Abstract
Cancer treatment still remains a challenge due to the several limitations of currently used chemotherapeutics, such as their poor pharmacokinetics, unfavorable chemical properties, as well as inability to discriminate between healthy and diseased tissue. Nanotechnology offered potent tools to overcome these limitations. Drug encapsulation within a delivery system permitted i) to protect the payload from enzymatic degradation/inactivation in the blood stream, ii) to improve the physicochemical properties of poorly water-soluble drugs, like paclitaxel, and iii) to selectively deliver chemotherapeutics to the cancer lesions, thus reducing the off-target toxicity, and promoting the intracellular internalization. To accomplish this purpose, several strategies have been developed, based on biological and physical changes happening locally and systemically as consequence of tumorigenesis. Here, we will discuss the role of inflammation in the different steps of tumor development and the strategies based on the use of nanoparticles that exploit the inflammatory pathways in order to selectively target the tumor-associated microenvironment for therapeutic and diagnostic purposes.
Keywords: Nanomedicine, Biomimetic nanoparticles, Inflammation, Active targeting, Cancer, Theranostic, Inflamed Vasculature
1. INTRODUCTION
Despite the multitude of chemotherapeutics already used in the clinic to fight many types of cancer, two main issues limit full pharmacological success. For one, intravenous (i.v.) administration of pharmaceutics suffers from several shortcomings including i) degradation by specific enzymes or physiologic conditions (e.g., lysosome acidic environment [1]), which alter pharmacological efficacy, and ii) poor biodistribution, which necessitates high drug doses to achieve therapeutically effective concentrations in the target tissue [1, 2], increasing the risk of extensive off-target effects [3, 4]. These factors hinder chemotherapeutic accumulation and efficacy, resulting in progressive drug resistance and short patient survival. In addition, for several drugs, unfavorable chemical properties (low or no solubility in biological fluids) require pharmaceutical formulations that are themselves toxic. For example, the marketed clinical formulation of paclitaxel, Taxol®, is one of the most effective drugs available to treat ovarian, breast, non-small cell lung cancer and AIDS-related Kaposi’s Sarcoma [5]. However, because of its insolubility in water and other solutions commonly used for intravenous injections, paclitaxel is dissolved in a 50/50 (v/v) mixture of Cremophor® EL (a surfactant) and dehydrated alcohol. This solution has been shown to cause severe hypersensitivity reactions, anemia and cardiovascular events, due to the toxic effects of Cremophor® EL [6]. The second main issue limiting therapeutic success stems from the inability of current chemotherapeutics to differentiate between healthy and cancerous tissue. This causes severe side effects [7], commonly including the loss of hair, bone marrow, and intestinal epithelial cells, which are all highly replicative cells. Other widely-used drugs are characterized by specific off-target toxicity (cardiotoxicity for anthracyclines [8, 9]).
Nanotechnologies offer potent tools to improve the physicochemical properties of chemotherapeutics, thereby reducing their systemic toxicity and increasing their therapeutic index, while retaining their pharmacological activity and increasing their accumulation at the disease site [10, 11]. Drug encapsulation and delivery using nanocarriers (a) prevents drug degradation and metabolic deactivation, and increases drug solubilization [4], thus improving its pharmacokinetics and biodistribution, (b) facilitates selective delivery of chemotherapeutics to cancer lesions through passive [enhanced permeation and retention effect (EPR)] or active (targeted particles) mechanisms, reducing off-target toxicity, and (c) improves intracellular penetration [2] of payload, overcoming multi drug resistance (MDR).
In order to selectively deliver chemotherapeutics to the cancer lesion, several strategies have been developed. Most of these rely on unique biological and physical features of the tumor microenvironment (e.g., leaky vasculature, compromised lymphatic drainage, angiogenesis, acidic pH, overexpression of cell membrane antigens) [12], which are exploited to gain access to the cancer cells. Recently, particular focus has been paid to the causal relationship between inflammation and cancer development [13, 14]. The local immune response and systemic inflammation have important roles in tumor progression, as well as patient survival in established cancers [21]. The study of inflammatory pathways activated during cancer progression has paved the way for novel therapeutic strategies based on the use of nanoparticles. Here, we will describe inflammation’s involvement in tumorigenesis, and will present how it can be exploited in order to selectively direct anti-cancer tools (both chemotherapeutics and diagnostic agents) to the tumor tissue.
2. ROLE OF INFLAMMATION DURING CANCER DEVELOPMENT
2.1. General overview
The connection between cancer and inflammation was observed for the first time about one and a half centuries ago [15]. During the past years, the role of inflammation in the origin of cancers has been better clarified and some mechanisms elucidated [16]. It is now well known that many of cancer’s environmental cues reside in chronic inflammation conditions consequent to chronic infections [16] and irritations [17], fat-based diet [18], alcohol abuse [19], tobacco smoking [20], and atmospheric pollutants [21] (Table 1 and Figure 1). Inflammation can impact every step of tumor development from initiation and promotion through metastasis and recurrence. An inflamed microenvironment can promote genomic instability, thus giving rise to the occurrence of genetic mutations [22]. Moreover, the chronic inflammatory environment is populated by innate immune cells. Most of these are macrophages [23] that, together with neutrophils, dendritic cells, mast cells, and T and B lymphocytes, infiltrate the tumor parenchyma. Cancer cells, stroma cells (e.g. fibroblasts and mesenchymal stem cells), and immune cells interact through direct contact and/or through production of signaling molecules, namely cytokine and chemokine. In particular, tumor-associated macrophages (TAMs) are known to secrete pro- angiogenic and pro-metastatic factors, to stimulate cell proliferation, and to inhibit cells’ apoptosis in the inflamed site [24]. Moreover, an abundant infiltration of TAMs is connected with poor prognosis in a variety of solid tumors [25, 26]. Macrophages with an alternative M2 phenotype are, in the majority of cases, associated with poor prognosis [27–29]. These macrophages, as opposed to classically activated M1 ones, typically express anti-inflammatory molecules (e.g. IL-10 or TGF-β). Moreover, they have been shown to be involved in the regulation of cancer stem cells (CSCs) functions and, consequently, to be involved in CSC-mediated oncogenesis, metastasization, and drug resistance [30, 31]. Thus, approaches that target TAMs polarization to fight tumors are considered a potential alternative to traditional therapies.
Table 1.
Chronic inflammatory conditions associated with cancer (Modified from reference 3 (Coussens et al. 2002))
| Inflammation types | Associated cancer |
|---|---|
| Asbestosis, Silicosis | Mesothelioma, lung carcinoma |
| Bronchitis | Lung carcinoma |
| Chronic Cystitis | Bladder carcinoma |
| Gingivitis, Lichen Planus | Oral squamous cell carcinoma |
| Chronic ulcerative colitis, Crohn’s Disease, Inflammatory Bowel Disease | Colorectal cancer |
| Reflux esophagitis | Esophageal carcinoma |
| Chronic pancreatitis | Pancreatic carcinoma |
| Skin inflammation | Melanoma |
Figure 1. Inflammation and cancer.
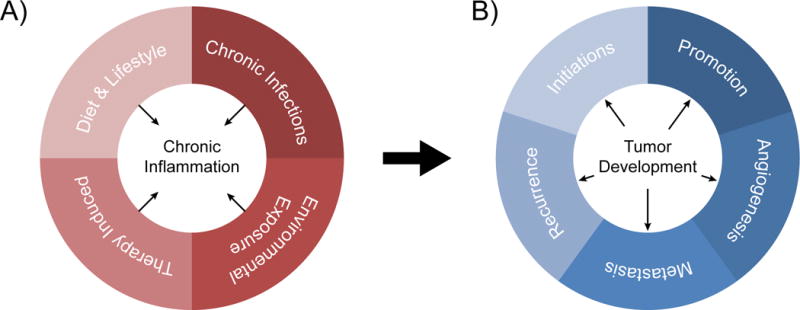
A) Microbial pathogens, dietary lifestyle, environmental phenomena, and tumor therapy can cause chronic inflammation. B) Chronic inflammation impacts all major stages of tumor development, from initiation to metastasis and recurrence.
2.2. Tumor initiation and promotion
Tumor initiation has for a long time been associated with an initial mutation of normal cells that, once mutated, acquired the ability to proliferate and survive longer than neighboring healthy cells. Later, it was recognized that more genetic events are needed for a malignant tumor’s initiation [32]. An inflamed microenvironment can promote tumor initiation in different ways, including the production of Reactive Oxygen Species (ROS) [33] and growth factors. Chronic inflammation is a source of mitochondrial and cytosolic ROS. ROS damage DNA, causing random mutations that transform normal cells into cells without control over proliferation and apoptosis. For this reason, ROS are considered one of the early origins of cancer [34]. Macrophages and inflammatory cells, in general, produce cytokines and growth factors that can mediate the acquisition of stem-like phenotypes by tumor cells, transforming them in CSCs. CSCs represent a population of cells within a tumor exhibiting features specific of stem cells, such as the ability of unlimited self-renewal and of multi linage differentiation. The presence of CSCs has been extensively investigated and revealed in many solid tumors [35] and they are believed to have a fundamental role in cancer therapy resistance and cancer recurrence after treatment. The production of TNF-α [36] and NF-κB [37], for instance, can activate Wnt/β-catenin signaling, one of the main CSC-regulating pathways. The production of cytokines is also involved in tumor promotion, the process which signals the evolution of a single cell to a fully-formed tumor bulk. Several mouse cancer models have been used to investigate this phenomenon and, in the majority of the cases, NF-κB and STAT3 are the main signals responsible for inflammation-mediated tumor promotion [38].
