAIT include weekly medication adjustments; structured modified dietary intervention with a hypocaloric (1,500–1,800 kcal) diet; up to 300 min/week of graded, balanced, and individualized exercise with emphasis on strength training; cognitive behavioral therapy; and group education. Antidiabetes medications were adjusted according to an algorithm designed to reduce or eliminate medications known to be associated with weight gain or hypoglycemia while initiating or increasing doses of medications that are weight neutral (10,13). None of the patients received anti-obesity medications during the study. A maintenance phase of individual monthly counseling is provided for the next 9 months, for a total intervention period of 1 year.
Randomization was computer-generated in centrally allocated blocks of four, stratified by BMI ≥35 and <35 kg/m2. Both groups returned to usual care following intervention and follow-up after 1 year was observational.
Metabolic Outcomes
Metabolic assessments were performed at baseline and repeated at 10% of initial body weight loss to obtain measurements at a comparable level of weight loss in both groups. If 10% weight loss did not occur by 3 months, metabolic assessments were performed at that time. Metabolic visits were also conducted at 12, 18, 24, and 36 months to obtain a time-based comparison. Assessments included weight, height, waist circumference, seated blood pressure using an automated device (BP742; Omron Healthcare), and medication doses. Clinical laboratory tests (performed by Quest Diagnostics) included HbA1c, fasting plasma glucose, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, microalbuminuria, renal function, liver function, and hematology. Body composition was assessed by bioelectrical impedance (TBF-215; Tanita Corporation). A 6-min walk test was performed. The UK Prospective Diabetes Study (UKPDS) Risk Engine was used to calculate risk of fatal and nonfatal cardiovascular events and stroke (14).
Patient-Reported Outcomes
Self-reported health outcomes were determined using the following validated instruments: 1) the 36-Item Short-Form (SF-36) survey, a generic health status instrument comprising two component scores (physical health and mental health) and eight scales (physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health) (15); 2) Impact of Weight on Quality of Life (IWQOL)-Lite, a 31-item disease-specific quality of life instrument comprising five scales (physical function, self-esteem, sexual life, public distress, and work), for which higher scores indicate greater impact (5); and 3) Problem Areas in Diabetes Survey (PAID), a 20-item questionnaire that assesses difficulty with diabetes self-management, emotional distress, eating behaviors, and other issues related to diabetes management, for which a higher score indicates more problems (16).
A structural equation model was developed to examine the effects of changes in weight and HbA1c on changes in the latent construct “quality of life.” Previous studies have demonstrated that improvements in glycemia, particularly reduction in HbA1c and glucose variability, lead to improved quality of life (17,18), but the relative contributions of weight loss versus improved glycemic control to the improvement in quality of life are not clear. Change in quality of life was measured as the differences between baseline and 1 year values in the total scores on the SF-36, PAID, and IWQOL instruments.
Statistical Analyses
The primary outcome was achievement of glycemic goal, defined as fasting plasma glucose levels <126 mg/dL and HbA1c <6.5% (48 mmol/mol) at 1 year of follow-up, regardless of whether patients were using pharmacological interventions. Longer time interval observational follow-up was conducted to assess durability of effects and emergent differences. The primary analysis was intention to treat and involved all randomly assigned patients who received at least one postrandomization assessment. Follow-up was prespecified to be censored at the time of bariatric surgery for those who were randomized to medical intervention but subsequently underwent surgery. Sample size was estimated assuming that RYGB would result in resolution of hyperglycemia in 80% of the patients and medical management in 20%. Twenty participants per group provided 97% power to detect a significant difference between groups with α = 0.05. Baseline results are presented as mean ± SD and outcome data as mean (95% CI) or median (interquartile range).
Dichotomous and continuous variables were analyzed using logistic regression and longitudinal linear mixed-effects models, respectively, to test the null hypotheses of equal resolution between groups of the respective outcome adjusting for baseline, unless noted otherwise. Dichotomous end points were considered to have not been attained if data were missing. Fisher exact test was used if fewer than five events were expected for a specific clinical end point. For the mixed-effects model, the fixed effects were “time” and “treatment group,” both of which were treated as categorical, and the random effect was “patient.” Where data are presented as a change from baseline, the baseline value was included as a covariate. Because our primary hypothesis involved comparisons between the two groups, we present the P value for the “treatment group” effect in the table and have used a footnote to designate when the “time × treatment” interaction effect was also significant. The within-group change from baseline was a prespecified contrast at each time point obtained from the mixed-effects model.
Results
Participants
A detailed description of the screening, enrollment, randomization, intervention, and retention procedures has previously been published (10). Briefly, 822 potential participants received a telephone screening interview, 148 subsequently attended an orientation session, and 93 underwent full medical screening. Forty-three participants were randomized to surgical (RYGB [n = 22]) or medical (intensive medical diabetes and weight management [IMWM] [n = 21]) therapy. Before any intervention, three participants withdrew consent, one received a diagnosis of breast cancer, and one received a diagnosis of severe depression; these five individuals were not included in the baseline data or any analyses. Nineteen patients were included for analysis in each group. Baseline demographics are presented in Table 1. The two groups were similar in age, sex distribution, race/ethnicity, proportion with BMI <35 kg/m2, and duration of diabetes.
Table 1.
