Abstract
Background
Understanding the neurobiological mechanisms that predict PTSD in recent trauma survivors is important for early interventions. Impaired inhibition of fear or behavioral responses is thought to be central to PTSD symptomatology, but its role in predicting PTSD is unknown. Here we examine whether brain function during response inhibition early after a civilian trauma can predict future PTSD symptoms.
Methods
Participants (original sample, N=27; replication sample, N=31) were recruited in the Emergency Department (ED) within 24h of trauma exposure. PTSD symptoms were assessed in the ED and 1, 3 and 6-months post-trauma. A Go/NoGo procedure in a 3T MRI scanner was used to measure neural correlates of response inhibition 1–2 months post-trauma. Elastic net regression was used to define the most optimal model to predict PTSD symptoms at 3-and 6-months among demographic, clinical and imaging measures.
Results
Less hippocampal activation was a significant predictor in the model predicting PTSD symptoms at 3-months (F(11,22)=4.33, p=0.01) and 6-months (F(9,19)=4.96, p=0.01). Other significant predictors in the model were race and pain level in the ED (3-months), and race and baseline depression symptoms (6-months). Using these predictors in a linear regression in the replication sample again resulted in significant models (F(3,23)=3.03, p=0.05; F(3,20)=5.74, p=0.007) with hippocampal activation predicting PTSD symptoms at 3-and 6-months.
Conclusions
Decreased inhibition-related hippocampal activation soon after trauma prospectively predicted greater future PTSD symptom severity. This finding may contribute to early identification of at-risk individuals and reveals potential targets for intervention or symptom prevention in the aftermath of trauma.
Keywords: Emergency department, functional magnetic resonance imaging (fMRI), hippocampus, longitudinal study, posttraumatic stress disorder (PTSD), predictive biomarkers, prospective study, response inhibition
Introduction
Up to 90% of the general population in the United States is exposed to a traumatic event at some point in their life (1). Traumatic events can include combat exposure, natural disasters or terrorist attacks, and life threatening accidents or assaults, many of which result in a need for emergency hospitalization. About 5–10% of trauma-exposed individuals develop posttraumatic stress disorder (PTSD) (2), therefore, one of the main goals of trauma research is to understand why some develop PTSD whereas others do not. Prospective studies are critical for improving our understanding of the neurobiological mechanisms that are associated with the development of PTSD in trauma survivors.
A specific and key impairment in PTSD is the inability to suppress a fear response in a safe environment (3). Although learning to associate a neutral cue with true danger is beneficial for survival, the ability to regulate behavioral and emotional responses in a safe environment is critical for ongoing health and wellbeing (4). The core symptoms of PTSD include excessive fear responses to trauma reminders, intrusive thoughts, and re-experiencing of the traumatic event despite being in another environment (4). Impaired fear inhibition has been demonstrated in both psychophysiological (5) and imaging studies of PTSD (6, 7). Individuals with PTSD show reduced vmPFC (7) and hippocampal recruitment during fear inhibition (6, 8), and these areas are activated together during extinction recall (9). The vmPFC regulates or inhibits the fear response (10, 11), whereas the hippocampus provides contextual information required for regulation of responses (10, 12, 13). Importantly, previous studies demonstrated that PTSD patients also showed reduced inhibition (14–16) and impaired processing of contextual cues (16, 17) during non-emotional response inhibition paradigms, indicating a more general inhibition and context processing deficit in PTSD. When using a simple Go/NoGo task with a red rectangle in the background as NoGo cue, brain regions associated with impaired fear inhibition and context processing in PTSD are hypoactive during response inhibition: in one study PTSD participants showed less vmPFC activation than trauma controls (15), and in a group of highly traumatized women decreased hippocampal activation correlated with more PTSD symptoms and less trait resilience (18). Moreover, hippocampal activation mediated the relationship between childhood trauma and PTSD symptoms versus resilience (18). Another study showed that inhibition of the startle response during early extinction was correlated with increased functional coupling of the hippocampus and vmPFC during NoGo relative to Go trials, as well as structural connectivity of white matter tracts connecting the hippocampus and the vmPFC (19). The hippocampus is important for modulation of behavior based on new sensory information or contextual cues (20), and previous findings suggest that increased hippocampal recruitment may be an important mechanism for coping with traumatic stress (18).
Previous studies have demonstrated that impaired inhibition in PTSD is not limited to fear regulation, and may therefore represent a more general deficit in PTSD (16, 21). Furthermore, impaired inhibition-related brain functioning in PTSD, particularly in the right inferior frontal gyrus (rIFG), did not improve with successful treatment (17). It was therefore suggested that impaired inhibition-related brain functioning may be a vulnerability factor for PTSD. This underscores the relevance of investigating neural correlates of response inhibition as potential biomarkers for PTSD development in the first months after trauma exposure. In a prospective fMRI study, prefrontal responses to threat stimuli were not predictive of later PTSD symptoms (24), however, no explicit response inhibition task was used. Another prospective study showed that decreased hippocampal activation during active down-regulation of negative emotional stimuli was related to increased PTSD symptoms (25). In the current study we aimed to probe brain regions associated with PTSD, and previously shown to be impaired in PTSD with this response inhibition task, i.e., the hippocampus and vmPFC. Furthermore, we included the rIFG as region of interest (ROI) as it has often been implicated in response inhibition (26), and whose function has been associated with PTSD (17).
The current prospective, longitudinal fMRI study recruited participants who were brought to a large level-I Emergency Department (ED) trauma center within 24h of experiencing a traumatic event. Functional MRI scans using a Go/NoGo response inhibition task were collected prior to PTSD diagnosis, 1–2 months post-trauma. Clinical data were collected in the ED at enrollment and 1, 3, and 6-months following trauma exposure. The data were used to investigate the hypothesis that response inhibition-related brain activation after trauma exposure predicts future PTSD symptoms. More specifically, we hypothesized that greater hippocampal, vmPFC and rIFG activation would predict fewer PTSD symptoms at 3-and 6-months post-trauma.
