Abstract
Objective
Microvascular pathophysiology which uniquely manifests as white matter (WM) abnormalities is often implicated in type 1 diabetes mellitus (T1DM)-related central nervous system (CNS) complications. The current study sought to identify regional WM abnormalities in young adults diagnosed with T1DM and further examine their association with cognitive and emotional dysfunction.
Research Design and methods
Diffusion tensor images (DTI) obtained from 34 young adults with T1DM for ≥ 15 years (mean duration, 20.9 years), and 16 age- and sex-matched healthy control subjects were analyzed using tract-based spatial statistics. Fractional anisotropy (FA) values of the whole brain were analyzed, and their associations with memory function and depressive symptoms were assessed.
Results
Whole brain voxel-wise analyses showed that T1DM-related FA reductions were most prominent within the fronto-temporo-parietal regions of the brain. Reduced FA values in the bilateral superior longitudinal fasciculi, at which group differences were most prominent, correlated with lower working memory performance in young adults with T1DM (left, P < .001; right, P = .009). Subsyndromal depressive symptoms were also associated with lower FA values in the right inferior fronto-occipital fasciculus (P = .004).
Conclusion
Widespread white matter microstructural abnormalities in the fronto-temporo-parietal brain regions, which are associated with emotional and cognitive dysfunction, may be a contributing factor to the neural mechanisms underlying T1DM-related CNS complications, thus affecting the quality of life in young adults with T1DM.
Keywords: Type 1 Diabetes Mellitus, White Matter, Connectivity, Diffusion Tensor Imaging, Cognition
INTRODUCTION
Long-term hyperglycemia in Type 1 diabetes mellitus (T1DM) has been considered an important contributing factor to diabetes-induced complications in the central nervous system (CNS) (1, 2). Emerging evidence suggests that structural and neurometabolic deficits in the fronto-temporo-parietal brain regions can lead to emotional and cognitive changes related to chronic T1DM (3–6). Given that the fronto-temporo-parietal regions are known as late-myelinating areas (7), these brain regions may be more vulnerable to detrimental effects of certain environmental factors as compared to other brain regions. In particular, it is suggested that the fronto-temporo-parietal regions may be more vulnerable during periods of active brain development such as throughout childhood and adolescence (7). Likewise, previous studies provide supporting evidence that the structural and neurometabolic alterations related to T1DM, which is usually diagnosed during childhood, may be prominent particularly in the fronto-tempo-parietal regions (3, 8–11). Considering the well-known microvascular alterations of white matter (WM) in prolonged hyperglycemic conditions (12–15), it is important to focus on the fronto-temporo-parietal WM regions in investigating T1DM-related brain structural abnormalities (16).
In the current study, we examined fractional anisotropy (FA), a sensitive marker for microstructural alterations in WM integrity, in both young adults with T1DM as well as healthy individuals using diffusion tensor imaging (DTI). We expected to observe T1DM-related deficits in the fronto-temporo-parietal WM regions (16). In addition, we focused on the working memory domain as a neurocognitive substrate of T1DM-related fronto-temporo-parietal WM deficits, considering its significant role in memory-related processes (14). As working memory performance appears vulnerable to diabetes-related metabolic changes (5, 6, 17, 18), it may improve with better glycemic control (19). We also examined relationships between the magnitude of FA reductions of the fronto-temporo-parietal association fibers and Hamilton Depression Rating Scale (HDRS) scores (20).
METHODS
Study Participants
Subjects were recruited from the Joslin Diabetes Center in Boston, Massachusetts. Details regarding this sample have been described in prior reports (3, 9, 21). Among the subjects who participated in previous magnetic resonance (MR) analyses (3, 9, 21), 34 young adults with T1DM (mean age [standard deviation, SD], 32.2 years [4.8]; 16 men and 18 women) and 16 age- and sex-matched healthy individuals (mean age [SD], 30.7 years [5.5]; 5 men and 11 women) whose DTI data were available comprised the study sample. The cohort characteristics, both demographic and clinical, were similar to those of previous studies (3, 9, 21) (Supplementary Table). DTI data were acquired at the Brain Imaging Center at the McLean Hospital in Belmont, Massachusetts. Further details of the inclusion and exclusion criteria are provided in the Supplementary Information, and were also described elsewhere (3, 9, 21).
All subjects provided written informed consent, as approved by the institutional committees on human subjects of the institution.
