Abstract
An artificial placenta (AP) utilizing venovenous extracorporeal life support (VV-ECLS) could represent a paradigm shift in the treatment of extremely premature infants. However, AP support could potentially alter cerebral oxygen delivery.
We assessed cerebral perfusion in fetal lambs on AP support using near-infrared spectroscopy (NIRS) and carotid arterial flow (CAF).
Fourteen premature lambs at EGA 130 days (term=145) underwent cannulation of the right jugular vein and umbilical vein with initiation of VV-ECLS. An ultrasonic flow probe was placed around the right carotid artery (CA) and a NIRS sensor was placed on the scalp. Lambs were not ventilated. CAF, percentage of regional saturation (rSO2) as measured by NIRS, hemodynamic data and blood gases were collected at baseline (native placental support) and regularly during AP support.
Fetal lambs were maintained on AP support for a mean of 55 ± 27 hours. Baseline rSO2 on native placental support was 40 ± 3%, compared to a mean rSO2 during AP support of 50 ± 11% (p=0.027). Baseline CAF was 27.4 ± 5.4 mL/kg/min compared to an average CAF of 23.7 ± 7.7 mL/kg/min during AP support. Cerebral fractional tissue oxygen extraction (FTOE) correlated negatively with CAF (r= −0.382, p<0.001) and mean arterial pressure (r= −0.425, p<0.001). FTOE weakly correlated with systemic O2 saturation (r=0.091, p=0.017).
Cerebral oxygenation and blood flow in premature lambs are maintained during support with an artificial placenta. Cerebral O2 extraction is inversely related to carotid flow and is weakly correlated with systemic O2 saturation.
Keywords: ECMO, extracorporeal membrane oxygenation, ECLS, extracorporeal life support, cerebral perfusion, prematurity
Introduction
The most common cause of death among preterm infants is respiratory failure, with extremely premature infants (<29 weeks estimated gestational age) at the greatest risk.1 Despite therapeutic advances such as low-pressure mechanical ventilation, high frequency oscillatory or jet ventilation, exogenous surfactant and extracorporeal membrane oxygenation (ECMO), morbidity and mortality remain high. Furthermore, these life-support strategies are suboptimal for extremely premature infants, as they are associated with pressure-related lung trauma and long-term morbidity.2, 3 The ideal strategy for the treatment of respiratory failure in extremely premature infants would be an Artificial Placenta (AP), which would recreate fetal physiology by utilizing extracorporeal life support (ECLS) for gas exchange, avoiding mechanical ventilation, and preserving fetal circulation.
In our laboratory, the AP utilizes venovenous (VV) ECLS with jugular drainage and umbilical vein reinfusion. We have previously demonstrated preservation of fetal circulation (patency of the ductus arteriosus), hemodynamic stability and gas exchange for 70 hours of AP support, and survival for up to 7 days.4, 5 However, we had not yet described cerebral blood flow or oxygen delivery, which would be required before use of the AP in clinical practice, similar to conventional ECLS.6, 7 This data can be obtained by several means. Near-infrared spectroscopy (NIRS) is an established method of measuring cerebral oxygenation in neonates on ECLS.8–10 Carotid and cerebrovascular blood flow can also be measured by ultrasonography with Doppler,11–13 and direct-contact carotid flow probes have even been used in lambs supported with VA-ECLS.14 The purpose of our study was to assess cerebral perfusion and oxygenation in this model of the AP using these technologies.
Methods
Operative Instrumentation
All experimental protocols were approved by our institution’s University Committee on the Use and Care of Animals. Pregnant ewes at EGA of 130–135 days (term=145 days; n=14) underwent laparotomy under general anesthesia. The uterus was exposed and a hysterotomy created to expose the fetus, after which buprenorphine 0.01 mg/kg was administered subcutaneously. After local injection of 1% lidocaine, the carotid sheaths were opened by surgical cut-down. The left carotid artery was ligated distally and a 5 Fr carotid arterial line (Arrow: Teleflex, Morrisville, NC, USA) was placed for preductal arterial blood sampling. Heparin 100 U/kg was injected IV, after which the right jugular vein was cannulated with a 10–12 Fr Bio-Medicus venous cannula (Medtronic, Minneapolis, MN). One of the umbilical veins (UV) was cannulated with a 10–12 Fr Bio-Medicus arterial cannula for reinfusion, and the remaining umbilical cord was ligated and divided. Lambs were then intubated, with the endotracheal tube filled with amniotic fluid and clamped.
