Abstract
OBJECTIVE
Connective tissue growth factor (CTGF), also known as CCN2, is a potent chemotactic and extracellular matrix-inducing matricellular protein that has been implicated in progression of inflammatory and fibroproliferative disorders. An emerging role of CTGF/CCN2 is that of a prosclerotic factor implicated in the development of cardiac disease. Our objective was to determine the role of CTGF/CCN2 as a predictor of cardiovascular events in type 2 diabetes in the Veterans Affairs Diabetes Trial (VADT) cohort.
RESEARCH DESIGN AND METHODS
Levels of CTGF/CCN2 were measured in 952 VADT patients a median of 1.9 years after entry into the study. Participants were followed for an average of 3.3 years for vascular outcomes. CTGF/CCN2 categories were defined as below the detectable limit (referent, 54.5%), lower half of detectable values (22.8%), and upper half of detectable values (22.7%). Hazard ratios (HRs) for cardiovascular end points in relation to CTGF/CCN2 categories were calculated by Cox proportional hazards models.
RESULTS
During follow-up, 4.8% had a myocardial infarction (MI), 6.9% had an MI or cardiovascular death, and 6.9% died. After adjustments by conventional risk factors, individuals in the highest category of CTGF/CCN2 were at higher risk of MI (HR 2.43 [95% CI 1.15, 5.14]), MI or cardiovascular death (HR 2.71 [95% CI 1.44, 5.08]), and all-cause mortality (HR 2.70 [95% CI 1.43, 5.08]) relative to individuals with CTGF below the detectable limit.
CONCLUSIONS
Our study indicates that high levels of CTGF/CCN2 predict future MI and cardiovascular death in patients with type 2 diabetes.
Introduction
Diabetes is associated with a number of metabolic and cardiovascular risk factors that contribute to a high rate of vascular events. The risk factors and mechanisms that contribute to the development of these complications are inadequately defined. In the clinical setting, we strive to maintain glycemic control, promote smoking cessation, and monitor and treat hypertension and hyperlipidemia to well-established goals. Nonetheless, it is clear that our current tools for risk assessment do not provide a complete picture, and as a result our treatment strategies are suboptimal, particularly with respect to macrovascular events in patients with established type 2 diabetes. This fact is underscored by recently released outcomes from the CSP-465 Veterans Affairs Diabetes Trial (VADT) (1,2), the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial (3), and the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial (4), all of which failed to show a significant benefit of tight glycemic control on cardiovascular events (5). In fact, the intensive glycemic control arm of ACCORD was stopped early owing to excess mortality (3). The mechanisms responsible for the initiation and progression of atherosclerosis are not completely defined. Early atherosclerotic lesions are characterized by endothelial dysfunction, accumulation of inflammatory cells, vascular smooth muscle cell proliferation, and transendothelial migration, as well as extracellular matrix deposition in the vessel wall (6). Moreover, the accelerated vascular pathology associated with diabetes is not fully explained by coexistence of traditional cardiovascular risk factors such as hypertension, dyslipidemia, and smoking.
Our data implicate a role for connective tissue growth factor (CTGF), also known as CCN2, as a mediator of diabetic vascular injury. CTGF is a member of the CCN family of matricellular proteins that has been implicated in the progression of the inflammatory process, response to injury, and wound healing (7,8). Dysregulation of CTGF/CCN2 expression has been linked to various fibroproliferative disorders including diabetes complications and atherosclerosis (8,9). An emerging role of CTGF/CCN2 is that of a prosclerotic factor implicated in the development of cardiac disease (10–15). CTGF/CCN2 expression is upregulated in cardiac tissue after myocardial infarction (MI) and has been shown to modulate cardiac function and tissue remodeling (16,17). Furthermore, CTGF/CCN2 was shown to be an effector of transforming growth factor (TGF)-β–mediated cardiac fibrosis, and the expression of CTGF in cardiac fibroblasts and cardiac myocytes was shown to be mediated via TGF-β (18–20). Moreover, CTGF/CCN2 has been shown to modulate the adverse effects of high glucose and free fatty acids on cardiomyocytes function such as hypertrophy and apoptosis (21). It is of interest to note here that CTGF/CCN2 promotes deposition of extracellular matrix proteins through interaction with other factors. For instance, CTGF regulates the fibrosis-associated renal lymphangiogensis in obstructed kidneys via induction and direct interaction with vascular endothelial growth factor C (22). In addition, the increased collagen deposition in lung fibrosis as a result of deletion of protein phosphatase and tensin homologue gene is mediated via increased expression of CTGF (23). At the molecular level, a number of factors have been shown to regulate the expression of CTGF/CCN2, including advanced glycation end products, sphingosine-1-phosphate, bradykinin, and LDL (24–29).
In earlier work, we reported that in type 1 diabetes, higher plasma CTGF/CCN2 levels were associated with increased common and internal carotid intima-media thickness, an established marker of subclinical atherosclerosis, as well as macroalbuminuria and hypertension (9). In the current study, we build on our prior work and examine the ability of CTGF to predict MI in patients with type 2 diabetes in the VADT.