Clinical and Cognitive Assessments
Clinical information on diabetic and metabolic characteristics of each subject was acquired from the medical records and laboratory tests. Long-term glycemic control levels were determined by averaging all available values of hemoglobin A1C (HbA1C) taken from the subject’s medical records, which were then grouped and time-weighted every 4 years since the onset of the illness (3, 9). Current HbA1C levels were measured approximately 30 minutes prior to the MR imaging. History of severe hypoglycemic episodes, as defined by the Diabetes Control and Complications Trial Research Group Criteria, as any hypoglycemic episode eliciting a coma, seizure, or unconsciousness, was obtained from medical records. The date of the initial diagnosis for T1DM was retrieved from either medical records or self-report.
The assessment of working memory function was performed using the Letter-Number Sequencing and the Spatial Span, which are subsets of the Wechsler Memory Scale (WMS)-III (22) that test verbal and visuospatial working memory, respectively. Immediate and delayed memory functions were also evaluated using the Paired Associates I and II subscales of the WMS-III, respectively (22). After adjusting for age, sex, and educational level, scores of each subtest were converted into z-scores using group means and standard deviations (SDs) of the control subjects.
MR Image Acquisition and Processing
All MR imaging was performed using a 1.5 Tesla GE whole body imaging system (Horizon LX, GE Medical systems, Milwaukee, WI, USA). Processing and analyses of DTI data were performed using the FMRIB Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl). The diffusion tensor was calculated at an individual voxel level to generate FA images. A nonlinear registration algorithm implemented in the tract-based spatial statistics (TBSS) was used to perform a whole brain voxel-wise statistical analysis of FA data (5, 23, 24).
The average FA values in the entire brain were extracted with the mask of the FA skeleton of each subject. The most probable anatomical localization of each cluster of group effects was determined based on the John Hopkins University (JHU) DTI-based white matter atlas provided by the FSL atlas tool. Among the clusters of group effects, those that fall within the fronto-temporal major association tracts, as defined in the JHU white matter tractography atlas, were considered as the regions of interest (ROIs). The FA values of these ROIs were extracted for further post-hoc analyses.
Details regarding MR image acquisition and processing are described in the Supplementary Information.
Statistical Analysis
Demographic and clinical variables were compared between young adults with T1DM and healthy individuals using the independent t-test or chi-square test.
For the voxel-wise comparisons of FA values, the general linear model was performed to examine the group effects (T1DM vs. control) while adjusting for age and sex. Between-group comparisons were performed by comparing T1DM with control, while regressing out the linear effects of age and sex as confounding variables. The number of random permutations was set to 5000. All statistical analyses were corrected for multiple comparisons using the threshold-free cluster enhancement (TFCE) approach (25). Results of the TFCE-threshold voxel clusters were deemed significant at P <.005.
For the secondary post-hoc analysis focusing on the fronto-temporo-parietal WM ROIs, the Pearson correlation analysis was carried out to examine the relationships between the mean FA of each ROI and age-, sex-, and educational level-adjusted z score for each memory function. Potential relationships between subsyndromal depressive symptoms measured by the HDRS and FA values of the ROIs were examined as well. We further examined the associations between diabetes-related characteristics including HbA1C levels and number of hypoglycemic events and FA values of the ROIs.
A two-tailed significance of P <.05 was considered as statistically significant. Data were analyzed using Stata SE, v11.0 (Stata Corp, College Station, TX).
RESULTS
Between-Group Differences in FA Values
There were no differences in demographic and clinical characteristics between the two groups except the performance on memory function (t = 2.23, P = .03)(Table 1).
TABLE 1.
