Abstract
Cellular effects of glucocorticoids can be separated into classical transcriptional regulation via activation of the canonical nuclear glucocorticoid receptor and rapid actions mediated by activation of one or more putative membrane-associated glucocorticoid receptors that regulate both transcriptional and non-transcriptional signaling. Dexamethasone-bovine serum albumin (Dex-BSA), is one of several membrane-limited steroid receptor agonists. Dex-BSA and other steroid conjugates such as corticosterone-, estradiol- and testosterone-BSA have been used to study rapid steroid effects initiated by a putative membrane receptor. The purity and stability of the steroid-BSA conjugate is crucial, therefore, since any steroid that is not bound to or that dissociates from the BSA conjugate could penetrate into the intracellular compartment and confound the experiment. We used fluorine NMR to determine if free Dex could be detected over time in a commercially available Dex-BSA dissolved in H2O. Non-covalently bound Dex was detected in the Dex-BSA solution, but the level of free Dex remained constant over time and over a range of temperatures, indicating that the free Dex was not a result of instability of the Dex-BSA conjugate. The free Dex was lost when the Dex-BSA was denatured and subjected to dialysis, which suggested that it was trapped in the Dex-BSA three-dimensional structure and not covalently bound to the BSA. The purified, renatured Dex-BSA retained its rapid activity, which confirmed that the observed effects of Dex-BSA are not caused by non- covalently-bound Dex. Therefore, the Dex contaminant found in the Dex-BSA solution is likely to be tightly, but non-covalently, bound to BSA, and the Dex-BSA activity remains membrane- limited. Our findings indicate that Dex-BSA remains a suitable membrane-restricted glucocorticoid receptor agonist, but suggest that denaturing purification is a useful control for the study of membrane-initiated steroid-BSA actions.
Keywords: corticosteroid, nongenomic, denaturation, dialysis, NMR, steroid
INTRODUCTION
Glucocorticoids are steroid hormones that regulate metabolic, immunological, and neurological processes [1–3] through transcriptional regulation of a multitude of genes [4]. Glucocorticoids are lipophilic hormones that can pass through the plasma membrane to bind to their cognate nuclear receptor, the glucocorticoid receptor (GR), which leads to nuclear translocation and dimerization of the receptor [5]. The cellular actions of glucocorticoids have been expanded recently to encompass rapid, transcription-independent effects on cellular function, including, for example, endocannabinoid and nitric oxide production [6] and regulation of ion channels [7]. The rapid nature and transcription independence of these glucocorticoid effects suggest actions at a plasma membrane-associated receptor. As with other steroid hormones, a membrane-impermeant GR ligand, dexamethasone (Dex) conjugated to bovine serum albumin (Dex-BSA), is often used to determine if glucocorticoids can act at a binding site constrained to the membrane. Dex-BSA was used in a companion study to test whether glucocorticoid restricted to the membrane signals to the intracellular GR to induce nuclear translocation and gene transcription in hypothalamic neurons (Rainville et al.). The Dex-BSA available from a commercial source is synthesized with between 8 and 40 molecules of Dex per BSA molecule, which raises the possibility that relatively high levels of free Dex may be present if the purity and/or the stability of the compound are not assured.
There has been little direct testing of the purity and stability of the Dex-BSA compound to date. Due to the fluorine atom in the Dex molecule, we were able to use 19F nuclear magnetic resonance (NMR) to detect non-covalently bound Dex [8] in a commercially available Dex-BSA compound. Additionally, we determined the biological relevance of a Dex contamination detected in the Dex-BSA in an assay of rapid glucocorticoid actions, and we describe a simple method for robust purification of the steroid-BSA conjugate via denaturing and dialysis. The purification methods discussed here can also be applied to other commonly used steroid conjugates, such as corticosterone-, estradiol- and testosterone-BSA.
EXPERIMENTAL
Nuclear Magnetic Resonance
All NMR experiments were performed on a Bruker 300 MHz NMR by BBO probe. A pulse sequence with inverse gated proton decoupling was used to acquire 19F NMR spectra at a frequency of 282.4 MHz. 500 scans were accumulated. All acquisition parameters, including receiver gain, were kept the same in order to compare the peak areas corresponding to Dex concentration. A 100 μM Dex solution (in deuterated DMSO) was an external standard to quantify the non-covalently bound Dex in 40 μM Dex-BSA.