Research Design and Methods
VADT Design and Population
The details of the VADT design have previously been described (1). Briefly, 1,791 veterans with type 2 diabetes and suboptimal glucose control were randomized in 20 participating sites to receive either intensive or standard glucose control. The goal for HbA1c levels was an absolute reduction of 1.5% in the intensive therapy group compared with the standard therapy group. All other modifiable cardiovascular risk factors were treated aggressively and uniformly in both arms of the study. All patients were treated according to guidelines of the American Diabetes Association for blood pressure, hypertension, diet, exercise, and diabetes education (30). All patients were prescribed aspirin, and all patients with elevated lipid levels were prescribed statins unless contraindicated. The study was approved by the institutional review board at each of the participating sites. All patients provided written informed consent.
Of the 1,791 VADT study participants, 995 patients from 17 of the participating sites, or approximately half from the standard arm and half from the intensive treatment arm, agreed to participate in a substudy focused on determining the association between specific biomarkers and macrovascular disease. The biochemical, physical, and demographic profiles of the 995 patients in the substudy did not differ significantly from those of the 796 not included in the substudy, with the exception of slightly lower age and LDL cholesterol and slightly higher triglyceride levels, as well as a higher prevalence of aspirin use at baseline in substudy participants compared with non–substudy participants (31). The study population for the current report consists of 952 of the 995 participants for whom samples were available for measurement of CTGF. In 43 patients, not enough plasma was collected to perform the measurements. Supplementary Fig. 1 includes a flow diagram of study participants.
Enrollment for the VADT study occurred from December 2000 to May 2003. Measurement of CTGF was performed on samples collected during a routine follow-up between July 2002 and March 2006, a median of 1.94 years (range 0–5.03) after participants’ baseline examination. Plasma samples were obtained after an overnight fast and stored at −80°C. Patients were followed until lost to follow-up, death, or May 2008. The median follow-up time after measurement of CTGF was 3.76 years (range 0–5.75). CTGF measurement for the current analysis occurred after all samples were collected. The baseline VADT cohort examination was standardized and included interviews, blood pressure measurements, anthropometric measurements, and fasting venipuncture (1).
CTGF/CCN2 Measurement
CTGF in plasma was measured with a sandwich ELISA that detects both intact CTGF and CTGF that has been proteolytically cleaved in the hinge region to release the N-fragment of CTGF (N+W-CTGF assay). The capture antibody is human anti-human CTGF–domain 1 (FibroGen, Inc., San Francisco, CA). A standard curve was prepared with rhCTGF (CTGF expressed in CHO cells and affinity purified with an anti-CTGF antibody column; FibroGen). After plate washing, 50 µL samples, standards, and controls was added along with 50 µL detection antibody (mouse anti-human CTGF–domain 2 antibody, 500 ng/mL; FibroGen, Inc.) for >1 h. Secondary antibody (100 µL, goat anti-mouse IgG(H+L)-AP, 1:2,000; Thermo Fisher Scientific, Waltham, MA) was added for 1 h. Stationary incubation at 37°C in 100 µL light-sensitive substrate buffer (0.1% diethanolamine in 1.1 mmol/L MgCl2 with 1 mg/mL para-nitrophenylphosphate substrate) for 5–10 min (depending on yellow color development) was followed by addition of 100 µL stop solution (10 N NaOH) to stabilize the colored product. Absorbance at 405 nm was acquired on a SpectraMax 340PC spectrophotometer and analyzed with SoftMax Pro 4.8 software (Molecular Devices, Sunnyvale, CA).
End Points
The primary end point for the VADT was the time to the first occurrence of any one of a composite of cardiovascular events. Each VADT event was adjudicated by an end point committee that used strict algorithms to define and document each event. The composite end point included documented MI; stroke; death from cardiovascular disease (CVD); new or worsening congestive heart failure; surgical intervention for cardiac, cerebrovascular, or peripheral vascular disease; inoperable coronary artery disease (CAD); and amputation for ischemic gangrene. Secondary outcomes included MI, CAD, death from CVD, and death from any cause. CAD included MI, coronary revascularization procedures, and clinically identified inoperable CAD. Cardiovascular death included sudden death as defined by the Framingham Heart Study, CAD, cerebrovascular accident, and other cardiovascular events (i.e., cardiomyopathy).
Statistical Analyses
Prospective analyses were carried out in which the plasma level of CTGF, as a biomarker, and cardiovascular end points, including MI and the composite end point, were the outcomes of interest. Since the CTGF assay was designed to measure levels above median values, slightly more than half (54.5%) of VADT participants had CTGF levels below the detectable level; therefore, for purposes of statistical analyses, individuals were categorized based on their CTGF level into one of three groups: those below the detectable limit, <7.8 ng/mL (54.5%); those with levels in the lower half of the detectable range, 7.8–13.4 ng/mL (22.8%); and those with levels in the upper half of the detectable range, >13.4–106 ng/mL (22.7%). Baseline clinical and demographic characteristics of the cohort are shown in Table 1, stratified by the three categories of CTGF level. Differences across categories of CTGF were tested using χ2 for categorical characteristics, while means, adjusted for age, ethnic minority status, and treatment arm, were determined for continuous variables using linear regression.
Table 1.