Demographic and clinical characteristics of young adults with type 1 diabetes mellitus and healthy individuals
| Characteristics | T1DM (n=34) |
Control (n=16) |
P value |
|---|---|---|---|
| Demographics | |||
| Mean age (SD), y | 32.2 (4.8) | 30.7 (5.5) | 0.34 |
| Female, n (%) | 18 (52.9) | 11 (68.8) | 0.29 |
| Educational level (SD), y | 16.0 (2.2) | 17.1 (2.0) | 0.10 |
| Medical characteristics | |||
| Lipids, mean (SD), mmol/L | |||
| HDL cholesterol | 1.45 (0.43) | 1.53 (0.38) | 0.53 |
| LDL cholesterol | 3.10 (1.17) | 2.65 (0.89) | 0.17 |
| Total cholesterol | 4.99 (1.55) | 4.56 (0.85) | 0.31 |
| Triglycerides | 2.21 (1.79) | 2.01 (0.90) | 0.68 |
| Blood pressure, mean (SD), mmHg | |||
| Systolic blood pressure | 122.3 (13.4) | 116.4 (13.1) | 0.15 |
| Diastolic blood pressure | 75.7 (8.8) | 72.4 (7.9) | 0.21 |
| Diabetes–specific clinical characteristics | |||
| Duration of illness (SD), y | 20.9 (3.4) | NA | NA |
| Age of onset (SD), y | 11.3 (4.6) | NA | NA |
| Lifetime average HbA1C (SD), % | 8.30 (1.04) | NA | NA |
| Lifetime average HbA1C (SD), mmol/mol | 67.25 (11.40) | NA | NA |
| No. of hypoglycemic episodes (SD) | 3.85 (6.53) | NA | NA |
| Current HbA1C (SD), % | 7.73 (1.17) | 5.08 (0.27) | <0.001 |
| Current HbA1C (SD), mmol/mol | 61.01 (12.78) | 31.97 (2.92) | <0.001 |
| Retinopathy level,* n (%) | |||
| No retinopathy | 23 (67.6) | NA | NA |
| Mild nonproliferative retinopathy | 8 (23.5) | NA | NA |
| Moderate nonproliferative retinopathy | 1 (2.9) | NA | NA |
| Proliferative retinopathy | 0 (0.0) | NA | NA |
| Behavioral characteristics | |||
| Memory function† | |||
| Immediate memory domain | −0.582 (0.978) | reference | 0.06 |
| Delayed memory domain | −0.625 (1.591) | reference | 0.16 |
| Working memory domain | −0.407 (0.849) | reference | 0.13 |
| Hamilton depression rating scale | 3.03 (3.60) | 2.50 (2.97) | 0.61 |
Group differences were tested by the independent t-test for continuous variables and by the χ2 tests for categorical variables
Data for 2 T1DM patients were unavailable.
Data are age, sex, and educational-level adjusted z scores which were calculated using group means and SDs of control subjects.
T1DM, type 1 diabetes mellitus; SD, standard deviation; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HbA1C, hemoglobin A1C; NA, not available or not applicable.
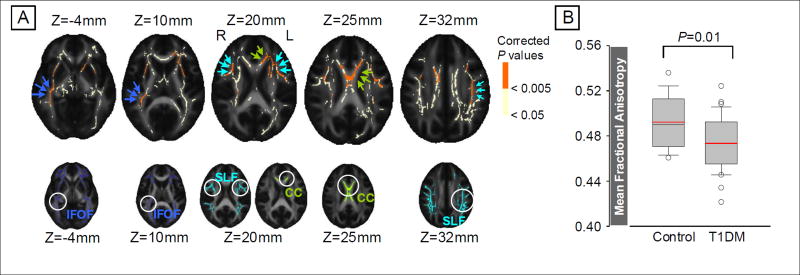
The voxel-wise group comparison demonstrated a wide range of brain regions with significant FA reductions in T1DM patients at TFCE-corrected P < .005 (Figure 1A). Major association fibers predominantly located in the bilateral fronto-temporal regions, including the superior longitudinal fasciculus (SLF), inferior fronto-occipital fasciculus (IFOF), and commissural fibers of the corpus callosum were affected. Information on the three clusters showing significant group effects (T1DM vs. control) in the voxel-wise analysis is presented in Table 2. The global mean FA values taken from the entire WM skeletons were lower in young adults with T1DM relative to healthy individuals after adjusting for age and sex (F1,46 = 7.26, P =.01)(Figure 1B).
FIGURE 1.
Voxel-wise analysis showing the WM regions of reduced FA values in young adults with T1DM relative to healthy individuals (A) and the comparison of global mean FA values between groups (B)
A: Profound FA value reductions in young adults with T1DM relative to healthy individuals were observed in the bilateral superior longitudinal fasciculi (cyan arrows of the upper row), the corpus callosum (green arrows of the upper row), and the right inferior fronto-occipital fasciculus (blue arrows of the upper row) at TFCE-corrected P <.005 (regions in orange color of the upper row). There was no regions with T1DM-related significant increase in FA values. Anatomical locations of the WM tracts based on the John Hopkins University DTI-based WM atlas are also overlaid on the brain templates at the same MNI coordinates for reference (lower row). TBSS results (upper row) and reference of anatomical WM tracts (lower row) were overlaid onto the FMRIB58 standard FA template.