Methods and Materials
Participants
Participants were recruited as part of a large prospective study conducted in the ED at Grady Memorial Hospital, the largest Level I Trauma center in Georgia (USA). Participants were eligible for the study if they suffered a DSM-IV-TR criterion-A trauma (27) in the past 24h. Exclusion criteria were a history of mania, schizophrenia, other psychosis, current suicidal ideation, suicide attempt in the last 3-months, current intoxication, or experienced loss of consciousness (>5 minutes) as a result of the trauma. Participants were excluded if they showed any impairment on the Glasgow Coma Scale (28) in order to exclude for traumatic brain injury (TBI). During their 1-month visit, MRI eligible participants were invited for a scan, which took place within 2 to 3 weeks of the 1-month visit, on average 54 days (SD=14, range: 26–93) after trauma exposure. Due to moving to another scan facility and updating scan parameters in midst of recruitment, the study resulted in two samples that were analyzed separately: an original sample (N=38), and a replication sample (N=39). After complete written and verbal description of the study, all participants provided written informed consent. The Institutional Review Board of Emory University, Georgia Institute of Technology, and the Research Oversight Committee of Grady Memorial Hospital approved the study procedures.
Clinical assessment
Baseline assessment in the ED included the Standardized Trauma Interview (STI), a 41-item interview on demographics, characteristics of the trauma, patient-rated severity, and social support (29). The Posttraumatic Stress Diagnostic Scale (PDS), a 49-item self-report measure, was used to measure lifetime trauma history and current PTSD symptoms related to trauma occurring before the current index event (30). Current depression symptoms were assessed using the 21-item self-report Beck Depression Inventory (BDI) (31). Trauma exposure during childhood was assessed using the Childhood Trauma Questionnaire (CTQ) (32), a 25-item questionnaire on trauma experienced before age 18. Follow-up assessments of PTSD symptoms were performed at 1, 3, and 6-months post-trauma using the Modified PTSD Symptom Scale (PSS) (33). The number of symptoms as measured with the PSS was used as the outcome variable in the analyses.
Functional MRI
Response Inhibition Task
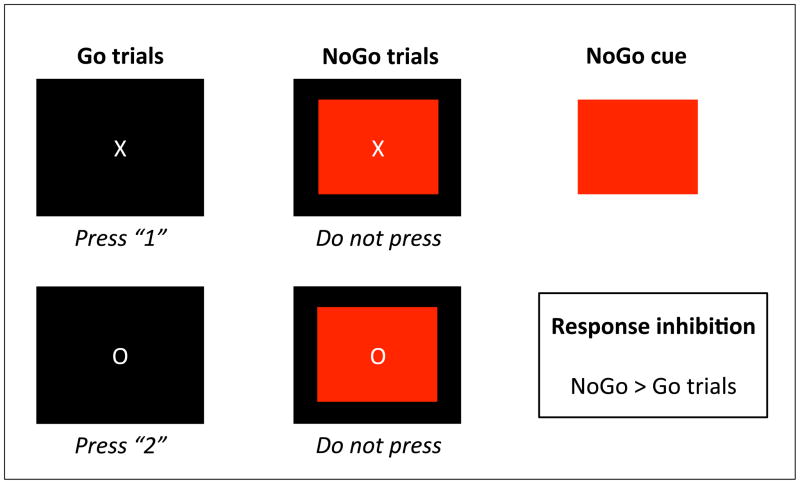
A Go/NoGo task was used to measure response inhibition (34). Participants were asked to respond to the Go-trials (white X or O on black screen), but to withhold their response to the NoGo-trials (red rectangle in the background of the X or O). All participants responded correctly to at least 75% of the Go and NoGo trials, and most participants had an accuracy score of 100% (Table 1). Only correct Go and NoGo trials were included in the analyses, and correct NoGo > correct Go was used as the contrast of interest in all analyses. The task is described in more detail in Figure 1.
Table 1.
Participant demographic and clinical data
| Original sample | Replication sample | |||
|---|---|---|---|---|
| N=27 | N=31 | Statistica | p-value | |
| Age, mean (SD) | 31.5 (10.3) | 36.9 (12.5) | t = −1.79 | 0.08 |
| Gender (% female) | 13/27 (48%) | 11/31 (35%) | χ2 = 0.95 | 0.33 |
| Race (%) | χ2 = 3.63 | 0.31 | ||
| Black | 17/27 (63%) | 26/31 (84%) | ||
| White | 6/27 (22%) | 3/31 (10%) | ||
| Mixed | 3/27 (11%) | 1/31 (3%) | ||
| Other | 1/27 (4%) | 1/31 (3%) | ||
| Education level (%) | χ2 = 3.26 | 0.52 | ||
| Master’s degree | 1/27 (4%) | 2/31 (6%) | ||
| Bachelor’s degree | 2/27 (7%) | 4/31 (13%) | ||
| Associate’s, some college | 15/27 (56%) | 10/31 (32%) | ||
| High school degree | 7/27 (26%) | 12/31 (39%) | ||
| Some high school | 2/27 (7%) | 3/31 (10%) | ||
| Type of trauma (%) | χ2 = 5.56 | 0.59 | ||
| Non-sexual assault | 1/27 (4%) | 1/31 (3%) | ||
| Motor vehicle collision | 19/27 (70%) | 19/31 (61%) | ||
| Motor cycle collision | 0/27 (0%) | 1/31 (3%) | ||
| Pedestrian vs. auto | 3/27 (11%) | 4/31 (13%) | ||
| Gunshot wound | 0/27 (0%) | 1/31 (3%) | ||
| Industrial/home accident | 0/27 (0%) | 3/31 (10%) | ||
| Bicycle accident | 2/27 (7%) | 1/31 (3%) | ||
| Sexual assault | 2/27 (7%) | 1/31 (3%) | ||
| Pain after trauma (0–10), mean (SD) | 5.12 (2.8) | 6.00 (2.5) | t = −1.23 | 0.23 |
| Patient-rated trauma severity (0–5), mean (SD) | 3.8 (1.1) | 4.2 (0.9) | t = −1.53 | 0.13 |
| Clinician-rated trauma severity (0–5), mean (SD) | 2.5 (0.8) | 2.6 (0.7) | t = −0.85 | 0.40 |
| Social support, mean (SD) | 2.8 (0.6) | 2.7 (0.6) | t =0.46 | 0.65 |
| Childhood trauma (CTQ),b mean (SD) | 37.8 (12.7) | 37.0 (14.3) | t =0.20 | 0.84 |
| Received treatment (%) | 7/27 (26%) | 4/31 (13%) | χ 2 = 1.59 | 0.21 |
| Days between trauma and scan, mean (SD) | 54.0 (14.3) | 53.9 (13.8) | t = 0.04 | 0.97 |
| Depression symptoms (BDI)c in the ED, mean (SD) | 8.6 (9.3) | 11.6 (11.6) | t = −1.07 | 0.29 |
| PTSD symptoms (PDS)d in the ED, mean (SD) | 4.6 (7.7) | 7.3 (7.4) | t = −1.29 | 0.20 |
| Meet DSM-IV criteria (%) | 2/27 (7%) | 3/31 (10%) | ||
| PTSD symptoms (PSS)e at 1-month, mean (SD) | 16.9 (12.8) | 15.0 (11.0) | t = 0.61 | 0.55 |
| Meet DSM-IV criteria (%) | 11/27 (41%) | 11/31 (35%) | ||
| PTSD symptoms (PSS)e at 3-months, mean (SD) | 12.7 (11.8) | 9.1 (8.5) | t =1.18 | 0.24 |
| Meet DSM-IV criteria (%) | 6/23 (26%) | 6/24 (25%) | ||
| PTSD symptoms (PSS)e at 6-months (mean, SD) | 10.0 (12.2) | 8.5 (8.3) | t = 0.46 | 0.65 |
| Meet DSM-IV criteria (%) | 5/20 (25%) | 4/21 (19%) | ||
| Percentage correct Go trials (mean, SD)) | 97.4% (4.8) | 98.2% (4.2) | t = 0.49 | 0.50 |
| Percentage correct NoGo trials (mean, SD) | 97.4% (4.8) | 99.4% (1.4) | t = −2.06 | 0.05 |
Statistical tests were performed to compare the 2 samples; Independent samples t-tests were conducted to compare means, and χ2tests were performed to compare proportions.