Clinical and demographic characteristics of the VADT population (n = 952) stratified by CTGF category
| Total population | CTGF categories (cut points), ng/mL |
P** | |||
|---|---|---|---|---|---|
| Below detectable limit <7.8 (n = 519) | ≥7.8–13.4 (n = 217) | >13.4–106 (n = 216) | |||
| Measured at VADT baseline | |||||
| Age (years)* | 59.7 (59.2, 60.3) | 57.9 (57.2, 58.6) | 61.1 (60.0, 62.2) | 62.7 (61.6, 63.8) | <0.0001 |
| Diabetes duration (years) | 11.4 (10.9, 11.9) | 10.5 (9.9, 11.1) | 11.5 (10.5, 12.5) | 13.5 (12.5, 14.5) | <0.0001 |
| Male* | 97.1 | 95.95 | 98.62 | 98.15 | 0.0835 |
| Non-Hispanic white* | 60.0 | 54.91 | 66.82 | 65.28 | 0.0021 |
| Intensive treatment* | 49.7 | 46.63 | 53.46 | 54.17 | 0.0866 |
| Prior vascular event* | 38.5 | 33.53 | 39.63 | 49.07 | 0.0004 |
| Current smoker* | 17.3 | 19.85 | 12.56 | 15.74 | 0.0472 |
| Hypertension* | 73.1 | 69.19 | 76.96 | 78.70 | 0.0105 |
| Exercise* | 44.5 | 43.91 | 45.83 | 44.44 | 0.8918 |
| Adherence to diet* | 46.7 | 45.95 | 47.69 | 47.69 | 0.8667 |
| Aspirin* | 91.8 | 92.26 | 90.74 | 91.63 | 0.7885 |
| Measured at time of CTGF measurement | |||||
| Statin* | 79.0 | 78.72 | 77.31 | 81.40 | 0.5662 |
| ACE* | 68.5 | 68.09 | 67.59 | 70.23 | 0.8099 |
| ARB* | 12.6 | 10.83 | 15.74 | 13.49 | 0.1681 |
| HbA1c (%) | 8.0 (7.9, 8.1) | 8.0 (7.9, 8.1) | 7.9 (7.8, 8.1) | 8.1 (7.9, 8.2) | 0.6426 |
| ACR‡ | 19 (17, 21) | 13 (11, 15) | 23 (18, 29) | 38 (30, 48) | <0.0001 |
| eGFR | 79.6 (78.1, 81.1) | 84 (83, 86) | 77 (74, 80) | 70 (67, 73) | <0.0001 |
| BMI (kg/m2) | 32.4 (32.0, 32.7) | 31.7 (31.3, 32.2) | 32.9 (32.2, 33.5) | 33.4 (32.7, 34.0) | <0.0001 |
| SB pressure (mmHg) | 127 (126, 128) | 126 (125, 128) | 128 (125, 130) | 130 (128, 132) | 0.0244 |
| DB pressure (mmHg) | 72.8 (72, 73) | 73 (72, 74) | 72 (71, 73) | 73 (71, 74) | 0.3449 |
| HDL cholesterol (mg/dL) | 38 (37, 39) | 39 (38, 40) | 37 (35, 38) | 37 (36, 39) | 0.0547 |
| LDL cholesterol (mg/dL) | 96 (94, 98) | 95 (92, 98) | 94 (89, 98) | 100 (96, 105) | 0.0487 |
| Triglycerides (mg/dL)‡ | 153 (148, 159) | 145 (138, 153) | 156 (145, 168) | 172 (160, 186) | 0.0012 |
Continuous characteristics are shown as means with associated 95% CIs, while categorical characteristics are shown as percentages. Characteristics of the study population adjusted for age, minority status, and treatment arm of the study. ACR, albumin-to-creatinine ratio; ARB, angiotensin II receptor blockers; DB, diastolic blood; SB, systolic blood.
*Unadjusted.
‡Owing to nonnormal distributions, geometric means are presented.
**χ2 for categorical variables and F test for continuous variables.
For cardiovascular time-to-event outcomes, Kaplan-Meier survival curves and Cox proportional hazards models were used to calculated hazard ratios (HRs) for end points of interest in relation to CTGF categories. Because CTGF levels were measured not at the baseline VADT examination but, instead, a median of 1.94 years later, left truncation was used to account for differences in time at risk; hence, a participant was considered at risk for a given event between measurement of CTGF levels and the end of VADT follow-up. Initial models examining the association between the three CTGF categories and each cardiovascular event of interest were assessed after age, ethnic minority, and treatment arm were controlled for. Secondary models additionally adjusted for prior cardiovascular event, hypertension status, and smoking status at date of randomization, as well as LDL cholesterol level, triglycerides, use of ACE inhibitors, and use of statins at time of CTGF measurement. The final model also adjusted for estimated glomerular filtration rate (eGFR) at time of CTGF measurement. These covariates were chosen a priori, since they represent either study design variables or established cardiovascular risk factors.
