Abstract
Schizophrenia (SCZ) is a neurodevelopmental psychiatric disorder, in which cognitive function becomes disrupted at early stages of the disease. Although the mechanisms underlying cognitive impairments remain unclear, N-methyl-D-aspartate receptors (NMDAR) hypofunctioning in the prefrontal cortex (PFC) has been implicated. Moreover, cognitive symptoms in SCZ are usually unresponsive to treatment with current antipsychotics and by onset, disruption of the dopamine system, not NMDAR hypofunctioning, dominates the symptoms. Therefore, treating cognitive deficits at an early stage is a realistic approach. In this study, we tested whether an early treatment targeting mGluR2 would be effective in ameliorating cognitive impairments in the methylazoxymethanol acetate (MAM) model of SCZ. We investigated the effects of an mGluR2 agonist/mGluR3 antagonist, LY395756 (LY39), on the NMDAR expression and function in juveniles, as well as cognitive deficits in adult rats after juvenile treatment. We found that gestational MAM exposure induced a significant decrease in total protein levels of the NMDAR subunit, NR2B, and a significant increase of pNR2BTyr1472 in the juvenile rat PFC. Treatment with LY39 in juvenile MAM-exposed rats effectively recovered the disrupted NMDAR expression. Furthermore, a subchronic LY39 treatment in juvenile MAM-exposed rats also alleviated the learning deficits and cognitive flexibility impairments when tested with a cross-maze based set-shifting task in adults. Therefore, our study demonstrates that targeting dysfunctional NMDARs with an mGluR2 agonist during the early stage of SCZ could be an effective strategy in preventing the development and progression in addition to ameliorating cognitive impairments of SCZ.
Keywords: mGluR2/3, NMDA receptor, prefrontal cortex, cognitive function, animal model, schizophrenia
Introduction
Schizophrenia (SCZ) is a neurodevelopmental disorder with prominent positive, negative, and cognitive symptoms occurring at various stages of the disease (van Os and Kapur, 2009; Insel, 2010). The onset of cognitive impairments typically arises during the early stages and lasts throughout the entire disease progression of SCZ. Cognitive impairments are the best predictor of long-term functional outcome (Cannon, 2015); however, the underlying mechanisms of cognitive dysfunction remain unclear. Nevertheless, N-methyl-D-aspartate receptors (NMDAR) hypofunctioning in the prefrontal cortex (PFC) has been strongly implicated in the pathology of SCZ. Currently, the widely used therapeutics for SCZ, including both typical and atypical antipsychotics, mainly target the dopamine system (Miyamoto et al., 2005; Howes and Kapur, 2009). Although antipsychotics are effective in ameliorating positive symptoms, these drugs are typically accompanied by strong side-effects and encompass limited efficacy in improving cognitive impairments (Miyamoto et al., 2005; Gomes et al., 2016). Therefore, there is an urgent need to search for other potential drug candidates for SCZ.
Pharmacologically targeting metabotropic glutamate receptor 2/3 (mGluR2/3) offers promising therapeutic effects to patients with SCZ (Moghaddam and Adams, 1998; Patil et al., 2007; Conn et al., 2009; Mezler et al., 2010; Kinon et al., 2011; Fell et al., 2012; Kinon et al., 2015). Recent studies have shown that compounds targeting mGluR2/3 are well-tolerated, exhibit few side-effects, and demonstrate a strong potential for alleviating cognitive impairments in both animal models and human subjects with SCZ (Helton et al., 1998; Spooren et al., 2002; Swanson et al., 2005; Fell et al., 2012; Harvey and Shahid, 2012). Specifically, activation of mGluR2/3 can decrease glutamate release pre-synaptically (Schoepp et al., 1999; Cartmell and Schoepp, 2000; Moghaddam, 2004), and simultaneously potentiate NMDAR function post-synaptically (Tyszkiewicz et al., 2004; Xi et al., 2011; Cheng et al., 2013; Trepanier et al., 2013; Wang et al., 2013; Li et al., 2015a; Li et al., 2015b; Engel et al., 2016).
However, our recent study demonstrated that LY395756 (LY39), a novel compound serving as both an mGluR2 agonist and mGluR3 antagonist, enhanced PFC NMDA receptor expression and function in the normal adult rat PFC, but fails to improve working memory and reverse MK801-induced working memory impairments (Li et al., 2015b). We speculate that as a neurodevelopmental disorder, a failure to correct synaptic disruption immediately following symptom onset would miss a critical treatment or prevention window for SCZ (Insel, 2010). In fact, the adolescence period has been identified as a vulnerability window and intervention time for treatment of SCZ (Gomes et al., 2016). Because NMDAR dysfunction occurs in the first (juvenile) stage of disease, early intervention would theoretically be more effective than treatment in later stages of the disorder. In this study, we tested this hypothesis by further examining the effects of LY39 on NMDAR expression and function in juvenile rats of the neurodevelopmental methylazoxymethanol acetate (MAM) model for SCZ. Our results showed that although LY39 had no effects on NMDAR expression and function in saline-treated animals or on neuronal excitability of prefrontal cortical neurons in vitro, treatment with LY39 during the juvenile period significantly reversed the misexpression of NMDAR subunits in MAM-treated rats. Importantly, treatment with LY39 in juveniles successfully reversed cognitive impairments in adults when examined on a cross-maze task. Our data provide novel insight for early intervention with mGluR2 activation for the treatment of cognitive impairments in SCZ.
Materials and Methods
Animals and Treatments
Animals used in this study were housed in a temperature-controlled room (21–23°C) on a 12 h light/dark cycle with food and water available ad libitum. All procedures were performed in accordance with the National Institutes of Health (NIH) animal use guidelines and were approved by the Institutional Animal Care and Use Committee of Drexel University College of Medicine.
Pregnant Sprague-Dawley dams at embryonic day 15 (E15, 250–280g) were purchased from the Charles River Laboratories (Wilmington, MA). Animals were given two days to habituate to the new environment, prior to experiments. Pregnant rats at E17 were injected (intraperitoneally, i.p.) with either neurotoxin MAM (25 mg/kg), used as a neurodevelopmental animal model for SCZ, or saline as a vehicle control, as we recently reported (Snyder et al., 2013). Pups were weaned and rehoused on postnatal day 21 (P21). For all experiments, animals aged P12–21 as juveniles, P28–45 as adolescents, and P90 as adults (Spear, 2000; Wang and Gao, 2009, 2010; Snyder et al., 2013).
To examine the acute effects of LY39 on NMDA receptor expression and function, LY39 at a dose of 0.3, 1.0, or 3.0 mg/kg (single dose, i.p.) was administered in juvenile rats. After one hour, PFC tissue was collected for Western blot analysis, with brain concentration of the compound peaking within 30 min of administration (Bond et al., 2000). For separate electrophysiology experiments, LY39 at a dose of 0.3 and 3.0 mg/kg (i.p.), respectively, was administered one hour before the animals were sacrificed for living slices. In both cases, saline solution (0.9 % sodium chloride) was used as vehicle control. For cross-maze, subchronic administration of LY39 or saline was given on five consecutive days with a single daily injection of 0.3 mg/kg (i.p.) from P21–P25 for both saline- and MAM-exposed male juveniles. Similarly, mPFC tissue was collected for Western blotting of NMDA protein levels one hour after performing the cross maze test. All animals were deeply anesthetized with Euthasol (0.2 ml/kg, Virbac Animal Health) and were immediately decapitated per the approved IACUC protocol for tissue collection. LY39 was purchased from Tocris Bioscience (Minneapolis, MN), and methylazoxymethanol acetate (MAM) was purchased from MRIGlobal Chemical Carcinogen Repository (Kansas City, MO).
Synaptic membrane protein collection
Animals were decapitated, and the brains were quickly removed. Brain regions containing the prelimbic area were dissected and homogenized in cold lysis buffer (in mM: 320 sucrose, 4 HEPES-NaOH buffer, pH 7.4, 2 EGTA, 1 sodium orthovanadate, 0.1 phenylmethylsulfonyl fluoride, 10 sodium fluoride, 10 sodium pyrophosphate, with 1 µg/ml leupeptin and 1 µg/ml aprotinin). Lysates were centrifuged at 1,000 g for 10 minutes at 4°C to remove large cell fragments and nuclear materials; the resulting supernatant was centrifuged again at 15,000 g for 15 min at 4°C to harvest cytoplasmic proteins in the supernatant. The pellet from this spin was resuspended in lysis buffer and centrifuged at 15,000 g for an additional 15 minutes at 4°C to produce synaptosomes. The synaptosomal fraction was then hypoosmotically lysed and centrifuged at 25,000 g for 30 minutes at 4°C to collect synaptosomal plasma membranes in the pellet. Lysis buffer was added to the pellet to make the final samples, which were then stored in −80°C for future use, or an aliquot was made and stored at −20°C for immediate use.
