Abstract
The human brain is a highly dynamic system with non-stationary neural activity and rapidly-changing neural interaction. Resting-state dynamic functional connectivity (dFC) has been widely studied during recent years, and the emerging aberrant dFC patterns have been identified as important features of many mental disorders such as schizophrenia (SZ). However, only focusing on the time-varying patterns in FC is not enough, since the local neural activity itself (in contrast to the inter-connectivity) is also found to be highly fluctuating from research using high-temporal-resolution imaging techniques. Exploring the time-varying patterns in brain activity and their relationships with time-varying brain connectivity is important for advancing our understanding of the co-evolutionary property of brain network and the underlying mechanism of brain dynamics. In this study, we introduced a framework for characterizing time-varying brain activity and exploring its associations with time-varying brain connectivity, and applied this framework to a resting-state fMRI dataset including 151 SZ patients and 163 age- and gender matched healthy controls (HCs). In this framework, 48 brain regions were first identified as intrinsic connectivity networks (ICNs) using group independent component analysis (GICA). A sliding window approach was then adopted for the estimation of dynamic amplitude of low-frequency fluctuation (dALFF) and dFC, which were used to measure time-varying brain activity and time-varying brain connectivity respectively. The dALFF was further clustered into six reoccurring states by the k-means clustering method and the group difference in occurrences of dALFF states was explored. Lastly, correlation coefficients between dALFF and dFC were calculated and the group difference in these dALFF-dFC correlations was explored. Our results suggested that 1) ALFF of brain regions was highly fluctuating during the resting-state and such dynamic patterns are altered in SZ, 2) dALFF and dFC were correlated in time and their correlations are altered in SZ. The overall results support and expand prior work on abnormalities of brain activity, static FC (sFC) and dFC in SZ, and provide new evidence on aberrant time-varying brain activity and its associations with brain connectivity in SZ, which might underscore the disrupted brain cognitive functions in this mental disorder.
1. Introduction
Characterizing brain dynamics from functional magnetic resonance imaging (fMRI) has advanced our knowledge of brain mechanisms (Calhoun et al., 2014; Hutchison et al., 2013). Time-varying patterns in functional connectivity (FC) are prominent in the resting-state, during which the mental activity is unconstrained (Deco et al., 2011; Hutchison et al., 2013). Studies in both animals and humans have demonstrated that rest brain is a highly dynamic system, which could be characterized by non-stationary spatial temporal functional organization and represented by ever-changing mental states at time scales ranging from milliseconds to hours (Allen et al., 2014; Hutchison et al., 2013; Liu et al., 2013; Marusak et al., 2017). Resting-state dynamic FC (dFC) might reflect brain’s evolving network configurations and might be associated with changing patterns of neural communication that subserve certain brain functions (Allen et al., 2013; Allen et al., 2011; Hutchison et al., 2015; Yu et al., 2015).
Despite great progress made on FC dynamics, there is still much work to do on time-varying patterns of brain. Research using high-temporal-resolution imaging techniques (such as electroencephalography [EEG]) has identified reoccurring microstates (by characterizing the changes in power of brain activity) (Lehmann 1990; Wackermann et al., 1993), which are related to spontaneous thoughts and mental processes (Lehmann et al., 1998). Spontaneous blood oxygenation-level dependent (BOLD) fluctuations are supposed to be generated from mental processes (Raichle et al., 2007), for which they might also have highly time-varying and reoccurring patterns linked to varied mental processes.
Recent advances in the field of dFC show that time-varying resting-state FC would be driven by the mental and vigilance states (Allen et al., 2014; Chang et al., 2010; Sakoğlu et al., 2010). Considering that both local BOLD activity and global FC are related to mental and cognitive processes (Britz et al., 2009; Hutchison et al., 2016; Marusak et al., 2017; McIntosh et al., 2008), it is reasonable to assume the possibility of associations between BOLD activity and FC along time. On the other hand, research show that many neural networks are adaptive networks, in which the change of network topology is linked to the change of the local activity (Gross et al., 2008). Since the brain involves an extensive network of inter-connected neurons which can be measured at the macro-scale with fMRI, such functional brain network might also self-organize as an adaptive network, with co-evolutionary local BOLD activity and FC.
Resting-state fMRI can characterize spontaneous brain activity and identify brain networks with covaried patterns, which make it a powerful technique for examining brain abnormalities in mental disorders without considering the difference in task performance between patients and controls (Bassett et al., 2012; Kühn et al., 2013; Pearlson et al., 2009). Schizophrenia (SZ) is a severe, chronic brain disorder whose symptoms can include delusions, disorganized thinking, hallucinations and social withdrawal (Calhoun et al., 2008; Endicott et al., 1978; Kay et al., 1987). The disruptions of variety of cognitive and emotional domains, such as attention (Braff 1993; Cornblatt et al., 1994), emotion (Brüne 2005; Edwards et al., 2002), and memory (Aleman et al., 1999; Saykin et al., 1991) are often observed in patients with SZ. Aberrant brain activity and FC in SZ are important neural signatures of this disorder, such as reduced amplitude of low-frequency fluctuation (ALFF) in cuneus (Hoptman et al., 2010; Turner et al., 2013) and dysconnectivity between thalamus and sensory regions (Calhoun et al., 2009; Kühn et al., 2013; Malaspina et al., 2004; Mingoia et al., 2012; Zhou et al., 2007). Increasing numbers of studies have focused on the abnormal patterns of dFC in SZ during recent years, which have revealed information not available in static FC (sFC) (Ma et al., 2014; Rashid et al., 2014). By clustering the dFC into five reoccurring dFC state, Damaraju and his colleagues found that SZ patients spent less time in states with strong FC. They also identified significant group difference in FC within sensory networks and in FC between thalamus and sensory networks in several dFC states (Damaraju et al., 2014). Similar dFC state analysis was applied on SZ and bipolar disorder dataset for investigating state-based FC difference (Rashid et al., 2014). Group differences between SZ patients and bipolar patients were found in patterns of FC involving the frontal and frontal-parietal regions at certain states. Dynamic FC features were also used for multi-classes classification. A recent study used the dFC states for the classification of SZ patients and bipolar patients and showed that the dFC features could improve the classification performance over the static FC features (Rashid et al., 2016). Brain graph properties were explored at the dynamic perspective as well and significant SZ related alteration of dynamic brain graph has been reported (Yu et al., 2015). However, until recently, previous work only focused on the aberrant patterns in static BOLD properties, sFC or dFC. Whether BOLD activity is time-varying and whether such time-varying patterns in brain BOLD activity are altered in this disorder remains poorly documented.
In this study, we hypothesized that 1) BOLD activity might be highly fluctuating and associated with changing brain connectivity over time during the resting-state; and 2) dynamic BOLD activity and its associations with dynamic brain connectivity might be altered in SZ. To verify this hypothesis, we proposed a framework for assessing the time-varying local BOLD activity (measured by dynamic amplitude of low-frequency fluctuation, dALFF) and investigating the associations between time-varying BOLD activity and time-varying brain connectivity (measured by correlation coefficient between dALFF and dFC). This framework was then applied to healthy controls (HCs) and SZ patients for the exploration of aberrant patterns in SZ. In this framework, spatial group independent component analysis (GICA) was first conducted to decompose the whole brain fMRI data into multiple functionally homogeneous regions (Calhoun et al., 2001; Calhoun et al., 2009; Kiviniemi et al., 2009). Secondly, a sliding window approach was adopted to estimate dALFF of brain regions and dFC between brain regions. Further, a k-means clustering method was used to identify patterns of dALFF that reoccur in time and across subjects. Finally, a dALFF-dFC correlation analysis was performed to investigate the associations between dALFF and dFC along time. The results showed that during the resting-state with eyes closed, BOLD activity comprises highly replicable dALFF states in a conceptual analogy to EEG microstates. HCs and SZ patients have significantly different occurrences of dALFF states. FC estimated at different dALFF states also differ, and such differences are coupled with alterations in ALFF. The dALFF and dFC are significantly correlated in time and, importantly, their associations are altered in SZ. Compared with HCs, SZ patients show lower or lost associations between dALFF of several important brain regions (such as thalamus and cuneus) and some dFC. Taken together, our findings suggested that the time-varying patterns in ALFF and their temporal associations with dFC could be potential neural biomarkers of SZ, which might provide new understanding of the pathophysiology of this disorder.