Appropriate interaction terms were used to determine whether treatment arm, ethnic minority status, or prior CVD event modified the relationship between CTGF categories and outcomes of interest. Potential effect modifiers were chosen a priori, since they represent either study design variables or established cardiovascular risk factors. The assumption of proportional hazards was evaluated by testing for interaction between the two CTGF index variables (i.e., defining the three CTGF categories) and continuous time variables. Reported P values are two sided with a type I error rate significance level of α = 0.05. HRs (95% CI) are displayed. All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC).
Results
At VADT baseline, the mean age of the study population was 59.7 years and the mean duration of diabetes was 11.4 years. Of the 952 participants studied, 97.1% were male, 60% were non-Hispanic white, 38.5% had experienced a prior event, and 49.7% were assigned to the VADT intensive treatment group. HbA1c levels, diastolic blood pressure, and HDL cholesterol remained similar across the three categories of CTGF after adjustment for age, ethnic minority status, and treatment arm of the study. Also, there was no association of CTGF categories with treatment for statins, ACE inhibitors, or aspirin. BMI, systolic blood pressure, albumin-to-creatinine ratio, eGFR, LDL cholesterol, and triglycerides were statistically significantly different among the three CTGF groups (Table 1). During the follow-up period, 46 had an MI and 66 experienced an MI or cardiovascular death after measurement of CTGF (Table 2).
Table 2.
VADT cardiovascular events after measurement of CTGF (total N = 952)
| Cardiovascular end points | N (%) |
|---|---|
| MI | 46 (4.83) |
| MI, procedure or inoperable | 105 (11.03) |
| MI or cardiovascular death | 66 (6.93) |
| Composite end point* | 166 (17.44) |
| Death | 66 (6.93) |
*Composite end point includes documented MI; stroke; death from cardiovascular causes; new or worsening congestive heart failure; surgical intervention for cardiac, cerebrovascular, or peripheral vascular disease; inoperable CAD; and amputation for ischemic gangrene.
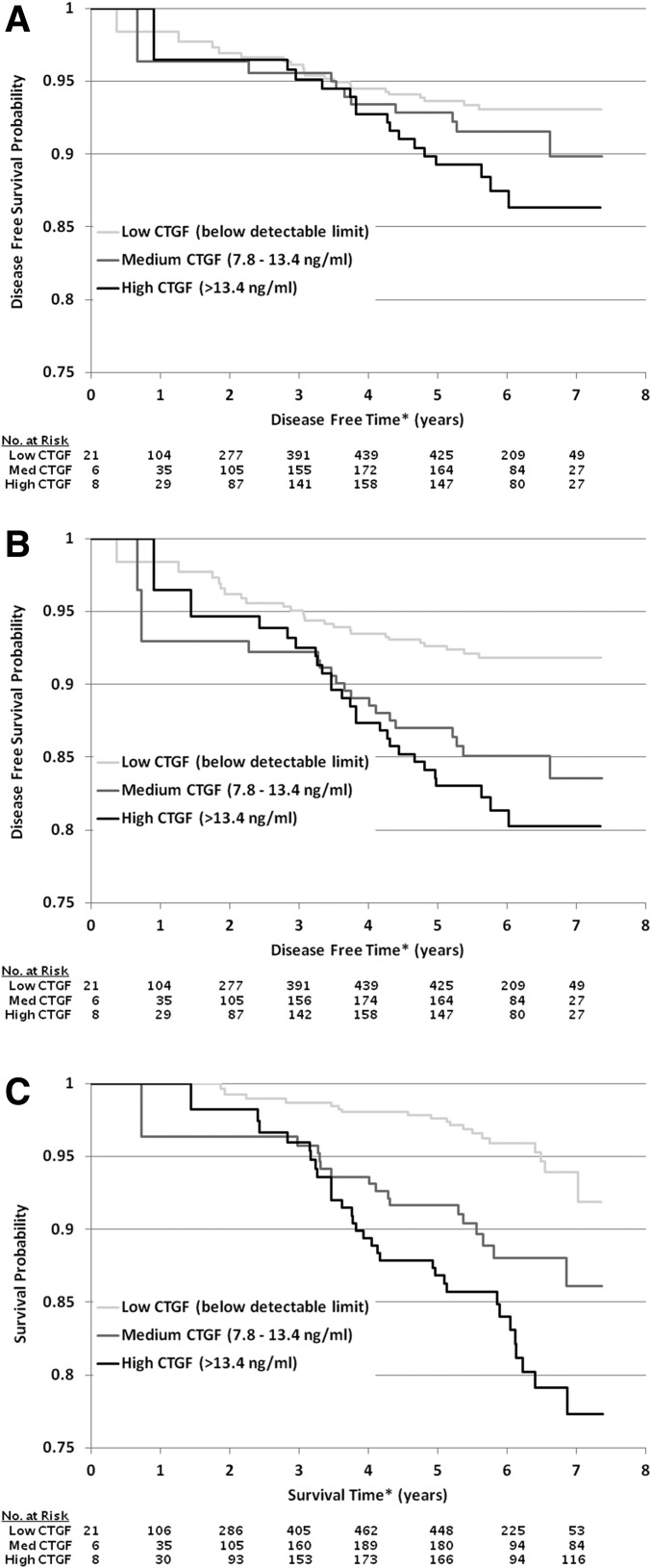
Figure 1 depicts Kaplan-Meier survival curves for MI (Fig. 1A), MI combined with cardiovascular death (Fig. 1B), and all-cause mortality (Fig. 1C). Cox proportional hazards models were used to examine the ability of CTGF to predict VADT macrovascular end points (Table 3). After adjustment for age, minority status, treatment arm, prior cardiovascular event, hypertension status and smoking status at date of randomization, as well as LDL cholesterol level, triglycerides, use of ACE inhibitors, and use of statins at time of CTGF measurement, individuals with CTGF level >13.4 ng/mL, compared with individuals with CTGF level <7.8 ng/mL, had significantly higher risk of MI (HR 2.36 [95% CI 1.16, 4.84]), MI or cardiovascular death (HR 2.76 [95% CI 1.51, 5.04]), and death from any cause (HR 3.02 [95% CI 1.66, 5.50]). This significantly increased risk was not observed in comparison of individuals with CTGF level between 7.8 and 13.4 ng/mL with individuals with CTGF level <7.8 ng/mL (Table 3). Moreover, additional adjustment for eGFR at time of the biomarker measurement had only a minimal impact on the HR for MI (HR 2.43 [95% CI 1.15, 5.14]) and MI or cardiovascular death (HR 2.71 [95% CI 1.44, 5.08]) and a slightly larger impact on the HR for all-cause mortality (HR 2.70 [95% CI 1.43, 5.08]). There was no evidence that study treatment arm, minority status, or prior history of a cardiovascular event modified the association between CTGF level and any of the macrovascular end points examined.