CTQ, Childhood Trauma Questionnaire; (32)
BDI, Beck Depression Inventory; (31)
PDS, Posttraumatic Stress Diagnostic Scale; (30)
PSS, Modified PTSD Symptom Scale.(33)
Figure 1. Go/NoGo task.
Response inhibition was measured using the Go/NoGo task that followed previous work by Leibenluft et al. (2007). On Go trials, a white X or O appeared on a black background for 1000ms. Participants were instructed to respond as fast as possible to this Go trial by pressing a 1 for X and 2 for O. However, one third of the trials was a NoGo trial (indicated by a red rectangle behind X or O), and participants were instructed to withhold their response. The stimulus event was followed by a jittered inter-trial interval ranging from 1250 to 2500ms, and a 500ms white fixation cross. The task consisted of 4 runs separated by three 30s rest periods. Each run comprised 26 “Go” trials, 13 “NoGo” trials, and 14 blank trials distributed randomly. Response inhibition was measured by subtracting correct Go trials from correct NoGo trials.
Brain Imaging Acquisition and Analyses
MRI scans for the 2 samples were collected at different scanners, using dissimilar scanner parameters, but both were Siemens 3.0-Tesla Magnetom Trio TIM whole-body MR scanners (Siemens, Malvern, PA) using a 12-channel head coil. Functional data were preprocessed and analyzed with SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Further information on data acquisition and analyses is described in the Supplement.
Group Analyses
The fMRI analyses were performed for the hippocampus, vmPFC and rIFG as ROIs. Functional, anatomically-constrained ROIs were created for each sample individually (35, 36). We used a p<0.05 threshold within the anatomically-defined (AAL atlas) bilateral hippocampus, vmPFC and rIFG to identify task-responsive voxels within this larger anatomical ROI. Mean contrast estimates for the NoGo>Go contrast were extracted from the resulting maps using SPM8, and were exported to IBM SPSS24.0. Missing data (only for CTQ and pain level) was imputed using mode.
First, correlation analyses were performed to investigate the relationship between hippocampal, vmPFC and rIFG contrast estimates and PTSD symptoms at 3-and 6-months. Second, elastic net regression was used to define the most optimal model to predict PTSD symptoms in the original sample. Group analyses were conducted using elastic net regularization procedures with SPSS default settings (37). For the predictors in the model, elastic net regression minimizes overfitting by penalizing coefficient estimates, thereby reducing the variance of estimates so that they are more stable and more generalizable to the larger population. Elastic net is particularly well suited to models wherein the predictors are highly correlated (38). PTSD symptoms at 3-and 6-months were used as continuous outcome measures in two different models. In addition to hippocampal, vmPFC or rIFG activation, models incorporated simultaneous estimation of numerous additional predictors that may influence the development of PTSD including demographics (age, gender, race, education), baseline symptoms (PTSD and depression), and childhood trauma (CTQ), patient-and clinician-rated severity, type of trauma, pain level in the ED, days between enrollment and scan, treatment, and social support (Table 1). The expected prediction error for each model was estimated with the .632 bootstrap (39). In accordance with the one-standard-error rule, the most parsimonious model within 1SE of the model with minimum expected prediction error was selected. Next, significant predictors resulting from this analysis were used in a linear regression model to predict PTSD symptoms at 3-and 6-months in the replication sample. Finally, correlation analyses and elastic net regression were performed to assess the relationship between PTSD symptoms at 1-month and hippocampal, vmPFC or rIFG activation.
Task-based whole brain activation for each group is presented in Supplemental Table S1. Additionally, to investigate the correlation between PTSD symptoms at 3-and 6-months and response inhibition-related activation outside the ROIs, we performed whole brain multiple regression analyses using PSS as a covariate. The resulting maps were tested for significance at a cluster-defining threshold of p<0.001 (as recommended by (40)), and a p<0.05 family-wise error (FWE)-corrected critical cluster size of k=41 was determined using SPM8 and a script (CorrClusTh.m; www2.warwick.ac.uk/fac/sci/statistics/staff/academic-research/nichols/scripts/spm).
Results
Participants
Demographic and clinical information is presented in Table 1.
Original Sample
Thirty-eight participants were scanned, but after excluding for head motion (>1mm/TR, N=5), falx calcification (N=3), technical issues during data collection (N=3), 27 participants (13 females) were included in the analyses. Twenty-three participants returned for their 3-month follow-up assessments, and 20 for their 6-month follow-up. At 6 months, 25% of participants met criteria for PTSD. Seven participants sought treatment from a psychologist, psychiatrist or mental health counselor between their index trauma and the 6-month follow-up. Use of treatment services was included in the statistical model.