Denaturing Purification
Dialysis was performed on Dex-BSA (40 μM) in HEPES buffer (10 μM) and Urea (8 M) using a Slide-A-Lyzer (Thermo) dialysis cassette with a 3,500 Da exclusion limit. The filled cassette was placed in 1 L HEPES buffer (10 mM) for two hours before changing to fresh HEPES (10 mM) for overnight dialysis. Dialyzed Dex-BSA was then analyzed by NMR and the same sample was used for subsequent GR trafficking experiments.
Cell Culture and Imaging
Immortalized hypothalamic cells mHypoE-N42 (N42) cells [9] were grown in DMEM 10% fetal bovine serum (FBS, Atlas Biologicals) and transfected with GR-GFP (a generous gift from Dr. Louis Muglia) using a Neon Transfection System (Invitrogen). 106 cells and 3.6 μg of plasmid DNA were pulsed twice in the transfection tip at 1400 mV for 10 ms. The transfected cells were seeded at 105 cells/mL and distributed into 35 mm culture dishes with a No. 0 cover glass bottom (MatTek) to adhere overnight. The media was then washed and changed to DMEM 5% charcoal-stripped FBS for 16 hours to eliminate any endogenous steroids prior to imaging on a laser confocal microscope (Nikon A1+). Using a pinhole size of 28 μm, images were acquired every 15 seconds before and after drug treatment. The Dex-BSA conjugate containing approximately 40 Dex molecules per BSA was obtained from Steraloids (Newport, RI). Purified Dex-BSA (100 nM final concentration) was added after 5 minutes and GR-GFP was tracked for an additional 25 minutes. NIS-Elements analysis software (Nikon) was used to determine average nuclear intensities of the GR-GFP signal, based on a designated nuclear region of interest, before and after drug treatment.
RESULTS
Non-covalently-bound Dex in Dex-BSA
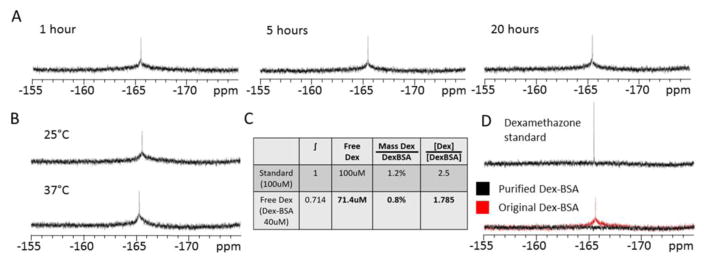
Using 19F NMR, a peak was observed at about -165 ppm in a sample of 40 μM Dex-BSA (Fig. 1). The narrow peak indicates that the corresponding compound can freely rotate, suggesting that it was caused by free Dex and not Dex covalently bound to BSA. To measure stability of Dex-BSA over time, the samples in D2O were measured by 19F NMR after 1, 5 and 20 hours at 25 C (Fig. 1A). The amplitude and shape of the peak at -165 ppm did not change over the 20-hour time span tested. Similarly, the NMR peak at -165 ppm did not change when the temperature was increased from 25°C to 37°C (Fig. 1B). These data indicate that the Dex-BSA compound is stable, and that Dex molecules covalently bound to BSA remain bound over an extended period in solution and at higher temperatures. To quantify the amount of free Dex contamination represented by the spectral peak at -165 ppm, the integral of the peak was calculated and was compared to a standard concentration of Dex (100 μM), which was a concentration estimated to be 6% of the mass of total Dex in the Dex-BSA sample. While the mass of the Dex contaminant was relatively small compared to the mass of Dex-BSA (0.8%), the molar concentration of the Dex contaminant was 1.785-fold greater than the Dex-BSA concentration (Fig 1C). The narrow peak of Dex observed in 19F NMR was not covalently bound to BSA, indicating it could be free in the solution. However, we found that no active Dex could be detected following size-exclusion filtration of the Dex-BSA (see accompanying paper, Rainville et al.), and that Dex-BSA had no effect on glucocorticoid response element (GRE)-induced transcription [10]. We then tested whether the resonance observed in the 19F NMR spectra is due to Dex that is restricted to, but non-covalently bound to, the Dex-BSA.
Figure 1.
Dex-BSA stability and effective purity. A. 19F NMR peaks (~-165 ppm) of non-covalently bound Dex in Dex-BSA (40 μM) solution are stable for 1, 5 and 20 hours. B. No change in the 19F NMR peak was seen between 25 C and 37 C. C. Non-covalently bound Dex was quantified by integration and comparison to Dex in DMSO (100 μM). Molecules of free Dex in Dex-BSA were calculated to be approximately 78.5% more abundant than Dex-BSA molecules. D. Denatured and dialysis-purified Dex-BSA showed no detectable peak at ~165 ppm.