2. Material and methods
2.1 Participants and fMRI dataset acquisition
Resting-state fMRI dataset from a total of 163 HCs (117 males, 46 females; mean age 36.9 and standard deviation of age 11.0) and 151 age- and gender-matched SZ patients (114 males, 37 females; mean age 37.8 and standard deviation of age 11.4) were used in present study. All participants were provided written, informed consent prior to scanning in accordance with the Internal Review Boards of corresponding institutions. All patients had chronic SZ and most of the participants were assessed with the CMINDS cognitive battery (included processing speed, attention vigilance, working memory, verbal learning, visual learning, reasoning problem solving and a composite score).
Participants were scanned during the eyes-closed rest condition at 7 different sites across the United States and passed data quality control. Images were collected on 3T Siemens Tim Trio Systems at six sites and on a 3T General Electric Discovery MR750 scanner at one site. All resting-state fMRI data were acquired using a standard gradient-echo echo planar imaging sequence with TE = 30 ms, TR = 2 s, FA = 77o, slice thickness = 4 mm, slice gap = 1 mm. The duration of each resting-state scan was 5 min 24 s (162 volumes).
2.2 Preprocessing
Data processing was performed using a combination of toolboxes, AFNI3 (https://afni.nimh.nih.gov), SPM12 (http://www.fil.ion.ucl.ac.uk/spm/) and GIFT4.0b (http://mialab.mrn.org/software/gift), and custom code written in MATLAB. We performed rigid body motion correction using the toolbox in SPM to correct subject head motion, followed by slice-timing correction to account for timing difference in slice acquisition. Then the fMRI data were despiked using AFNI3 3dDespike algorithm to mitigate the impact of outliers. The fMRI data were subsequently warped to a Montreal Neurological Institute (MNI) template and were resampled to 3 mm3 isotropic voxels. All functional images were smoothed using a Gaussian kernel (FWHM = 5 mm). Prior to GICA, time course of each voxel was variance normalized.
2.3 The framework to characterize dALFF and its relationship with dFC
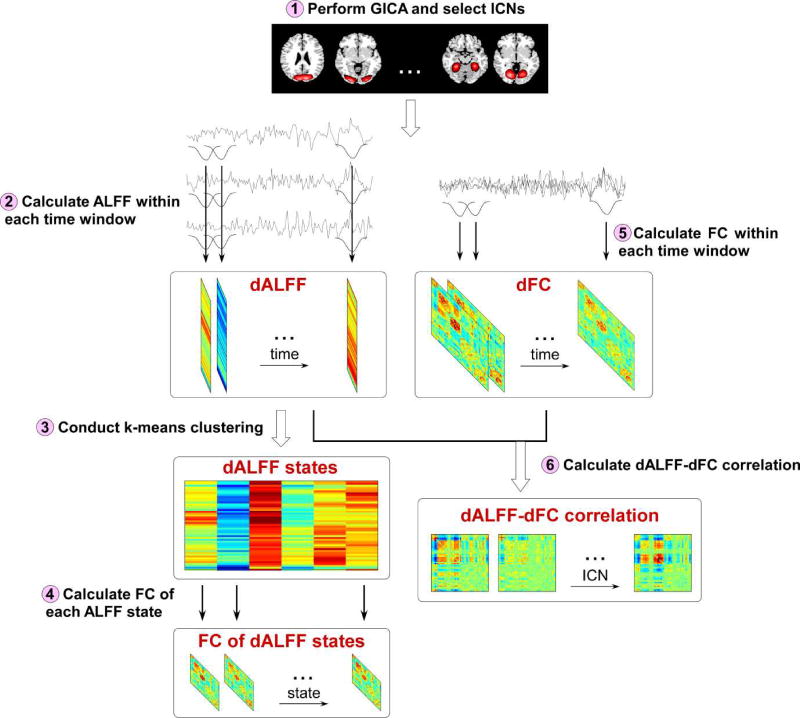
The proposed framework was illustrated using a flowchart in Figure 1. 6 major steps were included in this framework. Step 1) GICA was performed and ICNs were selected according to the spatial maps of components (details of GICA and ICNs selection were introduced in section 2.4); Step 2) dALFF of ICNs was calculated using a sliding window approach (details of dALFF calculation were introduced in section 2.5); Step 3) dALFF was clustered into 6 states using a k-means clustering method and the group difference (between HCs and SZ patients) in occurrences of dALFF states was examined (details of clustering and occurrences of states were introduced in section 2.6 and section 2.7); Step 4) FC of each dALFF state was calculated and the difference in ALFF and FC among states was examined (details of evaluating group difference in ALFF and FC among states were introduced in section 2.8); Step 5) dFC between ICNs was calculated using a sliding window approach (details of dFC calculation were introduced in section 2.9); Step 6) temporal correlation coefficient between dALFF and dFC was calculated and the group difference (between HCs and SZ patients) in dALFF-dFC correlations was examined (details of analysis of associations between dALFF and dFC were introduced in section 2.9).
Figure 1.
The flowchart for the exploration of dALFF and dALFF-dFC correlations.
2.4 Spatial GICA
Spatial GICA (Calhoun et al., 2012; Calhoun et al., 2001) was performed on the fMRI data using the GIFT toolbox. Principal components analysis (PCA) was first applied to reduce the subject specific data into 120 principal components which preserve more than 99% of the variance. Next, reduced data of all subjects were concatenated across time and decomposed into 100 independent components (ICs) using the infomax algorithm. The infomax ICA algorithm was repeated 10 times in ICASSO and the best run was selected to ensure the estimation stability. After estimating the aggregate spatial maps, spatiotemporal regression back reconstruction was performed to obtain the subject specific spatial maps and time courses (Erhardt et al., 2011). Additional post-processing steps were performed on the time courses of selected ICNs, which included: 1) detrending linear, quadratic, and cubic trends; 2) conducting multiple regressions of the 6 realignment parameters and their temporal derivatives; 3) despiking detected outliers; 4) low-pass filtering with cut-off frequency of 0.15 Hz.
2.5 Estimation of dALFF
In this study, we used ALFF to measure the pattern of local BOLD activity. ALFF is the square root of power spectrum integrated in a low-frequency range, which could capture the property of the most dominant frequency component during the resting-state (Zang et al., 2007; Zou et al., 2008). The dynamic patterns in ALFF were characterized by using a sliding window approach. The window was created by convolving a rectangle with a Gaussian (σ = 3 TRs). The window size was chosen as 20 TR (40s), because the window size in the range of 30 s to 1 min was shown to be a reasonable choice for capturing brain dynamics (Allen et al., 2014; Damaraju et al., 2014; de Lacy et al., 2017; Rashid et al., 2014; Shirer et al., 2012). We also examined the results with other window sizes, and they were provided in the supplementary materials. The window was slid along time in steps of 1 TR, resulting in W = 135 windows. dALFF of each ICN was estimated using the windowed data between the frequency band [0.025 – 0.15 Hz] using the REST toolbox (Zang et al., 2007). Each windowed data was first filtered (bandpass, [0.025 – 0.15 Hz]) to remove the effects of very low-frequency drift and high-frequency noise. Then the windowed data was transformed to a frequency domain and the power spectrum was estimated. The square root was calculated as each frequency of power spectrum. The ALFF of each window was obtained by averaging the square root across the given frequency band ([0.025 – 0.15 Hz]). dALFF estimates of all windows were finally concatenated to form an N×W dALFF matrix (where N denotes the number of ICNs and W denotes the number of windows) representing the changes in dALFF of ICNs as a function of time.
2.6 Clustering analysis
To assess the reoccurring dALFF patterns, a k-means clustering algorithm was performed on dALFF estimates of all subjects (Combining HC and SZ subjects). L1 norm was used as the distance function in k-means. A subset of windows with maximal variability in dALFF across components was chosen for the initial clustering. The number of cluster was determined as k = 6 using the elbow criterion, defined as the ratio of within-cluster to between-cluster distances (Allen et al., 2014). Our previous work has shown that the clustering patterns were consistent over a range of k from 5 to 7 (Allen et al., 2014; Damaraju et al., 2014; Marusak et al., 2017). The group centroids obtained by clustering exemplars were then used as a starting point to cluster all dALFF windows from all subjects.