2.3. Tumor angiogenesis
In 1986, Dovrak HF defined tumors as “wounds that don’t heal” [39]. Contrary to what happens during normal wound resolution, tumor development is in some way sustained by inflammatory cells. In the presence of a wound, macrophages, fibroblasts, and other key players in inflammation cooperatively lead to its resolution; instead in the case of mutated epithelial cells, inflammatory cells promote their replication and survival [39]. Inflammatory angiogenesis is involved in this process. Angiogenesis, the formation of new blood vessels originating from existing vasculature, represents one process contributing to tumor development, according to Hanahan and Weinberg’s famous article describing the hallmarks of cancer [32]. For this reason, angiogenesis is considered a potential therapeutic target; particularly, its suppression offers new therapeutic avenues. Even in the case of angiogenesis, TAM recruitment plays a fundamental role due to the fact that these cells produce pro-angiogenic molecules that boost the angiogenic switch [40]. TAMs are sensitive to the low oxygen tension (hypoxia) typical of tumor avascular regions and react by producing molecules such as the vascular endothelial growth factor A (VEGFA), considered one of the major pro-angiogenic cytokines [41–43]. The link between chronic inflammation and angiogenesis is not unidirectional; the borderline between cause and consequence is very thin and several mechanisms driving these two processes are still unclear [44]. It is clear that NF-κB and STAT3 regulate the majority of key angiogenic genes (CXCL8, interleukin-8, and hypoxia inducible factor 1 alpha), thus inactivation of these signals (together with depletion of TAM) is considered a potential target to reduce angiogenesis in the tumor environment [45].
2.4. Tumor metastasis and recurrence
Over 90% of cancer patients die due to the presence of metastasis, thus investigation into anti-metastasis therapy is extremely clinically relevant. This process requires collaboration between cancer, immune, and inflammatory cells. Tumor metastasis formation can be defined as a two-step process: first during “intravasation”, cancer cells leave the primary lesion in situ and start to circulate, then during “extravasation”, they exit the blood stream and colonize in another tissue. The mechanisms by which cancer cells enter blood vessels to circulate, then colonize in other sites are still not completely understood. One possible mechanism sees cancer cells recruited to distant tissues from the primary tumor, owing to the presence of chemokine gradients in those sites. The presence of an inflamed environment impacts metastases’ formation because the presence of pro-inflammatory cytokines in circulation induces the overexpression of ligands specific to cancer cell integrins on endothelial cells [46]. The presence of these ligands increases the probability that metastatic cells will adhere to the endothelium of secondary organs. Inflammatory cells, in particular TAMs, are believed to be responsible for tumor cell behavior in terms of migration, invasion and metastasis [24]. They produce cytokines and grow factors (e.g. TNF-α, IL-6, IL-1, TGFβ, and EGF) that stimulate the invasive motility of cancer cells [47]. For this reason, the density of TAMs in tumors is also associated with poor prognosis [48]. TAMs also produce protease and matrix metalloproteinases that degrade the basement membrane, thus generating channels that favor cancer cells invasion [49]. Conversely, depletion of macrophages from the basement membrane not only reduced the formation of mammary tumor lung metastases [49], but has also been shown to have anti-angiogenic and anti-metastatic effects on metastatic liver cancer [50].
3. TARGETING STRATEGIES THAT EXPLOIT THE INFLAMMATORY PROCESS
The past five years have seen considerable efforts in the development of technologies that aim to exploit properties typically representative in diseased tissue [51–53]. While traditional nanoparticles relied on the exploitation of the enhanced permeability and retention effect (i.e., passive nanoparticle targeting [3]), more recent efforts have focused on actively targeting inflamed vasculature [54, 55]. Specifically, this approach targets the endothelium of inflamed vasculature by modifying nanoparticle surfaces with targeting moieties for overexpressed angiogenic markers and antibodies against adhesion molecules. In this section, we will provide a brief overview of current technologies that target and exploit the inflamed environment of cancer diseases.
In the development of nanoparticles with increased affinity towards the vascular wall, substantial literature has demonstrated the importance of factoring in a particle’s shape to increase its contact and adhesion to the vessel wall. Particle shape and physicochemical properties have shown to have a major impact on particles hydrodynamics, in addition to the interactions with vascular targets [56–58]. For example, in a study comparing the specificity of polystyrene nanospheres and nanorods with equal volumes, nanorods displayed greater adhesion to microvascular networks, when functionalized with an agonist [59] antibody. Similar findings were observed in an in vivo biodistribution model where nanorods modified with Intercellular Adhesion Molecule 1 (ICAM-1) [60–63], an antibody specific to an endothelial molecule highly expressed in the lung and various cancers, exhibited a two-fold increase in lung accumulation when compared to similar nanospheres. Other works employing biocompatible and biodegradable porous silicon nanoparticles [64, 65] demonstrated that shape and size were both essential for increased accumulation in a melanoma-bearing mouse model [66]. This study compared variously-sized plateloid-shaped particles (600 × 200, 1000 × 400, and 1800 × 600 nm) and demonstrated that the smaller particles more readily accumulated in the liver and spleen while the larger particles deposited in the lungs. When surface-functionalized with a peptide critical for cell adhesion, these plateloid-shaped particles resulted in as much as an 8.1% accumulation in the tumor, with the highest tumor-to-liver accumulation ratio occurring with 1000 × 400 nm particles.
As previously mentioned, functionalizing the surface of a nanoparticle with an agonist antibody can increase accumulation in a variety of tumors and inflamed tumor stroma, regardless of the particle shape. A more recent strategy involves modifying the surface of previously studied nanoparticles with targeting antibodies or other biological moieties. In one study, researchers used the modular domain isolated from the leukocyte function associated antigen-1 (LFA-1) integrin as a surface modification for drug carriers [67]. LFA-1 has shown to be a critical molecule for T cell recruitment to inflamed tissue [68–70] and adhesion towards ICAM-1 [71]. Jin et al. demonstrated that modifying the surface of urethane acrylate non-ionomer drug carriers with the inserted (I) domain of the LFA-1 integrin, a 5.1-fold increase in nanoparticle accumulation was observed for subcutaneous xenograft ICAM-1-positive HeLa tumors. Encapsulation of paclitaxel, a taxane-classed drug used to treat various cancers, resulted in a significant inhibition of tumor growth in vivo while non-targeting particles showed no inhibition of tumor growth.
Other groups demonstrated success in the active targeting of tumor-associated inflammation through selectin-mediated homing. Selectins are almost exclusively found in endothelial and bone-marrow-derived cells and are identified by the cell in which they are found [72]. They are responsible for leukocyte homing and are critical in the recruitment of leukocytes during an inflammatory response. In a study employing the use of liposomes functionalized with Sialyl Lewis X tetrasaccharide, a carbohydrate epitope recognized by all selectins, it was found that when loaded with an anti-tumor therapeutic, merphalan, a significant increase in survival was observed in an adenocarcinoma-bearing mouse model [73]. E-selectin has also been hypothesized as playing a pivotal role in the recruitment of porous silicon particles, as witnessed by an upregulation of E-selectin five hours after mild hyperthermia treatment [74].
Similar to selectin functionalization, extensive work has also focused on incorporating antibodies on the particle surface. Vascular endothelium growth factor receptors (VEGFR) have been well-documented as an overexpressed receptor in angiogenesis during tumor formation [75]. Recent work has covalently linked an anti-VEGFR2 antibody to the surface of PEGylated liposomal doxorubicin [76]. In this study, surface functionalization with an anti-VEGFR2 antibody resulted in a significant reduction in blood microvessel density for three distinct tumor models (breast, pancreatic, and colorectal). Additionally, it was found that treatment localized in tumor cells in the pancreas, minimizing unwanted toxicity to exocrine pancreatic regions. In a similar study, functionalization of porous silicon with an anti-VEGFR2 antibody resulted in significantly higher adhesion of particles on inflamed endothelium in vitro [76]. Specifically, it was observed that functionalizing the porous silicon vector surface with VEGFR2 antibodies resulted in a 4-fold increase in endothelial cell adhesion under physiological flow conditions. In a breast cancer mouse model, a 3-fold increase was observed 2 h following administration. A study performed by our group employing VEGFR2-functionalized multistage nanovectors obtained similar results, demonstrating a 5-fold increase in tumor biodistribution [77]. In a comparable study employing the same technology, it was observed that modification of the porous silicon carrier’s surface with a meprin A antibody (meprin A is a protein overexpressed in colon cancer), resulted in a significant decrease in colorectal cancer, CaCo2, cell viability [78].
4. TREATMENT STRATEGIES EXPLOITING THE INFLAMMATORY PROCESS
The link between inflammation and cancer has led to the development of multiple nanomedicines which aim to reduce inflammation as a novel strategy to fight cancer. Some of these approaches exploit therapeutics that have been used for decades in other clinical applications, while others exploit recently gained knowledge concerning the role of macrophages in both inflammation and cancer.
The most regularly studied nanomedicine strategy to treat inflammation as a method to combat cancer is the loading and delivery of anti-inflammatory drugs. Anti-inflammatory drugs have been used for decades to treat inflammation in other clinical applications but with the increasingly recognized link between tumor-associated inflammation and cancer carcinogenesis, dissemination, and metastasis [79–81], those drugs are now being utilized as cancer therapies. Most anti-inflammatory drugs utilized in cancer nanomedicine are classified as either non-steroidal anti-inflammatory drugs (NSAIDs) or glucocorticosteroids.
4.1. NSAIDs
Researchers have found that blocking cyclooxygenase (COX) activity with NSAIDs like the ones discussed here have inhibited the growth of gliomas [81, 82], melanomas [83], ovarian tumors [84], colorectal cancer [85], prostate cancer [86], and osteosarcoma [87]. NSAIDs are currently the most highly-studied chemopreventive agents for many cancers, and have shown to modulate key inflammatory pathways, including the NF-κB pathway [88].