Figure 1.
Kaplan-Meier survival curves for incident MI (A), MI combined with cardiovascular death (B), and all-cause mortality (C). *The time axis starts at time of randomization, and left truncation is used to account for differences in time at risk, since CTGF was measured on samples collected a median of 1.94 years after randomization. Med, medium.
Table 3.
Adjusted HRs (95% CI) from Cox proportional hazards regression models for CTGF in relation to various outcomes* in the total population
| CTGF |
|||
|---|---|---|---|
| Model 1** | Model 2† | Model 3‡ | |
| MI | |||
| Below detectable limit | 1.00 | 1.00 | 1.00 |
| 7.8–13.4 ng/mL | 1.28 (0.59, 2.75) | 1.34 (0.61, 2.95) | 1.36 (0.62, 3.02) |
| >13.4 ng/mL | 2.24 (1.13, 4.45) | 2.36 (1.16, 4.84) | 2.43 (1.15, 5.14) |
| MI, procedure or inoperable disease | |||
| Below detectable limit | 1.00 | 1.00 | 1.00 |
| 7.8–13.4 ng/mL | 0.77 (0.45, 1.31) | 0.79 (0.46, 1.36) | 0.79 (0.46, 1.37) |
| >13.4 ng/mL | 1.40 (0.88, 2.23) | 1.32 (0.82, 2.13) | 1.32 (0.80, 2.17) |
| MI or cardiovascular death | |||
| Below detectable limit | 1.00 | 1.00 | 1.00 |
| 7.8–13.4 ng/mL | 1.78 (0.95, 3.33) | 1.63 (0.84, 3.18) | 1.62 (0.83, 3.17) |
| >13.4 ng/mL | 2.79 (1.55, 5.01) | 2.76 (1.51, 5.04) | 2.71 (1.44, 5.08) |
| Composite end point | |||
| Below detectable limit | 1.00 | 1.00 | 1.00 |
| 7.8–13.4 ng/mL | 1.02 (0.69, 1.53) | 0.94 (0.62, 1.42) | 0.93 (0.61, 1.42) |
| >13.4 ng/mL | 1.61 (1.11, 2.33) | 1.44 (0.99, 2.10) | 1.43 (0.97, 2.11) |
| All death | |||
| Below detectable limit | 1.00 | 1.00 | 1.00 |
| 7.8–13.4 ng/mL | 1.76 (0.90, 3.46) | 1.39 (0.68, 2.87) | 1.30 (0.62, 2.71) |
| >13.4 ng/mL | 3.30 (1.82, 5.99) | 3.02 (1.66, 5.50) | 2.70 (1.43, 5.08) |
*The number of events for each outcome for each model are as follows: MI, 46, 45, and 45 for model 1, model 2, and model 3, respectively; MI, procedure or inoperable disease, 105, 104, and 104; MI or cardiovascular death, 66, 63, and 63; composite end point, 166, 162, and 162; and all death, 66, 63, and 63.
**Adjusted for age, minority, and treatment arm.
†Additionally adjusted for prior cardiovascular event, hypertension status, and smoking status at date of randomization, as well as LDL, triglycerides, use of ACE inhibitors, and statin use at time of CTGF measurement.
‡Additionally adjusted for eGFR at time of CTGF measurement.