2.7 Occurrences of states
The aberrant patterns of dALFF in SZ were first investigated by examining the group difference in the occurrences of dALFF states. The occurrence of each dALFF state was measured by the number of time windows which were assigned to each state. The percentage occurrence of each dALFF state was calculated by dividing the number of total windows by the number of time windows which were assigned to each state. A two-sample t-test was performed to identify significant group difference in the occurrences of dALFF states. To investigate whether the occurrences of states are associated cognitive processes, we calculated the sample linear partial correlation coefficients between the occurrences and cognitive scores, controlled by several variables (the age and gender for HC and SZ group; age, gender and diagnosis for all subjects). We chose partial correlation as the measure of association between the atypical patterns and the cognitive score because we want to control the potential covariates (such as gender, age and diagnosis), which might have influences on the estimated relationships.
2.8 Difference in ALFF and FC among states
After identifying reoccurring patterns of dALFF, we sought to investigate whether FC changes among dALFF states and whether such changes in FC are coupled with the changes in ALFF. A subject-specific correlation coefficient matrix of each dALFF state was computed using the subject’s time points within the windows that were assigned to that state, as a representative pattern of FC of the subject for that state. Nonparametric repeated measures analysis of variance (ANOVA) was first conducted to test the difference in FC among states. If the difference is significant, then a post-hoc two-sample t-test was conducted element-wise to examine FC difference between each pair of states. To explore the potential coupling between ALFF and changes in FC among states, we chose thalamus as a seed region and investigated the difference in its ALFF and in its FC to sensory regions among states. Thalamus was chosen here because its BOLD activity and its FC to sensory networks are important correlates of SZ. The ALFF of thalamus was first averaged across windows of the same state, and a two-sample t-test was then conducted on the mean ALFF of thalamus to examine the ALFF difference between each pair of states. The two-sample t-test was also conducted on the mean thalamus FC with sensory regions, which was obtained by averaging all FC between thalamus and regions within auditory network (ADN), somatomotor network (SMN) and visual network (VSN), to examine the FC difference between each pair of states.
2.9 Analysis of associations between dALFF and dFC
Both ALFF and FC changed significantly among dALFF states, and more importantly, such changes were found to be coupled across states. To explore the detailed associations between ALFF and FC in time, we conducted following analysis. Firstly, dFC was estimated using a sliding window approach. The window parameters were exactly the same as those used in the dALFF estimation. The FC matrices were computed from the regularized precision matrix (inverse covariance matrix). The graphic LASSO algorithm was used for the estimation of the precision matrix. The L1 penalty was used for promoting sparsity in the precision matrix and the regularization parameter lambda λ was selected for each subject by cross-validation. For each subject, the FC estimates were concatenated to form an N×N×W dFC matrix representing the changes in FC between ICNs as a function of time.
The correlation coefficient between dALFF of each ICN and each dFC (dALFF-dFC correlation) was calculated to measure the association between ALFF and FC. A one sample t-test was applied to examine whether the dALFF-dFC correlations are significantly different from 0 (for both the HC and SZ groups). We categorized the dALFF-dFC correlations with group difference into three types: Type 1 (dALFF-dFC correlations with group difference significantly deviated from 0 for HC only), Type 2 (dALFF-dFC correlations with group difference significantly deviated from 0 for SZ only), and Type 3 (dALFF-dFC correlations with group difference are significantly deviated from 0 for both HC and SZ groups). Next, the group difference in the dALFF-dFC correlations was further explored via a twosample t-test. Finally, to assess whether the dALFF-dFC correlations are related to cognitive processes, we calculated sample linear partial correlation coefficients between Type 3 dALFF-dFC correlations and cognitive scores, controlled by several variables (the age and gender for HC and SZ group; age, gender and diagnosis for all subjects).
3. Results
3.1 Spatial GICA and selected ICNs
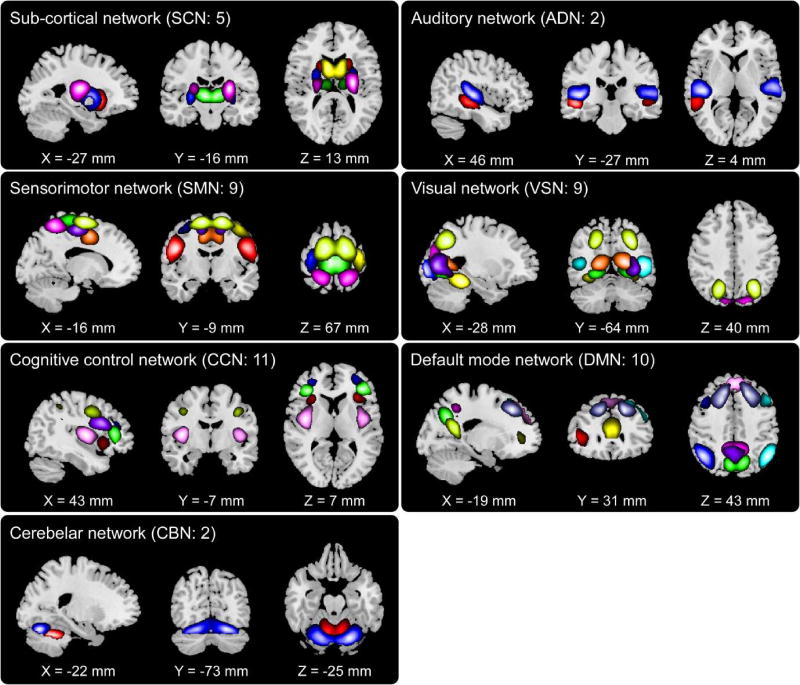
The spatial maps of 48 selected ICNs were depicted in Figure 2. In total N = 48 ICs were identified as ICNs by considering that their activation peaks fell on gray matter and low spatial over-lap with known vascular, ventricular, motion and some other artifacts. Based on prior knowledge of their functional meanings and the anatomical information, 48 ICNs were categorized into following 7 networks: subcortical network (SCN), ADN, VSN, SMN, cognitive control network (CCN), default-mode network (DMN), and cerebellar network (CBN). The detailed spatial map of each ICN was shown in supplementary materials Figure S1. Component labels and peak coordinates were provided in supplementary materials Table S1.
Figure 2.
spatial maps of the 48 selected ICNs, sorted into seven networks. Each color in the spatial maps corresponds to a different ICN.
3.2 dALFF and clustered dALFF states
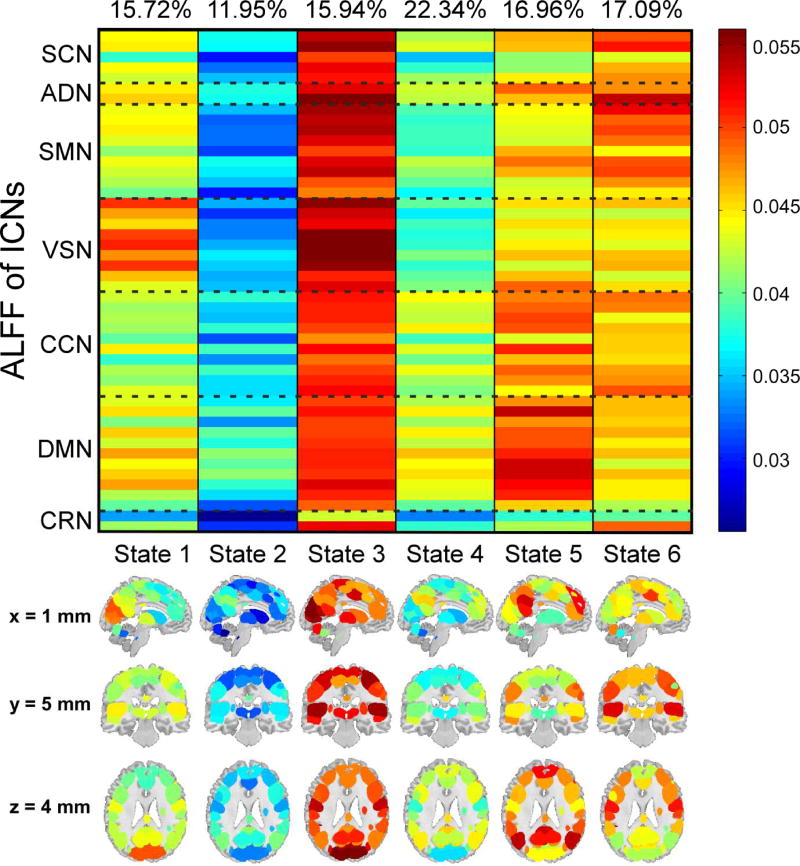
Figure 3 displayed the clustering results with k = 6 . Each column represented the centroid of a cluster, and reflected the ALFF activation patterns at one state. It could be observed that state 1, 3, 5, and 6 showed relative larger ALFF patterns, while state 2 and 4 showed relative smaller ALFF patterns. In state 1, visual regions had large ALFF. In contrast, other brain regions only had moderate ALFF. State 2 was a weak activation state, in which most of the brain regions had smallest ALFF. State 3 was the strongest activation state. Most of the brain regions, especially the regions within SCN, ADN, SMN and VSN, had the largest ALFF in this state. State 4 was another weak state with the second smallest ALFF patterns. State 5 and state 6 were two strong states with different ALFF patterns. In state 5, brain regions within CCN and DMN had relative larger ALFF compared with regions within other networks. In state 6, ALFF was larger in SCN, ADN and CRN.