AP Support
The extracorporeal circuit consisted of a low-resistance 0.5 m2 surface area polypropylene hollow fiber oxygenator/heat exchanger (Capiox RX05, Terumo Cardiovascular Systems, Ann Arbor, MI), a gravity-filled peristaltic roller pump (M-pump, MC3 Inc, Ann Arbor, MI) with a flow of 50–75 mL/kg/min, and ¼″ Tygon tubing (Saint-Gobain North America, Malverne, PA, USA). The sweep gas was comprised of a mixture of 70% O2 and 3% CO2 with balance nitrogen, and sweep flow was maintained at 1–3 L/min.
The lambs were incubated in a dry heated waterbed to help maintain core fetal temperature of 39 °C (normal lamb temperature is 38–40 °C) and heated via the heat exchanger in the AP circuit. Broad-spectrum antibiotics were given. Fetal volume status was maintained with a 10% dextrose / 0.225% NaCl solution at 4 mL/kg/hr. Additional boluses of colloids and crystalloids were given as needed for hypotension or low circuit flow. Lambs were transfused for hemoglobin levels <10 g/dL with blood procured from the native placenta at the time of delivery. Heparin was infused and titrated to a goal ACT of 180–200 s. Support was continued for up to 4 days, after which time the lamb was sacrificed. After sacrificed, brains were procured and examined for gross pathology. Brains were then sectioned for histopathologic analysis in the case of gross pathology.
Postoperative data collection
Fetal blood gases were collected hourly from the carotid artery and analyzed (ABL 505, Radiometer America, Cincinnati, OH). In addition, hemodynamics, blood glucose, temperature, and ACTs were recorded at regular intervals during AP support.
Cerebral blood flow and oxygenation
During the operative instrumentation, the right carotid artery was isolated, and a 2.5 mm ultrasonic flow probe (Transonic Systems Inc, Ithaca, NY) was placed around it for continuous carotid arterial flow (CAF) monitoring. Prior to cannulation or ligation of the umbilical cord, baseline CAF values were collected from experimental animals while still on native placental support. These values were also recorded every 30 minutes during artificial placental support and adjusted to body weight. The ratio of CAF to mean arterial pressure (MAP) was also calculated.
A transcranial Somanetics INVOS Cerebral Oximeter pediatric sensor (Somanetics Corporation, Troy, MI) was placed on the center of each lamb’s head to non-invasively monitor cerebral oxygenation – these probes remained in place for the entirety of the experiment without repositioning. NIRS measures reflect venous and arterial oxygenation in a ratio of approximately 84:16.15 The rSO2 values were used in conjunction with the oxygen saturation (SaO2) of blood drawn from the carotid artery to calculate the cerebral fractional tissue oxygen extraction (FTOE). FTOE, calculated as the ratio of brain oxygen consumption served as a relative measure of the balance between brain oxygen delivery and consumption (FTOE = (SaO2 - rSO2)/SaO2).16 Since rSO2 predominantly reflects venous oxygen saturation, an increase in FTOE reflects higher oxygen extraction, or increased oxygen consumption compared to oxygen delivery. Conversely, decreased FTOE implies decreased oxygen consumption relative to oxygen delivery.17, 18 As with baseline CAF, baseline NIRS values were collected from experimental animals before cannulation or umbilical cord ligation while still on native placental support. NIRS values were subsequently recorded every 30 minutes after initiation of AP support.
Statistical Analysis
Data were analyzed in each sample collection time as a separate data point. Values are expressed as mean ± standard deviation (SD). Statistical analysis was performed using SPSS (IBM, Armonk, NY), including paired t-tests and Pearson correlation coefficients. A p-value <0.05 was considered statistically significant.