Conclusions
We previously reported that in type 1 diabetes, increased plasma CTGF levels were associated with increased common and internal carotid intima-media thickness, an established marker of subclinical atherosclerosis, as well as macroalbuminuria and hypertension (9). In the current study, we report CTGF is a strong predictor of MI, MI combined with cardiovascular death, and all-cause mortality. The ability of CTGF to predict these end points remained after established cardiovascular risk factors were controlled for, including age, minority status, VADT treatment arm, history of CVD, history of hypertension, lipid levels, smoking status, use of ACE inhibitors, use of statins, and eGFR. Moreover, when incident MI was combined with procedure or inoperable disease (i.e., not limited to acute events), the HR declined and was not significant, indicating that CTGF may be related to acute events through a mechanism other than its association with atherosclerotic burden, for which CTGF has been postulated to be a plaque-stabilizing factor (12).
A major difference between the VADT and DCCT/EDIC (Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications) cohorts besides diabetes type (i.e., type 1 vs. type 2 diabetes) is the level of CVD at the time of measurement of CTGF. Patients enrolled in the VADT study were older, had a longer duration of diabetes and a high comorbidity burden, and were likely to have a history of CVD (i.e., 38.5%). DCCT/EDIC participants, on the other hand, were younger, had a shorter duration of diabetes and a low comorbidity burden, and had not had a cardiovascular event prior to enrollment. Results from both the DCCT/EDIC and VADT, which collectively include participants across the continuum of diabetes disease burden, indicate that CTGF may play a role not only in development of atherosclerosis but also in prediction of acute events and, as such, may have substantial value both as a biological marker of inflammation-induced tissue injury and as a therapeutic target.
The findings of the current study also point to a relationship between plasma CTGF levels and renal function. Our data indicated that the mean eGFR levels declined across increasing categories of CTGF. This is in line with our previous findings in which increased levels of plasma CTGF N-fragment were associated with decreased eGFR in patients with type 1 diabetes (9). The increase in plasma CTGF levels observed in patients with type 1 diabetes with overt albuminuria was not the result of a decrease in renal clearance of CTGF but, rather, was a result of increased production (9). It is important to point out that adjustment for eGFR had little impact on the HRs of CTGF between model 2 and model 3 (Table 3), indicating that renal function is not a confounder of the relationship between CTGF and outcomes of interests (i.e., MI, MI and cardiovascular death, all-cause mortality).
The increased risk of cardiovascular events seen in patients with type 2 diabetes is associated with a cluster of risk factors for cardiovascular and metabolic disorders that tend to coexist in these patients, including central adiposity, hypertension, and dyslipidemia (32). However, the complex signaling networks through which diabetes, hypertension, and dyslipidemia accelerate vascular damage and trigger acute vascular events are not well-defined. The modifiable factors engaged in these processes have yet to be identified, but there is evidence for promotion of chronic low-grade inflammation, oxidative stress, endothelial dysfunction, stimulation of proliferative/apoptotic pathways, and deposition of extracellular matrix. Importantly, inflammatory mediators and growth factors are increasingly recognized as key players in the pathogenesis of macrovascular disease (33).
Emerging studies indicate that CTGF/CCN2 is a pathogenic risk determinant acting along a causal pathway for development of CVD. CTGF is a secreted proadhesive matricellular protein that has been implicated in modulating inflammatory and fibroproliferative disorders (34). The expression of CTGF mRNA and protein levels were increased in vascular cells of advanced atherosclerotic lesions and were found to be aggregated predominantly in areas near the fibrous cap of the plaque, suggesting potential involvement in atherosclerotic plaque production (35,36). In addition, inhibition of CTGF in the cardiovascular system was shown to reverse tissue remodeling and the process of fibrosis (37). Moreover, plasma CTGF was linked to an increased risk of cardiovascular events and mortality in patients with atherosclerotic disease and was associated with plaque stabilization after stroke (12,15). Although the exact mechanisms through which CTGF mediates its inflammatory and fibrotic effects on the vasculature are not fully defined, engagement of all six members of integrin receptors and binding to heparin sulfate proteoglycans such as syndecan-4, LDL-related receptor protein, and the cation-independent mannose-6-phosphate receptor have been implicated as key factors through which CTGF transduces its cellular effects (38). It is of interest to point out here that CCN3 (nov), another member of the CCN family, has been shown to act downstream of TGF-β to inhibit the expression of CCN2 and, hence, attenuate deposition of extracellular matrix proteins (39,40). CCN3 functions as an endogenous negative modulator of the profibrotic actions of CCN2 in vivo to promote deposition of matrix proteins, thus providing a mechanistic pathway in which the activity of CCN2 can be regulated in disorders of fibrosis (39,40).
One limitation of our study is that the assay used to measure CTGF was designed to measure CTGF levels above median values in the population; hence, slightly more than half of the VATD population had CTGF levels below the detectable limit of the assay. Thus, we were forced to group all individuals below the detectable limit into a single category and were therefore unable to examine CTGF across a continuum of levels. Second, because the vascular disease burden was high among patients at enrollment into the VADT study, our measurement of CTGF, while occurring prior to development of acute cardiovascular events, likely represents CTGF measured when atherosclerotic burden was high. Hence, it is difficult to determine the temporal relationship between high plasma CTGF levels and development of atherosclerosis and whether CTGF levels were elevated because of the high burden of atherosclerosis or whether they played a causal role in the development of atherosclerosis. It is also difficult to know whether the association between CTGF and acute events only reflects an association between high atherosclerotic burden and increased risk of acute events or whether CTGF levels could also be related mechanistically to acute events. Our analysis, which reports an association with MI and MI combined with cardiovascular death, but not with MI combined with procedure or inoperable disease, suggests that CTGF levels may be related mechanistically to acute events. A third limitation is the somewhat low number of end points, with 46 events for MI and 66 events when MI is combined with cardiovascular death.