B: A box-and-whisker plot demonstrated a significant global mean FA reduction in young adults with T1DM relative healthy individuals while controlling for age and sex (F1,46=7.26, P=0.01). Red lines indicate the mean values of whole-brain mean FA in each group.
WM, white matter; CC, corpus callosum; DTI, diffusion tensor imaging; FA, fractional anisotropy; IFOF, inferior fronto-occipital fasciculus; MNI, Montreal Neurological Institute; SLF, superior longitudinal fasciculus; T1DM, type 1 diabetes mellitus; TBSS, tract-based spatial statistics; TFCE, threshold-free cluster enhancement
TABLE 2.
Detailed information on clusters showing reduced FA values in young adults with T1DM patients relative to healthy individuals
| Cluster Index |
Corresponding cortical area |
Corresponding white matter tract * | Number of voxels in the cluster |
MNI atlas coordinates (location of maximum Z- value) |
The mean FA value in the cluster (SD) |
Effect size† |
|||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| x | y | z | T1DM | Control | |||||
| 1 | Left frontal/parietal lobes | Superior longitudinal fasciculus, L | 4382 | −22 | 30 | 14 | 0.458 (0.028) | 0.504 (0.031) | 1.28 |
| Corpus callosum, body | |||||||||
| Corpus callosum, genu | |||||||||
| Anterior thalamic radiation, L | |||||||||
| 2 | Right frontal/temporal lobes | Inferior fronto-occipital fasciculus, R | 2000 | 37 | −12 | −12 | 0.495 (0.029) | 0.544 (0.031) | 1.31 |
| 3 | Right frontal lobe | Superior longitudinal fasciculus, R | 381 | 43 | 18 | 14 | 0.428 (0.031) | 0.482 (0.036) | 1.31 |
Significant clusters of voxels were calculated by the TBSS analysis for the differences of FA values between young adults with T1DM and healthy individuals, while adjusting for age and sex after the TFCE-correction for multiple comparisons at P<0.005 (regions in orange color of FA templates in Figure 1A). The minimum cluster size is greater than 100 voxels.
Most probable anatomical localizations of each cluster were determined based on the John Hopkins University DTI-based white matter atlas.
Cohen's d effect size for the T1DM group as having reduced FA values in comparison to the control group.
FA, fractional anisotropy; T1DM, type 1 diabetes mellitus; MNI, Montreal Neurological Institute; SD, standard deviation; L, left; R, right; TBSS, tract-based spatial statistics; TFCE, threshold-free cluster enhancement.
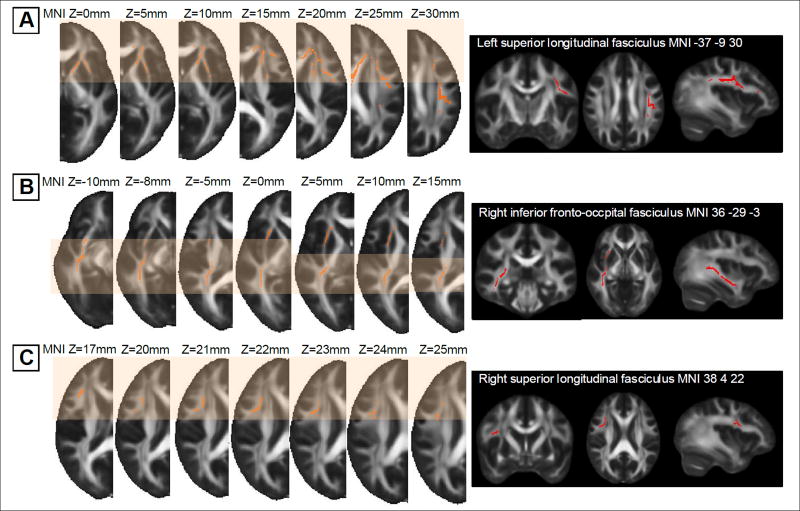
Among the significant clusters showing group effects, the voxels that fall within the association fibers connecting frontal and temporal cortices were selected as ROIs and the FA values extracted from each ROI were averaged for further analyses: left SLF ROI; right IFOF ROI; and right SLF ROI (Figure 2).
FIGURE 2.