Results
Lambs were successfully supported for 55±27 hours (range 10 to 92 hours) of support. Death was due to cardiovascular collapse, sepsis, or elective sacrifice. Mean body weight at the conclusion of all experiments was 4.3±0.6 kg (range 3.5–5.1 kg). Mean hemodynamics, carotid arterial blood gas values, and acid-base status were within the normal fetal range and stable throughout AP support [Table 1].
Baseline carotid arterial flow (CAF) as measured by Doppler before cannulation on native placental support was 25.1±4.5 mL/kg/min, compared to 23.7±7.7 mL/kg/min during AP support (p=0.47). Cerebral oxygenation (rSO2) values at baseline on native placenta support were 40±3%, compared to mean rSO2 during AP support of 50±11% (p=0.027). FTOE during AP support was 37±13% compared to 35±13% extraction with native placental support (p=0.69). Cerebral FTOE correlated negatively with CAF (r= −0.382, p<0.001) [Figure 1] and MAP (r= −0.425, p<0.001) [Figure 2]. Conversely, the CAF/MAP ratio correlated positively with pCO2 (r=0.252, p<0.001) [Figure 3].
Figure 1.
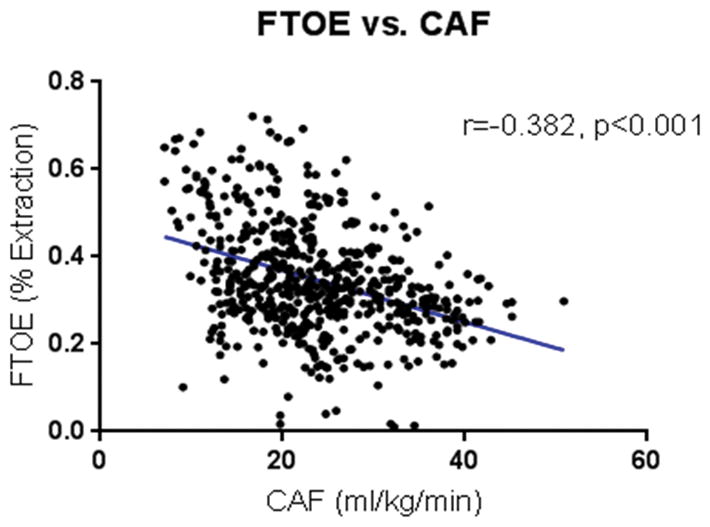
Fractional tissue oxygen extraction (FTOE) versus carotid arterial flow (CAF). FTOE decreased with increasing CAF.
Figure 2.
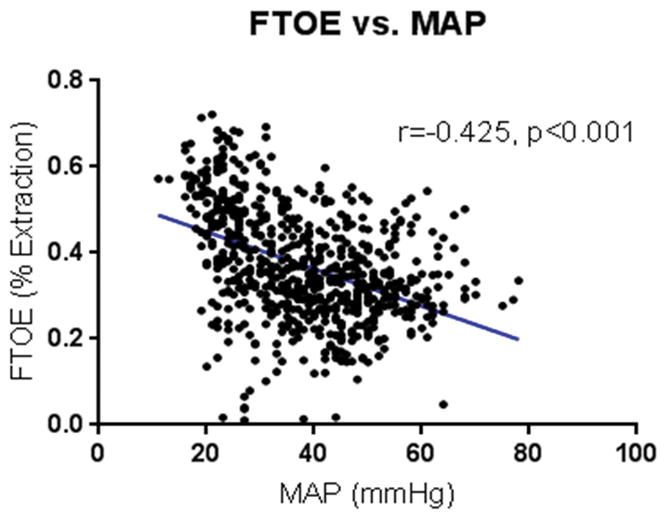
Fractional tissue oxygen extraction (FTOE) vs. mean arterial pressure (MAP). FTOE was found to decrease with increasing MAP.
Figure 3.