Western blotting
A bicinchoninic acid (BCA) protein assay was performed to determine protein concentration. Protein samples were prepared with 4× laemmli and lysis buffer, boiled for 5 minutes, and separated on a 7.5% SDS-PAGE gel. After electrophoresis, proteins were transferred to Immobilon PVDF membranes (Millipore, IPVH00010). The membrane was blocked with 5% nonfat milk and probed with primary antibodies at 4°C overnight. Each blot was used to probe multiple antibodies, including anti-mouse NR1 (Invitrogen, 32-0500, 1:5000), anti-rabbit NR2A (Millipore, 04-901, 1:2,500), anti-mouse NR2B (Millipore, 05-920, 1:2000), anti-rabbit pNR2B-Tyr1472 (Millipore, 454583, 1:1000), anti-rabbit pNR2B-Ser1303 (Millipore, 07-398, 1:1000), and anti-mouse actin (Sigma, A5316, 1:100,000) served as a loading control. The blots were incubated with horseradish peroxidase-coupled anti-rabbit or anti-mouse IgG secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) at room temperature for 1 hour. Signals were visualized using enhanced chemiluminescence (ECL Plus, Amersham Biosciences). Each group included at least 5 animals and samples from each animal were run at least 4 times to minimize inter-blot variance.
Electrophysiological recording in prefrontal cortical slices
Coronal slices of brain regions containing the prelimbic area (mPFC) were cut at 300 µm within an ice-cold oxygenated sucrose solution (in mM: 87 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 75 sucrose, 25 glucose, 0.5 CaCl2, 7.0 MgSO4, pH 7.4) using a Leica VT 1200S Vibratome (Leica Microsystems Inc., Buffalo Grove, IL). Slices were transferred to an incubation chamber, submerged in oxygenated Ringer’s solution (in mM: 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgSO4, 26 NaHCO3, and 10 dextroses, pH 7.4) at 37°C for one hour. The PFC slices were transferred into a heated (36°–37°C) recording chamber mounted on an Olympus BX51 upright microscope (Olympus America Inc., Center Valley, PA), where the slices were bathed in oxygenated Ringer’s solution. Neurons were visualized with infrared differential interference contrast via a Panasonic CCD video camera. Borosilicate glass pipettes (Harvard Apparatus, Holliston, MA) were pulled with a P-97 puller (Sutter Instrument, Novato, CA) such that the patch electrode had a resistance of 5–7 MΩ. The electrical signals were amplified and filtered at 1 kHz with a MultiClamp 700B amplifier, converted through a DigiData 1322A, and recorded through pCLAMP 9.2 software (Molecular Devices, Sunnyvale, CA).
Action potentials from layer 5 pyramidal neurons in the mPFC were recorded under current clamp mode with a current step protocol starting at −300 pA that increased from 20 steps at 50-pA increments at 1500 ms duration. The patch pipette was filled with a K+-gluconate intracellular solution (in mM: 120 K+-gluconate, 20 KCl, 4 Na2ATP, 0.3 Na2GTP, 5 Na2phosphocreatine, 0.1 EGTA, 10 HEPES, pH ~7.3). The access resistance of the recording was bridge-balanced, and the series resistance of the neuron was monitored and adjusted during recording through a −100 pA pulse at a duration of 150 ms. A baseline with vehicle was recorded, followed by bath application of LY39 at different concentrations of 0.1, 0.3, 1.0, 3.0, or 10.0 µM for at least 5 minutes before recording the action potentials.
Saline- and MAM-treated juvenile rats (P15–P25) were used to record NMDAR-mediated miniature excitatory postsynaptic currents (mEPSCs). One hour after a saline or LY39 injection, animals were anesthetized, and brains were removed. Cs+-intracellular solution (in mM: 110 D-gluconic acid, 110 CsOH, 10 CsCl2, 1 EGTA, 1 CaCl2, 10 HEPES, 1 Mg-ATP at pH 7.3 adjusted with CsOH and 5 QX-314) was used in order to block sodium and potassium channels. Under the voltage clamp mode, NMDAR-mEPSCs were recorded with the membrane potential held at +60 mV in the presence of TTX (0.5 µM), picrotoxin (100 µM) and AMPAR antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX, 20 µM). A 5-min stable baseline EPSC was recorded from each cell to ensure the reliability of the recording, and then the recording was continued for another 5 min under each condition. The series resistance was compensated and constantly monitored with an injection of −100 pA current pulse (150 ms duration).
MAM model of SCZ and cross-maze behavioral testing
Saline- and MAM-treated male juvenile rats (P21) were treated with LY39, and divided into four groups with initially 12 male rats in each group. The saline-saline (SS) and MAM-saline (MS) groups received five consecutive daily i.p. injection of saline from P21 to P25 between 10:00 and 11:00 AM, whereas the saline-LY39 (SL) and MAM-LY39 (ML) groups received daily injections of 0.3 mg/kg LY39 at the same time each day. The animals grew to adulthood (P90) for the behavioral test.
For the cross-maze test, adult saline- and MAM-treated animals were food restricted for two weeks before the cross-maze test. Twelve to fourteen grams of standard chow were provided daily. Animals were weighed 5 days/week, and they were maintained at no lower than 85% of their original body weight. A cross-maze box with four arms, including two white (smooth/rough) and two black (smooth/rough) arms, and four food wells at the ends of each arm, was used to assess PFC-dependent cognition (Stefani et al., 2003; Jeevakumar et al., 2015). During the task, frosted cheerios were used as the food reward. Animals were habituated to the maze for four consecutive days, which included exploration and getting accustomed to being placed in a start arm and turning right or left for a food reward. The set-shifting test is then conducted over two consecutive days. On the first day of testing (Set 1), rats are trained to a criterion performance level (8 consecutive successful trials) on brightness (black vs. white maze arms) discrimination. On the following day, the rats are trained to the alternative discrimination strategy, texture (rough vs. smooth arms, Set 2) for 80 trials, regardless of performance level. The outcome measure for day 1 is the number of trials and time to criterion, while these same parameters along with the total percentage of correct trials over 80 trials were evaluated for Set 2. Only animals that finished the task to completion, with a full set of results were included in the data analysis. Outliers were excluded from the data set, which included animals that fell three standard deviations from the mean and those that did not complete the task. This resulted in a n=8 per group.
Data analysis
For Western blotting, the relative expression of proteins was evaluated by measuring the optical density with the NIH Image J Pro software. The densitometry of total protein was normalized to actin, whereas phosphorylation of protein was normalized to its respective total protein level. Data that was non-normal based normality testing was further probed for possible outliers (z-score: ±3). Any identified outliers were excluded from the data set due to poor band quality or experimental errors.
For electrophysiological recordings, data were analyzed with Clampfit 9.2 (Molecular Devices, Sunnyvale, CA). To analyze the drug effects on neuronal excitability, a typical action potential was selected to create a sample template, and then spike numbers within each current step were automatically counted by the software. Similarly, a typical AMPAR-mediated sEPSC or an NMDAR-mediated mEPSC was selected to create a template, and then mEPSCs within each 5-min recording were detected by the Clampfit software with a template match threshold setting at a medium of 5 (range 1–9). All detected events were manually visualized and checked to ensure the reliability of the method. The mEPSC frequency (number of events) was normalized to events per second (Hz), and the mEPSC amplitude was measured from the onset to the peak of the events.
All data are presented as mean ± standard error. Data between two groups were analyzed with unpaired Student’s t-tests (with equal variances) or F-test (with unequal variances). Comparisons of multiple groups were carried out with ANOVA with SPSS followed by Tukey’s post hoc test to compare between individual groups. Statistical analysis was performed using GraphPad 5.0 (GraphPad Software Inc., La Jolla, CA) and a p-value <0.05 was considered statistically significant.