Replication Sample
Thirty-nine participants were scanned, of which 31 (11 females) were included in the analyses. Participants were excluded for head motion (N=5), falx calcification (N=1), technical issues (N=2), no behavioral response (N=1). At 3-months, 24 participants returned for their follow-up assessments, and 21 for their 6-month assessments. At 6-months, 19% met criteria for PTSD, and four participants sought treatment during the course of the study.
Functional MRI results
Original Sample
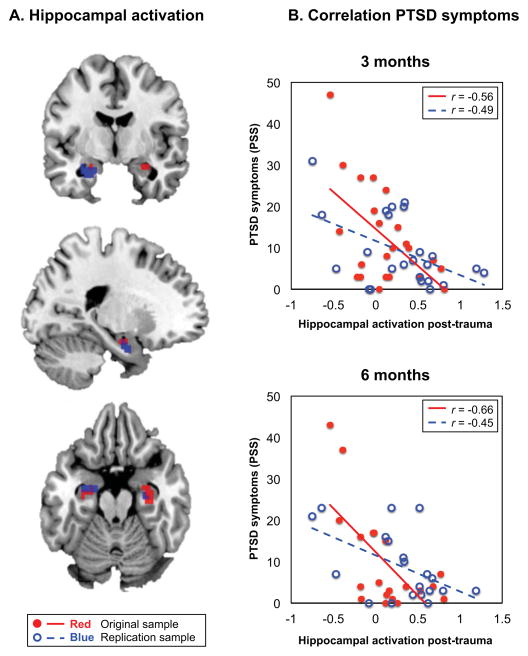
The functional hippocampal ROIs are presented in Figure 2a. The correlation analyses with PTSD symptoms at 3-and 6-months are shown in Figure 2b. Response inhibition-related hippocampal activation correlated significantly with PTSD symptoms at 3-months (r=−0.56, p=0.006) and 6-months (r=−0.66, p=0.002) 1. No significant correlations with the vmPFC or rIFG were observed.
Figure 2. Hippocampal activation predicts PTSD symptoms.
Figure 2a displays the activated voxels for the NoGo>Go inhibition contrast within the Automated Anatomical Labeling (AAL) atlas-defined bilateral hippocampus. The activated voxels for the original sample (N=27, k=16; in red) and the replication sample (N=31, k=45; in blue) were extracted for functional regions of interest (ROIs) analyses. Figure 2b shows the correlation between the contrast estimate in this functional hippocampal ROI (x-axis) and PTSD symptom score (y-axis) measured with the Modified PTSD Symptom Scale (PSS).(33) Hippocampal activation significantly correlated with PTSD symptoms at 3-months (original sample, p=0.006; replication sample, p=0.02) and 6-months (original sample, p=0.002; replication sample, p=0.04).
Lasso regression with elastic net was used to define the most optimal model to predict PTSD symptoms at 3-and 6-months using demographic, clinical and imaging measures. Table 2 displays the regression coefficients separately for all variables included in the most parsimonious models predicting PTSD symptoms at (a) 3-months and (b) 6-months. For the selected model predicting PTSD symptoms at 3-months, the apparent proportion of explained variance was 0.81 (F(11,22)=4.33, p=0.01). Hippocampal activation, race, and pain level in the ED were significant predictors, with less hippocampal activation, black/mixed race, and more pain in the ED predicting more PTSD symptoms at 3-months. At 6-months, the apparent proportion of explained variance for the selected model was 0.82 (F(9,19)=4.96, p=0.01). Less hippocampal activation, black/mixed race, and more depression symptoms in the ED predicted more PTSD symptoms at 6-months.
Table 2.
Regression coefficients for predicting PTSD symptoms
| Original sample: selected models using lasso regression with elastic net | |||||
|---|---|---|---|---|---|
| PTSD symptoms at 3-monthsa | |||||
|
| |||||
| Beta | SE | df | F | p-value | |
| Hippocampal activation | −0.26 | 0.10 | 1 | 6.18 | 0.03* |
| Race | 0.23 | 0.12 | 3 | 3.78 | 0.04* |
| Pain level in ED | 0.32 | 0.11 | 4 | 8.44 | 0.002* |
| Depression symptoms in ED (BDI) | 0.18 | 0.08 | 1 | 4.57 | 0.06 |
| PTSD symptoms in ED (PDS) | 0.10 | 0.14 | 1 | 0.51 | 0.49 |
| Treatment | 0.11 | 0.09 | 1 | 1.33 | 0.27 |
|
| |||||
| PTSD symptoms at 6-monthsb | |||||
|
| |||||
| Beta | SE | df | F | p-value | |
|
| |||||
| Hippocampal activation | −0.36 | 0.10 | 1 | 13.19 | 0.005* |
| Race | 0.25 | 0.13 | 3 | 3.80 | 0.05* |
| Pain level in the ED | 0.13 | 0.13 | 2 | 1.01 | 0.40 |
| Depression symptoms in ED (BDI) | 0.31 | 0.11 | 1 | 7.51 | 0.02* |
| PTSD symptoms in ED (PDS) | 0.13 | 0.15 | 1 | 0.75 | 0.41 |
| Days between enrollment and scan | −0.01 | 0.07 | 1 | 0.01 | 0.94 |
| Replication sample: using the selected significant predictors | ||||
|---|---|---|---|---|
| PTSD symptoms at 3-monthsc | ||||
|
| ||||
| Beta | SE | t | p-value | |
| Hippocampal activation | −0.52 | 3.25 | −2.71 | 0.01* |
| Race | −0.25 | 1.03 | −1.32 | 0.20 |
| Pain level in ED | −0.09 | 0.83 | −0.44 | 0.66 |
|
| ||||
| PTSD symptoms at 6-monthsd | ||||
|
| ||||
| Beta | SE | t | p-value | |
|
| ||||
| Hippocampal activation | −0.42 | 2.98 | −2.47 | 0.02* |
| Race | −0.24 | 0.89 | −1.33 | 0.20 |
| Depression symptoms in ED (BDI) | 0.44 | 0.12 | 2.50 | 0.02* |
Lasso regression with elastic net was performed in the original sample.
Regression coefficients for all variables included in the most optimal models to predict PTSD symptoms at 3-and 6-months are presented.
Significant factors (indicated by *) were included in linear regression models to predict PTSD symptoms at 3-and 6-months in the replication sample.
BDI, Beck Depression Inventory;23
PDS, Posttraumatic Stress Diagnostic Scale.22
Replication Sample
Response inhibition-related hippocampal activation in the replication sample also significantly correlated with PTSD symptoms at 3-months (r=−0.49, p=0.02) and 6-months (r=−0.45, p=0.04; Figure 2)1. No significant correlations with the vmPFC or rIFG were observed.