Figure 3.
Clustered dALFF states of all subjects. Each column represents the centroid of each cluster (dALFF states: 1~6). The percentage of occurrence of each state is list above each centroid.
3.3 Occurrences of dALFF states and their relationships with cognitive scores
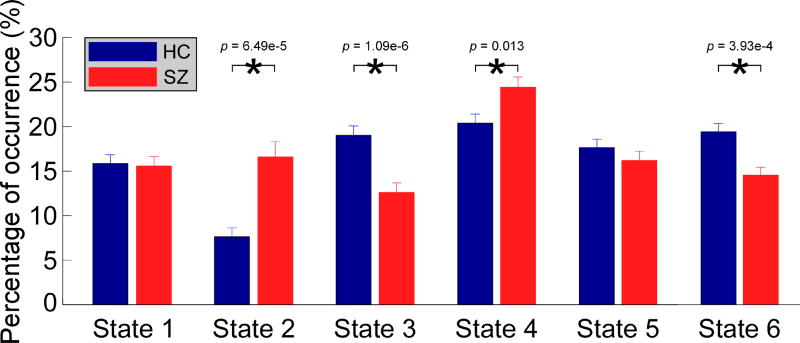
In Figure 4, group difference in occurrences of dALFF states was displayed. 4 out of 6 dALFF states have different occurrences between HCs and SZ patients. Compared with HCs, SZ patients had more occurrences in weak dALFF states (state 2: p = 6.48 × 10−5; state 4: p = 0.0130 ), and less occurrences in strong dALFF states (state 3: p = 1.09 × 10−6; state 6: p = 3.93 × 10−4 ). This finding is in line with previous work showing lower power of low-frequency fluctuations in SZ (Calhoun et al., 2008). There was no significant correlation between the occurrences of dALFF states and cognitive scores.
Figure 4.
Group difference in percentage occurrences of dALFF states. Bar represents the mean occurrence of each state, while the errorbar represents the standard error of mean of occurrence. Asterisks indicate p < 0.05 (FDR corrected).
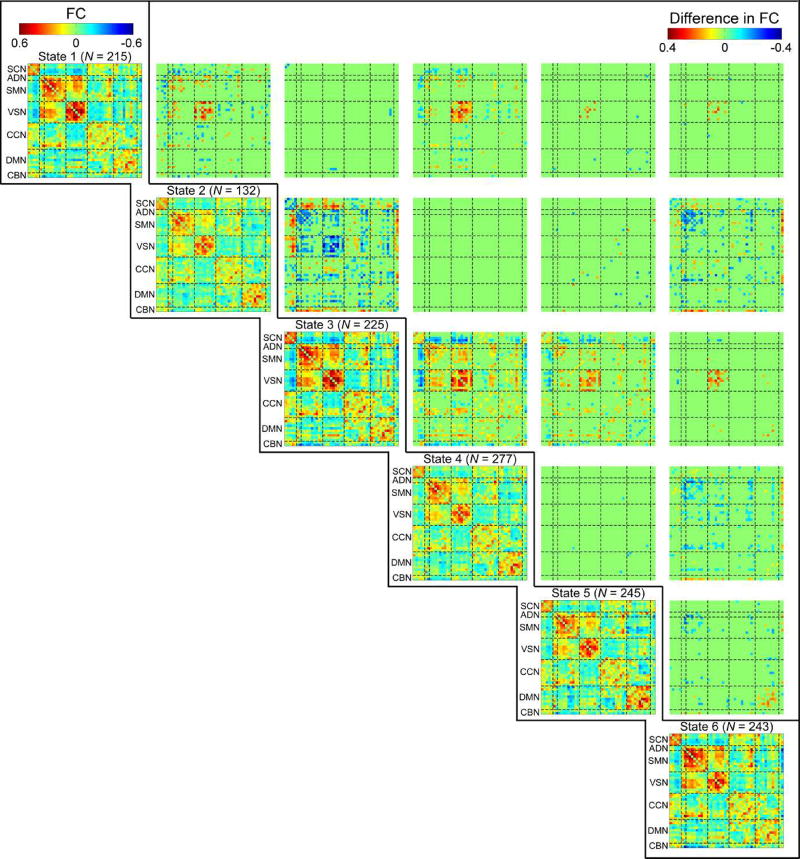
3.4 Difference in ALFF and FC among dALFF states
FC of each dALFF state and difference between them were displayed in Figure 6. Significantly different FC patterns could be observed among states. For example, compared with state 5, state 1 had stronger FC between regions within VSN and weaker FC between regions within DMN. FC of state 4 distinguished itself from FC of state 6 with respect to intra-network FC between ICNs within SMN, ADN and VSN. State 4 also showed relative smaller negative inter-network FC between SCN and SMN. State 2 and state 3 had most significant difference in their specific FC. State 2 had significantly weaker positive intra-network FC of SMN, ADN and VSN. In addition, positive internetwork FC between SMN and VSN, and negative inter-network FC between SMN and SCN were significantly weaker in state 2. We also examined group difference in FC of each dALFF state. It could be observed significantly different FC patterns in dALFF states, such as the hyperconnectivity between thalamus and sensory ICNs in patients with SZ at some states (dALFF state 1, 3, 4, and 6), which are consistent with previous results examining the group difference in state based FC (Damaraju et al., 2014). Detailed results of group difference in FC of dALFF states were provided in supplementary materials Figure S3.
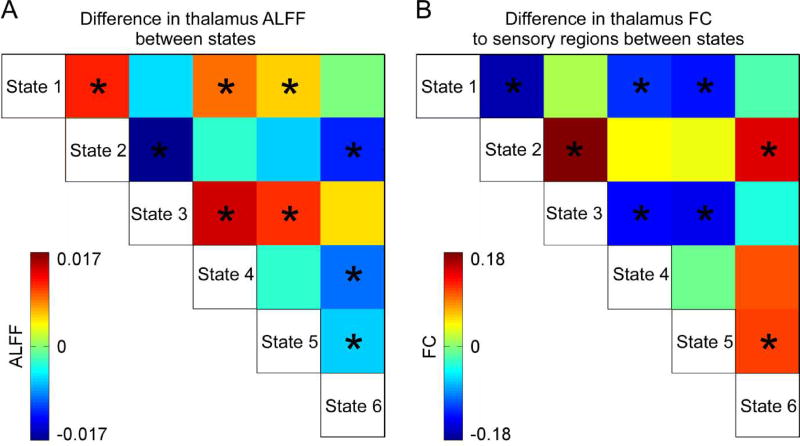
Figure 6.