Demographic, clinical, and laboratory data in the study subjects
| Baseline* |
1 year |
2 years |
3 years |
P | |||||
|---|---|---|---|---|---|---|---|---|---|
| RYGB | IMWM | RYGB | IMWM | RYGB | IMWM | RYGB | IMWM | ||
| N | 19 | 19 | |||||||
| Age, years | 50.7 ± 7.6 | 52.6 ± 4.3 | |||||||
| Sex | |||||||||
| Male | 6 (32) | 9 (47) | |||||||
| Female | 13 (68) | 10 (53) | |||||||
| Race/ethnicity | |||||||||
| White | 14 (74) | 10 (53) | |||||||
| African American | 3 (16) | 8 (42) | |||||||
| Asian | 1 (5) | 0 (0) | |||||||
| Hispanica | 1 (5) | 1 (5) | |||||||
| BMI <35 kg/m2 | 6 (32) | 7 (37) | |||||||
| Duration of diabetes, years | 10.6 ± 6.6 | 10.2 ± 6.1 | |||||||
| Clinical end points, n (%)b |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study-defined diabetes resolutionc | 11 (58) | 3 (16) | 7 (37) | 0 (0) | 8 (42) | 0 (0) | 0.005 | ||
| Meeting ADA treatment goals | |||||||||
| HbA1c <7.0% (<53 mmol/mol) | 15 (79) | 5 (26) | 13 (68) | 5 (26) | 11 (58) | 2 (11) | 0.011 | ||
| Direct LDL cholesterol <100 mg/dL | 15 (79) | 9 (47) | 10 (53) | 8 (42) | 8 (42) | 6 (32) | 0.300 | ||
| Systolic blood pressure <130 mmHg | 16 (84) | 11 (58) | 14 (74) | 5 (26) | 8 (42) | 6 (32) | 0.300 | ||
| Meeting all three goals | 11 (58) | 1 (5) | 7 (37) | 3 (16) | 2 (11) | 1 (5) | 0.293 | ||
| Normoglycemia | |||||||||
| HbA1c <6.0% (<42 mmol/mol) | 6 (32) | 0 (0) | 4 (21) | 0 (0) | 2 (11) | 0 (0) | 0.104 | ||
| Fasting plasma glucose <100 mg/dL | 14 (74) | 3 (16) | 8 (42) | 2 (11) | 7 (37) | 3 (16) | 0.216 | ||
| Meeting both criteria | 6 (32) | 0 (0) | 3 (16) | 0 (0) | 1 (5) | 0 (0) | 0.184 | ||
| Changes in clinical, metabolic, and laboratory measures, mean (95% CI)d |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Medications | |||||||||
| Antidiabetes | 2.3 ± 1.0 | 1.8 ± 0.8 | −1.6 (−2.0, −1.3) | 0.1 (−0.3, 0.4) | −1.4 (−1.8, −1.1) | 0.4 (0.0, 0.8) | −1.4 (−1.9, −1.0) | 0.7 (0.2, 1.2) | <0.001 |
| Antihypertensive | 1.6 ± 1.0 | 1.2 ± 1.0 | −0.8 (−1.2, −0.5) | 0.0 (−0.4, 0.3) | −1.0 (−1.3, −0.6) | 0.0 (−0.4, 0.4) | −0.9 (−1.3, −0.5) | 0.2 (−0.2, 0.7) | <0.001 |
| Lipid lowering | 0.9 ± 0.5 | 0.8 ± 0.5 | −0.6 (−0.8, −0.3) | 0.2 (0.0, 0.4) | −0.4 (−0.7, −0.2) | 0.0 (−0.3, 0.2) | −0.5 (−0.8, −0.3) | 0.0 (−0.2, 0.3) | <0.001e |
| Weight, kg | 104.6 ± 15.5 | 102.7 ± 17.0 | −27.9 (−30.2, −25.6) | −6.9 (−9.3, −4.6) | −26.3 (−29.6, −22.9) | −4.8 (−8.6, −1.0) | −24.9 (−29.5, −20.4) | −5.2 (−10.3, −0.2) | <0.001e |
| BMI, kg/m2 | 36.0 ± 3.5 | 36.5 ± 3.4 | −9.7 (−10.5, −8.8) | −2.3 (−3.1, −1.4) | −9.2 (−10.3, −8.0) | −1.6 (−2.9, −0.2) | −8.7 (−10.3, −7.1) | −1.8 (−3.5, 0.0) | <0.001e |
| Body composition | |||||||||
| Fat mass, kg | 45.5 ± 9.4 | 42.6 ± 9.8 | −22.6 (−25.0, −20.2) | −6.0 (−8.6, −3.4) | −21.4 (−24.6, −18.2) | −3.0 (−6.7, 0.7) | −19.9 (−24.0, −15.8) | −4.1 (−8.8, 0.6) | <0.001e |
| Lean mass, kg | 59.2 ± 14.1 | 60.1 ± 10.8 | −5.2 (−6.7, −3.6) | −1.3 (−2.9, 0.3) | −5.2 (−6.9, −3.5) | −1.9 (−3.8, 0.1) | −5.8 (−7.8, −3.8) | −1.3 (−3.5, 1.0) | 0.003e |
| Waist circumference, cm | 117.8 ± 14.9 | 114.1 ± 12.2 | −26.9 (−30.5, −23.4) | −6.4 (−10.1, −2.6) | −27.4 (−32.1, −22.6) | −5.4 (−10.9, 0.1) | −24.8 (−31.0, −18.6) | −1.0 (−8.2, 6.2) | <0.001e |
| Blood pressure | |||||||||
| Systolic, mmHg | 132.8 ± 10.5 | 126.3 ± 14.7 | −13.1 (−19.0, −7.1) | −1.6 (−7.9, 4.8) | −10.7 (−17.5, −3.8) | 4.2 (−4.1, 12.4) | −0.3 (−8.3, 7.8) | 9.7 (0.3, 19.1) | 0.011 |