In summary, this study demonstrates for the first time that high levels of CTGF/CCN2 predict future MI and cardiovascular death in patients with type 2 diabetes. Plasma CTGF/CCN2 levels warrant further study as a potential biomarker to be used for risk stratification with respect to prediction of cardiovascular events. Moreover, further understanding of the mechanisms responsible for the reported relationship between CTGF/CCN2 and acute vascular events may identify novel targets for therapeutic interventions.
Supplementary Material
Article Information
Acknowledgments. The authors thank the VADT Executive Committee for review and approval of the manuscript for submission.
Funding. This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute grants RO1-HL-077192 and PO1-HL-55782 and the Department of Veterans Affairs.
The funding sources of this study did not play a role in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the manuscript for publication. The content of this article does not represent the views of the Department of Veterans Affairs or the U.S. government.
Duality of Interest. K.E.L. reports personal fees and other from FibroGen, Inc., outside the submitted work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. K.J.H. analyzed data and wrote, reviewed, and edited the manuscript. M.A.J. reviewed the analytical plan and edited the manuscript. S.M.G. and D.K.L. oversaw the measurements of plasma CTGF and reviewed and edited the manuscript. K.E.L., M.F.L.-V., and L.M.L. reviewed and edited the manuscript. A.A.J. conceived the study, amassed the resulting data, and wrote, reviewed, and edited the manuscript. K.J.H. and A.A.J. had final responsibility for the decision to submit the manuscript for publication. A.A.J. was the underwriter of the study. K.J.H. and A.A.J. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 77th Scientific Sessions of the American Diabetes Association, San Diego, CA, 9–13 June 2017.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-2083/-/DC1.
Contributor Information
Collaborators: VADT Investigators, Maria F. Lopes-Virella, Kelly J. Hunt, Nathaniel L. Baker, Gabriel Virella, and Thomas Moritz
References
- 1.Abraira C, Duckworth W, McCarren M, et al.; VA Cooperative Study of Glycemic Control and Complications in Diabetes Mellitus Type 2 . Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications 2003;17:314–322 [DOI] [PubMed] [Google Scholar]
- 2.Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 5.Dluhy RG, McMahon GT. Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med 2008;358:2630–2633 [DOI] [PubMed] [Google Scholar]
- 6.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993;362:801–809 [DOI] [PubMed] [Google Scholar]
- 7.Perbal A, Perbal B. The CCN family of proteins: a 25th anniversary picture. J Cell Commun Signal 2016;10:177–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov 2011;10:945–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaffa AA, Usinger WR, McHenry MB, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Group . Connective tissue growth factor and susceptibility to renal and vascular disease risk in type 1 diabetes. J Clin Endocrinol Metab 2008;93:1893–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rickard AJ, Morgan J, Chrissobolis S, Miller AA, Sobey CG, Young MJ. Endothelial cell mineralocorticoid receptors regulate deoxycorticosterone/salt-mediated cardiac remodeling and vascular reactivity but not blood pressure. Hypertension 2014;63:1033–1040 [DOI] [PubMed] [Google Scholar]
- 11.Ponticos M. Connective tissue growth factor (CCN2) in blood vessels. Vascul Pharmacol 2013;58:189–193 [DOI] [PubMed] [Google Scholar]
- 12.Leeuwis JW, Nguyen TQ, Theunissen MG, et al. Connective tissue growth factor is associated with a stable atherosclerotic plaque phenotype and is involved in plaque stabilization after stroke. Stroke 2010;41:2979–2981 [DOI] [PubMed] [Google Scholar]
- 13.Koitabashi N, Arai M, Niwano K, et al. Plasma connective tissue growth factor is a novel potential biomarker of cardiac dysfunction in patients with chronic heart failure. Eur J Heart Fail 2008;10:373–379 [DOI] [PubMed] [Google Scholar]
- 14.Daniels A, van Bilsen M, Goldschmeding R, van der Vusse GJ, van Nieuwenhoven FA. Connective tissue growth factor and cardiac fibrosis. Acta Physiol (Oxf) 2009;195:321–338 [DOI] [PubMed] [Google Scholar]
- 15.Gerritsen KG, Falke LL, van Vuuren SH, et al.; SMART Study Group . Plasma CTGF is independently related to an increased risk of cardiovascular events and mortality in patients with atherosclerotic disease: the SMART study. Growth Factors 2016;34:149–158 [DOI] [PubMed] [Google Scholar]
