Abstract
Although B cells reactive with islet autoantigens are silenced by tolerance mechanisms in healthy individuals, they can become activated and contribute to the development of type 1 diabetes. We previously demonstrated that high-affinity insulin-binding B cells (IBCs) occur exclusively in the anergic (BND) compartment in peripheral blood of healthy subjects. Consistent with their activation early in disease development, high-affinity IBCs are absent from the BND compartment of some first-degree relatives (FDRs) as well as all patients with autoantibody-positive prediabetes and new-onset type 1 diabetes, a time when they are found in pancreatic islets. Loss of BND IBCs is associated with a loss of the entire BND B-cell compartment consistent with provocation by an environmental trigger or predisposing genetic factors. To investigate potential mechanisms operative in subversion of B-cell tolerance, we explored associations between HLA and non-HLA type 1 diabetes–associated risk allele genotypes and loss of BNDs in FDRs. We found that high-risk HLA alleles and a subset of non-HLA risk alleles (i.e., PTPN2 [rs1893217], INS [rs689], and IKZF3 [rs2872507]), relevant to B- and T-cell development and function are associated with loss of anergy. Hence, the results suggest a role for risk-conferring alleles in perturbation of B-cell anergy during development of type 1 diabetes.
Introduction
Type 1 diabetes is an autoimmune disease in which self-reactive lymphocytes destroy insulin-producing pancreatic β-cells. Although genetic variation is believed to be the major contributor to the risk of developing type 1 diabetes, environment also plays a contributing role. Together, these factors may impart their effects by compromising maintenance of immune tolerance in T cells and/or B cells, both of which are known to be essential in the pathogenesis of the disorder (1–4). Studies have shown that B cells likely act as antigen-presenting cells and autoantibody producers in type 1 diabetes (5,6). How self-reactive B cells, which normally are silenced in healthy individuals, become activated to participate in this disease is not known.
Previous studies have demonstrated that up to 70% of all B cells generated in the bone marrow are autoreactive (7). Autoreactive B cells are silenced by multiple mechanisms. Those reactive with highly avid self-antigens (e.g., cell surface proteins) undergo receptor editing in which they rearrange their antigen receptor light chains, modifying specificity (8). If this process fails to eliminate autoreactivity, cells can undergo apoptosis through a mechanism referred to as clonal deletion (9). Cells reactive with low-avidity autoantigens, even if they have high affinity, do not receive signals that are sufficiently strong to induce receptor editing or clonal deletion. These cells mature and proceed to the periphery where they are maintained in a state of unresponsiveness, termed anergy. Anergic B cells show evidence of previous antigen exposure, including downregulation of surface IgM, elevated basal calcium, and activation of negatively regulating signaling circuitry, but are refractory to further stimulation (10–12). Of note, studies in mice have demonstrated that anergy is rapidly reversed if autoantigen dissociates from the B-cell receptor (BCR), suggesting that this unresponsive state is maintained by a nondurable, presumably fragile, biochemical mechanism rather than by genetic reprogramming (13). Consistent with this mechanism, inhibitory signaling pathways are upregulated in anergic cells by protein phosphorylation (e.g., SHIP1, SHP-1) and microRNA regulation of effector expression (e.g., PTEN) (14,15). B-cell intrinsic expression of these regulatory phosphatases is required for maintenance of anergy (14). Additional genetic factors likely play a role in tuning B-cell responsiveness to antigen and maintenance of anergy. Obvious candidates reside among the products of gene alleles that have been shown to confer an increased risk of developing autoimmunity.
We previously examined the status of insulin-reactive B cells (IBCs) in peripheral blood of healthy individuals. We observed that B cells with high affinity for insulin occur in blood of healthy subjects where they are restricted in the anergic compartment (16). These cells are polyreactive, binding to lipopolysaccharide and chromatin as well as to insulin. Of note, they disappear from this compartment in subjects with islet autoantibody–positive and recent-onset type 1 diabetes as well as in a portion of healthy first-degree relatives (FDRs) (Fig. 1 and Supplementary Fig. 1). Preliminary studies in our laboratory have suggested that the disappearance of these cells reflects their relocalization to the pancreas and pancreatic lymph nodes. Specifically, IBCs are enriched among B cells in pancreatic islets of subjects with type 1 diabetes (M.J.S. and J.C.C., unpublished observations). However, we cannot rule out the possibility that these cells simply upregulate surface IgM and thus enter the mature naive compartment or do not enter the anergic compartment. To better understand what factors that contribute to the loss of B cells from the anergic compartment of blood early in type 1 diabetes, we explored the correlation between BND frequency among FDRs and high-risk HLA and non-HLA type 1 risk allele genotype.