Clusters showing significant FA value reductions in young adults with T1DM relative to healthy individuals (left column) and fronto-temporal white matter ROIs in each cluster (right column)
FA templates in the left column represent the clusters showing where FA values were significantly lower in young adults with T1DM relative to healthy individuals at corrected P <.005. Clusters in A, B, and C are mainly located on the left frontal, right temporal, right frontal regions (indicated by the shaded boxes), respectively, which also correspond to cluster indices 1, 2, and 3, in TABLE 2. Images in the right column in an orthogonal orientation (coronal, axial, and sagittal) display the fronto-temporal white matter ROIs (regions in red color), which were determined on the TBSS skeleton of each cluster (A, left superior longitudinal fasciculus ROI; B, right inferior fronto-occipital fasciculus ROI; C, right superior longitudinal fasciculus ROI).
FA, fractional anisotropy; MNI, Montreal Neurological Institute; ROIs, regions of interest; T1DM, type 1 diabetes mellitus; TBSS, tract-based spatial statistics
Secondary Clinical and Cognitive Correlations
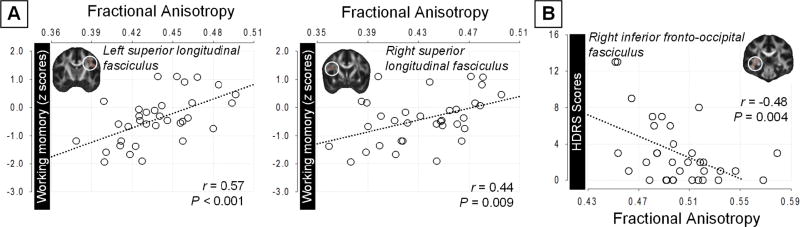
Of the secondary correlation analyses that were performed at the level of each memory domain (immediate, delayed, and working memory functions), working memory performance showed a positive correlation with FA values of the left SLF ROI (left SLF ROI, r = 0.57, P < .001; right SLF ROI, r = 0.44, P = .009) in young adults with T1DM (Figure 3). FA values of the right IFOF ROI were also positively correlated with working memory performance (r = .34, P = .049) in young adults with T1DM.
FIGURE 3. Correlations of FA values of each ROI with working memory function (A) and HDRS scores (B).
Scatter plots and regression lines (dotted lines) between FA values, extracted from white matter tract-specific ROI, and z scores for working memory (A), and the HDRS scores (B) in young adults with T1DM.
FA, fractional anisotropy; HDRS, Hamilton Depression Rating Scale; ROI, region of interest; T1DM, type 1 diabetes mellitus
Lower FA values of the right IFOF ROI were significantly associated with greater subsyndromal depressive symptoms in young adults with T1DM (r=-0.48, P =.004, Figure 3) but not in healthy individuals. FA values of the bilateral SLF were also correlated at a trend level with the HDRS scores (left SLF, r = −0.34, P = .050; right SLF, r = −0.23, P = .196) in the T1DM group, while this trend was not found in the control group.
The exploratory analyses were performed to examine the relationships between FA values in each ROI and diabetes-related clinical characteristics, including long-term glycemic control levels, the number of severe hypoglycemic episodes, age of onset, duration of illness, and current HbA1C levels, and the results are presented in the Supplementary Results.
DISCUSSION
In the current study of whole brain voxel-wise DTI analysis, young adults with T1DM demonstrated global deficits in microstructural WM integrity measured by mean FAs of the entire brain. Although there is marked heterogeneity in the methods of measurement, these findings are in alignment with previous reports regarding WM lesions in those with T1DM (5, 10, 16, 26–28). Specifically, we found that young adults with T1DM showed reduced FA values in major association fibers of the frontal, parietal, and temporal regions, as compared with healthy individuals. It is noteworthy that these deficits in WM tracts were observed in young adults with T1DM who were not yet diagnosed with any other apparent diabetes-induced micro or macrovascular diseases in the major organs. Furthermore, these results are in accordance with the previous report on the positive correlation between functional connectivity in the prefrontal cortex and cognition (29).
The current findings of region-specific T1DM-related WM alterations are consistent with those found in the previous reports on structural, neurometabolic, and functional abnormalities in the fronto-temporo-parietal areas of the brain in T1DM (3, 8–11, 16, 30). Considering that the onset of T1DM is typically during childhood and adolescence (31), metabolic alterations caused by T1DM may disrupt WM which is actively developing and myelinating during this period (6, 7, 32). In addition, regional characteristics of high insulin-receptor density and high cerebral energy requirement may also render the fronto-temporal areas to become more vulnerable to abnormal brain glucose levels (6). Notably, performance on working memory and subsyndromal depressive symptoms were correlated with WM microstructural alterations in the fronto-temporo-patietal regions in young adults with T1DM. Such findings provide evidence for the clinical and behavioral relevance of T1DM-related WM changes, given that memory and emotional regulation are higher cognitive functions which require network-based control of major cortical areas such as the prefrontal and temporal lobes (33).