Curcumin is a widely-used potent anti-inflammatory drug that has recently become a very popular option for cancer nanomedicine [89]. Various groups have studied the clinical potential of curcumin, along with other therapeutics, in multiple tumor models. Wei, et al. fabricated curcumin-conjugated cholesteryl-hyaluronic acid nanogels (CHA-CUR); the team found that treatment with CHA-CUR in a 4T1 murine breast cancer model resulted in up to 13-fold tumor suppression (based on treatment concentration [90]). In order to capitalize on possibly synergistic effects of curcumin with a commonly-used chemotherapy, Stigliano and coworkers co-loaded docetaxel (DTXL) and curcumin (CURC) in spherical polymeric nanoconstructs (SPNs) to treat glioblastoma multiforme [91]. Mice treated with DTXL+CURC SPNs exhibited 100% survival 90 days after injection versus 50% survival for those treated with DTXL SPNs and 0% survival for those treated with saline (all saline-treated animals died within 35 days). Interestingly, the authors did find that SPN accumulation in the tumor reduced from ≈4% of the injection dose to ≈2% of the injection dose after 2 weeks of treatment.
In an attempt to modulate the vascular deposition of circulating tumor cells (CTCs, believed to be the initiators of metastasis), another group loaded curcumin in lipid-polymer nanoparticles (NANOCurc [92]). Moderate doses of curcumin reduce the expression of ICAM-1 on human umbilical vein endothelial cells (HUVECs) and MUC-1 on cancer cells, both of which mediate CTC arrest on the vascular wall. Treating highly metastatic breast cancer cells (MDA-MB-231) and HUVECs alone with curcumin for 1h led to a ~50% reduction (p < 0.01) in vascular adhesion of the cancer cells. Upon treatment with NANOCurc, these same cancer cells exhibited ~70% reduced attachment to TNFα-induced inflamed HUVECs.
In a pooled analysis of 25,570 patients in 8 clinical trials, Rothwell, et al. found that daily aspirin use reduced deaths associated with several common cancers, including significant reductions in pancreatic and colorectal cancer deaths, with majority benefits seen after 5 years of daily treatment [93]. In an effort to improve the pharmacokinetics of aspirin through nanocarrier delivery, research groups have studied its encapsulation and ability to treat cancer. Thakkar et al. co-loaded ferulic acid (anti-oxidant, FA) and aspirin (ASP) into chitosan-coated solid lipid nanoparticles (c-SLNs) to treat pancreatic cancer. The group found that separately, the drugs had little to no effect on cancer cell viability, but together, the drugs elicited a 70% reduction in viability [94]. Upon oral administration in a PaCa-2 pancreatic tumor xenograft mice model, FA and ASP co-loaded c-SLNs suppressed tumor growth by 45% compared to the control (not statistically significant).
Ibuprofen is an easily accessed drug whose efficacy in vivo is insufficient due to its poor pharmacokinetic properties, therefore Cheng, et al. tested the improved efficacy of phospho-ibuprofen amid (PIA)-loaded nanocarriers in a model of human lung cancers [95]. PIA was 10-fold more potent than ibuprofen in inhibiting the growth of human non-small cell lung cancer cells. In addition, fluorescence imaging showed that liposomal PIA inhibited the growth of A549 xenografts by 95% (p < 0.001) relative to the vehicle control. Ketoprofen is a lesser-known, but still widely-used, COX inhibitor. Da Silveira, et al. found that ketoprofen-loaded nanocapsules (Keto-NC) decreased U251MG glioma cell viability by 25% and 45% following 48h and 72h of treatment, respectively [96]. Keto-NC treatment also resulted in a significant reduction in glioma tumor volume compared to control, NC, and Keto-free groups.
4.2. Glucocorticosteroids
Glucocorticosteroids are already often included in primary combination chemotherapy regimens to treat inflammatory carcinomas such as lymphocytic leukemia, Hodgkin’s and non-Hodgkin’s lymphomas, multiple myeloma, and breast cancer [97]. As a natural progression to its success in chemotherapy, many researchers are loading these steroids into nanocarriers to improve the drugs’ efficacy in the clinic. A simple design was utilized by Kroon, et al. who administered dexamethasone (DEX)-loaded liposomes to treat prostate cancer bone metastases in a mouse model [98]. At all dose levels, both free- and liposomal DEX significantly inhibited intra-bone tumor growth; liposomal DEX showed significantly less tumor growth than free-DEX for only one of the three tested drug concentrations. However, compared to the control group, liposomal DEX significantly inhibited tumor growth up to 26 days after initiation of treatment. Other groups have attempted to exploit the synergistic effects of steroids and chemotherapeutics by administering both within the same nanoformulation. D’Arrigo, et al. studied the effects of prednisolone-linked gellan gum nanohydrogels (Ge-Pred NH) loaded with paclitaxel (PCT) on cell lines for breast cancer and prostate cancer bone metastases [99]. Ge-Pred NH significantly reduced the cell viability of the pancreatic cancer cells (PC-3) in vitro compared to free PCT, however, the nanohydrogel therapy did not show as significant of results against the aggressive breast cancer cell line (MDA-MB-231).
4.3. M1/M2 Macrophage Reprogramming
Macrophages are associated with many disease conditions including inflammation, infection, atherosclerosis, lupus, cancer, and diabetes [100]. Within the biological spectrum involved in cancer, TAMs have been shown to contribute to migration [101], angiogenesis [102], and chemoresistance [103]. Macrophages exist in one of two phenotypes, M1 or M2. The pro-inflammatory M1 phenotype participates in anti-proliferative and cytotoxic activities by secreting reactive nitrogen and oxygen species and pro-inflammatory cytokines. In contrast, the M2 “repair” designation broadly refers to macrophages that function in constructive processes like wound healing, tissue repair, and those that turn off immune system activation by producing anti-inflammatory cytokines [104]. Macrophages which infiltrate into the tumor parenchyma initially have an M1 phenotype, but switch to an M2 phenotype after continued presence in the tumor microenvironment [105].
Reprogramming TAMs has been proposed as an effective strategy to inhibit tumor progression and metastasis [106, 107], and as such, many research groups have formulated nanocarriers capable of macrophage manipulation for cancer treatment. Song, et al. fabricated hyaluronic acid (HA)-coated, mannan-conjugated MnO2 nanoparticles (Man-HA-MnO2). The group attempted to utilize the ability of HA to reprogram anti-inflammatory, pro-tumoral M2 TAMs to pro-inflammatory, antitumor M1 macrophages to enhance MnO2 nanoparticles’ ability to lessen tumor hypoxia and modulate chemoresistance [108]. Treatment with Man-HA-MnO2 particles significantly increased tumor oxygenation and down-regulated hypoxia-inducible factor-1 (HIF-1α) and vascular endothelial growth factor (VEGF) in a breast tumor mouse model. Combination therapy with Man-HA-MnO2 NPs and doxorubicin inhibited tumor growth and tumor cell proliferation compared to chemotherapy alone. Finally, Man-HA-MnO2 treatment resulted in a decrease in M2/M1 ratio, indicating that the nanoparticles successfully guided TAMs away from the M2 phenotype, towards a tumor-inhibiting M1 phenotype.
4.4. Reprogramming Inflammatory Cells Using siRNA
Recently, because RNA aptamers can be exploited as therapeutics by introducing small interfering RNA (siRNA) molecules into the cellular cytosol in order to suppress disease-associated genes [109], many researchers are looking to use these molecules to alter tumor-inflammation associated cells as means of novel cancer therapy. However, siRNAs do not spontaneously enter unperturbed cells due to their relatively high molecular weight, negative charge, and high hydrophilicity [110]. Upon systemic administration, RNase degradation and reticuloendothelial system (RES)-mediated clearance remove the opportunity for siRNAs to elicit any therapeutic effect [111]. Therefore, encapsulation within a drug delivery system (i.e. nanocarriers) is necessary for siRNA to accomplish their therapeutic task [112, 113], with the additional potential to reduce side effects and immune stimulation [114].
In the context of inflammation in cancer, siRNA aptamers can be used to suppress the expression of pro-inflammatory signals in inflamed vasculature. For example, Leus, et al. delivered siRNA sequences to silence VE-cadherin in inflamed vasculature by loading the molecules in VCAM-1-targeted lipoplexes made from a proprietary lipid, SAINT-C18 [112]. After exposure to human aortic endothelial cells (HAECs) in vitro, VE-cadherin gene expression was down regulated by up to 60% by the siRNA-loaded lipoplexes. In primary human hepatic sinusoidal endothelial cells (HHSECs), the same particles caused an 85% down-regulation of VE-cadherin mRNA while particles loaded with control siRNA elicited no effect on VE-cadherin expression. Another research team loaded STAT3-targeting siRNA into E-selectin thioaptamer-conjugated multistage vesicles (ESTA-MSV) to treat breast cancer bone metastasis [115]. The group selected STAT3 as their target since the JAK2/STAT3 pathway plays an important role in breast cancer stem cell growth, and inhibition of said pathway has previously resulted in a decrease in the number of cancer stem cells, reducing the growth of primary breast cancer in murine tumor models [116]. The investigators found that treatment with siRNA-loaded ESTA-MSV caused a significant decrease in mamosphere forming efficiency compared to scramble siRNA-loaded ESTA-MSV. In addition, tumor-bearing mice treated with siRNA-loaded ESTA-MSV showed significantly higher survival rates than those treated with PBS and vehicle controls.