| Diastolic, mmHg | 81.7 ± 7.4 | 76.6 ± 8.8 | −5.3 (−8.6, −2.0) | −2.4 (−5.9, 1.1) | −4.3 (−7.8, −0.7) | 0.6 (−3.7, 4.9) | 2.0 (−2.0, 5.9) | −1.2 (−5.8, 3.5) | <0.001f |
| Physical fitness | |||||||||
| Distance walked in 6 min, m | 464 ± 56 | 467 ± 56 | 13 (−11, 37) | 25 (0, 50) | 34 (5, 62) | 32 (−1, 65) | 33 (−1, 68) | 37 (−4, 79) | 0.548 |
| Heart rate recovery at 1 min, bpm | 92.2 ± 15.2 | 87.5 ± 12.0 | −10.7 (−15.4, −6.1) | 0.9 (−4.0, 5.9) | −5.7 (−11.3, −0.2) | 2.7 (−4.0, 9.4) | −7.0 (−13.6, −0.4) | 1.4 (−6.9, 9.7) | 0.002 |
| 10-year UKPDS risk scores | |||||||||
| CHD, % | 9.8 ± 9.6 | 10.8 ± 6.9 | −4.1 (−5.7, −2.5) | 0.5 (−1.1, 2.0) | −2.6 (−4.3, −0.8) | −0.7 (−2.6, 1.2) | −2.5 (−4.3, −0.7) | 0.1 (−2.0, 2.1) | 0.009e |
| Fatal CHD, % | 6.5 ± 7.7 | 6.9 ± 4.9 | −3.0 (−4.3, −1.7) | 0.4 (−0.9, 1.8) | −1.8 (−3.3, −0.3) | 0.1 (−1.5, 1.7) | −1.5 (−3.2, 0.2) | 1.1 (−0.8, 2.9) | 0.012 |
| Stroke, % | 4.0 ± 4.1 | 4.0 ± 2.3 | −0.2 (−0.6, 0.2) | 0.6 (0.2, 1.0) | 0.6 (0.1, 1.1) | 1.2 (0.6, 1.8) | 1.4 (0.8, 2.0) | 2.2 (1.5, 2.9) | 0.024 |
| Fatal stroke, % | 0.6 ± 0.6 | 0.5 ± 0.3 | −0.1 (−0.2, 0.0) | 0.1 (0.0, 0.2) | 0.0 (−0.2, 0.1) | 0.2 (0.0, 0.4) | 0.2 (0.0, 0.3) | 0.5 (0.3, 0.7) | 0.004 |
| Laboratory measurements | |||||||||
| HbA1c, % | 8.24 ± 1.42 | 8.78 ± 1.02 | −1.97 (−2.52, −1.41) | −0.09 (−0.66, 0.47) | −1.91 (−2.49, −1.33) | −0.32 (−0.99, 0.35) | −1.79 (−2.38, −1.20) | −0.39 (−1.06, 0.28) | <0.001e |
| HbA1c, mmol/mol | 66.6 ± 15.5 | 72.5 ± 11.1 | −21.5 (−27.5, −15.4) | −1.0 (−7.2, 5.1) | −20.9 (−27.2, −14.5) | −3.5 (−10.8, 3.8) | −19.6 (−26.0, −13.1) | −4.3 (−11.6, 3.1) | <0.001e |
| Fasting plasma glucose, mg/dL | 132 ± 50 | 162 ± 54 | −47 (−62, −32) | −3 (−19, 13) | −47 (−64, −31) | −11 (−30, 8) | −46 (−62, −29) | −9 (−29, 10) | <0.001e |
| Total cholesterol, mg/dL | 154 ± 34 | 162 ± 39 | −4 (−18, 10) | 7 (−7, 22) | 7 (−8, 24) | −16 (−35, 3) | 5 (−12, 33) | −12 (−33, 9) | 0.022f |
| Direct LDL cholesterol, mg/dL | 88 ± 28 | 99 ± 29 | −6 (−18, 6) | 9 (−4, 21) | 5 (−9, 19) | −15 (−32, 2) | 3 (−13, 19) | −11 (−30, 8) | 0.037f |
| HDL cholesterol, mg/dL | 44 ± 10 | 39 ± 10 | 10 (7, 13) | 0 (−3, 4) | 12 (7, 16) | −1 (−6, 5) | 15 (10, 21) | 2 (−4, 9) | <0.001e |
| Triglycerides, mg/dL | 120 ± 66 | 156 ± 76 | −46 (−61, −30) | −6 (−23, 10) | −42 (−58, −25) | 6 (−13, 26) | −39 (−55, −22) | −22 (−42, −2) | <0.001e |
| Creatinine, mg/dL | 0.71 ± 0.14 | 0.86 ± 0.21 | −0.06 (−0.10, −0.02) | 0.00 (−0.04, 0.04) | −0.06 (−0.11, −0.01) | 0.07 (0.01, 0.13) | 0.03 (−0.03, 0.10) | 0.05 (−0.02, 0.12) | 0.011e |
| Urine albumin/creatinine, μg/mgg | 3 (0–7) | 3 (0–10) | 4 (2–7) | 3.5 (0–4) | 5 (2.5–15) | 6.5 (4–17) | 6 (3–9) | 6.5 (0–8) | 0.873h |
| Hematocrit, % | 36.8 ± 3.3 | 40.2 ± 4.3 | −2.4 (−3.6, −1.2) | 0.4 (−0.9, 1.6) | −3.0 (−4.4, −1.7) | 1.3 (−0.4, 2.9) | −2.6 (−4.2, −1.0) | 1.0 (−0.8, 2.9) | <0.001 |
| White blood count, ×106/mL | 6.8 ± 2.1 | 6.5 ± 1.8 | −0.9 (−1.4, −0.4) | 0.1 (−0.5, 0.6) | −1.1 (−1.7, −0.5) | 0.1 (−0.6, 0.9) | −0.9 (−1.6, −0.2) | 0.4 (−0.4, 1.2) | <0.001 |
| ALT, IU/L | 32 ± 16 | 27 ± 12 | −10 (−14, −7) | −5 (−9, −1) | −13 (−18, −9) | −9 (−14, −4) | −15 (−19, −10) | −7 (−12, −1) | 0.023 |
| AST, IU/L | 31 ± 22 | 23 ± 13 | −6 (−9, −2) | −4 (−7, 0) | −7 (−11, −4) | −8 (−12, −4) | −8 (−12, −5) | −6 (−11, −2) | 0.767 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
*Baseline data are mean ± SD, n (%), or median (interquartile range), unless otherwise stated.