Including all significant predictors defined in the original sample (Table 2) resulted in a significant model to predict PTSD symptoms at 3-months (F(3,23)=3.03, p=0.05, R2=0.31, R2adjusted=0.21) and 6-months (F(3,20)=5.74, p=0.007, R2=0.50, R2adjusted=0.42), with the hippocampus significantly predicting PTSD symptoms at 3-and 6-months.
PSS prior to scan
A significant correlation between PTSD symptoms at 1-month and hippocampal activation was observed in the original sample (r=−0.41, p=0.04), but not in the replication sample (r=−0.11, p=0.54). Using elastic net regression in the original sample revealed that hippocampal activation was not associated with PSS at 1-month, moreover, the overall model was not significant. No correlations were observed with the vmPFC or rIFG.
Whole Brain Analyses
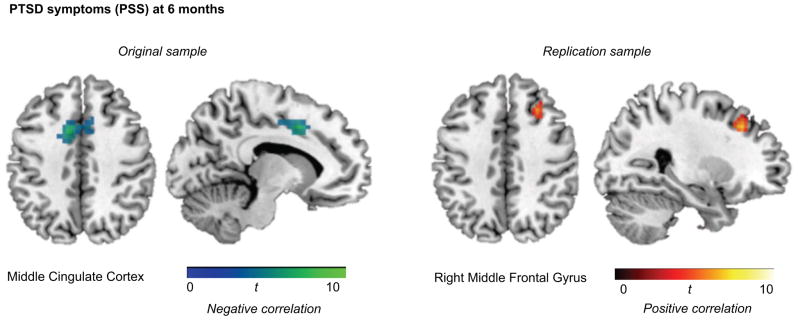
Figure 3 displays the results from the whole brain analyses (p<0.001, and a p<0.05 FWE-corrected cluster threshold of k=41) showing the negative correlation between PSS at 6-months and the mid cingulate cortex in the original sample, and the positive correlation between PSS at 6-months and the right middle frontal gyrus in the replication sample.
Figure 3. Whole brain correlations with PTSD symptoms.
Whole brain analyses (p<0.001, and FWE-corrected cluster threshold of k=41) for the correlation between response inhibition-related activation and PTSD symptoms are displayed for the original sample (left) and replication sample (right).
Discussion
The current prospective, longitudinal fMRI study aimed to identify neurobiological predictors for the development of PTSD by recruiting participants in the Emergency Department after a criterion-A trauma, scanning them 1–2 months later and assessing PTSD symptoms at 3-and 6-months post-trauma. We are the first to show that less anterior hippocampal activation predicted greater PTSD symptoms at 3-and 6-months post-trauma. Black/mixed race and more pain in the ED predicted more PTSD symptoms at 3-months, and black/mixed race and more baseline depression symptoms predicted more symptoms at 6-months. An out-of-sample test of these predictors in a replication sample showed again that reduced hippocampal activation significantly predicted PTSD symptoms at 3-and 6-months. These data show that increased response inhibition-related activation in the hippocampus predicts decreased risk for developing PTSD symptoms after trauma exposure. The implications of the study are that hippocampal function could serve as a target for early intervention in traumatized individuals.
The hippocampus is a particularly plastic and vulnerable region of the brain (41). Smaller hippocampal volume has been repeatedly associated with development (42) and persistence (43) of PTSD. This is the first study showing a role for hippocampal activation in the development of PTSD. Although the hippocampus is not typically linked to response inhibition and its role has mainly been demonstrated during contextual fear inhibition (6, 7, 9), we have previously demonstrated a relationship between hippocampal activation during response inhibition and PTSD symptoms (18, 19). Hippocampal functioning in Go/NoGo tasks has also been demonstrated in other studies: less hippocampal activation was shown in heavy drinkers compared to light drinkers (44), in violent adolescents (45), and in cannabis-using ADHD patients (46) relative to controls. Similarly, hippocampal activation during Go/NoGo was found to increase post-treatment in adolescents with bipolar disorder (47). Taken together, these findings suggest that lower levels of hippocampal involvement in response inhibition may contribute to transdiagnostic risk for psychiatric dysfunction, particularly for disorders with an impulsive motivational component. Additional basic research is needed in order to better define the specific cognitive functions performed by the hippocampus during response inhibition.
The hippocampus plays a key role in many aspects of cognition that are relevant to PTSD risk. The hippocampus is essential for the formation and retrieval of new memories, especially episodic memories consisting of autobiographical events and contextual information (48, 49). Impaired episodic memory of traumatic events is thought to contribute to the development of PTSD (50), and is related to hippocampal structure (51, 52) and function (53). Furthermore, the hippocampus plays a crucial role in contextual modulation of behavior by comparing new sensory inputs to existing representations (20). While we did not directly manipulate contextual cues, we likely tapped into the role of the hippocampus in processing information related to the NoGo trials (i.e., presence of a red background). As evident from animal research, the hippocampus is involved in occasion setting, where a contextual cue modulates discrimination between different stimuli (54, 55). Holland and colleagues suggested that hippocampal lesions hinder inhibitory learning about contextual and explicit cues (56), whereas dorsal hippocampal lesions in another study specifically impacted the processing of contextual cues (57). These studies emphasize the importance of the hippocampus in context processing and stimulus discrimination.
A recent perspective paper suggests that dysregulation within the context processing circuitry, consisting of the hippocampus and vmPFC, is a potentially key deficit in PTSD (21), and our findings support this growing literature. Another potential mechanism by which the hippocampus may participate in response inhibition is pattern separation, i.e., the conversion of comparable experiences or events into separate, non-overlapping representations (20). This hippocampus-dependent mechanism is fundamental to successful context processing, and impairment results in the inability to discriminate two similar yet different situations. Several studies have demonstrated that PTSD patients show impaired memory for specific patterns (58), poor processing of spatial cues (59), and impaired safety discrimination (3). The hippocampus is critical for the regulation of impulsivity. Hippocampal lesions in rats resulted in impulsive choice, reflected in a preference for an immediate small reward over a delayed, larger reward (60). Impulsive choice may explain reduced inhibition-related hippocampal activation seen in heavy drinkers (44), violent adolescents (45), and ADHD patients (46). Impulsivity has also been associated with the development of PTSD in both military (61) and civilian populations (62). There is clear evidence for the role of the hippocampus in PTSD risk, suggesting that better episodic memory and the ability to use and integrate contextual information may help an individual successfully regulate behavioral and emotional responses. Many previous studies have linked hippocampal structure to PTSD (e.g. (42, 43, 52)), and some have shown altered hippocampal activation (8, 18, 63). This is, however, the first study to examine hippocampus function prospectively in the early aftermath of trauma and show its critical role in the development of PTSD symptoms.