Denaturing purification removed free Dex from Dex-BSA
To determine if Dex was bound non-covalently to the Dex-BSA, we used a procedure to denature the Dex-BSA and release unbound Dex from any hydrophobic binding pockets formed by the folding structure of the Dex-BSA. The Dex-BSA was denatured in 8 M urea dissolved in 10 mM HEPES, and was then further purified by dialysis using a 3,500 Da exclusion limit to remove any unbound Dex and the Urea. The gradual removal of urea allows for BSA to return to its native confirmation, restoring membrane impermeability. The renatured Dex-BSA was then analyzed by 19F NMR. The renatured Dex-BSA showed no 19F NMR peak at -165 ppm (Fig. 1D), indicating that the free Dex contamination was removed from the Dex-BSA under denaturing conditions.
Dex-BSA retained rapid glucocorticoid actions following denaturation
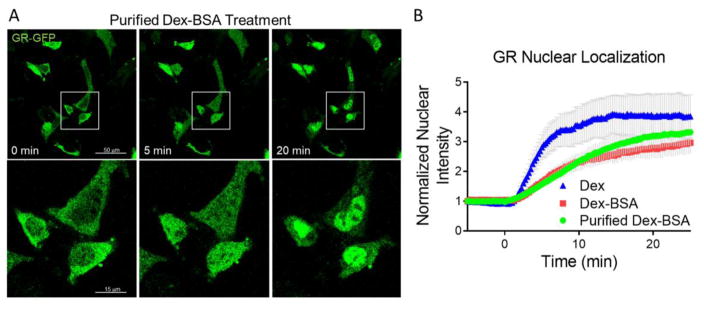
If the Dex-BSA lost its rapid actions following denaturation purification, this would suggest that the rapid effects were mediated by the free Dex contaminant within the Dex-BSA compound. We tested whether the purified Dex-BSA retained its membrane-initiated rapid actions using a GR nuclear translocation assay. We found that, like Dex, Dex-BSA (100 nM) induced the intracellular GR to rapidly translocate to the nucleus in N42 hypothalamic cells (Fig. 2B). Here, N42 hypothalamic cells that were transiently transfected with a GR-GFP construct were analyzed using live imaging under a confocal microscope (Nikon A1+) to track GR nuclear translocation in response to free Dex and to the denatured and purified Dex-BSA. The nuclear fluorescence intensity due to GR-GFP increased within 5 minutes of purified Dex-BSA application and the effect plateaued at 20 minutes (Fig. 2A). Frame-by-frame analysis revealed that the kinetics of the GR nuclear translocation induced by purified Dex-BSA was indistinguishable from that induced by unpurified Dex-BSA (Fig. 2B). These data confirm that the rapid effect of Dex-BSA on GR translocation is not a result of free Dex in the solution, but are due rather to the membrane-limited actions of Dex-BSA.
Figure 2.
Denaturation-purified Dex-BSA (100 nM) retained its effect on GR nuclear translocation. A. N42 cells expressing GR-GFP were treated with purified Dex-BSA and imaged at 0, 5, and 20 min. Lower panels show magnifications of the boxed regions in the upper panels. B. Time course of GR-GFP translocation to the nucleus, measured by fluorescence intensity in a nuclear region of interest. The kinetics of GR nuclear translocation induced by the unpurified (Dex-BSA) and the purified Dex-BSA were comparable to each other, but were both slower than the free Dex-induced nuclear translocation of GR (Dex).
DISCUSSION
The use of Dex-BSA as a membrane-limited glucocorticoid has been met with some skepticism, mainly for its assumed instability. Here we provide conclusive evidence that Dex-BSA is stable in solution over an extended period of time and at high temperature (i.e., body temperature). It was surprising, however, to learn that Dex-BSA is manufactured with a significant concentration of free Dex not covalently bound to the BSA, which we calculated to be 4.5% of the total Dex. While the chemical purity by mass (98.2%) of commercially available Dex-BSA is within what would appear to be acceptable standards, the molar concentration of Dex well exceeded that of Dex-BSA, by nearly 2-fold. If these Dex molecules were indeed free in solution and allowed to diffuse through the plasma membrane, any effects of the Dex-BSA could, in fact, be due to the free Dex.