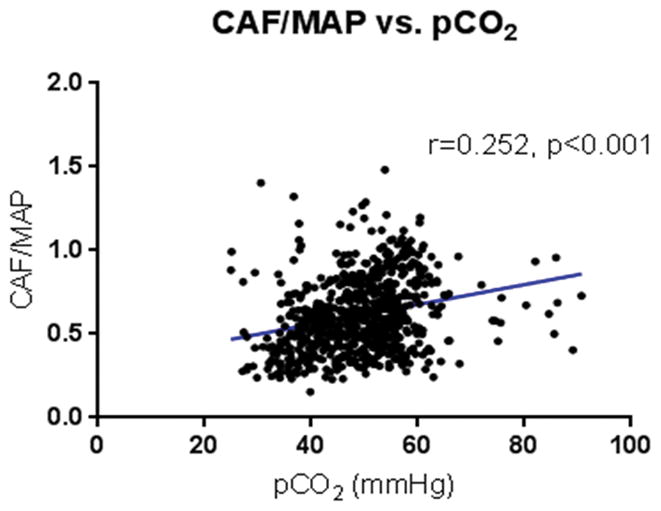
CAF/MAP ratio vs. pCO2. CAF/MAP ratio correlated positively with carotid arterial pCO2.
All lambs displayed spontaneous movement during AP support and retained baseline reflexes (corneal, suckling, and anal wink). On necropsy there was no gross or microscopic evidence of intracranial hemorrhage in any animals. However, one lamb did have a single 5 mm area of grossly soft parenchyma in the left frontal cortex and signs of neuronal death in this region on histopathology.
Discussion
We sought to establish the adequacy of cerebral blood flow and oxygenation during support with an artificial placenta in a premature lamb model. The means of assessment – near-infrared spectroscopy (NIRS) as a non-invasive continuous measurement of cerebral oxygenation, and carotid arterial blood flow by ultrasonography – are well-established.11–13, 15, 19, 20 During artificial placental support of 14 lambs for 10 to 92 hours, NIRS and CAF suggest that cerebral oxygen delivery is preserved.
Since the early days of ECLS, cerebral ischemia has been a feared complication.21–23 Cerebral perfusion depends on the mode of support; VA-ECLS entails ligation of the right carotid artery and jugular vein, and is directly associated with temporary reduction in flow to the right middle cerebral artery (RMCA) distribution right after initiation of ECLS, but this generally returns to normal.11–13, 24 VV-ECLS spares the carotid artery but requires ligation of the right jugular vein; cerebrovascular congestion from this ligation is associated with abnormalities of blood flow including decreased RMCA flow.12, 25, 26 Studies have not consistently shown that right-sided hypoperfusion occurs long-term, nor that it is associated with specific deficits.
Lambs supported by the AP are susceptible to similar alterations in cerebral perfusion since they undergo ligation of the right jugular vein. In this particular model it also involves ligation of the left carotid artery to allow for blood gas sampling. Cerebral blood flow in this model is therefore dependent on adequate cardiac function (as opposed to VA-ECLS, which provides an element of cardiac support in addition to gas exchange) and an intact Circle of Willis. Conceptually this places our model at the highest risk for cerebral hypoperfusion since it incorporates the drawbacks of both VA and VV ECLS [Figure 4]. Clinical application of the AP in human infants would preserve bilateral carotid circulation since the umbilical artery would be used for arterial access. Therefore, our observation that cerebral oxygenation and CAF are preserved in this high-risk model make the most conservative case for preservation of cerebral oxygen delivery with the AP. If cerebral hypertension became a clinical problem, a cephalad drainage catheter could be inserted to allow cerebral venous drainage.27
Figure 4.
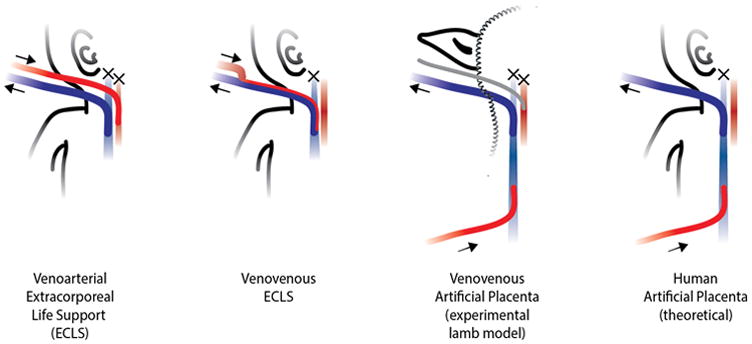
Schematics of the standard of care for a) venoarterial ECLS and b) venovenous ECLS; c) schematic of our experimental venovenous artificial placenta model, and d) schematic of an artificial placenta applied to humans (theoretical). Crosses represent ligation of a vessel.