Results
Prenatal MAM treatment induces NMDAR misexpression in the juvenile rat PFC
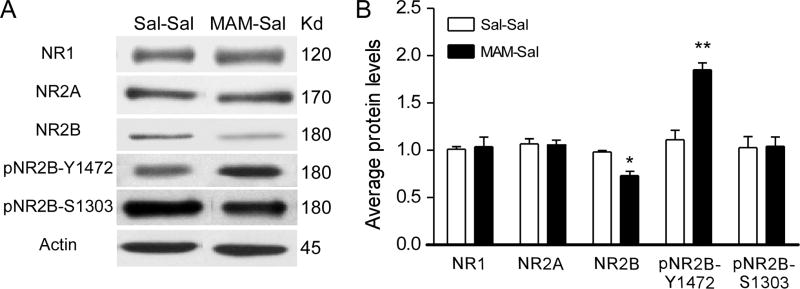
First, we examined protein levels of NMDAR subunits and two major phosphorylation sites of NR2B, pNR2BTyr1472 (Y1472) and pNR2BSer1303 (S1303). These two phosphorylation sites have been reported to be mainly involved in NMDAR synaptic insertion (Y1472) and functional enhancement (S1303) (Hallett et al., 2006; Chen and Roche, 2007; Li et al., 2009; Petralia et al., 2009; Mao et al., 2011). MAM- and Saline-treated pups at P21 were used for protein assessment. In PFC synaptosomal fractions of juvenile MAM animals, there was a significant decrease in NR2B protein levels and a significant increase in pNR2BTyr1472 (Y1472) compared to the saline control (n=3–5 rats for both Saline and MAM after 1 or 2 outliers were excluded from the analysis; t(8)=3.915, p=0.011 for NR2B and t(5)=5.537, p=0.003 for pNR2BTyr1472; Fig. 1). No difference in protein levels was found for NR1, NR2A or pNR2BSer1303 (S1303) (t(8)=0.110, p=0.918 for NR1; t(8)= − 0.590, p=0.587 for NR2A; t(5)=0.840, p=0.936 for pNR2B; respectively). These results indicated that MAM exposure induced an NMDAR misexpression in the PFC at the juvenile time point, which is consistent with our recent report in the hippocampus (Snyder et al., 2013). Furthermore, the misexpression seems to be selective to the NR2B subunit.
Figure 1.
Prenatal injection of MAM (25 mg/kg, i.p.) induced NMDARs misexpression in the juvenile rat PFC. A, Representative Western blots of NMDAR subunits and NR2B phosphorylation sites Y1472 and S1303. B, Summary histogram shows the protein levels of NMDAR subunits and phosphorylation of NR2B in synaptic plasma membrane fractions from saline- and MAM-exposed animals at P21. There was a significant decrease in NR2B and a significant increase in pNR2BY1472 in MAM-exposed rats (*p<0.05; **p<0.01 compared with Saline controls).
LY39 in Saline-treated animals has no effect on NMDAR expression but rescues the misexpression of NMDARs in MAM-exposed rat PFC
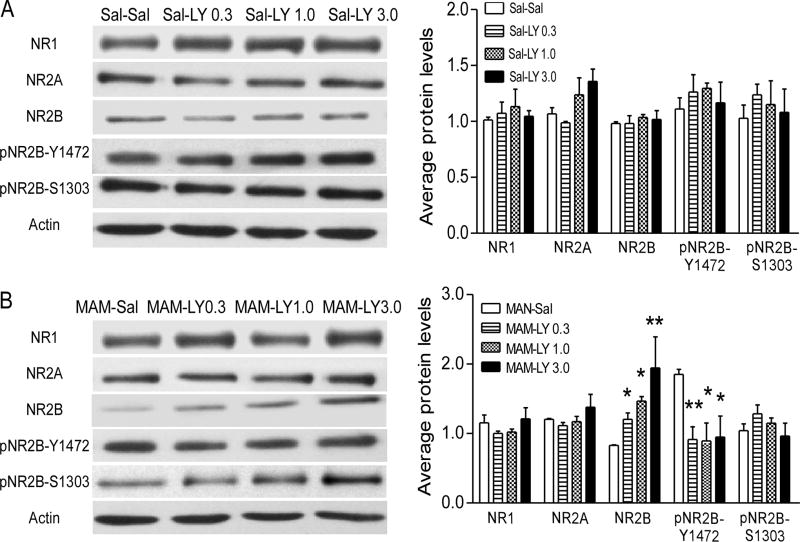
In our recent study, we reported that LY39 was very effective in regulating the expression and function of NMDARs in normal adult rat PFC in a dose-dependent manner (Li et al., 2015b). We, therefore, wondered whether LY39 had similar effects on NMDAR expression in juvenile saline-treated rats and whether this treatment was able to rescue the dysregulation of NMDARs in MAM-exposed rats. As shown in Figure 2A, we observed no significant effect of LY39 treatment in Sal or MAM on the expression of NR1 (Two-way ANOVA, main effect of exposure, F(1,26)=0.12, p=0.913) and no dose-dependent effect of LY39 treatment (main effect of dose, F(3,26)=0.667, p=0.580). While the protein level of NR2A showed a significant main effect of LY39 dose (Two-way ANOVA, F(3,26)=3.177, p=0.041), there was no exposure effect (Two-way ANOVA, F(1,26)=0.177, p=0.677). Post-hoc test exhibited a marginally significant change in Sal-exposed animals but not in the MAM-exposed group (Sal-exposed: One-way ANOVA, F(3,13)=3.381, p=0.0512. MAM-exposed: One-way ANOVA, F(3,13)=0.907, p=0.464). Interestingly, we found that both exposure and treatment had significant effects on the levels of NR2B (Two-way ANOVA, the main effect of exposure: F(1,26)=8.599, p=0.007; the main effect of dose: F(3,26)=5.803, p=0.004). Due to the significant interaction effect between exposure and dose (F(3,26)=4.999, p=0.007), we performed a simple-effect test. Statistic results indicated that in the MAM-exposed group, NR2B exhibited a significant dose-dependent increase (MAM-Sal vs MAM-LY0.3, p=0.039; vs MAM-LY1.0, p=0.002; vs MAM-LY3.0, 1.94±0.89, p<0.000). Meanwhile, treatment of LY39 at 3.0 mg/kg had significant stronger effect compared with 0.3 mg/kg (p=0.004) and 1.0 mg/kg (0.049). However, this effect was not found in the Sal-exposed group (p>0.05 for all). Also, we observed that 3.0 mg/kg LY39 had significantly stronger effect in the MAM-exposed group compared with a Sal-exposed group (p<0.0001). In contrast, both NR2B phosphorylation sites, Y1472 and S1303, were not affected by either exposure or different doses of LY39 (Y1472: Main effect of exposure, F(1,23)= 0.001, p=0.979, main effect of dose, F(3,23)=1.69, p=0.197, interaction effect, F(3,23)=2.768, p=0.065. S1303: Main effect of exposure, F(1, 23)=0.018, p=0.894, main effect of dose, F(3,23)=1.094, p=0.371, interaction effect, F(3,23)=0.116, p=0.950; Figure 2B). These data suggest that LY39 is effective in reversing juvenile NMDAR dysregulation in the PFC of MAM-exposed rats, and that juvenile rats exhibited a distinct sensitivity to LY39 in a state-dependent manner.
Figure 2.
In vivo treatment with LY39 exhibits no effects on prefrontal NMDAR subunit expression and phosphorylation in saline-treated juvenile rats, but significantly reverses the downregulation of prefrontal NR2B in the MAM-exposed rat. A, Representative Western blots and summary histograms of saline control and LY39 administration at 0.3, 1.0, or 3.0 mg/kg in saline-treated rats. LY39 at all three doses exhibited no effects on NMDAR subunit expression and NR2B phosphorylation (p>0.05 for all comparisons). B, Similar to those shown in Figure 1, NR2B and pNR2B-Y1472 in the MAM-SAL animals showed significant dysregulation compared to saline control, and LY39 reversed these dysregulations to baseline levels and rebound increases of NR2B at higher doses of 1.0 and 3.0 mg/kg (*p<0.05, **p<0.01). No significant differences were observed in NR1, NR2A and pNR2B-S1303. Note: Sal-Sal, Sal-LY0.3, Sal-LY1.0, Sal-LY3.0 account for Saline animals treated with Saline, 0.3 mg/kg LY39, 1.0 mg/kg LY39, and 3.0 mg/kg LY39, respectively; and MAM-Sal, MAM-LY0.3, MAM-LY1.0, MAM-LY3.0 mean MAM animals with these 4 treatments, respectively.