5. THERANOSTIC TOOLS THAT EXPLOIT THE INFLAMMATORY MICROENVIRONMENT
Theranostics are technologies that are able to diagnose and treat a disease using a single system. Recently, this topic has attracted interest from the scientific community in their efforts to improve current therapies for many diseases, especially cancer. Theranostic nano-platforms hold several advantages over typical diagnostic or therapeutic nanocarriers including more precise control of treatment, simplified efforts required by clinical staff, and ability to predict the effectiveness of the treatment based on the detected morphologic and biochemical features of the cancer lesions [117].
Fabrication of theranostic nanoparticles can occur in many different ways. The simplest way is to conjugate a diagnostic/imaging moiety or therapeutic payload to therapeutic or diagnostic nanoparticles, respectively. In some cases, diagnostic and therapeutic agents can be co-encapsulated in the same carrier. In others, carriers can be used to both detect the cancer lesion and provide a therapeutic effect. During the last decades, nanomedicine research has developed several platforms that can be classified as theranostic agents. The manipulation of specific materials at the nanoscale can combine unique detection tools and cytotoxic properties. This is the case of some metal nanoparticles designed for thermal ablation. For example, when gold nanoparticles are excited with a near infrared light (NIR) [118], they can locally generate heat, exploiting their surface plasmon resonance properties [119]. This approach showed promise because cancer cells exhibited greater sensitivity to temperature increases than healthy cells, providing a further mechanism of specificity towards diseased tissue [120]. In addition, to further enhance their therapeutic/diagnostic properties, particles can be surface-modified with molecules that favor passive (e.g. polyethylene glycol) or active (e.g. antibodies recognizing surface markers overexpressed by cancer cells) targeting of tumor lesions. The depth penetration of NIR light in biological tissue is considered to be around 1 cm [121], therefore this approach could be potentially used to treat relatively superficial tumors after systemic injection of gold nanoparticles [122].
For non-superficial tumors, gold nanoparticles can be easily detected through different imaging and diagnostic techniques including, but not limited to, two-photon luminescence imaging [123], and x-ray [124, 125] computed tomography, since they each show enhanced contrast properties compared to typical iodine agents [126]. To overcome their limitations due to encapsulation efficiency, multilayer carriers were fabricated to increase the payload volume. For example, Dr. Chen, et al, coupled gold nanorods with a coating of mesoporous silica to load doxorubicin. In addition, they showed that low intensity NIR irradiation efficiently triggered doxorubicin release. Further increases in the trigger intensity induced hyperthermia, while the mesoporous silica coating did not affect their diagnostic properties [127].
Similarly, the research team led by Dr. Zharov conjugated gold nanoparticles with TNF-α-loaded PEG [128]. Induced local inflammation was shown to increase the thermosensititvity of cancer cells [129–131]. The polymeric coating enhanced circulation and tumor targeting properties of the system, which showed good encapsulation efficiency of the therapeutic payload. Under NIR irradiation, the system generated heat that, in turn, favored release of the cytokine, nanobubble formation, and thermal explosion that synergistically provided a cytotoxic effect.
Iron oxide particles can provide similar advantages, as demonstrated by multiple groups [132]. A schematic of the typical structure of a theranostic agent is shown in Figure 2. The group of Drs. Daldrup-Link and Rao generated iron oxide nanoparticles able to respond to the proteolytic microenvironment of cancer lesions and selectively release therapeutic agents in the cancer lesion. These particles are surface-functionalized with a vascular disrupting agent, azademethylcolchicine, through a peptide sensitive to the enzymatic activity of matrix metalloproteinase-14 (MMP-14) [133]. The therapeutic properties of the particles are triggered only within the tumor microenvironment characterized by MMP-14 overexpression. On the other hand, the superparamagnetic properties of iron nanoparticles facilitates synergistic therapeutic and diagnostic properties through magnetic resonance imaging (MRI).
Figure 2. Scheme of theranostic agents.
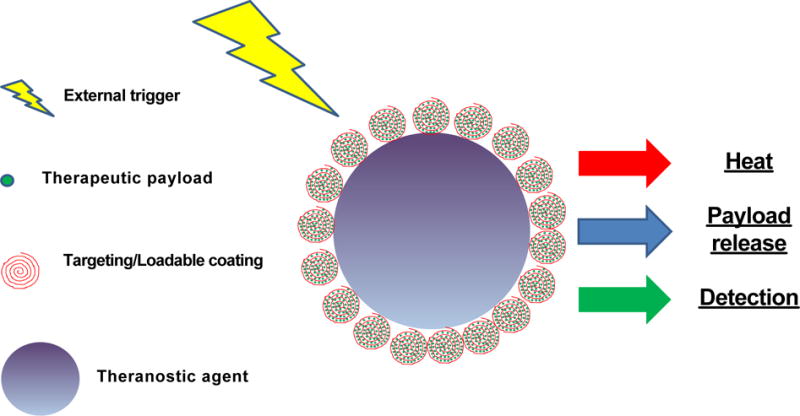
Multilayer theranostic agents are usually constituted by a core with imaging and therapeutic properties and a coating that increase loading capability of the therapeutic payload and can provide also targeting for cancer lesions. Usually the core is represented by metallic particles with NIR or contrast properties enable upon external treatment of generating heat that can favor, cancer cells killing and release of the therapeutic payload.
More recently, theranostics have been developed to detect and treat inflammatory conditions by exploiting pathological changes that occur during inflammation. Dr. Lee and collaorators developed a new micellar formulation able to detect H2O2 generated in inflamed tissues. While this molecule is universally recognized as a biological signal for intercellular communication, its overproduction during inflammation is associated with oxidative stress, aging, and loss of tissue function [134]. The system’s detection mechanism is based on co-encapsulation in the micellar composition of hydroxy-benzyl alcohol-incorporated copolyoxalate (HPOX) and a fluorescent dye able to produce peroxalate chemiluminescence in the presence of H2O2. During the reaction, HPOX releases hydroxybenzyl alcohol which works as an antioxidant, providing local curative activity.
Another delivery platform designed for targeting cancer-associated inflammation is represented by magnetoliposomes. These particles combine liposomes ability to deliver versatile payloads with the imaging properties of magnetite [135]. The system was designed to target inflammation associated with glioma by delivering omega-3 polyunsaturated fatty acid ethyl ester co-encapsulated in the lipid core with the magnetite for detection with MRI. Finally, many recent studies in theranostics for inflammation and cancer treatment are focused on imaging and targeting macrophages. These cells are crucial effectors in the development and amplification of inflammation and are universally recognized as an important component of the cancer microenvironment. While specific targeting towards tumor infiltrating macrophages still presents issues, theranostic approaches propose to overcome these limitations. Surface modification using dextran or glycan can induce a significant particle uptake by these cells, while the macrophage is killed with a cytotoxic agent or through radio-sensitization [136].
6. FUTURE PERSPECTIVES ON ENDOTHELIAL INFLAMMATION DELIVERY IN CANCER
Beyond targeting and treating inflammation, targeting the endothelium is a logical step to increase the delivery of therapeutics. Previous iterations of delivery platforms have focused on functionalizing nanovesicles with a monophasic approach that may attach, at best, two or three targeting moieties on the surface of the carrier. More recently, biomimetic medicine, or medicine that aims to mimic the natural characteristics of the body, has employed the use of whole cell membranes to increase targeting and prolong nanoparticle circulation by attempting to replicate the complex interactions of real cells with more fidelity than through ligand attachments. Because various biological barriers, when exposed to the physiological system, often encumber traditional nanoparticles, more complex nanoparticles are needed in order to reach tumors in sufficient quantities to elicit the desired therapeutic effect. The variety of incorporated key proteins from specific cell types, including leukocytes, platelets, and red blood cells [137–140], act as ready-made cloaks for nanoparticles that can be targeted based on the physiological functions of cells from which they are derived. Research groups following this line of reasoning, such as Tasciotti et al. [137], have demonstrated that applying purified leukocyte-derived membrane proteins onto the surface of porous silicon carriers significantly decreased opsonization and macrophage uptake of the particles. This surface modification conferred the carrier with over 150 leukocyte proteins [141], resulting in particle biocompatibility in a syngeneic environment in vitro and in vivo [142]. Additionally, functionalization of the carriers with leukocyte cellular membrane proteins showcased significant reduction in liver accumulation within the first 40 min of administration and significantly increased accumulation in a melanoma tumor mouse model in as little as 20 min mediated by the reduction of tight junctions in inflamed endothelia [143]. Work harnessing the favorable properties of platelets by integrating platelet membrane proteins within polymeric nanoparticles resulted in similarly avoided macrophage internalization while providing selective adhesion towards inflamed vasculature [144]. Specifically, platelet-cloaked nanoparticles demonstrated successful binding to a denuded artery in an angioplasty-induced arterial injury rat model. Particle accumulation was found on the luminal side above the muscle layer and remained at the injury site for 5 days.
Among the other cells that have been utilized through this approach are red blood cells (RBC). As was mentioned, RBCs are readily available cells from which membrane proteins can be derived and used to functionalize nanoparticles. Luk et al. [145] successfully used RBC membrane proteins to create nanocarriers that can deliver doxorubicin to a murine model of lymphoma, where injection of the particles elicited no immunogenic response while maintaining all drug delivery characteristics of the synthetic material. Other approaches, such as the one developed by Molinaro et al., were able to transfer over 300 membrane proteins deriving from leukocytes to the lipid vesicles, vastly surpassing the capability of protein functionalization with current liposomes by incorporating signals that allow for self-recognition and active targeting [146]. Despite the authors used these biomimetic nanovesicles, called Leukosomes, in a mouse model of localized inflammation, their description of the molecular pathways through which Leukosomes accumulate into the inflamed tissue (LFA1 and CD45-mediated accumulation [146]) gives reason of their potential employment for the selective delivery of therapeutics to the tumor-associated inflamed vasculature. In conjunction, the different approaches highlighted in this review mark a shift in the way drug delivery platforms are engineered with the immediate future pointing towards “biomimetic” approaches where hundreds of proteins or other signals can be functionalized onto a myriad of nano-constructs. This approach has the unique ability to combine knowledge gained from research on liposomes, many formulations of which have been approved by the Food and Drug Administration, significantly facilitating clinical translation for future technologies.