Our results indicate that CAF was near baseline values during AP support, despite the ligation of the contralateral carotid artery for a monitoring line. Additionally, cerebral rSO2 values were not significantly different from baseline levels that were collected from similarly-aged fetal lambs on native placental support, and approximate values that have been noted in premature sheep supported by mechanical ventilation.28 In normal human neonates, rSO2 rises during the first few minutes of life before reaching stable levels.29, 30 This is consistent with our observation that rSO2 was lower during native placental support, since more highly oxygenated blood is being delivered via the umbilical veins under artificial placental support.
This observation highlights the novelty of our experimental model and findings. The effects of ECLS on cerebral blood flow and oxygen delivery in newborn lambs have been previously studied by Hunter and colleagues. They reported that VV-ECLS had no impact on either variable. Similarly, carotid ligation only caused a brief decrease in right CBF that resolved in under one minute 31. It may not be surprising, then, that lambs in our study maintained cerebral oxygenation while undergoing VV-ECLS and contralateral carotid ligation. However, it is important to note the difference in our animal model, and the ECLS configuration used by the artificial placenta. Preterm lambs maintain fetal circulation immediately after delivery, with shunts including the foramen ovale and ductus arteriosus. Though the AP does utilize VV-ECLS, the umbilical vein reinfusion is paramount to its design, as it preferentially shunts oxygenated blood through the foramen ovale into the left heart, which from there supplies the premature brain. If we were to use conventional double lumen VV-ECLS as has previously been described, oxygenated blood would preferentially cross the tricuspid valve to be ejected into the pulmonary artery, through the ductus arteriosus, and to the descending aorta, thereby bypassing the cerebral circulation.
Making use of carotid arterial O2 saturation and rSO2, we calculated FTOE levels for a better understanding of cerebral oxygenation during AP support. “Luxury perfusion” (↑rSO2 with ↓FTOE) has been reported in sheep models of hypoxic-ischemic cerebral injury.32 FTOE values during AP support were very close to FTOE levels on native placental support, suggesting that the study animals did not experience luxury brain perfusion. Also, we found that FTOE decreased as carotid flow increased and was inversely correlated with MAP. These findings support the theory that our AP model allows the animal to maintain appropriate cerebrovascular autoregulation.
We also found that CAF/MAP correlated positively with pCO2. This observation aligns with previous studies in normal human physiology that demonstrate a positive correlation between carotid arterial blood flow and pCO2.33, 34 This finding also suggests that the CO2-based cerebrovascular auto-regulation mechanism remains intact during AP support.
Our study has several important limitations. Due to the complexity of animal care and our laboratory capabilities at the time, we were limited to collecting data every 30 minutes instead of continuously. This could have caused some variation due to artifact. Also, skull thickness in premature sheep mandated placement of the NIRS probe in midline skull, likely over the sagittal sinus. This would suggest that saturations may have reflected venous blood even more than that published 84%. Since we used late-term lambs to establish our VV-ECLS AP model, our findings may not translate directly to extremely premature human infants. Furthermore, clinical application of the AP may require several weeks of extracorporeal support, and further evaluation will need to be performed in significantly premature sheep long-term. We have established that a premature lamb at 118 days gestation correlates to a 24 week EGA human infant in terms of lung development.5 We therefore will need to repeat these studies in this more premature model to confirm similar findings at this earlier gestational age.