In vivo treatment of LY39 exhibited dose-dependent effects on NMDA-mEPSCs in Saline- and MAM-treated juvenile rats
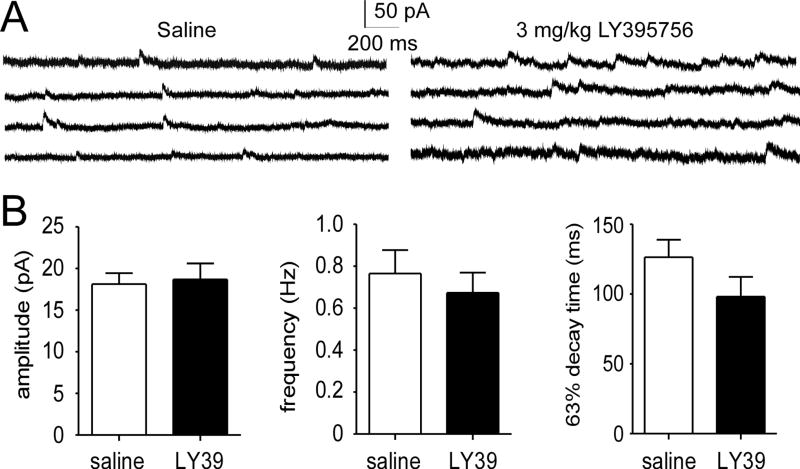
LY39 effectively reversed the downregulation of NR2B in MAM-exposed rats while exhibiting no effects on saline-treated animals. We examined this observation by the in vivo treatment of saline-treated juvenile rats with a high dose of LY39 (3.0 mg/kg) and then recorded NMDAR-mediated mEPSCs. Consistently, we found no significant differences in NMDA-mEPSC amplitude, frequency, and 63% decay time between the LY39- and saline-treated groups (n=14 for Sal-Sal and n=12 for Sal-LY39, t(24)=0.204, 0.123, 0.740 for amplitude, frequency, and 63% decay respectively; p>0.05 for all comparisons; Figure 3A and B). These results support our protein level data detected by Western blot (Figure 2).
Figure 3.
LY39 (3.0 mg/kg) in vivo treatment induced no significant changes in NMDA-mEPSC in layer 5 pyramidal neurons in the normal juvenile rat PFC. A, Representative NMDA-mEPSC traces of in vivo saline- and LY39-treated juvenile rats. B, Summary bar graphs of NMDA-mEPSC amplitude, frequency, and 63% decay time between in vivo saline-treated and LY39-treated groups. No significant differences were observed in all three parameters (n=14 for saline and n=12 for LY39; p>0.05 for all comparisons).
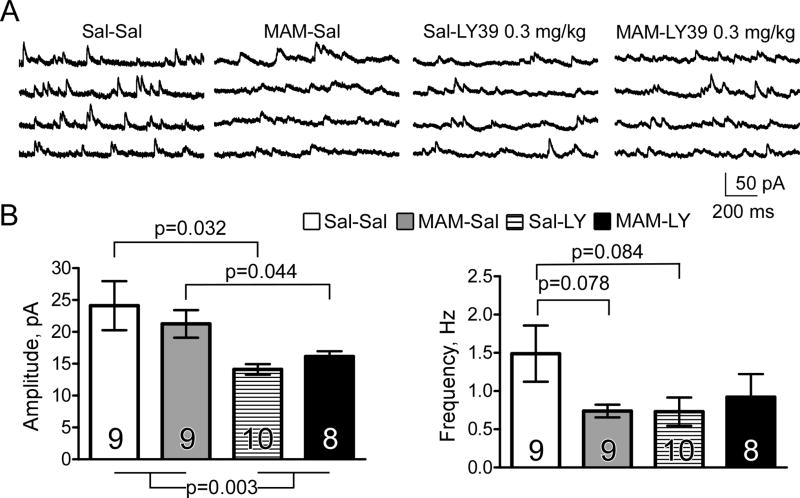
We further examined whether a lower dose of LY39 in vivo, which was able to revert NR2B protein loss in MAM animals would have different effects on NMDA-mEPSCs in Saline and MAM-treated animals. Prenatal MAM exposure did not affect the amplitude of NMDA-mEPSCs (Sal-Sal vs MAM-Sal; t(15)=0.626, p=0.541) but induced a trend of decrease in mEPSC frequency (t(15)=1.88, p=0.078). Surprisingly, we found that LY39 (0.3 mg/kg, i.p.) treatment for one hour significantly decreased the amplitude of NMDA-mEPSCs in both Saline and MAM animals (Two-Way ANOVA, treatment F(1,30)=10.28, p=0.003. Post-hoc: Sal-Sal vs Sal-LY: t(16)=2.54, p=0.032; MAM-Sal vs MAM-LY: t(14)=2.21, p=0.044; Figure 4). LY39 treatment also reduced the frequency of NMDA-mEPSCs in Saline animals, but the reduction was not statistically significant, and LY39 had no effect in MAM animals (Two-Way ANOVA, treatment F(1,30)=1.12, p=0.282. Post-hoc: Sal-Sal vs Sal-LY: t(16)=1.84, p=0.084; MAM-Sal vs MAM-LY: t(14)=−0.574, p=0.582; Fig. 4B). These findings are partially consistent with the protein levels and phosphorylation state of NMDAR subunits exhibited in Figures 1 and 2.
Figure 4.
LY39 (0.3 mg/kg, i.p.) in vivo treatment for one hour significantly decreases the amplitude and frequency of NMDA-mEPSCs in both saline and MAM animals. A, Representative NMDA-mEPSCs for each of four groups (Sal-Sal, MAM-Sal, Sal-LY39, MAM-LY39). Saline and LY39 injections (0.3 mg/kg, i.p.) were administered one hour before slice preparation for electrophysiology. B, Histograms depicting the effect of prenatal MAM exposure on the amplitude and frequency of NMDA-mEPSCs, as well as the effect of LY39 in both saline and MAM animals following one-hour exposure. LY39 treatment significantly decreases the amplitude of NMDA-mEPSCs, regardless of group (p<0.05 for both Sal-Sal and MAM-Sal animals) but the amplitude is not affected by MAM exposure (p>0.05 vs Sal-Sal). Prenatal MAM exposure and LY39 treatment reduce the frequency of NMDA-mEPSCs compared to Sal-Sal animals, but have no statistical significance (p>0.05 for both). LY39 treatment does not affect the frequency of NMDA-mEPSCs in MAM animals (p>0.05 MAM-SAL vs. MAM-LY39).
Subchronic treatment with LY39 in juveniles successfully reversed the learning deficits and cognitive flexibility impairment induced by prenatal MAM exposure in adulthood
SCZ is a chronic developmental disorder, with cognitive impairments usually presenting during the juvenile period and persisting into adulthood. Therefore, we proposed that juvenile MAM-treated rats would exhibit cognitive impairments in comparison to saline controls. Furthermore, since the administration of LY39 at a dose of 0.3 mg/kg effectively reversed the dysregulation of NMDAR protein levels induced by prenatal MAM exposure in the juveniles, we hypothesized that subchronic treatment of LY39 in juvenile MAM-exposed rats could be effective in reversing the cognitive impairment in adulthood. To test this possibility, we used a cross-maze based set-shifting task (Stefani et al., 2003) to examine whether deficits in cognitive flexibility could be restored by treatment with LY39 during the juvenile period.