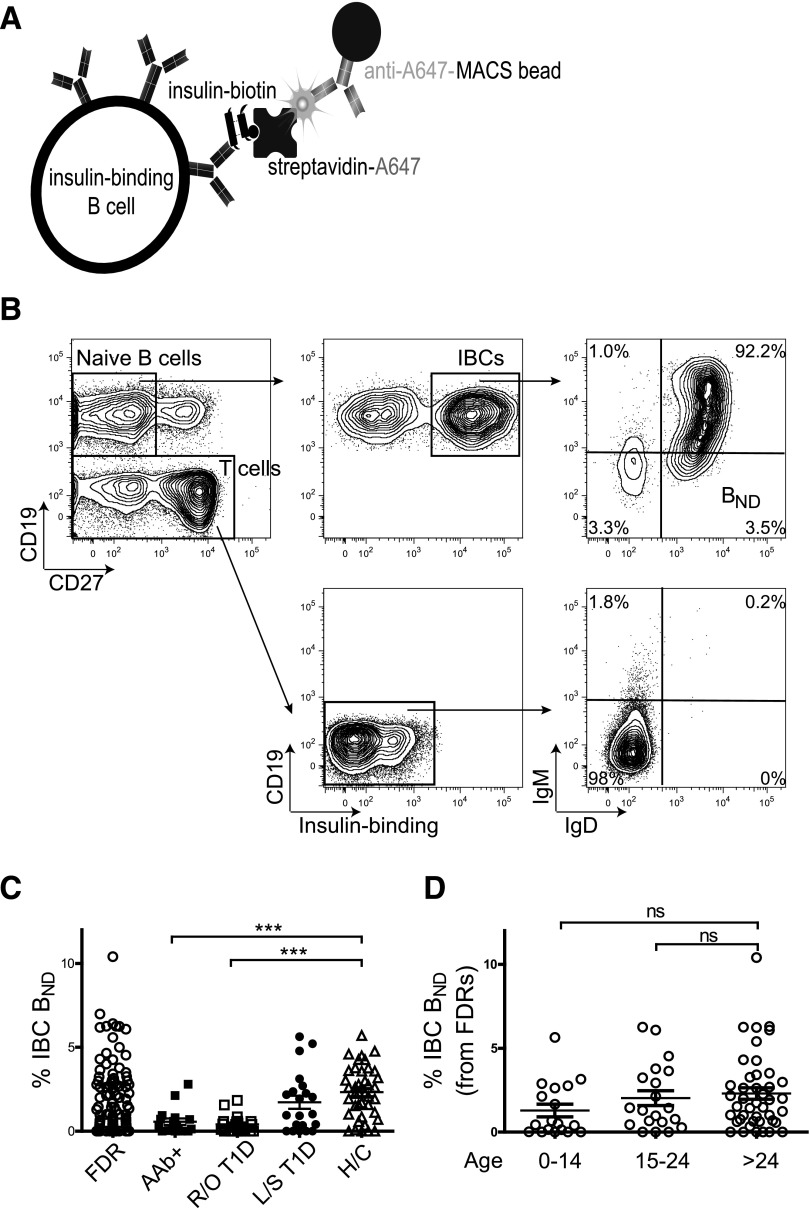
Figure 1.
Loss of IBC BND cells in subjects with autoantibody-positive (AAb+) prediabetes and recent-onset type 1 diabetes (R/O T1D) and some FDRs. A: Diagram of the adsorbent used to identify and enrich for IBCs. B: Representative gating strategy from a healthy control subject (H/C) to identify anergic (BND) IBCs. Gates for IgM and IgD are drawn on the basis of T cells (CD19−) and verified by using fluorescence minus one controls. C: BND cells (as a percentage of CD27− IBC-positive B cells) in peripheral blood of FDRs (n = 103); subjects with AAb+ prediabetes (n = 18), R/O T1D (n = 21), and long-standing type 1 diabetes (L/S T1D) (n = 21) and H/Cs (n = 49). D: BND cells (as percentage of CD27− IBC-positive B cells) in blood of FDRs of various age-groups (years). ***P < 0.001 by Student t test. ns, nonsignificant.