The derivation of proteins from patient samples, utilized by the biomimetic carriers discussed earlier, provides a platform to replicate complex signaling at the interface between nanocarriers and the endothelium in order to avoid foreign recognition and clearance, while accumulating in inflamed or diseased tissue. Possibilities for this platform are extensive as the cell source could be expanded to many different cell types and normal cell physiology used to modulate targeting to different organs. Wang et al. has shown examples of this reasoning by creating nanovectors coated with the membranes of activated leukocytes [147]; these particles showed enhanced homing to inflamed tissue in a lymphoma and breast cancer model. Using a combinatory approach, new levels of precision and sophistication in targeting different tissues can be achieved however, these approaches, while advantageous in their simplicity, capability of specialization, and efficiency, must be intelligently developed in conjunction with clinicians to design constructs that will be most beneficial in a clinical setting.
7. CONCLUSIONS
The cytotoxic nature of cancer therapeutics has presented a challenge since their inception and their therapeutic advantages are minimized by the adverse effects that result from systemic non-targeted delivery of chemotherapy. There is an inherent need to improve the toxicity of these drugs and this is the coupling with drug delivery could have the greatest advantage. An ideal drug delivery platform should limit the degradation/modification of drugs in the circulatory and monocyte phagocytic systems, thus increasing their biodistribution and pharmacokinetics, and reduces off-target effects while discerning between healthy and diseased organs. In this regard, nanotechnology has demonstrated increasing promise as multifunctional tool in achieving these requirements in addition to being optimal for transplantation [148]. However, although several strategies have been developed to optimize targeting and compatibility such as the use of natural components (e.g., ghee [149]) or components derived from nature, it is becoming increasingly clear that to achieve optimal biomimicry, a simple monophasic protein functionalization approach does not effectively recreate the complex interactions between cells and the host environment [150]. For example, autologous cell-derived protein cocktails to create biomimetic constructs may be a more advantageous strategy that not only increases efficacy of targeting for currently available drugs, but also expands the catalog of possible compounds. In addition, biomimetic constructs could be expanded to induce advantageous immunological responses, therefore combining immunotherapy and drug delivery in a single platform. Molecules such as curcumin show the importance of delivering the right molecule to the right area, rather than looking for the “magic bullet” to cure everything. As we strive to solve the problems in current cancer therapy, it seems that the more sophisticated systems needed to circumvent biological barriers using biomimetic approaches may provide a simple and powerful solution for targeted cancer therapy.
Acknowledgments
The authors acknowledge support from the National Institute of Health (1R56CA213859-01A1, 1R21CA173579-01A1 and 5U54CA143837 PSOC Pilot project), the Department of Defense (W81XWH-12-10414 BCRP Innovator Expansion), Cancer Prevention Research Institute of Texas (RP170466), George J. and Angelina P. Kostas Charitable Foundation, Brown Foundation Inc., William Randolph Hearst Foundation, The Regenerative Medicine Program Cullen Trust for Health Care, and CARIPARO Foundation Ricerca Pediatrica 2016–2018 Grant to E.T. and M.A.; R.M was supported by grant RF-2010-2305526; C.C. and A.P. were supported by grant RF-2010-2318372 from Italian Ministry of Health. We thank Associazione Bianca Garavaglia, Via C. Cattaneo, 8, 21052 Busto Arsizio Varese, Italy and Project CREME “Campania Research in Experimental Medicine” POR Campania FSE 2007/2013.
References
- 1.Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. Journal of Controlled Release. 2011;151(3):220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature nanotechnology. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 3.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9(8):615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 4.Svenson S. Clinical translation of nanomedicines. Current Opinion in Solid State and Materials Science. 2012;16(6):287–294. doi: 10.1016/j.cossms.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang JA, Anyarambhatla G, Ma L, Ugwu S, Xuan T, Sardone T, Ahmad I. Development and characterization of a novel Cremophor® EL free liposome-based paclitaxel (LEP-ETU) formulation. European journal of pharmaceutics and biopharmaceutics. 2005;59(1):177–187. doi: 10.1016/j.ejpb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Vader P, Fens MH, Sachini N, Van Oirschot BA, Andringa G, Egberts AC, Gaillard CA, Rasmussen JT, Van Wijk R, Van Solinge WW. Taxol®-induced phosphatidylserine exposure and microvesicle formation in red blood cells is mediated by its vehicle Cremophor® EL. Nanomedicine. 2013;8(7):1127–1135. doi: 10.2217/nnm.12.163. [DOI] [PubMed] [Google Scholar]
- 7.Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. European Journal of Pharmaceutical Sciences. 2013;48(3):416–427. doi: 10.1016/j.ejps.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Florescu M, Cinteza M, Vinereanu D. Chemotherapy-induced cardiotoxicity. Maedica (Buchar) 2013;8(1):59–67. [PMC free article] [PubMed] [Google Scholar]
- 9.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological reviews. 2004;56(2):185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 10.Cho K, Wang X, Nie S, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clinical cancer research. 2008;14(5):1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 11.Martinez JO, Evangelopoulos M, Bhavane R, Acciardo S, Salvatore F, Liu X, Ferrari M, Tasciotti E. Multistage Nanovectors Enhance the Delivery of Free and Encapsulated Drugs. Curr Drug Targets. 2015;16(14):1582–1590. doi: 10.2174/1389450115666141015113914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danhier F, Feron O, Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. Journal of Controlled Release. 2010;148(2):135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. The Lancet Oncology. 2014;15(11):e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 15.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? The lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 16.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124(4):823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Boyle P, Levin B. World cancer report 2008. IARC Press, International Agency for Research on Cancer; 2008. [Google Scholar]
- 18.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23(38):6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 19.Boffetta P, Hashibe M. Alcohol and cancer. The lancet oncology. 2006;7(2):149–156. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- 20.Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P, Boyle P. Tobacco smoking and cancer: A meta-analysis. International journal of cancer. 2008;122(1):155–164. doi: 10.1002/ijc.23033. [DOI] [PubMed] [Google Scholar]
- 21.Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer. Cancer. 2007;109(S12):2667–2711. doi: 10.1002/cncr.22655. [DOI] [PubMed] [Google Scholar]
- 22.Abbas T, Keaton MA, Dutta A. Genomic instability in cancer. Cold Spring Harbor perspectives in biology. 2013;5(3):a012914. doi: 10.1101/cshperspect.a012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Critical reviews in oncology/hematology. 2008;66(1):1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Zhang BC, Gao J, Wang J, Rao ZG, Wang BC, Gao JF. Tumor-associated macrophages infiltration is associated with peritumoral lymphangiogenesis and poor prognosis in lung adenocarcinoma. Medical Oncology. 2011;28(4):1447–1452. doi: 10.1007/s12032-010-9638-5. [DOI] [PubMed] [Google Scholar]
- 26.Lin EY, Pollard JW. Cancer and Inflammation: Novartis Foundation Symposium 256. Wiley Online Library; 2004. pp. 158–172. [PubMed] [Google Scholar]
- 27.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. European journal of cancer. 2006;42(6):717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Seminars in cancer biology. Elsevier; 2008. Vol. 18, pp 349–355. [DOI] [PubMed] [Google Scholar]
- 29.Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, Sakoda M, Ueno S, Natsugoe S, Takao S. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. Journal of Surgical Research. 2011;167(2):e211–e219. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 30.Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende CC. Stem cell marker CD133 affects clinical outcome in glioma patients. Clinical Cancer Research. 2008;14(1):123–129. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- 31.Pallini R, Ricci-Vitiani L, Banna GL, Signore M, Lombardi D, Todaro M, Stassi G, Martini M, Maira G, Larocca LM. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clinical Cancer Research. 2008;14(24):8205–8212. doi: 10.1158/1078-0432.CCR-08-0644. [DOI] [PubMed] [Google Scholar]