aHispanic subjects may be any race.
bP values represent comparison of proportions at 3 years (RYGB vs. IMWM) by Fisher exact test.
cPrimary end point, defined as proportion with HbA1c <6.5% and fasting plasma glucose <126 mg/dL with or without antidiabetes medication.
dP values represent differences between groups (RYGB vs. IMWM) from linear mixed-effects model adjusted for baseline values, unless otherwise noted.
eGroup × time interaction also significant at P < 0.05.
fP value represents group × time interaction; group effect was not significant.
gMedian and interquartile range provided due to skewed distribution.
hBy Kruskal-Wallis test for nonparametric data.
Primary End Point at 3 Years
After 3 years, eight patients (42%) in the RYGB group achieved study-defined glycemic goals (HbA1c <6.5% [<48 mmol/mol] and fasting plasma glucose <126 mg/dL), and seven of these eight patients were receiving no antidiabetes medications at that time. In contrast, no patients in the IMWM group achieved this glycemic end point (P = 0.005 vs. RYGB) (Table 1).
Clinical, Metabolic, and Laboratory Measures
In evaluation of additional key metrics of clinical interest, the surgical group also had a higher percentage of patients achieving HbA1c <7.0% (<53 mmol/mol) (58% vs. 11%; P = 0.011). Rates of LDL cholesterol <100 mg/dL, systolic blood pressure <130 mmHg, and additional definitions of normoglycemia based on fasting glucose or HbA1c alone were not different between groups (Table 1).
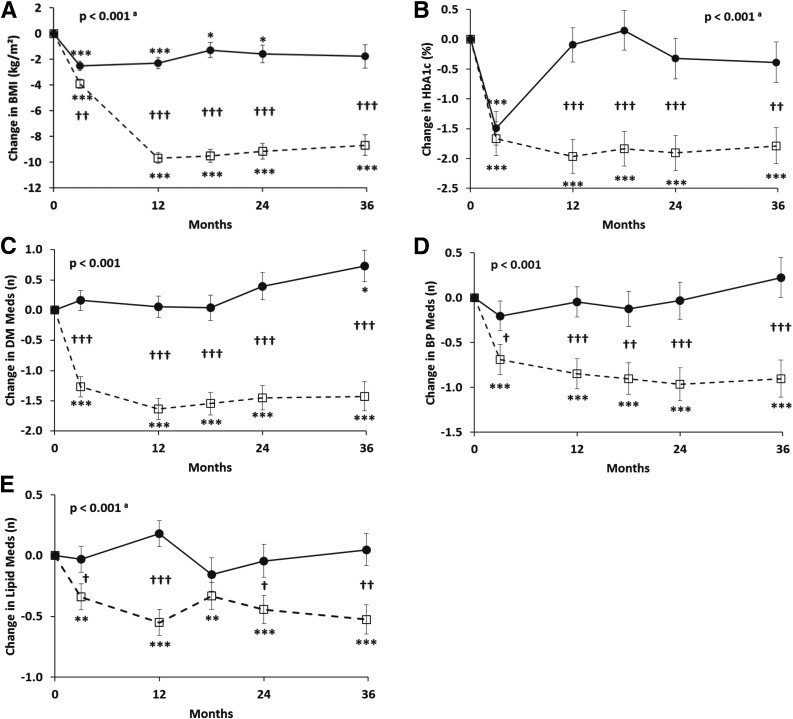
Changes in HbA1c and fasting plasma glucose were more favorable for RYGB versus IMWM at all time points. A change in HbA1c of −1.97% (95% CI −2.52, −1.41) (−21.5 mmol/mol [95% CI −27.5, −15.4]) was achieved by year 1 and sustained through year 3 (−1.79% [−2.38, −1.20]; −19.6 mmol/mol [−26.0, −13.1]) in the surgical group, while HbA1c in the medical weight loss group was not different from baseline at any time from year 1 through year 3 (Table 1 and Fig. 1).
Figure 1.
Changes (±SEM) in BMI (A), HbA1c (B), and antidiabetes (C), antihypertensive (D), and lipid-lowering (E) medications over 36 months in obese patients with type 2 diabetes randomized to RYGB (open squares with dashed lines) versus IMWM (filled circles with solid lines). P values represent overall differences between treatment groups by linear mixed-effects models adjusted for baseline values. atime × group interaction also significant at P < 0.05. *P < 0.05, **P < 0.01, ***P < 0.001 vs. baseline. †P < 0.05, ††P < 0.01, †††P < 0.001 between groups. DM Meds, antidiabetes medications; BP Meds, antihypertensive medications; Lipid Meds, lipid-lowering medications.