Decreased integrity of the SLF, a major WM association fiber connecting the prefrontal, parietal, and temporal lobes, is thought to be responsible for impaired working memory performance in several neuropsychiatric disorders (34). The current study suggests that WM tract disruption and its subsequent disruption in the network efficiency between the frontal, parietal, and temporal regions through the SLF may indeed contribute to decreased memory function, particularly in the working memory of young adults with T1DM.
Emotional disturbances including depression have been considered as the most important neurobehavioral complications in T1DM (35). The loss of WM integrity in several brain regions including the orbitofrontal, deep temporal, and cingulate cortices have frequently accompanied emotional dysregulation as well as subsequent development of depression (36). Considering that major WM tracts within the fronto-temporo-parietal regions are affected by metabolic dysfunction, it may also be expected that one's susceptibility to depression would be increased from such WM abnormalities as well. Although this needs to be confirmed in a relevant T1DM sample that is comorbid with current depression, inefficient connections between the prefrontal and deep temporal regions could potentially contribute to the development of depression in young adults with T1DM. Our assumption is supported by the observed correlation between reduced WM integrity localized on these prefronto-limbic areas and depressive symptoms, despite the fact that the level of depressive symptoms observed in the current sample was subclinical.
Microangiopathy constitutes one of the major pathophysiological mechanisms underlying T1DM-induced CNS complications (36, 37). Measurement of WM hyperintensities has therefore been focused in earlier neuroimaging studies on T1DM (5). However, results from our previous study using the focal WM hyperintensities did not show any significant increase in WM lesions in young adults with T1DM (21), despite their similarity in clinical characteristics to the current sample. This suggests that DTI may be better suited to detect subtle abnormalities of WM organization during the early stages of the disease.
Previous studies using multi-ROI and tractography approaches have demonstrated FA value reductions in the posterior WM tracts including optic radiations and posterior corona radiata (12, 13, 26, 38, 39). Although the current study also found an involvement of the left posterior WM tracts in young adults with T1DM, more prominent findings were observed in the fronto-temporo-parietal WM regions. It is noteworthy that sample heterogeneity as well as methodological differences across studies may have contributed to this discrepancy.
In addition, we found a negative relationship between current HbA1C and FA values in individuals with T1DM. This suggests a need for future longitudinal studies to examine whether improved glycemic controls may be associated with reversing the progression of white matter abnormalities.
Diabetes-specific clinical characteristics including long-term glycemic control levels, severe hypoglycemic episodes, and the duration of illness were not correlated with FAs in the current T1DM group (Supplementary Result). These findings contrast with previous reports on T1DM, in which negative correlations were found between the duration of illness (39, 40) or history of hyperglycemic events (26, 40) and FA values. This may be explained by considering that the young adults with T1DM in the current study had a narrower range of disease duration (mean disease duration [SD], 20.9 [3.4] years vs. 30.3 [10.8] years) (38) as well as a lower rate of microangiopathy (15) than those in previous studies. Such characteristics of our T1DM sample may have partially affected the possibility of detecting the above-mentioned correlation in the current study.
The interpretation of our study is limited primarily by a small sample size, although a large effect size of group-differences in FA value differences ensured adequate statistical power. Future longitudinal studies with a larger sample size of individuals diagnosed with T1DM would be necessary to provide important information regarding the natural progression of white matter changes related to T1DM, as well as its clinical implications.
Considering recent advances in DTI methodology, the use of 1.5 Tesla MR scanner with the 6 diffusion gradients could have limited opportunities for more sophisticated analyses of WM microstructures, even though higher magnetic fields may also raise more artifacts in DTI (41).
The results from the present study provide evidence of WM microstructural alterations that occur predominantly in the fronto-temporo-parietal regions in young adults with T1DM. These abnormalities in the fronto-temporo-parietal association fibers may be the underlying neuroanatomical basis for cognitive and emotional disturbances in young adults with T1DM. The current findings also suggest that DTI analysis allows for a sensitive determination of diabetes-induced WM structural integrity that may then facilitate earlier detection of T1DM-related CNS complications.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (DK–060754 and DK-084202); grant 2015M3C7A1028373 from the National Research Foundation of Korea; Institute for Information & communications Technology Promotion grant B0132-15-1001 from the MSIP; grant P30-DK-36836 (Joslin Diabetes and Endocrinology Research Center); the Joslin Diabetes Center Herbert Graetz Fund; and the Winthrop University Hospital Research Institute.