VmPFC functioning during the Go/NoGo task has previously been shown to be impaired in PTSD (15); however, here we did not show its involvement in predicting the development of PTSD. As demonstrated by Stevens and colleagues (64), vmPFC activation in PTSD may be specifically related to childhood trauma exposure. Also, the rIFG was not related to current or future PTSD symptoms. An explanation for this may be that in previous studies (16, 17) the rIFG was associated with PTSD during proactive inhibition, i.e., the anticipation of a stop signal, whereas the simpler task we used here did not include an anticipation component. Alternatively, the differences in study sample, type of trauma and duration of symptoms may explain the absence of rIFG findings in this study.
In the whole brain analyses, we observed a positive correlation with the right middle frontal gyrus, and a negative correlation between PTSD symptoms and inhibition-related activation in the middle cingulate cortex. The right middle frontal gyrus is activated during Go/NoGo tasks, particularly during more complex stimulus identification (65). The middle cingulate cortex is typically activated during motor tasks and is highly connected with the precentral gyrus (66), a motor area involved in the planning and execution of movements. Previous studies have observed a relationship between inhibition-related motor and frontal activation in PTSD patients (14–16), and here we show that reduced activation in motor control regions is also related to the development of PTSD. Furthermore, our results support the hypothesis from a pre/post-treatment study that showed reduced inhibition despite clinical improvement, and therefore postulated that it may represent a vulnerability factor for development of PTSD (17). However, the observed whole brain correlations were not the same across groups, and should therefore be interpreted with caution.
An important strength of this study is the inclusion of a replication sample. The hippocampus finding was replicated, even though MRI scans of the second sample were collected at a different scanner site, using different scan parameters, and despite the fact that the range of PTSD symptoms was smaller in the replication sample (6-months: 0–23) than in the original dataset (0–43). This demonstrates that the correlation was not dependent on more severe PTSD cases in the original sample. Hippocampal activation also predicted the development of mild PTSD symptoms, underscoring the robustness of inhibition-related hippocampal activation as a predictor, and the potential generalizability of this finding.
One of the limitations of this study is the sample size of each individual sample. There was a relatively high number of dropouts due to head motion, falx calcification and technical issues during data collection. However, as the numbers were comparable for the two samples and the final groups did not differ on demographic or clinical measures, we believe that the impact of this dropout on the results is negligible. A bigger sample is needed to appropriately power exploratory voxel-wise analyses of the whole brain. The majority of our population consisted of survivors of severe accidents; therefore, replication in military samples or individuals with high levels of interpersonal trauma is of interest. However, type of trauma did not influence PTSD outcome in our analyses. Another limitation of the current study is the timing of the MRI scan. The scan was collected on average 54 days after trauma, rather than immediately post-trauma. It is likely that some individuals have developed PTSD symptoms at this time, and PTSD severity may therefore correlate with hippocampal activation. Indeed, we observed a correlation between hippocampal activation and PTSD symptoms at 1-month in the original sample; however, this correlation was less strong than the correlations with PTSD symptoms at 3-and 6-months. Furthermore, this correlation was not observed in the replication sample, and was not associated with PTSD symptoms at 1-month in the elastic net regression. There are logistical and medical barriers to conducting scans immediately after trauma, especially if there was any injury to head or body. On the other hand, the current approach has the benefit of capturing any trauma-related neural changes, i.e., direct mechanical action, systemic effects, or neurocognitive influences. Future studies collecting data prior to trauma exposure are of high interest to determine pre-trauma vulnerability factors (67). However, scanning prior to trauma exposure, particularly in a civilian sample, is challenging and requires a very large sample. Finally, a more complex task with manipulation of contextual cues could possibly provide more insight in underlying processes, and moreover, may show a relationship with hippocampal functioning or PTSD symptoms. This is of high interest as it is not always feasible to scan recently traumatized individuals to assess risk for PTSD.
In conclusion, we showed that decreased hippocampal activation during response inhibition in the first months post-trauma significantly predicts an increased risk for the development of PTSD. Conversely, increased recruitment of the hippocampus may help an individual to successfully regulate behavioral and emotional responses, beneficial for coping with a traumatic experience. This finding may contribute to an early identification of trauma survivors at-risk for PTSD. Furthermore, the identification of specific mechanisms that affect risk for PTSD in the period after trauma exposure generates opportunities for the development of more specific targets for intervention and treatment, such as psychotherapy or pharmacological interventions aiming to increase hippocampal activation. Because of its inherent neural plasticity, the hippocampus may respond well to these interventions. Future studies targeting hippocampal-dependent functioning in the immediate aftermath of trauma are of high interest.
Supplementary Material
Supplemental Table S1. Whole brain NoGo>Go analyses
Acknowledgments
We would like to acknowledge Debra Houry, M.D., and Abigail Hankin-Wei, M.D. for their collaborative efforts on this study. We would also like to thank Alex O. Rothbaum, Thomas Crow, Heather Grinstead, Jessica Maples-Keller, Devika Fiorillo, Renuka Reddy, Zachary Clifford, Adam Munoz, Erin Lightman-Renner, Lydia Odenat, Loren M. Post, Liza C. Zwiebach, Kathryn Breazeale, Jessica Morgan, Natasha Mehta, Elicia D. Skelton, Taleesha S. Booker, Jonathan Zebrowski, Siddharta Kosaraju, and Ariella Dagi for their work in the Emergency Department recruiting and assessing participants.
Funding Support
This work was supported by funding from the National Institute of Mental Health (R01 MH094757 (KJR), R21 MH106902 (TJ), F32 MH101976 (JSS)), and by UL1 TR000424.