Weight, BMI, fat mass, lean mass, and waist circumference all decreased more after RYGB than medical management (Table 1). The reduction in weight reached its maximum at 1 year in both groups, but large differences in weight loss persisted through the end of the 3rd year (change of −24.9 kg [95% CI −29.5, −20.4] vs. −5.2 kg [−10.3, −0.2] at year 3 in RYGB vs. IMWM, respectively; P < 0.001).
The RYGB group also had greater improvements in triglycerides and HDL cholesterol, although change in LDL cholesterol was not different between groups (Table 1). The reduction in systolic blood pressure was greater after RYGB (P = 0.011), but diastolic blood pressure changes were more variable and did not differ between groups. The changes in projected 10-year risks of fatal and nonfatal coronary heart disease and stroke as determined by the UKPDS risk equation were all lower in the surgical group compared with the medical management group. The surgical group also had greater reductions in the number of antidiabetes, antihypertensive, and lipid-lowering medications (all P < 0.001) compared with IMWM.
Patient-Reported Outcomes
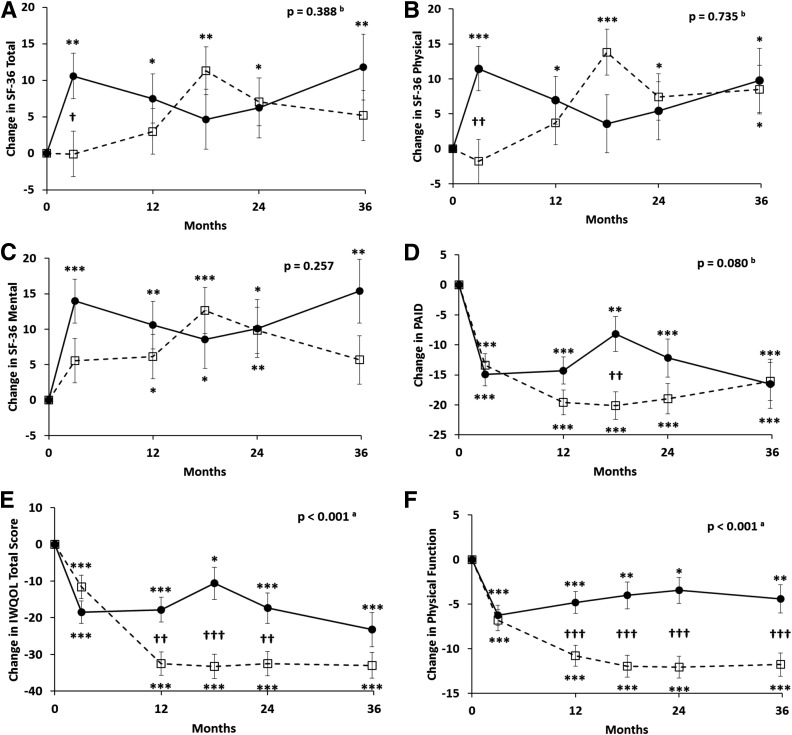
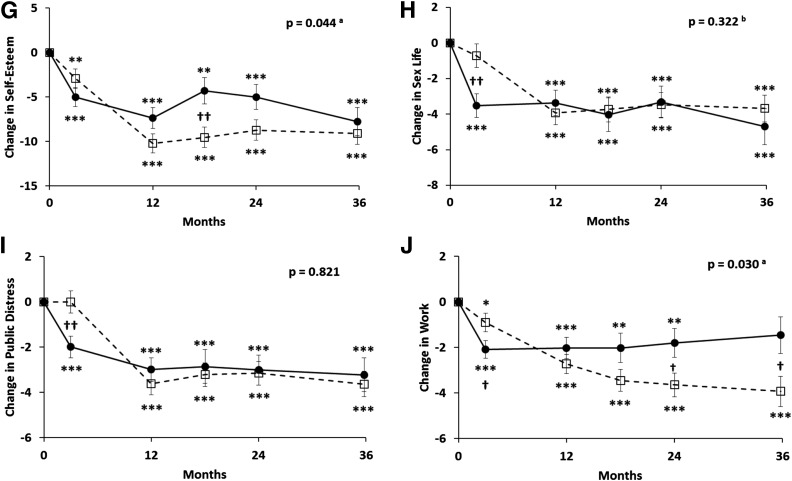
Self-reported health status assessed by the SF-36 showed that both groups had improvements in the total, physical component, and mental component scores over the 3-year follow-up, but there were no significant differences between groups (Fig. 2A–C). PAID improved in both groups, but again there were no differences between RYGB and IMWM (Fig. 2D). In contrast, there was significantly greater improvement exhibited for IWQOL after RYGB than after IMWM (P < 0.001) (Fig. 2E–J). This effect was largely due to changes in the physical function scale, with lesser beneficial changes also observed for self-esteem and work performance.
Figure 2.
Changes (±SEM) in SF-36 total score (SF-36 Total) (A), SF-36 physical (B), SF-36 mental (C), PAID (D), and IWQOL total score (E), and subscales of physical function (F), self-esteem (G), sex life (H), public distress (I), and work performance (J) over 36 months in obese patients with type 2 diabetes randomized to RYGB (open squares with dashed lines) versus IMWM (filled circles with solid lines). In A–C, an increase in score indicates improvement (better health status). In D–J, a decrease in score indicates improvement (fewer problems with diabetes or less impact of weight on quality of life). P values represent overall differences between treatment groups by linear mixed-effects model adjusted for baseline values. atime × group interaction also significant at P < 0.05. btime × group interaction significant at P < 0.05 but group effect not significant. *P < 0.05, **P < 0.01, ***P < 0.001 vs. baseline. †P < 0.05, ††P < 0.01, †††P < 0.001 between groups.