Research Design and Methods
Subject Selection and Peripheral Blood Processing
Peripheral blood was obtained with informed consent at the Barbara Davis Center for Childhood Diabetes by using protocols approved by the University of Colorado Institutional Review Board. Eligible subjects with type 1 diabetes were males or females who met the American Diabetes Association criteria for classification of disease. Insulin autoantibody (IAAs), GAD, IA2, and zinc transporter 8 antibody were assayed by radioimmunoassay, as previously described (17). Peripheral blood mononuclear cells from autoantibody-negative FDRs (n = 103); subjects with autoantibody-positive prediabetes (n = 18; identified in the Type 1 Diabetes TrialNet Natural History study), new-onset type 1 diabetes (n = 21; duration <12 months), and long-standing type 1 diabetes (n = 21; duration >12 months); and healthy age- and sex-matched control subjects (n = 49) were isolated from heparinized blood by Ficoll-Hypaque fractionation. DNA was extracted from the granulocyte layer by using the DNA Mini Kit (QIAGEN).
Flow Cytometric Analysis and Enrichment of IBCs
To maintain consistency of gating of flow cytometry data, each subject sample set was analyzed in parallel with an age- and sex-matched healthy control subject. The healthy control cells were used to construct gates, which were then copied to the FDR subject cells. Gating for IBC anergic (BND) cells was based on T cells (CD19−) and fluorescence minus one compensation controls. Peripheral blood mononuclear cells were stained in PBS, 1% BSA, and 0.02% sodium azide with human FcR Blocking Reagent (Miltenyi Biotec); 0.1 μg/106 cells insulin biotin; and mouse monoclonal anti-human antibodies against CD19-BV510 (BioLegend), CD27-PerCP or CD27-BUV395 (BioLegend), IgM-PE (SouthernBiotech), and IgD-fluorescein isothiocynate (BD Biosciences) or IgD-BV421 (BioLegend) for 20 min at 4°C. After washing, cells were fixed with 2% formaldehyde at 4°C followed by incubation with streptavidin-Alexa Fluor 647 for 20 min at 4°C. Cells were washed, suspended in magnetic-activated cell sorting (MACS) buffer (PBS, 0.5% BSA, 2 mmol/L EDTA) and incubated with anti-Cy5/anti-Alexa Fluor 647 microbeads (Miltenyi Biotec) for 15 min at 4°C. Samples were then passed over magnetized large separation columns (Miltenyi Biotec) and washed three times with 2 mL of MACS buffer, and bound cells were eluted with 6 mL of MACS buffer. Flow cytometry was performed with an LSR II (BD Biosciences) and data analyzed with FlowJo software version 8.8.4. Absolute cell counts were performed by using a hemocytometer (Thermo Fisher Scientific) to enumerate total lymphocytes. Total B-cell numbers were then determined by multiplying the frequency of CD19+ B cells determined by FACS by the total cell count determined by the hemocytometer. Absolute IBC numbers were determined by multiplying the frequency of IBCs in the CD19+ B-cell gate by the total B-cell count. Absolute IBC BND cells were determined by multiplying the frequency of CD27− IgMlo/− IgD+ cells within the IBC gate by the total IBC cell count.
HLA and Non-HLA Genotyping
By using established protocols (18), DNA from FDRs (n = 103) and healthy control subjects (n = 49) was HLA genotyped at the Autoantibody/HLA Service Center at the Barbara Davis Center for Childhood Diabetes. DNA from 60 FDRs was sent to the University of Florida Center for Pharmacogenomics for non-HLA genotyping. The purified DNA was then genotyped on a custom TaqMan single-nucleotide polymorphism (SNP) genotyping array (Thermo Fisher Scientific). All results from non-HLA genotyping are available in Supplementary Table 1.
Statistics
Data were analyzed by using GraphPad Prism software. One-way ANOVA followed by a Bonferroni multiple comparison posttest was used to determine significance of differences among the five patient groups, defined as P < 0.05. Mann-Whitney nonparametric unpaired t tests were used to determine differences in non-HLA genetic risk alleles.