A) Difference in ALFF of thalamus between each pair of states. B) Difference in the thalamus FC to sensory regions between each pair of states. Asterisks indicate p < 0.05 (FDR corrected).
The difference among states in thalamus’ ALFF and its FC to sensory regions was displayed in Figure 7A and 7B, respectively. FC between thalamus and sensory regions is identified to be negative for all dALFF states. Larger ALFF in thalamus was usually accompanied with stronger negative FC between thalamus and sensory regions, while smaller ALFF in thalamus was usually accompanied with weaker negative FC between thalamus and sensory regions. This result implied that changes in ALFF and FC might be coupled, and ALFF and FC might co-evolve in time.
Figure 7.
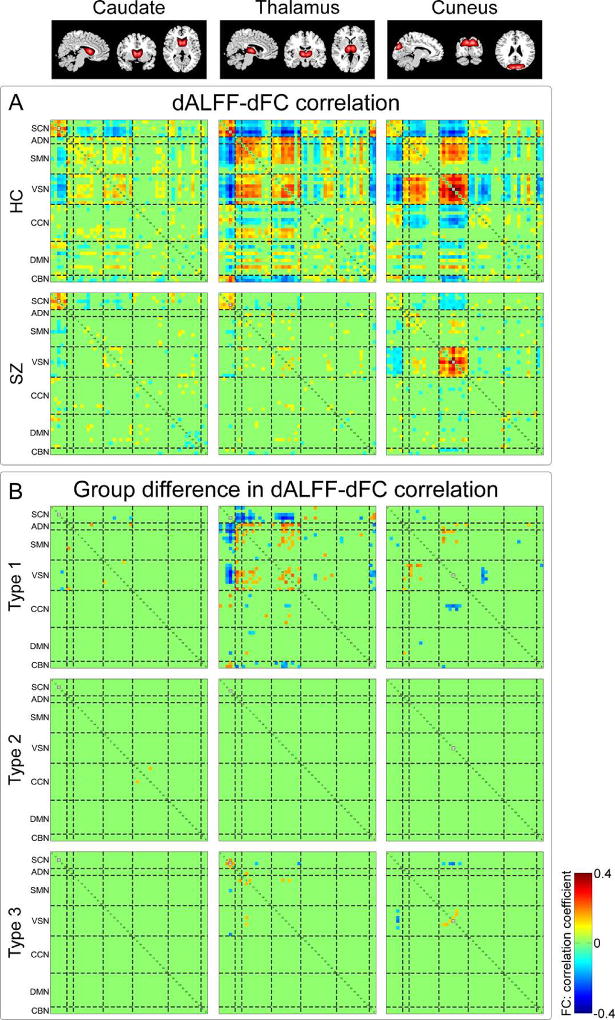
A) Correlations between dALFF of ICNs and dFC (dALFF-dFC correlations) for HCs and SZ patients. B) dALFF-dFC correlations with significant group difference. Type 1: dALFF-dFC correlations are only identified significant in HC group; type 2: dALFF-dFC correlations are only identified siginificant in SZ group; type 3: dALFF-dFC correlations are identified significant in both HC and SZ group. Three examples are presented: caudate nucleus, thalamus and cuneus. Significant correlation is corrected by FDR threshold (p < 0.05).
3.5 Associations between dALFF and dFC
Three example ICNs with significant dALFF-dFC correlations for HC and SZ group were displayed in Figure 8A: correlations between dALFF of caudate nucleus and dFC, correlations between dALFF of thalamus and dFC, and correlations between dALFF of cuneus and dFC. It could be observed that, 1) dALFF of one ICN was most likely to be correlated with this ICN related intra-network dFC; 2) dALFF of one ICN was not only correlated with this ICN related dFC, but also correlated with dFC between other ICNs; 3) dALFF was usually positively correlated with positive dFC and negatively correlated with negative dFC. We further examined whether dALFF-dFC correlations are alter in SZ. Several important ICNs were identified to have the most abnormal correlations with FC: thalamus, postcentral gyrus, paracentral lobule, lingual gyrus, cuneus, calcarine gyrus and caudate nucleus. The dALFF-dFC correlations with significant group difference were depicted in Figure 8B. It could be observed that most of the dALFF-dFC correlations with group difference were Type 1, which means that the major abnormality in dALFF-dFC correlations was the lost of associations between dALFF and dFC in SZ. Results of other ICNs with significantly aberrant dALFF-dFC correlations were provided in supplementary materials Figure S4. We also tested the reliability of dALFF-dFC correlations using different length of window sizes. We chose a shorter time window (16 TR) and a longer time window (24 TR) to compute the dALFF and dFC. Then dALFF-dFC correlations were calculated and the group difference was examined. The detailed results were provided in supplementary materials Figrue S5 and S6. We could obtain similar results by using these two different window sizes. Significantly aberrant dALFF-dFC correlations could still be identified in several ICNs, such as thalamus and cuneus.
Figure 8.
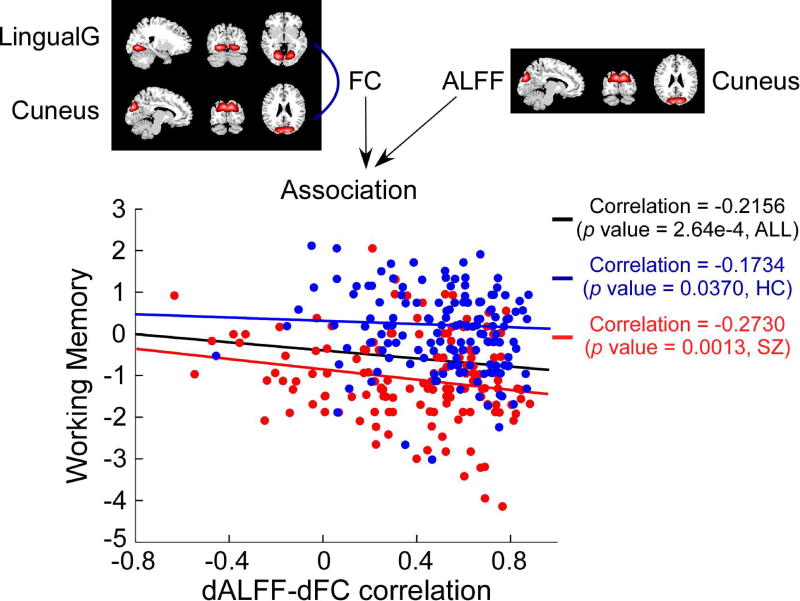
Scatter plot showing the relationship between a dALFF-dFC correlation and working memory score. The dALFF-dFC correlation is obained by calculating the correlation coefficient between dALFF of cuneus and cuneus’ FC to lingual gyrus. Black line: relationship across all subjects; blue line: relationship for HCs; red line: relationship for SZ patients.
dALFF-dFC correlations with Type 3 difference were then correlated with cognitive scores, for the purpose of investigating whether they are related to cognitive performance. A dALFF-dFC correlation was identified significantly correlated with cognitive score and the results were displayed in Figure 8. That is, the correlation between ALFF of cuneus and its FC to lingual gyrus was negatively correlated with score of working memory ( r = −0.2156, p < 2.64×10−4 , all subjects; r = −0.1734, p < 0.0370, HCs; r = −0.2730, p < 1.30×10−3, SZ patients).
4. Discussion
In this study, the reoccurring patterns of resting-state dALFF were first characterized and whether such patterns are altered in SZ was investigated. Then the existence of associations between dALFF and dFC was explored and the group difference in such dALFF-dFC correlations was examined. Our results showed that 1) individual subjects showed several highly reoccurring dALFF states during the resting-state; 2) compared with HCs, SZ patients had more occurrences in the states with weak ALFF and less occurrences in the states with strong ALFF; 3) dALFF of ICNs was associated with dFC between ICNs in time and some dALFF-dFC correlations were altered in SZ. Converging results suggest that the resting-state local brain activity would be highly fluctuating with reoccurring patterns and co-evolve with brain connectivity in time. Such dynamic patterns in brain activity and the co-evolutionary patterns might be related to cognitive performance and more importantly, be altered in SZ. They could serve as a promising biomarker for SZ. However, more work is needed to determine whether it is specific to this illness. Further investigation on the association between local and synchronized brain activity might lead to the better understanding of SZ and eventually diagnostic indicators.