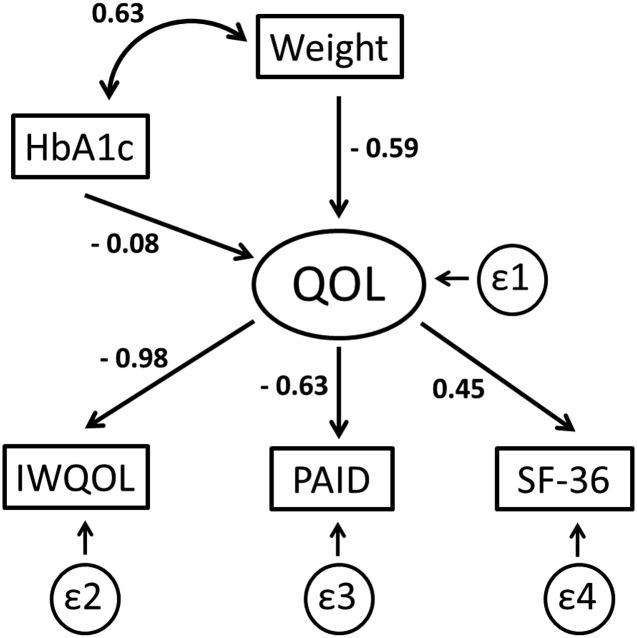
A structural equation model was constructed to examine the impact of changes in weight and HbA1c on the latent construct “quality of life,” as measured by changes in scores on the SF-36, PAID, and IWQOL (Fig. 3). The β-coefficients shown in Fig. 3 demonstrate that reduction in weight had a much greater impact on the improvement in perceived quality of life than reduction in HbA1c. Reduction in weight was also strongly associated with a lowering of HbA1c. Among the three primary instruments used to measure the latent construct, the strongest relationship was manifest as changes in IWQOL, with lesser contributions due to improvements in PAID and SF-36.
Figure 3.
Structural equation model depicting the relationship between predictor variables (change in HbA1c and change in weight) and the latent construct (change in quality of life [QOL]) from baseline to 1 year for all subjects in the study. Change in quality of life is measured by changes in patient responses to the IWQOL, PAID, and SF-36 instruments. Numbers represent standardized β-coefficients; ε1–ε4 represent error terms from the model. Negative β-coefficients indicate that a decrease in the measurement or score (weight, HbA1c, IWQOL, and PAID) was associated with improved quality of life.
Complications and Serious Adverse Events
Serious adverse events, defined by U.S. Food and Drug Administration criteria (see Supplementary Table 1), were more common among RYGB, including multiple gastrointestinal surgical procedures. A total of four patients had gastrointestinal surgeries. Two participants had one gastrointestinal surgical procedure each: one for cholecystectomy and the other for lysis of adhesions. Two patients had repeat gastrointestinal surgical procedures. One participant had two procedures for marginal ulceration and a cholecystectomy after a hospitalization for cholecystitis, and the other had two procedures for lysis of adhesions. One patient previously randomized to IMWM was successfully resuscitated from a witnessed cardiac arrest. There were no deaths in either group. All patients who experienced adverse events were included in the final analyses.
Conclusions
This study demonstrates that RYGB leads to clinical improvements—including a higher percentage of patients achieving nondiabetic range glycemia, greater improvement in HbA1c and fasting plasma glucose, and greater weight loss—over 3 years when compared with a medical diabetes- and weight-management program in surgically appropriate patients with type 2 diabetes and mild-to-moderate obesity. Additional cardiometabolic benefits include reduced systolic blood pressure and triglycerides and increased HDL cholesterol, with improvement in 10-year risks of fatal and nonfatal coronary heart disease and stroke. Importantly, all of these changes were accompanied by significant reductions in the number of antidiabetes, antihypertensive, and lipid-lowering medications. While both groups realized improvement in several patient-reported quality of life measures, the impact of weight on quality of life improved more after RYGB.
At enrollment, all study participants were receiving treatment for diabetes from a physician not connected to the randomized trial. Despite almost all patients using one or more diabetes medications, baseline HbA1c levels averaged 8.5% (69 mmol/mol), suggesting a need for additional intervention. Randomized comparative effectiveness trials help inform clinical decision making. Our current report provides evidence for durability of metabolic change after surgical compared with medical intervention and contributes to a growing body of observational (19–22) and small randomized (7–9,11,12,23) studies. Taken together, these studies support metabolic surgery as a treatment option for diabetes management, with better effects of surgery than medical management for glycemic control, weight loss, and potential reduction in complications associated with type 2 diabetes (rev. in 24,25). Importantly, these metabolic improvements occur in the setting of lower medication burden and low surgical risk.
Furthermore, approximately one-third of our study participants had class 1 obesity (BMI 30 to <35 kg/m2), for which data are sparse (26). Information on patients with low BMI—analyzed in the context of other randomized studies, each contributing small numbers of patients with lower-range BMI (27)—begins to provide level 1A evidence that surgery may be appropriate for type 2 diabetes management even when excess weight is less severe. These findings support the recent American Diabetes Association guideline to consider metabolic surgery in the treatment of obese patients with type 2 diabetes when hyperglycemia is inadequately medically controlled in appropriate surgical candidates (28).
Our medical intervention cohort achieved a sustained weight loss of 5.2 kg at 3 years, demonstrating the effectiveness of the medical intervention for obesity treatment. Sustained weight loss may have been achieved by lifestyle changes and/or use of newer diabetes medications that are weight neutral or promote weight loss. To date, multiple small randomized studies have compared metabolic surgery with a more intensive medical management than would typically be considered standard of care (7–9,11,12,23), suggesting that even greater benefits might be realized with surgery in the real-world setting. Reasons for recidivism in glycemic control in the setting of new effective medications for diabetes management are likely multifactorial, and more understanding of barriers to care is needed.