Results
As previously described and depicted in Fig. 1A, we used magnetic nanoparticles to enrich IBCs from the peripheral blood of healthy individuals and subjects along a continuum of diabetes development (16,19), including FDRs (n = 103); subjects with autoantibody-positive prediabetes (n = 18), recent-onset (<12 months) type 1 diabetes (n = 21), and long-standing (>12 months) type 1 diabetes (n = 21); and healthy control subjects (n = 49). We conducted a phenotypic analysis of IBCs to determine what proportion fell into the BND population (Fig. 1B). Phenotypically, BND cells appear as mature naive B cells but have downregulated surface IgM but retained IgD. We have previously reported that IBCs in the BND population have a very high affinity (∼6.6 × 10−10 mol/L) for insulin but are refractory to stimulation through BCR on the basis of decreased calcium flux and phosphorylation of Syk (16).
Although the absolute number (Supplementary Fig. 1) and frequency of total B cells and IBCs did not vary significantly among subject groups, we found that the anergic IBCs were absent, both in frequency and cell number, in blood of all subjects with autoantibody-positive prediabetes and recent-onset type 1 diabetes compared with those with long-standing diabetes and healthy control subjects (Fig. 1C and Supplementary Fig. 1). The observed loss of IBC BND cells from peripheral blood without a change in total IBC numbers is likely due to the very-low frequency of anergic cells within this population and variability in the number of total B cells among individuals. As previously shown, FDRs display heterogeneity in BND phenotype. We hypothesized that BND-low individuals may be at an increased risk for development of autoantibodies and, eventually, type 1 diabetes. To first rule out the possibility that loss of BND is simply a function of young age, we analyzed the frequency of BND cells in FDRs in various age-groups. As seen in Fig. 1D, no significant age-associated differences were found in BND frequency.
To understand what drives loss of IBC BND cells from peripheral blood and determine whether the low IBC BND phenotype of FDRs is associated with specific type 1 diabetes risk alleles, we HLA genotyped FDRs. FDRs who carried the high-risk HLA-DR3/4-DQ2/8 and HLA-DR4/4-DQ8/8 haplotypes uniformly had very-low IBC BND cell frequency in blood, whereas subjects heterozygous for the moderate-risk HLA-DR4-DQ8 and HLA-DR3-DQ2 genotypes showed similar levels as subjects who carried non–type 1 diabetes risk-associated HLA haplotypes (Fig. 2A). Of note, FDRs who carried the type 1 diabetes protective HLA-DQB1*0602 (DQ6) allele (20) had a significantly elevated frequency of IBC BND cells in blood, suggesting enhanced tolerance of autoreactive cells in these individuals. In particular, if high-risk type 1 diabetes HLA genotypes function in isolation (i.e., not influenced by other risk-conferring alleles or environmental factors) to regulate IBC levels, the effect of these genes might be seen in healthy control subjects. Although none of the control subjects we genotyped carried the high-risk HLA-DR3/4-DQ2/8 genotype, we saw a significant decrease in IBC BND frequency in carriers of the HLA-DR4/4-DQ8/8 genotype associated with a high risk for type 1 diabetes and a significant increase in IBC BND in carriers of the protective HLA-DQ6 allele (Fig. 2B).
Figure 2.
Loss of IBC BND cells is associated with high-risk type 1 diabetes HLA genotypes. A: Percentage of IBC BND cells in FDRs stratified by HLA genotypes (n = 103). B: Percentage of IBC BND cells in healthy control subjects (H/Cs) (n = 49) stratified by HLA genotypes. On the x-axis, HLA genotypes are listed from highest to lowest risk (left to right) for development of type 1 diabetes. *P < 0.05 by Student t test.
Finally, we explored whether non-HLA type 1 diabetes susceptibility risk-conferring alleles affect maintenance of B-cell anergy. We used an Immunochip to assay >50 non-HLA risk-associated SNPs. To best detect the effect of each allele, we analyzed only FDRs who were heterozygous for the HLA-DR3-DQ2 or non–type 1 diabetes–associated HLA alleles (n = 60) because these individuals were most heterogeneous in BND frequency. We reasoned that the impact of non-HLA risk alleles might be evident in this population. These studies indicated that loss of IBC BND cells is associated with polymorphisms in the INS (rs689), PTPN2 (rs1893217), and IKZF3 (rs2872507) genes. In addition, reduced IBC BND frequency was observed in carriers of the high-risk PTPN22 (rs2476601) SNP, although significance was not reached because of the low frequency of this allele in the subject group (Fig. 3).
Figure 3.