Although we demonstrated adequate cerebral perfusion with the AP, further studies will need to address cerebral function, evaluate white matter injury, and address the issue of intraventricular hemorrhage (IVH). Preliminary work in our laboratory demonstrate the feasibility of using amplitude-integrated electroencephalography to assess function and ability of post-mortem MRI to evaluate brain structure, myelination, and injury.35 Although IVH has rarely been observed in our heparinized AP experiments, the sheep is not a good model for IVH since the germinal matrix in present at approximately 70 days gestation.36 Clinical translation to premature infants already at risk for IVH will require elimination of heparin and development of non-thrombogenic surfaces.37, 38
Conclusion
Cerebral rSO2 and carotid arterial flow are maintained throughout AP support with VV-ECLS. This suggests that cerebral perfusion and oxygenation are maintained and adequate during AP support.
Table I.
Mean physiologic parameters on AP support
| Physiologic Parameters | Mean ± SD | Reference Values (range) |
|---|---|---|
| Heart Rate (beats/min) | 181 ± 30 | 150–250 |
| Mean Arterial Pressure (mmHg) | 38 ± 13 | 35–40 |
| Arterial partial pressure of carbon dioxide (pCO2) (mmHg) | 47 ± 9 | 40–50 |
| Arterial partial pressure of oxygen (pO2) (mmHg) | 38 ± 7 | 30–35 |
| pH | 7.27 ± 0.09 | 7.25–7.35 |
| Circuit Flow (mL/kg/min) | 73.5 ± 15.5 | 50–100 |
Acknowledgments
We wish to thank the Somanetics Corporation for their donation of equipment for this experiment. This work was supported by National Institutes of Health grant # 1R01HD073475-01A1, the Helen L. Kay Charitable Foundation, the Hartwell Foundation Biomedical Research Fellowship, and the University of Michigan Undergraduate Research Opportunity Program.
References
- 1.Greenough A, Murthy V. Respiratory problems in the premature newborn. Pediatric Health. 2009;3(3):241–249. [Google Scholar]
- 2.Sweet D, Carnielli V, Greisen G, et al. European Consensus Guidelines on the management of neonatal respiratory distress syndrome in preterm infants. Neonatology. 2010;97:402–417. doi: 10.1159/000297773. [DOI] [PubMed] [Google Scholar]
- 3.Bahrami K, Van Meurs K. ECMO for neonatal respiratory failure. Semin Perinatol. 2005;29:15–23. doi: 10.1053/j.semperi.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Gray BW, El-Sabbagh A, Zakem SJ, et al. Development of an artificial placenta V: 70 h veno-venous extracorporeal life support after ventilatory failure in premature lambs. Journal of pediatric surgery. 2013;48(1):145–153. doi: 10.1016/j.jpedsurg.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryner B, Gray B, Perkins E, et al. An extracorporeal artificial placenta supports extremely premature lambs for 1 week. Journal of pediatric surgery. 2015;50(1):44–49. doi: 10.1016/j.jpedsurg.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell LR, Bunyapen C, Holmes GL, et al. Right common carotid artery ligation in extracorporeal membrane oxygenation. J Pediatr. 1988;113:110–113. doi: 10.1016/s0022-3476(88)80543-2. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett RH, Toomasian J, Roloff D, et al. Extracorporeal Membrane Oxygenation (ECMO) in Neonatal Respiratory Failure 100 Cases. Ann Surg. 1986;204(3) doi: 10.1097/00000658-198609000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liem KD, Hopman JCW, Oeseburg B, et al. Cerebral Oxygenation and Hemodynamics During Induction of Extracorporeal Membrane Oxygenation as Investigated by Near Infrared Spectrophotometry. Pediatrics. 1995;95(4):555–561. [PubMed] [Google Scholar]
- 9.Papademetriou MD, Tachtsidis I, Elliot MJ, et al. Multichannel near infrared spectroscopy indicates regional variations in cerebral autoregulation in infants supported on extracorporeal membrane oxygenation. Journal of biomedical optics. 2012;17(6):067008. doi: 10.1117/1.JBO.17.6.067008. [DOI] [PubMed] [Google Scholar]