- 32.Hanahan D, Weinberg RA. The hallmarks of cancer. cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 33.Tafani M, Sansone L, Limana F, Arcangeli T, De Santis E, Polese M, Fini M, Russo MA. The Interplay of Reactive Oxygen Species, Hypoxia, Inflammation, and Sirtuins in Cancer Initiation and Progression. Oxidative Medicine and Cellular Longevity. 2015;2016 doi: 10.1155/2016/3907147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Biology and Medicine. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature reviews cancer. 2008;8(10):755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 36.Oguma K, Oshima H, Aoki M, Uchio R, Naka K, Nakamura S, Hirao A, Saya H, Taketo MM, Oshima M. Activated macrophages promote Wnt signalling through tumour necrosis factor-α in gastric tumour cells. The EMBO journal. 2008;27(12):1671–1681. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umar S, Sarkar S, Wang Y, Singh P. Functional cross-talk between β-catenin and NFκB signaling pathways in colonic crypts of mice in response to progastrin. Journal of Biological Chemistry. 2009;284(33):22274–22284. doi: 10.1074/jbc.M109.020941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bollrath J, Greten FR. IKK/NF-κB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO reports. 2009;10(12):1314–1319. doi: 10.1038/embor.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albini A, Tosetti F, Benelli R, Noonan DM. Tumor inflammatory angiogenesis and its chemoprevention. Cancer research. 2005;65(23):10637–10641. doi: 10.1158/0008-5472.CAN-05-3473. [DOI] [PubMed] [Google Scholar]
- 40.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue X-N, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer research. 2006;66(23):11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 41.Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, Morias Y, Movahedi K, Houbracken I, Schouppe E, Elkrim Y. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer research. 2014;74(1):24–30. doi: 10.1158/0008-5472.CAN-13-1196. [DOI] [PubMed] [Google Scholar]
- 42.Shieh YS, Hung YJ, Hsieh CB, Chen JS, Chou KC, Liu SY. Tumor-associated macrophage correlated with angiogenesis and progression of mucoepidermoid carcinoma of salivary glands. Annals of surgical oncology. 2009;16(3):751–760. doi: 10.1245/s10434-008-0259-6. [DOI] [PubMed] [Google Scholar]
- 43.Wu P. Cancer Related Inflammation and Tumor Angiogenesis. INTECH Open Access Publisher; 2012. [Google Scholar]
- 44.Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis. 2007;10(3):149–166. doi: 10.1007/s10456-007-9074-0. [DOI] [PubMed] [Google Scholar]
- 45.Gazzaniga S, Bravo AI, Guglielmotti A, van Rooijen N, Maschi F, Vecchi A, Mantovani A, Mordoh J, Wainstok R. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. Journal of Investigative Dermatology. 2007;127(8):2031–2041. doi: 10.1038/sj.jid.5700827. [DOI] [PubMed] [Google Scholar]
- 46.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 47.Hagemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, Klemm F, Pukrop T, Binder C, Balkwill FR. Macrophages induce invasiveness of epithelial cancer cells via NF-κB and JNK. The Journal of Immunology. 2005;175(2):1197–1205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- 48.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nature Reviews Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 49.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. The Journal of experimental medicine. 2001;193(6):727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang PY, Xu HX, Kong LQ, Wang L, Wu WZ, Tang ZY. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clinical Cancer Research. 2010;16(13):3420–3430. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez-Moure JS, Evangelopoulos M, Colvill K, Van Eps JL, Tasciotti E. Nanoantibiotics: a new paradigm for the treatment of surgical infection. Nanomedicine (Lond) 2017 doi: 10.2217/nnm-2017-0401. [DOI] [PubMed] [Google Scholar]
- 52.Xu R, Zhang G, Mai J, Deng X, Segura-Ibarra V, Wu S, Shen J, Liu H, Hu Z, Chen L, Huang Y, Koay E, Huang Y, Liu J, Ensor JE, Blanco E, Liu X, Ferrari M, Shen H. An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat Biotechnol. 2016;34(4):414–418. doi: 10.1038/nbt.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 54.Torchilin VP. Passive and active drug targeting: drug delivery to tumors as an example. Handb Exp Pharmacol. 2010;(197):3–53. doi: 10.1007/978-3-642-00477-3_1. [DOI] [PubMed] [Google Scholar]
- 55.Martinez JO, Brown BS, Quattrocchi N, Evangelopoulos M, Ferrari M, Tasciotti E. Multifunctional to multistage delivery systems: The evolution of nanoparticles for biomedical applications. Chin Sci Bull. 2012;57(31):3961–3971. doi: 10.1007/s11434-012-5387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SY, Ferrari M, Decuzzi P. Shaping nano-/micro-particles for enhanced vascular interaction in laminar flows. Nanotechnology. 2009;20(49):495101. doi: 10.1088/0957-4484/20/49/495101. [DOI] [PubMed] [Google Scholar]
- 57.Doshi N, Prabhakarpandian B, Rea-Ramsey A, Pant K, Sundaram S, Mitragotri S. Flow and adhesion of drug carriers in blood vessels depend on their shape: a study using model synthetic microvascular networks. J Control Release. 2010;146(2):196–200. doi: 10.1016/j.jconrel.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yazdi IK, Ziemys A, Evangelopoulos M, Martinez JO, Kojic M, Tasciotti E. Physicochemical properties affect the synthesis, controlled delivery, degradation and pharmacokinetics of inorganic nanoporous materials. Nanomedicine (Lond) 2015 doi: 10.2217/nnm.15.133. [DOI] [PubMed] [Google Scholar]
- 59.Kolhar P, Anselmo AC, Gupta V, Pant K, Prabhakarpandian B, Ruoslahti E, Mitragotri S. Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proc Natl Acad Sci U S A. 2013;110(26):10753–10758. doi: 10.1073/pnas.1308345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogawa Y, Hirakawa K, Nakata B, Fujihara T, Sawada T, Kato Y, Yoshikawa K, Sowa M. Expression of intercellular adhesion molecule-1 in invasive breast cancer reflects low growth potential, negative lymph node involvement, and good prognosis. Clin Cancer Res. 1998;4(1):31–36. [PubMed] [Google Scholar]
- 61.Kelly CP, O’Keane JC, Orellana J, Schroy PC, 3rd, Yang S, LaMont JT, Brady HR. Human colon cancer cells express ICAM-1 in vivo and support LFA-1-dependent lymphocyte adhesion in vitro. Am J Physiol. 1992;263(6 Pt 1):G864–870. doi: 10.1152/ajpgi.1992.263.6.G864. [DOI] [PubMed] [Google Scholar]
- 62.Tomita Y, Nishiyama T, Watanabe H, Fujiwara M, Sato S. Expression of intercellular adhesion molecule-1 (ICAM-1) on renal-cell cancer: possible significance in host immune responses. Int J Cancer. 1990;46(6):1001–1006. doi: 10.1002/ijc.2910460609. [DOI] [PubMed] [Google Scholar]
- 63.Shimoyama S, Gansauge F, Gansauge S, Widmaier U, Oohara T, Beger HG. Overexpression of intercellular adhesion molecule-1 (ICAM-1) in pancreatic adenocarcinoma in comparison with normal pancreas. Pancreas. 1997;14(2):181–186. doi: 10.1097/00006676-199703000-00011. [DOI] [PubMed] [Google Scholar]
- 64.Martinez JO, Boada C, Yazdi IK, Evangelopoulos M, Brown BS, Liu X, Ferrari M, Tasciotti E. Short and long term, in vitro and in vivo correlations of cellular and tissue responses to mesoporous silicon nanovectors. Small. 2013;9(9–10):1722–1733. doi: 10.1002/smll.201201939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinez JO, Evangelopoulos M, Chiappini C, Liu X, Ferrari M, Tasciotti E. Degradation and biocompatibility of multistage nanovectors in physiological systems. J Biomed Mater Res A. 2014;102(10):3540–3549. doi: 10.1002/jbm.a.35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van de Ven AL, Kim P, Haley O, Fakhoury JR, Adriani G, Schmulen J, Moloney P, Hussain F, Ferrari M, Liu X, Yun SH, Decuzzi P. Rapid tumoritropic accumulation of systemically injected plateloid particles and their biodistribution. J Control Release. 2012;158(1):148–155. doi: 10.1016/j.jconrel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park S, Kang S, Chen X, Kim EJ, Kim J, Kim N, Kim J, Jin MM. Tumor suppression via paclitaxel-loaded drug carriers that target inflammation marker upregulated in tumor vasculature and macrophages. Biomaterials. 2013;34(2):598–605. doi: 10.1016/j.biomaterials.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 68.Hogg N, Patzak I, Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat Rev Immunol. 2011;11(6):416–426. doi: 10.1038/nri2986. [DOI] [PubMed] [Google Scholar]
- 69.Giblin PA, Lemieux RM. LFA-1 as a key regulator of immune function: approaches toward the development of LFA-1-based therapeutics. Curr Pharm Des. 2006;12(22):2771–2795. doi: 10.2174/138161206777947731. [DOI] [PubMed] [Google Scholar]
- 70.Laberge S, Rabb H, Issekutz TB, Martin JG. Role of VLA-4 and LFA-1 in allergen-induced airway hyperresponsiveness and lung inflammation in the rat. Am J Respir Crit Care Med. 1995;151(3 Pt 1):822–829. doi: 10.1164/ajrccm.151.3.7881677. [DOI] [PubMed] [Google Scholar]
- 71.Makgoba MW, Sanders ME, Ginther Luce GE, Dustin ML, Springer TA, Clark EA, Mannoni P, Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- 72.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4(5):325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 73.Vodovozova EL, Moiseeva EV, Grechko GK, Gayenko GP, Nifant’ev NE, Bovin NV, Molotkovsky JG. Antitumour activity of cytotoxic liposomes equipped with selectin ligand SiaLe(X), in a mouse mammary adenocarcinoma model. Eur J Cancer. 2000;36(7):942–949. doi: 10.1016/s0959-8049(00)00029-0. [DOI] [PubMed] [Google Scholar]