Loss of IBC BND cells is associated with high-risk type 1 diabetes non-HLA genotypes. Percentage of IBC BND cells in FDRs who carry the risk allele for a particular SNP or do not. Risk alleles are in bold. ●, FDRs who carry the nonrisk allele; ○, FDRs who carry the risk allele. A total of 60 FDRs were analyzed for the presence or absence of each risk allele. *P < 0.05, **P < 0.01 by Student t test.
Discussion
Previous studies have shown that HLA class II is the major determinant of risk for type 1 diabetes development (21). Although both the HLA-DR4-DQ8 and HLA-DR3-DQ2 haplotypes confer the highest risk (22), development of IAAs is more frequent among subjects with HLA-DR4-DQ8 (23,24). Investigations also have demonstrated that the HLA-DQ8 molecule has an amino acid substitution at position 57 in the P9 pocket of the binding groove that creates a wider peptide-binding groove (25,26). Some argue that this substitution allows promiscuous binding and presentation of peptides, including those of insulin (26). Hence, the decreased frequency of anergic insulin-reactive B cells being closely associated with the DR4-DQ8 haplotype in FDRs is interesting. It is tempting to speculate that CD4+ T cells recognizing insulin peptides presented by IBCs may promote loss of anergy. However, in a parallel study, we found that unlike ex vivo IBCs from NOD mice, anergic IBCs from C57BL/6.H-2g7 do not process and display insulin 9-23 peptide in association with MHC class II (M.J.S. and J.C.C., unpublished observations). Thus, we hypothesize a two-step process in which B cells first lose anergy, gaining the ability to signal through the BCR and to process and present antigen, and are then further stimulated by T cells. These activated cells accumulate in pancreatic islets as shown by Leete et al. (27).
Consistent with the possibility that T cells promote departure of IBCs from the BND compartment is the association with SNP rs689 located in the highest risk-conferring non-HLA region INS. Carriers of this risk allele are believed to have decreased expression of insulin peptides in the thymus, leading to decreased negative selection of insulin-reactive T cells (28). In addition, a recent study found that SNP rs689 is significantly associated with development of IAAs, but not other autoantibodies, in patients with type 1 diabetes (29). Hence, it seems plausible that carriers of this polymorphism have increased numbers of peripheral insulin-reactive T cells that because they escaped central tolerance, could promote loss or prevent the establishment of B-cell anergy by providing the necessary T-cell help to an insulin-reactive B cell.
Departure of IBCs from the BND compartment also is associated with the PTPN2 SNP rs1893217. PTPN2 is broadly expressed in both B and T cells as well as in pancreatic β-cells. Studies have shown that PTPN2 has a range of functions, including negative regulation of JAK/STAT signaling (30) and dephosphorylation of Lck and Fyn after stimulation through the T-cell antigen receptor (31). In addition, the type 1 diabetes susceptibility PTPN2 SNP rs1893217 is associated with decreased interleukin-2 signaling in CD4+ T cells in healthy subjects (32). In subjects with type 1 diabetes, decreased interleukin-2 signaling has been shown to affect maintenance of FOXP3 expression in regulatory T (Treg) cells (33). Hence, it is plausible that the PTPN2 SNP rs1893217 could alter B-cell signaling, as it has been shown to do in T cells, or impair the development of Treg cells. The absence of Treg cells in the periphery has been shown to lead to the accumulation of autoreactive B cells (34) and loss of B-cell anergy (35). Consistent with this, studies in our laboratory have demonstrated that loss of IBC BND cells in FDRs is correlated with decreased frequency of CD4+CD25hiCD127lo Treg cells in the peripheral blood (Supplementary Fig. 2).
IKZF3 is a member of the Ikaros family of zinc-finger proteins that regulate B-cell proliferation and differentiation. Although not well studied in the context of type 1 diabetes, B cells in IKZF3-deficient mice have an activated phenotype (36), and the mice spontaneously develop human systemic lupus erythematosus–like features, including production of antinuclear antibodies and glomerulonephritis (37). Hence, loss of IBC BND cells in individuals carrying the type 1 diabetes risk-associated IKZF3 SNP could be due to changes in activation threshold caused by the polymorphism.