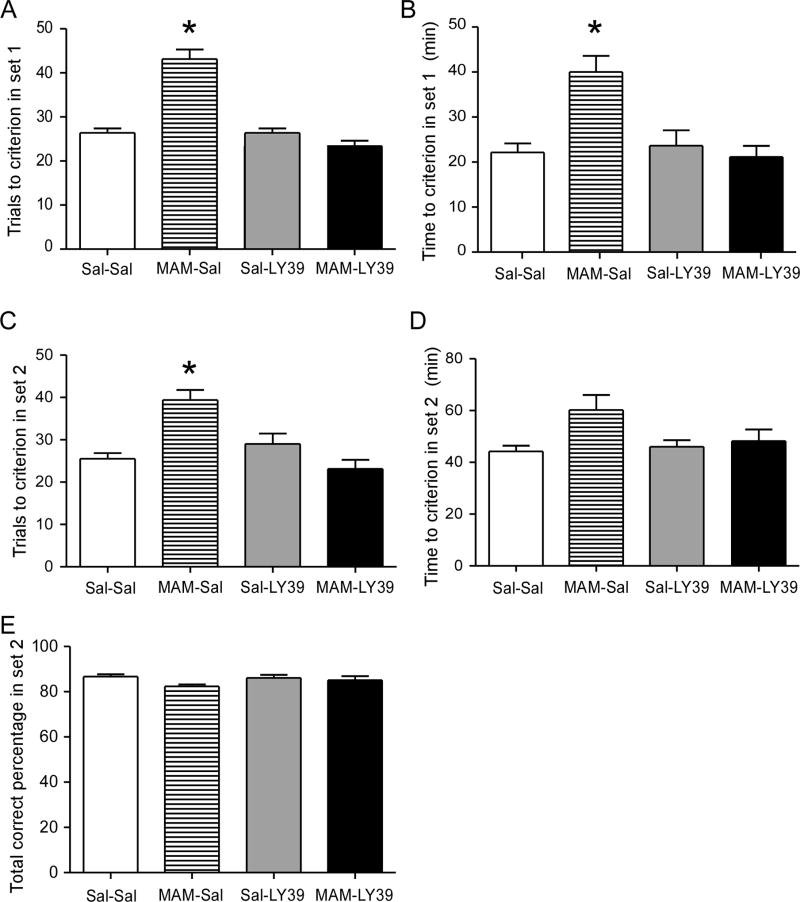
As shown in Figure 5, we recorded the results on both Set 1 and Set 2 experiments. In Set 1, MAM-saline rats required a significantly greater number of trials and more time to reach criterion, i.e., 8 consecutive correct trials (n=8 for all groups; F(1, 31)=34.5, p<0.05 for all comparisons; Fig. 5A and B), suggesting a learning deficit. With LY39 treatment, this impairment was effectively reversed (t(14)=1.872, p>0.05 MAM-LY vs. Sal-Sal). No significant differences were observed between saline-saline and saline-LY39 groups (t(14)=0.073, p>0.05). Since animals need to shift the reward discrimination from brightness (Set 1) to texture (Set 2), the performance in Set 2 is an indicator how efficiently animals can shift their strategy to obtain a reward (i.e., cognitive flexibility). MAM-saline animals required a greater number of trials to reach criterion in Set 2. This set-shifting deficit was ameliorated by treatment with LY39 (p>0.05 MAM-LY vs. Sal-Sal). However, MAM-saline rats did not show impairments in total time or total correct percentage across trials (Fig. 5C, D, E), suggesting that adult MAM-exposed rats did not exhibit a general disruption of performance. Therefore, these results suggest that MAM exposure induces an impairment of learning as well as cognitive flexibility that persists through adulthood, and juvenile LY39 treatment effectively rescues performance.
Figure 5.
Summary graphs of cross maze show an effective reversal of cognitive flexibility impairment by LY39 treatment in juveniles. A and B, MAM-Saline rats required a significantly greater number of trials and more time to reach criterion – 8 consecutive correct trials in Set 1, and 0.3 mg/kg in vivo LY39 treatment during the juvenile period completely reversed the disruption (n=8 for each group, *p<0.05). C, D, and E, MAM-Saline animals required a significantly greater number of trials to reach criterion but did not show impairment in total time and total correct percentage in Set 2. Treatment with 0.3 mg/kg LY39 in vivo during the juvenile period successfully reduced the trials to reach criterion to saline control level in the MAM-LY39 group (n=8 for each group, *p<0.05).
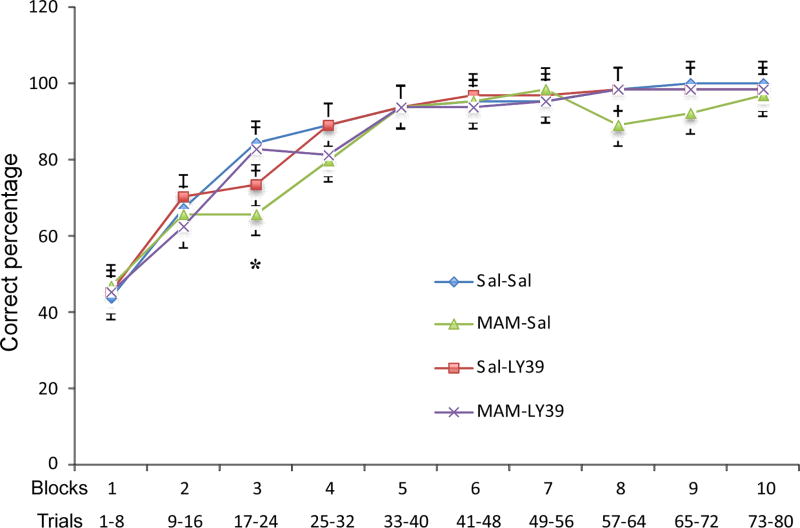
Furthermore, Figure 6 displays the animals’ learning progress and provides a clear illustration of their performance. The MAM-treated animals took a significantly longer time to reach criterion, demonstrating an average of 35–40 trials, whereas saline-treated animals reached criterion after only 25 trials (the third time bin). It is therefore expected to see a significant difference in the third block because this block contained trials 17 to 24 but no differences in performance after this point, since the total amount of correct trials was not significantly different (one-way ANOVA, F(1, 31)=2.420, Saline-Saline vs. Saline-LY39 p=0.008, vs. MAM-saline p=0.002, and vs. MAM-LY39 p>0.05; Fig. 6). There is significant improvement between each block from block 1 to block 4 in all four groups (p<0.05) and all animals reach >90% correct responding after block 4. However, in blocks 8 and 9, MAM-treated rats showed lower though not significantly reduced the correct percentage of responses compared with the other three groups. The specific mechanism of this phenomenon is unclear, but it is possible that MAM-treated rats had an increased preservation to a previously learned discrimination rule in Set 1 (Stefani et al., 2003).
Figure 6.
A summary graph of correct percentage in each block in Set 2 of the cross-maze. Set 2 contains 10 blocks, and each block contains 8 trials. MAM-Saline animals showed significantly lower correct percentage compared with Saline-Saline animals in block 3 (n=8 for each group, *p<0.05). No significant differences were found in other blocks among all groups (p>0.05).
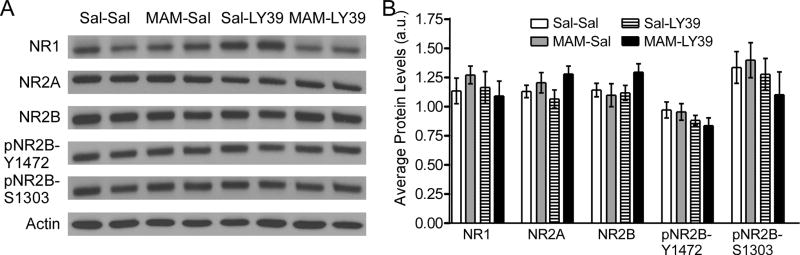
After behavioral testing, we collected the brain from all participating animals in order to assess protein levels of NMDAR subunits and phosphorylation at NR2B. As shown in Figure 7, in adulthood, MAM-exposed animals did not exhibit differences in levels of NMDAR subunits and phosphorylation sites of NR2B (NR1: t(8)=−1.03, p=0.335; NR2A: t(8)=−0.731, p=0.486; NR2B: t(8)=0.391, p=0.706; pNR2B-Y1472: t(8)=0.174, p=0.866; pNR2B-S1303: t(8)=−0.309, p=0.765). LY39 treatment during the juvenile stage also did not alter the protein levels of any NMDAR subunits or phosphorylation of NR2B in adult saline or MAM animals (Two-Way ANOVA treatment, NR1: F(1,16)=0.431, p=0.521; NR2A: F(1,16)=0.005, p=0.943; NR2B: F(1,16)=1.342, p=0.264; pNR2B-Y1472: F(1,16)=2.66, p=0.122; pNR2B-S1303: F(1,16)=1.29, p=0.273; Figure 7). Overall, protein expression of NR1, NR2A, NR2B, pNR2B-Y1472, and pNR2B-S1303 was comparable among the groups, neither affected by prenatal MAM-exposure nor by treatment with LY39 during juvenile development. These results suggest that NMDAR subunit expression in adult MAM rat PFC is normalized by either a compensative mechanism during development or the LY39 treatment during juvenile period.