- 74.Kirui DK, Mai J, Palange AL, Qin G, van de Ven AL, Liu X, Shen H, Ferrari M. Transient mild hyperthermia induces E-selectin mediated localization of mesoporous silicon vectors in solid tumors. PLoS One. 2014;9(2):e86489. doi: 10.1371/journal.pone.0086489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl 3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 76.Wicki A, Rochlitz C, Orleth A, Ritschard R, Albrecht I, Herrmann R, Christofori G, Mamot C. Targeting tumor-associated endothelial cells: anti-VEGFR2 immunoliposomes mediate tumor vessel disruption and inhibit tumor growth. Clin Cancer Res. 2012;18(2):454–464. doi: 10.1158/1078-0432.CCR-11-1102. [DOI] [PubMed] [Google Scholar]
- 77.Martinez JO, Evangelopoulos M, Karun V, Shegog E, Wang JA, Boada C, Liu X, Ferrari M, Tasciotti E. The effect of multistage nanovector targeting of VEGFR2 positive tumor endothelia on cell adhesion and local payload accumulation. Biomaterials. 2014;35(37):9824–9832. doi: 10.1016/j.biomaterials.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scavo MP, Gentile E, Wolfram J, Gu J, Barone M, Evangelopoulos M, Martinez JO, Liu X, Celia C, Tasciotti E, Vilar E, Shen H. Multistage vector delivery of sulindac and silymarin for prevention of colon cancer. Colloids Surf B Biointerfaces. 2015;136:694–703. doi: 10.1016/j.colsurfb.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 79.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 80.Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316(8):1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 81.Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Curr Pharm Des. 2012;18(26):3831–3852. doi: 10.2174/138161212802083707. [DOI] [PubMed] [Google Scholar]
- 82.Bernardi A, Braganhol E, Jager E, Figueiro F, Edelweiss MI, Pohlmann AR, Guterres SS, Battastini AM. Indomethacin-loaded nanocapsules treatment reduces in vivo glioblastoma growth in a rat glioma model. Cancer Lett. 2009;281(1):53–63. doi: 10.1016/j.canlet.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 83.Sanz-Motilva V, Martorell-Calatayud A, Nagore E. Non-steroidal anti-inflammatory drugs and melanoma. Curr Pharm Des. 2012;18(26):3966–3978. doi: 10.2174/138161212802083680. [DOI] [PubMed] [Google Scholar]
- 84.Culliton BJ. Hughes comments on Fredrickson’s leave. Science. 1987;236(4802):665. doi: 10.1126/science.3576189. [DOI] [PubMed] [Google Scholar]
- 85.Kraus S, Naumov I, Arber N. COX-2 active agents in the chemoprevention of colorectal cancer. Recent Results Cancer Res. 2013;191:95–103. doi: 10.1007/978-3-642-30331-9_5. [DOI] [PubMed] [Google Scholar]
- 86.Del Rosso A, Saldutto P, Di Pierro ED, Masciovecchio S, Galatioto GP, Vicentini C. Impacts of antibiotic and anti-inflammatory therapy on serum prostate specific antigen in asymptomatic men: our experience. Urologia. 2012;79(Suppl 19):37–40. doi: 10.5301/RU.2012.9364. [DOI] [PubMed] [Google Scholar]
- 87.Kamei S, Sakayama K, Tamashiro S, Aizawa J, Miyawaki J, Miyazaki T, Yamamoto H, Norimatsu Y, Masuno H. Ketoprofen in topical formulation decreases the matrix metalloproteinase-2 expression and pulmonary metastatic incidence in nude mice with osteosarcoma. J Orthop Res. 2009;27(7):909–915. doi: 10.1002/jor.20832. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Z, Rigas B. NF-kappaB, inflammation and pancreatic carcinogenesis: NF-kappaB as a chemoprevention target (review) Int J Oncol. 2006;29(1):185–192. [PubMed] [Google Scholar]
- 89.Naksuriya O, Okonogi S, Schiffelers RM, Hennink WE. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35(10):3365–3383. doi: 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- 90.Wei X, Senanayake TH, Bohling A, Vinogradov SV. Targeted nanogel conjugate for improved stability and cellular permeability of curcumin: synthesis, pharmacokinetics, and tumor growth inhibition. Mol Pharm. 2014;11(9):3112–3122. doi: 10.1021/mp500290f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stigliano C, Key J, Ramirez M, Aryal S, Decuzzi P. Radiolabeled polymeric nanoconstructs loaded with Docetaxel and Curcumin for cancer combinatorial therapy and nuclear imaging. Advanced Functional Materials. 2015;25(22):3371–3379. [Google Scholar]
- 92.Palange AL, Di Mascolo D, Carallo C, Gnasso A, Decuzzi P. Lipid-polymer nanoparticles encapsulating curcumin for modulating the vascular deposition of breast cancer cells. Nanomedicine. 2014;10(5):991–1002. doi: 10.1016/j.nano.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 94.Thakkar A, Chenreddy S, Wang J, Prabhu S. Ferulic acid combined with aspirin demonstrates chemopreventive potential towards pancreatic cancer when delivered using chitosan-coated solid-lipid nanoparticles. Cell Biosci. 2015;5:46. doi: 10.1186/s13578-015-0041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng KW, Nie T, Ouyang N, Alston N, Wong CC, Mattheolabakis G, Papayannis I, Huang L, Rigas B. A novel ibuprofen derivative with anti-lung cancer properties: synthesis, formulation, pharmacokinetic and efficacy studies. Int J Pharm. 2014;477(1–2):236–243. doi: 10.1016/j.ijpharm.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 96.da Silveira EF, Chassot JM, Teixeira FC, Azambuja JH, Debom G, Beira FT, Del Pino FA, Lourenco A, Horn AP, Cruz L, Spanevello RM, Braganhol E. Ketoprofen-loaded polymeric nanocapsules selectively inhibit cancer cell growth in vitro and in preclinical model of glioblastoma multiforme. Invest New Drugs. 2013;31(6):1424–1435. doi: 10.1007/s10637-013-0016-y. [DOI] [PubMed] [Google Scholar]
- 97.Coleman RE. Glucocorticoids in cancer therapy. Biotherapy. 1992;4(1):37–44. doi: 10.1007/BF02171708. [DOI] [PubMed] [Google Scholar]
- 98.Kroon J, Buijs JT, van der Horst G, Cheung H, van der Mark M, van Bloois L, Rizzo LY, Lammers T, Pelger RC, Storm G, van der Pluijm G, Metselaar JM. Liposomal delivery of dexamethasone attenuates prostate cancer bone metastatic tumor growth in vivo. Prostate. 2015;75(8):815–824. doi: 10.1002/pros.22963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.D’Arrigo G, Navarro G, Di Meo C, Matricardi P, Torchilin V. Gellan gum nanohydrogel containing anti-inflammatory and anti-cancer drugs: a multi-drug delivery system for a combination therapy in cancer treatment. Eur J Pharm Biopharm. 2014;87(1):208–216. doi: 10.1016/j.ejpb.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 100.Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE. Regulation of macrophage function in tumors: the multifaceted role of NF-kappaB. Blood. 2009;113(14):3139–3146. doi: 10.1182/blood-2008-12-172825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu CY, Xu JY, Shi XY, Huang W, Ruan TY, Xie P, Ding JL. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest. 2013;93(7):844–854. doi: 10.1038/labinvest.2013.69. [DOI] [PubMed] [Google Scholar]
- 102.Ono M. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Science. 2008;99(8):1501–1506. doi: 10.1111/j.1349-7006.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, Li H, Wang M, Yang J, Yi Q. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114(17):3625–3628. doi: 10.1182/blood-2009-05-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hazards of potent topical corticosteroids. Lancet. 1973;1(7808):870–871. [PubMed] [Google Scholar]
- 106.Eubank TD, Roberts RD, Khan M, Curry JM, Nuovo GJ, Kuppusamy P, Marsh CB. Granulocyte macrophage colony-stimulating factor inhibits breast cancer growth and metastasis by invoking an anti-angiogenic program in tumor-educated macrophages. Cancer Res. 2009;69(5):2133–2140. doi: 10.1158/0008-5472.CAN-08-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shiri S, Alizadeh AM, Baradaran B, Farhanghi B, Shanehbandi D, Khodayari S, Khodayari H, Tavassoli A. Dendrosomal curcumin suppresses metastatic breast cancer in mice by changing m1/m2 macrophage balance in the tumor microenvironment. Asian Pac J Cancer Prev. 2015;16(9):3917–3922. doi: 10.7314/apjcp.2015.16.9.3917. [DOI] [PubMed] [Google Scholar]
- 108.Song M, Liu T, Shi C, Zhang X, Chen X. Bioconjugated Manganese Dioxide Nanoparticles Enhance Chemotherapy Response by Priming Tumor-Associated Macrophages toward M1-like Phenotype and Attenuating Tumor Hypoxia. ACS Nano. 2016;10(1):633–647. doi: 10.1021/acsnano.5b06779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev. 2007;59(2–3):75–86. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8(2):129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Levin KE, Clark OH. The reasons for failure in parathyroid operations. Arch Surg. 1989;124(8):911–914. doi: 10.1001/archsurg.1989.01410080041006. discussion 914–915. [DOI] [PubMed] [Google Scholar]
- 112.Leus NG, Talman EG, Ramana P, Kowalski PS, Woudenberg-Vrenken TE, Ruiters MH, Molema G, Kamps JA. Effective siRNA delivery to inflamed primary vascular endothelial cells by anti-E-selectin and anti-VCAM-1 PEGylated SAINT-based lipoplexes. Int J Pharm. 2014;459(1–2):40–50. doi: 10.1016/j.ijpharm.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 113.Khaled SZ, Cevenini A, Yazdi IK, Parodi A, Evangelopoulos M, Corbo C, Scaria S, Hu Y, Haddix SG, Corradetti B, Salvatore F, Tasciotti E. One-pot synthesis of pH-responsive hybrid nanogel particles for the intracellular delivery of small interfering RNA. Biomaterials. 2016;87:57–68. doi: 10.1016/j.biomaterials.2016.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov. 2010;9(1):57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]