4.1 Aberrant dynamic ALFF states in schizophrenia
ALFF is a mathematical measure of signal power and it has been found to be higher in grey than in white matter (Biswal et al., 1995; Zang et al., 2007). Kiviniemi and colleagues showed that the neural activation in the visual cortex was related to ALFF around 0.034 Hz (Kiviniemi et al., 2000), which suggested ALFF to be a representation of regional spontaneous neuronal activity. ALFF is also found to be different among resting-state brain networks and be altered by mental diseases, such as SZ and attention deficit hyperactivity disorder (ADHD) (Hoptman et al., 2010; Zang et al., 2007; Zou et al., 2008). However, previous studies assumed ALFF is static throughout the entire scan, but ALFF is indeed time-varying because individuals are likely to engage in different mental processes which are associated with ALFF. It is important to investigate the temporal properties of abnormal local brain activity in patients’ brain. In this study, by using a sliding window approach and a k-means clustering, 6 reoccurring states with different ALFF patterns were identified in time. We found that both normal people and patients had reoccurring ALFF patterns during the resting-state. These reoccurring ALFF patterns were denoted as “dALFF states” in a conceptual analogy to the microstate of EEG. EEG microstates describe the short periods during which scalp topography remain relative stable (Lehmann 1990). We speculate that fMRI dALFF states and EEG microstates might capture similar physiological phenomena, although they persist for significantly different time-scale. Indeed, a previous study demonstrated that the EEG microstates time series showed dependencies over long time ranges and reveal scale-free, self-similar dynamics (Van de Ville et al., 2010). It is also widely reported that the fluctuations of EEG power are correlated with BOLD signal activity during the resting-state (Knyazev et al., 2011; Mantini et al., 2007; Wu et al., 2010). A recent simultaneous EEG and fMRI study found that a dynamic state with strong thalamocortical anticorrelations is associated with reduced EEG spectral alpha power and increased delta and theta power (Allen et al., 2017). In our study, FC patterns were found to be significantly different among dALFF states. Previous research suggested that changes of fMRI FC might reflect changes in neuronal synchrony, which may be driven largely by shifts in cognitive or vigilance states (Allen et al., 2013; Hutchison et al., 2013; Marusak et al., 2017; Shirer et al., 2012; Thompson et al., 2013). Thus it is possible that the dALFF states with different FC patterns are associated with the EEG microstates and represent the condition of neural assemblies that support different cognitive processes.
Although both normal people and patients have all these dALFF states during the resting-state, but the state occurrences were significantly different between groups. SZ patients had more occurrences in the states with weaker ALFF and fewer occurrences in the states with stronger ALFF. We also found that the dALFF averaged in time and static ALFF (sALFF) calculated using the whole scan are highly correlated (r > 0.9, supplementary materials Figure S2), which means that the dALFF could be a representation of sALFF in high temporal resolution. Previous studies focusing on the frequency power of resting-state BOLD signals showed that, SZ patients have smaller power of low-frequency fluctuations and larger power of high frequency fluctuations during the resting-state (Calhoun et al., 2008; Garrity et al., 2007). The underlying mechanism of such phenomenon is still unknown. Previous studies focusing on the brain activity in SZ usually assumed that brain activity patterns are constant in time and they speculated that such overall hypo-activity in SZ might be caused by the consistently lower low-frequency fluctuations in patients. However, our results provided evidence that the overall decreased low-frequency fluctuations in SZ are not caused by the consistently decreased low-frequency fluctuations during the entire scan, but due to that patients would spend more time in low ALFF states and less time in high ALFF states. Patients with SZ could also have high ALFF states as the HC did. More occurrences in states with smaller low-frequency power (weak dALFF states) and fewer occurrences in states with larger low-frequency power (strong dALFF states) would result in decreased averaged low-frequency power in SZ.
Significantly group difference in FC was identified in several dALFF states, which is consistent with the results in a previous dFC study (Damaraju et al., 2014). Our results showed that the FC was not only differernt between groups in each dALFF states, but also was significantly different among dALFF states. Considering the group difference in the occurrences of states, this finding might provide potential explanation of the observed dysconnectivity in SZ (Welsh et al., 2010). Hyperconnectivity between thalamus and sensory networks and hypoconnectivity within sensory networks have been widely reported in SZ (Damaraju et al., 2014; Woodward et al., 2012). In our study, we found that in state 2 and state 4, FC between thalamus and sensory networks is larger and FC within sensory networks is smaller, than FC in other states. SZ patients have more occurrences in these states might result in hyperconnectivity between thalamus and sensory networks and hypoconnectivity within sensory networks on average. These findings might also provide evidence of links between aberrant brain activity and dysfunction of brain network in SZ. The dysfunctional subcortical and sensory networks in SZ have been characterized in many studies (Braff 1993; Braff et al., 1990; Welsh et al., 2010) and it is suggested that such dysfunction might be caused by the hypoactivity of certain brain regions (Friston 2002; Pinault 2011).
4.2 Aberrant associations between dALFF and dFC in schizophrenia
It could be observed couplings between changes in ALFF and changes in FC among dALFF states. Moreover, significant associations between dALFF of ICN and dFC between ICNs were identified in both HCs and SZ patients. These findings suggest that resting-state brain functional network might be an adaptive network (Gross et al., 2008; Gross et al., 2009), in which the changes of brain activity are linked to the changes of brain connectivity. These results might also be supported by a previous study showing that the variation of the topology depends on the variations of the local regions in neural networks (Hopfield et al., 1983). An interesting observation in dALFF-dFC correlation is that dALFF of one ICN is not only correlated with its dFC to other ICNs, but also correlated with dFC between other ICNs. For example, thalamus’ dALFF is positively correlated with the dFC between regions within sensory networks. We speculate that activity of one brain region might influence or be influenced by other brain regions’ connectivity through its pathway to those regions so that the whole brain network could organize as a compact and efficient network.
We further found that such dALFF-dFC correlations are altered in SZ and most of the aberrant dALFF-dFC correlations are related to several ICNs, such as thalamus, caudate nucleus, and cuneus. These ICNs are mainly located in the subcortical and sensory networks, which are networks identified to be with both aberrant brain activity and FC in SZ. Most of the dALFF-dFC correlations with group difference are caused by the loss of dALFF-dFC correlations in SZ group. These findings might imply a disruption of the adaptive network property in brain with this disorder. By using graph theory based analysis, previous studies also identified consistently disrupted brain network properties or altered dynamics of brain network properties in patients with SZ (Bassett et al., 2012; Yu et al., 2015; Yu et al., 2011). A dALFF-dFC correlation between dALFF of cuneus and cuneus dFC to lingual gyrus was further found to be significantly negatively correlated with the score of working memory. The higher association is corresponding to the worse performance, and such phenomenon would be stronger in SZ group. The brain activity in cuneus and lingual gyrus, and their related brain connectivity have been shown to be associated with working memory performance (Hopfinger et al., 2000; Meda et al., 2009; Salmon et al., 1996). Our results suggest that the association between dALFF of cuneus and cuneus dFC to lingual gyrus might be also related to working memory process and such relationship might be altered in SZ.
4.3 Advantages of analysis of dALFF and its association with dFC
Studying dFC is becoming an important research topic for understanding novel correlates of mental processes and cognitive performance (Allen et al., 2014; Di et al., 2015; Hutchison et al., 2013). In our study, instead of focusing on the time-varying patterns of FC, we investigated the time-varying patterns of local brain activity directly, which is the source data used for FC estimation. By analyzing the dALFF, we could also observe highly reoccurring states in time. More importantly, our results on state specific FC showed similar group difference observed in previous dynamic FC studies, which suggest that the dynamic brain functional network could also be captured by using our dynamic brain activity estimation framework.
Given that both ALFF and FC fluctuate significantly and have highly reoccurring patterns during the resting-state, one could argue that there might be some relationships between the ALFF and FC in time. Investigating the associations between dALFF and dFC might improve our understanding of how brain dynamics exist and how they co-evolve in time. Also, the exploration of the group difference in such dALFF-dFC correlation might help answer why aberrant patterns are usually identified in both brain activity and FC in SZ. Our results suggest that the dALFF of local brain region might co-evolve with dFC between brain regions and such co-evolutionary patterns might be altered in SZ. The underlying mechanism of such abnormalities, whether and how they are related to the disrupted brain function in SZ still need to be explored.