Figure 7.
In adulthood, MAM-exposed animals do not exhibit differences in NMDAR subunits and phosphorylation sites of NR2B in the PFC. Juvenile LY39 treatment (0.3 mg/kg, i.p., daily for five consecutive days) does not alter NMDAR subunits or phosphorylation of NR2B in both adult saline and MAM animals tested in adulthood. A, Representative Western blots of Saline-Saline, MAM-Saline, Saline-LY39, and MAM-LY39 treated animals for NMDAR subunits NR1, NR2A, and NR2B and phosphorylation of NR2B at residues Y1472 and S1303. B, Summary histograms show that protein levels of NR1, NR2A, NR2B, pNR2B-Y1472, AND pNR2B-S1303 are not significantly affected by prenatal MAM-exposure, nor by treatment with LY39 during juvenile development in both saline and MAM animals (n=5 rats for each group, p>0.05 for all groups).
Discussion
In this study, we tested the hypothesis of whether early treatment with a novel mGluR2/3 targeting compound, LY39, would be effective in ameliorating cognitive impairment in a neurodevelopmental model of SCZ through correction of NMDAR dysfunction. We found that gestational MAM exposure induced a significant decrease of NR2B subunit expression and increased NR2B phosphorylation at residue Y1472 in the juvenile rat PFC. Treatment with LY39 did not induce any significant changes in NMDAR subunit expression or NR2B phosphorylation in the juvenile saline-treated rat PFC, but significantly reversed the total protein levels of NR2B and Y1472 phosphorylation in juvenile MAM-treated rat PFC. More importantly, subchronic treatment with LY39 in juveniles effectively rescued MAM-induced learning and cognitive flexibility deficits when the animals were tested in adulthood with a cross-maze based set-shifting task. In adulthood, MAM-induced NMDAR subunit levels and NR2B phosphorylation in the PFC were normalized following juvenile LY39 treatment.
Our findings are interesting and important in several aspects. First, our data provide solid evidence that targeting the glutamatergic system at an early stage of the disease is a plausible approach to prevent the progression of SCZ, especially in regards to learning and cognitive flexibility impairments that may be directly derived from the early dysfunction of NMDARs. SCZ is a neurodevelopmental disorder characterized by a dysfunctional neuronal circuitry and synaptic functioning that likely occurs during the early stages of the disease (Insel, 2010). By the time of diagnosable onset, typically due to the observable prominent psychosis, the neuronal circuit has been hard-wired (Cannon, 2015). For this reason, early intervention would be a plausible and therapeutically relevant approach. We tested this by treating juvenile MAM-exposed rats with LY39 and examining cognitive deficits in adulthood. We found that MAM-treated rats exhibited impaired learning as well as cognitive flexibility compared to the saline controls when tested with the cross-maze task. Interestingly, juvenile LY39 treatment completely corrected the cognitive deficits induced by MAM exposure. In this case, LY39 administration during the juvenile stage resulted in adult MAM animals behaving similarly to saline-treated controls for the behaviors tested with the cross-maze task.
Similar to the Wisconsin card sorting test used for patients, the cross-maze based set-shifting task is designed to test learning and cognitive flexibility in the face of changing dimensions (Stefani et al., 2003). In our study, MAM-treated animals required a greater number of trials and more time to learn the test in Set 1, and greater number of trials to shift this rule in Set 2, indicating deficits in both learning and cognitive flexibility. This finding is in agreement with disrupted PFC function in MAM-treated animals (Gourevitch et al., 2004; Flagstad et al., 2005; Lavin et al., 2005; Moore et al., 2006), and the increased perseveration with PFC lesions (Dias et al., 1996). MAM-treated rats had an increased perseveration to the previous dimension and therefore required more trials to shift to a new discrimination. However, there was no significant difference in correct percentage among the MAM-treated animals and the other groups, suggesting that the deficit in set-shifting ability was not due to a deficient generalized performance and memory capacity. Once MAM-treated rats acquired the skills, they can perform the task successfully afterward (Featherstone et al., 2007). LY39 treatment effectively reversed the learning deficit induced by MAM exposure, indicating a potential use of LY39 as an antipsychotic capable of treating learning and cognitive impairments. These results are in support of previous reports, in which mGluR2/3 agonists also reversed some cognitive deficits induced by NMDAR blockade in adult animals (Moghaddam and Adams, 1998; Nikiforuk et al., 2010; Meltzer et al., 2013; Valsamis et al., 2014).
Given the significant effects of mGluR2/3 agonists on NMDAR expression and function in the adult rat PFC (Xi et al., 2011; Li et al., 2015b), one of the major concerns of this undertaking was the potential side effects. However, LY39 did not have significant effects on the expression of NMDAR subunits and limited effects on NMDA-mEPSCs in saline-treated animals (Fig. 3 and 4). LY39 also had no effect on neuronal excitability in vitro in naïve juvenile rat PFC pyramidal neurons (Fig, S1 and S2)), indicating a limited negative effect. This finding appears to be contrary to our other finding in the adult rat PFC in which LY39 at a dose of 3.0 mg/kg induced significant increases in protein levels of NMDAR subunits and NR2B phosphorylation (Li et al., 2015b). The mechanism associated with this ineffective or limited action on the NMDAR in juvenile animals is not yet fully understood, but it is speculated to be attributable to the differential distribution of mGluR2 and mGluR3 in prefrontal synapses and receptor sensitivity to the drug across age cohorts (Petralia et al., 1996; Tamaru et al., 2001; Li et al., 2015a).
However, these findings are in agreement with our other results exhibited in Figures 1 and 2, at least partially. In Figure 2, LY39 had no effects on saline animals, but significantly increased NR2B protein and decreased Y1472 phosphorylation in a dose-dependent manner in MAM animals. This finding could explain why LY39 had a dose-dependent effect on NMDA-mEPSCs in saline animals. At a relatively low dose of 0.3 mg/kg, LY39 decreases glutamate release pre-synaptically, as indicated by a reduction of NMDA-mEPSC amplitude and frequency (Fig. 4); whereas at a high dose of 3 mg/kg, LY39 had no effects (Fig. 3). Reduced NR2B in MAM-Sal animals may boost Y1472 phosphorylation to increase synaptic insertion to the cell membrane as compensation for the loss of NMDAR function (Fig. 1), and thus LY39 exhibited no change in NMDA-mEPSC amplitude (post-synaptically) but a trend of decreased frequency due to a decreased number of NR2B-containing NMDARs in the synapses. LY39 at 0.3 mg/kg was unable to rescue the mEPSCs in MAM animals (MAM-LY39), which may be attributable to the reduced Y1472-mediated synaptic insertion (Fig. 2 and 4), even though it can increase the total protein level of NR2B in MAM animals (Fig. 2).
Another important finding is the similar protein levels of NMDAR subunits and phosphorylation among all groups in the adult rat PFC. This normalized NMDAR subunit expression and phosphorylation in the MAM rat PFC is consistent with our previous reports (Snyder et al., 2013) as well as others’ (Hradetzky et al., 2012) in both the hippocampus and PFC of MAM-exposed rats. More importantly, our findings indicate that the NMDARs misexpression and phosphorylation and NMDARs dysfunction observed in juvenile MAM animals did not persist into adulthood although the mechanism for this normalization remains unclear. One possibility is that the NMDAR expression and function are compensatively normalized during development. In this case, treatment with LY39 may not be a direct effector for restoring the learning and cognitive deficits but acts as a modulator of other signaling pathway(s) such as GSK3β, as we recently reported (Xing et al., 2016). In fact, our previous study has reported that in the adult animal model of MK801 treatment for SCZ, mGluR2/3 agonist LY379268 could affect the NMDAR expression and function via regulation of GSK3β signaling. GSK3β has been identified as the only required molecule for both LTP and LTD induction (Peineau et al., 2007; Peineau et al., 2008; Peineau et al., 2009), the neural correlates of cognitive function and memory. It is, therefore, possible that LY39 treatment directly regulates the activity of GSK3β, which in turn, regulates the expression and function of NMDARs, and thus affect the NMDAR-dependent LTP and LTD process (see Figure S8 in Xing et al 2016).