- 10.Tyree K, Tyree M, DiGeronimo R. Correlation of brain tissue oxygen tension with cerebral near-infrared spectroscopy and mixed venous oxygen saturation during extracorporeal membrane oxygenation. Perfusion. 2009;24(5):325–331. doi: 10.1177/0267659109353966. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto JS, Babcock DS, Brody AS, et al. Right common carotid artery ligation for extracorporeal membrane oxygenation: cerebral blood flow velocity measurement with Doppler duplex US. Radiology. 1990;175(3):757–760. doi: 10.1148/radiology.175.3.2188299. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda S, Aoyama M, Yamada Y, et al. Comparison of venoarterial versus venovenous access in the cerebral circulation of newborns undergoing extracorporeal membrane oxygenation. Pediatric surgery international. 1999;15(2):78–84. doi: 10.1007/s003830050521. [DOI] [PubMed] [Google Scholar]
- 13.Weber TR, Kountzman B. The effects of venous occlusion on cerebral blood flow characteristics during ECMO. J Pediatr Surg. 1996;31(8):1124–1127. doi: 10.1016/s0022-3468(96)90100-1. [DOI] [PubMed] [Google Scholar]
- 14.Stolar CJH, Reyes C. Extracorporeal membrane oxygenation causes significant changes in intracranial pressure and carotid artery blood flow in newborn lambs. Journal of Pediatric Surgery. 1988;23(12):1163–1168. doi: 10.1016/s0022-3468(88)80334-8. [DOI] [PubMed] [Google Scholar]
- 15.Watzman HM, Kurth CD, Montenegro LM, et al. Arterial and venous contributions to near-infrared cerebral oximetry. Anesthesiology. 2000;93(4):947–953. doi: 10.1097/00000542-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Toet MC, Lemmers PMA, van Schelven LJ, et al. Cerebral Oxygenation and Electrical Activity After Birth Asphyxia: Their Relation to Outcome. Pediatrics. 2006;117(2) doi: 10.1542/peds.2005-0987. [DOI] [PubMed] [Google Scholar]
- 17.Naulaers G, Morren G, Huffel SV, et al. Cerebral tissue oxygenation index in very premature infants. Arch Dis Child Fetal Neonatal Ed. 2002;87:F189–F192. doi: 10.1136/fn.87.3.F189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemmers PMA, Toet M, Schelven LJv, et al. Cerebral oxygenation and cerebral oxygen extraction in the preterm infant: the impact of respiratory distress syndrome. Exp Brain Res. 2006;173:458–467. doi: 10.1007/s00221-006-0388-8. [DOI] [PubMed] [Google Scholar]
- 19.Weiss M, Dullenkopf A, Kolarova A, et al. Near-infrared spectroscopic cerebral oxygenation reading in neonates and infants is associated with central venous oxygen saturation. Paediatric anaesthesia. 2005;15(2):102–109. doi: 10.1111/j.1460-9592.2005.01404.x. [DOI] [PubMed] [Google Scholar]
- 20.Franceschini MA, Boas DA, Zourabian A, et al. Near-infrared spiroximetry: noninvasive measurements of venous saturation in piglets and human subjects. Journal of applied physiology (Bethesda, Md : 1985) 2002;92(1):372–384. doi: 10.1152/jappl.2002.92.1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuda S, Aoyama M, Yamada Y, et al. Comparison of venoarterial versus venovenous access in the cerebral circulation of newborns undergoing extracorporeal membrane oxygenation. Pediatric surgery international. 1999;15:78–84. doi: 10.1007/s003830050521. [DOI] [PubMed] [Google Scholar]
- 22.Short BL. The effect of extracorporeal life support on the brain: a focus on ECMO. Seminars in perinatology. 2005;29(1):45–50. doi: 10.1053/j.semperi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Hofkosh D, Thompson AE, Nozza RJ, et al. Ten years of extracorporeal membrane oxygenation: neurodevelopmental outcome. Pediatrics. 1991;87(4):549–555. [PubMed] [Google Scholar]