We found that the free Dex, non-covalently bound to the Dex-BSA, is not responsible for the rapid effects of the Dex-BSA on GR nuclear trafficking, as denaturation-purified Dex-BSA retained the rapid effect. Because the Dex contaminant could only be removed with denaturing, it was likely trapped within hydrophobic pockets of the Dex-BSA molecule and was not biologically available to penetrate the membrane and activate the cytosolic GR. Commercial Dex-BSA remains, therefore, a suitable compound to study membrane-limited glucocorticoid actions. However, given the relative ease of purification of the Dex-BSA by denaturing and dialysis, future studies with the compound would benefit from the removal of the free Dex contaminant to avoid a potential confound, especially if there is variability in the stocks of the Dex-BSA obtained commercially.
Estradiol- and testosterone-BSA conjugates have also been shown to contain steroid monomers, which are released more readily under denaturing conditions [11]. Dialysis of the native sex steroid conjugates was ineffective in removing monomer contamination, but dialysis of denatured conjugates was not attempted in that study. These compounds have not been tested for stability over time, which is rendered difficult by their lack of fluorine substrate for the NMR biochemical analysis. Nevertheless, as shown here with the Dex-BSA compound, denaturation, dialysis and renaturation should provide a relatively easy and viable way of ensuring the purity and stability of any steroid-BSA conjugate, including corticosteroid and sex hormone conjugates.
Acknowledgments
This work was supported by NIH grant 2R01 MH066958. We thank Dr. Louis Muglia for his generous gift of the GR-GFP construct.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dinneen S, Alzaid A, Miles J, Rizza R. Metabolic effects of the nocturnal rise in cortisol on carbohydrate metabolism in normal humans. J Clin Invest. 1993;92:2283–90. doi: 10.1172/JCI116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017 doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed]
- 3.De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 4.Ritter HD, Mueller CR. Expression microarray identifies the unliganded glucocorticoid receptor as a regulator of gene expression in mammary epithelial cells. BMC Cancer. 2014;14:275. doi: 10.1186/1471-2407-14-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vernocchi S, Battello N, Schmitz S, Revets D, Billing AM, Turner JD, Muller CP. Membrane glucocorticoid receptor activation induces proteomic changes aligning with classical glucocorticoid effects. Mol Cell Proteomics. 2013;12:1764–79. doi: 10.1074/mcp.M112.022947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di S, Maxson MM, Franco A, Tasker JG. Glucocorticoids Regulate Glutamate and GABA Synapse-Specific Retrograde Transmission via Divergent Nongenomic Signaling Pathways. J Neurosci. 2009;29:393–401. doi: 10.1523/JNEUROSCI.4546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatterjee S, Sikdar SK. Corticosterone targets distinct steps of synaptic transmission via concentration specific activation of mineralocorticoid and glucocorticoid receptors. J Neurochem. 2014;128:476–90. doi: 10.1111/jnc.12478. [DOI] [PubMed] [Google Scholar]
- 8.Midelfart A, Dybdahl A, Müller N, Sitter B, Gribbestad IS, Krane J. Dexamethasone and Dexamethasone Phosphate Detected by1H and19F NMR Spectroscopy in the Aqueous Humour. Exp Eye Res. 1998;66:327–337. doi: 10.1006/exer.1997.0429. [DOI] [PubMed] [Google Scholar]
- 9.Belsham DD, Cai F, Cui H, Smukler SR, Salapatek AMF, Shkreta L. Generation of a Phenotypic Array of Hypothalamic Neuronal Cell Models to Study Complex Neuroendocrine Disorders. Endocrinology. 2004;145:393–400. doi: 10.1210/en.2003-0946. [DOI] [PubMed] [Google Scholar]
- 10.Strehl C, Gaber T, Löwenberg M, Hommes DW, Verhaar AP, Schellmann S, Hahne M, Fangradt M, Wagegg M, Hoff P, Scheffold A, Spies CM, Burmester GR, Buttgereit F. Origin and functional activity of the membrane-bound glucocorticoid receptor. Arthritis Rheum. 2011;63:3779–3788. doi: 10.1002/art.30637. [DOI] [PubMed] [Google Scholar]
- 11.De Goeij AFPM, Van Zeeland JK, Beek CJL, Bosman FT. Steroid-Bovine Serum Albumin conjugates: Molecular characterization and their interaction with andogen and estrogen receptors. J Steroid Biochem. 1986;24:1017–1031. doi: 10.1016/0022-4731(86)90355-9. [DOI] [PubMed] [Google Scholar]