Nevertheless, the rescue effect is likely due to decreased expression of mGluR2 in the PFC of the MAM-treated rats (Xing et al., unpublished observations). The lack of effect induced by LY39 on naïve or saline-treated animals is interesting and important; indicating that targeting mGluR2/3 or mGluR2 may have limited side-effects and would be well tolerated in SCZ patients, especially for early intervention. Indeed, this appears to be the case in clinical trials (Adams et al., 2013; Adams et al., 2014).
Studies on the effect of acute and chronic treatment of mGluR2/3 or mGluR2 agonists, as well as the effects on both normal and disease conditions, are still limited. A previous study reported that acute LY379268 treatment induced hyperlocomotor activity in phencyclidine and amphetamine models, but chronic treatment with LY379268 failed to replicate this effect (Galici et al., 2005). Given that drugs utilized in clinical patients are primarily administered chronically, whether the acute and subchronic effects of LY39 observed in this study can be replicated in chronic treatment remains to be explored. Several studies have already pointed out that mGluR2/3 agonists exhibit no significant effects on cognition in normal animals (Aultman and Moghaddam, 2001; Higgins et al., 2004; Schlumberger et al., 2009; Li et al., 2015b), although with NMDAR blockade, these drugs would be able to reverse certain cognitive deficits (Moghaddam and Adams, 1998; Nikiforuk et al., 2010). These findings are consistent with our results in which LY39 has limited effect in normal juvenile rats. This finding highlights a hypothesis that the utility of mGluR2/3 agonists on cognitive function may be specific to conditions associated with NMDAR dysfunction (Nikiforuk et al., 2010; Li et al., 2015a; Li et al., 2015b).
In summary, SCZ is a neurodevelopmental disorder with an onset during late adolescence and early adulthood. It is, therefore, necessary to identify and treat the prodromal phase of the illness, which usually occurs during the juvenile period. Our results show that LY39 administration during the juvenile period effectively reverses learning deficits in adulthood and provides promising insights into early intervention as an effective measure for treatment of cognitive impairments in SCZ. However, an important issue that should be considered is whether LY39 is also effective in dealing with cognitive dysfunction when treatment is conducted in older animals, such as adolescent, young adults, and even adult. To determine LY39’s prevention and treatment profiles, as well as the most effective therapeutic window of mGluR2/3 or mGluR2 agonists during different developmental periods, further studies are warranted.
Supplementary Material
Acknowledgments
This study was supported by grant R01MH085666 to W.J. Gao from the National Institutes of Health, USA; NSFC81271476 and 111 Project B13037 to F. Li, and NSFC81372107 to X.Q. Hu from the Natural Science Foundation of China.
Footnotes
Conflict of interest
The authors claim no any financial conflicts of interest.
References
- Adams DH, Zhang L, Millen BA, Kinon BJ, Gomez J-C. Pomaglumetad Methionil (LY2140023 Monohydrate) and Aripiprazole in Patients with Schizophrenia: A Phase 3, Multicenter, Double-Blind Comparison. Schizophrenia Research and Treatment. 2014;2014:758212. doi: 10.1155/2014/758212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DH, Kinon BJ, Baygani S, Millen BA, Velona I, Kollack-Walker S, Walling DP. A long-term, phase 2, multicenter, randomized, open-label, comparative safety study of pomaglumetad methionil (LY2140023 monohydrate) versus atypical antipsychotic standard of care in patients with schizophrenia. BMC Psychiatry. 2013;13:143. doi: 10.1186/1471-244X-13-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aultman JM, Moghaddam B. Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacology (Berl) 2001;153:353–364. doi: 10.1007/s002130000590. [DOI] [PubMed] [Google Scholar]
- Bond A, Jones NM, Hicks CA, Whiffin GM, Ward MA, O'Neill MF, Kingston AE, Monn JA, Ornstein PL, Schoepp DD, Lodge D, O'Neill MJ. Neuroprotective effects of LY379268, a selective mGlu2/3 receptor agonist: investigations into possible mechanism of action in vivo. J Pharmacol Exp Ther. 2000;294:800–809. [PubMed] [Google Scholar]
- Cannon TD. How Schizophrenia Develops: Cognitive and Brain Mechanisms Underlying Onset of Psychosis. Trends Cogn Sci. 2015 doi: 10.1016/j.tics.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J of Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Liu W, Duffney LJ, Yan Z. SNARE proteins are essential in the potentiation of NMDA receptors by group II metabotropic glutamate receptors. J Physiol. 2013;591:3935–3947. doi: 10.1113/jphysiol.2013.255075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends in pharmacological sciences. 2009;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Engel M, Snikeris P, Matosin N, Newell KA, Huang XF, Frank E. mGluR2/3 agonist LY379268 rescues NMDA and GABAA receptor level deficits induced in a two-hit mouse model of schizophrenia. Psychopharmacology (Berl) 2016;233:1349–1359. doi: 10.1007/s00213-016-4230-0. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Rizos Z, Nobrega JN, Kapur S, Fletcher PJ. Gestational methylazoxymethanol acetate treatment impairs select cognitive functions: parallels to schizophrenia. Neuropsychopharmacology. 2007;32:483–492. doi: 10.1038/sj.npp.1301223. [DOI] [PubMed] [Google Scholar]
- Fell MJ, McKinzie DL, Monn JA, Svensson KA. Group II metabotropic glutamate receptor agonists and positive allosteric modulators as novel treatments for schizophrenia. Neuropharmacology. 2012;62:1473–1483. doi: 10.1016/j.neuropharm.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Flagstad P, Glenthoj BY, Didriksen M. Cognitive deficits caused by late gestational disruption of neurogenesis in rats: a preclinical model of schizophrenia. Neuropsychopharmacology. 2005;30:250–260. doi: 10.1038/sj.npp.1300625. [DOI] [PubMed] [Google Scholar]
- Galici R, Echemendia NG, Rodriguez AL, Conn PJ. A selective allosteric potentiator of metabotropic glutamate (mGlu) 2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J Pharmacol Exp Ther. 2005;315:1181–1187. doi: 10.1124/jpet.105.091074. [DOI] [PubMed] [Google Scholar]
- Gomes FV, Rincon-Cortes M, Grace AA. Adolescence as a period of vulnerability and intervention in schizophrenia: Insights from the MAM model. Neurosci Biobehav Rev. 2016;70:260–270. doi: 10.1016/j.neubiorev.2016.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourevitch R, Rocher C, Le Pen G, Krebs MO, Jay TM. Working memory deficits in adult rats after prenatal disruption of neurogenesis. Behav Pharmacol. 2004;15:287–292. doi: 10.1097/01.fbp.0000135703.48799.71. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Spoelgen R, Hyman BT, Standaert DG, Dunah AW. Dopamine D1 activation potentiates striatal NMDA receptors by tyrosine phosphorylation-dependent subunit trafficking. J Neurosci. 2006;26:4690–4700. doi: 10.1523/JNEUROSCI.0792-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BH, Shahid M. Metabotropic and ionotropic glutamate receptors as neurobiological targets in anxiety and stress-related disorders: focus on pharmacology and preclinical translational models. Pharmacology, biochemistry, and behavior. 2012;100:775–800. doi: 10.1016/j.pbb.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ. Anxiolytic and side-effect profile of LY354740: a potent, highly selective, orally active agonist for group II metabotropic glutamate receptors. The Journal of pharmacology and experimental therapeutics. 1998;284:651–660. [PubMed] [Google Scholar]