- 24.O’Brien NF, Hall MW. Extracorporeal Membrane Oxygenation and Cerebral Blood Flow Velocity in Children. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2013;14(3) doi: 10.1097/PCC.0b013e3182712d62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor GA, Walker LK. Intracranial venous system in newborns treated with extracorporeal membrane oxygenation: Doppler US evaluation after ligation of the right jugular vein. Radiology. 1992;183(2):453–456. doi: 10.1148/radiology.183.2.1561349. [DOI] [PubMed] [Google Scholar]
- 26.O’Connor TA, Haney BM, Grist GE, et al. Decreased incidence of intracranial hemorrhage using cephalic jugular venous drainage during neonatal extracorporeal membrane oxygenation. Journal of pediatric surgery. 1993;28(10):1332–1335. doi: 10.1016/s0022-3468(05)80323-9. [DOI] [PubMed] [Google Scholar]
- 27.Skarsgard ED, Salt DR, Lee SK. Venovenous extracorporeal membrane oxygenation in neonatal respiratory failure: does routine, cephalad jugular drainage improve outcome? Journal of pediatric surgery. 2004;39(5):672–676. doi: 10.1016/j.jpedsurg.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 28.Barton SK, Moss TJ, Hooper SB, et al. Protective ventilation of preterm lambs exposed to acute chorioamnionitis does not reduce ventilation-induced lung or brain injury. PloS one. 2014;9(11):e112402. doi: 10.1371/journal.pone.0112402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pichler G, Avian A, Binder C, et al. aEEG and NIRS during transition and resuscitation after birth: promising additional tools; an observational study. Resuscitation. 2013;84(7):974–978. doi: 10.1016/j.resuscitation.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 30.Pichler G, Binder C, Avian A, et al. Reference ranges for regional cerebral tissue oxygen saturation and fractional oxygen extraction in neonates during immediate transition after birth. The Journal of pediatrics. 2013;163(6):1558–1563. doi: 10.1016/j.jpeds.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Hunter CJ, Blood AB, Bishai JM, et al. Cerebral blood flow and oxygenation during venoarterial and venovenous extracorporeal membrane oxygenation in the newborn lamb. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2004;5(5):475–481. doi: 10.1097/01.pcc.0000130992.73123.bc. [DOI] [PubMed] [Google Scholar]
- 32.Bennet L, Roelfsema V, Pathipati P, et al. Relationship between evolving epileptiform activity and delayed loss of mitochondrial activity after asphyxia measured by near-infrared spectroscopy in preterm fetal sheep. Journal of Physiology. 2006;572:141–154. doi: 10.1113/jphysiol.2006.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reivich M. Arterial PCO, and cerebral hemodynamics. American Journal of Pediatrics. 1964:206. doi: 10.1152/ajplegacy.1964.206.1.25. [DOI] [PubMed] [Google Scholar]
- 34.Kety SS, Schmidt CF. The Effects of Altered Arterial Tensions of Carbon Dioxide And Oxygen on Cerebral Blood Flow and Cerebral Oxygen Consumption of Normal Young Men. J Clin Invest. 1948;27(4):484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffith JL, Shimony JS, Cousins SA, et al. MR imaging correlates of white-matter pathology in a preterm baboon model. Pediatr Res. 2012;71(2):185–191. doi: 10.1038/pr.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balasubramaniam J, Del Bigio MR. Animal models of germinal matrix hemorrhage. J Child Neurol. 2006;21(5):365–371. doi: 10.1177/08830738060210050201. [DOI] [PubMed] [Google Scholar]
- 37.Major TC, Brant DO, Reynolds MM, et al. The attenuation of platelet and monocyte activation in a rabbit model of extracorporeal circulation by a nitric oxide releasing polymer. Biomaterials. 2010;31(10):2736–2745. doi: 10.1016/j.biomaterials.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Handa H, Brisbois EJ, Major TC, et al. In vitro and in vivo study of sustained nitric oxide release coating using diazeniumdiolate-doped poly(vinyl chloride) matrix with poly(lactide-co-glycolide) additive. Journal of Materials Chemistry B. 2013 doi: 10.1039/C3TB20277A. [DOI] [PMC free article] [PubMed] [Google Scholar]