Dr. Rothbaum has received funding from the Wounded Warrior Project, the Department of Defense (Clinical Trial Grant W81XWH-10-1-1045), NIMH (grant 1R01MH094757-01), a Brain and Behavior Research Foundation (NARSAD) Distinguished Investigator Grant, the McCormick Foundation, and Transcept Pharmaceuticals; she has served on an advisory board for Genentech, owns equity in Virtually Better, and has received royalties from Oxford University Press, Guilford, American Psychiatric Publishing, and Emory University. The other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
There are different approaches to define the hippocampus, and we therefore re-analyzed the data using the Hammers atlas as recommended by (57). Again, a significant correlation was demonstrated between bilateral hippocampal activation and PTSD symptoms in the original sample at 3-months (r=−0.482, p=0.020) and 6-months (r=−0.575, p=0.008), and in the replication sample at 3-months (r=−0.458, p=0.024) and 6-months (r=−0.442, p=0.045).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: The 1996 Detroit area survey of trauma. Archives of General Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Jovanovic T, Kazama A, Bachevalier J, Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62:695–704. doi: 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maren S, Phan KL, Liberzon I. The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, et al. Posttraumatic stress disorder may be associated with impaired fear inhibition: Relation to symptom severity. Psychiatry Research. 2009;167:151–160. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biological psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rougemont-Bücking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, et al. Altered Processing of Contextual Information during Fear Extinction in PTSD: An fMRI Study. CNS Neuroscience & Therapeutics. 2011;17:227–236. doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, et al. Impaired Contextual Modulation of Memories in PTSD: An fMRI and Psychophysiological Study of Extinction Retention and Fear Renewal. The Journal of Neuroscience. 2014;34:13435–13443. doi: 10.1523/JNEUROSCI.4287-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biological Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-Dependent Human Extinction Memory Is Mediated by a Ventromedial Prefrontal and Hippocampal Network. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- 13.Hermann A, Stark R, Milad MR, Merz CJ. Renewal of conditioned fear in a novel context is associated with hippocampal activation and connectivity. Soc Cogn Affect Neurosci. 2016 doi: 10.1093/scan/nsw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falconer E, Bryant R, Felmingham KL, Kemp AH, Gordon E, Peduto A, et al. The neural networks of inhibitory control in posttraumatic stress disorder. Journal of Psychiatry & Neuroscience: JPN. 2008;33:413–422. [PMC free article] [PubMed] [Google Scholar]
- 15.Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, et al. Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex. 2013;49:1884–1891. doi: 10.1016/j.cortex.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Rooij SJH, Rademaker AR, Kennis M, Vink M, Kahn RS, Geuze E. Impaired right inferior frontal gyrus response to contextual cues in male veterans with PTSD during inhibition. Journal of Psychiatry and Neuroscience. 2014;39:130223. doi: 10.1503/jpn.130223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Rooij SJH, Geuze E, Kennis M, Rademaker AR, Vink M. Neural correlates of inhibition and contextual cue processing related to treatment response in PTSD. Neuropsychopharmacology. 2015;40:667–675. doi: 10.1038/npp.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Rooij SJH, Stevens JS, Ely TD, Fani N, Smith AK, Kerley KA, et al. Childhood Trauma and COMT Genotype Interact to Increase Hippocampal Activation in Resilient Individuals. Frontiers in Psychiatry. 2016:7. doi: 10.3389/fpsyt.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fani N, King TZ, Brewster R, Srivastava A, Stevens JS, Glover E, et al. Fear-potentiated Startle During Extinction is Associated with White Matter Microstructure and Functional Connectivity. Cortex. 2015;64:249–259. doi: 10.1016/j.cortex.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15:1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberzon I, Abelson James L. Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron. 2016;92:14–30. doi: 10.1016/j.neuron.2016.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lommen MJJ, Engelhard IM, Sijbrandij M, van den Hout MA, Hermans D. Pre-trauma individual differences in extinction learning predict posttraumatic stress. Behaviour Research and Therapy. 2013;51:63–67. doi: 10.1016/j.brat.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Sijbrandij M, Engelhard IM, Lommen MJJ, Leer A, Baas JMP. Impaired fear inhibition learning predicts the persistence of symptoms of posttraumatic stress disorder (PTSD) Journal of Psychiatric Research. 2013;47:1991–1997. doi: 10.1016/j.jpsychires.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, et al. Amygdala Reactivity and Anterior Cingulate Habituation Predict Posttraumatic Stress Disorder Symptom Maintenance After Acute Civilian Trauma. Biological Psychiatry. doi: 10.1016/j.biopsych.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, et al. Amygdala Response to Negative Stimuli Predicts PTSD Symptom Onset following a Terrorist Attack. Depression and anxiety. 2014;31:834–842. doi: 10.1002/da.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Association AP. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 28.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 29.Rothbaum BO, Foa EB, Riggs DS, Murdock T, Walsh W. A prospective examination of post-traumatic stress disorder in rape victims. Journal of Traumatic Stress. 1992;5:455–475. [Google Scholar]
- 30.Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of posttraumatic stress disorder: The Posttraumatic Diagnostic Scale. Psychological assessment. 1997;9:445. [Google Scholar]
- 31.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 32.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 33.Foa EB, Tolin DF. Comparison of the PTSD Symptom Scale-Interview Version and the Clinician-Administered PTSD scale. J Trauma Stress. 2000;13:181–191. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- 34.Leibenluft E, Rich B, Vinton D, Nelson E, Fromm S, Berghorst L, et al. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. American Journal of Psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- 35.Mitsis GD, Iannetti GD, Smart TS, Tracey I, Wise RG. Regions of interest analysis in pharmacological fMRI: How do the definition criteria influence the inferred result? NeuroImage. 2008;40:121–132. doi: 10.1016/j.neuroimage.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 36.Degryse J, Seurinck R, Durnez J, Gonzalez-Castillo J, Bandettini PA, Moerkerke B. Introducing Alternative-Based Thresholding for Defining Functional Regions of Interest in fMRI. Frontiers in Neuroscience. 2017:11. doi: 10.3389/fnins.2017.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Kooij AJ, Meulman JJ. SPSS Inc, editor. Regression with Optimal Scaling. In: Meulman JJWJH, editor. SPSS Categories. Chicago: SPSS Inc; 1999. pp. 1–8.pp. 77–101.pp. 239–246. [Google Scholar]