Taken together, the current results indicate that loss of anergy in IBCs is associated with high-risk HLA and non-HLA type 1 diabetes genotypes. These risk alleles could act in a B-cell intrinsic fashion to undermine B-cell anergy, thereby enabling autoantigen presentation and, consequently, development of type 1 diabetes. Genetic risk could also act in a B-cell extrinsic fashion by allowing more insulin-reactive T cells to enter the periphery, increasing T-cell help. Finally, risk alleles may promote loss of Treg cells. Studies are under way to explore these possibilities, including experiments in reductionist models of the effect of the individual risk alleles on B-cell anergy in a fixed genetic background. In addition, we are undertaking longitudinal studies to determine whether disappearance of anergic cells from peripheral blood of FDRs is predictive of later development of autoantibodies and disease.
Supplementary Material
Article Information
Funding. This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DP3-DK-110845, U01-DK085509, and R01-DK-096492), National Institutes of Health Office of the Director (F30-OD-021477), and National Institute of Allergy and Infectious Diseases (R21-AI-124488, R01-AI-124487, P01-AI-42288).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.J.S. performed experiments for Figs. 1–3 and analyzed the data. M.J.S. and J.C.C. wrote the manuscript. M.R. was the study coordinator. C.W. assisted in the non-HLA genotyping. C.E.M. and M.A.A. designed and funded the genotyping SNP kit. C.E.M., M.A.A., P.A.G., and J.C.C. provided funding and reviewed and edited the manuscript. P.A.G. and J.C.C. designed the research. J.C.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-0937/-/DC1.
References
- 1.Fiorina P, Vergani A, Dada S, et al. Targeting CD22 reprograms B-cells and reverses autoimmune diabetes. Diabetes 2008;57:3013–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleffel S, Vergani A, Tezza S, et al. Interleukin-10+ regulatory B cells arise within antigen-experienced CD40+ B cells to maintain tolerance to islet autoantigens. Diabetes 2015;64:158–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010;464:1293–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodie GM, Wallberg M, Santamaria P, Wong FS, Green EA. B-cells promote intra-islet CD8+ cytotoxic T-cell survival to enhance type 1 diabetes. Diabetes 2008;57:909–917 [DOI] [PubMed] [Google Scholar]
- 5.Mariño E, Tan B, Binge L, Mackay CR, Grey ST. B-cell cross-presentation of autologous antigen precipitates diabetes. Diabetes 2012;61:2893–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orban T, Sosenko JM, Cuthbertson D, et al.; Diabetes Prevention Trial-Type 1 Study Group . Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:2269–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science 2003;301:1374–1377 [DOI] [PubMed] [Google Scholar]
- 8.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med 1993;177:999–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol 2004;5:645–650 [DOI] [PubMed] [Google Scholar]
- 10.O’Neill SK, Getahun A, Gauld SB, et al. Monophosphorylation of CD79a and CD79b ITAM motifs initiates a SHIP-1 phosphatase-mediated inhibitory signaling cascade required for B cell anergy. Immunity 2011;35:746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooke MP, Heath AW, Shokat KM, et al. Immunoglobulin signal transduction guides the specificity of B cell-T cell interactions and is blocked in tolerant self-reactive B cells. J Exp Med 1994;179:425–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodnow CC, Crosbie J, Adelstein S, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature 1988;334:676–682 [DOI] [PubMed] [Google Scholar]
- 13.Gauld SB, Benschop RJ, Merrell KT, Cambier JC. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol 2005;6:1160–1167 [DOI] [PubMed] [Google Scholar]
- 14.Getahun A, Beavers NA, Larson SR, Shlomchik MJ, Cambier JC. Continuous inhibitory signaling by both SHP-1 and SHIP-1 pathways is required to maintain unresponsiveness of anergic B cells. J Exp Med 2016;213:751–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Browne CD, Del Nagro CJ, Cato MH, Dengler HS, Rickert RC. Suppression of phosphatidylinositol 3,4,5-trisphosphate production is a key determinant of B cell anergy. Immunity 2009;31:749–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith MJ, Packard TA, O’Neill SK, et al. Loss of anergic B cells in prediabetic and new-onset type 1 diabetic patients. Diabetes 2015;64:1703–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steck AK, Zhang W, Bugawan TL, et al. Do non-HLA genes influence development