Cardiovascular disease remains a major cause of morbidity and mortality in obese patients with type 2 diabetes (29). Notably, estimated cardiovascular risk, calculated using the UKPDS risk engine designed for patients with type 2 diabetes (14), was lower after RYGB compared with IMWM, consistent with findings of large controlled outcome studies (17,30). Further studies are still warranted to provide long-term effectiveness and to measure microvascular and macrovascular outcomes.
The current results also expand our understanding of the potential benefits of bariatric surgery in obese type 2 diabetes on patient-centered outcomes. Many previous observational studies have demonstrated that weight loss achieved by diverse means—diet, lifestyle modification, or surgery—improves self-reported health status and quality of life (2). However, few, if any, of these studies have measured both health status and quality of life, and used both generic and disease-specific measures, within the context of a single randomized clinical trial.
In other published trials comparing bariatric procedures (RYGB, gastric sleeve, or gastric band) with lifestyle interventions, the SF-36 has typically demonstrated improvements in physical health and, less consistently, in mental health scores in both groups, although the benefits were often greater after surgery. We observed significant and comparable improvements in both intervention groups, which may be partially due to the intensive and comprehensive nature of our lifestyle program. It should be noted that the SF-36 generally has better discriminative properties (detecting differences between groups of people) than evaluative properties (detecting change over time), except when the change in health status is large, as it was in our study.
In contrast to the SF-36, the IWQOL is a disease-specific quality of life instrument that may be more sensitive to detecting change over time due to weight loss (5). All of the subscales improved in both groups, and the changes were significantly greater in RYGB versus medical and lifestyle intervention for physical functioning, self-esteem, and work performance. It is notable that the medical and lifestyle group achieved measurable benefit despite substantially smaller weight loss, suggesting that even modest weight loss improves the patients’ perception of their health and performance in personal, social, and work-related roles (31). Problem areas in diabetes also improved in both groups over the course of the study, with somewhat greater benefit in the RYGB group. However, these changes were not associated with changes in HbA1c, which was substantially better after surgery compared with the IMWM group. This lack of concordance between patients’ perception of the problems encountered in diabetes management and the actual results achieved in the IMWM group may be due to factors such as the changes in classes of antidiabetes medications used in the setting of increased availability of newer pharmacological agents or overall improvement in well-being and self-esteem achieved after the medical and lifestyle intervention itself.
When all of these measures were modeled simultaneously using a structural equation model, the reduction in weight had a much greater impact on quality of life than did improvement in HbA1c (Fig. 3). This is not entirely unexpected, as substantial loss of weight has beneficial effects on multiple aspects of physical and mental health, but does add further support to the use bariatric surgical procedures in diabetes management.
Adverse events were numerically more common after RYGB, but the number of participants and duration of follow-up limit interpretation of these risks. Our study is limited by the small sample size, intermediate duration of follow-up, heterogeneous population (both insulin and oral hypoglycemic agents), and few cardiovascular events. Participants in our study had relatively long duration of diabetes, high use of insulin, and high HbA1c at study entry—all consistent with lower likelihood of achieving remission after metabolic surgery (22,32).
Our study adds to a growing body of work showing metabolic and cardiovascular benefits of RYGB, even compared with an intensive multidisciplinary, multimodality medical diabetes and weight intervention. Our randomized trial also adds to the relatively scant existing data to support use of metabolic surgery in surgically appropriate patients with less severe-grade obesity and supports the recent American Diabetes Association guidelines to consider metabolic surgery in this setting. While surgery is not without adverse events, improved patient-reported outcomes provide further evidence that serious consideration be given to RYGB for treatment of diabetes in obese patients. Finally, our study demonstrates the relative importance of weight compared with other metabolic measures on the patient’s perception of quality of life.
Supplementary Material
Article Information
Acknowledgments. The authors thank the research assistants, postdoctoral fellows, and medical student who assisted with the study conduct (Katherine Kelly, Stephanie Worobey, Su-Ann Ding, Marlene Wewalka, and Jennifer Panosian, from Joslin Diabetes Center); members of the surgical team at Brigham and Women’s Hospital (David B. Lautz and Kerri Clancy), and the members of the Why WAIT program team at the Joslin Diabetes Center (Osama Hamdy, Ann Goebel-Fabbri, Nuha El Sayed, Iris Marquis, Jacqueline Shahar, Michael See, Gillian Arathuzik, Amanda Kirpitch, John Zrebiec, Pam Needle, Rebecca Lungo, and Joan Beaton).
Funding. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants RC1-DK-086918, R56-DK-095451, and P30-DK-03836; the Herbert Graetz Fund at Joslin Diabetes Center; and Patient-Centered Outcomes Research Institute grant CE-1304-6756. LifeScan, a division of Johnson & Johnson, provided home glucose-monitoring supplies; Nestle provided Boost; and Novo Nordisk provided drug supplies. A.B.G. received research support for this work in the form of supplies from LifeScan, Nestle, Novo Nordisk, and Cleveland Clinic (sponsored by Ethicon and Covidien).