References
- 1.Klein JP, Waxman SG. The brain in diabetes: molecular changes in neurons and their implications for end-organ damage. Lancet Neurol. 2003;2:548–54. doi: 10.1016/s1474-4422(03)00503-9. [DOI] [PubMed] [Google Scholar]
- 2.Semenkovich K, Bischoff A, Doty T, Nelson S, Siller AF, Hershey T, et al. Clinical presentation and memory function in youth with type 1 diabetes. Pediatr Diabetes. 2016;17:492–9. doi: 10.1111/pedi.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyoo IK, Yoon SJ, Musen G, Simonson DC, Weinger K, Bolo N, et al. Altered prefrontal glutamate-glutamine-gamma-aminobutyric acid levels and relation to low cognitive performance and depressive symptoms in type 1 diabetes mellitus. Arch Gen Psychiatry. 2009;66:878–87. doi: 10.1001/archgenpsychiatry.2009.86. [DOI] [PubMed] [Google Scholar]
- 4.McIntyre RS, Kenna HA, Nguyen HT, Law CW, Sultan F, Woldeyohannes HO, et al. Brain volume abnormalities and neurocognitive deficits in diabetes mellitus: points of pathophysiological commonality with mood disorders? Adv Ther. 2010;27:63–80. doi: 10.1007/s12325-010-0011-z. [DOI] [PubMed] [Google Scholar]
- 5.Musen G. Cognition and brain imaging in type 1 diabetes. Curr Diab Rep. 2008;8:132–7. doi: 10.1007/s11892-008-0024-z. [DOI] [PubMed] [Google Scholar]
- 6.Northam EA, Rankins D, Cameron FJ. Therapy insight: the impact of type 1 diabetes on brain development and function. Nat Clin Pract Neurol. 2006;2:78–86. doi: 10.1038/ncpneuro0097. [DOI] [PubMed] [Google Scholar]
- 7.Wozniak JR, Lim KO. Advances in white matter imaging: a review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neurosci Biobehav Rev. 2006;30:762–74. doi: 10.1016/j.neubiorev.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson SC, Blane A, Perros P, McCrimmon RJ, Best JJ, Wardlaw J, et al. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes. 2003;52:149–56. doi: 10.2337/diabetes.52.1.149. [DOI] [PubMed] [Google Scholar]
- 9.Musen G, Lyoo IK, Sparks CR, Weinger K, Hwang J, Ryan CM, et al. Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes. 2006;55:326–33. doi: 10.2337/diabetes.55.02.06.db05-0520. [DOI] [PubMed] [Google Scholar]
- 10.Northam EA, Rankins D, Lin A, Wellard RM, Pell GS, Finch SJ, et al. Central nervous system function in youth with type 1 diabetes 12 years after disease onset. Diabetes Care. 2009;32:445–50. doi: 10.2337/dc08-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wessels AM, Simsek S, Remijnse PL, Veltman DJ, Biessels GJ, Barkhof F, et al. Voxel-based morphometry demonstrates reduced grey matter density on brain MRI in patients with diabetic retinopathy. Diabetologia. 2006;49:2474–80. doi: 10.1007/s00125-006-0283-7. [DOI] [PubMed] [Google Scholar]
- 12.Aye T, Barnea-Goraly N, Ambler C, Hoang S, Schleifer K, Park Y, et al. White matter structural differences in young children with type 1 diabetes: a diffusion tensor imaging study. Diabetes Care. 2012;35:2167–73. doi: 10.2337/dc12-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnea-Goraly N, Raman M, Mazaika P, Marzelli M, Hershey T, Weinzimer SA, et al. Alterations in white matter structure in young children with type 1 diabetes. Diabetes Care. 2014;37:332–40. doi: 10.2337/dc13-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 15.van Duinkerken E, Schoonheim MM, Ijzerman RG, Klein M, Ryan CM, Moll AC, et al. Diffusion tensor imaging in type 1 diabetes: decreased white matter integrity relates to cognitive functions. Diabetologia. 2012;55:1218–20. doi: 10.1007/s00125-012-2488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siller AF, Lugar H, Rutlin J, Koller JM, Semenkovich K, White NH, et al. Severity of clinical presentation in youth with type 1 diabetes is associated with differences in brain structure. Pediatr Diabetes. 2016 doi: 10.1111/pedi.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaudieri PA, Chen R, Greer TF, Holmes CS. Cognitive function in children with type 1 diabetes: a meta-analysis. Diabetes Care. 2008;31:1892–7. doi: 10.2337/dc07-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naguib JM, Kulinskaya E, Lomax CL, Garralda ME. Neuro-cognitive performance in children with type 1 diabetes--a meta-analysis. J Pediatr Psychol. 2009;34:271–82. doi: 10.1093/jpepsy/jsn074. [DOI] [PubMed] [Google Scholar]