- 115.Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today. 2012;17(1–2):71–80. doi: 10.1016/j.drudis.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marotta LL, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, Bloushtain-Qimron N, Kim JJ, Choudhury SA, Maruyama R, Wu Z, Gonen M, Mulvey LA, Bessarabova MO, Huh SJ, Silver SJ, Kim SY, Park SY, Lee HE, Anderson KS, Richardson AL, Nikolskaya T, Nikolsky Y, Liu XS, Root DE, Hahn WC, Frank DA, Polyak K. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(-) stem cell-like breast cancer cells in human tumors. J Clin Invest. 2011;121(7):2723–2735. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen F, Ehlerding EB, Cai W. Theranostic nanoparticles. Journal of Nuclear Medicine. 2014;55(12):1919–1922. doi: 10.2967/jnumed.114.146019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. Journal of the American Chemical Society. 2006;128(6):2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 119.Akhter S, Ahmad MZ, Ahmad FJ, Storm G, Kok RJ. Gold nanoparticles in theranostic oncology: current state-of-the-art. Expert opinion on drug delivery. 2012;9(10):1225–1243. doi: 10.1517/17425247.2012.716824. [DOI] [PubMed] [Google Scholar]
- 120.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nature reviews Cancer. 2014;14(3):199–208. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 121.Hirsch LR, Stafford R, Bankson J, Sershen S, Rivera B, Price R, Hazle J, Halas NJ, West J. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proceedings of the National Academy of Sciences. 2003;100(23):13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nature Reviews Clinical Oncology. 2013;10(9):507–518. doi: 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Singh M, Harris-Birtill DC, Markar SR, Hanna GB, Elson DS. Application of gold nanoparticles for gastrointestinal cancer theranostics: a systematic review. Nanomedicine: Nanotechnology, Biology and Medicine. 2015;11(8):2083–2098. doi: 10.1016/j.nano.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 124.Cai QY, Kim SH, Choi KS, Kim SY, Byun SJ, Kim KW, Park SH, Juhng SK, Yoon KH. Colloidal gold nanoparticles as a blood-pool contrast agent for X-ray computed tomography in mice. Investigative radiology. 2007;42(12):797–806. doi: 10.1097/RLI.0b013e31811ecdcd. [DOI] [PubMed] [Google Scholar]
- 125.Kim D, Park S, Lee JH, Jeong YY, Jon S. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. Journal of the American Chemical Society. 2007;129(24):7661–7665. doi: 10.1021/ja071471p. [DOI] [PubMed] [Google Scholar]
- 126.Hainfeld J, Slatkin D, Focella T, Smilowitz H. Gold nanoparticles: a new X-ray contrast agent. The British journal of radiology. 2014 doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- 127.Zhang Z, Wang L, Wang J, Jiang X, Li X, Hu Z, Ji Y, Wu X, Chen C. Mesoporous Silica-Coated Gold Nanorods as a Light-Mediated Multifunctional Theranostic Platform for Cancer Treatment. Advanced Materials. 2012;24(11):1418–1423. doi: 10.1002/adma.201104714. [DOI] [PubMed] [Google Scholar]
- 128.Shao J, Griffin RJ, Galanzha EI, Kim JW, Koonce N, Webber J, Mustafa T, Biris AS, Nedosekin DA, Zharov VP. Photothermal nanodrugs: potential of TNF-gold nanospheres for cancer theranostics. Scientific reports. 2013;3 doi: 10.1038/srep01293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goel R, Shah N, Visaria R, Paciotti GF, Bischof JC. Biodistribution of TNF-α-coated gold nanoparticles in an in vivo model system. Nanomedicine. 2009;4(4):401–410. doi: 10.2217/nnm.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Visaria RK, Griffin RJ, Williams BW, Ebbini ES, Paciotti GF, Song CW, Bischof JC. Enhancement of tumor thermal therapy using gold nanoparticle–assisted tumor necrosis factor-α delivery. Molecular cancer therapeutics. 2006;5(4):1014–1020. doi: 10.1158/1535-7163.MCT-05-0381. [DOI] [PubMed] [Google Scholar]
- 131.Goel R, Swanlund D, Coad J, Paciotti GF, Bischof JC. TNF-α–based accentuation in cryoinjury—dose, delivery, and response. Molecular cancer therapeutics. 2007;6(7):2039–2047. doi: 10.1158/1535-7163.MCT-06-0676. [DOI] [PubMed] [Google Scholar]
- 132.Ho D, Sun X, Sun S. Monodisperse magnetic nanoparticles for theranostic applications. Accounts of chemical research. 2011;44(10):875–882. doi: 10.1021/ar200090c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ansari C, Tikhomirov GA, Hong SH, Falconer RA, Loadman PM, Gill JH, Castaneda R, Hazard FK, Tong L, Lenkov OD. Development of Novel Tumor-Targeted Theranostic Nanoparticles Activated by Membrane-Type Matrix Metalloproteinases for Combined Cancer Magnetic Resonance Imaging and Therapy. Small. 2014;10(3):566–575. doi: 10.1002/smll.201301456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cho S, Hwang O, Lee I, Lee G, Yoo D, Khang G, Kang PM, Lee D. Chemiluminescent and Antioxidant Micelles as Theranostic Agents for Hydrogen Peroxide Associated-Inflammatory Diseases. Advanced Functional Materials. 2012;22(19):4038–4043. [Google Scholar]
- 135.Calle D, Negri V, Ballesteros P, Cerdán S. Magnetoliposomes loaded with poly-unsaturated fatty acids as novel theranostic anti-inflammatory formulations. Theranostics. 2015;5(5):489. doi: 10.7150/thno.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Patel SK, Janjic JM. Macrophage targeted theranostics as personalized nanomedicine strategies for inflammatory diseases. Theranostics. 2014;5(2):150–172. doi: 10.7150/thno.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Enzo MV, Isenhart L, Ferrari M, Tasciotti E. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8(1):61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.He W, Frueh J, Wu Z, He Q. Leucocyte Membrane-Coated Janus Microcapsules for Enhanced Photothermal Cancer Treatment. Langmuir. 2016;32(15):3637–3644. doi: 10.1021/acs.langmuir.5b04762. [DOI] [PubMed] [Google Scholar]
- 139.Xuan M, Shao J, Dai L, Li J, He Q. Macrophage Cell Membrane Camouflaged Au Nanoshells for in Vivo Prolonged Circulation Life and Enhanced Cancer Photothermal Therapy. ACS Appl Mater Interfaces. 2016;8(15):9610–9618. doi: 10.1021/acsami.6b00853. [DOI] [PubMed] [Google Scholar]
- 140.Chen X, Wong R, Khalidov I, Wang AY, Leelawattanachai J, Wang Y, Jin MM. Inflamed leukocyte-mimetic nanoparticles for molecular imaging of inflammation. Biomaterials. 2011;32(30):7651–7661. doi: 10.1016/j.biomaterials.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Corbo C, Parodi A, Evangelopoulos M, Engler DA, Matsunami RK, Engler AC, Molinaro R, Scaria S, Salvatore F, Tasciotti E. Proteomic Profiling of a Biomimetic Drug Delivery Platform. Curr Drug Targets. 2015;16(13):1540–1547. doi: 10.2174/1389450115666141109211413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Evangelopoulos M, Parodi A, Martinez JO, Yazdi IK, Cevenini A, van de Ven AL, Quattrocchi N, Boada C, Taghipour N, Corbo C, Brown BS, Scaria S, Liu X, Ferrari M, Tasciotti E. Cell source determines the immunological impact of biomimetic nanoparticles. Biomaterials. 2016;82:168–177. doi: 10.1016/j.biomaterials.2015.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Palomba R, Parodi A, Evangelopoulos M, Acciardo S, Corbo C, de Rosa E, Yazdi IK, Scaria S, Molinaro R, Furman NE, You J, Ferrari M, Salvatore F, Tasciotti E. Biomimetic carriers mimicking leukocyte plasma membrane to increase tumor vasculature permeability. Sci Rep. 2016;6:34422. doi: 10.1038/srep34422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hu CM, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel SH, Zhu J, Shi W, Hofman FM, Chen TC, Gao W, Zhang K, Chien S, Zhang L. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Luk BT, Fang RH, Hu C-MJ, Copp JA, Thamphiwatana S, Dehaini D, Gao W, Zhang K, Li S, Zhang L. Safe and Immunocompatible Nanocarriers Cloaked in RBC Membranes for Drug Delivery to Treat Solid Tumors. Theranostics. 2016;6:1004–1011. doi: 10.7150/thno.14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Molinaro R, Corbo C, Martinez JO, Taraballi F, Evangelopoulos M, Minardi S, Yazdi IK, Zhao P, De Rosa E, Sherman MB, De Vita A, Toledano Furman NE, Wang X, Parodi A, Tasciotti E. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat Mater. 2016 doi: 10.1038/nmat4644. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang Q, Ren Y, Mu J, Egilmez NK, Zhuang X, Deng Z, Zhang L, Yan J, Miller D, Zhang HG. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer research. 2015;75(12):2520–2529. doi: 10.1158/0008-5472.CAN-14-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tasciotti E, Cabrera FJ, Evangelopoulos M, Martinez JO, Thekkedath UR, Kloc M, Ghobrial RM, Li XC, Grattoni A, Ferrari M. The Emerging Role of Nanotechnology in Cell and Organ Transplantation. Transplantation. 2016;100(8):1629–1638. doi: 10.1097/TP.0000000000001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Balasubramanian K, Evangelopoulos M, Brown BS, Parodi A, Celia C, Iman KY, Tasciotti E. Ghee Butter as a Therapeutic Delivery System. Journal of Nanoscience and Nanotechnology. 2017;17(2):977–982. doi: 10.1166/jnn.2017.12623. [DOI] [PubMed] [Google Scholar]
- 150.Evangelopoulos M, Tasciotti E. Bioinspired approaches for cancer nanotheranostics. Nanomedicine (Lond) 2017;12(1):5–7. doi: 10.2217/nnm-2016-0374. [DOI] [PubMed] [Google Scholar]