- 16.Panek AN, Posch MG, Alenina N, et al. Connective tissue growth factor overexpression in cardiomyocytes promotes cardiac hypertrophy and protection against pressure overload [published correction appears in PLoS One 2009;4]. PLoS One 2009;4:e6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koshman YE, Sternlicht MD, Kim T, et al. Connective tissue growth factor regulates cardiac function and tissue remodeling in a mouse model of dilated cardiomyopathy. J Mol Cell Cardiol 2015;89:214–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Accornero F, van Berlo JH, Correll RN, et al. Genetic analysis of connective tissue growth factor as an effector of transforming growth factor β signaling and cardiac remodeling. Mol Cell Biol 2015;35:2154–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol 2000;32:1805–1819 [DOI] [PubMed] [Google Scholar]
- 20.Chuva de Sousa Lopes SM, Feijen A, Korving J, et al. Connective tissue growth factor expression and Smad signaling during mouse heart development and myocardial infarction. Dev Dyn 2004;231:542–550 [DOI] [PubMed] [Google Scholar]
- 21.Wang X, McLennan SV, Allen TJ, Tsoutsman T, Semsarian C, Twigg SM. Adverse effects of high glucose and free fatty acid on cardiomyocytes are mediated by connective tissue growth factor. Am J Physiol Cell Physiol 2009;297:C1490–C1500 [DOI] [PubMed] [Google Scholar]
- 22.Kinashi H, Falke LL, Nguyen TQ, et al. Connective tissue growth factor regulates fibrosis-associated renal lymphangiogenesis. Kidney Int 2017;92:850–863 [DOI] [PubMed] [Google Scholar]
- 23.Parapuram SK, Thompson K, Tsang M, et al. Loss of PTEN expression by mouse fibroblasts results in lung fibrosis through a CCN2-dependent mechanism. Matrix Biol 2015;43:35–41 [DOI] [PubMed] [Google Scholar]
- 24.Twigg SM, Joly AH, Chen MM, et al. Connective tissue growth factor/IGF-binding protein-related protein-2 is a mediator in the induction of fibronectin by advanced glycosylation end-products in human dermal fibroblasts. Endocrinology 2002;143:1260–1269 [DOI] [PubMed] [Google Scholar]
- 25.Katsuma S, Ruike Y, Yano T, Kimura M, Hirasawa A, Tsujimoto G. Transcriptional regulation of connective tissue growth factor by sphingosine 1-phosphate in rat cultured mesangial cells. FEBS Lett 2005;579:2576–2582 [DOI] [PubMed] [Google Scholar]
- 26.Tan Y, Keum JS, Wang B, McHenry MB, Lipsitz SR, Jaffa AA. Targeted deletion of B2-kinin receptors protects against the development of diabetic nephropathy. Am J Physiol Renal Physiol 2007;293:F1026–F1035 [DOI] [PubMed] [Google Scholar]
- 27.El-Shewy HM, Sohn M, Wilson P, et al. Low-density lipoprotein induced expression of connective tissue growth factor via transactivation of sphingosine 1-phosphate receptors in mesangial cells. Mol Endocrinol 2012;26:833–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sohn M, Tan Y, Klein RL, Jaffa AA. Evidence for low-density lipoprotein-induced expression of connective tissue growth factor in mesangial cells. Kidney Int 2005;67:1286–1296 [DOI] [PubMed] [Google Scholar]
- 29.Sohn M, Tan Y, Wang B, Klein RL, Trojanowska M, Jaffa AA. Mechanisms of low-density lipoprotein-induced expression of connective tissue growth factor in human aortic endothelial cells. Am J Physiol Heart Circ Physiol 2006;290:H1624–H1634 [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association Standards of medical care for patients with diabetes mellitus. Diabetes Care 2002;25:213–229 [DOI] [PubMed] [Google Scholar]
- 31.Lopes-Virella MF, Hunt KJ, Baker NL, Virella G, Moritz T; VADT Investigators . The levels of MDA-LDL in circulating immune complexes predict myocardial infarction in the VADT study. Atherosclerosis 2012;224:526–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 33.Pradhan AD, Ridker PM. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J 2002;23:831–834 [DOI] [PubMed] [Google Scholar]
- 34.Kular L, Pakradouni J, Kitabgi P, Laurent M, Martinerie C. The CCN family: a new class of inflammation modulators? Biochimie 2011;93:377–388 [DOI] [PubMed] [Google Scholar]
- 35.Oemar BS, Lüscher TF. Connective tissue growth factor. Friend or foe? Arterioscler Thromb Vasc Biol 1997;17:1483–1489 [DOI] [PubMed] [Google Scholar]
- 36.Cicha I, Yilmaz A, Suzuki Y, et al. Connective tissue growth factor is released from platelets under high shear stress and is differentially expressed in endothelium along atherosclerotic plaques. Clin Hemorheol Microcirc 2006;35:203–206 [PubMed] [Google Scholar]
- 37.Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 2012;5(Suppl. 1):S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lau LF. Cell surface receptors for CCN proteins. J Cell Commun Signal 2016;10:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riser BL, Najmabadi F, Perbal B, et al. CCN3 (NOV) is a negative regulator of CCN2 (CTGF) and a novel endogenous inhibitor of the fibrotic pathway in an in vitro model of renal disease. Am J Pathol 2009;174:1725–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riser BL, Najmabadi F, Garchow K, Barnes JL, Peterson DR, Sukowski EJ. Treatment with the matricellular protein CCN3 blocks and/or reverses fibrosis development in obesity with diabetic nephropathy. Am J Pathol 2014;184:2908–2921 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.