- 19.Ryan CM, Freed MI, Rood JA, Cobitz AR, Waterhouse BR, Strachan MW. Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes Care. 2006;29:345–51. doi: 10.2337/diacare.29.02.06.dc05-1626. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinger K, Jacobson AM, Musen G, Lyoo IK, Ryan CM, Jimerson DC, et al. The effects of type 1 diabetes on cerebral white matter. Diabetologia. 2008;51:417–25. doi: 10.1007/s00125-007-0904-9. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Memory Scale. 3rd Ed. Psychological Corp.; San Antonio, Tx: 1997. [Google Scholar]
- 23.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nature Protocols. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- 25.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 26.Antenor-Dorsey JA, Meyer E, Rutlin J, Perantie DC, White NH, Arbelaez AM, et al. White matter microstructural integrity in youth with type 1 diabetes. Diabetes. 2013;62:581–9. doi: 10.2337/db12-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reijmer YD, Brundel M, de Bresser J, Kappelle LJ, Leemans A, Biessels GJ, et al. Microstructural white matter abnormalities and cognitive functioning in type 2 diabetes: a diffusion tensor imaging study. Diabetes Care. 2013;36:137–44. doi: 10.2337/dc12-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Elderen SG, Brandts A, van der Grond J, Westenberg JJ, Kroft LJ, van Buchem MA, et al. Cerebral perfusion and aortic stiffness are independent predictors of white matter brain atrophy in type 1 diabetic patients assessed with magnetic resonance imaging. Diabetes Care. 2011;34:459–63. doi: 10.2337/dc10-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saggar M, Tsalikian E, Mauras N, Mazaika P, White NH, Weinzimer S, et al. Compensatory Hyperconnectivity in Developing Brains of Young Children With Type 1 Diabetes. Diabetes. 2017;66:754–62. doi: 10.2337/db16-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolo NR, Musen G, Simonson DC, Nickerson LD, Flores VL, Siracusa T, et al. Functional Connectivity of Insula, Basal Ganglia, and Prefrontal Executive Control Networks during Hypoglycemia in Type 1 Diabetes. J Neurosci. 2015;35:11012–23. doi: 10.1523/JNEUROSCI.0319-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daneman D. Type 1 diabetes. Lancet. 2006;367:847–58. doi: 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- 32.Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacology, biochemistry, and behavior. 2007;86:189–99. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gazzaniga MS, Ivry RB, Mangun GR. Cognitive Neuroscience- the Biology of the Mind. 2nd Ed. W W Norton & Company; New York: 2002. [Google Scholar]
- 34.Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry. 2008;63:512–8. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson AM, Samson JA, Weinger K, Ryan CM. Diabetes, the brain, and behavior: is there a biological mechanism underlying the association between diabetes and depression? International review of neurobiology. 2002;51:455–79. doi: 10.1016/s0074-7742(02)51013-8. [DOI] [PubMed] [Google Scholar]
- 36.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 38.Franc DT, Kodl CT, Mueller BA, Muetzel RL, Lim KO, Seaquist ER. High connectivity between reduced cortical thickness and disrupted white matter tracts in long-standing type 1 diabetes. Diabetes. 2011;60:315–9. doi: 10.2337/db10-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kodl CT, Franc DT, Rao JP, Anderson FS, Thomas W, Mueller BA, et al. Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes. 2008;57:3083–9. doi: 10.2337/db08-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirchhoff BA, Jundt DK, Doty T, Hershey T. A longitudinal investigation of cognitive function in children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2017;18:443–9. doi: 10.1111/pedi.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood R, Bassett K, Foerster t, Spry C, Tong L. 1.5 tesla magnetic resonance imaging scanners compared with 3.0 tesla magnetic resonance imaging scanners: systematic review of clinical effectiveness. CADTH Technol Overv. 2012;2:e2201. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.