of persistent islet autoimmunity and type 1 diabetes in children with high-risk HLA-DR,DQ genotypes? Diabetes 2009;58:1028–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mychaleckyj JC, Noble JA, Moonsamy PV, et al.; T1DGC . HLA genotyping in the international Type 1 Diabetes Genetics Consortium. Clin Trials 2010;7(Suppl.):S75–S87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith MJ, Packard TA, O’Neill SK, et al. Detection and enrichment of rare antigen-specific B cells for analysis of phenotype and function. J Vis Exp 2017;(120):e55382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pugliese A, Gianani R, Moromisato R, et al. HLA-DQB1*0602 is associated with dominant protection from diabetes even among islet cell antibody-positive first-degree relatives of patients with IDDM. Diabetes 1995;44:608–613 [DOI] [PubMed] [Google Scholar]
- 21.Lambert AP, Gillespie KM, Thomson G, et al. Absolute risk of childhood-onset type 1 diabetes defined by human leukocyte antigen class II genotype: a population-based study in the United Kingdom. J Clin Endocrinol Metab 2004;89:4037–4043 [DOI] [PubMed] [Google Scholar]
- 22.Erlich H, Valdes AM, Noble J, et al.; Type 1 Diabetes Genetics Consortium . HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes 2008;57:1084–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schenker M, Hummel M, Ferber K, et al. Early expression and high prevalence of islet autoantibodies for DR3/4 heterozygous and DR4/4 homozygous offspring of parents with type I diabetes: the German BABYDIAB study. Diabetologia 1999;42:671–677 [DOI] [PubMed] [Google Scholar]
- 24.Kulmala P, Savola K, Reijonen H, et al.; Childhood Diabetes in Finland Study Group . Genetic markers, humoral autoimmunity, and prediction of type 1 diabetes in siblings of affected children. Diabetes 2000;49:48–58 [DOI] [PubMed] [Google Scholar]
- 25.Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature 1987;329:599–604 [DOI] [PubMed] [Google Scholar]
- 26.Horn GT, Bugawan TL, Long CM, Erlich HA. Allelic sequence variation of the HLA-DQ loci: relationship to serology and to insulin-dependent diabetes susceptibility. Proc Natl Acad Sci U S A 1988;85:6012–6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leete P, Willcox A, Krogvold L, et al. Differential insulitic profiles determine the extent of β-cell destruction and the age at onset of type 1 diabetes. Diabetes 2016;65:1362–1369 [DOI] [PubMed] [Google Scholar]
- 28.Vafiadis P, Bennett ST, Todd JA, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet 1997;15:289–292 [DOI] [PubMed] [Google Scholar]
- 29.Lempainen J, Härkönen T, Laine A, Knip M, Ilonen J; Finnish Pediatric Diabetes Register . Associations of polymorphisms in non-HLA loci with autoantibodies at the diagnosis of type 1 diabetes: INS and IKZF4 associate with insulin autoantibodies. Pediatr Diabetes 2013;14:490–496 [DOI] [PubMed] [Google Scholar]
- 30.Simoncic PD, Lee-Loy A, Barber DL, Tremblay ML, McGlade CJ. The T cell protein tyrosine phosphatase is a negative regulator of Janus family kinases 1 and 3. Curr Biol 2002;12:446–453 [DOI] [PubMed] [Google Scholar]
- 31.Wiede F, Shields BJ, Chew SH, et al. T cell protein tyrosine phosphatase attenuates T cell signaling to maintain tolerance in mice. J Clin Invest 2011;121:4758–4774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long SA, Cerosaletti K, Wan JY, et al. An autoimmune-associated variant in PTPN2 reveals an impairment of IL-2R signaling in CD4(+) T cells. Genes Immun 2011;12:116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long SA, Cerosaletti K, Bollyky PL, et al. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4(+)CD25(+) regulatory T-cells of type 1 diabetic subjects. Diabetes 2010;59:407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinnunen T, Chamberlain N, Morbach H, et al. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood 2013;121:1595–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonardo SM, De Santis JL, Malherbe LP, Gauld SB. Cutting edge: in the absence of regulatory T cells, a unique Th cell population expands and leads to a loss of B cell anergy. J Immunol 2012;188:5223–5226 [DOI] [PubMed] [Google Scholar]
- 36.Wang JH, Avitahl N, Cariappa A, et al. Aiolos regulates B cell activation and maturation to effector state. Immunity 1998;9:543–553 [DOI] [PubMed] [Google Scholar]
- 37.Sun J, Matthias G, Mihatsch MJ, Georgopoulos K, Matthias P. Lack of the transcriptional coactivator OBF-1 prevents the development of systemic lupus erythematosus-like phenotypes in Aiolos mutant mice. J Immunol 2003;170:1699–1706 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.