- 38.Zou H, Hastie T. Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2005;67:301–320. [Google Scholar]
- 39.van der Kooij AJ. Doctoral Thesis. Leiden University, Faculteit der Sociale Wetenschappen; 2007. Prediction accuracy and stability of regression with optimal scaling transformations. [Google Scholar]
- 40.Woo C-W, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. NeuroImage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McEwen BS. Stress and hippocampal plasticity. Annual Review of Neuroscience. 1999:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 42.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Rooij SJH, Kennis M, Sjouwerman R, Van Den Heuvel MP, Kahn RS, Geuze E. Smaller hippocampal volume as a vulnerability factor for the persistence of post-traumatic stress disorder. Psychological Medicine. 2015;45:2737–2746. doi: 10.1017/S0033291715000707. [DOI] [PubMed] [Google Scholar]
- 44.Ahmadi A, Pearlson GD, Meda SA, Dager A, Potenza MN, Rosen R, et al. Influence of Alcohol Use on Neural Response to Go/No-Go Task in College Drinkers. Neuropsychopharmacology. 2013;38:2197–2208. doi: 10.1038/npp.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiao Y, Mei Y, Du X, Xie B, Shao Y. Reduced brain activation in violent adolescents during response inhibition. Scientific Reports. 2016;6:21318. doi: 10.1038/srep21318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rasmussen J, Casey BJ, van Erp TGM, Tamm L, Epstein JN, Buss C, et al. ADHD and cannabis use in young adults examined using fMRI of a Go/NoGo task. Brain Imaging and Behavior. 2016;10:761–771. doi: 10.1007/s11682-015-9438-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diler RS, Segreti AM, Ladouceur CD, Almeida JRC, Birmaher B, Axelson DA, et al. Neural Correlates of Treatment in Adolescents with Bipolar Depression During Response Inhibition. Journal of Child and Adolescent Psychopharmacology. 2013;23:214–221. doi: 10.1089/cap.2012.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 49.Jin J, Maren S. Prefrontal-Hippocampal Interactions in Memory and Emotion. Frontiers in Systems Neuroscience. 2015;9:170. doi: 10.3389/fnsys.2015.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ehlers A, Clark DM. A cognitive model of posttraumatic stress disorder. Behaviour Research and Therapy. 2000;38:319–345. doi: 10.1016/s0005-7967(99)00123-0. [DOI] [PubMed] [Google Scholar]
- 51.Tischler L, Brand SR, Stavitsky K, Labinsky E, Newmark R, Grossman R, et al. The relationship between hippocampal volume and declarative memory in a population of combat veterans with and without PTSD. Annals of the New York Academy of Sciences. 2006:405–409. doi: 10.1196/annals.1364.031. [DOI] [PubMed] [Google Scholar]
- 52.Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, et al. MRI-Based Measurement of Hippocampal Volume in Patients With Combat-Related Posttraumatic Stress Disorder. The American journal of psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.St Jacques PL, Botzung A, Miles A, Rubin DC. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. Journal of Psychiatric Research. 2011;45:630–637. doi: 10.1016/j.jpsychires.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmajuk NA, Buhusi CV. Stimulus configuration, occasion setting, and the hippocampus(Article) Behavioral Neuroscience. 1997;111:235–258. doi: 10.1037//0735-7044.111.2.235. [DOI] [PubMed] [Google Scholar]
- 55.Myers CE, Gluck MA. Context, conditioning, and hippocampal rerepresentation in animal learning. Behavioral Neuroscience. 1994;108:835–847. doi: 10.1037//0735-7044.108.5.835. [DOI] [PubMed] [Google Scholar]
- 56.Holland PC, Lamoureux JA, Han JS, Gallagher M. Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus. 1999;9:143–157. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 57.Yoon T, Graham LK, Kim JJ. Hippocampal Lesion Effects on Occasion Setting by Contextual and Discrete Stimuli. Neurobiology of learning and memory. 2011;95:176–184. doi: 10.1016/j.nlm.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Šodić L, Antičević V, Britvić D, Ivkošić N. Short-term Memory in Croatian War Veterans with Posttraumatic Stress Disorder. Croatian medical journal. 2007;48:140–145. [PMC free article] [PubMed] [Google Scholar]
- 59.Gilbertson MW, Williston SK, Paulus LA, Lasko NB, Gurvits TV, Shenton ME, et al. Configural Cue Performance in Identical Twins Discordant for Posttraumatic Stress Disorder: Theoretical Implications for the Role of Hippocampal Function. Biological psychiatry. 2007;62:513–520. doi: 10.1016/j.biopsych.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheung THC, Cardinal RN. Hippocampal lesions facilitate instrumental learning with delayed reinforcement but induce impulsive choice in rats. BMC Neuroscience. 2005;6:36–36. doi: 10.1186/1471-2202-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.James LM, Strom TQ, Leskela J. Risk-taking behaviors and impulsivity among veterans with and without PTSD and mild TBI. Military Medicine. 2014;179:357–363. doi: 10.7205/MILMED-D-13-00241. [DOI] [PubMed] [Google Scholar]
- 62.Netto LR, Pereira JL, Nogueira JF, Cavalcanti-Ribeiro P, Santana RC, Teles CA, et al. Impulsivity is relevant for trauma exposure and PTSD symptoms in a non-clinical population. Psychiatry Research. 239:204–211. doi: 10.1016/j.psychres.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 63.Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, Elzinga BM, Sjoerds Z, et al. Increased anterior cingulate cortex and hippocampus activation in Complex PTSD during encoding of negative words. Social Cognitive and Affective Neuroscience. 2013;8:190–200. doi: 10.1093/scan/nsr084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stevens JS, Ely TD, Sawamura T, Guzman D, Bradley B, Ressler KJ, et al. Childhood maltreatment predicts reduced inhibition-related activity in the rostral anterior cingulate in PTSD, but not trauma-exposed controls. Depression and Anxiety. 2016 doi: 10.1002/da.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Criaud M, Boulinguez P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neuroscience & Biobehavioral Reviews. 2013;37:11–23. doi: 10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 66.Beckmann M, Johansen-Berg H, Rushworth MFS. Connectivity-Based Parcellation of Human Cingulate Cortex and Its Relation to Functional Specialization. The Journal of Neuroscience. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Wingen GA, Geuze E, Vermetten E, Fernández G. Perceived threat predicts the neural sequelae of combat stress. Molecular Psychiatry. 2011;16:664–671. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodionov R, Chupin M, Williams E, Hammers A, Kesavadas C, Lemieux L. Evaluation of atlas-based segmentation of hippocampi in healthy humans. Magnetic Resonance Imaging. 2009;27:1104–9. doi: 10.1016/j.mri.2009.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1. Whole brain NoGo>Go analyses