- Higgins GA, Ballard TM, Kew JN, Richards JG, Kemp JA, Adam G, Woltering T, Nakanishi S, Mutel V. Pharmacological manipulation of mGlu2 receptors influences cognitive performance in the rodent. Neuropharmacology. 2004;46:907–917. doi: 10.1016/j.neuropharm.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hradetzky E, Sanderson TM, Tsang TM, Sherwood JL, Fitzjohn SM, Lakics V, Malik N, Schoeffmann S, O'Neill MJ, Cheng TM, Harris LW, Rahmoune H, Guest PC, Sher E, Collingridge GL, Holmes E, Tricklebank MD, Bahn S. The methylazoxymethanol acetate (MAM-E17) rat model: molecular and functional effects in the hippocampus. Neuropsychopharmacology. 2012;37:364–377. doi: 10.1038/npp.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- Jeevakumar V, Driskill C, Paine A, Sobhanian M, Vakil H, Morris B, Ramos J, Kroener S. Ketamine administration during the second postnatal week induces enduring schizophrenia-like behavioral symptoms and reduces parvalbumin expression in the medial prefrontal cortex of adult mice. Behav Brain Res. 2015;282:165–175. doi: 10.1016/j.bbr.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Millen BA, Zhang L, McKinzie DL. Exploratory Analysis for a Targeted Patient Population Responsive to the Metabotropic Glutamate 2/3 Receptor Agonist Pomaglumetad Methionil in Schizophrenia. Biol Psychiatry. 2015;78:754–762. doi: 10.1016/j.biopsych.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Zhang L, Millen BA, Osuntokun OO, Williams JE, Kollack-Walker S, Jackson K, Kryzhanovskaya L, Jarkova N. A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. Journal of Clinical Psychopharmacology. 2011;31:349–355. doi: 10.1097/JCP.0b013e318218dcd5. [DOI] [PubMed] [Google Scholar]
- Lavin A, Moore HM, Grace AA. Prenatal disruption of neocortical development alters prefrontal cortical neuron responses to dopamine in adult rats. Neuropsychopharmacology. 2005;30:1426–1435. doi: 10.1038/sj.npp.1300696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ML, Hu XQ, Li F, Gao WJ. Perspectives on the mGluR2/3 agonists as a therapeutic target for schizophrenia: Still promising or a dead end? Prog Neuropsychopharmacol Biol Psychiatry. 2015a;60:66–76. doi: 10.1016/j.pnpbp.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ML, Yang SS, Xing B, Ferguson BR, Gulchina Y, Li YC, Li F, Hu XQ, Gao WJ. LY395756, an mGluR2 agonist and mGluR3 antagonist, enhances NMDA receptor expression and function in the normal adult rat prefrontal cortex, but fails to improve working memory and reverse MK801-induced working memory impairment. Experimental neurology. 2015b;273:190–201. doi: 10.1016/j.expneurol.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Xi D, Roman J, Huang YQ, Gao WJ. Activation of glycogen synthase kinase-3 beta is required for hyperdopamine and D2 receptor-mediated inhibition of synaptic NMDA receptor function in the rat prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:15551–15563. doi: 10.1523/JNEUROSCI.3336-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Guo ML, Jin DZ, Fibuch EE, Choe ES, Wang JQ. Post-translational modification biology of glutamate receptors and drug addiction. Front Neuroanat. 2011;5:19. doi: 10.3389/fnana.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Rajagopal L, Huang M, Oyamada Y, Kwon S, Horiguchi M. Translating the N-methyl-D-aspartate receptor antagonist model of schizophrenia to treatments for cognitive impairment in schizophrenia. Int J Neuropsychopharmacol. 2013;16:2181–2194. doi: 10.1017/S1461145713000928. [DOI] [PubMed] [Google Scholar]
- Mezler M, Geneste H, Gault L, Marek GJ. LY-2140023, a prodrug of the group II metabotropic glutamate receptor agonist LY-404039 for the potential treatment of schizophrenia. Curr Opin Investig Drugs. 2010;11:833–845. [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacol (Berl) 2004;174:39–44. doi: 10.1007/s00213-004-1792-z. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biological Psychiatry. 2006;60:253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforuk A, Popik P, Drescher KU, van Gaalen M, Relo AL, Mezler M, Marek G, Schoemaker H, Gross G, Bespalov A. Effects of a positive allosteric modulator of group II metabotropic glutamate receptors, LY487379, on cognitive flexibility and impulsive-like responding in rats. J Pharmacol Exp Ther. 2010;335:665–673. doi: 10.1124/jpet.110.170506. [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nature medicine. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Peineau S, Bradley C, Taghibiglou C, Doherty A, Bortolotto ZA, Wang YT, Collingridge GL. The role of GSK-3 in synaptic plasticity. Br J Pharmacol. 2008;153:S428–S437. doi: 10.1038/bjp.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peineau S, Nicolas CS, Bortolotto ZA, Bhat RV, Ryves WJ, Harwood AJ, Dournaud P, Fitzjohn SM, Collingridge GL. A systematic investigation of the protein kinases involved in NMDA receptor-dependent LTD: evidence for a role of GSK-3 but not other serine/threonine kinases. Mol Brain. 2009;2:22. doi: 10.1186/1756-6606-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JTR, Bortolotto ZA, Wang YT, Collingridge GL. LTP inhibits LTD in the hippocampus via regulation of GSK3[beta] Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Al-Hallaq RA, Wenthold RJ. Trafficking and Targeting of NMDA Receptors. Boca Raton FL: Taylor & Francis Group, LLC; 2009. [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, MGLUR2 and MGLUR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Schlumberger C, Schafer D, Barberi C, More L, Nagel J, Pietraszek M, Schmidt WJ, Danysz W. Effects of a metabotropic glutamate receptor group II agonist LY354740 in animal models of positive schizophrenia symptoms and cognition. Behav Pharmacol. 2009;20:56–66. doi: 10.1097/FBP.0b013e3283242f57. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Snyder MA, Adelman AE, Gao W-J. Gestational methylazoxymethanol exposure leads to NMDAR dysfunction in hippocampus during early development and lasting deficits in learning. Neuropsychopharmacology. 2013;38:328–340. doi: 10.1038/npp.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Schoeffter P, Gasparini F, Kuhn R, Gentsch C. Pharmacological and endocrinological characterisation of stress-induced hyperthermia in singly housed mice using classical and candidate anxiolytics (LY314582, MPEP and NKP608) European journal of pharmacology. 2002;435:161–170. doi: 10.1016/s0014-2999(01)01562-x. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci. 2003;117:728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- Trepanier C, Lei G, Xie YF, Macdonald JF. Group II metabotropic glutamate receptors modify N-methyl-D-aspartate receptors via Src kinase. Sci Rep. 2013;3:926. doi: 10.1038/srep00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyszkiewicz JP, Gu Z, Wang X, Cai X, Yan Z. Group II metabotropic glutamate receptors enhance NMDA receptor currents via a protein kinase C-dependent mechanism in pyramidal neurones of rat prefrontal cortex. J Physiol. 2004;554:765–777. doi: 10.1113/jphysiol.2003.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsamis B, Chang M, Typlt M, Schmid S. Activation of mGluR2/3 receptors in the ventro-rostral prefrontal cortex reverses sensorimotor gating deficits induced by systemic NMDA receptor antagonists. Int J Neuropsychopharmacol. 2014;17:303–312. doi: 10.1017/S1461145713001041. [DOI] [PubMed] [Google Scholar]
- van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- Wang HX, Gao WJ. Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacol. 2009;34:2028–2040. doi: 10.1038/npp.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Gao WJ. Development of calcium-permeable AMPA receptors and their correlation with NMDA receptors in fast-spiking interneurons of rat prefrontal cortex. J Physiol. 2010;588:2823–2838. doi: 10.1113/jphysiol.2010.187591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M-J, Li Y-C, Snyder MA, Wang H, Li F, Gao W-J. Group II metabotropic glutamate receptor agonist LY379268 regulates AMPA receptor trafficking in prefrontal cortical neurons. PLoS ONE. 2013;8:e61787. doi: 10.1371/journal.pone.0061787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi D, Li Y-C, Snyder MA, Gao RY, Adelman AE, Zhang W, Shumsky JS, Gao W-J. Group II metabotropic glutamate receptor agonist ameliorates MK-801-induced dysfunction of NMDA receptors via Akt/GSK-3beta pathway. Neuropsychopharmacol. 2011;36:1260–1274. doi: 10.1038/npp.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing B, Li YC, Gao WJ. GSK3beta hyperactivity during an early critical period impairs prefrontal synaptic plasticity and induces lasting deficits in spine morphology and working memory. Neuropsychopharmacology. 2016;41:3003–3015. doi: 10.1038/npp.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.