4.4 Limitations and future directions
In this study, we used a sliding window approach with the window size as 20 TRs for the estimation of dALFF and dFC. The window size of 40 s was selected as a reasonable choice for estimating FC dynamics, because previous studies, including our own, have shown that changes of FC are not particularly sensitive to a window size in the range of 20s to 1 min (Allen et al., 2014; Chang et al., 2010; Li et al., 2014). However, since our current work is also focusing on dALFF, it is not clear whether such window size would be optimal for capturing the dynamics of brain activity. We have tested the reliability of the analysis using different window sizes. The dALFF and dFC were estimated with the window size from 16 TRs to 24 TRs. Consistent with previous studies on dFC, shorter window results in higher variability in dynamic patterns while larger window over smoothes the estimates. We also repeated the association analysis on dALFF and dFC obtained by sliding window approaches with 16 TRs and 24 TRs, and the overall results are similar. Therefore, we assume that 20 TRs window size could provide a good trade-off between the ability to resolve brain dynamics. Previous studies also raised the question that typical resting-state acquisition parameters might not be optimized for the exploration of dynamic patterns of FC and activity (Hutchison et al., 2013). It is still an open question how the scan length influences the dynamic analysis of connectivity and activities. Most of the dynamic studies still used the dataset with typical acquisition parameters and identified valuable dynamic patterns in both FC and brain activity. For example, Liu and Duyn used a dataset from 1000 Functional Connectomes Project with only 123 time points and successfully identified highly re-occurring co-activation patterns of brain activity (Liu et al., 2013). Rashid and her colleagues applied dFC clustering analysis on a dataset with the scan length of 5 min 15 s and found valuable biomarkers in dFC for the classification of SZ and bipolar patients (Rashid et al., 2016). Our current used dataset has already been used in a previous dFC study and atypical dFC patterns in SZ have been identified (Damaraju et al., 2014). Although we believe that current fMRI dataset could provide sufficient information for performing our dynamic analysis, it should be noted that a longer scan with higher temporal resolution would be better for studying the dynamic patterns in both FC and brain activity.
Previous neuroimaging research identified reoccurring FC patterns by using a sliding window estimation approach and a k-mean clustering approach (Allen et al., 2014; Damaraju et al., 2014; Marusak et al., 2017). In our study, we also identified reoccurring patterns of brain activity. Although we could observe that FC of dALFF states has similar patterns with clustered FC states, the relationships between clustered dALFF states and clustered dFC states have not been explored. Whether and how the reoccurring FC states and reoccurring ALFF states are related to each other should be considered and investigated in future, so as to advance the understanding of associations between brain activity and brain connectivity.
Significant correlations between dALFF and dFC were found in this study, which imply the links between local BOLD activity and their FC. Although mounting evidence showed relationships between local activity and FC dynamics (Musso et al., 2010; Van de Ville et al., 2010), it is still unclear whether such links are of specific properties of neural network (Gross et al., 2008), or just merely inherent relationships between signals themselves and their connectivity (Hutchison et al., 2013). In addition, it is important to explore whether the observed aberrant associations between dALFF and dFC are due to the medication. For example, we could focus on the first-episode SZ patients in the future. Furthermore, since such associations were evaluated by correlation coefficient, a mathematic metric without directions, it is hard to determine whether aberrant brain activity causes aberrant FC or aberrant FC causes aberrant brain activity. The causal relationships between dALFF and dFC could be investigated in our future work.
Supplementary Material
Figure 5.
Diagonal: FC of dALFF states for all subjects. The above numbers show the count of subjects that have at least 10 time window in each state. Off-diagonal: FC difference among dALFF states. Significant FC difference is corrected by FDR (p < 0.05) for multiple comparison.
Acknowledgments
This work is supported by the National Institutes of Health (NIH) grants (R01EB006841, R01REB020407 and P20GM103472 PI: Calhoun; R37 MH43775 PI: Pearlson) and National Science Foundation (NSF) grant 1539067.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Allen E, Eichele T, Wu L, Calhoun V. EEG signatures of functional connectivity states. Hum Brain Mapp. 2013 doi: 10.1007/s10548-017-0546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E, Damaraju E, Eichele T, Wu L, Calhoun V. EEG Signatures of Dynamic Functional Network Connectivity States. Brain Topogr. 2017:1–16. doi: 10.1007/s10548-017-0546-2. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Havlicek M, Rachakonda S, Fries J, Kalyanam R. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex. 2014;24:663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Nelson BG, Mueller BA, Camchong J, Lim KO. Altered resting state complexity in schizophrenia. NeuroImage. 2012;59:2196–2207. doi: 10.1016/j.neuroimage.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn Reson Med. 1995;34:537–541. doi: 10.3410/f.714597885.790202808. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia: human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL. Information processing and attention dysfunctions in schizophrenia. Schizophr Bull. 1993;19:233–259. doi: 10.1093/schbul/19.2.233. [DOI] [PubMed] [Google Scholar]
- Britz J, Landis T, Michel CM. Right parietal brain activity precedes perceptual alternation of bistable stimuli. Cereb Cortex. 2009;19:55–65. doi: 10.1093/cercor/bhn056. [DOI] [PubMed] [Google Scholar]
- Brüne M. Emotion recognition, ‘theory of mind,’ and social behavior in schizophrenia. Psychiatry Res. 2005;133:135–147. doi: 10.1016/j.psychres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar J. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008;29:828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA. Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum Brain Mapp. 2008;29:1265–1275. doi: 10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Eichele T, Pearlson G. Functional brain networks in schizophrenia: a review. Front Hum Neurosci. 2009;3:17. doi: 10.3389/neuro.09.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, Adalı T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. NeuroImage. 2009;45:S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T. Multisubject independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery. IEEE Rev Biomed Eng. 2012;5:60–73. doi: 10.1109/RBME.2012.2211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Miller R, Pearlson G, Adalı T. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron. 2014;84:262–274. doi: 10.1016/j.neuron.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. Time–frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage. 2010;50:81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull. 1994;20:31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- Damaraju E, Allen E, Belger A, Ford J, McEwen S, Mathalon D, Mueller B, Pearlson G, Potkin S, Preda A. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin. 2014;5:298–308. doi: 10.1016/j.nicl.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lacy N, Doherty D, King B, Rachakonda S, Calhoun V. Disruption to control network function correlates with altered dynamic connectivity in the wider autism spectrum. Neuroimage Clin. 2017;15:513–524. doi: 10.1016/j.nicl.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Di X, Fu Z, Chan SC, Hung YS, Biswal BB, Zhang Z. Task-related functional connectivity dynamics in a block-designed visual experiment. Front Hum Neurosci. 2015;9:543. doi: 10.3389/fnhum.2015.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin Psychol Rev. 2002;22:789–832. doi: 10.1016/S0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. 2011;32:2075–2095. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Dysfunctional connectivity in schizophrenia. World Psychiatry. 2002;1:66–71. doi: [PMC free article] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Gross T, Blasius B. Adaptive coevolutionary networks: a review. J R Soc Interface. 2008;5:259–271. doi: 10.1098/rsif.2007.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross T, Sayama H. Adaptive networks. Adaptive Networks, Springer; 2009. pp. 1–8. [Google Scholar]