Duality of Interest. Covidien provided funds for the surgical costs of participants with BMI <35 kg/m2 who were randomized to undergo surgery. D.C.S. serves on advisory panels for GI Windows and Medtronic. A.B.G. serves on advisory panels for Boston Heart Diagnostics Corporation, Baranova, and Kowa. A.B.G. currently has a position with the Novartis Institutes for Biomedical Research that did not begin until after the manuscript was completed. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. D.C.S. contributed to the study concept and design, analyzed data, contributed to the discussion, and wrote, reviewed, and edited the manuscript. F.H. contributed to the study concept and design, collected data, and reviewed the manuscript. K.F. collected data. A.V. collected data and reviewed the manuscript. A.B.G. contributed to the study concept and design, obtained study funding, supervised study operations, collected data, contributed to the discussion, and wrote, reviewed, and edited the manuscript. D.C.S. and A.B.G. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
Clinical trial reg. no. NCT01073020, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-0487/-/DC1.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 2013;368:1613–1624 [DOI] [PubMed] [Google Scholar]
- 2.Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet 2015;115:1447–1463 [DOI] [PubMed] [Google Scholar]
- 3.Wing RR; Look AHEAD Research Group . Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med 2010;170:1566–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlsson J, Taft C, Rydén A, Sjöström L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes 2007;31:1248–1261 [DOI] [PubMed] [Google Scholar]
- 5.Kolotkin RL, Crosby RD, Williams GR, Hartley GG, Nicol S. The relationship between health-related quality of life and weight loss. Obes Res 2001;9:564–571 [DOI] [PubMed] [Google Scholar]
- 6.Kroes M, Osei-Assibey G, Baker-Searle R, Huang J. Impact of weight change on quality of life in adults with overweight/obesity in the United States: a systematic review. Curr Med Res Opin 2016;32:485–508 [DOI] [PubMed] [Google Scholar]
- 7.Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg 2015;150:931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings DE, Arterburn DE, Westbrook EO, et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia 2016;59:945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding SA, Simonson DC, Wewalka M, et al. Adjustable gastric band surgery or medical management in patients with type 2 diabetes: a randomized clinical trial. J Clin Endocrinol Metab 2015;100:2546–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg 2014;149:716–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schauer PR, Bhatt DL, Kirwan JP, et al.; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med 2017;376:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamdy O, Mottalib A, Morsi A, et al. Long-term effect of intensive lifestyle intervention on cardiovascular risk factors in patients with diabetes in real-world clinical practice: a 5-year longitudinal study. BMJ Open Diabetes Res Care 2017;5:e000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens RJ, Kothari V, Adler AI, Stratton IM; United Kingdom Prospective Diabetes Study (UKPDS) Group . The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56). Clin Sci (Lond) 2001;101:671–679 [PubMed] [Google Scholar]
- 15.McHorney CA, Ware JE Jr., Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994;32:40–66 [DOI] [PubMed] [Google Scholar]
- 16.Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas In Diabetes (PAID) questionnaire. Diabet Med 2003;20:69–72 [DOI] [PubMed] [Google Scholar]
- 17.Testa MA, Simonson DC. Health economic benefits and quality of life during improved glycemic control in patients with type 2 diabetes mellitus: a randomized, controlled, double-blind trial. JAMA 1998;280:1490–1496 [DOI] [PubMed] [Google Scholar]
- 18.Testa MA, Gill J, Su M, Turner RR, Blonde L, Simonson DC. Comparative effectiveness of basal-bolus versus premix analog insulin on glycemic variability and patient-centered outcomes during insulin intensification in type 1 and type 2 diabetes: a randomized, controlled, crossover trial. J Clin Endocrinol Metab 2012;97:3504–3514 [DOI] [PubMed] [Google Scholar]
- 19.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753–761 [DOI] [PubMed] [Google Scholar]
- 20.Sjöström L, Lindroos AK, Peltonen M, et al.; Swedish Obese Subjects Study Scientific Group . Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–2693 [DOI] [PubMed] [Google Scholar]
- 21.Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA 2015;313:62–70 [DOI] [PubMed] [Google Scholar]
- 22.Purnell JQ, Selzer F, Wahed AS, et al. Type 2 diabetes remission rates after laparoscopic gastric bypass and gastric banding: results of the Longitudinal Assessment of Bariatric Surgery Study. Diabetes Care 2016;39:1101–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015;386:964–973 [DOI] [PubMed] [Google Scholar]
- 24.Rubino F, Nathan DM, Eckel RH, et al.; Delegates of the 2nd Diabetes Surgery Summit . Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care 2016;39:861–877 [DOI] [PubMed] [Google Scholar]
- 25.Schauer PR, Mingrone G, Ikramuddin S, Wolfe B. Clinical outcomes of metabolic surgery: efficacy of glycemic control, weight loss, and remission of diabetes. Diabetes Care 2016;39:902–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maggard-Gibbons M, Maglione M, Livhits M, et al. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA 2013;309:2250–2261 [DOI] [PubMed] [Google Scholar]
- 27.Cummings DE, Cohen RV. Bariatric/metabolic surgery to treat type 2 diabetes in patients with a BMI <35 kg/m2. Diabetes Care 2016;39:924–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Diabetes Association Obesity management for the treatment of type 2 diabetes. Sec. 7. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(Suppl. 1):S57–S63 [DOI] [PubMed] [Google Scholar]
- 29.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 2014;370:1514–1523 [DOI] [PubMed] [Google Scholar]
- 30.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012;307:56–65 [DOI] [PubMed] [Google Scholar]
- 31.Delahanty LM. The Look AHEAD study: implications for clinical practice go beyond the headlines. J Acad Nutr Diet 2014;114:537–542 [DOI] [PubMed] [Google Scholar]
- 32.Hatoum IJ, Blackstone R, Hunter TD, et al. Clinical factors associated with remission of obesity-related comorbidities after bariatric surgery. JAMA Surg 2016;151:130–137 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.