- Hopfield JJ, Feinstein DI, Palmer RG. Unlearning Has a Stabilizing Effect in Collective Memories. Nature. 1983;304:158–159. doi: 10.1038/304158a0. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Zuo X-N, Butler PD, Javitt DC, D'Angelo D, Mauro CJ, Milham MP. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr Res. 2010;117:13–20. doi: 10.1016/j.schres.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn JH, Glover GH, Gonzalez-Castillo J. Dynamic functional connectivity: promise, issues, and interpretations. NeuroImage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum Brain Mapp. 2013;34:2154–2177. doi: 10.1002/hbm.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Morton JB. Tracking the brain's functional coupling dynamics over development. J Neurosci. 2015;35:6849–6859. doi: 10.1523/JNEUROSCI.4638-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Morton JB. It's a matter of time: reframing the development of cognitive control as a modification of the brain's temporal dynamics. Dev Cogn Neurosci. 2016;18:70–77. doi: 10.1016/j.dcn.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Flszbein A, Opfer LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Jauhiainen J, Tervonen O, Pääkkö E, Oikarinen J, Vainionpää V, Rantala H, Biswal B. Slow vasomotor fluctuation in fMRI of anesthetized child brain. Magn Reson Med. 2000;44:373–378. doi: 10.1002/1522-2594. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Starck T, Remes J, Long X, Nikkinen J, Haapea M, Veijola J, Moilanen I, Isohanni M, Zang YF. Functional segmentation of the brain cortex using high model order group PICA. Hum Brain Mapp. 2009;30:3865–3886. doi: 10.1002/hbm.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyazev GG, Slobodskoj-Plusnin JY, Bocharov AV, Pylkova LV. The default mode network and EEG alpha oscillations: an independent component analysis. Brain research. 2011;1402:67–79. doi: 10.1016/j.brainres.2011.05.052. [DOI] [PubMed] [Google Scholar]
- Kühn S, Gallinat J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr Bull. 2013;39:358–365. doi: 10.1093/schbul/sbr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann D. Past, present and future of topographic mapping. Brain Topogr. 1990;3:191–202. doi: 10.1007/BF01128876. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Strik W, Henggeler B, König T, Koukkou M. Brain electric microstates and momentary conscious mind states as building blocks of spontaneous thinking: I. Visual imagery and abstract thoughts. Int J Psychophysiol. 1998;29:1–11. doi: 10.1016/S0167-8760(97)00098-6. [DOI] [PubMed] [Google Scholar]
- Li X, Zhu D, Jiang X, Jin C, Zhang X, Guo L, Zhang J, Hu X, Li L, Liu T. Dynamic functional connectomics signatures for characterization and differentiation of PTSD patients. Hum Brain Mapp. 2014;35:1761–1778. doi: 10.1002/hbm.22290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Duyn JH. Time-varying functional network information extracted from brief instances of spontaneous brain activity. Proc. Natl. Acad. Sci. U.S.A. 2013;110:4392–4397. doi: 10.1073/pnas.1216856110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Calhoun VD, Phlypo R, Adalı T. Dynamic changes of spatial functional network connectivity in healthy individuals and schizophrenia patients using independent vector analysis. NeuroImage. 2014;90:196–206. doi: 10.1016/j.neuroimage.2013.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Harkavy-Friedman J, Corcoran C, Mujica-Parodi L, Printz D, Gorman JM, Van Heertum R. Resting neural activity distinguishes subgroups of schizophrenia patients. Biol Psychiatry. 2004;56:931–937. doi: 10.1016/j.biopsych.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. U.S.A. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Calhoun VD, Brown S, Crespo LM, Sala-Hamrick K, Gotlib IH, Thomason ME. Dynamic functional connectivity of neurocognitive networks in children. Hum Brain Mapp. 2017;38:97–108. doi: 10.1002/hbm.23346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Kovacevic N, Itier RJ. Increased brain signal variability accompanies lower behavioral variability in development. PLoS Comput Biol. 2008;4:e1000106. doi: 10.1371/journal.pcbi.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Stevens MC, Folley BS, Calhoun VD, Pearlson GD. Evidence for anomalous network connectivity during working memory encoding in schizophrenia: an ICA based analysis. PLoS One. 2009;4:e7911. doi: 10.1371/journal.pone.0007911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingoia G, Wagner G, Langbein K, Maitra R, Smesny S, Dietzek M, Burmeister HP, Reichenbach JR, Schlösser RG, Gaser C. Default mode network activity in schizophrenia studied at resting state using probabilistic ICA. Schizophr Res. 2012;138:143–149. doi: 10.1016/j.schres.2012.01.036. [DOI] [PubMed] [Google Scholar]
- Musso F, Brinkmeyer J, Mobascher A, Warbrick T, Winterer G. Spontaneous brain activity and EEG microstates. A novel EEG/fMRI analysis approach to explore resting-state networks. NeuroImage. 2010;52:1149–1161. doi: 10.1016/j.neuroimage.2010.01.093. [DOI] [PubMed] [Google Scholar]
- Pearlson G, Calhoun VD. Convergent approaches for defining functional imaging endophenotypes in schizophrenia. Front Hum Neurosci. 2009;3:37. doi: 10.3389/neuro.09.037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D. Dysfunctional thalamus-related networks in schizophrenia. Schizophr Bull. 2011;37:238–243. doi: 10.1093/schbul/sbq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Rashid B, Damaraju E, Pearlson GD, Calhoun VD. Dynamic connectivity states estimated from resting fMRI Identify differences among Schizophrenia, bipolar disorder, and healthy control subjects. Front Hum Neurosci. 2014;8:897. doi: 10.3389/fnhum.2014.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid B, Arbabshirani MR, Damaraju E, Cetin MS, Miller R, Pearlson GD, Calhoun VD. Classification of schizophrenia and bipolar patients using static and dynamic resting-state fMRI brain connectivity. NeuroImage. 2016;134:645–657. doi: 10.1016/j.neuroimage.2016.04.051. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoğlu Ü, Pearlson GD, Kiehl KA, Wang YM, Michael AM, Calhoun VD. A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia. MAGN RESON MATER PHY. 2010;23:351–366. doi: 10.1007/s10334-010-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon E, Van der Linden M, Collette F, Delfiore G, Maquet P, Degueldre C, Luxen A, Franck G. Regional brain activity during working memory tasks. Brain. 1996;119:1617–1625. doi: 10.1093/brain/119.5.1617. doi: 10.1.1.917.8794. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P. Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48:618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Shirer W, Ryali S, Rykhlevskaia E, Menon V, Greicius M. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Magnuson ME, Merritt MD, Schwarb H, Pan WJ, McKinley A, Tripp LD, Schumacher EH, Keilholz SD. Short-time windows of correlation between large-scale functional brain networks predict vigilance intraindividually and interindividually. Hum Brain Mapp. 2013;34:3280–3298. doi: 10.1002/hbm.22140. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JA, Damaraju E, Van Erp TG, Mathalon DH, Ford JM, Voyvodic J, Mueller BA, Belger A, Bustillo J, McEwen SC. A multi-site resting state fMRI study on the amplitude of low frequency fluctuations in schizophrenia. Front Neurosci. 2013;7:137. doi: 10.3389/fnins.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Ville D, Britz J, Michel CM. EEG microstate sequences in healthy humans at rest reveal scale-free dynamics. Proc. Natl. Acad. Sci. U.S.A. 2010;107:18179–18184. doi: 10.1073/pnas.1007841107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackermann J, Lehmann D, Michel CM, Strik WK. Adaptive Segmentation of Spontaneous Eeg Map Series into Spatially Defined Microstates. Int J Psychophysiol. 1993;14:269–283. doi: 10.1016/0167-8760(93)90041-M. [DOI] [PubMed] [Google Scholar]
- Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36:713–722. doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–1099. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Eichele T, Calhoun VD. Reactivity of hemodynamic responses and functional connectivity to different states of alpha synchrony: a concurrent EEG-fMRI study. NeuroImage. 2010;52:1252–1260. doi: 10.1016/j.neuroimage.2010.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Sui J, Rachakonda S, He H, Pearlson G, Calhoun VD. Altered small-world brain networks in temporal lobe in patients with schizophrenia performing an auditory oddball task. Front Syst Neurosci. 2011;5:7. doi: 10.3389/fnsys.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Erhardt EB, Sui J, Du Y, He H, Hjelm D, Cetin MS, Rachakonda S, Miller RL, Pearlson G. Assessing dynamic brain graphs of time-varying connectivity in fMRI data: application to healthy controls and patients with schizophrenia. NeuroImage. 2015;107:345–355. doi: 10.1016/j.neuroimage.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Zou Q-H, Zhu C-Z, Yang Y, Zuo X-N, Long X-Y, Cao Q-J, Wang Y-F, Zang Y-F. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J. Neurosci. Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.